Abstract
Atrial fibrillation (AF) is the most common cardiac arrhythmia encountered in clinical practice, which is associated with substantial risk of stroke and thromboembolism. As an arrhythmia that is particularly common in the elderly, it is an important contributor towards morbidity and mortality. Ventricular rate control has been a preferred and therapeutically convenient treatment strategy for the management of AF. Recent research in the field of rhythm control has led to the advent of newer antiarrhythmic drugs and catheter ablation techniques as newer therapeutic options. Currently available antiarrhythmic drugs still remain limited by their suboptimal efficacy and significant adverse effects. Catheter ablation as a newer modality to achieve sinus rhythm (SR) continues to evolve, but data on long-term outcomes on its efficacy and mortality outcomes are not yet available. Despite these current developments, rate control continues to be the front-line treatment strategy, especially in older and minimally symptomatic patients who might not tolerate the antiarrhythmic drug treatment. This review article discusses the current evidence and recommendations for ventricular rate control in the management of AF. We also highlight the considerations for rhythm control strategy in the management of patients of AF.
Keywords: Atrial fibrillation, rate control, beta-blockers
Atrial fibrillation (AF) is the most common cardiac arrhythmia encountered in clinical practice.1 It has been estimated that >3 million people in the US and >4.5 million in the EU have paroxysmal or persistent AF.2–4 AF is associated with an approximately fivefold increased risk of stroke,5 threefold risk of heart failure,6 diminished quality of life7 and increased healthcare costs.8,9 Ventricular rate control strategy has been a traditional front-line and well-tolerated therapeutic option for the management of patients with AF. Although seemingly preferable in terms of potential advantages related to improved cardiac function and avoidance of unfavourable atrial electrical and mechanical remodelling, the currently available rhythm control options have important limitations. Antiarrhythmic drugs are considered first-line agents for rhythm control and the currently available drugs have limited efficacy in terms of achievement and maintenance of sinus rhythm (SR). Even in a clinical trial with particularly favourable results associated with the use of antiarrhythmic drugs, AF recurred in about 35 % of the patients in the amiodarone arm; the rates of recurrence of AF were even higher with the use of sotalol and propafenaone (63 %).10 Recent data from the Permanent Atrial fibriLLAtion Outcome Study Using Dronedarone on Top of Standard Therapy (PALLAS) trial and other studies on the utility of dronedarone as a newer antiarrhythmic agent also suggest its limited efficacy and significant potential for adverse events.11–13
Catheter ablation, although generally superior to antiarrhythmic drug therapy in achieving SR, still remains limited by non-trivial recurrence rates for AF as well as the potential for procedural complications and the lack of currently available data on very long-term efficacy and mortality.14–18 In the meantime, it continues to be an area of intensive research in terms of its role as an evolving therapeutic option to achieve sinus rhythm.
In this review article we aim to discuss recommendations for ventricular rate control strategy in the management of patients with AF based on currently available data and the relevance of a rhythm control strategy with the advent of recent treatment advances in the field of antiarrhythmic drug therapy and catheter ablation.
Rate Control
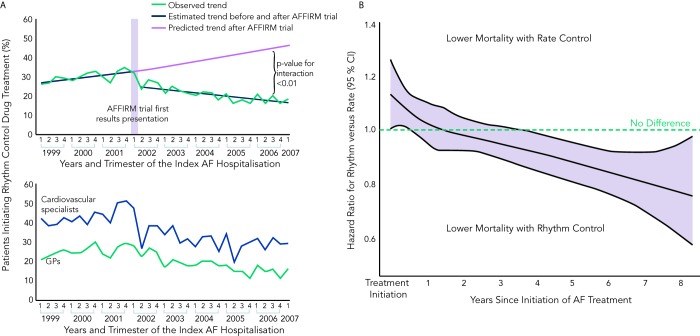
Ventricular rate control has been an area of continuous investigation over the years. Until the results of Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) trial were published, rhythm control was widely believed to be a superior therapy with the rationale of SR being associated with better exercise tolerance, superior symptom control and possible decrease in morbidity (see Figure 1).19 Despite these potential benefits of rhythm control, rate control was found to be non-inferior to rhythm control in the AFFIRM trial, with a trend toward reduced mortality with rate control. Results from the Rate Control Versus Electric Cardioversion for Persistent Atrial Fibrillation (RACE) study also confirmed that a rate control strategy is as effective as rhythm control in management of AF and offers potential advantages such as a lower risk of adverse drug effects, cost-effectiveness and decreased incidence of hospitalisations.20,21 Various meta-analyses have shown that a rate control strategy is at least as effective as rhythm control in patients with AF when comparing endpoints such as cardiovascular and all-cause mortality.22–25 It should be noted, however, that the majority of patients in these trials were elderly patients without highly symptomatic AF. A recently published meta-analysis of multiple randomised clinical trials comparing rate versus rhythm control strategies concluded that the incidence of stroke, systemic embolism, heart failure and myocardial infarction were similar between the two groups. The rhythm control strategy was found to be associated with a generally higher incidence of hospitalisations as compared with rate control. This could be potentially attributed to several factors such as necessity for achieving rhythm control by cardioversion in a monitored setting, adverse effects and arrhythmias secondary to the use of antiarrhythmic drugs.26 In a prospective cost-efficacy analysis on patients enrolled in the How to Treat Chronic Atrial Fibrillation (HOT CAFÉ) trial, a rhythm control strategy was found to be associated with increased overall healthcare costs and resource utilisation. This was attributed predominantly to the hospitalisations related to cardioversion, which generated not only direct procedure-related costs but also consultation costs.27,28 The trend of increased hospitalisations with a rhythm control strategy was also seen in other major trials (AFFIRM, RACE and Pharmacological Intervention in Atrial Fibrillation [PIAF]).19,20,29 Following the publication of these favourable results for rate control over rhythm control, there was a measurable increase in the prescription of rate control medications and decline in the use of rhythm control therapies for the management of AF (see Figure 2A).29–33
Figure 1: Cumulative Mortality from Any Cause in the Rhythm Control and the Rate Control Group in the AFFIRM Trial.
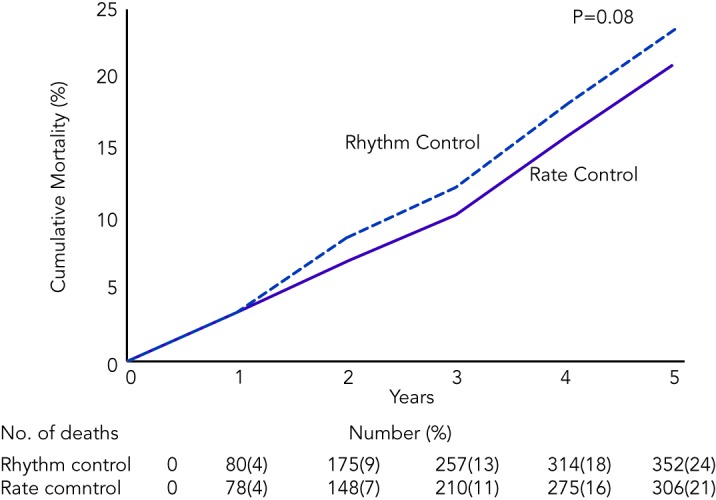
Reproduced with permission from Wyse, et al., 2002.19
Figure 2: A) Time Trends in the Prescription Patterns of Rhythm Control Medications After the Publication of the AFFIRM Trial in a Large Registry of Canadian Patients B) Effect of Rate Control and Rhythm Control Strategies on All-cause Mortality in this Group of Patients.
AF = atrial fibrillation; AFFIRM = Atrial Fibrillation Follow up-Investigation of Rhythm Management; CI = confidence interval. B) Reproduced with permission from Ionscu-Ittu, et al., 2012.33
Atrioventricular (AV) node ablation followed by permanent pacemaker implantation remains a viable treatment strategy for AF associated with difficult to control rapid ventricular rates. It was investigated by Brignole et al. in two randomised clinical studies involving patients with severely symptomatic paroxysmal AF, and patients with chronic AF and heart failure, respectively.34,35 This strategy principally relies on the fact that AV node ablation is very effective in controlling the ventricular response to AF, especially in patients who do not respond well to the pharmacological rate control strategy. AV node ablation has been demonstrated to be highly effective in controlling AF symptoms, improving quality of life and general wellbeing.36–39 The use of this strategy is further supported by its impact on decreasing healthcare costs, which are accounted for by a significant decrease in hospitalisations, outpatient visits and antiarrhythmic drug use.40
Despite these favourable results of AV nodal ablation and pacemaker implantation in patients with refractory AF, it is important to be cognisant of its limitations. In patients with paroxysmal AF who exhibit variable periods of SR, a single chamber (ventricular) permanent pacing system may lead to worsening of functional status due to the loss of AV synchrony and abnormal ventricular activation. In such patients who may have self-limiting episodes of AF, a dual-chamber, rate-modulated (DDDR) pacemaker with an autonomic mode switch capability would be more useful so that in the absence of AF, AV synchrony is maintained.41,42 The clinical studies by Brignole et al. also concluded that patients with chronic AF and co-existing heart failure might benefit from Ventricular Rate-modulated Pacing (VVIR) mode of pacing.34,35 Concerns raised by the results of the Dual chamber and VVI Implantable Defibrillator (DAVID) study regarding the role of right ventricular pacing as a causative factor in increasing the risk of heart failure admissions and the superior outcomes of biventricular pacing demonstrated by the left ventricular-based cardiac stimulation Post AV nodal ablation Evaluation (PAVE) study favours the use of biventricular pacemaker in patients with impaired left ventricular function, which is not attributed to tachycardia who undergo AV nodal ablation.43,44 Another limitation of this strategy is the lack of elimination of the thromboembolic risk because AF is still maintained in such patients. Finally, AV nodal ablation typically leaves patients pacemaker-dependent, and failure of the pacing system in these patients, although rare, can be potentially catastrophic.
Rhythm Control
Despite the therapeutic convenience and potentially lower adverse events associated with the rate control strategy, there are several arguments in favour of a rhythm control strategy. Although none of the major randomised controlled trials (RCTs) comparing rate control versus rhythm control demonstrated a net benefit of rhythm control in patients with AF, a recent, large population-based registry of 26,130 Canadian patients suggested superiority in overall mortality with the use of rhythm control strategy after several years of follow-up (see Figure 2B).33 In another recently published observational study, rhythm control was found to be associated with lower rates of stroke and transient ischaemic attack (TIA) in patients with AF who were at moderate and high-risk of stroke.45 Whether these registry studies have identified real and important advantages of medical rhythm control versus rate control, which were not found in the RCTs, or whether the registries were confounded by the inherent limitations of non-randomised study design, remains unknown. A meta-analysis of various RCTs comparing rate control with rhythm control demonstrated that rhythm control was superior to rate control for prevention of all-cause mortality in patients younger than 65 years.26 This difference in the results of RCTs and observational studies could be potentially explained by multiple factors, including underrepresentation of younger patients in the RCTs, and unidentified differences in co-morbidities in patients in the population-based observational studies. The follow-up period of patients in the observational studies was comparatively longer than the RCTs, which could be yet another contributing factor to the discrepancies in these results.
In the Atrial Fibrillation and Congestive Heart Failure (AF-CHF) trial, which was designed to investigate the effect of underlying rhythm on the quality of life and functional capacity in patients with AF and congestive heart failure, a higher proportion of time spent in SR was associated with gains in functional status and improved quality of life, although the rhythm control strategy was not found to be superior to rate control in this study.46 To further support this concept, a post hoc analysis of the AFFIRM trial demonstrated that the presence of SR (as opposed to a rhythm control strategy) was associated with a lower risk of death, while the use of rhythm control drugs was associated with worsened mortality, and these two effects appeared to counterbalance, resulting in roughly similar mortality for rate versus rhythm control.47 Similar results were also observed in the Danish Investigations of Arrhythmia and Mortality on Dofetilide (DIAMOND) trial, which showed that patients who had SR either with or without antiarrhythmic drug therapy had a better prognosis compared with patients who continued to have AF.48
The results of RCTs comparing rate control with rhythm control should therefore not be interpreted as demonstrating a lack of benefit of sinus rhythm, but rather that the currently available antiarrhythmic medications are limited in their efficacy and have adverse effects, which make their routine use as first-line therapy a less favourable therapeutic option for many AF patients, particularly those with minimal symptoms when treated with adequate rate control. These data also suggest that younger patients who were not well-represented in the RCTs may derive greater benefit from a rhythm control strategy, likely by prevention of long-term complications of AF. The concept of ‘AF begets AF’ can be kept in mind while making decisions for such patients, and the early period of paroxysmal AF may represent a window of opportunity to establish SR in these patients.49–51 The presence of AF not only predisposes to an increased risk of systemic thromboembolism but also has been demonstrated to be an important risk factor for endothelial dysfunction and inflammation, which can be potentially reversed by restoration of SR.52–55 Although a rate control strategy might be simpler to achieve in comparison to rhythm control, it generally will not prevent the progression of paroxysmal to persistent AF.56,57 This could be attributed to a potentially beneficial role of rhythm control to reverse the electrical and structural remodelling responsible for initiation and perpetuation of AF.58–60
A risk stratification scheme HATCH score (Hypertension: one point, Age >75 years: one point, TIA or Stroke: two points, Chronic obstructive pulmonary disease: one point, Heart failure: two points) has been developed to predict progression of paroxysmal to persistent AF. This score may be used for an early selection of patients for a rhythm control strategy in an effort to prevent disease progression. If a high score is found, then clinicians should recognise that long-term sinus rhythm maintenance will be more difficult to achieve, and rate control may be more appropriate.61 Development of safer antiarrhythmic drugs with improved efficacy remains an elusive target for the pharmaceutical industry, but would greatly aid in expansion of rhythm control strategy to a diverse patient population. Catheter ablation remains an alternative rhythm control strategy, which substitutes the upfront procedural risks for the long-term risk of antiarrhythmic drugs.
In the recently published ThermoCool-AF trial involving patients with paroxysmal AF, catheter ablation was demonstrated to be clearly superior to antiarrhythmic drugs for the endpoints of overall rhythm control, freedom from symptoms and overall quality of life.62 In regard to hard endpoints such as mortality and stroke, the ongoing multicentre Catheter Ablation versus Antiarrhythmic drug therapy for Atrial fibrillation (CABANA) trial will provide crucial data on the efficacy and safety of catheter ablation for achieving rhythm control and further aid in the treatment decision of rate versus rhythm control.63,64
Targets for Rate Control
Ventricular rate control in patients with AF has dual advantages – reduction in symptoms and improvement in cardiac efficiency.
Uncontrolled ventricular rates have multiple adverse effects such as worsening of symptoms, decrease in stroke volume and development of tachycardia-induced cardiomyopathy and congestive heart failure.65,66 In the AFFIRM trial, adequate rate control was defined as an average heart rate ≤80 beats per minute (bpm) at rest and a maximum heart rate ≤110 bpm during either a six-minute walk or moderate exercise,19 whereas the standard for rate control in the RACE trial was more lenient, requiring only a resting heart rate <100 bpm. Analyses of the RACE and the AFFIRM trials suggested that the subgroup of patients with resting heart rates above 100 bpm had worse outcomes compared with patients with slower rates, but it was not clear whether this was due to the impact of better rate control, or reflected co-morbidities resulting in both higher heart rates and worse outcomes. In the subsequent randomised Rate control Efficacy in Permanent Atrial Fibrillation (RACE II) trial; a lenient rate control strategy (resting heart rate <110 bpm) was demonstrated to be as effective as strict rate control (resting heart rate <80 bpm and heart rate during moderate exercise <110 bpm) for the composite outcomes of cardiovascular mortality, hospitalisations for heart failure, stroke, systemic embolism, bleeding and life-threatening arrhythmia.67 In a retrospective analysis of four studies involving AF and heart failure patients with cardiac resynchronisation therapy defibrillator devices, a poorly controlled heart rate (rate >90 bpm) was found to be strongly associated with an increased risk of hospitalisation and adverse events (hazard ratio [HR]: 5.9, p<0.001).68
In a recently published analysis of the RACE II trial, three subgroups of rate control (‘successful’ strict rate: average 72 ± 7 bpm, ‘failed’ strict rate: average 86 ± 14 bpm and lenient rate: average 93 ± 8 bpm) were compared for the composite primary endpoints of cardiovascular morbidity and mortality, and no difference was observed in the three groups, and quality of life was also found to be comparable between groups.69 A strict rate control therapy could also prove to be time-consuming, necessitating more outpatient visits and more combinations and higher dosages of rate control drugs (see Figure 3).19,20,69,70
Figure 3: Impact of Intensity of Rate Control on Cardiovascular Morbidity and Mortality in an Analysis of the RACE II Trial.
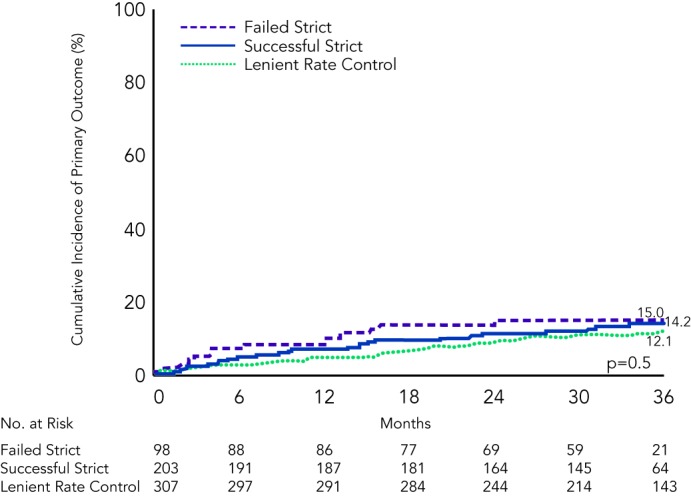
‘Successful strict rate control’: average 72 + 7 bpm, ‘failed strict rate control’: average 86 + 14 bpm and ‘lenient rate control’: average 93 + 8 bpm. Reproduced with permission from Groenveld, et al., 2013.69
The interpretation of data from RCTs should, however, be individualised to patients in clinical practice; optimal heart rate control may vary on the basis of symptom burden and presence of co-morbidities, and patients with heart failure may represent a special group for whom strict rate control may be particularly beneficial. Additionally, it is possible that the impact of the adequacy of rate control may take many years to manifest and may not have been fully captured in trials such as RACE II, which followed patients for up to three years. If a patient remains symptomatic despite a lenient rate control or if there is a higher predisposition to develop cardiomyopathy, then lower heart rate targets may be indicated.
Methods of Achieving Rate Control in Patients with Atrial Fibrillation
In the AFFIRM trial, beta (β)-blockers (with or without digoxin) were found to have an overall success rate of approximately 70 % for achieving rate control when used either alone or in combination with digoxin, which was superior to the 54 % success rate of calcium channel antagonists (with or without digoxin).19,70 Recently published results from the RATe control in Atrial Fibrillation (RATAF) study contradicted the findings of the AFFIRM trial in regard to the comparative efficacy of β-blockers versus calcium blockers for rate control. RATAF trial was a prospective cross-over study comparing four drug regimens (diltiazem 360 milligrams (mg)/day, verapamil 240 mg/day, metoprolol 100 mg/day and carvedilol 25 mg/day) to reduce ventricular rate in patients with permanent AF. RATAF showed that calcium blockers (particularly diltiazem at 360 mg/day) were superior to the other drugs tested for achieving 24-hour heart rate control and also fared better in terms of control of symptom frequency and severity.71 Current guidelines recommend use of either a β-blocker or non-dihydropyridine calcium channel antagonist as a first-line agent for rate control in patients with AF.2 For many patients, either class of drugs will be acceptable, although for patients with certain co-morbidities, one drug class will clearly be preferred over the other (β-blockers preferred for heart failure patients, for example, and calcium blockers preferred for patients with bronchospasm).
Digoxin as a single agent has not been found to be as effective as β-blockers or calcium blockers in controlling ventricular rates in patients with AF; its efficacy is further reduced in the states of high sympathetic tone, which could be a precipitating factor in certain forms of paroxysmal AF or during exercise. The use of digoxin as a single agent should be reserved for sedentary patients or those with left ventricular systolic dysfunction who can be adequately rate controlled with digoxin alone (although systolic dysfunction may indicate potential benefit from β-blockade).2,72–74 Concern about digoxin use for rate control has been increased recently by newly published data from the AFFIRM trial, which revealed a significant increase in all-cause mortality with the use of digoxin. In a subgroup analysis of patients without congestive heart failure (CHF), digoxin was found to be associated with a 37 % increase in mortality. It should be noted, however, that this was not based on randomisation to digoxin or placebo, and there could be unidentified differences in the patients who received digoxin in the AFFIRM trial contributing to this outcome.75 Digoxin can be considered in combination with another rate control agent in patients with AF and left ventricular systolic dysfunction and patients who fail to achieve adequate rate control with a single agent.2,73,74
Intravenous (IV) amiodarone may be particularly effective as a rate control medication in critically ill patients who develop uncontrolled and haemodynamically compromising high ventricular rates during AF despite the use of conventional agents or patients who are intolerant to the conventional rate controlling agents.76 The rate controlling effects of amiodarone could be potentially attributed to its calcium channel blocking as well to its antiadrenergic properties.77
Ideally the selection of a drug for rate control should be made on the basis of results of RCTs, patient preference and presence of co-morbidities. It should also be important to bear in mind that these medications are not mutually exclusive; although a significant number of patients respond to monotherapy, a combination of rate control agents can be considered in patients who fail to achieve an adequate rate control with a single agent. Various interactions among these drugs, and the effect of electrolyte imbalance and fluid status of the patient should also be kept in mind while prescribing combinations of these medications, and careful attention should be paid to patients given both β-blockers and calcium blockers, as severe bradycardia can ensue.
Conclusions and Recommendations
Atrial fibrillation remains a common public health problem responsible for increasing morbidity, mortality and healthcare costs. Despite advancements in the field of rhythm control and availability of newer antiarrhythmics, rate control continues to be a preferred and therapeutically convenient option in patients who are older, minimally symptomatic and those who might not tolerate the adverse effects of currently available antiarrhythmic drugs. Catheter ablation is increasingly being used as an effective rhythm control strategy for many patients with drug-refractory AF, but there are not yet definitive data available in regard to hard endpoints such as mortality and stroke, which would allow ablation to be recommended as first-line therapy for the majority of patients with AF. Rhythm control may be preferred in younger patients, those who might benefit from prevention of the progression of AF and patients who remain symptomatic despite an optimal rate control. Management decisions can be guided by the available literature, physician preference and the symptom burden of the patients. Currently available data (see Table 1) supports the use of lenient rate control as a front-line strategy over strict rate control for most patients treated with rate control, although heart failure patients may benefit from more aggressive rate control targets.
Table 1: Comparison of Rate Control versus Rhythm Control as Management Strategies for Atrial Fibrillation.
| Therapeutic Strategy | Advantages | Disadvantages |
|---|---|---|
| Rate control | • Therapeutically convenient • Less exposure to drug toxicity • Preferred in older, minimally symptomatic AF • Optimal rate control adequate to decrease hospitalisation • Cost-effective |
• No effect on disease progression • May not be beneficial in highly symptomatic patients |
| Rhythm control | • Prevents disease progression
• Avoids unfavourable electrical and structural remodelling • Potentially preferable in younger patients • Better quality of life |
• Exposure to adverse effects of antiarrhythmic drugs (or risks of ablation procedures)
• Generally less cost-effective |
AF = atrial fibrillation.
References
- 1.Chugh SS, Blackshear JL, Shen WK et al. Epidemiology and natural history of atrial fibrillation: clinical implications. J Am Coll Cardiol. 2001;37:371–78. doi: 10.1016/s0735-1097(00)01107-4. [DOI] [PubMed] [Google Scholar]
- 2.Fuster V, Rydén LE, Cannom DS et al. 2011 ACCF/AHA/ HRS focused updates incorporated into the ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J Am Coll Cardiol. 2011;57:e101–98. doi: 10.1016/j.jacc.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Go AS, Hylek EM, Phillips KA et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. JAMA. 2001;285:2370–75. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 4.Stewart S, Murphy NF, Walker A et al. Cost of an emerging epidemic: an economic analysis of atrial fibrillation in the UK. Heart. 2004;90:286–92. doi: 10.1136/hrt.2002.008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–88. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 6.Krahn AD, Manfreda J, Tate RB et al. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-Up study. Am J Med. 1995;98:476–84. doi: 10.1016/S0002-9343(99)80348-9. [DOI] [PubMed] [Google Scholar]
- 7.Dorian P, Paquette M, Newman D et al. Quality of life improves with treatment in the Canadian Trial of Atrial Fibrillation. Am Heart J. 2002;143:984–90. doi: 10.1067/mhj.2002.122518. [DOI] [PubMed] [Google Scholar]
- 8.Woodchis WP, Bhatia RS, Leblanc K et al. A review of the cost of atrial fibrillation. Value Health. 2012;15:240–8. doi: 10.1016/j.jval.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Wolowacz SE, Samuel M, Brennan VK et al. The cost of illness of atrial fibrillation: a systemic review of the recent literature. Europace. 2011;13:1375–85. doi: 10.1093/europace/eur194. [DOI] [PubMed] [Google Scholar]
- 10.Roy D, Talajic M, Dorian P et al. Amiodarone to prevent recurrence of atrial fibrillation. Canadian Trial of Atrial Fibrillation Investigators. N Engl J Med. 2000;342:913–20. doi: 10.1056/NEJM200003303421302. [DOI] [PubMed] [Google Scholar]
- 11.Connolly SJ, Camm AJ, Halperin JL et al. Dronedarone in high-risk permanent atrial fibrillation. N Engl J Med. 2011;365:2268–76. doi: 10.1056/NEJMoa1109867. [DOI] [PubMed] [Google Scholar]
- 12.Löbe S, Salmáš J, John S et al. Usefulness of Dronederone in patients with atrial arrhythmias. Am J Cardiol. 2013;111:1311–4. doi: 10.1016/j.amjcard.2012.12.057. [DOI] [PubMed] [Google Scholar]
- 13.Said SM, Esperer HD, Kluba K Efficacy and safety profile of dronedarone in clinical practice. Results of the Magdeburg Dronedarone registry (MADRE study). Int J Cardiol. 2012. [Epub ahead of print]. [DOI] [PubMed]
- 14.Pappone C, Vicedomini G, Augello G et al. Radiofrequency catheter ablation and antiarrhythmic drug therapy: a prospective, randomized, 4-year follow-up trial: the APAF study. Circ Arrhythm Electrophysiol. 2011;4:808–14. doi: 10.1161/CIRCEP.111.966408. [DOI] [PubMed] [Google Scholar]
- 15.Cappato R, Calkins H, Chen SA et al. Updated worldwide survey on the methods efficacy and safety of catheter ablation of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:32–8. doi: 10.1161/CIRCEP.109.859116. [DOI] [PubMed] [Google Scholar]
- 16.Maan A, Shaikh AY, Mansour M et al. Complications from catheter ablation of atrial fibrillation: a systemic review. Crit Pathw Cardiol. 2011;10:76–83. doi: 10.1097/HPC.0b013e318224b7bd. [DOI] [PubMed] [Google Scholar]
- 17.Blandino A, Toso E, Scaglione M Long-Term Efficacy and Safety of Two Different Rhythm Control Strategies in Elderly Patients with Symptomatic Persistent Atrial Fibrillation. J Cardiovasc Electrophysiol. 2013. [Epub ahead of print]. [DOI] [PubMed]
- 18.Shah AN, Mittal S, Sichrovsky TC et al. Long-term efficacy following successful pulmonary vein isolation: pattern and prediction of very late occurrence. J Cardiovasc Electrophysiol. 2008;19:661–7. doi: 10.1111/j.1540-8167.2008.01101.x. [DOI] [PubMed] [Google Scholar]
- 19.Wyse DG, Waldo AL, DiMarco JP et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–33. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 20.Van Gelder IC, Hagens VE, Bosker HA et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1834–40. doi: 10.1056/NEJMoa021375. [DOI] [PubMed] [Google Scholar]
- 21.Hagens VE, Vermeulen KM, TenVergert EM et al. Rate control is more cost- effective than rhythm control for patients with persistent atrial flbrillation--results for the RAte Control versus Electric cardioversion (RACE) study. Eur Heart J. 2004;25:1542–9. doi: 10.1016/j.ehj.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 22.Kumana CR, Cheung BM, Cheung GT et al. Rhythm vs. rate control of atrial fibrillation meta- analysed by numbers needed to treat. Br J Clin Pharmacol. 2005;60:347–54. doi: 10.1111/j.1365-2125.2005.02449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Denus S, Sanoski CA, Carlsson J et al. Rate vs rhythm control in patients with atrial fibrillation: a meta- analysis. Arch Intern Med. 2005;165:258–62. doi: 10.1001/archinte.165.3.258. [DOI] [PubMed] [Google Scholar]
- 24.Caldiera D, David C, Sampaio C. Rate vs rhythm control in patients with atrial fibrillation and heart failure: a systemic review and meta-analysis of randomised controlled trials. Eur J Intern Med. 2011;22:448–55. doi: 10.1016/j.ejim.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Caldeira D, David C, Sampaio C. Rate versus rhythm control in atrial fibrillation and clinical outcomes: updated systemic review and meta- analysis of randomized controlled trials. Arch Cardiovasc Dis. 2012;105:226–38. doi: 10.1016/j.acvd.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Chatterjee S, Sardar P, Lichstein E et al. Pharmacologic rate versus rhythm control strategies in atrial fibrillation: an updated comprehensive review and meta- analysis. Pacing Clin Electrophysiol. 2013;36:122–33. doi: 10.1111/j.1540-8159.2012.03513.x. [DOI] [PubMed] [Google Scholar]
- 27.Pietrasik A, Kosior DA, Niewada M et al. The cost comparison of rhythm and rate control strategies in persistent atrial fibrillation. Int J Cardiol. 2007;118:21–7. doi: 10.1016/j.ijcard.2006.03.085. [DOI] [PubMed] [Google Scholar]
- 28.Opolski G, Torbicki A, Kosior DA et al. Rate control vs rhythm control in patients with nonvalvular persistent atrial fibrillation: the result of the Polish How to Treat Chronic Atrial Fibrillation (HOT CAFE) Study. Chest. 2004;126:476–86. doi: 10.1378/chest.126.2.476. [DOI] [PubMed] [Google Scholar]
- 29.Hohnloser SH, Kuck KH, Lilienthal J. Rhythm or rate control in atrial fibrillation--Pharmacological intervention in Atrial Fibrillation (PIAF): a randomised trial. Lancet. 2000;356 ((9244)):1789–94. doi: 10.1016/s0140-6736(00)03230-x. [DOI] [PubMed] [Google Scholar]
- 30.Pilote L, Eisenberg MJ, Essebag V Temporal Trends in Medications Use and Outcomes in Atrial Fibrillation. Can J Cardiol. 2013. [Epub ahead of print]. [DOI] [PubMed]
- 31.Andrade JG, Connolly SJ, Dorian P et al. Antiarrhythmic use from 1991 to 2007: insights from the Canadian Registry of Atrial Fibrillation (CARAF I and II). Heart Rhythm. 2010;7:1171–7. doi: 10.1016/j.hrthm.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 32.Martin-Doyle W, Essebag V, Zimetbaum P, Reynolds MR. Trends in US hospitalization rates and rhythm control therapies following publication of the AFFIRM and RACE trials. J Cardiovasc Electrophysiol. 2011;22:548–53. doi: 10.1111/j.1540-8167.2010.01950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ionscu-Ittu R, Abrahamowicz M, Jackevicius CA et al. Comparative effectiveness of rhythm control vs rate control drug treatment effect on mortality in patients with atrial fibrillation. Arch Intern Med. 2012;172:997–1004. doi: 10.1001/archinternmed.2012.2266. [DOI] [PubMed] [Google Scholar]
- 34.Brignole M, Giafranchi L, Menozzi C et al. Assessment of atrioventricular junction ablation and DDDR mode-switching pacemaker versus pharmacological treatments in patients with severely symptomatic paroxysmal atrial fibrillation: a randomized controlled study. Circulation. 1997;96:2617–24. doi: 10.1161/01.cir.96.8.2617. [DOI] [PubMed] [Google Scholar]
- 35.Brignole M, Menozzi C, Gianfranchi L et al. Assessment of atrioventricular junction ablation and VVIR pacemaker versus pharmacological treatment in patients with heart failure and chronic atrial fibrillation: a randomized controlled study. Circulation. 1998;98:953–60. doi: 10.1161/01.cir.98.10.953. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez LM, Smeets JL, Xie B et al. Improvement in left heart function by ablation of atrioventricular nodal conduction in selected patients with lone atrial fibrillation. Am J Cardiol. 1993;72:1137–41. doi: 10.1016/0002-9149(93)90982-i. [DOI] [PubMed] [Google Scholar]
- 37.Lee SH, Chen SA, Tai C T et al. Comparison of quality of life and cardiac performance after complete atrioventricular junction modification in patients with medically refractory atrial fibrillation. J Am Coll Cardiol. 1998;31:637–44. doi: 10.1016/s0735-1097(97)00530-5. [DOI] [PubMed] [Google Scholar]
- 38.Kay GN, Ellenbogen KA, Giudici M et al. The Ablate and Pace Trial: a prospective study of catheter ablation of the AV conduction system and permanent pacemaker implantation for treatment of atrial fibrillation. J Interv Card Electrophysiol. 1998;2:121–35. doi: 10.1023/a:1009795330454. [DOI] [PubMed] [Google Scholar]
- 39.Fitzpatrick AD, Kourouyan HD, Siu A et al. Quality of life and outcomes after radiofrequency His-bundle catheter ablation and permanent pacemaker implantation: impact of treatment in paroxysmal and established atrial fibrillation. Am Heart J. 1996;131:499–507. doi: 10.1016/s0002-8703(96)90528-1. [DOI] [PubMed] [Google Scholar]
- 40.Jensen SM, Bergfeldt L, Rosenqvist M. Long-term follow-up of patients treated by radiofrequency ablation of atrioventricular junction. Pacing Clin Electrophysiol. 1995;18 ((9 Pt 1)):1609–14. doi: 10.1111/j.1540-8159.1995.tb06982.x. [DOI] [PubMed] [Google Scholar]
- 41.Schuchert A, van Langen H, Michels K, Meinertz T. DDD(R) HYS pacing with autonomic mode switch in patients with paroxysmal atrial fibrillation following AV nodal ablation. Cardiology. 1997;88:323–7. doi: 10.1159/000177353. [DOI] [PubMed] [Google Scholar]
- 42.Marshall HJ, Harris ZI, Griffth MJ, Gammage MD. Atrioventricular nodal ablation and implantation of mode switching dual chamber pacemakers: effective treatment for drug refractory paroxysmal atrial fibrillation. Heart. 1998;79:543–7. doi: 10.1136/hrt.79.6.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilkoff BL, Cook JR, Epstein AE et al. Dual-chamber pacing or ventricular backup pacing in patients with implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA. 2002;288:3115–23. doi: 10.1001/jama.288.24.3115. [DOI] [PubMed] [Google Scholar]
- 44.Doshi RN, Daoud EG, Fellows C et al. Left ventricular-based cardiac stimulation post AV nodal ablation evaluation (the PAVE study) J Cardiovasc Electrophysiol. 2005;16:1160–5. doi: 10.1111/j.1540-8167.2005.50062.x. [DOI] [PubMed] [Google Scholar]
- 45.Tsadok MA, Jackevicius CA, Essebag V et al. Rhythm versus rate control therapy and subsequent stroke or transient ischemic attack in patients with atrial fibrillation. Circulation. 2012;126:2680–7. doi: 10.1161/CIRCULATIONAHA.112.092494. [DOI] [PubMed] [Google Scholar]
- 46.Suman-Horduna I, Roy D, Frasure-Smith N et al. Quality of life and functional capacity in patients with atrial fibrillation and congestive heart failure. J Am Coll Cardiol. 2013;61:455–60. doi: 10.1016/j.jacc.2012.10.031. [DOI] [PubMed] [Google Scholar]
- 47.Corley SD, Epstein AE, DiMarco JP et al. Relationship between sinus rhythm, treatment and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2004;109:1509–13. doi: 10.1161/01.CIR.0000121736.16643.11. [DOI] [PubMed] [Google Scholar]
- 48.Torp-Pedersen C, Møller M, Bloch-Thomson PE et al. Dofetilide in patients with congestive heart failure and left ventricular dysfunction. Danish Investigations of Arrhythmia and Mortality on Dofetilide Study Group. N Engl J Med. 1999;341:857–65. doi: 10.1056/NEJM199909163411201. [DOI] [PubMed] [Google Scholar]
- 49.Lu Z, Scherlag BJ, Lin J et al. Atrial fibrillation begets atrial fibrillation: autonomic mechanism for atrial electrical remodeling induced by short-term rapid atrial pacing. Circ Arrhythm Electrophysiol. 2008;1:184–92. doi: 10.1161/CIRCEP.108.784272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rostock T, Steven D, Lutomsky B et al. Atrial fibrillation begets atrial fibrillation in the pulmonary veins on the impact of atrial fibrillation on the electrophysiological properties of the pulmonary veins on the impact of atrial fibrillation on the electrophysiological properties of the pulmonary veins in humans. J Am Coll Cardiol. 2008;51:2153–60. doi: 10.1016/j.jacc.2008.02.059. [DOI] [PubMed] [Google Scholar]
- 51.Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atria fibrillation A study in awake chronically instrumented goats. Circulation. 1995;92:1954–68. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 52.Lim HS, Willoughby SR, Schultz C et al. Effect of atrial fibrillation on atrial thrombogenesis in humans: Impact of rate and rhythm. J Am Coll Cardiol. 2013;61:852–60. doi: 10.1016/j.jacc.2012.11.046. [DOI] [PubMed] [Google Scholar]
- 53.Willoughby SR, Roberts-Thomson RL, Lim HS et al. Atrial platelet reactivity in patients with atrial fibrillation. Heart Rhythm. 2010;7:1178–83. doi: 10.1016/j.hrthm.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 54.Yoshino S, Yoshikawa A, Hamasaki S Atrial fibrillation-induced endothelial dysfunction improves after restoration of sinus rhythm. Int J Cardiol. 2012. [Epub ahead of print]. [DOI] [PubMed]
- 55.Skalidis EI, Zacharis EA, Tsetis DK et al. Endothelial cell function during atrial fibrillation and after restoration of sinus rhythm. Am J Cardiol. 2007;99:1258–62. doi: 10.1016/j.amjcard.2006.12.044. [DOI] [PubMed] [Google Scholar]
- 56.De Vos CB, Breithardt G, Camm AJ et al. Progression of atrial fibrillation in the REgistry on Cardiac rhythm disORDers assessing the control of Atrial fibrillation cohort: clinical correlates and the effect of rhythm-control therapy. Am Heart J. 2012;163:887–93. doi: 10.1016/j.ahj.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 57.Camm AJ, Breithardt G, Crinjs H et al. Real-life observations of clinical outcomes with rhythm- and rate-control therapies for atrial fibrillation RECORDAF (Registry on Cardiac Rhythm Disorders Assessing the Control of Atrial Fibrillation). J Am Coll Cardiol. 2011;58:493–501. doi: 10.1016/j.jacc.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 58.Hobbs WJ, Flynn S, Todd DM et al. Reversal of atrial electrical remodeling after cardioversion of persistent atrial fibrillation in humans. Circulation. 2000;101:1145–51. doi: 10.1161/01.cir.101.10.1145. [DOI] [PubMed] [Google Scholar]
- 59.Riatt MH, Kusumoto W, Giraud G, McAnulty JH. Reversal of electrical remodeling after cardioversion of persistent atrial fibrillation. J Cardiovasc Electrophysiol. 2004;15:507–12. doi: 10.1046/j.1540-8167.2004.03217.x. [DOI] [PubMed] [Google Scholar]
- 60.Yu WC, Lee SH, Tai C T et al. Reversal of atrial electrical remodeling following cardioversion of long-standing atrial fibrillation in man. Cardiovasc Res. 1999;42:470–6. doi: 10.1016/s0008-6363(99)00030-9. [DOI] [PubMed] [Google Scholar]
- 61.de Vos CB, Pisters R, Nieuwlaat R et al. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J Am Coll Cardiol. 2010;55:725–31. doi: 10.1016/j.jacc.2009.11.040. [DOI] [PubMed] [Google Scholar]
- 62.Wilber DJ, Pappone C, Neuzil P et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA. 2010;303:333–40. doi: 10.1001/jama.2009.2029. [DOI] [PubMed] [Google Scholar]
- 63.ClinicalTrials.gov, Catheter Ablation vs Anti-arrhythmic Drug Therapy for Atrial Fibrillation Trial (CABANA), 2013. Available at: http://clinicaltrials.gov/show/NCT00911508. (accessed 10 May 2013).
- 64.Cleland JG, Coletta A P, Buga L et al. Clinical trials update from the American College of Cardiology meeting 2010: DOSE, ASPIRE, CONNECT, STICH, STOP-AF, CABANA, RACE II, EVEREST II, ACCORD, NAVIGATOR. Eur J Heart Fail. 2010;12:623–9. doi: 10.1093/eurjhf/hfq083. [DOI] [PubMed] [Google Scholar]
- 65.Fujino T, Yamashita T, Suzuki S et al. Characteristics of congestive heart failure accompanied by atrial fibrillation with special reference to tachycardia-induced cardiomyopathy. Circ J. 2007;71:936–40. doi: 10.1253/circj.71.936. [DOI] [PubMed] [Google Scholar]
- 66.Calò L, De Ruvo E, Sette A et al. Tachycardia-induced cardiomyopathy: mechanism of heart failure and clinical implications. J Cardiovasc Med (Hagerstown) 2007;8:138–43. doi: 10.2459/01.JCM.0000260841.30415.62. [DOI] [PubMed] [Google Scholar]
- 67.Van Gelder IC, Groenveld H F, Crinjs HJ et al. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362:1363–73. doi: 10.1056/NEJMoa1001337. [DOI] [PubMed] [Google Scholar]
- 68.Sarkar S, Koehler J, Crossley GH et al. Burden of atrial fibrillation and poor rate control detected by continuous monitoring and the risk of heart failure hospitalization. Am Heart J. 2012;164:616–24. doi: 10.1016/j.ahj.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 69.Groenveld H F, Tijssen JG, Crinjs HJ et al. Rate control efficacy in permanent atrial fibrillation: Successful and failed strict rate control against a background of lenient rate control: Data from RACE II (Rate Control Efficacy in Permanent Atrial fibrillation). J Am Coll Cardiol. 2013;61:741–8. doi: 10.1016/j.jacc.2012.11.038. [DOI] [PubMed] [Google Scholar]
- 70.Olshansky B, Rosenfeld LE, Warner AL et al. The atrial fibrillation follow up investigation of rhythm management (AFFIRM) study: approaches to control rate in atrial fibrillation. J Am Coll Cardiol. 2004;43:1201–8. doi: 10.1016/j.jacc.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 71.Ulimoen SR, Enger S, Carlson J et al. Comparison of four single-drug regimens on ventricular rate and arrhythmia-related symptoms in patients with permanent atrial fibrillation. Am J Cardiol. 2013;111:225–30. doi: 10.1016/j.amjcard.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 72.Gillis AM, Verma A, Talajic M et al. Canadian Cardiovascular Society atrial fibrillation guidelines 2010: rate and rhythm management. Can J Cardiol. 2011;27:47–59. doi: 10.1016/j.cjca.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 73.Skanes AC, Healey JS, Cairns JA et al. Focused 2012 update of the Canadian Cardiovascular Society atrial fibrillation guidelines: recommendations for stroke prevention and rate/ rhythm control. Can J Cardiol. 2012;28:125–36. doi: 10.1016/j.cjca.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 74.Fauchier L, Grimard C, Pierre B et al. Comparison of beta blocker and digoxin alone and in combination for management of patients with atrial fibrillation and heart failure. Am J Cardiol. 2009;103:248–54. doi: 10.1016/j.amjcard.2008.09.064. [DOI] [PubMed] [Google Scholar]
- 75.Whitbeck MG, Charnigo RJ, Khairy P Increased mortality among patients taking digoxin- analysis from the AFFIRM study. Eur Heart J. 2012. [Epub ahead of print]. [DOI] [PubMed]
- 76.Clemo H F, Wood MA, Gilligan DM, Ellenbogen KA. Intravenous amiodarone for acute heart rate control in the critically ill patient with atrial tachyarrhythmias. Am J Cardiol. 1998;81:594–8. doi: 10.1016/s0002-9149(97)00962-4. [DOI] [PubMed] [Google Scholar]
- 77.Singh BH, Sarma JSM Singh BN, Dzau VJ, Vanhoutte PM, Woosley RL. Amiodarone and amiodarone derivatives In: Cardiovascular Pharmacology and Therapeutics New York, US: Churchill Livingstone. 1994. pp. 689–709.