Abstract
Atrial fibrillation is the most common clinically significant cardiac arrhythmia, increasing the risk of stroke, heart failure and morbidity and mortality. Current therapies, including rate control and rhythm control by antiarrhythmic drugs or ablation therapy, are moderately effective but far from optimal. Gene therapy has the potential to become an attractive alternative to currently available therapies for atrial fibrillation. Various gene transfer vectors have been developed for cardiovascular disease with viral vectors being most widely used due to their high efficiency. Several gene delivery methods have been employed on different therapeutic targets. With increasing understanding of arrhythmia mechanisms, novel therapeutic targets have been discovered. This review will evaluate state-of-art gene therapy strategies and approaches including sinus rhythm restoration and ventricular rate control that could eventually prevent or eliminate atrial fibrillation in patients.
Keywords: Atrial fibrillation, arrhythmia, heart, gene therapy, vector
In the United States, atrial fibrillation (AF) is the most common sustained cardiac arrhythmia affecting approximately six million patients and contributing to a greatly increased risk of stroke, heart failure (HF) and overall morbidity and mortality.1,2 The prevalence of AF is increasing as the average age of the population increases.3,4
Currently available therapies for AF are suboptimal. Therapeutic options include sinus rhythm restoration and/or ventricular rate control. Both are achieved by pharmacological or ablation therapy. Efficacy is limited, and the risk of adverse effects to therapy is increased in patients with long-standing persistent AF and comorbidities that commonly accompany AF, such as HF or lung disease.5 Antiarrhythmic drug use has potential risks such as pro-arrhythmia and non-cardiovascular toxicities.6–8 AF ablation therapy has become increasingly popular, but efficacy is limited, with high recurrence rates requiring repeated procedures.9,10 Severe complications (including mortality of 0.1 % in a recent survey) remain a persistent problem for AF ablation.11 Limitations in currently available therapies dictate a need for novel and more effective therapies.
Cardiovascular gene therapy has the potential to expand treatment options for AF. Recent improvements in gene transfer vectors and delivery methods and a deeper understanding of molecular mechanisms of AF increase the probability that gene therapy will successfully translate to a clinically viable therapy over the next few years. In this review, we will analyse the available vectors and delivery methods for myocardial gene therapy and evaluate the current state-of-the-art for AF gene therapy.
Clinical Perspective
At the current time, gene transfer in several preclinical models has been shown to successfully control ventricular rate or restored sinus rhythm during atrial fibrillation (AF), strengthening the rationale for future use of gene therapy to treat atrial fibrillation.
Continuing advances in vector technology with clinically favourable attributes, development of minimally invasive gene delivery methods, and novel gene therapy targets increases the likelihood of successful, future translation of AF gene therapy to the clinic.
Gene therapy for AF has the potential for tremendous patient benefit, but it is not without potential risks. Formal preclinical testing and well thought out clinical trials are prerequisite before clinical release of a gene therapeutic.
General Principles of Myocardial Gene Transfer
Gene therapy is the delivery of functional genes into a target cell or tissue for the treatment or prevention of disease. Three major components for successful gene therapy are the selection of a gene transfer vector, a delivery method and a therapeutic gene target.
Vectors
Generally, vectors for gene delivery fall into one of two different kinds: viral or nonviral vectors. Nonviral vectors are DNA plasmids, alone or in combination with adjuncts that improve delivery.12,13 The advantage of naked DNA (plasmid with no complexing agent) is its inherent simplicity. DNA in the form of a plasmid is relatively easy to grow, purify and use, and it has documented acceptance by regulatory bodies. The major problem with nonviral vectors is inadequate transfection efficiency.14 Most studies that have assessed in vivo gene delivery with nonviral vectors have shown very limited uptake by target cells, with at most a few percent of target cells expressing the transgene. Complexing agents (lipid, carbohydrate or protein coatings) increase delivery to a limited degree, but these agents also increase toxicity. Some physical methods (electroporation, ultrasound disruption of microbubbles) have been developed to enhance gene transfer, but the efficiency in vivo still remains low.14–18
Viral vectors are more frequently used for cardiovascular diseases due to their superior efficiency in cellular uptake and gene expression compared with nonviral vectors. The viral vector genome is altered by removing genes essential for virus replication so that viral vectors can only grow under special, supported circumstances and not in the target tissue (except conditionally replicating adenoviruses used in cancer gene therapy that are not relevant to this AF discussion). The three most commonly used viruses for cardiovascular applications are adenoviruses (Ad), adeno-associated viruses (AAV) and lentiviruses.
Ad are double-stranded DNA viruses with a 35 kb genome. First generation Ad have deletions of a limited number of viral genes, preventing virus replication and creating space for gene insertions up to 10 kb. Helper-dependent Ad vectors have the entire viral genome removed. Ad vectors have been widely used in the myocardial gene transfer literature, mainly due to their high transduction efficiency in cardiac myocytes and capability of generating peak expression over a short time period. The main disadvantage of Ad is the ability to elicit a profound immune response from the recipient. This immune response limits duration of gene expression to 2–4 weeks in vivo, depending on the target organ and transgene.19 Immune responses to Ad can cause organ damage and systemic inflammatory responses.20 Ad vectors have been used in a number of myocardial gene therapy clinical trials that have not yet shown efficacy (due in large part to limitations in delivery) but that also have not shown any detectable toxicity.21–23
AAV is a small virus with a linear 5 kb single-stranded DNA genome containing two genes: rep and cap.24 Recombinant AAVs have many desirable properties for cardiac gene transfer, including long-term (potentially permanent) gene expression and a relatively limited immune or inflammatory response by the host organism.25 Among the reported AAV serotypes, AAV1, 6, 8 and 9 are most commonly used for cardiac gene therapy.26,27 AAV gene therapy was initially tested on rodents, where dense cardiac delivery was demonstrated. It is not nearly as effective in large mammals as it is in mice, but it appears sufficient to alter the phenotype in large mammalian models of disease.28,29 The principal advantage of AAV is the possibility of permanent gene expression. Cardiac studies have been limited, but gene expression in skeletal muscle persisted over one year after injection in a haemophilia clinical trial.30 Preclinical large mammalian models with various targets, delivery techniques and transgenes have reported stable expression persisting for several years after gene transfer.31–33 The principal disadvantage of AAV vectors is the limited insert size, preventing use with some ion channels or other large genes. Recent cardiac studies have shown efficacy of AAV gene therapy in large mammalian models and in a human HF clinical trial.28,34
Lentiviruses are human immunodeficiency virus-based members of the retrovirus family. They are enveloped RNA viruses capable of packaging approximately 10 kb of genetic information. Unlike other retroviruses, lentivirus can transduce non-dividing cells, including cardiomyocytes. Transfection efficiency for lentivirus vectors is similar to AAV.30 Long-term stable gene expression is another similarity between lentiviruses and AAV, although by a different mechanism. Lentiviruses actually integrate into the host genome allowing permanent expression. A potential risk for lentiviruses is the possibility of mutagenesis related to the insertion site. Another significant limitation is the inability of current technology to concentrate them to levels necessary for intracoronary delivery. Lentivirus vectors have been used for intramyocardial injection in large mammalian models of cardiac disease. The feasibility of using lentivirus vectors in situations needing widespread cardiac delivery has not yet been verified and no clinical trials using lentivirus vectors for cardiac disease have been attempted to date.
Gene Delivery Methods
Several gene delivery strategies have been developed and verified for the large mammalian heart (see Figure 1). For AF therapy, the areas of therapeutic interest are primarily broad atrial delivery for sinus rhythm restoration and atrioventricular (AV) nodal delivery for rate control. Methods to deliver a gene transfer vector to these regions reported in preclinical AF models include direct myocardial injection followed by electroporation, epicardial gene painting and intracoronary catheterisation.31–34 Some methods such as intravenous injection and tail vein injection have been reported in mice, but these have not shown viability in large mammals.35,36
Figure 1: Gene Delivery Methods for Therapeutic Targets Relevant To Atrial Fibrillation.
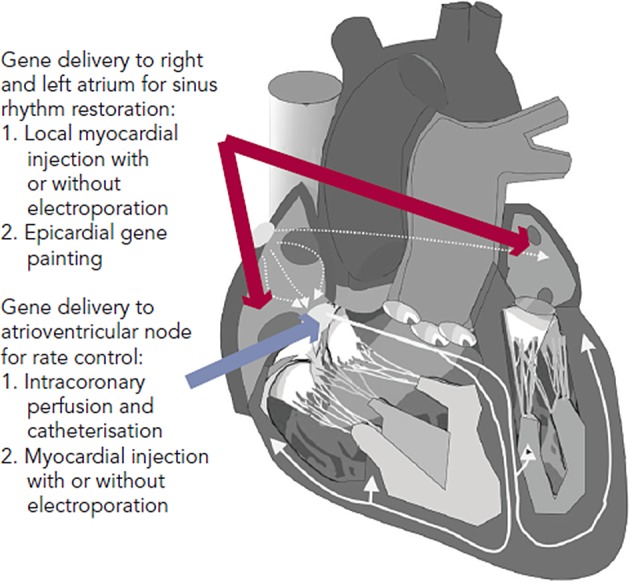
Direct myocardial injection is one of the simplest methods for effective cardiac gene delivery. Both naked DNA plasmids and viral vectors have been tested with this method.37,38 Intramyocardial injection leads to focused, high-density gene expression, but gene delivery is limited to the tissue volume within a few millimetres of the needle track.39,40 Thus, multiple injection sites would be required to achieve sufficient gene delivery in the large mammalian heart, which increases both the risk of adverse events during the procedure and the probability of heterogeneous gene expression. Injection-related tissue damage also has a risk of triggering an acute inflammatory response.41,42
In order to enhance efficiency, electroporation has been used immediately after plasmid or virus injection. Since its initial trial on skeletal muscle, electroporation-mediated nonviral gene therapy has improved efficiency in both small and large mammalian hearts in vivo.15,43–45 Thomas et al. and Aistrup et al. have demonstrated viability of direct myocardial injection with epicardial electroporation for gene delivery to the atria.34,46–48 In their studies, epicardial electroporation increased the efficiency from ~10 % to ~50 % in both atria. The drawbacks of this hybrid injection/electroporation approach include possible fibrillation of the heart if the pulse is not synchronised and challenges to coordinate the injection with the placement of the electrodes for routine clinical use.15 Targeting for direct injection has been reported with various imaging modalities, such as magnetic resonance imaging (MRI), cardiac CARTO-NOGA mapping (Biosense Webster Inc, CA, US) and ultrasound. These have not yet been reported in AF studies, but they could potentially be adapted for atrial or AV nodal gene delivery.49–52
To date, the atrial epicardial gene painting method is the only reported widespread atrial gene transfer method that achieves dense, transmural, homogenous atrial expression without affecting ventricular cardiomyocytes.33 Gene painting involves applying a mixture of poloxamer gel, dilute trypsin and gene transfer vector to the atrial epicardial surface. Poloxamer gel is used to increase virus contact time with the atria and trypsin increases virus penetration. The main technical difficulty to translating painting is the current need for open access to the atrial epicardial surface. In clinical settings, this delivery method could potentially be performed during open cardiac surgery or cardiac allografting.53 Modifications to create a minimally invasive method for this technique have not yet been reported.
Intracoronary perfusion is an attractive method for either whole heart or targeted AV nodal gene delivery, but it is not effective for isolated atrial gene delivery due to the lack of sufficient atrial vasculature.54 The advantages of this approach include minimal invasiveness and delivery of vectors using clinically standard equipment. The main disadvantage of delivery by intracoronary perfusion is the limited efficacy of whole heart delivery in spite of several reports that have outlined various parameters that can optimise gene transfer.55–59 To date, the best available method requires nitroglycerin, adenosine, vascular endothelial growth factor and low calcium administration to increase transvascular access, and simultaneous coronary arterial and venous delivery to increase target area. Sasano et al. used these methods with Ad vectors and achieved 80 % transfer to the anterior-septal left ventricle.32 A modification to achieve dense, whole heart delivery has not yet been reported.
Gene delivery methods are arguably the principal limitation to clinical translation of AF gene therapy. Myocardial injection and intracoronary infusion have been used in angiogenesis and heart failure clinical trials.60,61 As noted above, these methods would be limited for widespread, specific atrial delivery in the clinical setting. The epicardial painting method has potential for clinical application during cardiac surgery, and the development of a minimally invasive version could potentially lead to use in non-surgical AF therapy.
Gene Therapy Targets and Strategies for Ablation of Atrial Fibrillation
A key consideration for developing AF therapies is the arrhythmia mechanism. Both triggered activity and reentry have been implicated for provoking AF onset. Multiple lines of evidence support reentry as the dominant mechanism for sustaining AF.5,62 Approaches for sinus rhythm restoration include reentry-disrupting interventions such as prolongation of atrial action potential duration (APD), improvement of atrial conduction and trigger disruption by prevention of calcium leak from the sarcoplasmic reticulum. Rate control has targeted the AV node with overexpression of inhibitory G proteins, suppression of stimulatory G proteins, or introduction of a calcium channel inhibiting protein. Table 1 presents various gene targets and strategies for AF therapy.
Table 1: Gene Therapy Targets and Strategies for Ablation of Atrial Fibrillation.
| Study | Type of Strategy | Transgene or Target | Findings | Species | Vector | Delivery Method |
| Rhythm Control | ||||||
| Kikuchi et al. 200533 | Reentry-disrupting interventions by prolongation of APD | Human KCNH2-G628S | Atrial APD prolongation. No ventricular effects. Arrhythmia suppression not tested. | Swine | Ad | Epicardial painting |
| Atrial APD prolongation eliminated burst pacing-induced AF | Swine | Ad | Epicardial painting | |||
| Amit et al.201063 | Canine | Swine | Ad | Myocardial injection and electroporation | ||
| Soucek et al.201246 | KCNH2-G627S | |||||
| Igarashi et al. 201264 | Reentry-disrupting interventions by improvement of atrial conduction velocity | GJA1, GJA5 | Improved atrial conduction and prevented AF | Swine | Ad | Epicardial painting |
| Bikou et al. 201134 | GJA1 | Swine | Ad | Myocardial injection and electroporation | ||
| Liu et al. 200873 | HDAC class I and II inhibitor | HDAC inhibition reduced atrial arrhythmia inducibility and atrial fibrosis | Transgenic mice | NA | ||
| Trappe et al. 201347 | CASP3 inhibitor | Knockdown of caspase 3 suppressed or delayed sustained AF by reducing atrial cardiomyocyte apoptosis | Swine | Ad | Myocardial injection and electroporation | |
| Li et al. 201276 and 201493 | Trigger-disruption by preventing Ca2+ leak from SR | CAMK2D inhibitor | Inhibition of CaMKII phosphorylation of RyR2 prevented AF induction | Transgenic mice | NA | |
| Rate Control | ||||||
| Donahue et al. 200031 | Modification of AV node of AV node | GNAI2 | Suppressed AV conduction and reduced ventricular rate by 20 % in anesthetised animals | Swine | Ad | Intracoronary perfusion and catheterisation |
| Bauer et al. 200480 | GNAI2-Q205L | Suppressed AV conduction and reduced ventricular rate ~20 % in alert animals | Swine | Ad | Intracoronary perfusion and catheterisation | |
| Lugenbiel et al. 201282 | Modification of SA node | GNAS siRNA | Suppression of Gαs protein expression reduced ventricular rate by 8–17 % during SR | Swine | Ad | Myocardial injection and electroporation, |
| Murata et al. 200481 | Suppress L-type Calcium channel | GEM | Gem gene transfer to AV node reduced the heart rate by 20 % during AF | Swine | Ad | Intracoronary catheterisation |
AF = atrial fibrillation; SR = sinus rhythm, Ad = Adenovirus; AV = atrioventricular; APD = action potential duration; NA = not available.
Restoration of Sinus Rhythm
A principal element of AF electrical remodelling is the shortening of APD, which favours the maintenance of reentrant circuits. A logical approach would be to prevent AF by increasing the reentrant path length to prolong APD. Kikuchi et al. and Amit et al. demonstrated that gene transfer of the dominant negative mutation KCNH2 (G628S) blocked the IKr current and prolonged atrial APD.33 Animals receiving this gene by the epicardial gene painting method were resistant to atrial burst pacing-induced AF.63 The extent of APD prolongation and AF resistance correlated with gene expression. The dominant negative character of the mutation allowed it to suppress the endogenous, presumably normal, KCNH2 expressed in the atria. Soucek et al. performed a comparable study confirming that inhibition of KCNH2 function could prevent AF. They used the canine analogue of the channel (CERG-G627S) delivered to pigs using the injection/ electroporation method.46
A potentially complementary strategy is to prevent or reverse impaired intra-atrial conduction associated with AF or other diseases affecting the atria. One method to improve conduction is to target disease-related gap junction remodelling. Igarashi et al. found that atrial conduction impairment correlated with connexin (Cx) expression, phosphorylation and intercalated disk localisation. Using the atrial epicardial painting method, they showed not only that Cx43 gene transfer could reverse the conduction defect, but also that Cx40 gene transfer could replace the lost Cx43 and prevent AF.64 Bikou et al. additionally showed that Cx43 gene transfer using the injection/electroporation method improved atrial conduction and prevented AF.34
A possible approach to suppress conduction heterogeneity is to target atrial structural remodelling. Atrial apoptosis, inflammation and fibrosis are near universal findings, not only in AF, but also in diseases that support development of AF (e.g. HF, hypertension).65–67 Experimental studies indicate that AF can be prevented by suppression of fibrotic pathways including the renin–angiotensin system, transforming growth factor-β1, and other pathways relevant to inflammation and oxidative stress.68–72 As an example, histone deacetylase inhibition inhibited atrial fibrosis and reduced AF vulnerability in transgenic mice.73 No studies with gene therapy in clinically relevant models have yet been reported for prevention or reversal of atrial fibrosis, but this area holds promise.
AF is also linked to cardiomyocyte apoptosis, which leads to a reduction in conduction velocity. In vivo gene transfer with Ad-siRNA-Cas3 to knockdown caspase 3 has suppressed apoptosis, improved conduction velocity and delayed onset of AF, but didn’t alter myocardial fibrosis significantly.47
Calcium leak from the sarcoplasmic reticulum (SR) through ryanodine receptors (RYRs) potentially plays an important role in triggered activity that initiates AF.74 One strategy to target calcium leak from the SR is to reduce calcium/calmodulin-dependent protein kinase II (CaMKII) activity.75 Inhibition of CaMKII decreased phosphorylation of RyR2 and prevented induction of AF in FKBP12.6 knockout mice.76 Long-term inhibition of CaMKII prevented AF in CREM mice.77 A limitation of these data is that they are all from various transgenic mouse models. AF mechanisms are likely to differ in mice where triggered activity may play a more prominent role. Further investigations in preclinical models are required for a thorough understanding of the various roles of triggering and sustaining mechanisms in maintaining AF.
A strategy explored for protection against vagal-induced AF was administration of genes encoding the C-terminal fragment of Gαi and Gαo. The strategy competitively inhibited interaction between endogenous Gαi and Gαo with the muscarinic receptor, attenuating the effects of parasympathetic stimulation. Investigators delivered plasmids by direction injection followed by electroporation. Afterwards, they checked AF inducibility with vagal stimulation or carbachol administration. The combination of Gαi plus Gαo had similar effects on APD shortening when compared with Gαi alone, but had improved AF prevention effects suggesting a mechanism that involved more than the observed APD effects.18
Rate Control
AF normally results in an elevated ventricular rate. Rate controlling drugs are largely successful mainstays of therapy, but their use is limited in some patients by inadequate efficacy or intolerable side effects.78,79 Patients with AV node ablation are permanently dependent on pacemaker, and they have loss of synchronous left ventricular contraction that can be partially relieved by biventricular pacing, again requiring more implanted hardware. As a proof-of-concept, the inhibitory G-protein α-subunit (Gαi2) was incorporated into Ad and transferred to porcine AV node. The heart rate during AF after Gαi2 gene transfer reduced 20 %.31 In a follow-up study, the heart rate reduction with wild-type Gαi2 was lost when the animals were awake. A constitutively active Gαi2 mutation (cGi) caused a similar rate reduction that was impervious to animal arousal.80 A similar approach introduced Gem, an L-type calcium channel blocking G protein into the pig AV node and reduced ventricular rate.81
An alternative approach for AV nodal therapy is down-regulation of the stimulatory G protein α subunit. Lugenbeil et al. found that RNA interference-mediated inhibition of Gαs expression decreased ventricular rate by 20 %.82 In a separate study, they transferred this gene to the sinoatrial node and achieved an 8–17 % decrease in sinus rate.48 This strategy may well be complementary with the reported Gαi approach since the two proteins relay opposite signalling pathways in AV nodal cells. Gαs transmits β adrenergic signalling by increasing adenylate cyclase activity and downstream effects through protein kinase A. Gαi transmits cholinergic and purinergic signalling that decrease adenylate cyclase activity, antagonising the β-adrenergic effects in the AV node.
RNA interference is evolving to be a promising method for therapeutic silencing of protein-coding genes.83 MicroRNAs (MiRs) that inhibit expression of several proteins have been described. MiRs have been proven in various systems to be critical contributors to the pathophysiology of AF, either directly by modulation of ion channels and connexins, or indirectly by affecting fibrosis.84 MiR-1 is related to potassium channel and conduction velocity.85 MiR-21 and MiR-101 are associated with cardiac fibrosis.86,87 MiR-26 and MiR-328 contribute to the electrical remodelling of AF.88,89 MiRs are a potential gene therapy target for AF. This area is developing rapidly although it is still in an early stage.
Conclusions
Gene therapy approaches for AF are all currently at the preclinical development stage. Translation to clinical trial and practice is a long and difficult process. Clinical trials have demonstrated excellent long-term safety but limited efficacy for gene therapy in other cardiovascular diseases.22,23,60,61,90 The existing obstacles include lack of efficient, safe and clinically relevant delivery approaches and lack of vectors with high transfer efficiency and long-term regulated expression.91,92
Improvements in gene transfer vectors and delivery methods (including development of minimally invasive delivery methods) will further increase the likelihood of successful transfer of animal studies to clinical science. A better understanding of AF mechanisms in humans (particularly those with persistent AF complicated by heart failure or other cardiac diseases) is needed to refine therapeutic targets. Appropriate animal models with established AF need to be developed to study for longer time to insure effectiveness and durability of gene therapy. Overall, gene therapy for atrial fibrillation holds the potential to become the paradigm for clinical treatment but extensive further development is needed to reach this goal.
References
- 1.Khairy P, Nattel S. New insights into the mechanisms and management of atrial fibrillation. Can Med Assoc J. 2002;167:1012–20. [PMC free article] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL et al. Heart Disease and Stroke Statistics-2014 Update A Report From the American Heart Association. Circulation. 2014;129:E28–292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miyasaka Y, Barnes ME, Gersh BJ et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–25. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 4.Go AS, Hylek EM, Phillips KA et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–5. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 5.Nattel S, Burstein B, Dobrev D. Atrial Remodeling and Atrial Fibrillation Mechanisms and Implications. Circ Arrhythm Electrophysiol. 2008;1:62–73. doi: 10.1161/CIRCEP.107.754564. [DOI] [PubMed] [Google Scholar]
- 6.Connolly SJ, Camm AJ, Halperin JL et al. Dronedarone in high- risk permanent atrial fibrillation. N Engl J Med. 2011;365:2268–76. doi: 10.1056/NEJMoa1109867. [DOI] [PubMed] [Google Scholar]
- 7.Echt DS, Liebson PR, Mitchell LB et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324:781–8. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 8.Zimetbaum P. Antiarrhythmic Drug Therapy for Atrial Fibrillation. Circulation. 2012;125:381–89. doi: 10.1161/CIRCULATIONAHA.111.019927. [DOI] [PubMed] [Google Scholar]
- 9.Rostock T, Salukhe TV, Steven D et al. Long-term single- and multiple-procedure outcome and predictors of success after catheter ablation for persistent atrial fibrillation. Heart Rhythm. 2011;8:1391–7. doi: 10.1016/j.hrthm.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 10.Dobrev D, Carlsson L, Nattel S. molecular targets for atrial fibrillation therapy. Nat Rev Drug Discov. 2012;11:275–91. doi: 10.1038/nrd3682. [DOI] [PubMed] [Google Scholar]
- 11.Cappato R, Calkins H, Chen SA et al. Prevalence and causes of fatal outcome in catheter ablation of atrial fibrillation. J Am Coll Cardiol. 2009;53:1798–803. doi: 10.1016/j.jacc.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 12.Lin H, Parmacek MS, Morle G et al. Expression of recombinant genes in myocardium in vivo after direct injection of DNA. Circulation. 1990;82:2217–21. doi: 10.1161/01.cir.82.6.2217. [DOI] [PubMed] [Google Scholar]
- 13.Nabel EG, Plautz G, Nabel GJ. -specific gene expression in vivo by direct gene transfer into the arterial wall. Science. 1990;249:1285–8. doi: 10.1126/science.2119055. [DOI] [PubMed] [Google Scholar]
- 14.Chen ZY, Lin Y, Yang F et al. Gene therapy for cardiovascular disease mediated by ultrasound and microbubbles. Cardiovascular ultrasound. 2013;11:11. doi: 10.1186/1476-7120-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hargrave B, Downey H, Strange R, Jr et al. Electroporation- mediated gene transfer directly to the swine heart. Gene Ther. 2013;20:151–7. doi: 10.1038/gt.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen S, Shohet RV, Bekeredjian R et al. Optimization of ultrasound parameters for cardiac gene delivery of adenoviral or plasmid deoxyribonucleic acid by ultrasound-targeted microbubble destruction. J Am Coll Cardiol. 2003;42:301–8. doi: 10.1016/s0735-1097(03)00627-2. [DOI] [PubMed] [Google Scholar]
- 17.Yockman JW, Kastenmeier A, Erickson HM et al. Novel polymer carriers and gene constructs for treatment of myocardial ischemia and infarction. J Control Release. 2008;132:260–6. doi: 10.1016/j.jconrel.2008.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aistrup GL, Cokic I, Ng J et al. Targeted nonviral gene- based inhibition of Galpha(i/o)-mediated vagal signalling in the posterior left atrium decreases vagal-induced atrial fibrillation. Heart Rhythm. 2011;8:1722–9. doi: 10.1016/j.hrthm.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang YP, Nunes FA, Berencsi K et al. Cellular-Immunity to Viral-Antigens Limits E1-Deleted Adenoviruses for Gene- Therapy. Proc Natl Acad Sci U S A. 1994;91:4407–11. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marshall E. therapy on trial. Science. 2000;288:951–7. [PubMed] [Google Scholar]
- 21.Gupta R, Tongers J, Losordo DW. studies of angiogenic gene therapy. Circ Res. 2009;105:724–36. doi: 10.1161/CIRCRESAHA.109.200386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hedman M, Muona K, Hedman A et al. Eight-year safety follow-up of coronary artery disease patients after local intracoronary VEGF gene transfer. Gene Ther. 2009;16:629–34. doi: 10.1038/gt.2009.4. [DOI] [PubMed] [Google Scholar]
- 23.Kukula K, Chojnowska L, Dabrowski M et al. Intramyocardial plasmid-encoding human vascular endothelial growth factor A165/basic fibroblast growth factor therapy using percutaneous transcatheter approach in patients with refractory coronary artery disease (VIF-CAD) Am Heart J. 2011;161:581–9. doi: 10.1016/j.ahj.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 24.Berns KI. replication. Microbiol Rev. 1990;54:316–29. doi: 10.1128/mr.54.3.316-329.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wasala NB, Shin JH, Duan DS. evolution of heart gene delivery vectors. Journal of Gene Medicine. 2011;13:557–65. doi: 10.1002/jgm.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zacchigna S, Zentilin L, Giacca M. -associated virus vectors as therapeutic and investigational tools in the cardiovascular system. Circ Res. 2014;114:1827–46. doi: 10.1161/CIRCRESAHA.114.302331. [DOI] [PubMed] [Google Scholar]
- 27.Palomeque J, Chemaly ER, Colosi P et al. Efficiency of eight different AAV serotypes in transducing rat myocardium in vivo. Gene Ther. 2007;14:989–97. doi: 10.1038/sj.gt.3302895. [DOI] [PubMed] [Google Scholar]
- 28.Pleger ST, Shan C, Ksienzyk J et al. Cardiac AAV9-S100A1 gene therapy rescues post-ischemic heart failure in a preclinical large animal model. Sci Transl Med. 2011;3:92ra64. doi: 10.1126/scitranslmed.3002097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawase Y, Ly HQ, Prunier F et al. Reversal of cardiac dysfunction after long-term expression of SERCA2a by gene transfer in a pre-clinical model of heart failure. J Am Coll Cardiol. 2008;51:1112–9. doi: 10.1016/j.jacc.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 30.Fleury S, Simeoni E, Zuppinger C et al. Multiply attenuated, self-inactivating lentiviral vectors efficiently deliver and express genes for extended periods of time in adult rat cardiomyocytes in vivo. Circulation. 2003;107:2375–82. doi: 10.1161/01.CIR.0000065598.46411.EF. [DOI] [PubMed] [Google Scholar]
- 31.Donahue JK, Heldman AW, Fraser H et al. Focal modification of electrical conduction in the heart by viral gene transfer. Nat Med. 2000;6:1395–8. doi: 10.1038/82214. [DOI] [PubMed] [Google Scholar]
- 32.Sasano T, Kikuchi K, McDonald AD et al. Targeted high- efficiency, homogeneous myocardial gene transfer. J Mol Cell Cardiol. 2007;42:954–61. doi: 10.1016/j.yjmcc.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kikuchi K, McDonald AD, Sasano T et al. Targeted modification of atrial electrophysiology by homogeneous transmural atrial gene transfer. Circulation. 2005;111:264–70. doi: 10.1161/01.CIR.0000153338.47507.83. [DOI] [PubMed] [Google Scholar]
- 34.Bikou O, Thomas D, Trappe K et al. Connexin 43 gene therapy prevents persistent atrial fibrillation in a porcine model. Cardiovasc Res. 2011;92:218–25. doi: 10.1093/cvr/cvr209. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi H, Carbonaro D, Pepper K et al. Neonatal gene therapy of MPS I mice by intravenous injection of a lentiviral vector. Mol Ther. 2005;11:776–89. doi: 10.1016/j.ymthe.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 36.Gregorevic P, Blankinship MJ, Allen JM et al. Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat Med. 2004;10:828–34. doi: 10.1038/nm1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guzman RJ, Lemarchand P, Crystal RG et al. Efficient Gene- Transfer into Myocardium by Direct-Injection of Adenovirus Vectors. Circ Res. 1993;73:1202–07. doi: 10.1161/01.res.73.6.1202. [DOI] [PubMed] [Google Scholar]
- 38.Wright MJ, Wightman LML, Lilley C et al. In vivo myocardial gene transfer: Optimization, evaluation and direct comparison of gene transfer vectors. Basic Res Cardiol. 2001;96:227–36. doi: 10.1007/s003950170053. [DOI] [PubMed] [Google Scholar]
- 39.Kass-Eisler A, Falck-Pedersen E, Alvira M et al. Quantitative determination of adenovirus-mediated gene delivery to rat cardiac myocytes in vitro in vivo. Proc Natl Acad Sci U S A. 1993;90:11498–502. doi: 10.1073/pnas.90.24.11498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.French BA, Mazur W, Geske RS et al. Direct in-Vivo Gene- Transfer into Porcine Myocardium Using Replication-Deficient Adenoviral Vectors. Circulation. 1994;90:2414–24. doi: 10.1161/01.cir.90.5.2414. [DOI] [PubMed] [Google Scholar]
- 41.Li JJ, Ueno H, Pan Y et al. Percutaneous Transluminal Gene- Transfer into Canine Myocardium in-Vivo by Replication- Defective Adenovirus. Cardiovasc Res. 1995;30:97–105. [PubMed] [Google Scholar]
- 42.Solheim S, Seljeflot I, Lunde K et al. Inflammatory responses after intracoronary injection of autologous mononuclear bone marrow cells in patients with acute myocardial infarction. Am Heart J. 2008;155(55):e1–9. doi: 10.1016/j.ahj.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 43.Ayuni EL, Gazdhar A, Giraud MN et al. In vivo electroporation mediated gene delivery to the beating heart. PloS one. 2010;5:e14467. doi: 10.1371/journal.pone.0014467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mir LM, Bureau MF, Gehl J et al. High-efficiency gene transfer into skeletal muscle mediated by electric pulses. Proc Natl Acad Sci U S A. 1999;96:4262–67. doi: 10.1073/pnas.96.8.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marshall WG, Boone BA, Burgos JD et al. Electroporation- mediated delivery of a naked DNA plasmid expressing VEGF to the porcine heart enhances protein expression. Gene Ther. 2010;17:419–23. doi: 10.1038/gt.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soucek RD, Thomas K, Kelemen K et al. Genetic suppression of atrial fibrillation using a dominant-negative ether-a-go-go- related gene mutant. Heart Rhythm. 2012;9:265–72. doi: 10.1016/j.hrthm.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 47.Trappe K, Thomas D, Bikou O et al. Suppression of persistent atrial fibrillation by genetic knockdown of caspase 3: a pre-clinical pilot study. Eur Heart J. 2013;34:147–57. doi: 10.1093/eurheartj/ehr269. [DOI] [PubMed] [Google Scholar]
- 48.Lugenbiel P, Bauer A, Kelemen K et al. Biological Heart Rate Reduction Through Genetic Suppression of G alpha(s) Protein in the Sinoatrial Node. J Am Heart Assoc. 2012;1 doi: 10.1161/JAHA.111.000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang X, Atalar E. -guided gene therapy. FEBS letters. 2006;580:2958–61. doi: 10.1016/j.febslet.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 50.Banovic M, Ostojic MC, Bartunek J et al. Brachial approach to NOGA-guided procedures: electromechanical mapping and transendocardial stem-cell injections. Texas Heart Institute journal / from the Texas Heart Institute of St. Luke’s Episcopal Hospital, Texas Children’s Hospital. 2011;38:179–82. [PMC free article] [PubMed] [Google Scholar]
- 51.David AL, Peebles DM, Gregory L et al. Clinically applicable procedure for gene delivery to fetal gut by ultrasound-guided gastric injection: toward prenatal prevention of early-onset intestinal diseases. Hum Gene Ther. 2006;17:767–79. doi: 10.1089/hum.2006.17.767. [DOI] [PubMed] [Google Scholar]
- 52.Voges J, Reszka R, Gossmann A et al. Imaging-guided convection-enhanced delivery and gene therapy of glioblastoma. Annals of neurology. 2003;54:479–87. doi: 10.1002/ana.10688. [DOI] [PubMed] [Google Scholar]
- 53.Ly H, Kawase Y, Yoneyama R et al. Gene therapy in the treatment of heart failure. Physiology. 2007;22:81–96. doi: 10.1152/physiol.00037.2006. [DOI] [PubMed] [Google Scholar]
- 54.Greener I, Donahue JK. therapy strategies for cardiac electrical dysfunction. J Mol Cell Cardiol. 2011;50:759–65. doi: 10.1016/j.yjmcc.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Donahue JK, Kikkawa K, Johns DC et al. Ultrarapid, highly efficient viral gene transfer to the heart. Proc Natl Acad Sci USA. 1997;94:4664–8. doi: 10.1073/pnas.94.9.4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boekstegers P, von Degenfeld G, Giehrl W et al. Myocardial gene transfer by selective pressure-regulated retroinfusion of coronary veins. Gene Ther. 2000;7:232–40. doi: 10.1038/sj.gt.3301079. [DOI] [PubMed] [Google Scholar]
- 57.Bridges CR, Burkman JM, Malekan R et al. Global cardiac- specific transgene expression using cardiopulmonary bypass with cardiac isolation. Ann Thorac Surg. 2002;73:1939–46. doi: 10.1016/s0003-4975(02)03509-9. [DOI] [PubMed] [Google Scholar]
- 58.Breil I, Koch T, Belz M et al. Effects of bradykinin, histamine and serotonin on pulmonary vascular resistance and permeability. Acta Physiol Scand. 1997;159:189–98. doi: 10.1046/j.1365-201X.1997.549324000.x. [DOI] [PubMed] [Google Scholar]
- 59.Hajjar RJ, Schmidt U, Matsui T et al. Modulation of ventricular function through gene transfer in vivo. Proc Natl Acad Sci U S A. 1998;95:5251–56. doi: 10.1073/pnas.95.9.5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Penn MS, Mendelsohn FO, Schaer GL et al. An open-label dose escalation study to evaluate the safety of administration of nonviral stromal cell-derived factor-1 plasmid to treat symptomatic ischemic heart failure. Circ Res. 2013;112:816–25. doi: 10.1161/CIRCRESAHA.111.300440. [DOI] [PubMed] [Google Scholar]
- 61.Jessup M, Greenberg B, Mancini D et al. Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+- ATPase in patients with advanced heart failure. Circulation. 2011;124:304–13. doi: 10.1161/CIRCULATIONAHA.111.022889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mandapati R, Skanes A, Chen J et al. Stable microreentrant sources as a mechanism of atrial fibrillation in the isolated sheep heart. Circulation. 2000;101:194–99. doi: 10.1161/01.cir.101.2.194. [DOI] [PubMed] [Google Scholar]
- 63.Amit G, Kikuchi K, Greener ID et al. Selective Molecular Potassium Channel Blockade Prevents Atrial Fibrillation. Circulation. 2010;121:2263–70. doi: 10.1161/CIRCULATIONAHA.109.911156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Igarashi T, Finet JE, Takeuchi A et al. Connexin Gene Transfer Preserves Conduction Velocity and Prevents Atrial Fibrillation. Circulation. 2012;125(216):U103. doi: 10.1161/CIRCULATIONAHA.111.053272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee KW, Everett TH, Rahmutula D et al. Pirfenidone prevents the development of a vulnerable substrate for atrial fibrillation in a canine model of heart failure. Circulation. 2006;114:1703–12. doi: 10.1161/CIRCULATIONAHA.106.624320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anyukhovsky EP, Sosunov EA, Plotnikov A et al. Cellular electrophysiologic properties of old canine atria provide a substrate for arrhythmogenesis. Cardiovascular research. 2002;54:462–69. doi: 10.1016/s0008-6363(02)00271-7. [DOI] [PubMed] [Google Scholar]
- 67.Li DS, Fareh S, Leung TK et al. Promotion of atrial fibrillation by heart failure in dogs - Atrial remodeling of a different sort. Circulation. 1999;100:87–95. doi: 10.1161/01.cir.100.1.87. [DOI] [PubMed] [Google Scholar]
- 68.Everett THT, Olgin JE. fibrosis and the mechanisms of atrial fibrillation. Heart Rhythm. 2007;4:S24–7. doi: 10.1016/j.hrthm.2006.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Burstein B, Nattel S. fibrosis: Mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. 2008;51:802–09. doi: 10.1016/j.jacc.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 70.Verheule S, Sato T, Everett T et al. Increased vulnerability to atrial fibrillation in transgenic mice with selective atrial fibrosis caused by overexpression of TGF-beta 1. Circ Res. 2004;94:1458–65. doi: 10.1161/01.RES.0000129579.59664.9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Freestone B, Beevers DG, Lip GYH. The renin-angiotensin- aldosterone system in atrial fibrillation: a new therapeutic target? J Hum Hypertens. 2004;18:461–65. doi: 10.1038/sj.jhh.1001694. [DOI] [PubMed] [Google Scholar]
- 72.Van Wagoner DR. Oxidative Stress and Inflammation in Atrial Fibrillation: Role in Pathogenesis and Potential as a Therapeutic Target. J Cardiovasc Pharmacol. 2008;52:306–13. doi: 10.1097/FJC.0b013e31817f9398. [DOI] [PubMed] [Google Scholar]
- 73.Liu F, Levin MD, Petrenko NB et al. Histone-deacetylase inhibition reverses atrial arrhythmia inducibility and fibrosis in cardiac hypertrophy independent of angiotensin. J Mol Cell Cardiol. 2008;45:715–23. doi: 10.1016/j.yjmcc.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dobrev D, Voigt N, Wehrens XHT. ryanodine receptor channel as a molecular motif in atrial fibrillation: pathophysiological and therapeutic implications. Cardiovasc Res. 2011;89:734–43. doi: 10.1093/cvr/cvq324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rokita AG, Anderson ME. Therapeutic Targets in Cardiology Arrhythmias and Ca2+/Calmodulin-Dependent Kinase II (CaMKII) Circulation. 2012;126:2125–39. doi: 10.1161/CIRCULATIONAHA.112.124990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li N, Wang TN, Wang W et al. Inhibition of CaMKII Phosphorylation of RyR2 Prevents Induction of Atrial Fibrillation in FKBP12.6 Knockout Mice. Circ Res. 2012;110(465):U208. doi: 10.1161/CIRCRESAHA.111.253229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Heijman J, Voigt N, Wehrens XH et al. Calcium dysregulation in atrial fibrillation: the role of CaMKII. Front Pharmacol. 2014;5:30. doi: 10.3389/fphar.2014.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kwaku KF. therapy for rate control in atrial fibrillation: a new approach to an old problem. Circulation. 2006;113:2474–6. doi: 10.1161/CIRCULATIONAHA.106.626689. [DOI] [PubMed] [Google Scholar]
- 79.Boriani G, Biffi M, Diemberger I et al. Rate control in atrial fibrillation: choice of treatment and assessment of efficacy. Drugs. 2003;63:1489–509. doi: 10.2165/00003495-200363140-00005. [DOI] [PubMed] [Google Scholar]
- 80.Bauer A, McDonald AD, Nasir K et al. Inhibitory G protein overexpression provides physiologically relevant heart rate control in persistent atrial fibrillation. Circulation. 2004;110:3115–20. doi: 10.1161/01.CIR.0000147185.31974.BE. [DOI] [PubMed] [Google Scholar]
- 81.Murata M, Cingolani E, McDonald AD et al. Creation of a genetic calcium channel blocker by targeted gem gene transfer in the heart. Circ Res. 2004;95:398–405. doi: 10.1161/01.RES.0000138449.85324.c5. [DOI] [PubMed] [Google Scholar]
- 82.Lugenbiel P, Thomas D, Kelemen K et al. Genetic suppression of Galphas protein provides rate control in atrial fibrillation. Basic Res Cardiol. 2012;107:265. doi: 10.1007/s00395-012-0265-5. [DOI] [PubMed] [Google Scholar]
- 83.McCaffrey AP, Meuse L, Pham TTT et al. Gene expression - RNA interference in adult mice. Nature. 2002;418:38–9. doi: 10.1038/418038a. [DOI] [PubMed] [Google Scholar]
- 84.Dobrev D. Is altered atrial microRNA-ome a critical contributor to the pathophysiology of atrial fibrillation? Basic Res Cardiol. 2012. p. 107. [DOI] [PubMed]
- 85.Yang BF, Lin HX, Xiao JN et al. The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat Med. 2007;13:486–91. doi: 10.1038/nm1569. [DOI] [PubMed] [Google Scholar]
- 86.Adam O, Lohfelm B, Thum T et al. Role of miR-21 in the pathogenesis of atrial fibrosis. Basic Res Cardiol. 2012;107 doi: 10.1007/s00395-012-0278-0. [DOI] [PubMed] [Google Scholar]
- 87.Pan ZW, Sun XL, Shan HL et al. MicroRNA-101 Inhibited Postinfarct Cardiac Fibrosis and Improved Left Ventricular Compliance via the FBJ Osteosarcoma Oncogene/ Transforming Growth Factor-beta 1 Pathway. Circulation. 2012;126:840–50. doi: 10.1161/CIRCULATIONAHA.112.094524. [DOI] [PubMed] [Google Scholar]
- 88.Lu YJ, Zhang Y, Wang N et al. MicroRNA-328 Contributes to Adverse Electrical Remodeling in Atrial Fibrillation. Circulation. 2010;122:2378–87. doi: 10.1161/CIRCULATIONAHA.110.958967. [DOI] [PubMed] [Google Scholar]
- 89.Luo XB, Pan ZW, Shan HL et al. MicroRNA-26 governs profibrillatory inward-rectifier potassium current changes in atrial fibrillation. Journal of Clinical Investigation. 2013;123:1939–51. doi: 10.1172/JCI62185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stewart DJ, Kutryk MJ, Fitchett D et al. VEGF gene therapy fails to improve perfusion of ischemic myocardium in patients with advanced coronary disease: results of the NORTHERN trial. Mol Ther. 2009;17:1109–15. doi: 10.1038/mt.2009.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Katz MG, Fargnoli AS, Pritchette LA et al. Gene delivery technologies for cardiac applications. Gene Ther. 2012;19:659–69. doi: 10.1038/gt.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hedman M, Hartikainen J, Yla-Herttuala S. Progress and prospects: hurdles to cardiovascular gene therapy clinical trials. Gene Ther. 2011;18:743–9. doi: 10.1038/gt.2011.43. [DOI] [PubMed] [Google Scholar]
- 93.Li N, Chiang DY, Wang S et al. Ryanodine receptor-mediated calcium leak drives progressive development of an atrial fibrillation substrate in a transgenic mouse model. Circulation. 2014;129:1276–85. doi: 10.1161/CIRCULATIONAHA.113.006611. [DOI] [PMC free article] [PubMed] [Google Scholar]


