Abstract
The closed chest convergent procedure is a multidisciplinary approach to atrial fibrillation (AF) treatment. Epicardial posterior left atrial (PLA) ablation is performed by a cardiac surgeon using a transdiaphragmatic endoscope, immediately followed by percutaneous pulmonary vein (PV) isolation performed by a cardiac electrophysiologist. Interim outcomes for the treatment of non-paroxysmal AF (NPAF) were evaluated based on peri-procedural safety and complications, freedom from recurrent AF, and need for cardioversion or repeat catheter ablation at three, six and 12 months post-procedure. A total of 43 patients (86 % NPAF) underwent the convergent procedure. Patients were 84 % male, with mean age 58.6 ± 8.7 years. Mean AF duration was 45.4 ± 40.3 months. Pre-procedure left atrium (LA) volumetric data using cardiac magnetic resonance imaging (MRI) or computed tomography (CT) was available for 30 patients (70 %). Average LA volume was 155.5 ± 48.4 millilitres (ml); two-thirds of patients had a LA volume >130 ml. There was no operative or peri-operative mortality. Sinus rhythm (SR) was recorded at three months in 31 of 39 (79 %) patients, at six months in 24 of 27 (89 %) patients and at 12 months in nine patients. The convergent procedure is a safe and effective option for both PV isolation and PLA substrate ablation in NPAF patients. Long-term follow-up is required and randomised clinical trials warranted.
Keywords: Atrial fibrillation, ablation, convergent procedure, epicardial–endocardial ablation
Atrial fibrillation (AF) is the most common cardiac arrhythmia worldwide. In 2010, the prevalence of AF in the US was estimated at 5.9 million.1 The decision to pursue treatment to maintain sinus rhythm is driven by disabling symptoms related to AF; including palpitations, dyspnoea, fatigue, stroke and congestive heart failure. Percutaneous catheter-based ablation is an established therapy for symptomatic paroxysmal AF resistant to antiarrhythmic medications. However, treatment of non-paroxysmal atrial fibrillation (NPAF), including persistent and long-standing persistent AF, is much less effective.2 Endovascular catheter ablation for NPAF is limited by short- and long-term recurrent AF and lower arrhythmia-free survival compared with catheter ablation for paroxysmal atrial fibrillation (PAF).3,4 Particular patient characteristics, including continuous AF greater than six months duration and enlarged left atrial volume, may predict lower success rates with endovascular catheter-based ablation.5 These patients may experience post-ablation recurrent AF due to the inability to effectively perform substrate ablation in addition to pulmonary vein isolation.
Open chest and thoracoscopic epicardial surgical ablation techniques have been developed to address technical limitations of endovascular catheter ablation. However, these surgical approaches have disadvantages including peri-operative morbidity, extended hospitalisation and recovery, post-operative pain, inability to confirm bidirectional block of the pulmonary veins, and inability to reach endovascular sites to treat left or right atrial flutter.6,7
The convergent procedure was designed to address the limitations of both catheter and surgical ablation by offering a synergistic, multidisciplinary approach that does not require sternotomy, thoracotomy or thoracic ports.8 Closed chest, endoscopic epicardial ablation is combined with standard endocardial catheter ablation techniques to isolate the pulmonary veins (PVs) and ablate the posterior left atrium (PLA) substrate that may promote and/or sustain AF. Convergent epicardial–endocardial ablation is performed within the electrophysiology (EP) laboratory. Its closed chest access allows for maintenance of therapeutic peri-procedural anticoagulation and minimises post-procedure pain and length of stay. The epicardial ablation catheter used in the convergent procedure has been demonstrated to create continuous, transmural linear lesions in a canine model,9 and offers the advantage of PLA substrate ablation with unipolar radiofrequency (RF) energy directed anteriorly, thus minimising damage to the oesophagus and other posterior structures. We detail our single-centre experience with the convergent procedure for treatment of NPAF in a predominantly NPAF population with left atrial enlargement.
Methods
Study Population
Forty-three patients with predominantly disabling NPAF resistant to class I or class III antiarrhythmic medications with no prior history of open chest cardiac surgery underwent ablation therapy between June 2010 and February 2013. The convergent procedure was recommended after consultation with both a cardiac electrophysiologist and a cardiac surgeon based upon one or more of the following:
persistent or long-standing persistent AF;
enlarged left atrium;
structural heart disease; or
prior failed endovascular catheter ablation of PAF/NPAF.
Convergent Procedure Description
The patients were prepped and draped in the standard sterile fashion. Cardiac computed tomography (CT) or magnetic resonance imaging (MRI) as well as transesophageal echocardiogram (TEE) were performed pre-procedure. A subxiphoid incision was made to access the pericardial space through a transdiaphragmatic pericardial window, created through the central tendon of the diaphragm. A 20 millimetre (mm) cannula was inserted through a transdiaphragmatic, pericardial window to allow direct access and visualisation of the PLA.10 Epicardial ablation was accomplished with the Numeris® Guided Coagulation Device (nContact Inc, Morrisville, North Carolina, US). Vacuum was used to ensure constant contact with epicardial tissue. Saline perfusion, controlled by the vacuum, cooled the device and epicardium to ensure localised efficient ablation. After completion of epicardial ablation, a pericardial drain was placed to mitigate the risk of tamponade during endocardial ablation and during the peri-operative period.
The endocardial component of the procedure was initiated immediately after completion of epicardial PLA ablation. Bilateral femoral venous access was obtained. A diagnostic 20-pole recording catheter was advanced to the anterolateral right atrium and coronary sinus. Double transseptal access was obtained under intracardiac echocardiography guidance. Heparin was administered throughout left-sided catheterisation to ensure activated clotting time (ACT) remained between 300 and 400 seconds. Electroanatomic mapping (NAVx, St. Jude Medical, St Paul, MN, US) was used to create geometry and voltage maps to guide endocardial ablation. Bidirectional block (entrance and exit block) was confirmed in each pulmonary vein in all patients. After completion of epicardial and endocardial lesions, atrial burst pacing was performed during intravenous isoproterenol infusion (up to 15 micrograms per minute [mcg/min]) to assess atrial arrhythmia inducibility. A schematic lesion pattern from epicardial–endocardial ablation is shown in Figure 1.
Figure 1: Schematic of Convergent Procedure.
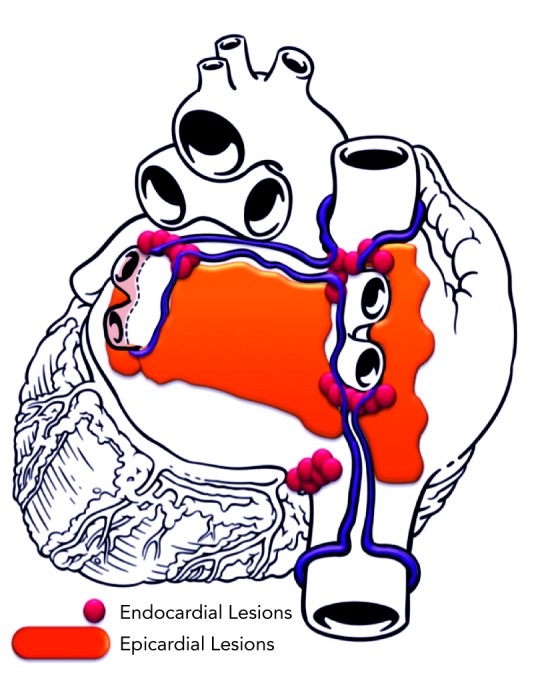
Post-procedure Patient Management
All patients were discharged on antiarrhythmic drug (AAD) therapy. AAD status, including the need for new or escalating therapy, was recorded at each follow-up. Anticoagulants were instituted post-procedure and continued for at least six months. Patients taking warfarin who had anticoagulation discontinued pre-procedure were bridged with Heparin until therapeutic anticoagulation was obtained post-procedure. Patients taking newer oral anticoagulants (NOACs), including dabigatran and rivaroxaban, had their anticoagulant held 24 hours before and resumed the night of the procedure.
Monitoring
Patients were scheduled for routine follow-up and 12-lead electrocardiography (ECG) at three, six and 12 months post-procedure. Additionally, a two-week cardiac event monitor was placed at six months to assess AF/atrial flutter (AFL) and atrial tachycardia (AT) burden. Subsequent AF treatments including changes in AAD therapy, repeat catheter ablation and need for electrical cardioversion were recorded at each follow-up visit.
Statistical Analysis
Data was reported as mean ± standard deviation (SD) for quantitative variables; and counts and percentages for categorical measures.
Results
Forty-three patients undergoing the convergent procedure were included. The mean age was 58.6 ± 8.7 years and 84 % were male. 86 % (37/43) of patients had NPAF with an average AF duration of 45.4 ± 40.3 months. Pre-procedural LA volumetric data by either cardiac MRI or CT was available for 70 % of patients with an average LA volume of 155.5 ± 48.4 ml. Patient demographics are detailed in Table 1.
Table 1: Patient Demographics.
| Characteristic | All Subjects n=43 |
|---|---|
| Male, n (%) | 36 (84 %) |
| Age (years), Mean (SD) Range | 58.6 (8.7) 38, 74 |
| Pre-operative LA volume (ml), Mean (SD) Range | 155.5 (48.4) 109, 342 |
| Pre-operative LVEF, Mean (SD) Range | 55 % (12 %) 20 %, 75 % |
| Atrial fibrillation type, N Paroxysmal AF Non-Paroxysmal AF | 6 (14 %) 37 (86 %) |
| AF duration (months), Mean (SD) | 45.4 (40.3) |
| Range | 4, 180 |
AF = atrial fibrillation; LA = left atrium; LVEF = left ventricular ejection fraction; ml = millilitres; SD = standard deviation.
All procedures were performed in a single day. High density LA voltage maps following epicardial ablation confirmed extensive posterior substrate ablation (<0.2 millivolts [mV]) in all patients; a representative voltage map is shown in Figure 2. Extent of epicardial ablation anterior to PVs varied with pericardial reflection anatomy. Epicardial ablation alone isolated at least one PV in 12 patients (28 %); the left inferior PV was isolated in 92 % of these patients. Near isolation (single RF ablation at one endocardial location resulting in bidirectional block) was observed in five patients (12 %). Remaining PVs were isolated with segmental, predominantly anterior endocardial ablation. Time from first endocardial ablation until completion of all endocardial lesions was 107 ± 59 minutes (min). Mean fluoroscopy time was 32.1 ± 10.8 min while radiation dose averaged 590 ± 241 milligray (mGy). Nine patients converted from AF to sinus rhythm (SR) during ablation (three during epicardial and six during endocardial ablation). Of the six patients who converted to sinus rhythm during endocardial ablation, four converted to sinus rhythm with cavotricuspid isthmus ablation after the convergent procedure resulted in a change in rhythm from AF to typical atrial flutter. The remaining two patients converted to sinus rhythm during endocardial pulmonary vein isolation.
Figure 2: Voltage Map Showing Electrical Silence of the Posterior Left Atrium after Convergent Epicardial–Endocardial Ablation.
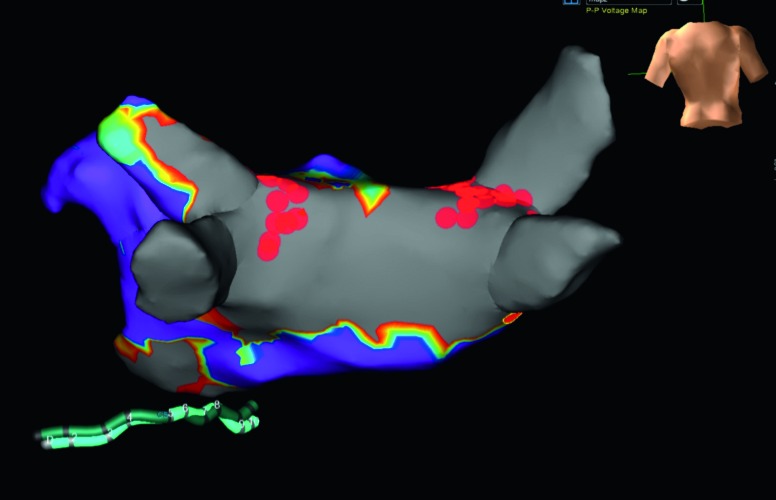
There were no major adverse cardiovascular events, including peri-operative or late mortality, stroke, transient ischaemic attack, pericardial tamponade, pericardial effusion, phrenic nerve injury, atrial-oesophageal fistula, myocardial infarction, high grade heart block or excessive bleeding requiring transfusion. Non-steroidal anti-inflammatory drug (NSAID) and/or corticosteroid therapy was administered during hospitalisation and after hospital discharge in 19 patients to manage transient pericarditis. All episodes resolved within seven days of treatment and no recurrent pericarditis has been observed.
Figure 3 shows outcomes based on three and six month follow-up. Freedom from AF/AFL/AT was documented in 31 of 39 (79 %) patients at three months, and 24 of 27 (89 %) patients at six months. Of the nine patients who have reached 12-month follow-up, all have remained in sinus rhythm without documented AF/AFL/AT recurrence.
Figure 3: Interim Outcomes at Three and Six Month Follow-up.
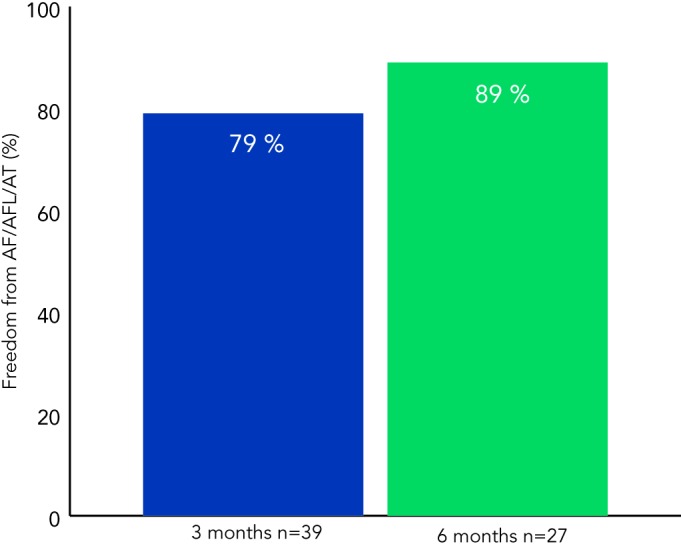
AF = atrial fibrillation; AFL = atrial flutter; AT = atrial tachycardia.
Discussion
Catheter-based ablation of NPAF with associated left atrial anatomic and electrical abnormality is less effective compared with catheter ablation of paroxysmal AF. These more complex patient populations require a safe and effective procedural option to restore sinus rhythm without subsequent interventions.
Enthusiasm for surgical ablation techniques that access the heart through chest incisions or ports has been tempered by extended hospitalisation, post-procedure pain, prolonged recovery and substantial morbidity. On-pump surgical ablation has been associated with a 28.0 % major adverse event rate including 1.7 % peri-operative mortality; the off-pump major adverse event rate has been reported at 14.0 % with 0.5 % peri-operative mortality; and both observed an 11.0 % rehospitalisation rate within the peri-operative period.6 Open surgical ablation has not obtained widespread adoption due to these limitations.
The percutaneous nature of catheter ablation overcomes many deficiencies of surgical ablation. However, endocardial ablation catheters direct energy away from the heart, increasing the potential to damage adjacent structures including the oesophagus; whereas epicardial procedures direct energy into the heat sink of the heart and reduce risk of damage to surrounding structures. Major adverse events during catheter ablation occur in 4–9 % of cases based on multicentre data from US national databases with in-hospital or peri-operative mortality of 0.24–0.96 %.11–14 While catheter ablation has become a primary treatment modality for paroxysmal AF, success has been less favourable for NPAF. Patients often require multiple interventions over a short period of time and outcomes are not sustained long-term.2,4,15 The deficiencies in catheter ablation outcomes are accentuated in patients with enlarged atria; success in patients with LA volume >135 ml was reported to be 18 % irrespective of AF type.5
The convergent epicardial–endocardial AF ablation procedure was designed to provide a safe and effective treatment option for patients with refractory NPAF, including those with enlarged atria and/or underlying structural heart abnormalities. Table 2 details published or presented data from five convergent ablation studies; outcomes demonstrate consistency in the ability to maintain sinus rhythm in NPAF patients at one-year post-procedure.
Table 2: Published and Presented Convergent Procedure Outcomes.
| Reference (# Subjects) | % NPAF | Type of Monitoring | Freedom from AF/AFL/AT (Follow-up) | Repeat Ablations | Adverse Event Rate |
|---|---|---|---|---|---|
| Civello16 (n=104) | 73 % | 72 hours to 14 days Holter | 92.0 % (8.0 months) | 4 % | 5.8 % |
| Gilligan17 (n=39) | 79 % | 72 hours Holter | 94.0 % (12.6 months) | 6 % | 2.6 % |
| Golden18 (n=61) | 88 % | 72 hours Holter | 79.0 % (11.0 months) | 8 % | 3.3 % |
| Gersak10 (n=50) | 94 % | Reveal® XT | 91.0 % (12.0 months) | 2 % | 10.0 % |
| Gehi8 (n=101) | 83 % | 24 hour Holter | 70.5 %* (12.0 months) | 6 % | 6.0 % |
AF = atrial fibrillation; AFL = atrial flutter; AT = atrial tachycardia; NPAF = Non-paroxysmal atrial fibrillation. *Arrhythmia free survival.
While long-term data is needed to further determine the durability of treatment, initial results are promising. A prospective study in a NPAF population with implanted loop recorders that underwent the convergent procedure demonstrated sinus rhythm maintenance in 88 % and 87 % of patients at one and two years, respectively.10
Quality of life has been shown to significantly improve after the convergent procedure; an observation that was durable over 12-month follow-up.8 In this cohort, arrhythmia-free survival after a single procedure was achieved in 66.3 % of patients; and after accounting for the 6.0 % repeat ablation procedures, arrhythmia-free survival was 70.5 %.8
The outcomes presented in this paper show promising initial results in treating NPAF patients with enlarged atria, consistent with those published or presented previously. Our single-centre experience has demonstrated that both components of the procedure can be performed safely and efficiently without major adverse cardiac events. The lack of AF recurrence in patients with a left atrial volume <130 ml and the high interim success in patients with substantially enlarged atria highlight the applicability of treatment to the more complex patients that may not be optimal for endovascular catheter ablation alone. Whether the outcomes are the product of creating more definitive linear lesions, electrically silencing the posterior left atrium, or ablating focal AF drivers requires more investigation.
Multidisciplinary integration and coordination is inherent in the convergent procedure. The elimination of any chest incisions, avoidance of lung deflation, and simplification of the procedure by not dissecting the pericardial to left atrium attachments enable combining epicardial and endocardial ablation within EP standard practice. The closed chest epicardial and endocardial components are performed within the same anaesthetic setting, optimising patient care and integrating hospital operations, while minimising surgical risk. This multidisciplinary solution encourages the establishment of centres of excellence capable of addressing patient and payer demands while providing management options for all AF patient populations.
Convergent procedure outcomes from this and previous initiatives show that cardiac surgeons and electrophysiologists can collaborate to combine best practices for the management of patients with complex AF aetiologies. While further study remains necessary, the initial results have been encouraging and the procedure well-tolerated. Randomised clinical trials are warranted to compare treatment of NPAF with the convergent procedure versus endovascular catheter ablation alone.
References
- 1.Miyasaka Y, Barnes ME, Gersh BJ. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota 1980 to 2000 and implications on the projections for future prevalence. Circulation. 2006;114:119–25. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 2.Sorgente A, Tung P, Wylie J, Josephson ME. Six year follow-up after catheter ablation of atrial fibrillation: a palliation more than a true cure. Am J Cardiol. 2012;109:1179–86. doi: 10.1016/j.amjcard.2011.11.058. [DOI] [PubMed] [Google Scholar]
- 3.Chao TF, Ambrose K, Tsao HM et al. Relationship between the CHADS(2) score and risk of very late recurrences after catheter ablation of paroxysmal atrial fibrillation. Heart Rhythm. 2012;9:1185–91. doi: 10.1016/j.hrthm.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Chao TF, Tsao HM, Lin YJ et al. Clinical outcome of catheter ablation in patients with nonparoxysmal atrial fibrillation: results of 3-year follow-up. Circ Arrhythm Electrophysiol. 2012;5:514–20. doi: 10.1161/CIRCEP.111.968032. [DOI] [PubMed] [Google Scholar]
- 5.Helms AS, West JJ, Patel A et al. Relation of left atrial volume from three-dimensional computed tomography to atrial fibrillation recurrence following ablation. Am J Cardiol. 2009;103:989–93. doi: 10.1016/j.amjcard.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 6.Ad N, Suri RM, Gammie JS et al. Surgical ablation of atrial fibrillation trends and outcomes in North America. J Thorac Cardiovasc Surg. 2012;144:1051–60. doi: 10.1016/j.jtcvs.2012.07.065. [DOI] [PubMed] [Google Scholar]
- 7.Abo-Salem E, Paone RF, Nugent K Stand Alone Surgical Ablation for Atrial Fibrillation. J Card Surg. 2013. [Epub ahead of print]. [DOI] [PubMed]
- 8.Gehi AK, Mounsey JP, Pursell I et al. Hybrid epicardial-endocardial ablation using a pericardioscopic technique for the treatment of atrial fibrillation. Heart Rhythm. 2013;10:22–8. doi: 10.1016/j.hrthm.2012.08.044. [DOI] [PubMed] [Google Scholar]
- 9.Garrett HE, Porter C, Fonger JD et al. Evaluation of an Unipolar RF Coagulation System for Epicardial AF Ablation in Chronic GLP Canine Models. J Innov Card Rhythm Man. 2012;3:1042–8. [Google Scholar]
- 10.Gersak B, Pernat A, Robic B, Sinkovec M. Low rate of atrial fibrillation recurrence verified by implantable loop recorder monitoring following a convergent epicardial and endocardial ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2012;23:1059–66. doi: 10.1111/j.1540-8167.2012.02355.x. [DOI] [PubMed] [Google Scholar]
- 11.Shah RU, Freeman J V, Shilane D et al. Procedural complications, rehospitalizations and repeat procedures after catheter ablation for atrial fibrillation. J Am Coll Cardiol. 2012;59:143–9. doi: 10.1016/j.jacc.2011.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellis ER, Culler SD, Simon AW, Reynolds MR. Trends in utilization and complications of catheter ablation of atrial fibrillation in Medicare beneficiaries. Heart Rhythm. 2009;6:1267–73. doi: 10.1016/j.hrthm.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piccini JP, Sinner MF, Greiner MA et al. Outcomes of medicare beneficiaries undergoing catheter ablation for atrial fibrillation. Circulation. 2012;126:2200–7. doi: 10.1161/CIRCULATIONAHA.112.109330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kneeland PP, Fang MC. Trends in catheter ablation for atrial fibrillation in the United States. J Hosp Med. 2009;4:E1–5. doi: 10.1002/jhm.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tilz RR, Rillig A, Thum AM et al. Catheter ablation of longstanding persistent atrial fibrillation: 5-year outcomes of the Hamburg Sequential Ablation Strategy. J Am Coll Cardiol. 2012;60:1921–9. doi: 10.1016/j.jacc.2012.04.060. [DOI] [PubMed] [Google Scholar]
- 16.Civello K, Smith A, Boedefeld WM. Combined Endocardial/ Epicardial Ablation for AF: Single Center Experience in 100+ Consecutive Patients. Our Lady of Lake Hospital, Baton Rouge, LA. Presented at: HRS Scientific Sessions, Boston, Massachusetts, 11 May 2012.
- 17.Gilligan DM, Joyner CA, Bundy GM. Collaboration of Cardiac Surgery and Electrophysiology to Treat Atrial Fibrillation: Convergent Procedure Outcomes from A Single Center. Levinson Heart Hospital, CJW Medical Center, Richmond, VA. Presented at: HRS Scientific Sessions, Boston, Massachusetts, 12 May 2012.
- 18.Golden K, Olson J, Walts P Clinical outcomes of a new Epicardial/Endocardial ablation procedure (Convergent) for the Treatment of Atrial Fibrillation. St Vincent Hospital, Indianapolis, IN. Presented at: HRS Scientific Sessions, Boston, Massachusetts, 11 May 2012.


