Abstract
Scar-related reentry is the most common mechanism of monomorphic ventricular tachycardia (VT) in patients with structural heart disease. Catheter ablation has assumed an increasingly important role in the management of VT in this setting, and has been shown to reduce VT recurrence and implantable cardioverter defibrillator (ICD) shocks. The approach to mapping and ablation will depend on the underlying heart disease etiology, VT inducibility and haemodynamic stability. This review explores pre-procedural planning, approach to ablation of both mappable and unmappable VT, and post-procedural testing. Future developments in techniques and technology that may improve outcomes are discussed.
Keywords: Ventricular tachycardia, catheter ablation, entrainment mapping, pace mapping, substrate modification, core isolation, epicardial ablation, septal ablation, surgical ablation
Scar-related reentrant ventricular tachycardia (VT) may be present in a variety of structural heart disease (SHD) phenotypes. In this setting, VT circuits are comprised of viable myocytes separated by fibrosis, allowing for the slow conduction needed to facilitate reentry.1,2 Aetiologies of fibrosis include ischaemic heart disease (IHD), inflammatory conditions, infiltrative cardiomyopathy, dilated cardiomyopathy (DCM), hypertrophic cardiomyopathy and arrhythmogenic right ventricular (RV) dysplasia.
Implantable cardioverter defibrillators (ICDs) are the mainstay of therapy for the prevention of sudden cardiac death in patients with SHD.3 However, ICD shocks are associated with diminished quality of life and increased mortality.4–6 Anti-arrhythmic drugs (AADs) have an important role in shock reduction; however these agents often have limited efficacy and significant side-effects.7,8
Catheter ablation has assumed an increasingly important role in the management of VT. In patients with IHD and drug-refractory VT, ablation has been shown to reduce arrhythmia recurrence and ICD therapies.9–11 Patients who have VT rendered non-inducible by an ablation procedure have a lower VT recurrence rate and mortality compared with those who still have inducible arrhythmias after the ablation.12 Catheter ablation has also been shown to be effective in the treatment of VT storm in patients with SHD receiving chronic AAD therapy.13
In the setting of non-ischaemic SHD, catheter ablation outcome varies according to the nature of the underlying heart disease, with a greater need for epicardial mapping and ablation, higher recurrence rate and more AAD use in long-term follow-up.14–16 For the most part, VT ablation remains underutilised and some patients may benefit from earlier intervention.17
Although ablation also has an important role in the management of patients with idiopathic VT, this review will focus on ablation of scar-related reentrant VT, the most common mechanism of monomorphic VT in patients with SHD.
Pre-procedural Planning
Heart failure optimisation is important for decreasing the risk of haemodynamic deterioration during the procedure. This occasionally requires pre-operative assessment of ventricular filling pressures and intravenous (IV) diuresis. When feasible, AADs should be held prior to ablation for a minimum of five half-lives to allow for induction and targeting of all potential VTs. For patients at high risk for VT during the interim or patients requiring amiodarone, bridging with IV lidocaine in a monitored setting has been our standard practice. If severe peripheral vascular disease is suspected, imaging should be performed to guide decisions regarding retrograde aortic versus transseptal left ventricular access, as well as to whether percutaneous left ventricular assist devices (LVADs) can be safely introduced via the femoral artery.
Electrocardiographic Characterisation
Twelve-lead electrocardiograms (ECGs) of all clinical VTs are important in localising VT exit sites, identifying potential ablation targets and directing the best ablation strategy including possible epicardial access. In the setting of non-ischaemic IHD, morphological criteria suggesting an epicardial exit include the presence of Q waves in leads where they do not belong; lead I for basal anterolateral scar (coupled with the absence of inferior Q waves) and leads II and aVF for basal inferior scar. Other criteria based on the identification and quantification of slow conduction in the initial portion of the QRS includes a longer pseudo-delta wave, larger maximum deflection index and longer QRS duration. These criteria are not as sensitive or specific as morphological criteria for the identification of epicardial origin.18 Of note, in the setting of IHD, neither morphological nor quantitative criteria have been shown to reliably predict an epicardial VT exit site.19
In the absence of ECG data, ICD electrograms (EGMs) of the clinical event can be used to confirm that VT occurring spontaneously is consistent with induced VTs in the lab.
Pre-procedural Imaging
Pre-procedural echocardiography is routinely performed to assess biventricular function, elucidate aortic valve pathology prior to retrograde LV access and exclude intramural LV thrombus. Consideration may also be given to the assessment of coronary anatomy, especially when mapping during VT in patients with IHD is desired and exercise testing is equivocal or not easily performed. Of note, patients with coronary artery disease may still exhibit a non-ischaemic substrate / aetiology for VT, as suggested by the presence of multiple VTs of basal origin.20
Further assessment of the location and extent of scar can be performed with magnetic resonance imaging (MRI), and may help in procedural planning and characterising the appropriate target for ablation especially in patients with non-ischaemic SHD. In experienced centres, cardiac MRI can be safely performed in patients with implanted devices,21 although interpretation may be limited by image distortion due to device artifact.22 Patients with non-ischaemic SHD typically exhibit one of two scar patterns on MRI, namely basal anteroseptal and inferolateral.23 This has important implications for the ablation procedure, with a higher need for epicardial access in the antero- or inferolateral subtype and the higher recurrence rate in the anteroseptal (mid-myocardial) subtype.24
Procedural Setup
Our preferred practice is to perform VT ablation with conscious sedation due to several potential advantages including avoidance of arrhythmia suppression by anaesthetic agents, maintenance of higher blood pressure during mapping and faster recovery times. Potential disadvantages include patient discomfort and inadvertent patient movement that can alter the electroanatomical map. If the need for epicardial access arises, general anaesthesia is preferred in order to maximise safety and minimise patient discomfort.
Access to the LV can be obtained via retrograde aortic, transseptal or, rarely, transapical approaches. It has been our routine practice to utilise retrograde access except in patients with aortic valve pathology, significant atheroma / calcification in the ascending aorta or severe peripheral arterial disease. In those patients in whom epicardial access is planned, full sternal preparation and setup for coronary angiography are also performed.
In the setting of baseline haemodynamic compromise, or if mapping during haemodynamically unstable VT is anticipated, consideration may be given to the use of mechanical assist devices. Intra-aortic balloon pumps (IABPs) have a limited role in maintaining haemodynamic stability in the setting of VT and low cardiac output,25 and have not been shown to improve procedural outcomes. Percutaneous LVADs allow for end-organ perfusion during long periods of tachycardia. They have been shown to be safe and effective in supporting haemodynamic status during the procedure, potentially reducing ablation time and hospital length of stay.26,27 There are associated costs and potential morbidities with the use of such devices, and these must be weighed against the benefits. To date no improvement in arrhythmia outcome with these devices has been documented.
Substrate Characterisation
Prior to ablation, detailed endocardial and/or epicardial electroanatomical voltage mapping are routinely performed during normal sinus or paced rhythm. Conventionally, normal endocardial bipolar voltage is defined as >1.5 millivolts (mV); normal epicardial bipolar voltage is defined as >1 mV. Bipolar signal amplitude <0.5 mV correlates with dense fibrosis on surgical pathology. Intermediate values are indicative of border zones.28,29
Unipolar endocardial voltage (recorded between the tip of the ablation catheter and Wilson’s central terminal) can also be assessed for characterisation of mid-myocardial or epicardial scar.30 Confirmation of adequate contact with the ventricular myocardium is important when assessing signal amplitude, and tools such as intracardiac echocardiography (ICE) or contact-force sensing can be helpful.
Markers of abnormal or slow conduction, usually present in and around the area of scar, are identified and tagged during mapping. These include late potentials (LPs), which are low-amplitude EGMs occurring after the end of the surface QRS, and multicomponent or fractionated EGMs.31,32 Of note, mapping of abnormal EGMs during RV pacing may be more sensitive than during normal sinus rhythm (NSR) or biventricular pacing due to a change in the direction of the activation wave front (see Figure 1).33
Figure 1: Important Mapping Concepts.
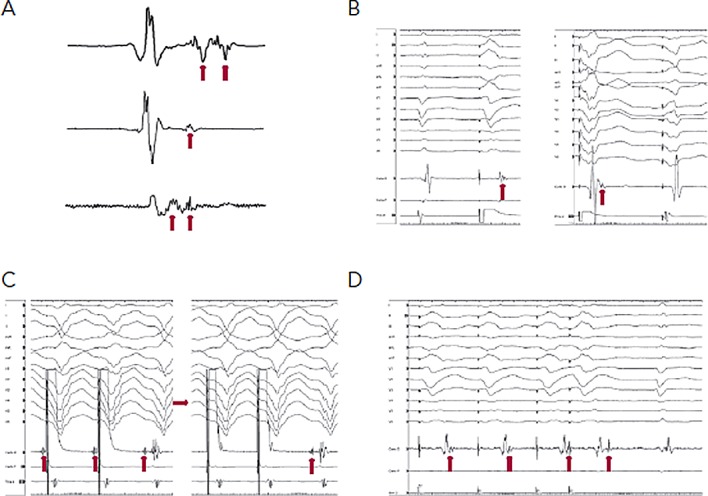
A) Sinus rhythm electrograms (EGMs) demonstrating late potentials and multicomponent EGMs (arrows). B) Right ventricular pacing utilised to expose late potentials (arrows) hidden within the QRS during sinus rhythm (left panel) and biventricular pacing (right panel). C) Entrainment mapping with capture of the far field EGM (left panel). A reduction in pacing output (right panel) results in successful capture of the local EGM (arrow) and evidence of concealed entrainment. D) EGMs recorded from abnormal substrate are poorly coupled to the rest of the myocardium. RV pacing results in separation of the late potential (hidden within the sinus QRS beat), and extrastimulus results in further delay.
Following substrate characterisation, VT induction is attempted (Figure 2). This involves programmed stimulation and burst pacing, with the possible use of isoproterenol. When VT is induced, a recording of the device EGM at 25 mm/second sweep speed is compared with the EGM recorded during the clinical VT episode. The degree of haemodynamic stability during VT will determine the type of mapping that will be pursued prior to ablation. Factors associated with haemodynamically tolerated VT include preserved cardiac output, shorter duration of VT episode, longer VT cycle length and VT induction in a consciously sedated patient.
Figure 2: Approach to Ventricular Tachycardia Ablation.
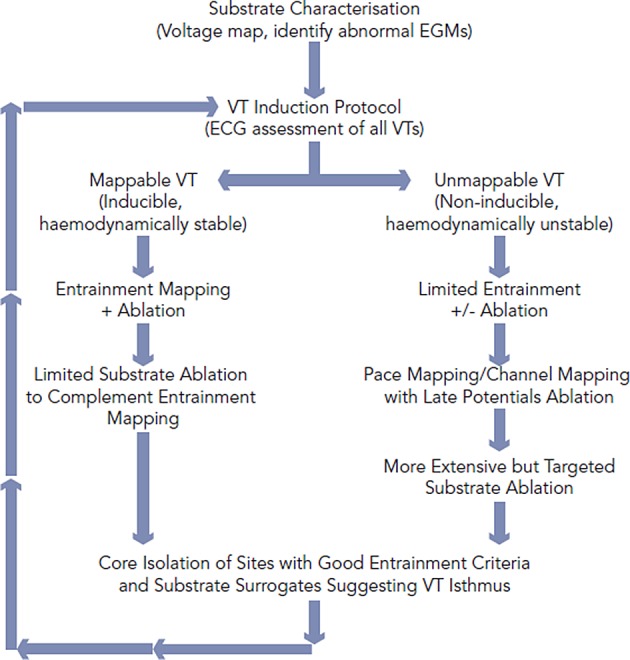
The approach to VT ablation depends on VT inducibility and haemodynamic stability. Although considerable overlap, patients with mappable VT undergo targeted substrate modification following entrainment-guided ablation. Patients with unmappable VT undergoing limited entrainment (if possible) prior to more extensive substrate ablation. Substrate ablation may be guided by pacemapping, channel assessment and location of late potentials. When possible the core of the VT substrate which houses the machinery for the VT circuit based on entrainment and substrate assessment will be isolated to achieve endpoint beyond non-inducibility.
Ablation of Mappable VT
During haemodynamically tolerated scar-related VT, the onset of the surface QRS corresponds temporally to the emergence of the diastolic VT circuit from the scar. Hence, activation mapping can be performed as a rapid method of tracing back the activation wave front within normal myocardium to the vicinity of the VT exit site. Within the area of scar, progressively earlier diastolic activation may be recorded. Mid-diastolic signals are of specific importance as they may represent potential regions of slow-conduction vital to maintaining the VT circuit, where ablation can result in tachycardia termination. However the mere presence of diastolic activation during VT does not guarantee an appropriate ablation target.
Entrainment mapping (see Figure 3), or continuous resetting of the reentrant VT circuit, is designed to identify sites that are vital to maintaining the VT circuit. During haemodynamically tolerated VT, sites with diastolic activation are chosen for entrainment pacing. To minimise temporal changes in the excitable gap of the reentrant circuit, entrainment is performed only 10–20 milliseconds faster than the tachycardia cycle length (TCL). Ensuring local capture by reducing the pacing output may be necessary to avoid capturing far field EGMs (see Figure 1) and ensure a more accurate response to pacing that can be used to identify a VT isthmus site amenable to more limited ablation.
Figure 3: Entrainment Mapping and Ablation During Ventricular Tachycardia.
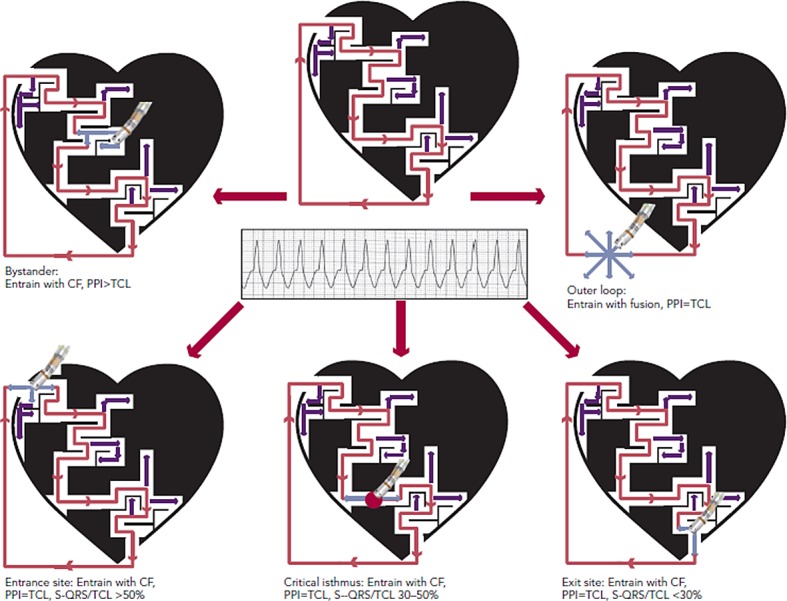
CF = constant fusion, PPI = post-pacing interval, TCL = tachycardia cycle length, S-QRS = stimulus-to-QRS interval. Dense scar is depicted in black with a conducting channel of viable myocardium. Active VT circuit is depicted with red arrows and bystander areas with purple arrows. Pacing is performed faster than the TCL (pale blue arrows), and the response indicates the relationship of the pacing location to the VT circuit. Ablation delivered at the isthmus (red dot) results in VT termination.
Pacing within the protected VT circuit results in a paced QRS morphology that is identical to the spontaneous VT. This is termed concealed fusion, and occurs when the pacing wave front collides antidromically with the propagating VT wave front, while the orthodromic wave front activates the ventricle in an identical manner to the spontaneous VT. The mere presence of concealed fusion is not synonymous with a critical VT circuit element, since “blind end” channels or “bystanders” are often present; these channels are adjacent to, but not part of, the VT circuit. To prove that a specific site is an integral part of the reentrant circuit (i.e. entrance, isthmus or exit site), the post-pacing interval (PPI) should approximate the tachycardia cycle length (TCL). Also in these locations, the stimulus-to-QRS interval (S-QRS) will approximate the EGM-to-QRS.34 Once a protected part of the circuit is identified, the S-QRS timing indicates the conduction time between the pacing site and the exit of the circuit. A shorter S-QRS would suggest a site closer to the exit, whereas a long S-QRS indicates a site closer to the entrance.
Ablation lesions delivered at critical components of the VT circuit are most predictive of VT termination and rendering it noninducible.35 Other phenomena such as termination of VT by a non-captured pacing stimulus or by mechanical catheter pressure may indicate mapping at a site integral for VT propagation. Reasons why ablation at these areas may not be successful include a broad isthmus or the involvement of mid-myocardial/epicardial substrate that extends beyond the lesion size created by currently available catheters.
Ablation of Unmappable VT
Although entrainment mapping is the preferred method for characterising the VT circuit, haemodynamic instability during tachycardia and the need to induce clinical VT may make this approach challenging, particularly in the setting of general anaesthesia. The presence of multiple VT morphologies also makes entrainment mapping challenging. In patients with well-tolerated clinical VT, 74 % will have at least one unmappable VT induced at electrophysiology (EP) study.36 In the irrigated VT ablation trial, 54 % of induced VT morphologies were unmappable, most commonly due to haemodynamic instability.9 Alternative ablation methods utilise mapping and ablation in sinus or paced rhythm (see Figure 4), and have shown comparable efficacy to entrainment mapping.37
Figure 4: Mapping and Ablation During Normal Sinus Rhythm.
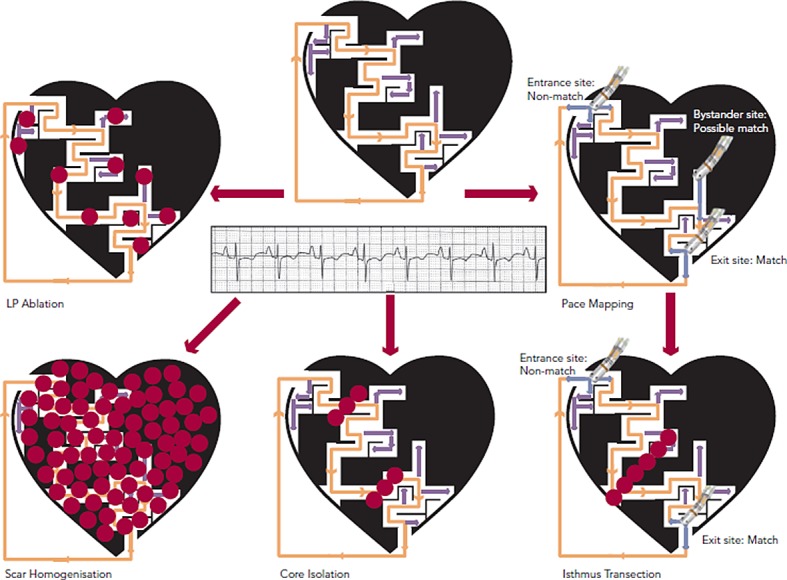
LP= late potentials. Dense scar is depicted in black with a conducting channel of viable myocardium. Inactive VT circuit is depicted with orange arrows and bystander areas with light purple arrows. Different approaches to mapping and ablation include LP ablation, pace mapping with isthmus identification and transection, scar homogenisation and core isolation. Ablation (red dots) is targeted at interrupting the VT circuit.
Channel Identification and Empiric Lines
Attempts have been made to identify conducting channels within scar. In sinus rhythm, such channels may serve as a zone of slow conduction for reentrant VT. They can be identified by reducing the usual voltage cutoff to identify regions of relatively preserved voltage within the dense scar.38–40 Ablation transecting these channels has been proposed as a substrate modification strategy. We have shown that only 30 % of these channels actually predicted a VT isthmus site, although the predictability increased to 85 % for channels that contain isolated LPs.39 However, only 44 % of mappable VTs were associated with any identified channel. Thus, the use of channel identification alone to characterise VT circuits has a low sensitivity and specificity.
Other reports regarding ablation of VT during sinus rhythm investigated the utility of empiric ablation lines. Such lines can be either single or multiple, and transect from the middle of the scar to the border zones.28 Other investigators studied the utility of shorter lines projecting from an area identified as an isthmus or a good pace map.41 Although such ablation lesions may appear on the electroanatomical maps as a line that transects myocardial tissue, assessment of block is rarely performed. More recent studies have utilised more extensive substrate modification strategies in an effort to further reduce VT recurrence.
Substrate Modification
During substrate modification, abnormal EGMs including LPs and fractionated EGMs in and around the scar are targeted in an attempt to ablate all potential channels that can support VT reentry. This approach has been studied extensively in patients with scar-related VT and has shown improved long-term prognosis.42–44 Due to the probabilistic nature of substrate modification, it is accepted that a large amount of ablation will be required. In fact, some centres have suggested a more extensive endocardial and epicardial scar ablation strategy in patients with IHD, termed scar homogenisation.45 Whether this is necessary or superior to the standard endocardial approach requires further investigation.
There are caveats to substrate-guided ablation. The presence of abnormal EGMs does not necessarily predict involvement in the VT circuit. Thus, because elimination of all such EGMs is preferred with this approach, it does require extensive ablation and longer procedural times. It can be difficult to eliminate abnormal potentials, even with long ablation lesions. In addition, standard mapping may fail to detect all abnormal EGMs.42 Detailed “remapping” at the end of the procedure is also frequently performed in order to confirm complete elimination of the specifically targeted arrhythmogenic substrate.
Pace Mapping
Pace mapping involves pacing from areas of abnormal EGMs in and around the scar in an attempt to match the clinical VT morphology, and can help approximate the anatomic VT location. Pacing from within the scar can also identify slow-conduction channels, marked by a prolonged S-QRS.46
VT exit site pacing will yield a “matched” QRS with a short S-QRS. Isthmus site pacing close to the exit will yield a “matched” QRS with a long S-QRS. Entrance site pacing, however, will frequently yield a “non-matched” QRS as the stimulus wave front may also exit antidromically in a different manner from the VT.47 Recently, it has been recognised that an abrupt transition between a paced-QRS that matches the clinical VT (exit site) and a non-matched paced-QRS (entrance site) can identify an isthmus.48 Typical isthmus sites are located in areas with low EGM voltages (< 1.5 mV EGM), with dimensions ranging from 21-59 mm length by 15-47 mm width. Once characterised, isthmus transection with ablation frequently eliminates the targeted VT.
There are important caveats to pace mapping. Although pace mapping may approximate the location of the exit site of the circuit, it does not distinguish isthmus sites from bystander sites, which can only be done with entrainment mapping. In addition, pacing at an entrance site, although potentially a successful ablation site, may yield a “non-matched” QRS. This is in contrast to entrainment, where concealed fusion can be observed all along the isthmus including the entrance location, but with differing S-QRS.
In addition, varying the pacing rate may alter the paced-QRS morphology.49 To minimise this, pacing at VT TCL is suggested. We also recommend using the lowest possible pacing output to reduce the possibility of far field capture.
Scar or VT Core Isolation
In addition to pace mapping and substrate modification, recent techniques involving electrical isolation of the scar have been developed. In patients with IHD, electrical isolation of the entire low-voltage area with a circumferential line along the border zone was associated with a reduction in VT recurrence.50 There is concern about the haemodynamic consequence of extensively ablating in proximity to normal myocardium at the edge of scar.
In our institution, we have focused on electrical isolation of the core portion of the scar that is involved in the VT circuit, rather than the entire scar. In addition to the endpoint of clinical VT non-inducibility, the ability to demonstrate entrance and exit block from the isolated area of scar may improve ablation outcome. Further studies are needed to validate this approach and compare it with other commonly used techniques.
Post-procedural Testing
Following ablation, programmed stimulation is performed with a goal of clinical VT non-inducibility.51 If non-clinical VTs are induced, the decision to perform further ablation is weighed against the risk of a longer procedure time with potential haemodynamic consequences from fluid overload. It is our practice to try to eliminate all inducible VTs if patients remain haemodynamically stable and are tolerating the procedure well.
At our institution, non-invasive programmed stimulation (NIPS) is performed for all patients with SHD via the implanted ICD approximately 48 hours post-VT ablation while withholding AADs. Despite VT non-inducibility at the end of the procedure some patients may still have VT inducible at NIPS, and those have a higher risk of long-term recurrence, including with VT storm.52 In patients with inducible clinical VT at NIPS, consideration is given to repeat the ablation prior to discharge. In those patients with inducible non-clinical VTs, the 12-lead morphology and device EGMs of these VTs are stored for use and review in case of recurrence. Results of the NIPS can also guide device programming and management of AADs.
Special Situations
Patients with non-ischaemic SHD such as DCM may exhibit epicardial or mid-myocardial substrate. Pre-procedural MRI imaging, 12-lead ECG morphology of the clinical VT and or unipolar voltage mapping may identify such substrate.18,53 An endocardial unipolar signal amplitude of <8.3 mV suggests mid-myocardial or epicardial scar.30 Typically, scar in these patients is found along the basal lateral epicardium or the septal mid-myocardium.24 Epicardial VT ablation is rarely required in post-infarct patients.54
Epicardial Ablation
Our standard practice is to reverse anticoagulation prior to epicardial access. Consideration may be given to upfront epicardial access if there is a clear indication (such as previously failed endocardial ablation). Once epicardial access is obtained, as described by Sosa and colleagues,55 the ablation approach is similar to endocardial ablation. Due to the presence of epicardial fat and coronary vessels, abnormal EGMs are defined not just by low voltage, but also by the presence of fractionation and LPs.29
Prior to applying ablation lesions, a safe distance must be confirmed from the coronary arteries (via coronary angiography) and the phrenic nerve (via high-output pacing from the ablation catheter). With few exceptions, ablation at sites within 5 mm of a major coronary artery is generally avoided. Different strategies for phrenic nerve protection during ablation have been described, including epicardial balloon inflation and instillation of air/saline into the pericardial space (see Figure 5).56
Figure 5: Electroanatomic Mapping in a Patient with Ventricular Tachycardia and Underlying Dilated Cardiomyopathy.
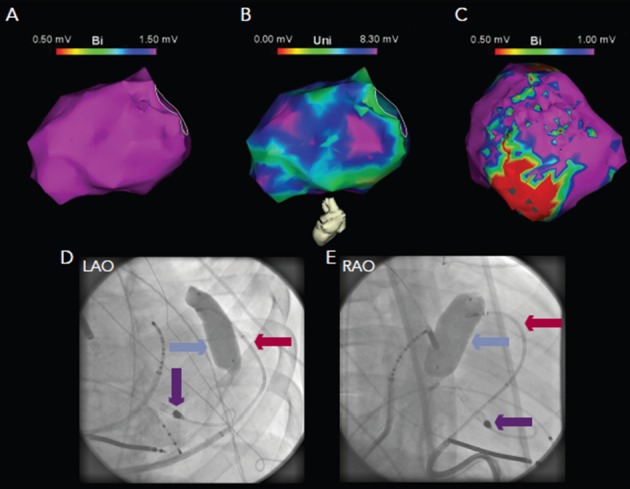
Endocardial mapping reveals normal bipolar (panel A) but abnormal unipolar voltage (panel B), suggestive of mid-myocardial or epicardial scar. Epicardial bipolar mapping (panel C) reveals extensive scar substrate. Due to phrenic nerve capture in this area, inflation of a contrast-filled balloon (blue arrow) in the epicardium was utilised to separate the phrenic nerve from the epicardial surface, with the ablation catheter (red arrow) in contact with the epicardium below the balloon (panels D and F). A left ventricular assist device was used to facilitate mapping and ablation (purple arrow).
Surgical backup is recommended for all epicardial cases in the event of uncontrolled bleeding due to ventricular laceration or vascular damage. In the event of an operative repair, surgical epicardial cryoablation targeting regions of visible epicardial scar or guided by prior mapping data is feasible.57
Septal Ablation
Septal VT substrate poses a particular challenge in patients with DCM.58 Due to septal thickness, endocardial ablation may have difficulty penetrating deep into the septal scar. In addition, potential damage to the conduction system should be considered with the possible need for permanent pacing. In cases of intraseptal VT morphologies refractory to conventional ablation parameters, our approach has been the application of long (2–3 minutes) ablation lesions on both sides of the septum targeting areas of unipolar abnormality. Alternative approaches such as trans-coronary ethanol infusion, bipolar ablation on both sides of the septum and needle ablation catheters have been described.59,60
Surgical VT Ablation
In rare cases where patients have failed multiple percutaneous VT ablation attempts and are also not candidates for cardiac transplant, surgical VT ablation can also be offered.57 In these cases, detailed characterisation of the underlying substrate and identification of the critical components of the VT circuit is performed in the EP lab. Following this, radiofrequency (RF) ablation lesions that were partially successful are targeted in the operating room by cryoablation, accomplishing much larger lesions.57
Future Directions
Advances in mapping tools such as multipolar catheters may decrease procedural times and improve the ability to detect and target abnormal EGMs. The utilisation of contact-force sensing may also improve myocardial contact to enable better ablation penetration and reduce the risk of VT recurrence. Improvement of percutaneous LVADs may allow their safe use in a larger subset of patients in order to reduce filling pressures and facilitate entrainment mapping. Randomised clinical trials and large volume centre registries comparing novel ablation endpoints (e.g. scar homogenisation or core isolation) will hopefully provide improved procedural outcome and guide clinicians to the best ablation strategy, particularly in patients with non-ischaemic substrate.
Acknowledgements
This manuscript was supported in part by the F Harlan Batrus Research Fund.
References
- 1.de Bakker JM, Coronel R, Tasseron S et al. Ventricular tachycardia in the infarcted, Langendorff-perfused human heart: role of the arrangement of surviving cardiac fibers. J Am Coll Cardiol. 1990;15:1594–1607. doi: 10.1016/0735-1097(90)92832-m. [DOI] [PubMed] [Google Scholar]
- 2.de Bakker JM, van Capelle FJ, Janse MJ et al. Slow conduction in the infarcted human heart. “Zigzag” course of activation. Circulation. 1993;88:915–26. doi: 10.1161/01.cir.88.3.915. [DOI] [PubMed] [Google Scholar]
- 3.Zipes DP, Camm AJ, Borggrefe M et al. ACC/AHA/ESC 2006 guidelines for management of patients With ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (writing committee to develop guidelines for management of patients With ventricular arrhythmias and the prevention of sudden cardiac death): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e385–484. doi: 10.1161/CIRCULATIONAHA.106.178233. [DOI] [PubMed] [Google Scholar]
- 4.Ingles J, Sarina T, Kasparian N, Semsarian C et al. Psychological wellbeing and posttraumatic stress associated with implantable cardioverter defibrillator therapy in young adults with genetic heart disease. Int J Cardiol. 2013;168:3779–84. doi: 10.1016/j.ijcard.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Sweeney MO. The contradiction of appropriate shocks in primary prevention ICDs: increasing and decreasing the risk of death. Circulation. 2010;122:2638–41. doi: 10.1161/CIRCULATIONAHA.110.000208. [DOI] [PubMed] [Google Scholar]
- 6.Pacifico A, Ferlic LL, Cedillo-Salazar FR et al. Shocks as predictors of survival in patients with implantable cardioverter-defibrillators. J Am Coll Cardiol. 1999;34:204–10. doi: 10.1016/s0735-1097(99)00142-4. [DOI] [PubMed] [Google Scholar]
- 7.Bardy GH, Lee KL, Mark DB et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–37. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 8.Connolly SJ, Dorian P, Roberts RS et al. Comparison of beta-blockers, amiodarone plus beta-blockers, or sotalol for prevention of shocks from implantable cardioverter defibrillators: the OPTIC Study: a randomized trial. JAMA. 2006;295:165–71. doi: 10.1001/jama.295.2.165. [DOI] [PubMed] [Google Scholar]
- 9.Stevenson WG, Wilber DJ, Natale A et al. Irrigated radiofrequency catheter ablation guided by electroanatomic mapping for recurrent ventricular tachycardia after myocardial infarction: the multicenter thermocool ventricular tachycardia ablation trial. Circulation. 2008;118:2773–82. doi: 10.1161/CIRCULATIONAHA.108.788604. [DOI] [PubMed] [Google Scholar]
- 10.Reddy VY, Reynolds MR, Neuzil P et al. Prophylactic catheter ablation for the prevention of defibrillator therapy. N Engl J Med. 2007;357:2657–65. doi: 10.1056/NEJMoa065457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuck K-H, Schaumann A. et al. Catheter ablation of stable ventricular tachycardia before defibrillator implantation in patients with coronary heart disease (VTACH): a multicentre randomised controlled trial. Lancet. 2010;375:31–40. doi: 10.1016/S0140-6736(09)61755-4. VTACH study group. [DOI] [PubMed] [Google Scholar]
- 12.Ghanbari H, Baser K, Yokokawa M et al. Non-induciblity in post Infarction VT as an end point for VT ablation and its effects on outcomes: a meta-analysis. Circ Arrhythm Electrophysiol. 2014;7:677–83. doi: 10.1161/CIRCEP.113.001404. [DOI] [PubMed] [Google Scholar]
- 13.Carbucicchio C, Santamaria M, Trevisi N et al. Catheter ablation for the treatment of electrical storm in patients with implantable cardioverter-defibrillators: short- and long-term outcomes in a prospective single-center study. Circulation. 2008;117:462–9. doi: 10.1161/CIRCULATIONAHA.106.686534. [DOI] [PubMed] [Google Scholar]
- 14.Piers SRD, Leong DP, van Huls, van Taxis CFB et al. Outcome of ventricular tachycardia ablation in patients with nonischemic cardiomyopathy: the impact of noninducibility. Circ Arrhythm Electrophysiol. 2013;6:513–21. doi: 10.1161/CIRCEP.113.000089. [DOI] [PubMed] [Google Scholar]
- 15.Tokuda M, Tedrow UB, Kojodjojo P et al. Catheter ablation of ventricular tachycardia in nonischemic heart disease. Circ Arrhythm Electrophysiol. 2012;5:992–1000. doi: 10.1161/CIRCEP.112.971341. [DOI] [PubMed] [Google Scholar]
- 16.Dinov B, Fiedler L, Schönbauer R et al. Outcomes in catheter ablation of ventricular tachycardia in dilated nonischemic cardiomyopathy compared with ischemic cardiomyopathy: results from the Prospective Heart Centre of Leipzig VT (HELP-VT) Study. Circulation. 2014;129:728–36. doi: 10.1161/CIRCULATIONAHA.113.003063. [DOI] [PubMed] [Google Scholar]
- 17.Frankel DS, Mountantonakis SE, Robinson MR et al. Ventricular tachycardia ablation remains treatment of last resort in structural heart disease: argument for earlier intervention. J Cardiovasc Electrophysiol. 2011;22:1123–8. doi: 10.1111/j.1540-8167.2011.02081.x. [DOI] [PubMed] [Google Scholar]
- 18.Vallès E, Bazan V, Marchlinski FE. ECG criteria to identify epicardial ventricular tachycardia in nonischemic cardiomyopathy. Circ Arrhythm Electrophysiol. 2010;3:63–71. doi: 10.1161/CIRCEP.109.859942. [DOI] [PubMed] [Google Scholar]
- 19.Martinek M, Stevenson WG, Inada K et al. QRS characteristics fail to reliably identify ventricular tachycardias that require epicardial ablation in ischemic heart disease. J Cardiovasc Electrophysiol. 2012;23:188–93. doi: 10.1111/j.1540-8167.2011.02179.x. [DOI] [PubMed] [Google Scholar]
- 20.Aldhoon B, Tzou WS, Riley MP et al. Nonischemic cardiomyopathy substrate and ventricular tachycardia in the setting of coronary artery disease. Heart Rhythm. 2013;10:1622–7. doi: 10.1016/j.hrthm.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 21.Nazarian S, Hansford R, Roguin A et al. A prospective evaluation of a protocol for magnetic resonance imaging of patients with implanted cardiac devices. Ann Intern Med. 2011;155:415–24. doi: 10.7326/0003-4819-155-7-201110040-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mesubi O, Ahmad G, Jeudy J et al. Impact of ICD artifact burden on late gadolinium enhancement cardiac MR imaging in patients undergoing ventricular tachycardia ablation. Pacing Clin Electrophysiol. 2014;37:1274–83. doi: 10.1111/pace.12405. [DOI] [PubMed] [Google Scholar]
- 23.Piers SRD, Tao Q, van Huls, van Taxis CFB et al. Contrast-enhanced MRI-derived scar patterns and associated ventricular tachycardias in nonischemic cardiomyopathy: implications for the ablation strategy. Circ Arrhythm Electrophysiol. 2013;6:875–83. doi: 10.1161/CIRCEP.113.000537. [DOI] [PubMed] [Google Scholar]
- 24.Oloriz T, Silberbauer J, Maccabelli G et al. Catheter ablation of ventricular arrhythmia in nonischemic cardiomyopathy: anteroseptal versus inferolateral scar sub-types. Circ Arrhythm Electrophysiol. 2014;7:414–23. doi: 10.1161/CIRCEP.114.001568. [DOI] [PubMed] [Google Scholar]
- 25.Papaioannou TG, Stefanadis C. Basic principles of the intraaortic balloon pump and mechanisms affecting its performance. ASAIO J. 2005;51:296–300. doi: 10.1097/01.mat.0000159381.97773.9b. [DOI] [PubMed] [Google Scholar]
- 26.Miller MA, Dukkipati SR, Mittnacht AJ et al. Activation and entrainment mapping of hemodynamically unstable ventricular tachycardia using a percutaneous left ventricular assist device. J Am Coll Cardiol. 2011;58:1363–71. doi: 10.1016/j.jacc.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 27.Aryana A, Gearoid O’Neill P, Gregory D et al. Procedural and clinical outcomes after catheter ablation of unstable ventricular tachycardia supported by a percutaneous left ventricular assist device. Heart Rhythm. 2014;11:1122–30. doi: 10.1016/j.hrthm.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 28.Marchlinski FE, Callans DJ, Gottlieb CD, Zado E. Linear ablation lesions for control of unmappable ventricular tachycardia in patients with ischemic and nonischemic cardiomyopathy. Circulation. 2000;101:1288–96. doi: 10.1161/01.cir.101.11.1288. [DOI] [PubMed] [Google Scholar]
- 29.Cano O, Hutchinson M, Lin D et al. Electroanatomic substrate and ablation outcome for suspected epicardial ventricular tachycardia in left ventricular nonischemic cardiomyopathy. J Am Coll Cardiol. 2009;54:799–808. doi: 10.1016/j.jacc.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 30.Hutchinson MD, Gerstenfeld EP, Desjardins B et al. Endocardial unipolar voltage mapping to detect epicardial ventricular tachycardia substrate in patients with nonischemic left ventricular cardiomyopathy. Circ Arrhythm Electrophysiol. 2011;4:49–55. doi: 10.1161/CIRCEP.110.959957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bogun F, Good E, Reich S et al. Isolated potentials during sinus rhythm and pace-mapping within scars as guides for ablation of post-infarction ventricular tachycardia. J Am Coll Cardiol. 2006;47:2013–9. doi: 10.1016/j.jacc.2005.12.062. [DOI] [PubMed] [Google Scholar]
- 32.Kühne M, Abrams G, Sarrazin J-F et al. Isolated potentials and pace-mapping as guides for ablation of ventricular tachycardia in various types of nonischemic cardiomyopathy. J Cardiovasc Electrophysiol. 2010;21:1017–23. doi: 10.1111/j.1540-8167.2010.01756.x. [DOI] [PubMed] [Google Scholar]
- 33.Arenal A, Glez-Torrecilla E, Ortiz M et al. Ablation of electrograms with an isolated, delayed component as treatment of unmappable monomorphic ventricular tachycardias in patients with structural heart disease. J Am Coll Cardiol. 2003;41:81–92. doi: 10.1016/s0735-1097(02)02623-2. [DOI] [PubMed] [Google Scholar]
- 34.Waldo AL, Henthorn RW. Use of transient entrainment during ventricular tachycardia to localize a critical area in the reentry circuit for ablation. Pacing Clin Electrophysiol. 1989;12:231–44. doi: 10.1111/j.1540-8159.1989.tb02652.x. [DOI] [PubMed] [Google Scholar]
- 35.Stevenson WG, Friedman PL, Sager PT et al. Exploring postinfarction reentrant ventricular tachycardia with entrainment mapping. J Am Coll Cardiol. 1997;29:1180–9. doi: 10.1016/s0735-1097(97)00065-x. [DOI] [PubMed] [Google Scholar]
- 36.Callans DJ, Zado E, Sarter BH et al. Efficacy of radiofrequency catheter ablation for ventricular tachycardia in healed myocardial infarction. Am J Cardiol. 1998;82:429–32. doi: 10.1016/s0002-9149(98)00353-1. [DOI] [PubMed] [Google Scholar]
- 37.Volkmer M, Ouyang F, Deger F et al. Substrate mapping vs. tachycardia mapping using CARTO in patients with coronary artery disease and ventricular tachycardia: impact on outcome of catheter ablation. Europace. 2006;8:968–76. doi: 10.1093/europace/eul109. [DOI] [PubMed] [Google Scholar]
- 38.Arenal A, del Castillo S, Gonzalez-Torrecilla E et al. Tachycardia-related channel in the scar tissue in patients with sustained monomorphic ventricular tachycardias: influence of the voltage scar definition. Circulation. 2004;110:2568–74. doi: 10.1161/01.CIR.0000145544.35565.47. [DOI] [PubMed] [Google Scholar]
- 39.Mountantonakis SE, Park RE, Frankel DS et al. Relationship between voltage map “channels” and the location of critical isthmus sites in patients with post-infarction cardiomyopathy and ventricular tachycardia. J Am Coll Cardiol. 2013;61:2088–95. doi: 10.1016/j.jacc.2013.02.031. [DOI] [PubMed] [Google Scholar]
- 40.Hsia HH, Lin D, Sauer WH et al. Anatomic characterization of endocardial substrate for hemodynamically stable reentrant ventricular tachycardia: identification of endocardial conducting channels. Heart Rhythm. 2006;3:503–12. doi: 10.1016/j.hrthm.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 41.Soejima K, Suzuki M, Maisel WH et al. Catheter ablation in patients with multiple and unstable ventricular tachycardias after myocardial infarction: short ablation lines guided by reentry circuit isthmuses and sinus rhythm mapping. Circulation. 2001;104:664–9. doi: 10.1161/hc3101.093764. [DOI] [PubMed] [Google Scholar]
- 42.Vergara P, Trevisi N, Ricco A et al. Late potentials abolition as an additional technique for reduction of arrhythmia recurrence in scar related ventricular tachycardia ablation. J Cardiovasc Electrophysiol. 2012;23:621–7. doi: 10.1111/j.1540-8167.2011.02246.x. [DOI] [PubMed] [Google Scholar]
- 43.Jaïs P, Maury P, Khairy P et al. Elimination of local abnormal ventricular activities: a new end point for substrate modification in patients with scar-related ventricular tachycardia. Circulation. 2012;125:2184–96. doi: 10.1161/CIRCULATIONAHA.111.043216. [DOI] [PubMed] [Google Scholar]
- 44.Arenal Á, HernÁndez J, Calvo D et al. Safety, long-term results, and predictors of recurrence after complete endocardial ventricular tachycardia substrate ablation in patients with previous myocardial infarction. Am J Cardiol. 2013;111:499–505. doi: 10.1016/j.amjcard.2012.10.031. [DOI] [PubMed] [Google Scholar]
- 45.Di Biase L, Santangeli P, Burkhardt DJ et al. Endo-epicardial homogenization of the scar versus limited substrate ablation for the treatment of electrical storms in patients with ischemic cardiomyopathy. J Am Coll Cardiol. 2012;60:132–41. doi: 10.1016/j.jacc.2012.03.044. [DOI] [PubMed] [Google Scholar]
- 46.Stevenson WG, Sager PT, Natterson PD et al. Relation of pace mapping QRS configuration and conduction delay to ventricular tachycardia reentry circuits in human infarct scars. J Am Coll Cardiol. 1995;26:481–8. doi: 10.1016/0735-1097(95)80026-d. [DOI] [PubMed] [Google Scholar]
- 47.Nayyar S, Wilson L, Ganesan AN et al. High-density mapping of ventricular scar: a comparison of ventricular tachycardia (VT) supporting channels with channels that do not support VT. Circ Arrhythm Electrophysiol. 2014;7:90–8. doi: 10.1161/CIRCEP.113.000882. [DOI] [PubMed] [Google Scholar]
- 48.de Chillou C, Groben L, Magnin-Poull I et al. Localizing the critical isthmus of postinfarct ventricular tachycardia: the value of pace-mapping during sinus rhythm. Heart Rhythm. 2014;11:175–81. doi: 10.1016/j.hrthm.2013.10.042. [DOI] [PubMed] [Google Scholar]
- 49.Goyal R, Harvey M, Daoud EG et al. Effect of coupling interval and pacing cycle length on morphology of paced ventricular complexes. Implications for pace mapping. Circulation. 1996;94:2843–9. doi: 10.1161/01.cir.94.11.2843. [DOI] [PubMed] [Google Scholar]
- 50.Tilz RR, Makimoto H, Lin T et al. Electrical isolation of a substrate after myocardial infarction: a novel ablation strategy for unmappable ventricular tachycardias—feasibility and clinical outcome. Europace. 2014;16:1040–52. doi: 10.1093/europace/eut419. [DOI] [PubMed] [Google Scholar]
- 51.Aliot EM, Stevenson WG, Almendral-Garrote JM et al. EHRA/ HRS expert consensus on catheter ablation of ventricular arrhythmias: developed in a partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC), and the Heart Rhythm Society (HRS); in collaboration with the American College of Cardiology (ACC) and the American Heart Association (AHA) Heart Rhythm. 2009;6:886–933. doi: 10.1016/j.hrthm.2009.04.030. [DOI] [PubMed] [Google Scholar]
- 52.Frankel DS, Mountantonakis SE, Zado ES et al. Noninvasive programmed ventricular stimulation early after ventricular tachycardia ablation to predict risk of late recurrence. J Am Coll Cardiol. 2012;59:1529–35. doi: 10.1016/j.jacc.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 53.Bazan V, Gerstenfeld EP, Garcia FC et al. Site-specific twelve-lead ECG features to identify an epicardial origin for left ventricular tachycardia in the absence of myocardial infarction. Heart Rhythm. 2007;4:1403–10. doi: 10.1016/j.hrthm.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 54.Sarkozy A, Tokuda M, Tedrow UB et al. Epicardial ablation of ventricular tachycardia in ischemic heart disease. Circ Arrhythm Electrophysiol. 2013;6:1115–22. doi: 10.1161/CIRCEP.113.000467. [DOI] [PubMed] [Google Scholar]
- 55.Sosa E, Scanavacca M, d’ Avila A, Pilleggi F. A new technique to perform epicardial mapping in the electrophysiology laboratory. J Cardiovasc Electrophysiol. 1996;7:531–6. doi: 10.1111/j.1540-8167.1996.tb00559.x. [DOI] [PubMed] [Google Scholar]
- 56.Di Biase L, Burkhardt JD, Pelargonio G et al. Prevention of phrenic nerve injury during epicardial ablation: comparison of methods for separating the phrenic nerve from the epicardial surface. Heart Rhythm. 2009;6:957–61. doi: 10.1016/j.hrthm.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 57.Anter E, Hutchinson MD, Deo R et al. Surgical ablation of refractory ventricular tachycardia in patients with nonischemic cardiomyopathy. Circ Arrhythm Electrophysiol. 2011;4:494–500. doi: 10.1161/CIRCEP.111.962555. [DOI] [PubMed] [Google Scholar]
- 58.Haqqani HM, Tschabrunn CM, Tzou WS et al. Isolated septal substrate for ventricular tachycardia in nonischemic dilated cardiomyopathy: incidence, characterization, and implications. Heart Rhythm. 2011;8:1169–76. doi: 10.1016/j.hrthm.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 59.Tokuda M, Sobieszczyk P, Eisenhauer AC et al. Transcoronary ethanol ablation for recurrent ventricular tachycardia after failed catheter ablation: an update. Circ Arrhythm Electrophysiol. 2011;4:889–96. doi: 10.1161/CIRCEP.111.966283. [DOI] [PubMed] [Google Scholar]
- 60.Sapp JL, Beeckler C, Pike R et al. Initial human feasibility of infusion needle catheter ablation for refractory ventricular tachycardia. Circulation. 2013;128:2289–95. doi: 10.1161/CIRCULATIONAHA.113.003423. [DOI] [PubMed] [Google Scholar]


