Abstract
Ablation of atrial fibrillation (AF) is an established treatment option for symptomatic patients refractory to antiarrhythmic medication. In patients with paroxysmal AF, ablation can be offered as first-line therapy when performed in an experienced centre. The accepted cornerstone for all ablation strategies is isolation of the pulmonary veins. However, it is still challenging to achieve contiguous, transmural, permanent lesions using radio-frequency current (RFC) based catheters in conjunction with a three-dimensional mapping system and the learning curve remains long. These limitations have kindled interest in developing and evaluating novel catheter designs that incorporate alternative energy sources. Novel catheters include balloon-based ablation systems, incorporating different energy modalities such as laser (Heartlight™, CardioFocus, Marlborough, MA, US), RFC (Hot Balloon Catheter, Hayama Arrhythmia Institute, Kanagawa, Japan) and cryo-energy (ArcticFront, Medtronic, Inc., Minneapolis, MN, US). While the cryoballoon (CB) and the radiofrequency hot balloon (RHB) are single-shot devices, the endoscopic ablation system (EAS) allows for point-by-point ablation. The CB and EAS are well established as safe, time-efficient and effective ablation tools. Initial studies using the RHB could also demonstrate promising results. However, more data are required.
Keywords: Atrial fibrillation, ablation, pulmonary vein isolation, balloon catheter, cryoballoon, endoscopic ablation system, hot balloon
Atrial fibrillation (AF) is the most common cardiac arrhythmia affecting 1.5–2 % of the general population.1 In the current clinical guidelines, catheter ablation is recommended in addition to drug-based antiarrhythmic therapy for patients suffering from symptomatic, drug-refractory AF. However, catheter ablation, if performed at an experienced centre, may also serve as first-line therapy in patients with paroxysmal AF (PAF).1 Currently, pulmonary vein isolation (PVI) is accepted as the cornerstone of the ablative strategy in patients with PAF but also in persistent forms of AF. Recent evidence from the STAR-AF II study presented at ESC, demonstrated that PVI alone proved to be non-inferior when compared with more extensive ablation strategies such as ablation of complex fractionated atrial electrograms (CFAE) or linear lesions in addition to PVI in patients with persistent AF.2 However, achieving contiguous, transmural, permanent lesions using radio-frequency current (RFC) based catheters in conjunction with a three-dimensional mapping system, remains a real challenge with a long learning curve.
These limitations have led to the development and evaluation of novel catheter designs incorporating alternative energy sources. These catheter designs include RFC-based spiral mapping and ablation catheters (PVACTM, Medtronic, Inc., Minneapolis, MN, USA; nMARQTM, Biosense Webster, Inc., Diamond Bar, CA, USA), as well as balloon-based ablation systems utilising different energy modalities such as laser (HeartlightTM, CardioFocus, Marlborough, MA, USA), RFC (Hot Balloon Catheter, Hayama Arrhythmia Institute, Kanagawa, Japan) and cryo-energy (ArcticFront, Medtronic, Inc., Minneapolis, MN, USA). The cryoballoon (CB) and endoscopic ablation system (EAS) are now well established as safe, clinically effective and time-efficient ablation tools. The radiofrequency hot balloon (RHB) has also shown potential for safe and effective PVI, although more data are needed.
Cryoballoon-based Pulmonary Vein Isolation
The most established balloon-based ablation system is the CB and the first-generation CB was introduced approximately 10 years ago. It consists of a non-compliant balloon which is available in two different diameters (23 mm and 28 mm) and utilises N2O as the refrigerant. A stiff wire or modified spiral mapping catheter (AchieveTM, Medtronic Inc., Minneapolis, MN, US) is inserted via a central lumen of the catheter shaft, allowing safe manipulation within the left atrium and stable balloon-positioning along the ostium of the target PV. The first-generation CB utilised four injection jets for balloon cooling at a rather proximal position of the CB. Consequently, the zone of maximal cooling was along the balloon equator, sparing the distal tip.
Using the first-generation CB and a freeze cycle duration of 300 ms, successful PVI was typically followed by a bonus freeze cycle of the same duration. Multiple studies demonstrated high acute success rates of 92–100 % after PVI using the first-generation CB,3,4 and the learning curve was short as demonstrated in the Sustained Treatment of Paroxysmal Atrial Fibrillation (STOP-AF) study.5 The one-year clinical success rate after PVI using the first-generation CB was 73 %.4 However, upon longer follow-up in a prospective, observational study by Vogt et al., rates of freedom from atrial tachyarrhythmias dropped to 62 % after a single procedure and 76 % after multiple procedures during 30 months of clinical follow-up.6 Similarly, Neumann et al. reported a 5-year single-procedure clinical success rate of 53 %. In summary, 5-year clinical success rates are comparable to the long-term 47 % success rates obtained after RFC-based ablation.7,8 A high rate of electrical PV reconduction was demonstrated in patients with AF recurrence after first-generation CB-based PVI9 and the use of more than one bonus freeze cycle did not result in improved clinical outcomes.10 Regarding the safety profile, ablation using the first-generation CB was associated with a low rate of PV stenosis (0.9 %), cardiac tamponade (0.57 %) and clinically apparent transient ischaemic attack (TIA) or stroke (0.32 %).3 The incidence of transient or persistent right phrenic nerve paralysis (PNP) as a characteristic complication of balloon-based PVI was reported to be 6.4 %, while the rate of persistent PNP (≥1 year) was considerably lower at 0.37 %.3 The incidence of oesophageal thermal injury was between 0 % and 17 %, depending on balloon size.11,12 An atrio-oesophageal fistula after first-generation CB-based PVI was reported as a case report.13
The second-generation CB (ArcticFront AdvanceTM, Medtronic Inc., Minneapolis, MN, US) introduced in 2012 features a modified refrigerant injection system with eight injection jets located at a more distal balloon position. The result is more homogeneous cooling of the complete distal balloon hemisphere including the distal tip (see Figure 1). Similar to the first-generation CB, the rate of acute PVI is 99–100 %.14–16 However, recently published single-centre studies demonstrate a superior 1-year success rate ranging from 80 % to 86 %.15–17 With regards to complications using the second-generation CB, an initial report described an incidence of PNP of 19.5 %.18 In contrast, a study from our centre reported a rate of 3.5 %, which is in line with the rate of PNP using the first-generation CB.19 The incidence of ablation-related oesophageal thermal lesions using the 28 mm second-generation CB increased to 12–19 %.20,21 Safety cut-offs demonstrating a high sensitivity and specificity have been developed to prevent thermal lesion formation.20,21 Prospective studies are needed to validate these safety cut-offs.
Figure 1: The Second-generation Cryoballoon.

A. Offers a more homogeneous cooling of the distal hemisphere and distal balloon tip compared with the first-generation CB; B. Increased incidence of oesophageal thermal lesions using the second-generation 28 mm cryoballoon. Modified from Metzner et al.20
As demonstrated in another interesting study, the rate of real-time recordings from the Achieve catheter increased from 49 % using the first-generation CB to 76 % with the second-generation CB.22 Visualising PV recordings during cryoablation is a prerequisite for an individualised ablation strategy that takes into account the time taken to isolate the target PV. The ongoing ‘FIRE AND ICE’ trial (NCT01490814) is the first to compare the acute and long-term efficacy as well as the safety profile of the second-generation CB with conventional RFC ablation in a prospective, randomised, multicentre fashion. Recruitment is expected to be completed in early 2015. This study will help to clarify the future role of the second-generation CB for catheter ablation of PAF.
Endoscopic Pulmonary Vein Isolation
The EAS is a balloon-based ablation system incorporating a titratable laser energy source and a miniature 2F endoscope, allowing for a spectacular view into the target PV (see Figure 2). The first-generation EAS consisted of a non-compliant balloon available in three sizes 20, 25, and 30 mm diameter) filled and flushed with heavy water (D2O). In addition to the embedded endoscope, the central balloon shaft contained the laser source, which could be titrated from 6.3 W/cm to 7.6 W/cm. Energy was applied via two separate laser arcs of 90° or 150°. In early studies, acute PVI was achieved in 91 % of targeted PVs.23 The AF recurrence rate after 1 year of clinical follow-up was 40 %. However, since the laser arc spanned at least 90°, optimal balloon-to-PV contact was a prerequisite for effective and safe energy transfer. In addition, optimal ostial sealing was hampered by the noncompliant characteristics of the balloon.
Figure 2: Endoscopic View of a Pair of Left-sided Pulmonary Veins With a Visible Laser (green) Along the Anterior Portion of the Pulmonary Vein Ostium.
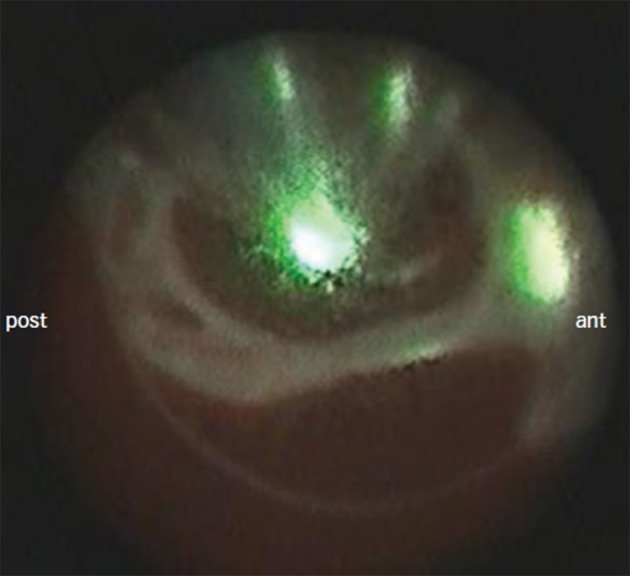
ant = anterior; post = posterior.
The current generation EAS consists of a compliant balloon adjustable in size from 9–35 mm that easily adapts to the individual PV diameter. The laser arc covers 30° of a complete circle, facilitating an individualised ablation line design in a point-by-point fashion. In addition, the current EAS offers energy titration in five steps ranging from 5.5 W to a maximum of 12 W. Higher energy levels are typically applied along the anterior left atrial wall, which is characterised by thicker cardiac tissue, and lower energy levels are used along the thinner posterior wall. Titrating down energy levels along the posterior wall is essential due to the close proximity of extra-cardiac anatomical structures such as the oesophagus.
Table 1: Specific Characteristics of Balloon-based Ablation Systems.
| Cryoballoon | Endoscopic Ablation System | Radiofrequency Hot Balloon | |
| Compliant balloon | no (23 mm or 28 mm) | yes (9–35 mm) | yes (25–35 mm) |
| Energy source | N2O | laser | radiofrequency current |
| Titratable energy source | no | yes | yes |
| ‘Over-the-wire’ | yes | no | yes |
| Single-shot device | yes | no | yes |
| Individual ablation line design | no | yes | no |
Initial studies demonstrated the feasibility of EAS-guided PVI with convincing acute and mid-term clinical efficacy and a favourable safety profile.24 The rate of acute PVI using the EAS ranges from 98 % to 100 %.24–26 The high rate of acute PVI translated into an 86 % durable isolation rate after three months as assessed by Dukkipati et al.25 However, applied energy levels in the initial studies were not standardised and influenced by parameters such as balloon-to-tissue contact, the target site along the PV or by the proximity of extra-cardiac structures such the oesophagus or the phrenic nerve. Consequently, our group systematically evaluated the effect of three different energy settings (posterior 5.5 W/ anterior 7 W vs 7 W/8.5 W vs 8.5 W/10 W) on the acute procedural efficacy and safety in a cohort of 30 patients. The use of higher energy levels (8.5 W/10 W) was associated with a significant increase in the rate of acute PVI after a purely visually-guided ablation circle, thus reducing the need for time-consuming gap-mapping and re-ablation. At the same time, the application of higher energy settings did not compromise the safety profile.27
A similar study was performed by Bordignon et al. investigating the effect of energy titration on clinical efficacy and safety in a cohort of 60 patients with AF.28 The authors compared a low dose (LD) protocol (30 patients, 5.5–8.5 W) with a high dose (HD) protocol (30 patients, >8.5 W) and found that the rate of electrical PVI after completion of a purely visually-guided ablation circle was higher in the HD group compared with the LD group (89 % vs 69 %), that the proportion of patients in whom all PVs were isolated after a single ablation circle per PV was higher in the HD group (70 % vs 39 %), and that the recurrence rate of AF was lower in the HD group during a median follow-up of 311 (261–346) days (17 % vs 40 %).
A comparable rate of oesophageal thermal lesions in patients treated with the EAS (18 %) and those treated with RF energy (15 %) was observed.29 However, the quality of lesions differed in that ulcerations were found in 57 % of the EAS group and in none of the RFC group. Furthermore, in a multicentre analysis of 200 patients the incidence of PNP was 2.5 % and the incidence of pericardial tamponade 2 %.30 However, no stroke, TIA, atrio-oesophageal fistula or significant PV stenosis occurred. In another study from our centre, the incidence of silent cerebral ischaemic lesions after endoscopic ablation was evaluated and reported at 11.4 %, which was not statistically different from irrigated RF (18.2 %) or cryoablation (5 %).31
One-year clinical follow-up data from a prospective, multicentre study in patients with PAF showed a single-procedure clinical success rate of 63 % off anti-arrhythmic medication.26 These results were confirmed by Dukkipati et al. reporting a 1-year success rate off anti-arrhythmic drugs of 60.2 % after one or two procedures.30 Furthermore, Sediva et al. performed a clinical follow-up of 48 months and showed that even 75 % of patients with PAF remained in stable SR after EAS-guided ablation.32
Future modifications of the system may include electrodes on the balloon surface to provide real-time electrical information from the PVs, fluorescence techniques to show transmurality of lesions, and/ or an adjustable laser arc size. A study in the US recently completed enrolment comparing endoscopic EAS ablation with RF-based ablation in a multicentre, prospective, randomised fashion (NCT01456000). First results are expected in early 2015.
Hot Balloon-based Pulmonary Vein Isolation
The RHB (Hayama Arrhythmia Institute, Kanagawa, Japan) consists of a compliant balloon (diameter 25–35 mm) which is introduced into and manipulated within the LA via a 13F steerable transseptal sheath. The catheter shaft houses two lumen. A guide-wire allowing for safe manipulation (‘over-the-wire’ technique) is introduced via one lumen, while a composite of contrast medium and saline is injected via the second lumen. The RHB is inflated and positioned along the respective PV and after verification of optimal PV occlusion the inner fluid is heated up to 70–75°C via a RFC generator with a maximal output of 200 W. The RHB temperature is automatically regulated by RFC energy output at a preselected value.33–35 During ablation, cooling of the oesophagus is performed according to the intraluminal oesophageal temperature measured with a temperature probe. PN pacing is continuously performed during ablation along the septal PVs.
Initial studies could assess a promising acute efficacy in combination with a beneficial safety profile. In a study performed by Sohara et al. 100 patients with PAF or persistent AF were treated by RHB-based PVI and an additional LA box lesion. Acute ablation success was achieved in all patients and after 1-year clinical follow-up 92 patients were in stable SR off antiarrhythmic drugs. No atrial-to-oesophageal fistula and no permanent PN-palsy occurred.36 In a recent study a reduced incidence of oesophageal thermal injury was demonstrated if the oesophagus was actively cooled when the endoluminal temperature exceeded 39°C.37
Conclusions
The second-generation CB as well as the EAS and the RHB are innovative balloon-based ablation systems that have proven safe and clinically effective. Ongoing studies such as the FIRE AND ICE trial comparing the systems to RFC-based ablation will further clarify their role in the treatment of AF.
References
- 1.Camm AJ, Lip GY, De Caterina R et al. 2012 focused update of the ESC guidelines for the management of atrial fibrillation: an update of the 2010 ESC guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33(21):2719–47. doi: 10.1093/eurheartj/ehs253. [DOI] [PubMed] [Google Scholar]
- 2.Verma A, Sanders P, Macle L et al. Substrate and trigger ablation for reduction of atrial fibrillation trial-part II (STAR AF II): design and rationale. Am Heart J. 2012;164(1):1–6. doi: 10.1016/j.ahj.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Andrade JG, Khairy P, Guerra PG et al. Efficacy and safety of cryoballoon ablation for atrial fibrillation: a systematic review of published studies. Heart Rhythm. 2011;8:1444–51. doi: 10.1016/j.hrthm.2011.03.050. [DOI] [PubMed] [Google Scholar]
- 4.Chun KR, Schmidt B, Metzner A et al. The “single big cryoballoon” technique for acute pulmonary vein isolation in patients with paroxysmal atrial fibrillation: a prospective observational single centre study. Eur Heart J. 2009;30:699–709. doi: 10.1093/eurheartj/ehn570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Packer DL, Kowal RC, Wheelan KR et al. STOP AF Cryoablation Investigators. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial. J Am Coll Cardiol. 2013;61:1713–23. doi: 10.1016/j.jacc.2012.11.064. [DOI] [PubMed] [Google Scholar]
- 6.Vogt J, Heintze J, Gutleben KJ et al. Long-term outcomes after cryoballoon pulmonary vein isolation: results from a prospective study in 605 patients. J Am Coll Cardiol. 2013;61(16):1707–12. doi: 10.1016/j.jacc.2012.09.033. [DOI] [PubMed] [Google Scholar]
- 7.Neumann T, Wójcik M, Berkowitsch A et al. Cryoballoon ablation of paroxysmal atrial fibrillation: 5-year outcome after single procedure and predictors of success. Europace. 2013;15(8):1143–9. doi: 10.1093/europace/eut021. [DOI] [PubMed] [Google Scholar]
- 8.Ouyang F, Tilz R, Chun J et al. Long-term results of catheter ablation in paroxysmal atrial fibrillation: lessons from a 5-year follow-up. Circulation. 2010;122(23):2368–77. doi: 10.1161/CIRCULATIONAHA.110.946806. [DOI] [PubMed] [Google Scholar]
- 9.Fürnkranz A, Chun KR, Nuyens D et al. Characterization of conduction recovery after pulmonary vein isolation using the “single big cryoballoon” technique. Heart Rhythm. 2010;7(2):184–90. doi: 10.1016/j.hrthm.2009.10.038. [DOI] [PubMed] [Google Scholar]
- 10.Chun KR, Fürnkranz A, Köster I et al. Two versus one repeat freeze-thaw cycle(s) after cryoballoon pulmonary vein isolation: the alster extra pilot study. J Cardiovasc Electrophysiol. 2012;23(8):814–9. doi: 10.1111/j.1540-8167.2012.02315.x. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed H, Neuzil P, d’Avila A et al. The esophageal effects of cryoenergy during cryoablation for atrial fibrillation. Heart Rhythm. 2009;6(7):962–9. doi: 10.1016/j.hrthm.2009.03.051. [DOI] [PubMed] [Google Scholar]
- 12.Fürnkranz A, Chun KR, Metzner A et al. Esophageal endoscopy results after pulmonary vein isolation using the single big cryoballoon technique. J Cardiovasc Electrophysiol. 2010;21(8):869–74. doi: 10.1111/j.1540-8167.2010.01739.x. [DOI] [PubMed] [Google Scholar]
- 13.Stöckigt F, Schrickel JW, Andrié R, Lickfett L. Atrioesophageal fistula after cryoballoon pulmonary vein isolation. J Cardiovasc Electrophysiol. 2012;23(11):1254–7. doi: 10.1111/j.1540-8167.2012.02324.x. [DOI] [PubMed] [Google Scholar]
- 14.Chierchia GB, Di Giovanni G, Sieira-Moret J et al. Initial experience of three-minute freeze cycles using the second-generation cryoballoon ablation: acute and short-term procedural outcomes. J Interv Card Electrophysiol. 2014;39:145–51. doi: 10.1007/s10840-013-9855-x. [DOI] [PubMed] [Google Scholar]
- 15.Fürnkranz A, Bordignon S, Schmidt B et al. Improved procedural efficacy of pulmonary vein isolation using the novel second-generation cryoballoon. J Cardiovas Electrophysiol. 2013;24:492–7. doi: 10.1111/jce.12082. [DOI] [PubMed] [Google Scholar]
- 16.Metzner A, Reissmann B, Rausch P et al. One-year clinical outcome after pulmonary vein isolation using the second-generation 28-mm cryoballoon. Circ Arrhythm Electrophysiol. 2014;7(2):288–92. doi: 10.1161/CIRCEP.114.001473. [DOI] [PubMed] [Google Scholar]
- 17.Ciconte G, Chierchia GB, De Asmundis C et al. Spontaneous and adenosine-induced pulmonary vein reconnection after cryoballoon ablation with the second-generation device. J Cardiovasc Electrophysiol. 2014;25(8):845–51. doi: 10.1111/jce.12421. [DOI] [PubMed] [Google Scholar]
- 18.Casado-Arroyo R, Chierchia GB, Conte G et al. Phrenic nerve paralysis during cryoballoon ablation for atrial fibrillation: a comparison between the first- and second-generation balloon. Heart Rhythm. 2013;10(9):1318–24. doi: 10.1016/j.hrthm.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Metzner A, Rausch P, Lemes C et al. The incidence of phrenic nerve injury during pulmonary vein isolation using the second-generation 28 mm cryoballoon. J Cardiovasc Electrophysiol. 2014;25:466–70. doi: 10.1111/jce.12358. [DOI] [PubMed] [Google Scholar]
- 20.Metzner A, Burchard A, Wohlmuth P et al. Increased incidence of esophageal thermal lesions using the second-generation 28 mm cryoballoon. Circ Arrhythm Electrophysiol. 2013;6(4):769–75. doi: 10.1161/CIRCEP.113.000228. [DOI] [PubMed] [Google Scholar]
- 21.Fürnkranz A, Bordignon S, Schmidt B et al. Luminal esophageal temperature predicts esophageal lesions after second-generation cryoballoon pulmonary vein isolation. Heart Rhythm. 2013;10(6):789–93. doi: 10.1016/j.hrthm.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 22.Fürnkranz A, Bordignon S, Schmidt B et al. Improved procedural efficacy of pulmonary vein isolation using the novel second-generation cryoballoon. J Cardiovasc Electrophysiol. 2013;24(5):492–7. doi: 10.1111/jce.12082. [DOI] [PubMed] [Google Scholar]
- 23.Reddy VY, Neuzil P, Themistoclakis S et al. Visually-guided balloon catheter ablation of atrial fibrillation: experimental feasibility and first-in-human multicenter clinical outcome. Circulation. 2009;120(1):12–20. doi: 10.1161/CIRCULATIONAHA.108.840587. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt B, Metzner A, Chun KR et al. Feasibility of circumferential pulmonary vein isolation using a novel endoscopic ablation system. Circ Arrhythm Electrophysiol. 2010;3(5):481–8. doi: 10.1161/CIRCEP.110.954149. [DOI] [PubMed] [Google Scholar]
- 25.Dukkipati SR, Neuzil P, Kautzner J et al. The durability of pulmonary vein isolation using the visually guided laser balloon catheter: Multicenter results of pulmonary vein remapping studies. Heart Rhythm. 2012;9(6):919–25. doi: 10.1016/j.hrthm.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 26.Metzner A, Wissner E, Schmidt B et al. Acute and long-term clinical outcome after endoscopic pulmonary vein isolation: results from the first prospective, multicenter study. J Cardiovasc Electrophysiol. 2013;24(1):7–13. doi: 10.1111/j.1540-8167.2012.02427.x. [DOI] [PubMed] [Google Scholar]
- 27.Metzner A, Wissner E, Schoonderwoerd B et al. The influence of varying energy settings on efficacy and safety of endoscopic pulmonary vein isolation. Heart Rhythm. 2012;9(9):1380–5. doi: 10.1016/j.hrthm.2012.03.059. [DOI] [PubMed] [Google Scholar]
- 28.Bordignon S, Chun KR, Gunawardene M et al. Energy titration strategies with the endoscopic ablation system: lessons from the high-dose vs. low-dose laser ablation study. Europace. 2013;15(5):685–9. doi: 10.1093/europace/eus352. [DOI] [PubMed] [Google Scholar]
- 29.Metzner A, Schmidt B, Fuernkranz A et al. Esophageal temperature change and esophageal thermal lesions after pulmonary vein isolation using the novel endoscopic ablation system. Heart Rhythm. 2011;8(6):815–20. doi: 10.1016/j.hrthm.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 30.Dukkipati SR, Kuck KH, Neuzil P et al. Pulmonary vein isolation using a visually guided laser balloon catheter: the first 200-patient multicenter clinical experience. Circ Arrhythm Electrophysiol. 2013;6(3):467–72. doi: 10.1161/CIRCEP.113.000431. [DOI] [PubMed] [Google Scholar]
- 31.Wissner E, Metzner A, Neuzil P et al. Asymptomatic brain lesions following laserballoon-based pulmonary vein isolation. Europace. 2014;16(2):214–9. doi: 10.1093/europace/eut250. [DOI] [PubMed] [Google Scholar]
- 32.Sedivá L, Petrů J, Skoda J et al. Visually guided laser ablation: a single-centre long-term experience. Europace. 2014;16(12):1746–51. doi: 10.1093/europace/euu168. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka K, Satake S, Saito S et al. A new radiofrequency thermal balloon catheter for pulmonary vein isolation. J Am Coll Cardiol. 2001;38:2079–86. doi: 10.1016/s0735-1097(01)01666-7. [DOI] [PubMed] [Google Scholar]
- 34.Sohara H, Satake S, Tanaka K, Watanabe Y. Simultaneous pulmonary vein and adjacent leftatrium ablation using a radiofrequency hot balloon catheter to treat atrial fibrillation: feasibility, safety, and long-term results in an initial far eastern clinical trial. Europace. 2006;8(Suppl I):264. [Google Scholar]
- 35.Sohara H, Takeda H, Ueno H, Satake S. Monitoring the esophageal temperature during hot balloon catheter ablation for atrial fibrillation to avoid asymptomatic esophageal ulcer. Circulation J. 2008;72(Suppl I):711. [Google Scholar]
- 36.Sohara H, Takeda H, Ueno H et al. Feasibility of the radiofrequency hot balloon catheter for isolation of the posterior left atrium and pulmonary veins for the treatment of atrial fibrillation. Circ Arrhythm Electrophysiol. 2009;2(3):225–32. doi: 10.1161/CIRCEP.108.817205. [DOI] [PubMed] [Google Scholar]
- 37.Sohara H, Satake S, Takeda H et al. Prevalence of esophageal ulceration after atrial fibrillation ablation with the hot balloon ablation catheter: what is the value of esophageal cooling? J Cardiovasc Electrophysiol. 2014;25(7):686–92. doi: 10.1111/jce.12394. [DOI] [PubMed] [Google Scholar]


