Abstract
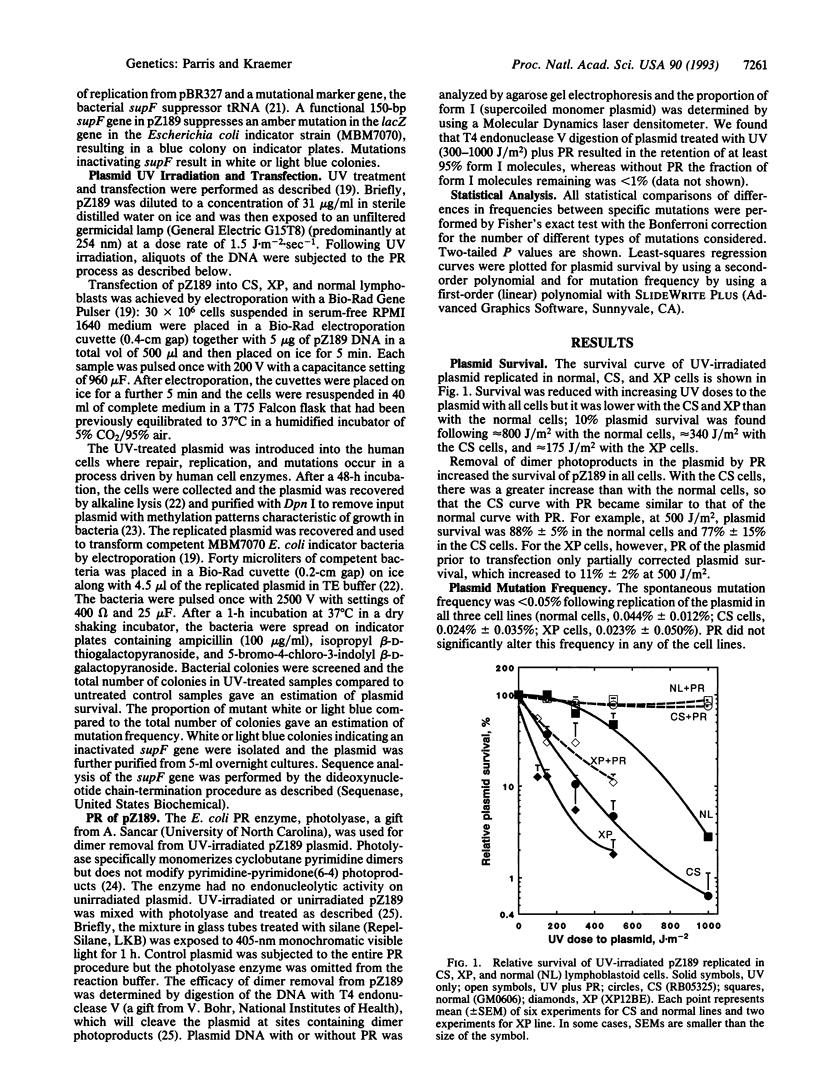
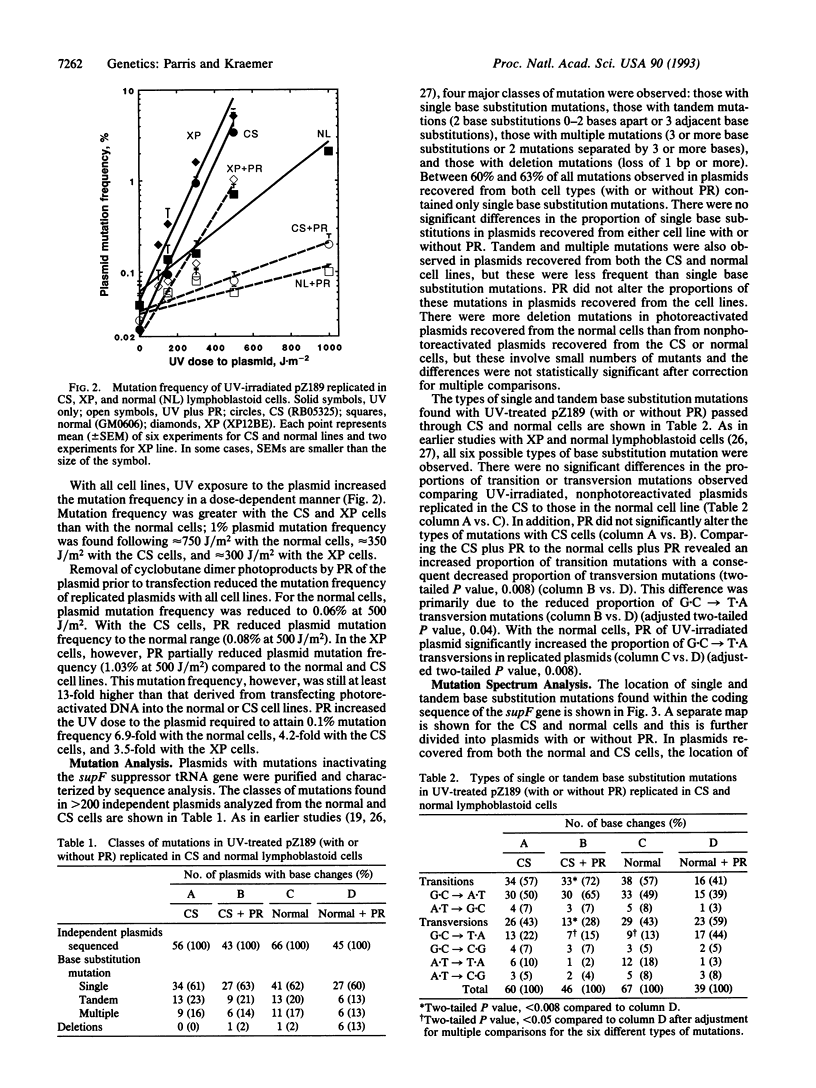
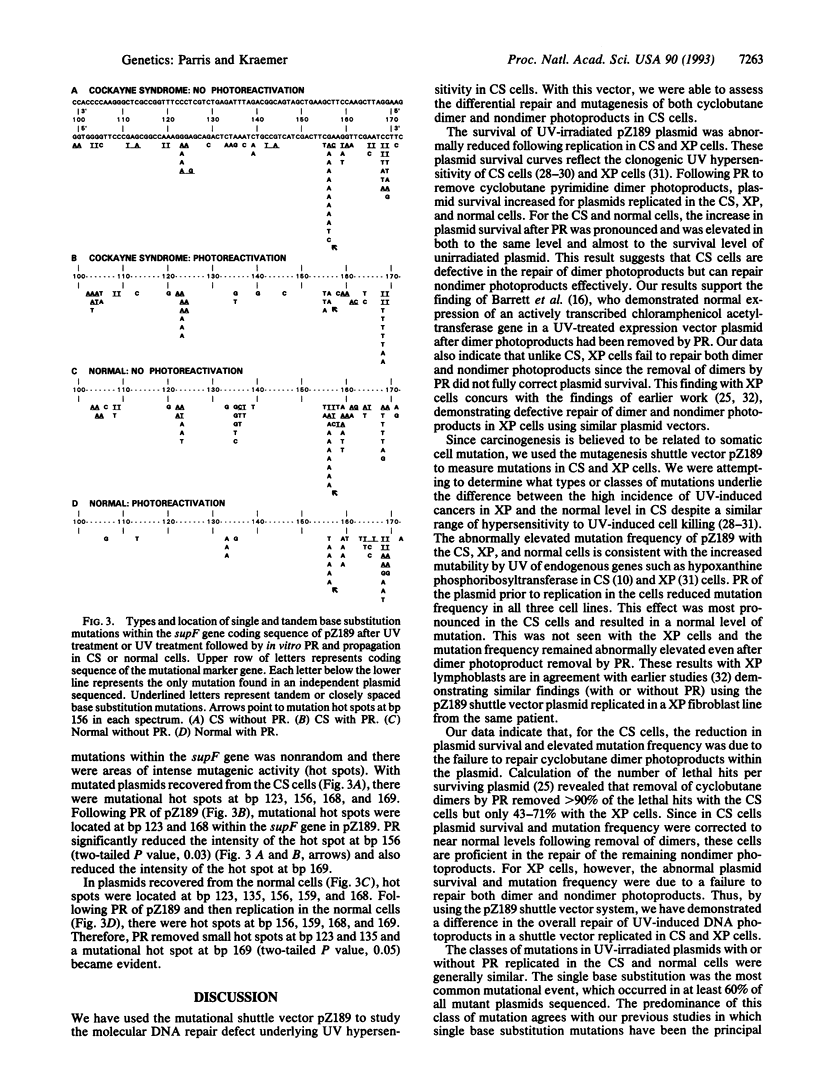
We compared the contribution to mutagenesis in Cockayne syndrome (CS) cells of the major class of UV photoproducts, the cyclobutane pyrimidine dimer, to that of other DNA photoproducts by using the mutagenesis shuttle vector pZ189. Lymphoblastoid cell lines from the DNA repair-deficient disorders CS and xeroderma pigmentosum (XP) and a normal line were transfected with UV-treated pZ189. Cyclobutane dimers were selectively removed before transfection by photoreactivation (PR), leaving nondimer photoproducts intact. After UV exposure and replication in CS and XP cells, plasmid survival was abnormally reduced and mutation frequency was abnormally elevated. After PR, plasmid survival increased and mutation frequency in CS cells decreased to normal levels but remained abnormal in XP cells. Sequence analysis of > 200 mutant plasmids showed that with CS cells a major mutational hot spot was caused by unrepaired cyclobutane dimers. These data indicate that with both CS and XP cyclobutane dimers are major photoproducts generating reduced plasmid survival and increased mutation frequency. However, unlike XP, CS cells are proficient in repair of nondimer photoproducts. Since XP but not CS patients have a high frequency of UV-induced skin cancers, our data suggest that prevention of UV-induce skin cancers is associated with proficient repair of nondimer photoproducts.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews A. D., Barrett S. F., Yoder F. W., Robbins J. H. Cockayne's syndrome fibroblasts have increased sensitivity to ultraviolet light but normal rates of unscheduled DNA synthesis. J Invest Dermatol. 1978 May;70(5):237–239. doi: 10.1111/1523-1747.ep12541383. [DOI] [PubMed] [Google Scholar]
- Barrett S. F., Robbins J. H., Tarone R. E., Kraemer K. H. Evidence for defective repair of cyclobutane pyrimidine dimers with normal repair of other DNA photoproducts in a transcriptionally active gene transfected into Cockayne syndrome cells. Mutat Res. 1991 Nov;255(3):281–291. doi: 10.1016/0921-8777(91)90032-k. [DOI] [PubMed] [Google Scholar]
- Brash D. E., Franklin W. A., Sancar G. B., Sancar A., Haseltine W. A. Escherichia coli DNA photolyase reverses cyclobutane pyrimidine dimers but not pyrimidine-pyrimidone (6-4) photoproducts. J Biol Chem. 1985 Sep 25;260(21):11438–11441. [PubMed] [Google Scholar]
- Brash D. E., Seetharam S., Kraemer K. H., Seidman M. M., Bredberg A. Photoproduct frequency is not the major determinant of UV base substitution hot spots or cold spots in human cells. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3782–3786. doi: 10.1073/pnas.84.11.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredberg A., Kraemer K. H., Seidman M. M. Restricted ultraviolet mutational spectrum in a shuttle vector propagated in xeroderma pigmentosum cells. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8273–8277. doi: 10.1073/pnas.83.21.8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumback R. A., Yoder F. W., Andrews A. D., Peck G. L., Robbins J. H. Normal pressure hydrocephalus. Recognition and relationship to neurological abnormalities in Cockayne's syndrome. Arch Neurol. 1978 Jun;35(6):337–345. doi: 10.1001/archneur.1978.00500300011002. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. DNA repair in man. Birth Defects Orig Artic Ser. 1989;25(2):61–82. [PubMed] [Google Scholar]
- Deschavanne P. J., Chavaudra N., Fertil B., Malaise E. P. Abnormal sensitivity of some Cockayne's syndrome cell strains to UV- and gamma-rays. Association with a reduced ability to repair potentially lethal damage. Mutat Res. 1984 Feb;131(2):61–70. doi: 10.1016/0167-8817(84)90012-9. [DOI] [PubMed] [Google Scholar]
- Glickman B. W., Schaaper R. M., Haseltine W. A., Dunn R. L., Brash D. E. The C-C (6-4) UV photoproduct is mutagenic in Escherichia coli. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6945–6949. doi: 10.1073/pnas.83.18.6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoar D. I., Waghorne C. DNA repair in Cockayne syndrome. Am J Hum Genet. 1978 Nov;30(6):590–601. [PMC free article] [PubMed] [Google Scholar]
- Jiang N., Taylor J. S. In vivo evidence that UV-induced C-->T mutations at dipyrimidine sites could result from the replicative bypass of cis-syn cyclobutane dimers or their deamination products. Biochemistry. 1993 Jan 19;32(2):472–481. doi: 10.1021/bi00053a011. [DOI] [PubMed] [Google Scholar]
- Kraemer K. H., Lee M. M., Scotto J. Xeroderma pigmentosum. Cutaneous, ocular, and neurologic abnormalities in 830 published cases. Arch Dermatol. 1987 Feb;123(2):241–250. doi: 10.1001/archderm.123.2.241. [DOI] [PubMed] [Google Scholar]
- Kraemer K. H., Seidman M. M. Use of supF, an Escherichia coli tyrosine suppressor tRNA gene, as a mutagenic target in shuttle-vector plasmids. Mutat Res. 1989 Mar-May;220(2-3):61–72. doi: 10.1016/0165-1110(89)90011-0. [DOI] [PubMed] [Google Scholar]
- Lehmann A. R., Kirk-Bell S., Arlett C. F., Harcourt S. A., de Weerd-Kastelein E. A., Keijzer W., Hall-Smith P. Repair of ultraviolet light damage in a variety of human fibroblast cell strains. Cancer Res. 1977 Mar;37(3):904–910. [PubMed] [Google Scholar]
- Lehmann A. R., Kirk-Bell S., Mayne L. Abnormal kinetics of DNA synthesis in ultraviolet light-irradiated cells from patients with Cockayne's syndrome. Cancer Res. 1979 Oct;39(10):4237–4241. [PubMed] [Google Scholar]
- Lehmann A. R. Three complementation groups in Cockayne syndrome. Mutat Res. 1982 Dec;106(2):347–356. doi: 10.1016/0027-5107(82)90115-4. [DOI] [PubMed] [Google Scholar]
- Marshall R. R., Arlett C. F., Harcourt S. A., Broughton B. A. Increased sensitivity of cell strains from Cockayne's syndrome to sister-chromatid-exchange induction and cell killing by UV light. Mutat Res. 1980 Jan;69(1):107–112. doi: 10.1016/0027-5107(80)90180-3. [DOI] [PubMed] [Google Scholar]
- Mayne L. V., Lehmann A. R. Failure of RNA synthesis to recover after UV irradiation: an early defect in cells from individuals with Cockayne's syndrome and xeroderma pigmentosum. Cancer Res. 1982 Apr;42(4):1473–1478. [PubMed] [Google Scholar]
- Mitchell D. L., Nairn R. S. The biology of the (6-4) photoproduct. Photochem Photobiol. 1989 Jun;49(6):805–819. doi: 10.1111/j.1751-1097.1989.tb05578.x. [DOI] [PubMed] [Google Scholar]
- Muriel W. J., Lamb J. R., Lehmann A. R. UV mutation spectra in cell lines from patients with Cockayne's syndrome and ataxia telangiectasia, using the shuttle vector pZ189. Mutat Res. 1991 Mar;254(2):119–123. doi: 10.1016/0921-8777(91)90002-7. [DOI] [PubMed] [Google Scholar]
- Nance M. A., Berry S. A. Cockayne syndrome: review of 140 cases. Am J Med Genet. 1992 Jan 1;42(1):68–84. doi: 10.1002/ajmg.1320420115. [DOI] [PubMed] [Google Scholar]
- Norris P. G., Arlett C. F., Cole J., Lehmann A. R., Hawk J. L. Abnormal erythemal response and elevated T lymphocyte HRPT mutant frequency in Cockayne's syndrome. Br J Dermatol. 1991 May;124(5):453–460. doi: 10.1111/j.1365-2133.1991.tb00625.x. [DOI] [PubMed] [Google Scholar]
- Otsuka F., Tarone R. E., Cayeux S., Robbins J. H. Use of lymphoblastoid cell lines to evaluate the hypersensitivity to ultraviolet radiation in Cockayne syndrome. J Invest Dermatol. 1984 May;82(5):480–484. doi: 10.1111/1523-1747.ep12260999. [DOI] [PubMed] [Google Scholar]
- Parris C. N., Kraemer K. H. Ultraviolet mutagenesis in human lymphocytes: the effect of cellular transformation. Exp Cell Res. 1992 Aug;201(2):462–469. doi: 10.1016/0014-4827(92)90295-j. [DOI] [PubMed] [Google Scholar]
- Peak M. J., Peak J. G. Solar-ultraviolet-induced damage to DNA. Photodermatol. 1989 Feb;6(1):1–15. [PubMed] [Google Scholar]
- Peden K. W., Pipas J. M., Pearson-White S., Nathans D. Isolation of mutants of an animal virus in bacteria. Science. 1980 Sep 19;209(4463):1392–1396. doi: 10.1126/science.6251547. [DOI] [PubMed] [Google Scholar]
- Perera M. I., Um K. I., Greene M. H., Waters H. L., Bredberg A., Kraemer K. H. Hereditary dysplastic nevus syndrome: lymphoid cell ultraviolet hypermutability in association with increased melanoma susceptibility. Cancer Res. 1986 Feb;46(2):1005–1009. [PubMed] [Google Scholar]
- Protić-Sabljić M., Kraemer K. H. Reduced repair of non-dimer photoproducts in a gene transfected into xeroderma pigmentosum cells. Photochem Photobiol. 1986 May;43(5):509–513. doi: 10.1111/j.1751-1097.1986.tb09528.x. [DOI] [PubMed] [Google Scholar]
- Seetharam S., Kraemer K. H., Waters H. L., Seidman M. M. Mutational hotspot variability in an ultraviolet-treated shuttle vector plasmid propagated in xeroderma pigmentosum and normal human lymphoblasts and fibroblasts. J Mol Biol. 1990 Apr 5;212(3):433–436. doi: 10.1016/0022-2836(90)90319-H. [DOI] [PubMed] [Google Scholar]
- Seetharam S., Kraemer K. H., Waters H. L., Seidman M. M. Ultraviolet mutational spectrum in a shuttle vector propagated in xeroderma pigmentosum lymphoblastoid cells and fibroblasts. Mutat Res. 1991 Jan;254(1):97–105. doi: 10.1016/0921-8777(91)90045-q. [DOI] [PubMed] [Google Scholar]
- Seidman M. M., Dixon K., Razzaque A., Zagursky R. J., Berman M. L. A shuttle vector plasmid for studying carcinogen-induced point mutations in mammalian cells. Gene. 1985;38(1-3):233–237. doi: 10.1016/0378-1119(85)90222-7. [DOI] [PubMed] [Google Scholar]
- Seidman M. The development of transient SV40 based shuttle vectors for mutagenesis studies: problems and solutions. Mutat Res. 1989 Mar-May;220(2-3):55–60. doi: 10.1016/0165-1110(89)90010-9. [DOI] [PubMed] [Google Scholar]
- Tatsumi K., Toyoda M., Hashimoto T., Furuyama J., Kurihara T., Inoue M., Takebe H. Differential hypersensitivity of xeroderma pigmentosum lymphoblastoid cell lines to ultraviolet light mutagenesis. Carcinogenesis. 1987 Jan;8(1):53–57. doi: 10.1093/carcin/8.1.53. [DOI] [PubMed] [Google Scholar]
- Venema J., Mullenders L. H., Natarajan A. T., van Zeeland A. A., Mayne L. V. The genetic defect in Cockayne syndrome is associated with a defect in repair of UV-induced DNA damage in transcriptionally active DNA. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4707–4711. doi: 10.1073/pnas.87.12.4707. [DOI] [PMC free article] [PubMed] [Google Scholar]


