Abstract
The effect of monovalent cations (Li+, K+, NH4+, Na+) on the water structure in aqueous chloride and acetate solutions was characterized by oxygen K-edge X-ray absorption spectroscopy (XAS), X-ray emission spectroscopy, and resonant inelastic X-ray scattering (RIXS) of a liquid microjet. We show ion- and counterion dependent effects on the emission spectra of the oxygen K-edge, which we attribute to modifications of the hydrogen bond network of water. For acetates, ion pairing with carboxylates was also probed selectively by XAS and RIXS. We correlate our experimental results to speciation data and to the salting-out properties of the cations.
I. INTRODUCTION
Since the pioneering experiments of Hofmeister on precipitation of egg white proteins,1 the capability of ions to salt-in or salt-out proteins in solution has been thoroughly investigated.2–4 However, the origin of the so-called “Hofmeister effect” still remains under discussion and different salting mechanisms have been proposed.3 More particularly, it was suggested that modification of the hydrogen bond structure of water molecules induced by ions is responsible for changes in macromolecule solubility.5 According to this picture, ions were classified into “chaotropes” and “kosmotropes” depending on their salting abilities.2,6,7 Nevertheless, later studies based on pump-probe spectroscopy,8 pressure perturbation calorimetry,9 or X-ray absorption spectroscopy (XAS)10 demonstrated that ions have a minimal impact on the water structure beyond their first hydration shell. Such conclusions were later questioned by neutron diffraction results.11,12
Other factors which may induce ion-specific effects are the direct ion-macromolecule interactions in water.13–15 Depending on the ions, ion pairing strength with functional groups of the macromolecules may vary as demonstrated by XAS on carboxylate groups with different cations for example.16,17 One question which remains largely unexplored is the link between ion-pairing and water structure. It has been shown that long range ion cooperative effects may impact the water molecule dynamics, in particular in ionic solutions where ions and counterions are strongly hydrated.18–20 Recently, Xie and Gao suggested that ion-counterions cooperative effects should be taken into account to explain Hofmeister effects.21 However, scarce experimental data are currently available on the link between ion-counterions interactions and water structure.
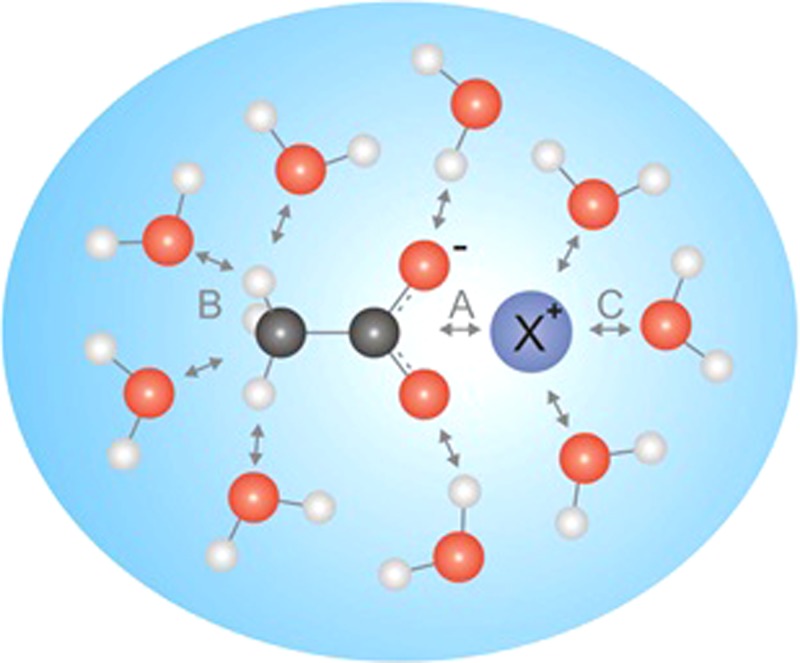
In the present study, acetates were chosen as model molecules to mimic carboxylate functional groups of proteins. Ion pairing between acetates molecules and different cations are compared to water-structure modifications in these ionic aqueous solutions using soft X-ray spectroscopies. For comparison, the effect on water structure of aqueous chloride solutions is also characterized. The different electronic interactions between cations, water and acetate molecules discussed in this study are summarized in Fig. 1.
FIG. 1.
Competing interactions in aqueous acetate solutions: ion pairing between carboxylate functional group and X+ cation (A), acetate-water (B), and X+ cation-water interactions (C).
XAS, X-ray Emission Spectroscopy (XES), and Resonant Inelastic X-ray Scattering (RIXS) are complementary methods that can be applied on liquids using a microjet to reveal information about water structure or ion pairing with acetate when the oxygen K-edge is probed. For the total fluorescence yield (TFY) mode of XAS and XES/RIXS, a core-hole is created and the emission caused by the resulting relaxation processes is detected. Thus, these methods allow to obtain a snapshot of the configurations in the liquid phase during the core-hole lifetime, which is in the case of the here probed oxygen in the order of 3–4 fs,22 whereas reorientation times in water lie in the picosecond regime.23
Previous reports have shown that XES is sensitive to modification of the hydrogen bond network of water molecules.24–26 Therefore, we first applied this technique on chloride 1M aqueous solutions of monovalent cations (Li+, K+, NH4+, Na+) to probe the effect of these salts on the emission signal from the oxygen of the water molecules, which we correlate to changes in the hydrogen bond network. In the second step, we studied with this approach the respective acetate solutions. In the third step, ion pairing between the same cations and acetate molecules were probed in 1M acetate aqueous solutions by XAS and RIXS. These methods allow a selective probing of the acetate's oxygen since the carboxylate feature is energetically separated from water contribution on the XA spectra16 and can be excited resonantly to obtain RIXS spectra associated to acetate.27,28
II. MATERIALS AND METHODS
Chloride and acetates 1M aqueous solutions were prepared from pure salts (LiCl > 99% (Sigma Aldrich), Li Acetate 99.95% (Sigma Aldrich), NaCl: 99.5% (Sigma Aldrich), Na Acetate > 99.0% (Sigma Aldrich), NH4Cl > 99.5% (Fluka), NH4 Acetate > 98.0% (Fluka), KCl:1.04933.0500 (Merck) K Acetate: > 99.5% (Merck)), and ultrapure water (0.05 μS). XAS and XES measurements were carried out at the U41-PGM undulator beamline of the BESSY II third-generation synchrotron facility of the Helmholtz Zentrum Berlin using the liquid microjet technique in the LiXEdrom setup described in details elsewhere.29 The XA spectra of the liquid samples were recorded in the TFY mode using a 5 × 5 mm2 GaAsP photodiode (Hamamatsu GaAsP G1127-4) in a 45° angle with respect to the beamline. The XE measurements were performed using a MCP-fluorescent screen-CCD detector (Gamma Data) in combination with a grating (1200 lines/mm, blazing 400–1000 eV, 7.5 m grating radius) in Rowland-geometry with the liquid microjet as source in slit-less configuration and in a 90° angle with respect to the beamline. The sample is run in a liquid micro-jet using glass nozzles of 16–22 μm diameter with a flow rate of 0.8–1.0 ml/min after filtration through a 2 μm pore size membrane. Measurements were performed at a chamber pressure of approximately 10−5 millibars. Energy calibration for XES and RIXS was done with respect to the feature from elastic scattering.
III. RESULTS AND DISCUSSION
A. Aqueous chloride solutions: Effects on the hydrogen bond network of water
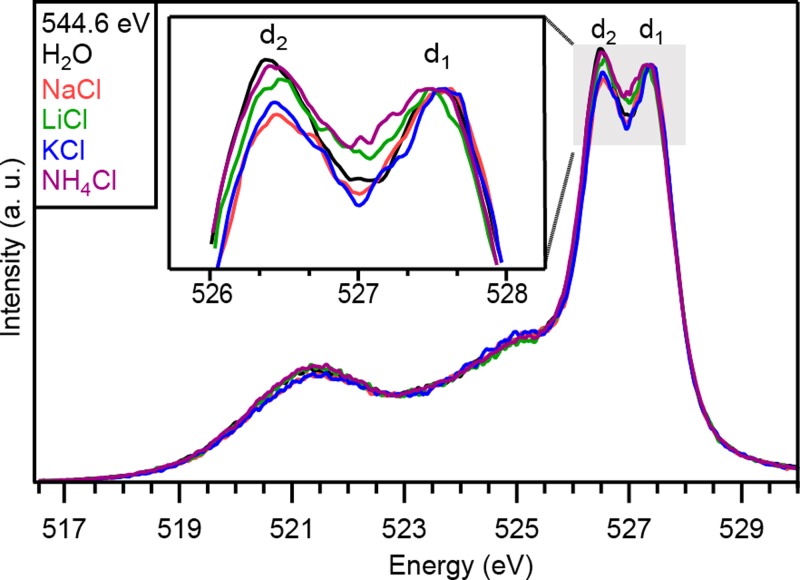
First, cation–water interactions were investigated in 1M aqueous chloride solutions for four different cations (Na+, Li+, K+, NH4+). It was previously reported that the oxygen K-edge XA spectra of aqueous ionic solutions show minor perturbations induced by different monovalent cations.10 The respective XE spectra presented in Fig. 2 indicate a clear dependence on the different cations in the chloride solutions.
FIG. 2.
XE spectra of 1M NaCl, LiCl, KCl, NH4Cl aqueous solutions for excitation energies of 544.6 eV. XES spectra are normalized on the d1 peak. Inset shows a magnified view of the d2/d1 region.
The overall shape of the XE spectra is similar to previous reports.24–26 In particular, the double peak related to the 1b1 molecular orbital (MO) of water at 527.0 eV, separated into the d1 and d2 components, is observed.24–26,30 The physical origin of the splitting is still under debate and several interpretations have been proposed so far. Fuchs et al. have proposed that these two features are the superposition of two spectra related to H2O and OH− molecules.24 Another interpretation was given by Tokushima et al. who suggested that different local hydrogen bond structures could lead to two superposed spectral components with a relative energy shift.25 Finally, Odelius described these features by ab initio simulations taking into account core-hole excited-state dynamics.30 Despite the discussion about its origin, latter experiments demonstrated that these features are sensitive to the hydrogen bond network of water molecules.26,31,32 More precisely, an increase of the number of broken hydrogen bonds has been related to a decrease of the relative intensity of the d2 peak compared to the d1 peak. In this work, the relative intensity between these two peaks will be interpreted as a probe of relative changes in the number of broken hydrogen bonds. Since a quantitative correlation of the intensity of these features to the number of hydrogen bonds is still a topic of ongoing research, we refer here only to relative changes between different samples of equal concentration.
For the aqueous chloride solutions, a cation-dependent ratio between the d1 and d2 peaks is observed (Fig. 2). In comparison to the spectrum of pure water, the d2 component is reduced in intensity for all shown ionic solutions. According to the interpretation described above, all these cations (together with Cl−) decrease the number of hydrogen bonds compared to bulk water. More precisely, the following order is observed for a decreasing disruption of the hydrogen bond network: Na+ > K+ > Li+ > NH4+. This order corresponds to the salting-out series first proposed by Randall and Failey.3,4,33 Consequently, stronger reduction of water's hydrogen bonds may be related to higher salting-out capabilities, in agreement with simulations performed by Zangi.34
Except for Li+, this ordering follows the classical picture that smaller ions induce more hydrogen-bond breaking due to a stronger electrostatic ordering of water molecules around the cations.7 The singular behavior of Li+ has already been reported in other studies7,35,36 and may be explained by a lower coordination number of Li+ compared to the other ions, leading to a lower number of water molecules directly affected by the presence of the ion.37,38
B. Aqueous acetate solutions: Effects on the hydrogen bond network of water
In the second step, the chloride anions were replaced by acetate anions. In order to study the modifications on the hydrogen bond network caused by this exchange, XES measurements were recorded on the acetate solutions.
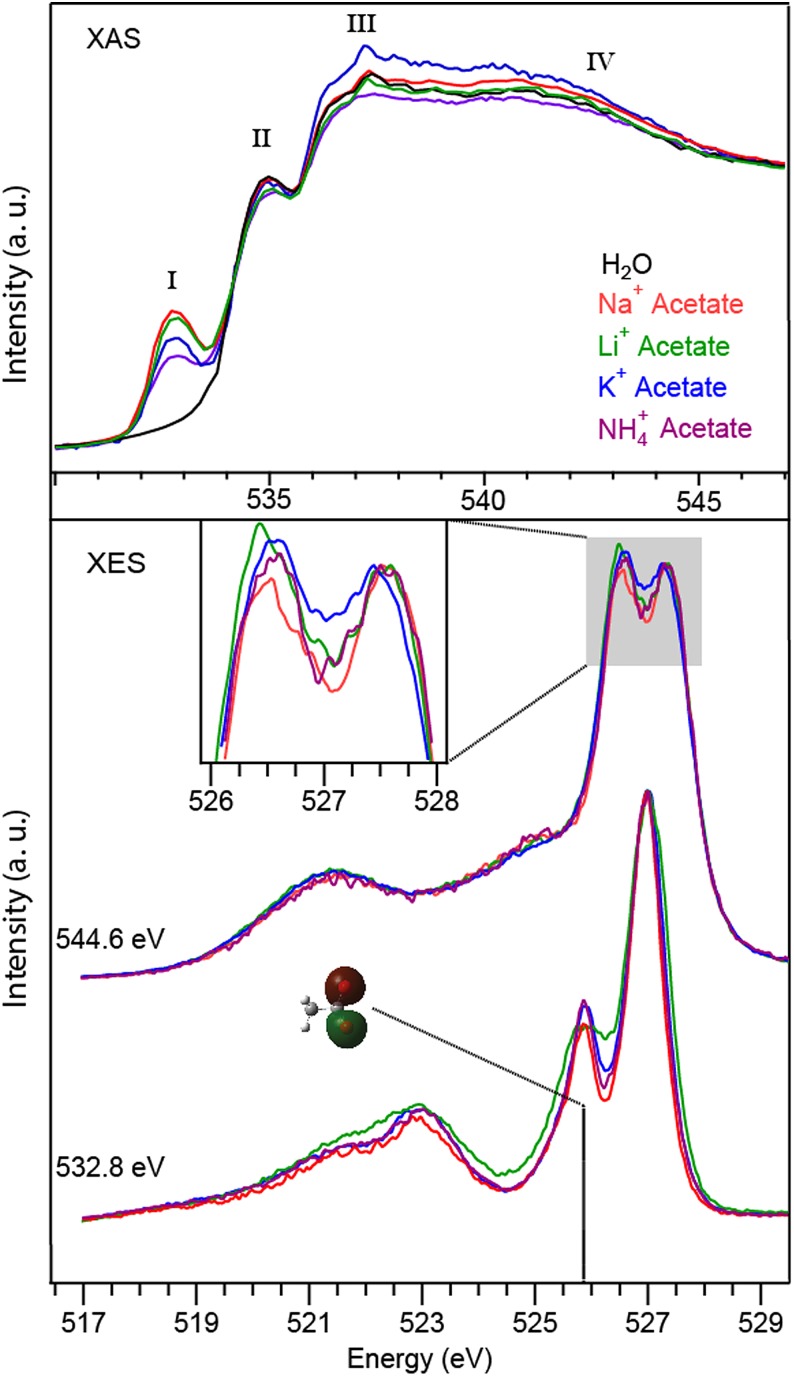
In Fig. 3, the XA, XE, and RIXS spectra of acetate solutions are shown, of which we discuss here first the XE spectra in the middle excited non-resonantly at 544.6 eV. Upon exciting non-resonantly at 544.6 eV, spectra that are dominated by the emission signal from the oxygen of water are recorded. Note that also contributions from the oxygen of acetate have to be taken into account. Considering a 1M concentration of acetate, the estimated acetate contribution lies in the range of less than 4%, which should in the first approximation contribute equally to each spectrum, since all the spectra have the same acetate concentration. Accordingly, we discuss in the following only relative spectral changes between different acetate solutions. As in the case of aqueous chloride solutions, cation-dependent changes in intensity of the d2 component with respect to d1 are observed in the XE spectra. According to these results, the number of hydrogen bonds decreases in the acetate solutions depending on the cation in the following order: Li+ > K+ > NH4+ > Na+. Note that this order is different from the one with chloride solutions which will be discussed later.
FIG. 3.
Oxygen O1s XAS spectra of acetate with the counter cations Na+, Li+, K+, NH4+ in 1M aqueous solution (top). Spectra resulting from the resonant excitation of the carboxylate's oxygen (532.8 eV) and XES spectra excited with 544.6 eV are shown in the bottom. The RIXS spectrum at 532.8 eV is normalized to the feature at 526.9 eV. The XES spectra (544.6 eV) are normalized on the d1 peak. The inset shows a magnified view of the d2/d1 region.
C. Aqueous acetate solutions: Ion-ion interaction
In addition, we studied the ion-ion interaction between the acetates and the respective counter ions. A selective probing of the carboxylate oxygen and its interaction with the different cations is achieved here via XAS and RIXS. Fig. 3 (top) shows oxygen K-edge XA spectra of 1M aqueous acetate solutions for the same cations (Na+, Li+, K+, NH4+) compared with the spectrum of pure water. The spectra are normalized to the background signal before and after the absorption edge (530.0 eV and 547.0 eV) in order to take into account the photon flux variations from the synchrotron.
The O1s XA spectra of the acetate solutions are constituted of a first peak (I) at 532.5 eV, which is specific to carboxylate groups, in addition to characteristic features of water XA fluorescence, namely, the pre-(II), main-(III), and post-(IV) edge peak at about 535.0 eV, 537.5 eV, and 542.0 eV, respectively. Peak I originates from the O1s core-level excitation of an electron into the π* character LUMO state of the carboxyl group of acetate.16 The obtained spectra show the intensity of the carboxylate feature of acetate decreasing for different cations in a sequence Na+ > Li+ > K+ > NH4+. This order is in accordance with former work performed with a flow cell.16 Higher peak intensity was previously interpreted in terms of stronger ion pairing of the ions with the carboxylate group of acetate in aqueous solution, following the rule of matching water affinities.16 The strong ion pairing of Na+ with carboxylate groups of proteins was also reported before,39 justifying the use of acetate as a model molecule for proteins. Due to strong X-ray absorption of water molecules, XAS features of liquid water are largely affected by saturation effects in the microjet.40 Note, however, that the saturation effect does not impact the ordering of the feature related to the carboxyl group of acetate because it appears below the absorption edge of water molecules.
Note that even though the intensity order of the spectral features is reproduced compared to previous measurements on a flow cell, changes in the peak intensity ratio are observed upon comparing the data obtained from the micro-jet to the flow-cell measurements. These differences could stem, e.g., from interactions between sample and the hydrophilic Si3N4 membranes, oxidation effects of the membrane or radiation damage induced by the slower flow in the flow cell.
Probing the carboxylate groups may give insights on the nature of the ion pairing between acetates and cations. At an excitation energy of 532.8 eV, the 1s→π*C=O absorption of carboxylate groups can be probed selectively.27,41 The resulting RIXS spectra shown in Fig. 3 (bottom) demonstrate that the oxygen MOs are differently affected by the various cations in water. The characteristic emission signature is dominated by two sharp emission features at 525.8 eV and 526.9 eV, which have been assigned to contributions from out-of-plane and in-plane oxygen lone-pair orbitals.27,41 The other features below 523.0 eV are furthermore related to MOs distributed on the methyl group. In a previous work, a general sensitivity of the 525.8 eV acetate feature was found upon varying the counter ion.28 Upon correlating the interaction strength of the various counterions revealed via XAS to the intensity of the 525.8 eV acetate feature, originating from the acetate's sigma type orbitals, we can conclude that an increasing interaction strength between acetate and counterion leads to an intensity decrease of the peak at 525.8 eV, as seen in Fig. 3. This assignment is in agreement with the peak evolution related to previous work performed on zinc acetate solutions.28
Accordingly, the RIXS spectra obtained here allow an ordering of the interaction strength of the cations with the carboxylate group in the following order: Na+ ≈ Li+ > K+ ≥ NH4+. These results are in relatively good agreement with the ordering derived from the XA data from our measurements on the O K-edge or from carbon K-edge results reported by Uejio et al.17 Upon comparing the different spectra, variations in the peak width are observed for the Li+ acetate. This could be due to an additional interaction between the Li+ ions and the hydrophobic part of the acetate. Based on molecular dynamics simulations, Thomas and Elcock have shown that after addition of methane in an aqueous lithium chloride solution, the formation of Li+-methane complexes could be thermodynamically favorable.36 The complex formation was only predicted in the specific case of Li+ cations. In such complexes, stronger electronic interaction between Li+ and MOs delocalized over the whole molecule are expected. MOs involving electrons closer to the hydrophobic part of the acetate are more affected by Li+ which would explain why the XE spectrum recorded at 532.8 eV is different from other cations.
D. Correlation between ion pairing and hydrogen bonds
From the experimental results presented above the following information was obtained. Whereas with Cl− counterions the following order of decreasing the number of hydrogen bonds in dependence of the counterion is observed: NH4+ < Li+ < K+ < Na+, in acetate solutions, a change of this order is observed: Li+ < K+ < NH4+ < Na+. Furthermore, direct ion pairing between acetates and ions was probed selectively by XAS and RIXS and was found to increase in the sequence: NH4+ < K+ < Li+ < Na+. In order to draw a comprehensive picture from these results, the speciation in each solution was derived from the Hydra database and visualized with the Medusa software42 (see supplementary material43). According to this database, in a 1M chloride concentration 100% of NH4+, Li+, and Na+, and 85% of K+ prevail as hydrated ions. In the acetate solutions a fraction of 50% Li+(CH3COO−), 30% Na+(CH3COO−), and 30% K+(CH3COO−) prevails, whereas for NH4+, different to assumptions in a previous study,16 no pairing with the acetate ions is documented according to this database.
Our results can be unified to the following picture: independent from the counterion (Cl− or CH3COO−), NH4+ is in aqueous solution mainly surrounded by water. This is in agreement with the experimental observation that the interaction between NH4+ and acetate is the weakest of all the investigated ions. The experimentally observed changes in the order of hydrogen bond disruption of NH4+ with respect to Li+ and K+ when replacing Cl− by CH3COO− should be therefore correlated to specific interactions between the Li+ and K+ and their respective counterions. The disruptive effect of the Li+ and K+ ions on the hydrogen bond network, as determined from chloride solutions where they are mainly solvated, is reduced in the acetate solution. Accordingly, upon pairing with acetate the disruptive effect of the ions on the hydrogen bond network is lowered. Na+ has the strongest hydrogen bond breaking effect of the chloride solutions as well as of the acetate solutions, whereas it also shows the strongest interaction with the acetate.
Our measurements in chloride solutions allow to directly draw conclusion on the ion effects on the hydrogen bond structure (note however, that also the Cl− always contributes), whereas for the interpretation of the XE spectra obtained from acetate solutions the effects of hydrogen bond disruption and ion pairing contribute both. A strongly debated issue in literature is whether the disruption of hydrogen bonds or ion pairing is the main origin of salting-out effects.2,4,13 Assuming that ion pairing is the main cause of salting-out, one would expect (according to our XAS and RIXS results) that sodium and lithium have roughly the same salting-out properties. This is not the case since sodium cations are generally considered as good salting-out agents while lithium cations have limited salting-out capabilities.2,4 On the other hand, the salting-out order matches well with the cations order of hydrogen bond disruption in chloride solutions. The combination of these results suggest accordingly that the salting-out effect is rather based on the cation-induced hydrogen bond network disruption than on the ion pairing strength. Note, however, that for a more detailed interpretation a clear determination of the physical origin of the splitting of the 1b1 feature is desirable. Furthermore, this could possibly allow insights to ultrafast dynamics in aqueous ion solutions. As mentioned above, the probing time of the here applied methods XAS, XES/RIXS is determined by the core-hole lifetime of the oxygen (3–4 fs22). Ultrafast core-hole induced dynamics within this lifetime could play an important role.23 As the detuning below the absorption onset effectively reduces the duration of the scattering process44 also time resolved information could be obtained in the future via a RIXS mapping approach.
IV. CONCLUSIONS
In conclusion, we characterized modification of the hydrogen bond network of water by monovalent cations in aqueous chloride and acetate solutions by XES in a microjet based on the variations of the d2/d1 ratio. According to this approach the number of water hydrogen bonds decreases in chloride solutions according to the order: NH4+ < Li+ < K+ < Na+. In acetate solutions, the number of hydrogen bonds decreases according to: Li+ > K+ > NH4+ > Na+. Furthermore, direct ion pairing between acetates and ions was probed selectively by XAS and RIXS and was found to increase in the sequence: NH4+ < K+ < Li+ < Na+. The present results show that the water hydrogen-bond network is affected by cations and that the changes are cation specific. Ion pairing tends to reduce the cation-induced disruption of the hydrogen bond network. For more detailed interpretations of the effects of the ions on the XE spectra of water an agreement on the physical origin of the splitting of the 1b1 feature has to be established.
The comparison of our in situ characterization of both hydrogen bond network and ion pairing with salting-out ordering reported elsewhere indicates that salting-out could rather be induced by disruption of the hydrogen bond network by cations than by ion pairing although considerable uncertainty remains and further work is clearly warranted.
ACKNOWLEDGMENTS
This work was supported by the Helmholtz-Gemeinschaft via the young investigator fund VH-NG-635 and the European Research Council Grant No. 279344. K.M.L. is grateful to the financial support by the Helmholtz Postdoc Grant PD-059. T.P. would like to acknowledge the Alexander von Humboldt Foundation for financial support.
REFERENCES
- 1. Hofmeister F., Arch. Exp. Pathol. Pharmakol. 25, 1–30 (1888). 10.1007/BF01838161 [DOI] [Google Scholar]
- 2. Marcus Y., Chem. Rev. 109, 1346–1370 (2009). 10.1021/cr8003828 [DOI] [PubMed] [Google Scholar]
- 3. Grover P. K. and Ryall R. L., Chem. Rev. 105, 1–10 (2005). 10.1021/cr030454p [DOI] [PubMed] [Google Scholar]
- 4. Lo Nostro P. and Ninham B. W., Chem. Rev. 112, 2286–2322 (2012). 10.1021/cr200271j [DOI] [PubMed] [Google Scholar]
- 5. Collins K. D. and Washabaugh M. W., Q. Rev. Biophys. 18, 323 (1985). 10.1017/S0033583500005369 [DOI] [PubMed] [Google Scholar]
- 6. Gurney R. W., Ionic Processes in Solution (McGraw-Hill, New York, 1953). [Google Scholar]
- 7. Hribar B., Southall N. T., Vlachy V., and Dill K. A., J. Am. Chem. Soc. 124, 12302–12311 (2002). 10.1021/ja026014h [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Omta A. W., Kropman M. F., Woutersen S., and Bakker H. J., Science 301, 347–349 (2003). 10.1126/science.1084801 [DOI] [PubMed] [Google Scholar]
- 9. Batchelor J. D., Olteanu A., Tripathy A., and Pielak G. J., J. Am. Chem. Soc. 126, 1958–1961 (2004). 10.1021/ja039335h [DOI] [PubMed] [Google Scholar]
- 10. Cappa C. D., Smith J. D., Messer B. M., Cohen R. C., and Saykally R. J., J. Phys. Chem. B 110, 5301–5309 (2006). 10.1021/jp054699c [DOI] [PubMed] [Google Scholar]
- 11. Mancinelli R., Botti A., Bruni F., Ricci M. A., and Soper A. K., Phys. Chem. Chem. Phys. 9, 2959–2967 (2007). 10.1039/b701855j [DOI] [PubMed] [Google Scholar]
- 12. Mancinelli R., Botti A., Bruni F., Ricci M. A., and Soper A. K., J. Phys. Chem. B 111, 13570–13577 (2007). 10.1021/jp075913v [DOI] [PubMed] [Google Scholar]
- 13. Zhang Y. and Cremer P. S., Curr. Opin. Chem. Biol. 10, 658–663 (2006). 10.1016/j.cbpa.2006.09.020 [DOI] [PubMed] [Google Scholar]
- 14. Gurau M. C., Lim S.-M., Castellana E. T., Albertorio F., Kataoka S., and Cremer P. S., J. Am. Chem. Soc. 126, 10522–10523 (2004). 10.1021/ja047715c [DOI] [PubMed] [Google Scholar]
- 15. Schwartz C. P., Uejio J. S., Duffin A. M., England A. H., Kelly D. N., Prendergast D., and Saykally R. J., Proc. Natl. Acad. Sci. U. S. A. 107, 14008–14013 (2010). 10.1073/pnas.1006435107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aziz E. F., Ottosson N., Eisebitt S., Eberhardt W., Jagoda-Cwiklik B., Vácha R., Jungwirth P., and Winter B., J. Phys. Chem. B 112, 12567–12570 (2008). 10.1021/jp805177v [DOI] [PubMed] [Google Scholar]
- 17. Uejio J. S., Schwartz C. P., Duffin A. M., Drisdell W. S., Cohen R. C., and Saykally R. J., Proc. Natl. Acad. Sci. U. S. A. 105, 6809–6812 (2008). 10.1073/pnas.0800181105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tielrooij K. J., Garcia-Araez N., Bonn M., and Bakker H. J., “ Cooperativity in ion hydration,” Science 328, 1006–1009 (2010). 10.1126/science.1183512 [DOI] [PubMed] [Google Scholar]
- 19. Tielrooij K. J., van der Post S. T., Hunger J., Bonn M., and Bakker H. J., J. Phys. Chem. B 115, 12638–12647 (2011). 10.1021/jp206320f [DOI] [PubMed] [Google Scholar]
- 20. Pastorczak M., van der Post S. T., and Bakker H. J., Phys. Chem. Chem. Phys. 15, 17767–17770 (2013). 10.1039/c3cp52419a [DOI] [PubMed] [Google Scholar]
- 21. Xie W. J. and Gao Y. Q., J. Phys. Chem. Lett. 4, 4247–4252 (2013). [DOI] [PubMed] [Google Scholar]
- 22. Neeb M., Rubensson J. E., Biermann M., and Eberhardt W., J. Electron Spectrosc. Relat. Phenom. 67, 261–274 (1994). 10.1016/0368-2048(93)02050-V [DOI] [Google Scholar]
- 23. Laage D., Stirnemann G., Sterpone F., Rey R., and Hynes J. T., Annu. Rev. Phys. Chem. 62, 395–416 (2011). 10.1146/annurev.physchem.012809.103503 [DOI] [PubMed] [Google Scholar]
- 24. Fuchs O., Zharnikov M., Weinhardt L., Blum M., Weigand M., Zubavichus Y., Bär M., Maier F., Denlinger J., Heske C. et al. , Phys. Rev. Lett. 100, 027801 (2008). 10.1103/PhysRevLett.100.027801 [DOI] [PubMed] [Google Scholar]
- 25. Tokushima T., Harada Y., Takahashi O., Senba Y., Ohashi H., Pettersson L. G. M., Nilsson A., and Shin S., Chem. Phys. Lett. 460, 387–400 (2008). 10.1016/j.cplett.2008.04.077 [DOI] [Google Scholar]
- 26. Lange K. M., Könnecke R., Soldatov M., Golnak R., Rubensson J.-E., Soldatov A., and Aziz E. F., Angew. Chem. 123, 10809–10813 (2011). 10.1002/ange.201104161 [DOI] [PubMed] [Google Scholar]
- 27. Tokushima T., Horikawa Y., Harada Y., Takahashi O., Hiraya A., and Shin S., Phys. Chem. Chem. Phys. 11, 1679–1682 (2009). 10.1039/b818812b [DOI] [PubMed] [Google Scholar]
- 28. Golnak R., Atak K., Suljoti E., Hodeck K. F., Lange K. M., Soldatov M. A., Engel N., and Aziz E. F., Phys. Chem. Chem. Phys. 15, 8046–8049 (2013). 10.1039/c3cp50686j [DOI] [PubMed] [Google Scholar]
- 29. Lange K. M., Könnecke R., Ghadimi S., Golnak R., Soldatov M. A., Hodeck K. F., Soldatov A., and Aziz E. F., Chem. Phys. 377, 1–5 (2010). 10.1016/j.chemphys.2010.08.023 [DOI] [Google Scholar]
- 30. Odelius M., Phys. Rev. B 79, 144204 (2009). 10.1103/PhysRevB.79.144204 [DOI] [Google Scholar]
- 31. Lange K. M., Soldatov M., Golnak R., Gotz M., Engel N., Könnecke R., Rubensson J.-E., and Aziz E. F., Phys. Rev. B 85, 155104 (2012). 10.1103/PhysRevB.85.155104 [DOI] [Google Scholar]
- 32. Arai H., Horikawa Y., Sadakane K., Tokushima T., Harada Y., Senba Y., Ohashi H., Takata Y., and Shin S., Phys. Chem. Chem. Phys. 14, 1576–1580 (2012). 10.1039/c2cp23276f [DOI] [PubMed] [Google Scholar]
- 33. Randall M. and Failey C. F., Chem. Rev. 4, 285–290 (1927). 10.1021/cr60015a004 [DOI] [Google Scholar]
- 34. Zangi R., J. Phys. Chem. B 114, 643–650 (2010). 10.1021/jp909034c [DOI] [PubMed] [Google Scholar]
- 35. Thomas A. S. and Elcock A. H., J. Am. Chem. Soc. 129, 14887–14898 (2007). 10.1021/ja073097z [DOI] [PubMed] [Google Scholar]
- 36. Thomas A. S. and Elcock A. H., J. Chem. Theory Comput. 7, 818–824 (2011). 10.1021/ct100521v [DOI] [PubMed] [Google Scholar]
- 37. Olsher U., Izatt R. M., Bradshaw J. S., and Dalley N. K., Chem. Rev. 91, 137–164 (1991). 10.1021/cr00002a003 [DOI] [Google Scholar]
- 38. Mähler J. and Persson I., Inorg. Chem. 51, 425–438 (2012). 10.1021/ic2018693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vrbka L., Vondrásek J., Jagoda-Cwiklik B., Vácha R., and Jungwirth P., Proc. Natl. Acad. Sci. U. S. A. 103, 15440–15444 (2006). 10.1073/pnas.0606959103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Näslund L.-A., Edwards D. C., Wernet P., Bergmann U., Ogasawara H., Pettersson L. G. M., Myneni S., and Nilsson A., J. Phys. Chem. A 109, 5995–6002 (2005). 10.1021/jp050413s [DOI] [PubMed] [Google Scholar]
- 41. Horikawa Y., Tokushima T., Harada Y., Takahashi O., Chainani A., Senba Y., Ohashi H., Hiraya A., and Shin S., Phys. Chem. Chem. Phys. 11, 8676–8679 (2009). 10.1039/b910039c [DOI] [PubMed] [Google Scholar]
- 42. Puigdomenech I., Hydra/Medusa Chemical Equilibrium Database and Plotting Software, in, freely downloadable software at www.kemi.kth.se/medusa/, KTH Royal Institute of Technology (2004).
- 43. See supplementary material at http://dx.doi.org/10.1063/1.4884600E-SDTYAE-1-004403 for ion speciation calculations.
- 44. Rubensson J.-E., J. Electron Spectrosc. Relat. Phenom. 110, 135–151 (2000). 10.1016/S0368-2048(00)00161-4 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- See supplementary material at http://dx.doi.org/10.1063/1.4884600E-SDTYAE-1-004403 for ion speciation calculations.