Abstract
We report time-resolved X-ray absorption measurements after photolysis of carbonmonoxy myoglobin performed at the LCLS X-ray free electron laser with nearly 100 fs (FWHM) time resolution. Data at the Fe K-edge reveal that the photoinduced structural changes at the heme occur in two steps, with a faster (∼70 fs) relaxation preceding a slower (∼400 fs) one. We tentatively attribute the first relaxation to a structural rearrangement induced by photolysis involving essentially only the heme chromophore and the second relaxation to a residual Fe motion out of the heme plane that is coupled to the displacement of myoglobin F-helix.
I. INTRODUCTION
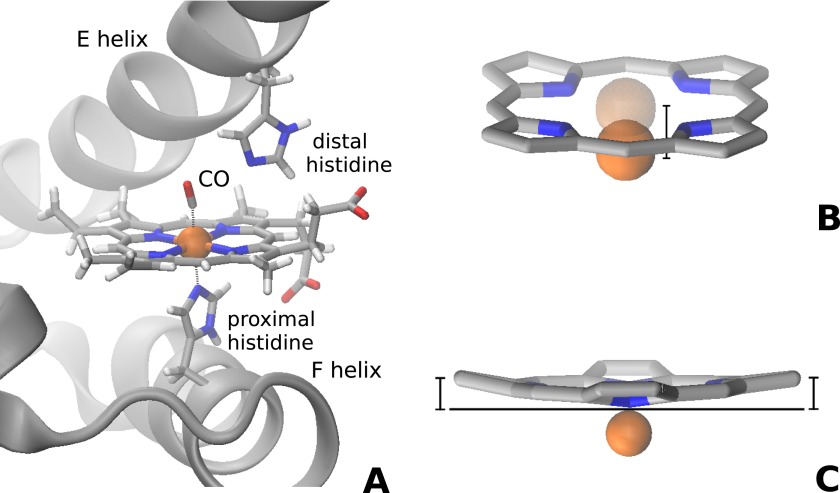
Investigating chemical reactions in the femtosecond timescale with direct structural sensitive techniques is one of the scientific challenges that can now be approached thanks to the advent of X-ray free electron lasers (XFELs). Ultrafast nuclear and electronic rearrangements of transition metal complexes occurring after photoinduced spin crossover transitions have recently been reported both for molecular complexes dissolved in water (Bressler et al., 2009; Lemke et al., 2013; and Zhang et al., 2014) or in the crystalline state (Cammarata et al., 2014). In the case of proteins, ultrafast nuclear motions localized at the active site level similar to those observed in transition metal complexes are expected to drive biologically relevant conformational changes of the entire protein (Ansari et al., 1985). Indeed, the propagation of the perturbation from photoexcited chromophores to the global polypeptide chain conformation has been recently observed through XFEL time-resolved X-ray scattering both in a bacterial reaction center (Arnlund et al., 2014) and in the hemeprotein myoglobin (Mb) (Levantino et al., 2015). However, in view of the limited spatial resolution of X-ray solution scattering (Cammarata et al., 2008), these studies were not able to provide information on the time evolution of the chromophore structural rearrangement that occurs after light absorption and precedes the polypeptide response (Martin and Vos, 1992). In the case of Mb, one of the most extensively studied systems in biology (Frauenfelder et al., 2003), information relevant to ultrafast structural changes of the heme chromophore has been obtained through time-resolved spectroscopy (Martin et al., 1983; Findsen et al., 1985; Petrich et al., 1988; Zhu et al., 1994; Franzen et al., 1995a; Mizutani and Kitagawa, 2001; and Sato et al., 2007). In particular, it is well-known that the bond between the protein and physiologically relevant diatomic ligands (O2, CO or NO) can be photolyzed within tens of fs from heme light absorption (Petrich et al., 1988). In carbonmonoxy Mb (MbCO), the heme is 6-coordinate with the low spin Fe(II) at its center coordinating four nitrogen atoms of the porphyrin, one nitrogen atom of the proximal histidine (His 93) side chain, and the carbon atom of the CO ligand (Figure 1(a)). After photolysis, the Fe(II) spin changes from S = 0 to S = 2, and the heme adopts a domed structure with the Fe moving out of the mean heme plane towards the proximal histidine (Figure 1(b)) (Teng et al., 1994; Schlichting et al., 1994).
FIG. 1.
Schematics of myoglobin active site. (a) In MbCO the Fe(II) at the heme center coordinates the four pyrrole nitrogens, the Nε atom of the proximal histidine, and the carbon atom of the CO ligand. After photolysis, the Fe(II) spin changes from S = 0 to S = 2 and the heme adopts a domed structure: (b) the Fe(II) moves out of the mean heme plane and (c) the porphyrin pyrrole rings tilt slightly with respect to the mean heme plane.
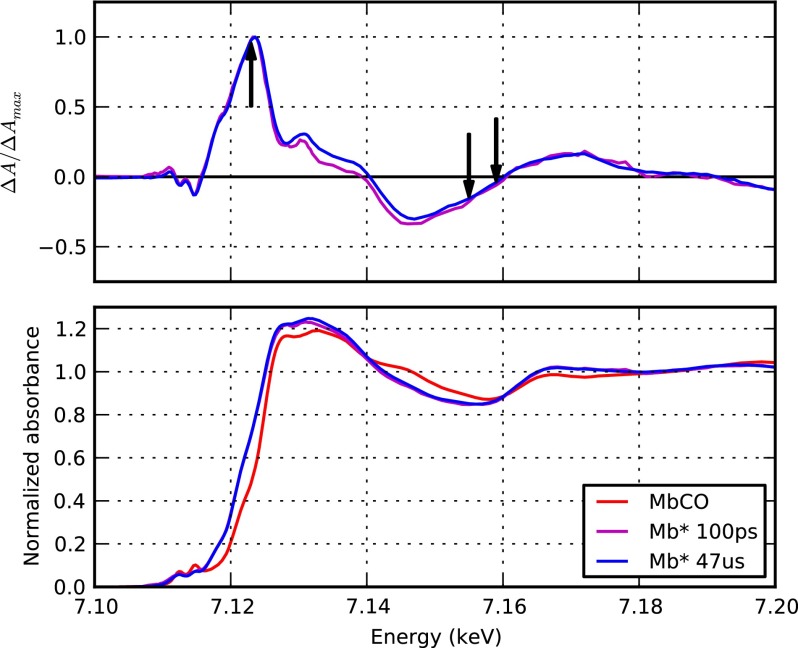
The heme doming structural change can be directly monitored through resonance Raman spectroscopy since the Raman active Fe-His stretching vibration is resonantly coupled to heme absorption (Bangcharoenpaurpong et al., 1984) and its intensity is proportional to the distance of the Fe(II) with respect to the heme macrocycle plane (Stavrov, 1993). Time-resolved resonance Raman investigations have demonstrated that heme doming occurs within 700 fs (Franzen et al., 1995a; 1995b; and Kruglik et al., 2010). However, the temporal evolution of the iron out-of-plane motion could not be resolved in those experiments in view of the time resolution of those experiments. An experimental technique potentially able to monitor the structural changes at the heme with fs time resolution is X-ray absorption spectroscopy (XAS). Indeed, X-ray Absorption Near Edge Structure (XANES) spectroscopy is very sensitive to both the electronic distribution and the position of the nuclei around the absorbing metal (Lima et al., 2014; Shelby et al., 2014). In particular, it has been shown that the Fe(II) K-edge XANES spectrum of MbCO photoproduct is sensitive to the distances and bond angles around the heme iron (Della Longa et al., 2001; Arcovito et al., 2005) and that one of the most relevant parameters affecting the spectrum shape is the distance between the Fe and the Nε of the proximal histidine (Arcovito et al., 2005). Time-resolved XAS has been used in the past to investigate the structural dynamics of Mb. Pioneering work by Mills et al. (1984) showed that within 300 μs from photolysis of MbCO, the Fe K-edge energy position (∼7.1 keV) is red-shifted by ∼3 eV with respect to the MbCO equilibrium spectrum. Many years later, Wang et al. repeated the same experiment with a slightly shorter time resolution (100 μs) but obtaining much higher signal-to-noise ratio XANES spectra thanks to the ∼100 times higher repetition rate of photolysis employed (Wang et al., 2005). Their data show that the Fe K-edge XANES spectrum at 100 μs from photolysis of MbCO is characterized by a relative broad pre-edge peak associated to weak Fe 1s to 3d transitions typical of high spin Fe(II) systems. Full advantage of the time resolution available at third generation X-ray synchrotron sources was recently exploited by Chergui group (Lima et al., 2011; Lima et al., 2012) and the Chen group (Stickrath et al., 2013). However, no significant difference between the 100 ps spectrum and those measured at longer time delays could be observed (Stickrath et al., 2013) (Figure 2), indicating that nuclear and electronic rearrangements at the heme level are already completed within 100 ps, in agreement with time-resolved resonance Raman results (Franzen et al., 1995a; 1995b; Mizutani and Kitagawa, 2001; Sato et al., 2007; and Kruglik et al., 2010).
FIG. 2.
XANES spectra of myoglobin at the Fe K-edge. XANES spectra obtained by Stickrath et al. (2013) at the advanced photon source synchrotron are reported. Bottom panel: the steady-state MbCO spectrum (red) is compared with the photoproduct (Mb*) spectra at 100 ps (magenta) and at 47 μs (blue) from photolysis of MbCO (all data were provided by Prof. Lin Chen, Northwestern University). Upper panel: normalized difference spectra calculated from the data reported in the lower panel show that no significant change is observed between 100 ps (magenta) and 47 μs (blue) from photolysis; black arrows indicate the X-ray energies of 7.123, 7.155, and 7.159 keV at which XFEL time-scans have been recorded during the LCLS experiment.
In order to track in real-time, the structural transition taking place at the heme after photolysis of MbCO, we have performed a time-resolved X-ray absorption experiment at the Linear Coherent Light Source (LCLS) XFEL. By exploiting the femtosecond X-ray pulses available at the LCLS, we have been able to monitor the time-evolution of the Fe K-edge absorption signal at selected energies. Our data show that the heme structural transition consists of two steps, with an initial response (∼70 fs) associated to a partial heme doming and a slower component (∼400 fs) that we tentatively attribute to a residual Fe out-of-plane motion that is slaved to the displacement of Mb F-helix.
II. EXPERIMENTAL METHODS
A. Sample preparation and handling
The sample was a solution of horse MbCO in CO saturated 0.1 M phosphate buffer at pH 7.4. The protein concentration was 5.6 mM (∼100 mg/ml). A few molar excess of sodium dithionite was anaerobically added to the solution while the sample was saturated with CO to ensure full reduction of the heme iron and in order to minimize residuals traces of dissolved molecular oxygen. An aliquot (∼10 ml) of the MbCO solution was anaerobically transferred to a vial connected to a closed loop liquid circulation system that was previously flushed thoroughly with N2 and then with CO. A slight positive pressure of CO gas was maintained in the sample reservoir during the experiments. The liquid circulation system was connected to a capillary nozzle which generated a stable 100 μm round liquid jet inside a He purged sample chamber. The flow rate was set to 2 ml/min. The optical absorption spectrum of microliter samples was measured before, after, and during the X-ray absorption data collection to ensure that close to 100% of the myoglobin molecules were in the MbCO ligation state.
B. Time-resolved X-ray absorption setup
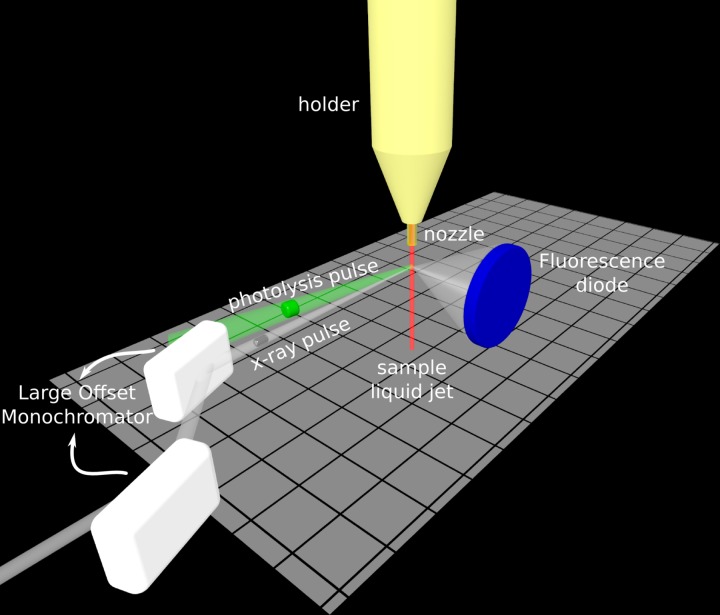
Time-resolved X-ray absorption measurements at the Fe K-edge have been performed at the XPP endstation of the LCLS (Figure 3). The 100 μm liquid jet was vertically oriented with respect to both the incoming optical and X-ray beams. Photolysis of the bond between Mb and CO was achieved with linearly polarized optical pulses (∼70 fs FWHM; 538 nm; 20 μJ per pulse, vertically polarized) focused to a 250 μm (FWHM) circular spot at the sample. This corresponds to an estimated average of 2.2 photons absorbed per heme at each photolysis pulse. A nearly collinear (∼1°) geometry between optical and X-rays beams was adopted. X-rays of ∼1 eV bandwidth were selected from the FEL spectrum using a Si(111) double-crystal monochromator (so called Large Offset Double Crystal Monochromator). This monochromator relies on two independent crystals with an horizontal offset of 600 mm thus allowing the XPP instrument in a location to remain in place (in the 600 mm offset position) while three instruments located in the far experimental hall of LCLS take the beam. This setup, while allowing to rapidly switch between beamlines and even to perform different experiments at the same time (multiplexed mode), has the disadvantage of not being suitable for rapidly changing the X-ray energy (Zhu et al., 2012), and thus no time-resolved X-ray spectra could be measured during our available beamtime. The X-ray beam was focused downstream the sample by means of Be lenses, the beamsize at the sample position was ∼30 μm (FWHM). The X-ray pulses duration was ∼30 fs (FWHM) and the estimated number of photons is of the order of 4 × 1010 for the intense shots at the sample position. The X-ray absorption of the sample was monitored in fluorescence mode (Jaklevic et al., 1993) by measuring the total fluorescence signal in the vicinity of the Fe K-edge. The X-ray fluorescence signal was collected with a Si diode (Canberra FD450–18-300RM, diameter 2.54 cm) positioned at a 90° angle in the horizontal plane with respect to the X-ray beam propagation direction (detector-sample distance of ∼5 cm). Diode nonlinearity was corrected using the same procedure described by Lemke et al. (2013). Moreover, by using the recently developed XPP timing tool, the collected data have been time sorted on the basis of independent shot-by-shot measurements of the arrival times of pump and probe pulses (Harmand et al., 2013). This correction resulted in ∼10 fs effective time jitter between optical and X-ray pulses, which is significantly shorter than the intrinsic uncorrected shot-to-shot time jitter (∼300 fs). The main contribution to the overall time resolution of our experiment, which we estimate to be ∼125 fs, is the velocity mismatch of the optical and X-ray beams through the 100 μm thick liquid jet (∼100 fs).
FIG. 3.
Schematic representation of the experimental setup. A ∼100 μm thick liquid jet of the sample (a solution of ∼5.6 mM MbCO in 0.1 M phosphate buffer at pH 7.4, in red) was produced with a homemade nozzle. The sample was photoexcited with ∼70 fs optical laser pulses at 538 nm (green beam) and probed with ∼30 fs monochromatic X-ray pulses (white beam) in nearly collinear (∼1°) geometry (the angle between the pump and the probe beams is exaggerated in the figure for the sake of clarity). The large offset monochromator of XPP is sketched in white (Zhu et al., 2012). An X-ray diode (in blue) was used to measure the total fluorescence yield signal as a function of the delay between pump and probe pulses.
During the time-resolved data collection, we continuously monitored the ground state fluorescence signal (Ioff) by dropping the photolysis pulse every 17 shots. This allows to track with high fidelity relative changes (Ion/Ioff) in that it minimizes the effects of slow drifts (e.g., X-ray beam pointing). Data have been analyzed using a script based on the freely available ixppy package (https://github.com/htlemke/ixppy).
III. RESULTS
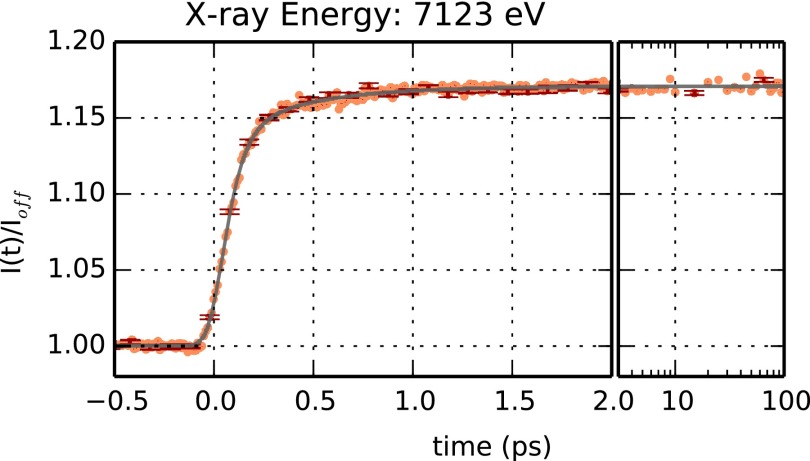
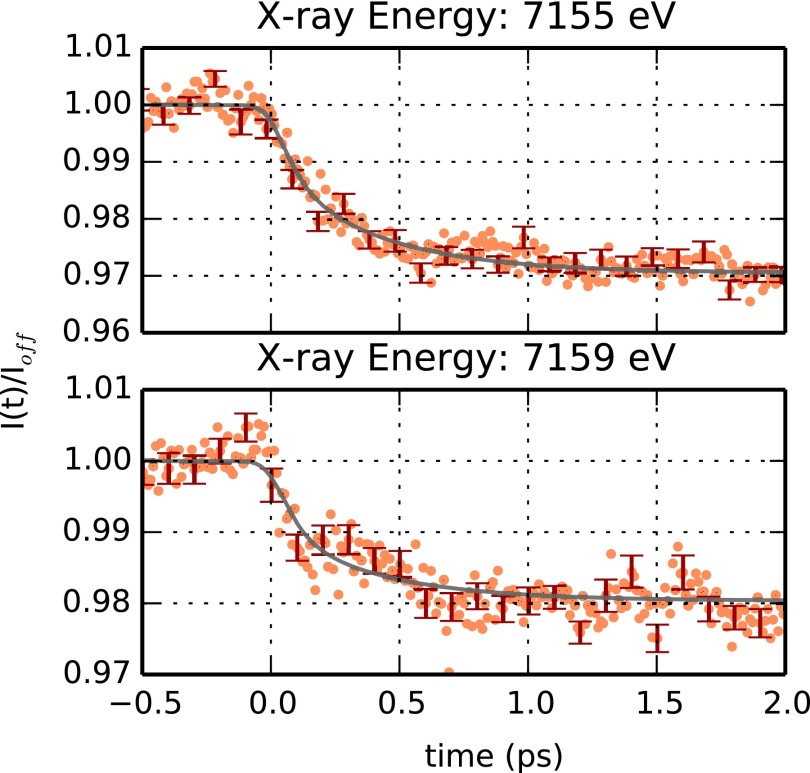
The time evolution of the sample X-ray absorption near the Fe K-edge has been monitored at selected energies (7123, 7155, and 7159 eV). No full XANES spectra could be measured during the experiment in view of the large offset monochromator available during our beamtime (see Methods). Figure 4 reports the signal obtained at an X-ray energy of 7123 eV, which corresponds to the average Fe K-edge position and to the maximum of the deoxyMb-MbCO XANES difference spectrum (Lima et al., 2011). Structural changes at the level of the heme chromophore, such as the elongation of the bonds between the Fe ion and the pyrrole N atoms are expected to induce a shift in the Fe K-edge position that can be probed by monitoring the time evolution of the absorption at 7123 eV. Figure 4 shows that, indeed, a large signal change is observed in the subpicosecond timescale, as hinted by previous optical spectroscopy studies (Martin et al., 1983; Petrich et al., 1988; Franzen et al, 1995a; 1995b; Mizutani and Kitagawa, 2001; Sato et al., 2007; and Kruglik et al., 2010). Such ultrafast signal change could not be observed in previous time-resolved X-ray absorption studies performed at synchrotrons, in view of the significantly lower (∼3 orders of magnitude) time resolution of those experiments. The sudden increase of the signal around time t = 0 indicates that we are observing the contribution of relaxation processes having a characteristic time longer than the effective time resolution of the measurement. Indeed, the data in Figure 4 can be accurately described in terms of the sum of two exponential processes having characteristic times of (73 ± 5) fs and (400 ± 25) fs, respectively. Both exponentials have been convoluted with a Gaussian instrument response function having a (53 ± 2) fs width (corresponding to 125 fs FWHM). The faster exponential process accounts for ∼80% (±3%) of the observed signal increase, indicating that significant electronic and nuclear rearrangements around the iron occur at ∼70 fs from photolysis of MbCO. These results are compatible also with the signal evolution obtained with X-ray energies of 7155 and 7159 eV (Figure 5). These two X-ray energies correspond to emitted photoelectrons having an energy in the 30–40 eV region for which the mean free path is close to a minimum (Muller et al., 1982; Tanuma et al., 1994), thus having the highest sensitivity to the position of iron nearest neighbour atoms. Since the signal-to-noise ratio at these energies is quite low, the fitting parameters are less reliable than those obtained from the signal at 7123 eV. However, as shown by the continuous line in Figure 5, the same relaxation times are able to describe satisfactorily the observed time evolutions obtained at 7155 and 7159 eV. Indeed, by using only the relative weight of the exponentials as a free fitting parameter, one can satisfactorily reproduce the experimental data. The relative weight of the fast component is found to be 0.40 (±0.03) and 0.48 (±0.04) for 7155 and 7159 eV, respectively. Within the signal-to-noise ratio of our data, there is no evidence of any relevant further signal evolution at times longer than ∼1 ps, indicating that the most significant structural changes around the Fe(II) take place in the subpicosecond timescale. Moreover, no periodic modulation of the X-ray absorption signal is evident in our data. Although the time-resolution of our experiment is sufficiently high to observe the iron-histidine (∼220 cm−1) and/or the heme doming (∼50 cm−1) vibrational modes (Zhu et al., 1994), we cannot exclude that a small amplitude modulation is within the noise level of our data. More experiments with significantly improved statistics would be needed to test whether this is the case or not.
FIG. 4.
Time dependence of the absorption at the Fe K-edge (7123 eV) after photolysis of MbCO. The ratio between the total fluorescence signal, I(t), and the average signal in the absence of photolysis, Ioff, is plotted as a function of time (orange symbols). Left panel: data between −0.5 and 2 ps. Right panel: data between 2 and 100 ps plotted in a logarithmic time scale. Negative times correspond to X-ray pulses arriving at the sample before the optical pulses. Errorbars (corresponding to one standard deviation) are shown every 10 experimental points (red symbols). The experimental time evolution was fitted in terms of the convolution between a normalized Gaussian function and the sum of two exponential functions (black line) (Lemke et al., 2013). The fitting parameters for the two exponential functions are τ1 = 73 ± 5 fs (80 ± 3%) and τ2 = 400 ± 20 fs (20 ± 3%); the Gaussian width is 53 ± 2 fs (RMS).
FIG. 5.
Time dependence of the absorption at 7155 and 7159 eV after photolysis of MbCO. The ratio between the total fluorescence signal, I(t), and the average signal in the absence of photolysis, Ioff, is plotted as a function of time (orange symbols) between −0.5 and 2 ps. Errorbars (corresponding to one standard deviation) are shown every 10 experimental points in red. The time evolutions observed at 7155 eV (upper panel) and at 7159 eV (bottom panel) can be satisfactorily described using the same exponential characteristic times obtained from the fit of the data obtained a 7123 eV. The relative weight of the 73 fs component with respect to the 400 fs one, are 40 ± 3% and 48 ± 4% for the data at 7155 and 7159 eV, respectively.
IV. DISCUSSION
The ∼70 fs relaxation time obtained in the present experiment is shorter than the ∼170 fs characteristic timescale of the signal change in iron coordination complexes (Bressler et al., 2009; Lemke et al., 2013) at the same X-ray photon energy and attributed to the structural change accompanying the iron low-spin to high-spin transition, but is longer than a quarter-period of the Fe-His stretching vibration (νFe-His ∼ 220 cm−1, corresponding to an oscillation period of ∼150 fs) observed in both equilibrium deoxygenated Mb and photolyzed MbCO (Findsen et al., 1985; Franzen et al., 1995a). Moreover, this relaxation time is close to predictions based on theoretical calculations and molecular dynamics (MD) simulations (Henry et al., 1985; Petrich et al., 1991; and Li et al., 1993). Indeed, by combining quantum mechanical calculations to model the 5-coordinate heme metastable state generated right after photolysis with MD simulations on both heme model compounds or entire protein models, an iron out-of-plane motion with an average relaxation half-time of ∼50 fs was invariably obtained. In light of our recent time-resolved X-ray scattering data (Levantino et al., 2015) and of the observation of a ∼70 fs relaxation phase in the present experiment, we propose that the ∼400 fs relaxation is a residual iron out-of-plane motion that is coupled to the motion of the F helix of Mb polypeptide chain. Indeed, the ∼70 fs relaxation is likely to result in the elongation of the bonds between the iron and the pyrrole N atoms and in a corresponding compression of the iron-histidine bond; this latter compression is expected to trigger the F helix motion, thus initiating Mb proteinquake (Ansari et al., 1985). The F helix motion occurs at ∼400 fs from photolysis and propagates to the global protein structure within 1 ps (Levantino et al., 2015), in agreement with the observation of a fully relaxed Fe-His stretching mode within ∼700 fs from photolysis of MbCO (Franzen et al., 1995a; 1995b). It must be noted that previous time-resolved optical absorption spectroscopy experiments have observed a single ∼300 fs relaxation process, which has been attributed to the (de)population of different possible intermediates with partially domed heme (Martin et al., 1983; Petrich et al., 1988; and Franzen et al., 2001). Ye et al. (2002) interpreted alternatively this relaxation as an evidence of the 5-coordinate heme vibrational cooling based on a comparison with results obtained on photoexcited deoxyMb. Our data cannot rule out this alternative interpretation, although simulative (Henry et al., 1986; Sagnella and Straub, 2001; and Zhang et al., 2007), theoretical (Li and Champion, 1994), and time-resolved Raman (Lingle et al., 1991; Mizutani and Kitagawa, 1997) investigations point to a heme cooling process occurring after several picoseconds from photolysis of MbCO.
In order to fully characterize the structural dynamics of the heme, the extent of motion and changes in electronic configuration involved during the first (∼70 fs) and the second (∼400 fs) relaxation steps, full femtosecond XANES spectra (over a 70–100 eV range) would be needed. Indeed, different hypotheses on the attribution of the observed X-ray absorption signal time evolutions to specific structural entities could be verified only through an in depth analysis of XANES spectra as it has been shown for Mb equilibrium states or cryogenic photoproducts (Della Longa et al., 2001; Arcovito et al., 2005; and Lima et al., 2014). The work presented here is a first step in the direction of elucidating the ultrafast structural changes occurring after photoexcitation of a macromolecule chromophore and demonstrates that the significantly improved time resolution (∼100 fs) now available at XFELs may allow to monitor electronic and nuclear rearrangements at chemical relevant timescales, thus extending and complementing time-resolved solution (Levantino et al., 2015) and crystallography studies (Schotte et al., 2003; Srajer and Royer, 2012).
ACKNOWLEDGMENTS
Portions of this research were carried out at the Linac Coherent Light Source (LCLS) at the SLAC National Accelerator Laboratory. LCLS is an Office of Science User Facility operated for the U.S. Department of Energy Office of Science by Stanford University. M.C. acknowledges the PEPS project “SASLELX” of the CNRS and the University of Rennes 1 for financial support. M.L. acknowledges the financial support of the University of Palermo (FFR program 2012/2013 and CoRI program 2013). The authors would like to thank Lin Chen (Northwestern University) for kindly providing the data shown in Figure 2.
Contributed paper, published as part of the special topic issue “Invited Papers of the 2nd International BioXFEL Conference,” Ponce, Puerto Rico, January 2015.
References
- 1. Ansari, A. , Berendzen, J. , Bowne, S. F. , Frauenfelder, H. , Iben, I. E. , Sauke, T. B. , Shyamsunder, E. , and Young, R. D. , “ Protein states and proteinquakes,” Proc. Natl. Acad. Sci. U.S.A. 82, 5000–5004 (1985). 10.1073/pnas.82.15.5000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arcovito, A. , Lamb, D. , Nienhaus, G. , Hazemann, J. , Benfatto, M. , and Della Longa, S. , “ Light-induced relaxation of photolyzed carbonmonoxy myoglobin: A temperature-dependent x-ray absorption near-edge structure (XANES) study,” Biophys. J. 88(4), 2954–2964 (2005). 10.1529/biophysj.104.054973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arnlund, D. , Johansson, L. C. , Wickstrand, C. , Barty, A. , Williams, G. J. , Malmerberg, E. , Davidsson, J. , Milathianaki, D. , DePonte, D. P. , Shoeman, R. L. , Wang, D. , James, D. , Katona, G. , Westenhoff, S. , White, T. A. , Aquila, A. , Bari, S. , Berntsen, P. , Bogan, M. , Driel, T. B. V. , Doak, R. B. , Kjær, K. S. , Frank, M. , Fromme, R. , Grotjohann, I. , Henning, R. , Hunter, M. S. , Kirian, R. A. , Kosheleva, I. , Kupitz, C. , Liang, M. , Martin, A. V. , Nielsen, M. M. , Messerschmidt, M. , Seibert, M. M. , Sjöhamn, J. , Stellato, F. , Weierstall, U. , Zatsepin, N. A. , Spence, J. C. H. , Fromme, P. , Schlichting, I. , Boutet, S. , Groenhof, G. , Chapman, H. N. , and Neutze, R. , “ Visualizing a protein quake with time-resolved x-ray scattering at a free-electron laser,” Nat. Methods 11, 923–926 (2014). 10.1038/nmeth.3067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bangcharoenpaurpong, O. , Schomacker, K. T. , and Champion, P. M. , “ A resonance Raman investigation of myoglobin and hemoglobin,” J. Am. Chem. Soc. 106, 5688–5698 (1984). 10.1021/ja00331a045 [DOI] [Google Scholar]
- 5. Bressler, C. , Milne, C. , Pham, V.-T. , Elnahhas, A. , van der Veen, R. M. , Gawelda, W. , Johnson, S. , Beaud, P. , Grolimund, D. , Kaiser, M. , Borca, C. N. , Ingold, G. , Abela, R. , and Chergui, C. , “ Femtosecond XANES study of the light-induced spin crossover dynamics in an iron(II) complex,” Science 323, 489–492 (2009). 10.1126/science.1165733 [DOI] [PubMed] [Google Scholar]
- 6. Cammarata, M. , Levantino, M. , Schotte, F. , Anfinrud, P. A. , Ewald, F. , Choi, J. , Cupane, A. , Wulff, M. , and Ihee, H. , “ Tracking the structural dynamics of proteins in solution using time-resolved wide-angle x-ray scattering,” Nat. Methods 5, 881–886 (2008). 10.1038/nmeth.1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cammarata, M. , Bertoni, R. , Lorenc, M. , Cailleau, H. , Di Matteo, S. , Mauriac, C. , Matar, S. F. , Lemke, H. , Chollet, M. , Ravy, S. , Laulhé, C. , Létard, J. F. , and Collet, E. , “ Sequential activation of molecular breathing and bending during spin-crossover photoswitching revealed by femtosecond optical and x-ray absorption spectroscopy,” Phys. Rev. Lett. 113, 227402 (2014). 10.1103/PhysRevLett.113.227402 [DOI] [PubMed] [Google Scholar]
- 8. Della Longa, S. , Arcovito, A. , Girasole, M. , Hazemann, J. L. , and Benfatto, M. , “ Quantitative analysis of x-ray absorption near edge structure data by a full multiple scattering procedure: The Fe-CO geometry in photolyzed carbonmonoxy-myoglobin single crystal,” Phys. Rev. Lett. 87, 155501 (2001). 10.1103/PhysRevLett.87.155501 [DOI] [PubMed] [Google Scholar]
- 9. Findsen, E. W. , Scott, T. W. , Chance, M. R. , Friedman, J. M. , and Ondrias, M. R. , “ Picosecond time-resolved Raman studies of photodissociated carboxymyoglobin,” J. Am. Chem. Soc. 107(11), 3355–3357 (1985). 10.1021/ja00297a056 [DOI] [Google Scholar]
- 10. Franzen, S. , Bohn, B. , Poyart, S. , and Martin, J.-L. , “ Evidence for sub-picosecond heme doming in hemoglobin and myoglobin: A time-resolved resonance Raman comparison of carbonmonoxy and deoxy species,” Biochemistry 34, 1224–1237 (1995a). 10.1021/bi00004a016 [DOI] [PubMed] [Google Scholar]
- 11. Franzen, S. , Bohn, B. , Poyart, S. , DePillis, G. , Boxer, S. G. , and Martin, J.-L. , “ Functional aspects of ultra-rapid heme doming in hemoglobin, myoglobin, and the myoglobin mutant H93G,” J. Biol. Chem. 270, 1718–1720 (1995b). 10.1074/jbc.270.4.1718 [DOI] [PubMed] [Google Scholar]
- 12. Franzen, S. , Kiger, L. , Poyart, C. , and Martin, J.-L. , “ Heme photolysis occurs by ultrafast excited state metal-to-ring charge transfer,” Biophys. J. 80, 2372–2385 (2001). 10.1016/S0006-3495(01)76207-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frauenfelder, H. , McMahon, B. H. , and Fenimore, P. W. , “ Myoglobin: The hydrogen atom of biology and a paradigm of complexity,” Proc. Natl. Acad. Sci. U.S.A. 100, 8615–8617 (2003). 10.1073/pnas.1633688100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harmand, M. , Coffee, R. , Bionta, M. R. , Chollet, M. , French, D. , Zhu, D. , Fritz, D. M. , Lemke, H. T. , Medvedev, N. , Ziaja, B. , Toleikis, S. , and Cammarata, M. , “ Achieving few-femtosecond time-sorting at hard x-ray free-electron lasers,” Nat. Photonics 7, 215–218 (2013). 10.1038/nphoton.2013.11 [DOI] [Google Scholar]
- 15. Henry, E. R. , Levitt, M. , and Eaton, W. A. , “ Molecular dynamics simulation of photodissociation of carbon monoxide from hemoglobin,” Proc. Natl. Acad. Sci. U.S.A. 82, 2034–2038 (1985). 10.1073/pnas.82.7.2034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Henry, E. R. , Eaton, W. A. , and Hochstrasser, R. M. , “ Molecular dynamics simulations of cooling in laser-excited heme proteins,” Proc. Natl. Acad. Sci. U.S.A. 83, 8982–8986 (1986). 10.1073/pnas.83.23.8982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jaklevic, J. , Kirby, J. A. , Klein, M. P. , Robertson, A. S. , Brown, G. S. , and Eisenberger, P. , “ Fluorescence detection of EXAFS: sensitivity enhancement for dilute species and thin films,” Solid State Commun. 88, 1105–1108 (1993). 10.1016/0038-1098(93)90303-5 [DOI] [Google Scholar]
- 18. Kruglik, S. G. , Yoo, B.-K. , Franzen, S. , Vos, M. H. , Martin, J.-L. , and Negrerie, M. , “ Picosecond primary structural transition of the heme is retarded after nitric oxide binding to heme proteins,” Proc. Natl. Acad. Sci. U.S.A. 107, 13678–13683 (2010). 10.1073/pnas.0912938107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lemke, H. T. , Bressler, C. , Chen, L. X. , Fritz, D. M. , Gaffney, K. J. , Galler, A. , Gawelda, W. , Haldrup, K. , Hartsock, R. W. , Ihee, H. , Kim, J. , Kim, K. H. , Lee, J. H. , Nielsen, M. M. , Stickrath, A. B. , Zhang, W. , Zhu, D. , and Cammarata, M. , “ Femtosecond x-ray absorption spectroscopy at a hard x-ray free electron laser: Application to spin crossover dynamics,” J. Phys. Chem. A 117, 735–740 (2013). 10.1021/jp312559h [DOI] [PubMed] [Google Scholar]
- 20. Levantino, M. , Schirò, G. , Lemke, H. T. , Cottone, G. , Glownia, J. M. , Zhu, D. , Chollet, M. , Ihee, H. , Cupane, A. , and Cammarata M., “ Ultrafast myoglobin structural dynamics observed with an x-ray free electron laser,” Nat. Commun. 6, 6772 (2015). 10.1038/ncomms7772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li, H. , Elber, R. , and Straub, J. E. , “ Molecular dynamics simulation of No recombination to myoglobin mutants,” J. Biol. Chem. 268, 17908–17916 (1993), available at http://www.jbc.org/content/268/24/17908.long. [PubMed] [Google Scholar]
- 22. Li, P. and Champion, P. M. , “ Investigations of the thermal response of laser-excited biomolecules,” Biophys. J. 66, 430–436 (1994). 10.1016/S0006-3495(94)80793-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lima, F. A. , Milne, C. J. , Amarasinghe, D. C. V. , Rittmann-Frank, M. H. , van der Veen, R. M. , Reinhard, M. , Pham, V.-T. , Karlsson, S. , Johnson, S. L. , Grolimund, D. , Borca, C. , Huthwelker, T. , Janousch, M. , Van Mourik, F. , Abela, R. , and Chergui, M. , “ A high-repetition rate scheme for synchrotron-based picosecond laser pump x-ray probe experiments on chemical and biological systems in solution,” Rev. Sci. Instrum. 82, 063111 (2011). 10.1063/1.3600616 [DOI] [PubMed] [Google Scholar]
- 24. Lima, F. A. , Milne, C. J. , Rittmann-Frank, M. H. , van der Veen, R. M. , Reinhard, M. , Penfold, T. J. , Benfatto, M. , and Chergui, M. , “ X-ray absorption studies of the photo-induced structural changes of myoglobin in physiological,” Research in Optical Sciences, OSA Technical Digest 2012, paper IW2D.6, 2012.
- 25. Lima, F. A. , Penfold, T. J. , van der Veen, R. M. , Reinhard, M. , Abela, R. , Tavernelli, I. , Rothlisberger, U. , Benfatto, M. , Milne, C. J. , and Chergui, M. , “ Probing the electronic and geometric structure of ferric and ferrous myoglobins in physiological solutions by Fe K-edge absorption spectroscopy,” Phys. Chem. Chem. Phys. 16(4), 1617–1631 (2014). 10.1039/C3CP53683A [DOI] [PubMed] [Google Scholar]
- 26. Lingle, R. , Xu, X. , Zhu, H. , Yu, S.-C. , and Hopkins, J. B. , “ Picosecond Raman study of energy flow in a photoexcited heme protein,” J. Phys. Chem. 95, 9320–9331 (1991). 10.1021/j100176a053 [DOI] [Google Scholar]
- 27. Martin, J.-L. , Migus, A. , Poyart, C. , Lecarpentier, Y. , Astier, R. , and Antonetti, A. , “ Femtosecond photolysis of co-ligated protoheme and hemoproteins: Appearance of deoxy species with a 350-fsec time constant,” Proc. Natl. Acad. Sci. U.S.A. 80, 173–177 (1983). 10.1073/pnas.80.1.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Martin, J. L. and Vos, M. H. , “ Femtosecond biology,” Annu. Rev. Biophys. Biomol. Struct. 21, 199–222 (1992). 10.1146/annurev.bb.21.060192.001215 [DOI] [PubMed] [Google Scholar]
- 29. Mills, D. M. , Lewis, A. , Harootunian, A. , Huang, J. , and Smith, B. , “ Time-resolved x-ray absorption spectroscopy of carbon monoxide-myoglobin recombination after laser photolysis,” Science 223, 811–813 (1984). 10.1126/science.223.4638.811 [DOI] [PubMed] [Google Scholar]
- 31. Mizutani, Y. and Kitagawa, T. , “ Direct observation of cooling of heme upon photodissociation of carbonmonoxy myoglobin,” Science 278, 443–446 (1997). 10.1126/science.278.5337.443 [DOI] [PubMed] [Google Scholar]
- 32. Mizutani, Y. and Kitagawa, T. , “ Ultrafast structural relaxation of myoglobin following photodissociation of carbon monoxide probed by time-resolved resonance Raman spectroscopy,” J. Phys. Chem. B 105, 10992–10999 (2001). 10.1021/jp010923w [DOI] [Google Scholar]
- 34. Muller, J. E. , Jepsen, O. , and Wilkins, J. W. , “ X-ray absorption spectra: K-edges of 3d transition metals, L-edges of 3d and 4d metals, and M-edges of Palladium,” Solid State Commun. 42, 365–368 (1982). 10.1016/0038-1098(82)90154-5 [DOI] [Google Scholar]
- 35. Petrich, J. W. , Poyart, S. , and Martin, J.-L. , “ Photophysics and reactivity of heme proteins: A femtosecond absorption study of hemoglobin, myoglobin, and protoheme,” Biochemistry 27, 4049–4060 (1988). 10.1021/bi00411a022 [DOI] [PubMed] [Google Scholar]
- 36. Petrich, J. W. , Lambry, J.-C. , Kuczera, K. , Karplus, M. , Poyart, C. , and Martin, J.-L. , “ Ligand binding and protein relaxation in heme proteins: A room temperature analysis of NO geminate recombination,” Biochemistry 30, 3975–3987 (1991). 10.1021/bi00230a025 [DOI] [PubMed] [Google Scholar]
- 37. Sagnella, D. E. and Straub, J. E. , “ Directed energy ‘funneling’ mechanism for heme cooling following ligand photolysis or direct excitation in solvated carbonmonoxy myoglobin,” J. Phys. Chem. B 105, 7057–7063 (2001). 10.1021/jp0107917 [DOI] [Google Scholar]
- 38. Sato, A. , Gao, Y. , Kitagawa, T. , and Mizutani, Y. , “ Primary protein response after ligand photodissociation in carbonmonoxy myoglobin,” Proc. Natl. Acad. Sci. U.S.A. 104, 9627–9632 (2007). 10.1073/pnas.0611560104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schlichting, I. , Berendzen, J. , Phillips, G. N. , and Sweet, R. M. , “ Crystal structure of photolysed carbonmonoxy-myoglobin,” Nature 371, 808–812 (1994). 10.1038/371808a0 [DOI] [PubMed] [Google Scholar]
- 40. Schotte, F. , Lim, M. , Jackson, T. A. , Smirnov, A. V. , Soman, J. , Olson, J. S. , Phillips, Jr., G. N. , Wulff, M. , and Anfinrud, P. A. , “ Watching a protein as it functions with 150-ps time-resolved x-ray crystallography,” Science 300, 1944–1947 (2003). 10.1126/science.1078797 [DOI] [PubMed] [Google Scholar]
- 41. Shelby, M. L. , Mara, M. W. , and Chen, L. X. , “ New insight into metalloporphyrin excited state structures and axial ligand binding from x-ray transient absorption spectroscopic studies,” Coord. Chem. Rev. 277, 291–299 (2014). 10.1016/j.ccr.2014.05.025 [DOI] [Google Scholar]
- 42. Srajer, V. and Royer, W. E. , “ Time-resolved x-ray crystallography of heme proteins,” Methods Enzymol. 437, 379–395 (2012). 10.1016/S0076-6879(07)37019-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stavrov, S. S. , “ The effect of iron displacement out of the porphyrin plane on the resonance Raman spectra of heme proteins and iron porphyrins,” Biophys. J. 65, 1942–1950 (1993). 10.1016/S0006-3495(93)81265-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stickrath, A. B. , Mara, M. W. , Lockard, J. V. , Harpham, M. R. , Huang, J. , Zhang, X. , Attenkofer, K. , and Chen, L. X. , “ Detailed transient heme structures of Mb-CO in solution after CO dissociation: An x-ray transient absorption spectroscopic study,” J. Phys. Chem. B 117, 4705–4712 (2013). 10.1021/jp3086705 [DOI] [PubMed] [Google Scholar]
- 45. Tanuma, S. , Powell, C. J. , and Penn, D. R. , “ Calculations of electron inelastic mean free paths,” Surf. Interface Anal. 21, 165–176 (1994). 10.1002/sia.740210302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Teng, T.-Y. , Srajer, V. , and Moffat, K. , “ Photolysis-induced structural changes in single crystal of carbonmonoxy myoglobin at 40 K,” Nat. Struct. Biol. 1, 701–705 (1994). 10.1038/nsb1094-701 [DOI] [PubMed] [Google Scholar]
- 47. Wang, H. , Peng, G. , and Cramer, S. P. , “ Document x-ray absorption spectroscopy of biological photolysis products: Kilohertz photolysis and soft x-ray applications,” J. Electron. Spectrosc. Relat. Phenom. 143, 1–7 (2005). 10.1016/j.elspec.2004.10.011 [DOI] [Google Scholar]
- 48. Ye, X. , Demidov, A. , and Champion, P. M. , “ Measurements of the photodissociation quantum yields of MbNO and MbO2 and the vibrational relaxation of the six-coordinate heme species,” J. Am. Chem. Soc. 124, 5914–5924 (2002). 10.1021/ja017359n [DOI] [PubMed] [Google Scholar]
- 49. Zhang, Y. , Fujisaki, H. , and Straub, J. E. , “ Molecular dynamics study on the solvent dependent heme cooling following ligand photolysis in carbonmonoxy myoglobin,” J. Phys. Chem. B 111, 3243–3250 (2007). 10.1021/jp065877k [DOI] [PubMed] [Google Scholar]
- 50. Zhang, W. , Alonso-Mori, R. , Bergmann, U. , Bressler, C. , Chollet, M. , Galler, A. , Gawelda, W. , Hadt, R. J. , Hartsock, R. W. , Kroll, T. , Kjær, K. S. , Kubiček, K. , Lemke, H. T. , Liang, H. W. , Meyer, D. A. , Nielsen, M. M. , Purser, C. , Robinson, J. S. , Solomon, E. I. , Sun, Z. , Sokaras, D. , van Driel, T. B. , Vankó, G. , Weng, T. C. , Zhu, D. , and Gaffney, K. J. , “ Tracking excited-state charge and spin dynamics in iron coordination complexes,” Nature 509, 345–348 (2014). 10.1038/nature13252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhu, L. , Sage, J. T. , and Champion, P. M. , “ Observation of coherent reaction dynamics in heme proteins,” Science 266, 629–632 (1994). 10.1126/science.7939716 [DOI] [PubMed] [Google Scholar]
- 52. Zhu, D. , Cammarata, M. , Feldkamp, J. M. , Fritz, D. M. , Hastings, J. B. , Lee, S. , Lemke, H. T. , Robert, A. , Turner, J. L. , and Feng, Y. , “ A single-shot transmissive spectrometer for hard x-ray free electron lasers,” Appl. Phys. Lett. 101, 034103 (2012). 10.1063/1.4736725 [DOI] [Google Scholar]