Abstract
Background: older people often experience complex problems. Because of multiple problems, care for older people in general practice needs to shift from a ‘problem-based, disease-oriented’ care aiming at improvement of outcomes per disease to a ‘goal-oriented care’, aiming at improvement of functioning and personal quality of life, integrating all healthcare providers. Feasibility and cost-effectiveness of this proactive and integrated way of working are not yet established.
Design: cluster randomised trial.
Participants: all persons aged ≥75 in 59 general practices (30 intervention, 29 control), with a combination of problems, as identified with a structured postal questionnaire with 21 questions on four health domains.
Intervention: for participants with problems on ≥3 domains, general practitioners (GPs) made an integrated care plan using a functional geriatric approach. Control practices: care as usual.
Outcome measures: (i) quality of life (QoL), (ii) activities of daily living, (iii) satisfaction with delivered health care and (iv) cost-effectiveness of the intervention at 1-year follow-up.
Trial registration: Netherlands trial register, NTR1946.
Results: of the 11,476 registered eligible older persons, 7,285 (63%) participated in the screening. One thousand nine hundred and twenty-one (26%) had problems on ≥3 health domains. For 225 randomly chosen persons, a care plan was made. No beneficial effects were found on QoL, patients' functioning or healthcare use/costs. GPs experienced better overview of the care and stability, e.g. less unexpected demands, in the care.
Conclusions: GPs prefer proactive integrated care. ‘Horizontal’ care using care plans for older people with complex problems can be a valuable tool in general practice. However, no direct beneficial effect was found for older persons.
Keywords: primary care, integrated care, proactive care, aged, older people
Introduction
With the ageing population, an ever increasing number of older people with multiple health problems will be depending on health care. In recent decades, health care has tended to be organised by means of problem-based, disease-oriented care programs. However, one disease and/or its intervention could influence the diagnosis, impact or treatment of another disease. These interactions between diseases and their treatment complicate the determination of disease-specific treatment goals. Thus, for older persons with multiple health problems, this model does not suffice [1, 2].
In these older patients, illness presentation and the consequences of disease are better clarified with integrative disease models rather than by simple medical models [1]. Since well-being and providing for oneself without assistance from others is of increasing importance for older patients with multiple health problems [3], care for older people needs to shift from problem-based, disease-oriented care aiming at improvement of outcomes per disease to goal-oriented, integrated care. An integrated, care model, aiming at global health outcomes, is more suitable than a model mainly aiming at improving individual disease outcomes [4]. The problems older people face are not always known to care providers. The general practitioner (GP) may sometimes suspect the presence of some of these problems, but usually only acts on demand. Therefore, this model of care should be provided in a proactive way to set and prioritise goals together with the patient and to empower the patient to reach these goals.
Although this shift in care model sounds ideal in theory, actual implementation in primary care can be difficult. Models that have been investigated range from light interventions to intensive guided care [5–7]. However, until now, no important positive effects have been shown. Also, although some studies examined cost savings, to our knowledge few studies have evaluated cost-effectiveness.
Two systematic reviews reported on complex or multi-factorial interventions to prevent functional decline and hospitalisation. The review of Beswick et al. showed an effect on nursing home admission, hospital admission and physical functioning, and the review of Lin et al. showed no benefit on hospitalisations and institutionalisations and a small effect on functional ability. The effect of complex interventions could probably be larger if the people who are most likely to benefit are targeted. Simple and effective screening is therefore needed [8]. In the above-mentioned two systematic reviews, the included studies used several selection tools to target the interventions, such as the opinion of the physician about the person's health, computer-assisted predictive index for risk of hospitalisation and functional decline, and tests for physical functioning. Often somatic or physical problems were used to select people for interventions. As we found in our research that complex problems, i.e. the accumulation and interaction of different health problems, also psychological and social health problems, lead to a decline in physical, mental and social functioning [9], in our study we aimed our intervention at those who have problems in several different health domains. Also the intervention targets multiple health domains.
The ISCOPE (Integrated Systematic Care for Older PEople) study aims to assess the effectiveness and cost-effectiveness of a simple structural monitoring system to detect the deterioration in somatic, functional, mental or social health of individuals aged 75 and over followed by the execution of a care plan for those people with a combination of somatic, functional, mental and social problems. The ISCOPE study operationalises horizontal care by developing a care plan for older persons with complex problems, i.e. a combination of functional, somatic (health and illness), mental and/or social problems. The care plan focuses on function rather than on disease and aims to restore, maintain or maximise functional independence, or to compensate for loss of autonomy by appropriate support (functional approach). Although the approach is functional, underlying disease can still be a focus of attention. The goals, wishes and expectations of the older person are the starting point for the care plan [10]. To identify older persons with complex problems proactively, the ISCOPE study uses a simple structural screening and monitoring system to detect deterioration in somatic, functional, mental or social health of individuals aged ≥75, and brings this to the attention of the GP.
Materials and methods
Design overview
The study is an observer-blinded cluster randomised controlled trial with randomisation at the level of the general practice. To avoid contamination, we used a complete consent pre-randomisation design [11, 12]. The Medical Ethical Committee of the Leiden University Medical Center approved the study. The study was registered in the Netherlands Trial Register (NTR1946).
Setting and participants
Recruitment of general practices
All 560 GPs in the region of Leiden, the Netherlands, were invited to participate. In the initial invitation letter, we provided as little information as possible about the intervention to prevent behavioural change in the control group of GPs [13]. Before inviting the older people to participate, we asked GPs to classify all enlisted older people into three categories according to their own perception: (i) not vulnerable, (ii) possibly vulnerable and (iii) vulnerable. The GPs were asked to classify their older people according to their perception of vulnerability of the older person to capture characteristics of the non-responder group. GPs were not provided with a specific definition of vulnerability. Instead, they were asked to indicate ‘in their opinion’ which of their patients were considered vulnerable in the context of this study [14].
Recruitment of participants
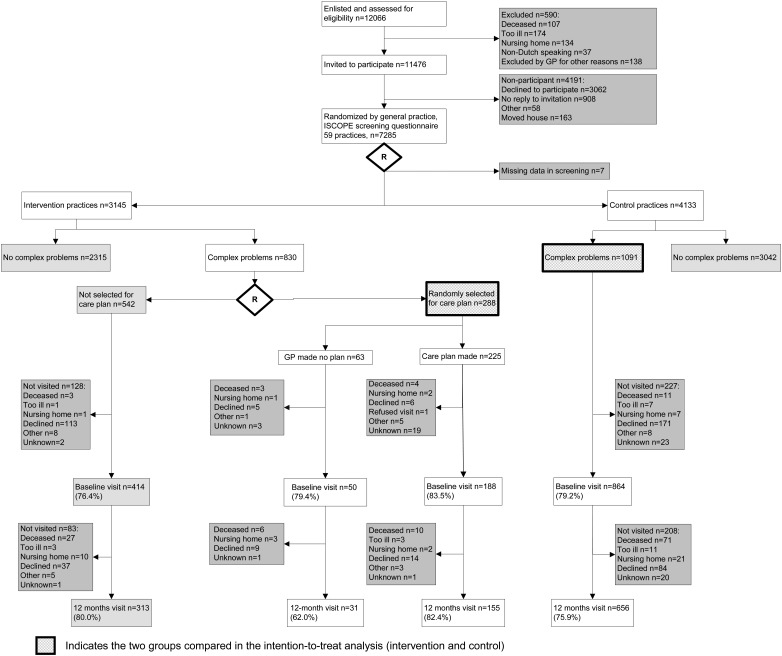
In the Netherlands, all community-dwelling persons are registered at a GP. During the inclusion period (September 2009 to September 2010), all persons aged ≥75 received an invitation by mail from their GP to participate in the study. The GPs excluded people with terminal illness or an expected life expectancy of ≤3 months. Also included with this invitation were a screening questionnaire (Supplementary data, File 1, available in Age and Ageing online) addressing four domains of health and an informed consent form. All older people who participated in the screening provided written informed consent. After 3 weeks, the non-responders were contacted again by telephone and, if required, were assisted by telephone or visited at home to fill in the screening questionnaire. A total of 7,285 older persons responded by sending in the complete screening questionnaire (Figure 1).
Figure 1.
Flowchart of participants in the study.
Randomisation and interventions
Training of GPs
After randomisation (after screening), the GPs and practice nurses of the intervention practices were trained (during two sessions of 3 h each) by a GP specialised in geriatric care to deliver proactive integrated care, including designing, conducting and adjusting a care plan (Box 1, Supplementary data, File 2, available in Age and Ageing online). The GPs practiced care plan making during the training period with two or three older patients within their own practice. During the intervention, the GPs had the possibility to consult a GP with special post-graduate training in geriatrics and they received an extra training of 3 h on resources and organisation of care to older people in primary care.
Box 1. Training for both GPs and practice nurses together.
| Session 1 | Theory on care plans using a functional integrative approach. Practicing care plans for own patient and discussing care plans in the group. Planning for the 10 care plans in the study. |
| Session 2 | Discuss care plans for own patients. Plan organising the intervention in own practice, i.e. allocate responsibilities, care plan making and registering, organising multidisciplinary meetings, evaluating care plans, organising a list of community resources for older people. |
| Session 3 | Develop an overview of local resources for own region together with practice nurse. Discussion on fall interventions with occupational therapist. |
Screening questionnaire
The screening questionnaire contained questions on four domains of health: functional, somatic (health and illness), mental and social and each domain contained 4–9 questions [9]. A positive answer to two or more questions in a domain led to a positive score on the domain. The questions were derived from existing validated questionnaires [15–18] and were based on predictors related to functional decline [19–22]. The screening questionnaire captures accumulation and interaction of deficits [9]. Individuals with problems on three as well as four domains were classified as having complex problems which is associated with poor outcomes on disability, feelings of loneliness, health-related quality of life and GP contact time [9]. In the intervention practices, the GPs received the results of the screening questionnaire of their own patients. In the control practices, GPs did not receive feedback about the screening questionnaire and provided care as usual to their older patients.
Care plan
In the intervention practices, the GP or the practice nurse (under supervision of the GP) made an integrated care plan for participants with complex problems. This care plan consisted of two steps. The first was an inventory of the existing health problems using problem areas as introduced by Bangma, stemming from Dutch rehabilitation medicine: somatic, activities of daily living (ADL), social, mental and communicative problems [23]. The wishes and expectations of the older person about goals to be achieved were explored in a dialogue with the participant and informal caregiver(s). Subsequently, a care plan was made, taking the priorities and goals of the older person and informal caregiver as a starting point (Supplementary data, File 3, available in Age and Ageing online). The GP/practice nurse, together with the older person, formulated actions to be taken and evaluation plans for follow-up. Other care professionals were involved where needed (multidisciplinary consultation). For the purpose of the present study, to be able to complete the care plans within the set time period of the study, the participating GPs made care plans for a maximum of 10 randomly chosen participants with complex problems. These care plans were made in a time period of 2–3 months. For the other selected participants, care plans could be made after the first 10 randomly chosen participants. For the participants with complex problems who were not selected, usual care was provided. These participants were not included in the final analysis.
Outcomes and follow-up
At baseline and at 1-year follow-up, participants were visited by a research nurse to measure outcomes. At 6 months post-baseline, the EQ-5D was sent by mail.
To show the outcome of the screening, a comparison was made between participants with complex problems and participants without complex problems with data from the EPR and data from the questionnaires.
Primary outcome
The primary outcomes were quality of life at 1-year follow-up and competence in ADL. Quality of life was measured by Cantril's ladder that has steps ranging from 0 to 10 [24]. ADL was measured with the Groningen Activities Restriction Scale (GARS) [25]. The GARS is a questionnaire that assesses disabilities in competence in nine BADL items and nine instrumental activities of daily living (IADL) items. (range 18–72, higher score means more disability).
These primary outcome measures were chosen, because our ultimate goal was to improve functioning and the ability to live independently. As we expected improvement of functioning might not always be feasible to achieve but quality of life can still be influenced, we chose ADL functioning as well as quality of life as primary outcomes.
Secondary outcomes
Because we used a comprehensive intervention, we used a wide array of secondary outcomes.
As secondary outcomes, we examined satisfaction with delivered care of the older persons, the GPs and informal caregivers.
Older people
Participants were asked to indicate their satisfaction with and confidence in their GP, pharmacist, specialist, physiotherapist, hospital and home care on a 5-point Likert scale. The five levels of satisfaction were then dichotomised into ‘satisfied’ (including very satisfied, satisfied and neutral) and ‘dissatisfied’ (including dissatisfied and very dissatisfied) [26]. Answers to the questions regarding confidence were dichotomised in the same way.
General practitioners
Outcomes related to the experiences of the GPs were established with questionnaires and focus groups. At baseline and at 1-year follow-up, questionnaires were sent to GPs in the intervention group asking them about the overview of care needs, stability in the care situation and (expected) improvement in the care situation for each participant for whom they had made a care plan (answers on a 10-point scale). To evaluate the experiences of the GPs with the screening and care plans, two focus groups were organised (each with four GPs). In both groups, three GPs had extra staff available to enable them to place more focus on the care of their older patients. The first group consisted of GPs who did not manage or only partly managed to complete care plans, and the second group consisted of GPs who completed all the care plans.
Informal caregivers
At baseline and at 1-year follow-up, informal caregivers were sent a questionnaire about the amount of time spent on care for the older participant (hours per week spent on household activities, personal care and activities outside the house), the burden of this care (score 0–10) and their quality of life (score 0–10) [27].
Other secondary outcomes
Cognitive function was measured with the Mini-Mental State Examination (MMSE) with scores ranging from 0 to 30 points (higher score means less cognitive problems) [28]. Depressive symptoms were assessed with the 15-item Geriatric Depression Scale (GDS-15), with scores ranging from 0 (optimal) to 15 (higher score means more depression) [29]. The GDS-15 was administered only to participants with an MMSE score ≥18 points. Self-rated health was measured using the question ‘How do you rate your health in general?’ on a 5-point scale ranging from 1 to 5, and the question ‘How do you rate your health compared to one year ago?’ on a 5-point scale ranging from 1 to 5. Self-rated loneliness was assessed with the Loneliness Scale of De Jong Gierveld (DJG), an 11-item questionnaire covering both emotional loneliness (6 items) and social loneliness (5 items) that were specifically developed for use in elderly populations (higher score means more social problems) [18].
Since the study was aimed at identifying older people with complex problems, we also evaluated combined outcomes indicating complexity. We used the total score on the ISCOPE screening questionnaire and a combined Z-score of the GARS score (functional domain), self-rated health (somatic domain), GDS score (mental domain) and DJG score (social domain).
Process evaluation and contents of the care plan
The content of the care plans is described by the median number of defined problems, goals and actions (with inter-quartile range; IQR), the percentage of problems, goals and actions, and the ‘level’ of functional approach used in the description of the problem-goal-action sequence: handicap/limitation, complaint/symptom, disease/diagnosis, other. To categorise the problems, type of goals and type of actions we used a partly deductive (start with predefined categories based on anatomic areas for the problems) and partly inductive (include extra categories) process.
Sample size
The required sample size was based on the change in BADL. In the Leiden 85-plus Study, we found a decrease of ∼1.4 points per year, with a standard deviation (SD) of 3 [21, 30]. Based on this result, we decided on a change of 1.0 point as a clinically relevant difference. With a power of 85%, a significance level of 0.05, we needed a sample size of 163 patients per group (IBM SPSS Sample Power 3). To take cluster randomisation into account, we used the following formula: ESS = mk/(1 + ICC(m − 1)) with m = number of vulnerable elderly of 75 years and older in a general practice, k = number of practices, ESS = ‘effective sample size’ as calculated as if we use randomisation on a patient level [31]. Assuming that ∼10 participants with complex problems would be feasible per general practice, an intra-cluster correlation coefficient (ICC) of 0.05 [31], and also taking dropout into account, it was estimated that ∼60 general practices should be recruited.
Analysis non-response screening
To compare responders (with and without complex problems) and non-responders, we used patient data from two health centres: a rural and a city GP health centre (total of 629 patients) who participated in the ISCOPE study. Anonymous data from the electronic patient records (EPR) were available for participants and non-participants. A comparison was made of socio-demographic data, diseases (International Classification of Primary Care (ICPC) codes), medication, use of care and the GP's appraisal of vulnerability. For each health domain, corresponding items in the EPR were compared (e.g. for the functional domain, the number of home visits and number of referrals to physiotherapy were compared, and for the somatic domain, the number of prevalent diseases (ICPC-codes) was compared).
Statistical analysis
Descriptive statistics were used to compare baseline characteristics of the participants in the intervention and control practices. Means and SD are used for continuous variables that were normally distributed and medians with IQR for continuous variables that were not normally distributed. Proportions are used to describe differences in categorical variables. In both groups, mortality differences between participants with complex problems were analysed using Cox regression analysis. Differences in median scores between baseline and follow-up in the GP questionnaire were tested with Mann–Whitney U tests, because incomplete scoring in the GP questionnaires prevented a paired analysis,
The primary analysis focused on the difference in Cantril's ladder score and ADL score (GARS BADL and IADL) between participants with complex problems for whom a care plan should have been made (intervention group) compared with all participants with complex problems in the care as usual practices (control group).
Analyses were performed on an intention-to-treat basis and per protocol basis. A sensitivity analysis for effectiveness was performed in the group of participants with problems in four domains. A linear mixed model (LMM) analysis was used, correcting for age, sex, baseline scores and clustering of patients per general practice. The model included a variable for time of measurement (baseline and 1-year follow-up) and a variable for allocation (with value 0 in control patients and value 1 at 1-year follow-up in intervention group patients). The estimate for time of measurement shows the change in score for the control group. The estimate for the allocation shows the difference in change in score between the intervention and control group. Because we assumed that the intervention would have no effect on mortality (which was confirmed by the analysis on mortality) and we were exploring the effect in those who survived, participants who died during follow-up were excluded from the primary outcome analyses. The LMM analysis corrects for outcomes missing at random.
Analysis of focus groups
Focus group interviews were recorded and transcribed verbatim. We used thematic analysis involving coding, categorising and theme identification. All incentives and barriers for screening and care plans mentioned in the verbatim reports were coded and analysed independently by two researchers (J.W.B., W.d.E.).
Economic evaluation
The cost-effectiveness of the intervention from a societal perspective during the 1-year follow-up was analysed (Supplementary data, File 4, available in Age and Ageing online).
Role of the funding source
This study was funded by ZonMw, the Netherlands Organisation for Health Research and Development: ZonMw No. 311,060,201. The sponsor had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; and preparation, review or approval of the manuscript.
Results
Participants
General practitioners
Of the 560 GPs approached, 104 (19%) working in 59 general practices agreed to participate. Concern about the workload was the main reason for non-participation (28%); also, 4% had just left the practice, 4% had very few older people enlisted, 7% were not interested in the project and 4% already implemented an intervention in the care for their older patients. The remaining practices had other reasons for non-participation or did not respond (34%).
The participating practices were representative for the urbanised area in the vicinity of the city of Leiden (the Netherlands).
Older people
The participation of older persons is shown in Figure 1. Of the 12,066 registered people aged ≥75, 590 (5%) were not eligible, because they were deceased (0.9%), too ill (1.4%), admitted to a nursing home (1.1%), non-Dutch speaking (0.3%) or for other reasons (1.1%).
Screening
Of the 11,476 registered eligible older persons, 7,285 (63%) participated in the screening and 4,191 (37%) declined or did not respond for other reasons. One-third of the population (2,240, 31%) was assisted by a relative (n = 1,396, 19%) or a research nurse (n = 844, 12%).
Non-response analysis
Non-responders are compared with responders (both non-complex and complex) in Table 1. Non-responders had the same number of disease and prescriptions but were more vulnerable according to the GP than non-complex responders. Complex responders had more disease and prescriptions, and were more vulnerable according to GP than both other groups. Healthcare use in non-responders was similar to non-complex responders. Complex responders had more GP consultations, GP home visits, physiotherapy and primary mental health care than both other groups.
Table 1.
Non-response in screening
| Responders |
Non-responder, n = 271 | P value non-responder versus non-complex responders | P value non-responder versus complex responders | |||
|---|---|---|---|---|---|---|
| Total, n = 358 | No complex problems, n = 280 | Complex problems,a n = 75 | ||||
| Age (median, IQR) | 80 (77–83) | 79 (77–82) | 83 (78–86) | 81 (78–85) | <0.001 | 0.103 |
| Male sex (%) | 42.3 | 46.1 | 28.0 | 33.9 | 0.004 | 0.331 |
| Living alone (%) | 20.0 | 17.8 | 28.0 | 24.4 | 0.059 | 0.530 |
| Number of diseases (median, IQR) | 2 (1–3) | 2 (1–3) | 3 (2–5) | 2 (1–4) | 0.045 | <0.001 |
| Vulnerable according to GP (%) | 19.8 | 13.7 | 42.7 | 25.4 | 0.001 | 0.004 |
| Number of prescriptions (median, IQR) | 9 (5–13) | 8 (4–12) | 13 (8–19) | 8 (4–12) | 0.946 | <0.001 |
| Healthcare use last year | ||||||
| Consultations GP (median, IQR) | 8 (4–12) | 7 (4–11) | 12 (6–17) | 7 (4–12) | 0.486 | <0.001 |
| Home visits GP last year, yes/no (%) | 36.9 | 29.3 | 65.3 | 44.6 | <0.001 | 0.002 |
| Out of hours contact GP, yes/no (%) | 13.0 | 10.7 | 21.3 | 14.8 | 0.154 | 0.171 |
| Referrals (median, IQR) | 1 (0–3) | 1 (0–2) | 2 (1–4) | 1 (0–2) | 0.734 | <0.001 |
| Physiotherapy, yes/no (%) | 15.5 | 13.2 | 24.0 | 9.2 | 0.138 | 0.001 |
| Mental health care, yes/no (%) | 1.9 | 2.6 | 0.0 | 3.4 | 0.671 | 0.132 |
aThree missing values.
Screening results
Table 2 shows the characteristics and screening results of participants who returned the screening questionnaire in the 30 intervention practices and of participants in the 29 control practices. Median age, sex, income, living circumstances and outcomes of the screening questionnaire were similar. Overall, 26% of the participants had complex problems. Participants with complex problems have a poorer score on all questionnaire outcomes compared with participants without complex problems (Supplementary data, Tables, available in Age and Ageing online).
Table 2.
Characteristics and screening results of participants who returned the screening questionnaire
| Intervention group (30 practices), n = 3,145 | Control group (29 practices) n = 4,133 | |
|---|---|---|
| Age in years: median (IQR) | 80.5 (77.7; 84.5) | 81.3 (77.9; 85.8) |
| Sex (female) | 1,913 (60.9) | 2,551 (61.7) |
| Only state pension | 209 (15.4) | 266 (15.0) |
| Living alone | 698 (51.4) | 1,001 (56.4) |
| Complex problems | 830 (26.5) | 1,091 (26.4) |
| ≥2 problems in the domain | ||
| Functional domain | 785 (25.0) | 1,012 (24.6) |
| Somatic domain | 1,648 (52.7) | 2,124 (51.8) |
| Mental domain | 1,411 (45.0) | 1,906 (46.2) |
| Social domain | 1,040 (33.2) | 1,362 (33.0) |
n (%) unless stated otherwise.
Participants with complex problems
Non-response analysis of participants with complex problems
At baseline, there were no significant differences in sex and reasons for non-participation between non-participants in the intervention group and non-participants in the control group (Figure 1; P values, see Supplementary data, Tables, available in Age and Ageing online). The median age of the non-participants in the control group was higher than that in the intervention group (84.2 versus 82.5 years; P = 0.038); at the follow-up measurement, this was 83.1 versus 84.1; P = 0.536.
Comparison of outcomes between intervention and control groups
Intra-cluster correlation coefficient
After analysis of the data, we found an ICC of 0.002. Post hoc, a power of 85% was calculated for this study.
Patient outcomes
In total, 288 participants with complex problems were randomly selected for a care plan out of the intervention practices. Table 3 shows the characteristics of the participants with complex problems in the intervention and control practices. Participants in the intervention practices selected for a care plan (n = 288) were similar to those who were not selected for a care plan (Table 3).
Table 3.
Baseline characteristics of participants with complex problems
| Intervention group |
Control group n = 1,091 | ||
|---|---|---|---|
| Not selected for care plan, n = 542 | Selected for care plan, n = 288 | ||
| Age in yearsa | 82.7 (79.2; 87.1) | 82.0 (78.8; 86.9) | 83.7 (79.8; 88.0) |
| Sex (% female) | 374 (69.0) | 208 (72.5) | 788 (72.2) |
| Score on four domains of screening questionnaire (%) | 189 (34.9) | 92 (31.9) | 359 (32.9) |
| >4 medications (%) | 423 (78.2) | 233 (80.9) | 808 (74.1) |
| Cantril's laddera | 7 (6–8) | 7 (6–8) | 7 (6–8) |
| GARS total scorea | 37 (29; 47) | 36 (27; 45) | 37 (29; 46) |
| BADL subscale scorea | 11 (9; 15) | 11 (9; 15) | 11 (9; 15) |
| IADL subscale scorea | 19 (25; 33) | 18 (25; 30) | 20 (26; 32) |
| MMSE scorea | 27 (25; 29) | 28 (26; 29) | 27 (25; 29) |
| GDS scorea | 3 (1; 5) | 2 (1; 4) | 3 (1; 5) |
| DJG scorea | 4 (1; 6) | 3 (1; 5) | 4 (1; 6) |
GARS, Groningen Activity Restriction Scale; BADL, basic activities of daily living; IADL, instrumental activities of daily living; MMSE, Mini Mental State Examination; GDS, Geriatric Depression scale; DJG, De Jong-Gierveld Loneliness scale.
a(median, IQR).
During the 1-year follow-up, 19 (6.6%) participants in the intervention group and 87 (8.0%) in the control group died (P = 0.479).
Table 4 presents the primary and secondary outcomes for the intention-to-treat analysis. In the control group, the change in GARS score at 1-year follow-up (3.5; 95% confidence interval (CI): 3.0; 4.0) shows that this group is deteriorating rapidly. There was no difference in change in the score on Cantril's ladder or GARS score (total, BADL or IADL) between participants who were randomised to have a care plan made in the intervention group and participants with complex problems in the control group. Also, there was no difference in secondary patient outcomes. In a per protocol analysis, i.e. in the intervention group only including participants for whom a care plan was made, no difference was found between the two groups (Supplementary data, Tables, available in Age and Ageing online). A sensitivity analysis in participants with problems in four domains showed similar results.
Table 4.
Outcomes of the intention-to-treat analysis adjusted for age at screening, sex and baseline score
| Outcome | Change in 1-year follow-up for control group (n = 1,091) | P-value | Extra change in intervention group compared with control group (n = 288) | P-value |
|---|---|---|---|---|
| Primary outcomes | ||||
| Cantril's ladder | −0.2 (−0.3; 0.0) | 0.004 | 0.0 (−0.2; 0.2) | 0.823 |
| GARS total score | 3.5 (3.0; 4.0) | <0.001 | −0.6 (−1.7; 0.5) | 0.299 |
| GARS subscale BADL | 1.4 (1.1; 1.7) | <0.001 | −0.2 (−0.9; 0.4) | 0.450 |
| GARS subscale IADL | 2.1 (1.8; 2.4) | <0.001 | −0.4 (−1.1; 0.3) | 0.238 |
| Secondary outcomes | ||||
| MMSE | −0.7 (−1.0; −0.5) | <0.001 | 0.4 (0.0; 0.9) | 0.066 |
| GDS-15 | 0.1 (−0.1; 0.3) | 0.168 | 0.0 (−0.4; 0.4) | 0.916 |
| DJG | −0.1 (−0.3; 0.1) | 0.410 | −0.1 (−0.5; 0.3) | 0.661 |
| Total score ISCOPE screening | −0.6 (−0.8; −0.4) | <0.001 | −0.3 (−0.8; 0.1) | 0.141 |
| Combined outcome (Z-scores) | −0.5 (−0.6; −0.4) | <0.001 | 0.0 (−0.3; 0.3) | 0.845 |
Satisfaction with care
Older people's satisfaction with care
During the 1-year follow-up, the number of people satisfied with the GP increased in the intervention group (from 96.8 to 97.8%, P = 0.218) and decreased in the control group (from 94.5 to 93.7%, P = 0.039). No difference between the two groups was observed on confidence in the GP or on satisfaction with and confidence in the other care providers.
GPs' satisfaction with care
GPs returned baseline questionnaires for 202 participants with a care plan and returned 1-year follow-up questionnaires for 146 of these participants. GPs reported an improvement in the overview of care needs [from median 7.0 (IQR 6.0–8.0) to 8.0 (IQR 7.0–9.0); P < 0.001] and experienced more stability in the care situation [from median 7.0 (IQR 6.0–8.0) to 8.0 (IQR 7.0–8.0); P < 0.001]. Although baseline expectations for improvement in the care situation were low, the reported actually experienced improvement at 1-year follow-up was good [median 5.5 (IQR 2.0–7.0) and 7.0 (IQR 6.0–8.0), respectively; P < 0.001].
In the focus groups, the GPs felt that new information had emerged from the screening, indicating (in particular) their possible ‘blind spot’ for mental and social issues. Some GPs feared that ‘medicalisation’ was stimulated by the screening. GPs experienced more control over the care situation and were more aware of the functioning and wishes of the older people. However, they found the protocolised way of working difficult and suggested that it was perhaps more suited to the practice nurse. Some GPs preferred to focus on the medical task. Organising multidisciplinary consultations was found to be cumbersome.
Informal caregivers
Of the 269 responding informal caregivers (40 in the intervention group, 143 in the control group), the majority were children of the older person (60 and 59%, respectively); their median age was 61 (IQR 51–70) years and 62 (IQR 56–75) years, respectively; and in the intervention group, 35% was male compared with 28% in the control group. The intervention group spent a median number of 5 (IQR 2–20) hours per week on household activities, 3 (2–14) on personal care and 4 (2–6) hours on outside activities. For the control group, this was 5 (IQR 3–12), 3 (IQR 1–10) and 3 (IQR 1–7), respectively. Between baseline and 1-year follow-up, there was no significant difference between the groups in the (change in) time spent on care for the older participant, the burden felt by the informal caregiver and their quality of life (Supplementary data, Tables, available in Age and Ageing online).
Process evaluation and content of care plan
A total of 288 randomly chosen participants with complex problems were assigned to receive a care plan. Of these, in 7% (n = 20) the GP indicated that the drafted care plan was not carried out due to death, referral to a nursing home, moving house, etc. In 15% (n = 43), the GP did not prepare the care plan due to time constraints or other logistic problems. Three GPs did not manage to make any care plan at all. The median number of problems, goals and actions in the care plans was 3 (IQR 2–4), 4 (2–5) and 3 (2–5), respectively. The five most prevalent problems were depressive complaints (20% of patients), loneliness/isolation (19%), decreased mobility (19%), memory complaints (17%) and hearing complaints (12%). The five most prevalent actions were action by the patient or informal caregiver (13% of actions), such as engaging in social activities, referral to another physician (9%), further diagnostic interventions (9%), frequent check-up by the GP (7%) and optimising the medication (7%). In the problem–goal–action sequences, 46% of descriptions were expressed as handicap/limitation, 46% as complaint/symptom and 8% as disease/diagnosis.
Economic evaluation
Costs were estimated at €236 per care plan (Supplementary data, File 4, available in Age and Ageing online), which includes training of GPs and practice nurses (16%), screening (21%), making the care plan (30%) and carrying out the care plan (34%). These care plan costs constituted only 1.3% of the total healthcare costs during the 1-year follow-up. No differences were found in the use of other types of health care or in total healthcare costs.
Discussion
This study evaluated a proactive horizontal approach by the GP for older patients, consisting of a brief (postal) screening questionnaire and making an integrated care plan for (some) patients with complex problems. The GPs had successfully taken on the functional approach, as seen from the contents of the care plans. GPs experienced better overview of care needs and more stability, e.g. less unexpected care demands, in the care for individual patients with complex problems. Older patients with complex problems were already largely satisfied with the care offered by their GP, and the change in satisfaction was therefore small. Nevertheless, no significant improvement was found in quality of life or functional status after 1-year follow-up in participants with complex problems in the intervention group compared with those in the usual care group. In addition, there was no significant difference in change of somatic problems and mental/social functioning, or in complexity. Except for the care plans, patients in the intervention group had the same amount of healthcare use and costs as the control group.
Comparison with literature
Two systematic reviews evaluated studies on complex interventions (with individualised assessment and provision, or referral to appropriate care) to prevent functional decline in older people [5, 6]. Although one review showed a reduction in admissions to hospitals/nursing homes, a reduction in falls and an improvement in functioning, the effects were only modest [5]. Moreover, the effects were mainly in studies conducted before 1993, suggesting that modification of care after 1993 was of little additional value; this idea has recently been confirmed [32]. The more recent review showed small effects on functioning, but mainly in studies performed in the USA and not in non-US countries [6]. This latter review showed no effects on hospitalisation, institutionalisation or mortality; in addition, due to significant heterogeneity between the reviewed studies, the net benefit could not be determined [6]. Two other systematic reviews on comprehensive care programs for people with multi-morbidity were also unable to determine net benefit due to heterogeneity [33, 34]. The present study also examined the cost-effectiveness and preferences of GPs and older persons which, until now, have scarcely been investigated.
Strengths and limitations
The ISCOPE study was performed in a large number of practices in urbanised and sub-urbanised areas, involving single-handed practices and group practices, thereby guaranteeing generalisability to other practices in the Netherlands. Outcomes were measured during home visits, thereby increasing the reliability and completeness of the measurements. Because ∼37% of the older people did not participate in the ISCOPE study, this could have led to a selection of healthier (or perhaps less healthy) older persons or people more likely to accept the suggested intervention. A non-response evaluation showed that non-participants were slightly more vulnerable than the participants; this difference might decrease with more extensive reminding procedures.
Although only three GPs were unable to make any care plan, we have no data on the fidelity to the intervention after the initial establishment of the care plan. This might have been an issue contributing to the lack of effect.
We were unable to obtain repeated assessments over a longer period. Repeated assessments might have amplified the proactive aspect of the intervention, possibly leading to a detectable effect on the outcomes.
Possible reasons for no effect in functioning
There are a few possible reasons for the lack of effect on the functioning of older persons in the present study. First, many changes in the care for older people in primary care have been instigated since the early 1990s [5]. The present study started in a climate of growing interest in preventive and proactive care for older people, which ensured enthusiasm among participating GPs; government and care professionals were already moving in this direction. In 2007, the National Program for Elderly Care was set up, aiming to improve the quality of care for older people by developing coherent care better suited to the individual needs of older persons (http://www.nationaalprogrammaouderenzorg.nl/english/the-national-care-for-the-elderly-programme/). Although this climate of change implies that GPs were keen to participate in this study, no extra provisions (i.e. financial compensation to implement proactive care for older people) were yet in place; this keeps the risk of contamination in the usual care practices low. However, GPs (also in the control group) might have had an increased awareness of the need to work proactively for their older patients, as policy reports on this subject were issued in 2007 and 2010 (mission statements of the Dutch College of General Practice (DCGP) (http://nhg.artsennet.nl/English.htm) and of the Royal Dutch Medical Association (http://knmg.artsennet.nl/Over-KNMG/About-KNMG.htm), and a guideline for the care for older people in general practice issued by the DCGP (http://nhg.artsennet.nl/English.htm).
Second, unsolicited care programs or other devices might not work, because those who would expect benefit from the offered service or device have already obtained it [35–37]. In this study, because the initiative for a care plan did not originate from the participant, executing the plan did not bring the desired changes.
Third, interventions targeted at specific risk factor management may be more effective than organisational interventions with a broader focus [33, 34]. Indeed, the focus of our intervention may have been too broad and may have diluted the effect of each particular outcome measure, thus reducing the power to detect a difference. For this reason, we used quality of life as primary outcome, and we also investigated combined scores of questionnaires as a secondary outcome; however, this also revealed no effect of the intervention.
Fourth, the intervention may not have been sufficiently intense to be able to cause effect. However, one meta-analysis demonstrated that the intensity of the complex interventions made no difference to its effectiveness [5]. Possibly, the participants were relatively healthy with little room for improvement. However, a sensitivity analysis in the group with problems in four domains also failed to show an effect.
Five, a change in approach in organisation and delivery of care does not necessarily lead to effects on the level of functioning or quality of life of the patient. The two meta-analyses showed no positive effect on functioning of any of the interventions, possibly due to the use of non-responsive ADL and IADL instruments [5, 6]. Perhaps outcomes related to healthcare delivery, such as patient experience with continuity of care [38], or more individualised outcomes such as goal attainment scaling [39], might have elicited more response than traditional outcome measures of functioning [40–43]. Unfortunately, at the start of the present study, these latter outcomes were not widely available for practical use in research with community-dwelling older people but might be promising for use in future studies.
Six, a randomised trial may not be appropriate for this sort of interventions as the services offered comprise a complex mix of uncontrollable variables embedded in a social process, more than a treatment program alone [44]. The success of the offered services depends on factors other than functioning (such as building a relationship with the client, the perceived need for care, past experiences with health care, etc.) which cannot be measured or controlled in such a way as to meet the requirements of randomised controlled trials. The increase in satisfaction with care among both the older people and the GPs might reflect these factors. Although failure to show an effect should not be used solely as an excuse to discontinue the service, it remains important to find evidence regarding the cost-effectiveness of new ways of working.
Implications
The question remains as to which outcomes will convince healthcare professionals and policymakers in their decision-making regarding implementation of an intervention. The present study showed no beneficial effect on functioning and quality of life of older persons or on healthcare costs; therefore, this integrated care model cannot be recommended for this particular goal. Nevertheless, in the Netherlands, healthcare organisation for older people in general practice has assumed its own momentum. GPs are increasingly interested and motivated to implement proactive care to prevent functional decline in vulnerable older persons [45] and see this as an improvement of their care. Since 2011, health insurers in the Netherlands have provided funding to GPs to innovate services towards proactive care for older people, encompassing two main elements: case finding and care plans. This lack of congruence between research and policy is an issue that should receive more attention.
Integrative and proactive care for older community-dwelling people will probably be an essential instrument in primary care to be able to manage the care since (in the present political climate) the need to cut costs results in more older people living independently in the community rather than in care homes, but still requiring health care. This study also shows that GPs working with a proactive care plan report the benefit of increased stability in the care of older persons. We think that horizontal care using care plans for older people with complex problems can be a valuable tool in general practice. However, since no direct beneficial effect was found for older persons, based on this study, we cannot recommend this intervention to improve patient outcomes in general practice.
Key points.
Older people have multiple problems that are not always known to their GP.
Proactive, integrated care for older people could improve independence and quality of life but is not common in general practice.
This study investigates feasibility and cost-effectiveness of this way of working.
This improves satisfaction of GPs about the care.
No effect on outcomes in older people is shown.
Supplementary data
Supplementary data mentioned in the text are available to subscribers in Age and Ageing online.
Conflicts of interest
None declared.
Funding
This study was funded by ZonMw, the Netherlands, Organization for Health Research and Development: ZonMw No. 311060201. The sponsor had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; and preparation, review or approval of the manuscript.
Ethical approval
The medical ethical committee of Leiden University Medical Center approved the study in 2009.
Supplementary Material
Acknowledgements
The authors thank the participating practices (GPs, practice nurses and staff), and all the participants and research nurses for their contribution and commitment to the study. We also thank Dr A.W. Wind (coordinator of the ‘Post-graduate training for GPs with special interest in the geriatrics' of the Dutch College of General Practitioners, organised by the Department of Public Health and Primary Care. Leiden University Medical Center) for her contribution to the design of the intervention.
References
- 1.Fried LP, Storer DJ, King DE, Lodder F. Diagnosis of illness presentation in the elderly. J Am Geriatr Soc 1991; 39: 117–23. [DOI] [PubMed] [Google Scholar]
- 2.Tinetti ME. Performance-oriented assessment of mobility problems in elderly patients. J Am Geriatr Soc 1986; 34: 119–26. [DOI] [PubMed] [Google Scholar]
- 3.Fried TR, McGraw S, Agostini JV, Tinetti ME. Views of older persons with multiple morbidities on competing outcomes and clinical decision-making. J Am Geriatr Soc 2008; 56: 1839–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Maeseneer J, Boeckxstaens P. James Mackenzie Lecture 2011: multimorbidity, goal-oriented care, and equity. Br J Gen Pract 2012; 62: e522–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beswick AD, Rees K, Dieppe P, et al. Complex interventions to improve physical function and maintain independent living in elderly people: a systematic review and meta-analysis. Lancet 2008; 371: 725–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin JS, Whitlock EP, Eckstrom E, et al. Challenges in synthesizing and interpreting the evidence from a systematic review of multifactorial interventions to prevent functional decline in older adults. J Am Geriatr Soc 2012; 60: 2157–66. [DOI] [PubMed] [Google Scholar]
- 7.Boult C, Reider L, Frey K, et al. Early effects of ‘Guided Care’ on the quality of health care for multimorbid older persons: a cluster-randomized controlled trial. J Gerontol A Biol Sci Med Sci 2008; 63: 321–7. [DOI] [PubMed] [Google Scholar]
- 8.Stott DJ, Langhorne P, Knight PV. Multidisciplinary care for elderly people in the community. Lancet 2008; 371: 699–700. [DOI] [PubMed] [Google Scholar]
- 9.Van Houwelingen AH, Den Elzen WPJ, Le Cessie S, Blom JW, Gussekloo J. Consequences of interaction of functional, somatic, mental and social problems in community-dwelling older people. PLoS One 2015; 10: e0121013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Junius-Walker U, Stolberg D, Steinke P, et al. Health and treatment priorities of older patients and their general practitioners: a cross-sectional study. Qual Prim Care 2011; 19: 67–76. [PubMed] [Google Scholar]
- 11.Schellings R, Kessels AG, ter Riet G, et al. Indications and requirements for the use of prerandomization. J Clin Epidemiol 2009; 62: 393–9. [DOI] [PubMed] [Google Scholar]
- 12.Schellings R, Kessels AG, Ter Riet G, et al. Members of research ethics committees accepted a modification of the randomized consent design. J Clin Epidemiol 2005; 58: 589–94. [DOI] [PubMed] [Google Scholar]
- 13.Smelt AF, van der Weele GM, Blom JW, Gussekloo J, Assendelft WJ. How usual is usual care in pragmatic intervention studies in primary care? An overview of recent trials. Br J Gen Pract 2010; 60: e305–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drewes YM, Blom JW, Assendelft WJ, Stijnen T, den Elzen WP, Gussekloo J. Variability in vulnerability assessment of older people by individual general practitioners: a cross-sectional study. PLoS One 2014; 9: e108666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weeks SK, McGann PE, Michaels TK, Penninx BW. Comparing various short-form Geriatric Depression Scales leads to the GDS-5/15. J Nurs Scholarsh 2003; 35: 133–7. [DOI] [PubMed] [Google Scholar]
- 16.Steverink S, Slaets JP, Schuurmans H, Van Lis M. Measuring frailty: developing and testing the GFI (Groningen frailty Indicator). Gerontologist 2001; 41: 236–7. [Google Scholar]
- 17.McCusker J, Bellavance F, Cardin S, Trepanier S. Screening for geriatric problems in the emergency department: reliability and validity. Identification of Seniors at Risk (ISAR) Steering Committee. Acad Emerg Med 1998; 5: 883–93. [DOI] [PubMed] [Google Scholar]
- 18.de Jong Gierveld J, Kamphuis FH. The development of a Rasch-type loneliness-scale. Appl Psychol Meas 1985; 9: 289–99. [Google Scholar]
- 19.Stek ML, Gussekloo J, Beekman AT, van Tilburg W, Westendorp RG. Prevalence, correlates and recognition of depression in the oldest old: the Leiden 85-plus study. J Affect Disord 2004; 78: 193–200. [DOI] [PubMed] [Google Scholar]
- 20.Artero S, Touchon J, Ritchie K. Disability and mild cognitive impairment: a longitudinal population-based study. Int J Geriatr Psychiatry 2001; 16: 1092–7. [DOI] [PubMed] [Google Scholar]
- 21.Drewes YM, den Elzen WP, Mooijaart SP, et al. The effect of cognitive impairment on the predictive value of multimorbidity for the increase in disability in the oldest old: the Leiden 85-plus Study. Age Ageing 2011; 40: 352–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perissinotto CM, Stijacic Cenzer I, Covinsky KE. Loneliness in older persons a predictor of functional decline and death. Arch Intern Med 2012; 172: 1078–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hertogh CMPM, Deerenberg-Kessler W, Ribbe MW. The problem-oriented multidisciplinary approach in Dutch nursing home care. Clin Rehabil 1996; 10: 135–42. [Google Scholar]
- 24.Cantril H. The Pattern of Human Concerns. New Brunswick, NJ: Rutgers University Press, 1965. [Google Scholar]
- 25.Kempen GI, Miedema I, Ormel J, Molenaar W. The assessment of disability with the Groningen Activity Restriction Scale. Conceptual framework and psychometric properties. Soc Sci Med 1996; 43: 1601–10. [DOI] [PubMed] [Google Scholar]
- 26.Collins K, O'Cathain A. The continuum of patient satisfaction—from satisfied to very satisfied. Soc Sci Med 2003; 57: 2465–70. [DOI] [PubMed] [Google Scholar]
- 27.Lutomski JE, Baars MA, Schalk BW, et al. TOPICS-MDS Consortium. The development of the Older Persons and Informal Caregivers Survey Minimum DataSet (TOPICS-MDS): a large-scale data sharing initiative. PLoS One 2013; 8: e81673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189–98. [DOI] [PubMed] [Google Scholar]
- 29.D'Ath P, Katona P, Mullan E, Evans S, Katona C. Screening, detection and management of depression in elderly primary care attenders. I: the acceptability and performance of the 15 item Geriatric Depression Scale (GDS15) and the development of short versions. Fam Pract 1994; 11: 260–6. [DOI] [PubMed] [Google Scholar]
- 30.de Ruijter W, Westendorp RG, Macfarlane PW, Jukema JW, Assendelft WJ, et al. The routine electrocardiogram for cardiovascular risk stratification in old age: the Leiden 85-plus study. J Am Geriatr Soc 2007; 55: 872–7. [DOI] [PubMed] [Google Scholar]
- 31.Killip S, Mahfoud Z, Pearce K. What is an intracluster correlation coefficient? Crucial concepts for primary care researchers. Ann Fam Med 2004; 2: 204–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Metzelthin SF, van Rossum E, de Witte LP, et al. Effectiveness of interdisciplinary primary care approach to reduce disability in community dwelling frail older people: cluster randomised controlled trial. BMJ 2013; 347: f5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Bruin SR, Versnel N, Lemmens LC, et al. Comprehensive care programs for patients with multiple chronic conditions: a systematic literature review. Health Policy 2012; 107: 108–45. [DOI] [PubMed] [Google Scholar]
- 34.Smith SM, Soubhi H, Fortin M, Hudon C, O'Dowd T. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database Syst Rev 2012; 4: CD006560. [DOI] [PubMed] [Google Scholar]
- 35.de Craen AJ, Gussekloo J, Blauw GJ, Willems CG, Westendorp RG. Randomised controlled trial of unsolicited occupational therapy in community-dwelling elderly people: the LOTIS trial. PLoS Clin Trials 2006; 1: e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Weele GM, de Waal MW, van den Hout WB, et al. Effects of a stepped-care intervention programme among older subjects who screened positive for depressive symptoms in general practice: the PROMODE randomised controlled trial. Age Ageing 2012; 41: 482–8. [DOI] [PubMed] [Google Scholar]
- 37.Gussekloo J, de Bont LE, von Faber M, et al. Auditory rehabilitation of older people from the general population—the Leiden 85-plus study. Br J Gen Pract 2003; 53: 536–40. [PMC free article] [PubMed] [Google Scholar]
- 38.Uijen AA, Schellevis FG, van den Bosch WJ, et al. Nijmegen Continuity Questionnaire: development and testing of a questionnaire that measures continuity of care. J Clin Epidemiol 2011; 64: 1391–9. [DOI] [PubMed] [Google Scholar]
- 39.Rockwood K, Stolee P, Fox RA. Use of goal attainment scaling in measuring clinically important change in the frail elderly. J Clin Epidemiol 1993; 46: 1113–8. [DOI] [PubMed] [Google Scholar]
- 40.Steenbeek D, Gorter JW, Ketelaar M, Galama K, Lindeman E. Responsiveness of Goal Attainment Scaling in comparison to two standardized measures in outcome evaluation of children with cerebral palsy. Clin Rehabil 2011; 25: 1128–39. [DOI] [PubMed] [Google Scholar]
- 41.Rockwood K, Howlett S, Stadnyk K, et al. Responsiveness of goal attainment scaling in a randomized controlled trial of comprehensive geriatric assessment. J Clin Epidemiol 2003; 56: 736–43. [DOI] [PubMed] [Google Scholar]
- 42.Rockwood K, Fay S, Song X, MacKnight C, Gorman M; Video-Imaging Synthesis of Treating Alzheimer's Disease (VISTA) Investigators. Attainment of treatment goals by people with Alzheimer's disease receiving galantamine: a randomized controlled trial. CMAJ 2006; 174: 1099–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wright JG, Young NL. The patient-specific index: asking patients what they want. J Bone Joint Surg Am 1997; 79: 974–83. [DOI] [PubMed] [Google Scholar]
- 44.Clark J. Preventive home visits to elderly people. Their effectiveness cannot be judged by randomised controlled trials. BMJ 2001; 323: 708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Drewes YM, Koenen JM, de Ruijter W, et al. GPs’ perspectives on preventive care for older people: a focus group study. Br J Gen Pract 2012; 62: e765–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.