Abstract
Potential crosslinks between inflammation and leukaemia have been discussed for some time, but experimental evidence to support this dogma is scarce. In particular, it is important to understand the mechanisms responsible for potential upregulation of proto-oncogenic growth factor expressions by inflammatory mediators. Here, we investigated the ability of the highly inflammatory cytokine interleukin-1 beta (IL-1β) to induce the production of stem cell factor (SCF), which is a major hematopoietic growth factor that controls the progression of acute myeloid leukaemia upon malignant transformation of haematopoietic myeloid cells. We found that human IL-1β induced the expression/secretion of SCF in MCF-7 human epithelial breast cancer cells and that this process depended on the hypoxia-inducible factor 1 (HIF-1) transcription complex. We also demonstrated a crucial role of the phosphatidylinositol-3 kinase (PI-3K)/mammalian target of rapamycin (mTOR) pathway in IL-1β-induced HIF-1α accumulation in MCF-7 cells. Importantly, mTOR was also found to play a role in IL-1β-induced SCF production. Furthermore, a tendency for a positive correlation of IL-1β and SCF levels in the plasma of healthy human donors was observed. Altogether, our results demonstrate that IL-1β, which normally bridges innate and adaptive immunity, induces the production of the major haematopoietic/proleukaemic growth factor SCF through the PI-3K/mTOR pathway and the HIF-1 transcription complex. These findings strongly support a cross-talk between inflammation and acute myeloid leukaemia.
Keywords: Inflammation, Stem cell factor, Leukaemia, Hypoxia
Introduction
Stem cell factor (SCF) is a cytokine produced by epithelial/endothelial cells and fibroblasts which plays a crucial role in haematopoiesis, melanogenesis and leukaemia progression.1 It is recognized by the Kit receptor, also known as CD117,1,2 which is expressed in different types of non-differentiated haematopoietic cells including myeloblasts and promonoblasts.1 Importantly, human myeloid leukaemia cells and mast cells continue expressing the Kit receptor and their proliferation is upregulated by SCF.1,2,3 Increased expression of SCF during inflammatory responses could explain the promotion of existing leukaemic processes.
Recent evidence demonstrated that expression of the SCF gene is directly regulated by the hypoxia-inducible factor 1 (HIF-1) transcription complex.4 HIF-1 is a heterodimeric complex containing constitutive beta and inducible alpha subunits. HIF-1α is a limiting factor which determines activation of the HIF-1 transcription complex. This complex controls cellular adaptation to low oxygen availability (hypoxia).5 Recent observations have also revealed that innate immune responses induced by pathogen-associated molecular patterns and inflammatory cytokines trigger HIF-1 activation in immune cells via differential mechanisms.6,7,8 HIF-1 is crucial for cellular adaptation to inflammatory stress since it controls glycolysis, angiogenesis and cell adhesion on the genomic level.9 In theory, this mechanism could be responsible for triggering inflammatory activation of SCF production in the target cells.
It was recently reported that exposure of human lung-derived fibroblasts to the highly inflammatory cytokine interleukin-1 beta (IL-1β) leads to SCF expression.10,11,12 It was also found that this process is controlled by the transcription factor NF-κB.11,12 However, there is still a lack of experimental evidence regarding the biochemical mechanisms responsible for controlling SCF production induced by inflammation and IL-1β in particular. The potential mechanisms of inflammatory expression of SCF therefore still need further elucidation.
Here, we report that IL-1β induces the production of SCF in MCF-7 human epithelial breast cancer cells. This process depends on IL-1β-induced HIF-1α accumulation/HIF-1 activation. HIF-1 activity due to stimulation of the cells with IL-1β was comparable with exposure to classic HIF-1 inducers, such as hypoxia, cobalt chloride and the proteasomal inhibitor MG-132. IL-1β-induced SCF production in MCF-7 cells was attenuated by silencing HIF-1α expression using specific siRNA. Using pharmacological inhibitors we also demonstrated a crucial role for the phosphatidylinositol-3 kinase (PI-3K)/mammalian target of rapamycin (mTOR) pathway in IL-1β-induced HIF-1α accumulation in MCF-7 cells. An important role of mTOR in the translation of SCF mRNA upregulated by the HIF-1 transcription complex was also shown. Finally, a tendency for a positive correlation of IL-1β and SCF levels in plasma of healthy human donors was observed.
Materials and methods
Materials
RPMI-1640 medium, foetal calf serum and supplements, DOTAP transfection reagent, rapamycin, LY294002, rottlerin and other pharmacological inhibitors were purchased from Sigma (Suffolk, UK). Maxisorp microtitre plates were obtained from Nunc (Roskilde, Denmark). Mouse monoclonal antibodies to HIF-1α, mTOR and β-actin as well as rabbit polyclonal antibody against phospho-S2448 mTOR were obtained from Abcam (Cambridge, UK). Goat anti-mouse and goat anti-rabbit fluorescence dye-labelled antibodies were purchased from Li-Cor (Lincoln, NE, USA). ELISA-based assay kits for the detection of SCF and IL-1β were purchased from R&D Systems (Abingdon, UK). Human recombinant IL-1β and SCF were produced by Dr Varani (Bellinzona, Switzerland). All other chemicals were of the highest grade of purity and commercially available.
Expression of IL-1β and SCF
IL-1β was expressed in Escherichia coli Rosetta-gami cells with a pET21 vector (Novagen, Schaffhausen, Switzerland) and purified by ammonium sulfate precipitation followed by ion exchange and size exclusion chromatography. Human SCF protein was produced in E. coli and purified following published protocols.20 As a final step for the production of both proteins , possible endotoxin contaminants were further removed by ionic exchange chromatography. The expressed proteins did not contain any contaminants and displayed biological activity comparable to that observed using commercially available IL-1β and SCF (obtained from R&D Systems). The quality of the purified proteins was also verified by NMR spectroscopy and mass spectrometry.
MCF-7 breast cancer cells and THP-1 human myeloid cells
MCF-7 human breast adenocarcinoma cells and THP-1 human leukaemia monocytic macrophages were obtained from the European Collection of Cell Cultures (Salisbury, UK). Cells were grown in RPMI 1640 media supplemented with 10% foetal calf serum, penicillin (50 IU/ml) and streptomycin sulphate (50 µg/ml). MCF-7 cells were used as the main model since, physiologically, breast cancer epithelial cells constantly interact with a large number of premature blood cells. They express IL-1 receptor type I and are capable of releasing SCF and therefore are considered as an excellent model to investigate the biochemical mechanisms of IL-1β-induced SCF production.
Whole-cell extracts were prepared using 0.05 M Tris lysis buffer containing 150 mM NaCl, 5 mM ethylenediamine tetraacetate, 0.5% nonidet-P 40 and 1 mM phenylmethylsulfonyl fluoride (supplied immediately before use).
Transfer of HIF-1α siRNA into MCF-7 cells
We used a HIF-1α-specific siRNA (target sequence: ugu gag uuc gca ucu uga u dtdt) localized at position 146 bases downstream of the HIF-1α start codon.21 This is a recognized HIF-1α-specific siRNA sequence which demonstrates high specificity to HIF-1α mRNA and has been successfully employed before.21,22 Transfection into MCF-7 cells was performed using DOTAP reagent according to the manufacturer's protocol. The knockdown efficiency observed was 56%±9%.
Human plasma from healthy donors
Buffy coats were purchased from the UK National Health Service Blood and Transplant Service and were originally acquired from healthy blood donors undergoing routine blood donation following ethical approval (NHS-REC ref. number: 07/Q1206/3). Blood plasma was obtained during routine Ficoll-density separation of leukocytes and erythrocytes and subjected for measurement of SCF and IL-1β by ELISA.
Western blot analysis
Intracellular HIF-1α and SCF levels were determined using Western blot analysis and β-actin staining was employed to assess equal loading, as previously described.3 Li-Cor (Lincoln, NE, USA) goat secondary antibodies, conjugated with fluorescent dyes, were used according to the manufacturer's protocol in order to visualize the proteins of interest using the Li-Cor Odyssey imaging system. Quantitative analysis of Western blot data was performed using Odyssey Image Studio Lite 3.1 software and values were normalized against respective β-actin expressions.
Detection of HIF-1 DNA-binding activity
HIF-1 DNA-binding activity was analysed using the method previously described.3 Briefly, a 96-well Maxisorp microtitre plate was coated with streptavidin and blocked with BSA; 2 pmol/well biotinylated 2 hypoxia responsive element (HRE)-containing oligonucleotide were then immobilized by 1 h incubation at room temperature. After washing five times with TBST buffer (10 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.05% Tween-20), the plate was incubated for 1 h with 20 µl/well of cell lysate at room temperature. Following a washing step (five times with TBST buffer), the plate was then incubated with mouse anti-HIF-1α antibodies (1∶1000 in TBS plus 2% BSA). After 1 h incubation at room temperature, the plate was washed (five times with TBST buffer) and incubated with HRP-labelled rabbit anti-mouse IgG (1∶1000) in TBST buffer. After further extensive washing with TBST, bound secondary antibodies were detected using the peroxidase reaction (orthophenylenediamine/H2O2). Reactions were quenched after 10 min with an equal volume of 1 M H2SO4 and colour development was measured in a microplate reader (absorbance at 492 nm).
Detection of phospho-S2448 mTOR in cell lysates
We employed an ELISA assay to analyse mTOR S2448 phosphorylation as described before.22 Briefly, plates were coated with mouse anti-mTOR, blocked with 1% BSA, and cell lysates were then added to the wells and kept at room temperature for at least 2 h under constant agitation. After extensive washing with TBST buffer, anti-phospho-S2448 mTOR antibodies were added and the plate was incubated for 2 h at room temperature (with constant agitation). After washing 5 times with TBST buffer the plate was then incubated with 1∶1000 HRP-labelled goat anti-rabbit IgG in TBST buffer and, after extensive washing with TBST, bound secondary antibodies were detected by the peroxidase reaction (orthophenylenediamine/H2O2). Reactions were quenched after 10 min with an equal volume of 1 M H2SO4 and colour development was measured using a microplate reader (absorbance at 492 nm).
Measurement of HIF-1α and SCF mRNA levels by quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR)
Total RNA was isolated using a GenElute mammalian total RNA miniprep kit. After decontamination (the kit (Sigma) was used according to the manufacturer's protocol) we performed a HIF-1α mRNA RT-PCR in accordance with the manufacturer's protocol. Quantitative RT-PCR was then performed using the following primers: HIF-1α, 5′-CTCAAAGTCGGACAGCCTCA-3′, 5′-CCCTGCAGTAGGTTTCTGCT-3′ SCF, 5′-ACTGACTCTGGAATCTTTCTCAGG-3′, 5′-GATGTTTTGCCAAGTCATTGTTGG-3′ actin, 5′-TGACGGGGTCACCCACACTGTGCCCATCTA-3′, 5′-CTAGAAGCATTTGCGGTCGACGA-TGGAGGG-3′.22,23 Reactions were performed using a LightCycler 480 RT-PCR system and respective SYBR Green I Master kit (Roche, Burgess Hill, UK). Analyses were performed according to the manufacturer's protocol and values representing HIF-1α and SCF mRNA levels were normalized against those of actin. We also performed a contamination control and, following decontamination, samples were immediately subjected to quantitative RT-PCR.
Detection of SCF, IL-1β and VEGF
SCF, IL-1β and VEGF concentrations in cell culture medium (supernatants) and human blood plasma (SCF and IL-1β) were analysed by commercial ELISA kits (R&D Systems) according to the manufacturer's protocols.
Detection of phosphatidylinositol 3 kinase (PI-3K) activity
PI-3K activity was analysed using an established protocol.24,25,26 Briefly, cell lysates were incubated for 30 min with 30 µl 0.1 mg/ml substrate (PI-4,5-diphosphate was used as the substrate in a form of an emulsion which was prepared using bidistilled water; sonication for 1 h in a water bath sonicator is recommended) in kinase assay buffer. The latter was prepared from 20 mM Tris (pH 7.5), 100 mM NaCl, 0.5 mM ethylenediamine tetraacetate, 8 mM MgCl2 and 40 µM ATP in a total volume of 100 µl at 37 °C with constant agitation. Reactions were terminated by adding 1 ml of mixture hexane/isopropanol (13∶7, v/v) and 0.2 ml of a mixture of 2 M KCl/HClconc (8∶0.25, v/v). Samples were then vortexed and organic phases were washed with HCl (0.5 ml; 0.1 M). This was followed by detection of phosphate groups using molybdenum reagent25,27 containing two parts of 30 g/l (NH4)6Mo7O24, five parts of H2SO4 (140 ml of H2SO4 conc was diluted to 900 ml with bidistilled water), two parts of 54 g/l ascorbic acid and one part of 1.36 g/l potassium antimonyl-tartrate.26 In case of very high or low absorbance, PI-3,4,5-triphosphate was separated by thin-layer chromatography using silica gel thin-layer chromatography plates (chromatographic mixture of 2 M acetic acid/isopropanol (1∶2, v/v) has to be applied) and detected using molybdenum reagent.
Analysis of HIF-1α prolyl hydroxylase (PHD) activity
To detect HIF-1α PHD activity, we used a peptide-based assay as described before.27,28 HIF-1α-free cell lysates were used to avoid the impact of hydroxylation of intracellular HIF-1α. Lysates of non-treated and treated THP-1 cells were incubated for 1 h in 96-well Maxisorp microtitre plates. These plates were coated with HIF-1α capture antibody and blocked with BSA as described before.27 Upon completion of the incubation lysates were used for the PHD activity assay.
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) cell viability assay
Cell viability was analysed using the Promega (Southampton, UK) MTS cell viability assay kit according to the manufacturer's protocol.
In-cell assay of SCF bound to THP-1 cells
In-cell assays were performed as previously described.29 Cells, after respective treatments, were centrifuged for 5 min at 300g, washed with fresh RPMI media containing FCS and antibiotics and then exposed to 2 µg/ml anti-SCF antibody for 2 h. This was followed by centrifugation (5 min, 300g) and then resuspension in media. The cells were then incubated for 2 h with a fluorescently-labelled dye secondary antibody (Li-Cor, Cambridge, UK). Following centrifugation and washing, cells were then transferred into the wells of a 96-well plate which was then scanned by the Odyssey machine (Li-Cor, Cambridge, UK). Results were quantified using Odyssey software.
Statistical analysis
Each experiment was performed at least three times and statistical analysis was conducted using a two-tailed Student's t-test. Differences were considered as significant at P<0.01.
Results
IL-1β induces SCF production and HIF-1α accumulation in MCF-7 human epithelial breast cancer cells
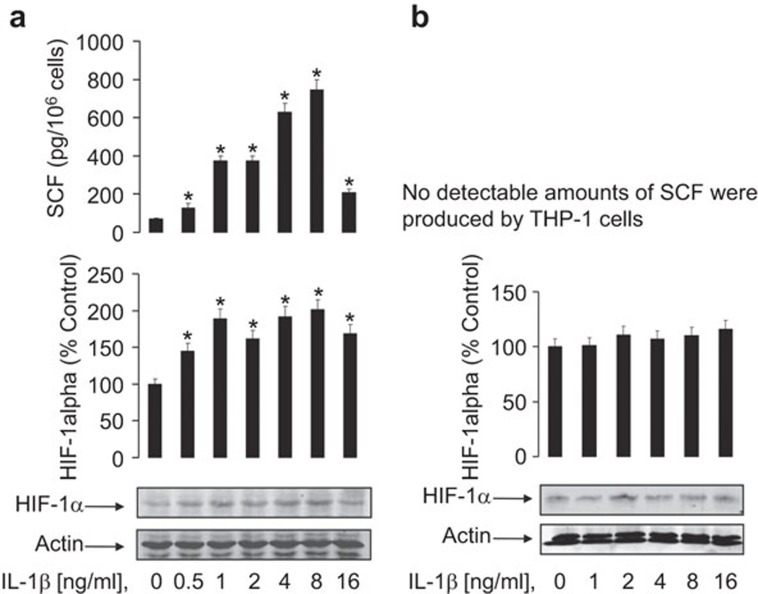
We investigated the ability of the highly inflammatory cytokine IL-1β to induce SCF expression and secretion in MCF-7 human epithelial breast cancer cells. MCF-7 cells were exposed to increasing concentrations of IL-1β for 24 h followed by detection of SCF release and HIF-1α accumulation. It was found that IL-1β induced both SCF release and HIF-1α accumulation (the optimal concentration of the cytokine was 8 ng/ml, Figure 1a). As a negative control, we used THP-1 human myeloid leukaemia cells which express around 20 molecules per cell of IL-1β receptor type 1 and do not produce SCF.13,14 THP-1 cells were exposed to the same concentrations of IL-1β as MCF-7 for 24 h. A minor increase in HIF-1α accumulation was observed (normally, only a very high concentration of IL-1β could induce HIF-1α accumulation in these cells).15 No detectable amounts of SCF were produced (Figure 1b).
Figure 1.
IL-1β induces SCF production and HIF-1α accumulation in MCF-7 cells in a concentration-dependent manner. (a) MCF-7 cells were exposed to increasing concentrations of IL-1β for 24 h followed by detection of SCF release and HIF-1α accumulation. (b) THP-1 human myeloid leukaemia cells were treated in the same way and were used as a negative control cell line which does not produce SCF. Western blot data show one representative experiment of three that produced similar results and were quantitatively analysed. Quantitative data are shown as mean values±s.d. of at least three individual experiments; *P<0.01 vs. control. HIF, hypoxia-inducible factor; SCF, stem cell factor.
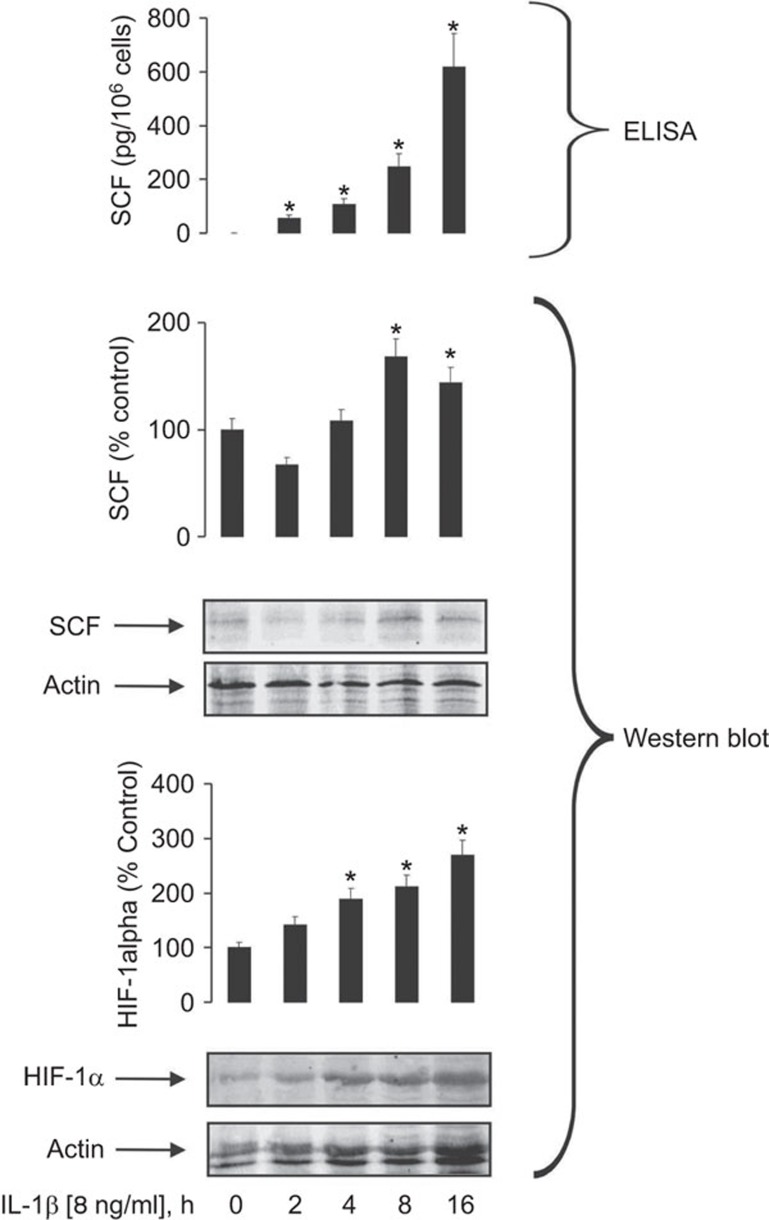
Using an optimal concentration of IL-1β (8 ng/ml), we next investigated the dynamics of its downstream effects. We exposed MCF-7 cells to the cytokine for 2, 4, 8 and 16 h and then studied SCF release and intracellular accumulation as well as HIF-1α levels, which showed a time-dependent increase in response (Figure 2).
Figure 2.
IL-1β-induced SCF production by the MCF-7 cells is a time-dependent process. MCF-7 cells were exposed to 8 ng/ml IL-1β for different periods of time followed by analysis of intracellular HIF-1α and SCF accumulation (Western blot) and SCF release (ELISA). Western blot data show one representative experiment of three which demonstrated similar results and were quantitatively analysed. Quantitative data are shown as mean values±s.d. for n=3; *P<0.01 vs. control. HIF, hypoxia-inducible factor; SCF, stem cell factor.
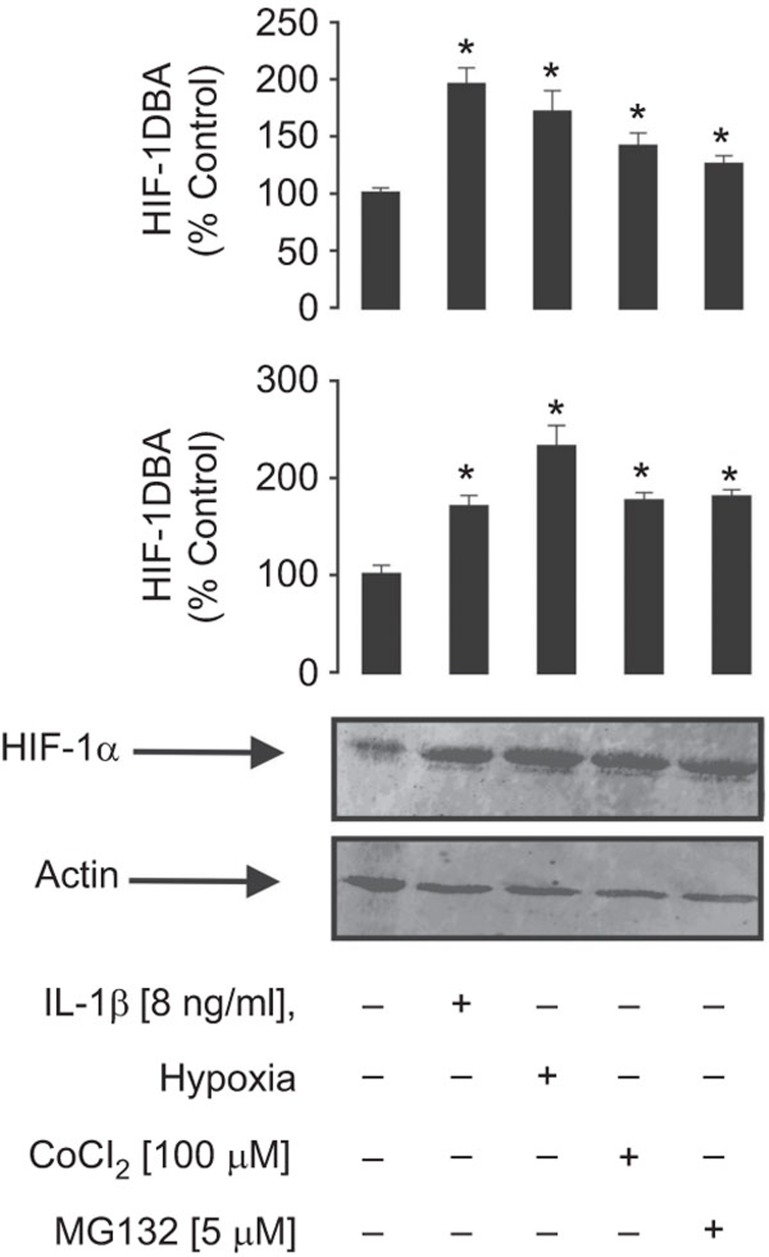
We then asked whether the observed IL-1β-induced HIF-1α accumulation is comparable with exposure to classical HIF-1α-inducing stimuli such as hypoxia (3% oxygen), cobalt chloride (100 µM) and the proteasomal degradation inhibitor MG-132 (5 µM). We found that both HIF-1α accumulation and HIF-1 DNA-binding activity induced by IL-1β were indeed comparable to those observed upon exposure of MCF-7 cells to ‘classic stimuli' (Figure 3).
Figure 3.
The level of IL-1β-induced HIF-1α accumulation in MCF-7 cells is comparable with those observed for classic HIF-1 activators. MCF-7 cells were exposed for 4 h to 8 ng/ml IL-1β, hypoxia (3% oxygen), cobalt chloride (100 µM) or MG132 (5 µM) followed by detection of HIF-1α accumulation and HIF-1 DBA as outlined in the ‘Materials and methods' section. Western blot data show one representative experiment of three that gave similar results and were quantitatively analysed. Quantitative data are shown as mean values±s.d. of n=3; *P<0.01 vs. control. DBA, DNA-binding activity; HIF, hypoxia-inducible factor.
The HIF-1 transcription complex is crucial for IL-1β-induced SCF expression in MCF-7 cells
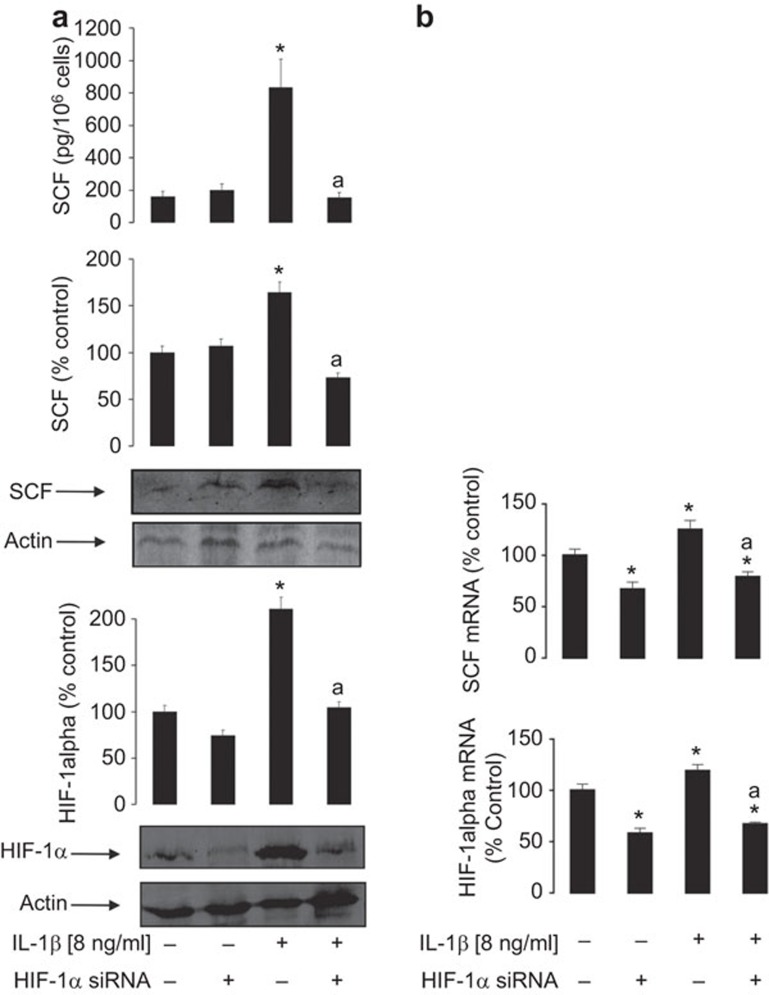
To investigate the role of HIF-1 in IL-1β-induced SCF expression in MCF-7 cells, we used normal and HIF-1α knockdown MCF-7 cells. We found that, in HIF-1α knockdown cells, IL-1β was not able to induce SCF protein expression and release upon 24 h of exposure to 8 ng/ml IL-1β (Figure 4a). Importantly, qRT-PCR analysis demonstrated that 6 h of exposure of MCF-7 cells to 8 ng/ml IL-1β increased both HIF-1α and SCF mRNA levels. Both types of upregulation were attenuated in HIF-1α knockdown MCF-7 cells (Figure 4b).
Figure 4.
The HIF-1 transcription complex is crucial for IL-1β-induced SCF expression in MCF-7 cells. (a) Normal and HIF-1α knockdown MCF-7 cells were exposed to 8 ng/ml IL-1β for 24 h followed by detection of intracellular HIF-1α/SCF levels as well as SCF release. (b) Normal and HIF-1α knockdown MCF-7 cells were exposed to 8 ng/ml IL-1β for 6 h followed by analysis of HIF-1α and SCF mRNA levels by qRT-PCR. Each sample was subjected to a contamination control through running qRT-PCR immediately after the decontamination procedure. No response was detected during 55 amplification cycles, indicating that contamination levels were close to zero (and are therefore not shown in the main figure). Western blot data show one representative experiment of 3–7 that gave similar results and were quantitatively analysed. Quantitative data are shown as mean values±s.d. of 3–7 individual experiments; *P<0.01 vs. control; aP<0.01 vs. IL-1β treatment. HIF, hypoxia-inducible factor; SCF, stem cell factor.
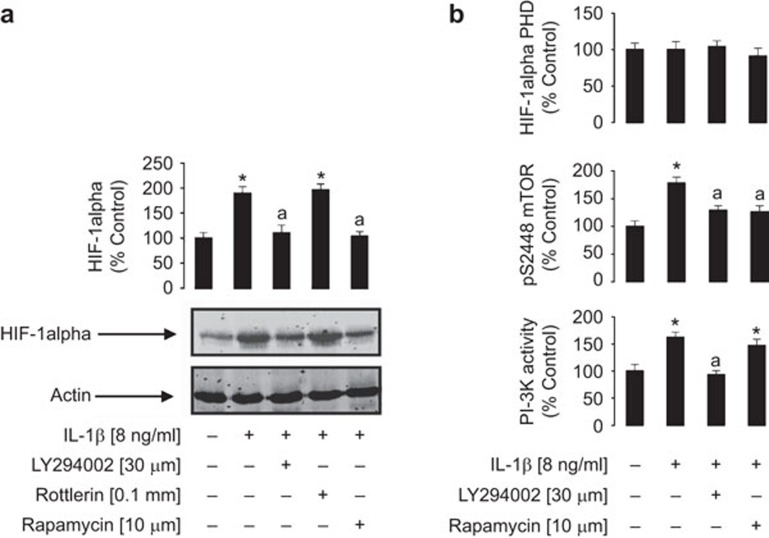
The PI-3K/mTOR pathway, but not HIF-1α PHD, is involved in IL-1β-induced HIF-1α accumulation and SCF expression
We investigated the mechanisms which support IL-1β-induced HIF-1α accumulation in MCF-7 breast cancer cells. Cells were pre-treated with pharmacological inhibitors of intracellular signalling enzymes which could be involved in mTOR activation (30 µM LY294002, a PI-3K inhibitor, or 0.1 mM Rottlerin, a PKCδ inhibitor) for 1 h and compared to the mTOR inhibitor rapamycin (10 µM). This was followed by 4 h of exposure to IL-1β (8 ng/ml). We observed that LY294002 and rapamycin, but not rottlerin, attenuated IL-1β-induced HIF-1α accumulation in MCF-7 cells suggesting crucial involvement of the PI-3K/mTOR pathway (Figure 5a). This is consistent with previous observations in other cell types.16 Importantly, a significant increase in PI-3K activity and phosphorylation of mTOR at S2448 were observed in MCF-7 cells treated with IL-1β. PI-3K activation was attenuated by LY294002 but not by rapamycin, while mTOR S2448 phosphorylation was attenuated by both inhibitors (Figure 5b). No changes were observed in HIF-1α PHD activity under any conditions, suggesting that HIF-1α prolyl hydroxylation is not affected by any of the above treatments (Figure 5b). Subsequently, analogous experiments were performed using 1 mM N-acetylcystein (an antioxidant), 10 µM PD098059 (an inhibitor of the extracellular signal-regulating kinase, ERK) and 100 µM N-monomethyl-arginine (NMMA; a nitric oxide synthase inhibitor). However, neither of these agents reduced IL-1β-induced HIF-1α accumulation (data not shown) suggesting that redox/NO-dependent mechanisms and the ERK pathway are not involved in this process.
Figure 5.
The PI-3K/mTOR pathway is crucially involved in IL-1β-induced HIF-1α accumulation in MCF-7 cells. Cells were pre-treated with inhibitors for 1 h (as indicated) and subsequently exposed to 8 ng/ml IL-1β for 4 h. The procedure was followed by Western blot analysis of HIF-1α accumulation (a) and detection of PI-3K and HIF-1α PHD activities as well as phospho-S2448 mTOR deposition (b). HIF-1α Western blot data show one representative experiment of three similar results and were quantitatively analysed. Quantitative data are shown as mean values±s.d. of at least three individual experiments; results represent percentage values compared to the control. *P<0.01 vs. control; aP<0.01 vs. IL-1β treatment. HIF, hypoxia-inducible factor; mTOR, mammalian target of rapamycin; PHD, prolyl hydroxylase; PI-3K, phosphatidylinositol-3 kinase.
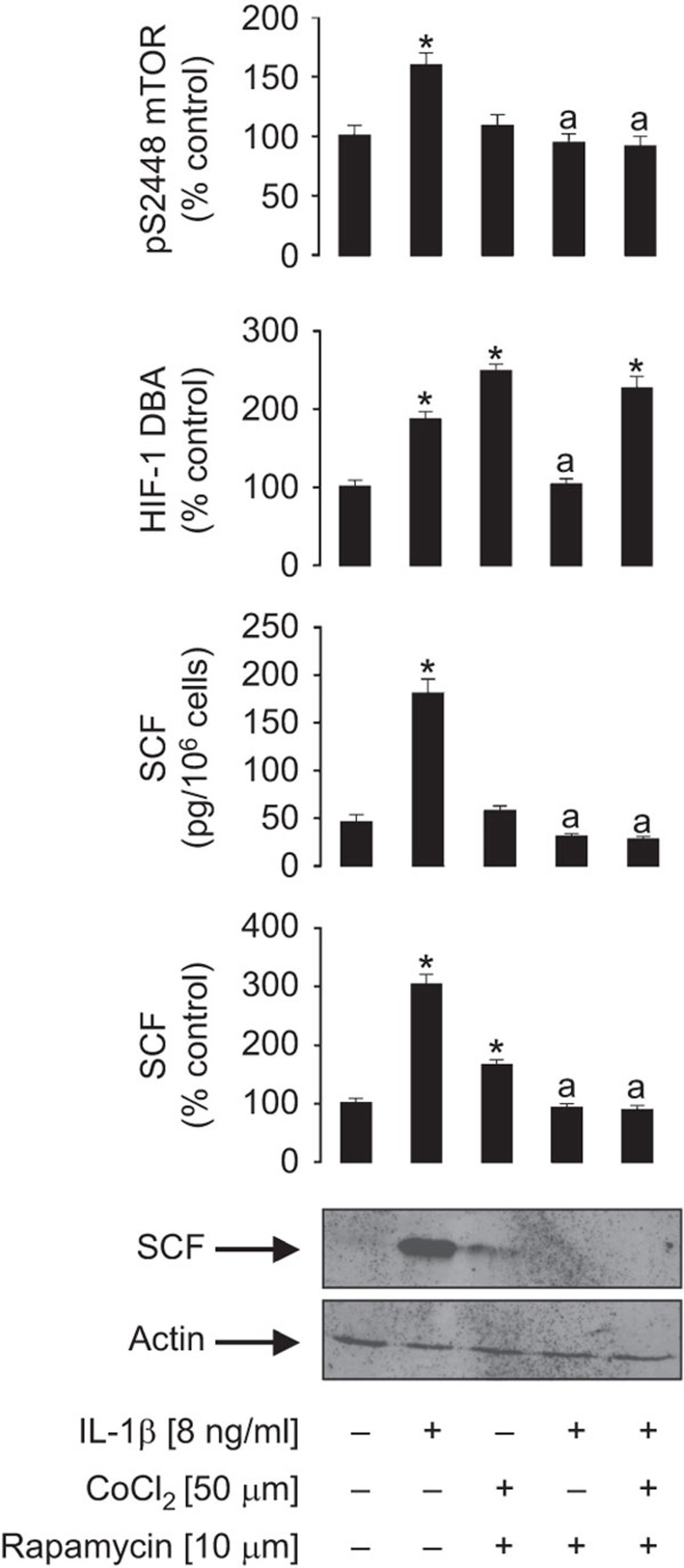
We also investigated whether mTOR is only directly involved in IL-1β-induced HIF-1α accumulation or whether it also contributes to the translation of SCF. For this purpose, we pre-treated MCF-7 cells with 10 µM rapamycin followed by 4 h exposure to 8 ng/ml IL-1β in the absence or presence of 50 µM CoCl2 to maintain HIF-1 activity in the case of inhibition of mTOR with rapamycin. HIF-1 DNA-binding activity and S2448 mTOR phosphorylation were monitored (Figure 6). The presence of CoCl2 prevented HIF-1 inactivation caused by rapamycin, but did not prevent the attenuation of IL-1β-induced SCF intracellular accumulation and release (Figure 6). This suggests that mTOR is also involved in SCF production. It is important to mention that traces of SCF were detected in the cells cotreated with CoCl2 and rapamycin alone, while SCF was not detectable in the cells stimulated with IL-1β in the presence of CoCl2 and rapamycin. This is most likely due to a high dependence of the cells on glycolysis during inflammatory stimulation (with IL-1β in our case).9 Glycolysis depends on both HIF-1-controlled transcription and mTOR-induced translation processes. Therefore, glycolysis was limited upon costimulation of the cells with IL-1β and rapamycin, thus reducing the energy potential of the cells. As shown in our previous reports, this effect downregulates the expression of growth factors/cytokines.7,8,9
Figure 6.
mTOR is crucially involved in IL-1β-induced SCF production. MCF-7 cells were pre-treated for 1 h with 10 µM rapamycin followed by 4 h exposure to 8 ng/ml IL-1β in the absence or presence of 50 µM CoCl2 to maintain HIF-1 activity in the case of mTOR inhibition with rapamycin. HIF-1 DNA-binding activity and S2448 mTOR phosphorylation were monitored as described in the ‘Materials and methods' section. Western blot data show one representative experiment of three that gave similar results and were quantitatively analysed. Quantitative data are shown as mean values±s.d. of at least three individual experiments; *P<0.01 vs. control; aP<0.01 vs. IL-1β. HIF, hypoxia-inducible factor; mTOR, mammalian target of rapamycin; SCF, stem cell factor.
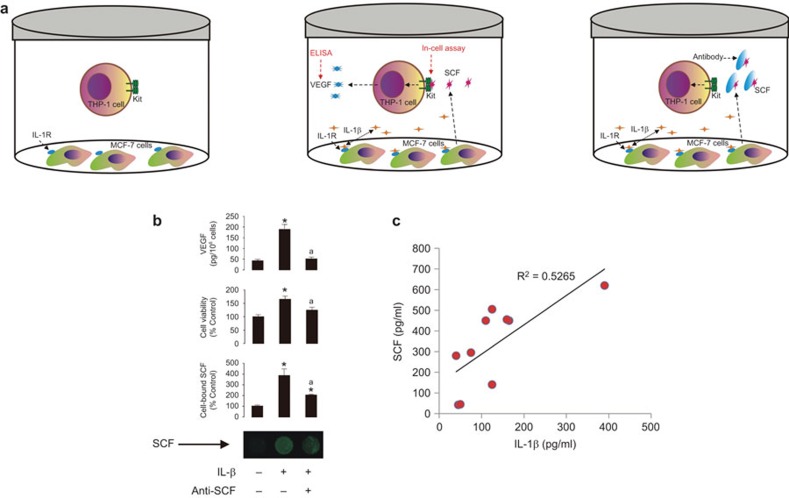
The effects observed are reproducible in a cell coculture system
We sought to reproduce the effects described above in a coculture model containing SCF producing cells (MCF-7 cells) and myeloid cells expressing the Kit receptor (THP-1 cells). Cells were cocultured at a ratio of three MCF-7 cells to one THP-1 cell and exposed to 8 ng/ml IL-1β for 24 h with or without 2 µg/ml SCF neutralizing antibody (R&D Systems, Abingdon, UK) which was added 30 min prior to IL-1β. After 24 h THP-1 cells and medium were subjected to further analysis. Equal amounts of cells from each treatment were analysed for the presence of SCF on their surface using an in-cell assay. The levels of VEGF released (as a biological response of THP-1 cells to treatment with SCF3) were analysed in the medium using ELISA (Figure 7a). SCF was detected on the surface of THP-1 cells when IL-1β was present in the coculture. This effect was reduced by the SCF neutralizing antibody (Figure 7b). Using MTS assays we found that, in the presence of IL-1β, THP-1 cells significantly proliferated more rapidly, an effect which was also reduced by the SCF neutralizing antibody. VEGF levels in the cell culture medium where higher in cocultures containing IL-1β, but they were significantly lowered by the presence of SCF (Figure 7b). This experiment confirms our basic conclusions reported above.
Figure 7.
IL-1β and SCF crosslinks using an in vitro coculture system and human plasma in vivo. (a) MCF-7 cells were cocultured with THP-1 cells at a ratio of 3∶1. Cocultures were exposed to 8 ng/ml IL-1β for 24 h in the absence or presence of 2 µg/ml human SCF neutralizing antibody which was added 30 min before IL-1β. (b) MTS cell viability and in-cell assay of SCF binding to THP-1 cells and VEGF release as outlined in Materials and Methods. (c) IL-1β and SCF levels were analysed in the plasma of ten healthy human donors by ELISA. Correlation analysis was performed using MS Excel software. Imaging data show one representative experiment of three that gave similar results and were quantitatively analysed. Quantitative data are shown as mean values±s.d. of at least three individual experiments; *P<0.01 vs. control; aP<0.01 vs. IL-1β. MTS, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium; SCF, stem cell factor.
IL-1β and SCF levels positively correlate in human plasma in vivo
We measured the levels of IL-1β and SCF levels in the plasma of healthy human donors as outlined in the section on ‘Materials and methods'. A clear tendency for a positive correlation between the levels of IL-1β and SCF was observed (Figure 7c) and could be considered as an indirect confirmation in vivo of the effects which we observed using cells in vitro.
Discussion
While an association between inflammation and oncogenesis has long been appreciated, there is comparatively little experimental evidence to actually support this concept.17,18 It is, however, of considerable importance to establish whether inflammatory mediators are able to upregulate the expressions of proto-oncogenic growth factors and elucidate the mechanisms involved. Although IL-1β is known to induce SCF production by human lung-derived fibroblasts10,11 the mechanisms have not been resolved except for the involvement of NF-kB transcription factor in the upregulation of SCF mRNA levels.10,11,12 However, it has been shown that the SCF gene contains hypoxia-responsive elements in its promoter region4 and we demonstrated that IL-1β (as well as other inflammatory stimuli) activates the HIF-1 transcription complex which is also an upstream regulator of SCF production.4 Furthermore, NF-κB is known to promote non-hypoxic upregulation of HIF-1 on a transcriptional level, and thus could actually crosslink the two pathways. Because of this, we aimed to study the role of the HIF-1 transcription complex and associated pathways in IL-1β-induced SCF production.
SCF is a major haematopoietic growth factor which crucially controls the progression of acute myeloid leukaemia upon malignant transformation of hematopoietic cells of myeloid lineage. This cytokine is also responsible for mast cell development and maintaining the function of mature mast cells of different phenotypes.19 Given the importance of mast cells in contributing to allergic inflammation, regulation of SCF production by IL-1β would suggest an important allergo-oncological axis.
In the present study, we employed MCF-7 human breast cancer epithelial cells which are capable of producing SCF and express IL-1β receptor type 1, which mediates the intracellular pro-inflammatory reactions induced by this cytokine. Since pro-inflammatory stimuli, such as IL-1β, provoke major alterations in intracellular signalling networks and cell metabolism, this requires target cells to adapt to inflammatory stress. This process depends majorly on the activation of the HIF-1 transcription complex which has over 40 target genes that control, for example, glycolysis, angiogenesis and cell adhesion. The promoter region of the SCF gene contains hypoxia-responsive elements; HIF-1 cognate sequences. We therefore investigated the effects of IL-1β on SCF expression and HIF-1α accumulation in MCF-7 cells and found that IL-1β induces both SCF expression and release as well as HIF-1α accumulation in a concentration-dependent manner. Eight ng/ml was found to be the optimal cytokine concentration and both processes took take place in a time-dependent manner. Importantly, the levels IL-1β-dependent induction of HIF-1α accumulation/HIF-1 DNA-binding activity was comparable with those induced by classic triggers of HIF-1 activation (3% hypoxia, CoCl2 or use of the proteasome inhibitor MG132).
Since the promoter region of the SCF gene contains hypoxia-responsive elements,4 we investigated the role of the HIF-1 transcription complex in IL-1β-induced SCF expression using normal and HIF-1α knockdown MCF-7 cells exposed to IL-1β. We discovered that HIF-1α knockdown cells were unable to upregulate SCF expression and release in response to stimulation with IL-1β. This was observed on all three levels—transcription, intracellular protein production and secretion of SCF.
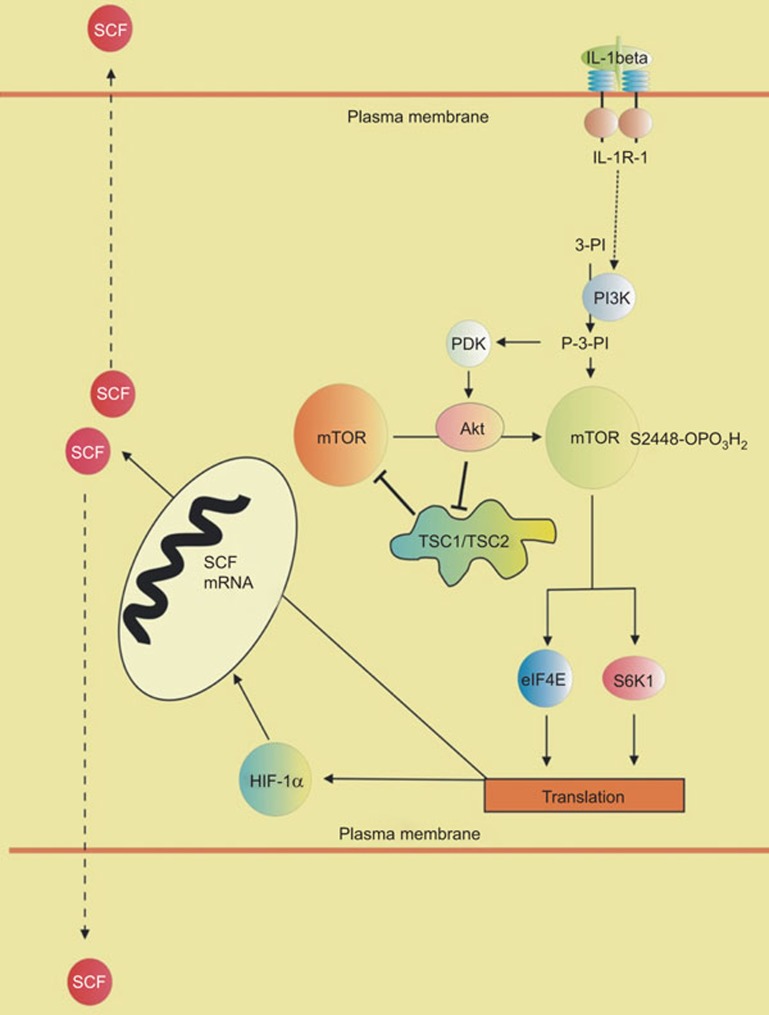
Experiments with enzyme assays and pharmacological inhibitors clearly demonstrated the involvement of the PI-3K/mTOR pathway in IL-1β-induced HIF-1 activation. Importantly, HIF-1α prolyl hydroxylation had no input, which is consistent with previous observations in other cell lines.16 However, attenuation of mTOR activity downregulates the translation of a number of signalling proteins and SCF might not be an exception in this case. Experiments with MCF-7 cells, where mTOR was blocked by rapamycin and HIF-1 was kept active with the help of CoCl2, demonstrated that mTOR is critical for SCF production. Experiments with normal and HIF-1α knockdown MCF-7 cells confirmed a crucial involvement of HIF-1 in IL-1β-induced SCF expression, while further experiments also suggested a critical role of mTOR at the translational level. A summary of these conclusions is outlined in Figure 8.
Figure 8.
Crucial involvement of the PI-3K/mTOR pathway and HIF-1 transcription complex in IL-1β induced SCF production and secretion. eIF4E, eukaryotic translation initiation factor 4E; HIF, hypoxia-inducible factor; mTOR, mammalian target of rapamycin; PDK, phosphatidylinositol (P-3-PI)-dependent kinase; PI-3K, phosphatidylinositol-3 kinase; SCF, stem cell factor; S6K1, ribosomal protein S6 kinase beta-1; TSC, tuberous sclerosis complex.
Our results were verified using a coculture system containing MCF-7 and THP-1 cells. It was clearly shown that SCF released by MCF-7 cells in response to stimulation with IL-1β was biologically active since it interacted with THP-1 cells and induced VEGF release. The fact that all these effects were not completely attenuated by SCF neutralizing antibody (despite significant reductions) is possibly due to the fact that each resting THP-1 cell expresses approximately 20 molecules of the IL-1 receptor.14 Thus, IL-1β-mediated effects could also occur in addition to those caused by SCF, which would not be attenuated by SCF neutralizing antibodies. Additionally, our results received a partial confirmation in vivo in experiments with human blood samples where there was a clear tendency for a correlation between IL-1β and SCF levels in plasma.
Altogether, our results demonstrate that the highly inflammatory cytokine IL-1β, which crucially bridges innate and adaptive immunity, induces the production of the major haematopoietic/proleukaemic growth factor SCF. The process depends on the HIF-1 transcription complex and is controlled by the PI-3K/mTOR pathway. These observations underline important crosslinks between IL-1β-dependent host immune responses, including autoimmune disorders and allergies, and leukaemia progression as well as haematopoiesis.
References
- 1Broudy VC. Stem cell factor and hematopoiesis. Blood 1997; 90: 1345–1364. [PubMed] [Google Scholar]
- 2Lee SJ, Yoon JH, Song KS. Chrysin inhibited stem cell factor (SCF)/c-Kit complex-induced cell proliferation in human myeloid leukemia cells. Biochem Pharmacol 2007; 74: 215–225. [DOI] [PubMed] [Google Scholar]
- 3Gibbs BF, Yasinska IM, Oniku AE, Sumbayev VV. Effects of stem cell factor on hypoxia-inducible factor 1 alpha accumulation in human acute myeloid leukaemia and LAD2 mast cells. PLoS One 2011; 6: e22502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4Han ZB, Ren H, Zhao H, Chi Y, Chen K, Zhou B et al. Hypoxia-inducible factor (HIF)-1 alpha directly enhances the transcriptional activity of stem cell factor (SCF) in response to hypoxia and epidermal growth factor (EGF). Carcinogenesis 2008; 29: 1853–1861. [DOI] [PubMed] [Google Scholar]
- 5Semenza GL. HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol Med 2002; 8: S62–S67. [DOI] [PubMed] [Google Scholar]
- 6Sumbayev VV. LPS-induced Toll-like receptor 4 signalling triggers cross-talk of apoptosis signal-regulating kinase 1 (ASK1) and HIF-1alpha protein. FEBS Lett 2008; 582: 319–326. [DOI] [PubMed] [Google Scholar]
- 7Lall H, Coughlan K, Sumbayev VV. HIF-1alpha protein is an essential factor for protection of myeloid cells against LPS-induced depletion of ATP and apoptosis that supports Toll-like receptor 4-mediated production of IL-6. Mol Immunol 2008; 45: 3045–3049. [DOI] [PubMed] [Google Scholar]
- 8Nicholas SA, Sumbayev VV. The involvement of hypoxia-inducible factor 1 alpha in Toll-like receptor 7/8-mediated inflammatory response. Cell Res 2009; 19: 973–983. [DOI] [PubMed] [Google Scholar]
- 9Sumbayev VV, Nicholas SA. Hypoxia-inducible factor 1 as one of the “signaling drivers” of Toll-like receptor-dependent and allergic inflammation. Arch Immunol Ther Exp (Warsz) 2010; 58: 287–294. [DOI] [PubMed] [Google Scholar]
- 10da Silva CA, Kassel O, Mathieu E, Massard G, Gasser B, Frossard N. Inhibition by glucocorticoids of the interleukin-1beta-enhanced expression of the mast cell growth factor SCF. Brit J Pharmacol 2002; 135: 1634–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11Da Silva CA, Heilbock C, Kassel O, Frossard N. Transcription of stem cell factor (SCF) is potentiated by glucocorticoids and interleukin-1beta through concerted regulation of a GRE-like and an NF-kappaB response element FASEB J 2003; 17: 2334–2336. [DOI] [PubMed] [Google Scholar]
- 12Reber L, Vermeulen L, Haegeman G, Frossard N. Ser276 phosphorylation of NF-kB p65 by MSK1 controls SCF expression in inflammation. PLoS One 2009; 4: e4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13Sims JE, Gayle MA, Slack JL, Alderson MR, Bird TA, Giri JG et al. Interleukin 1 signaling occurs exclusively via the type I receptor. Proc Natl Acad Sci USA 1993; 90: 6155–6159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Nehme A, Edelman J. Dexamethasone inhibits high glucose-, TNF-alpha-, and IL-1beta-induced secretion of inflammatory and angiogenic mediators from retinal microvascular pericytes. Invest Ophthalmol Vis Sci 2008; 49: 2030–2038. [DOI] [PubMed] [Google Scholar]
- 15Sumbayev VV, Yasinska IM, Garcia CP, Gilliland D, Lall GS, Gibbs BF et al. Gold nanoparticles downregulate interleukin-1beta-induced pro-inflammatory responses. Small 2013; 9: 472–477. [DOI] [PubMed] [Google Scholar]
- 16Jung YJ, Isaacs JS, Lee S, Trepel J, Neckers L. IL-1b mediated up-regulation of HIF-1a via an NFkB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. FASEB J 2003; 17: 2115–2117. [DOI] [PubMed] [Google Scholar]
- 17Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001; 357: 539–545. [DOI] [PubMed] [Google Scholar]
- 18Coussens LM, Werb Z. Inflammation and cancer. Nature 2002; 420: 860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19Frenz AM, Gibbs BF, Pearce FL. The effect of recombinant stem cell factor on human skin and lung mast cells and basophil leukocytes. Inflamm Res 1997; 46: 35–39. [DOI] [PubMed] [Google Scholar]
- 20Wang C, Liu J, Wang L, Geng X. Solubilization and refolding with simultaneous purification of recombinant human stem cell factor. Appl Biochem Biotechnol 2008; 144: 181–189. [DOI] [PubMed] [Google Scholar]
- 21Hanze J, Eul BG, Savai R, Krick S, Goyal P, Grimminger F, Seeger W, Rose F. RNA interference for HIF-1 inhibits its downstream signalling and affects cellular proliferation. Biochem Biophys Res Commun 2003; 312: 571–577. [DOI] [PubMed] [Google Scholar]
- 22Yasinska IM, Gibbs BF, Lall GS, Sumbayev VV. The HIF-1 transcription complex is essential for translational control of myeloid hematopoietic cell function by maintaining mTOR phosphorylation. Cell Mol Life Sci 2014; 71: 699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23Wiesner C, Nabha SM, dos Santos EB, Yamamoto H, Meng H, Melchior SW et al. C-kit and its ligand stem cell factor: potential contribution to prostate cancer bone metastasis. Neoplasia 2008; 10: 996–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24Abooali M, Lall GS, Coughlan K, Lall HS, Gibbs BF, Sumbayev VV. Crucial involvement of xanthine oxidase in the intracellular signalling networks associated with human myeloid cell function. Sci Rep 2014; 4: 6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25Sumbayev VV. PI3 kinase and direct S-nitrosation are involved in down-regulation of apoptosis signal-regulating kinase 1 during LPS-induced Toll-like receptor 4 signalling. Immunol Lett 2008; 115: 126–130. [DOI] [PubMed] [Google Scholar]
- 26Schneiter R, Daum G. Analysis of yeast lipids. In: Methods in Molecular Biology, 313: Yeast Protocols. 2nd ed. Totowa, NJ: Humana Press Inc., 2006: 75–84. [DOI] [PubMed] [Google Scholar]
- 27Oehme F, Jonghaus W, Narouz-Ott L, Huetter J, Flamme I. A nonradioactive 96-well plate assay for the detection of hypoxia-inducible factor prolyl hydroxylase activity. Anal Biochem 2004; 330: 74–80. [DOI] [PubMed] [Google Scholar]
- 28Nicholas SA, Sumbayev VV. The role of redox-dependent mechanisms in the downregulation of ligand-induced Toll-like receptors 7, 8 and 4-mediated HIF-1 alpha prolyl hydroxylation. Immunol Cell Biol 2010; 88: 180–186. [DOI] [PubMed] [Google Scholar]
- 29Nicholas SA, Oniku AE, Sumbayev VV. Myeloid cell death associated with Toll-like receptor 7/8-mediated inflammatory response. Implication of ASK1, HIF-1 alpha, IL-1 beta and TNF-alpha. Mol Immunol 2010; 48: 240–247. [DOI] [PubMed] [Google Scholar]