The paucity of naturally occurring CD4+FoxP3+ regulatory T cells (Tregs) and difficulties in the identification and isolation of such cells are major obstacles in the study of human Tregs. The availability of human Treg-like cell lines is likely to facilitate a better understanding of the molecular basis of Treg function and aid the discovery of pharmacological reagents to regulate Treg activity in a disease state. In this study, we examined the Treg phenotype and the immune suppressive effect of a human T-cell leukemia virus type 1 (HTLV-1) infected cell line, MT-2. The results clearly demonstrated that the MT-2 cell line has the phenotypic and functional characteristics of human Tregs and is a useful tool in the study of human Tregs.
The MT-2 cell line was derived from normal human cord leukocytes of a healthy donor by co-cultivation with leukemic cells from an adult T-cell leukemia (ATL) patient.1 It has been shown that MT-2 cells express FoxP3, and when mixed at a ratio of 1∶2 (suppressors/responders), MT-2 cells inhibited the proliferative response of purified human peripheral blood (PB) CD4+CD25− cells stimulated with dendritic cells and anti-CD3 Ab.2 However, the Treg phenotypic markers and relative suppressive activity of MT-2 cells on both proliferation and cytokine production of cocultured responder cells need to be further evaluated.
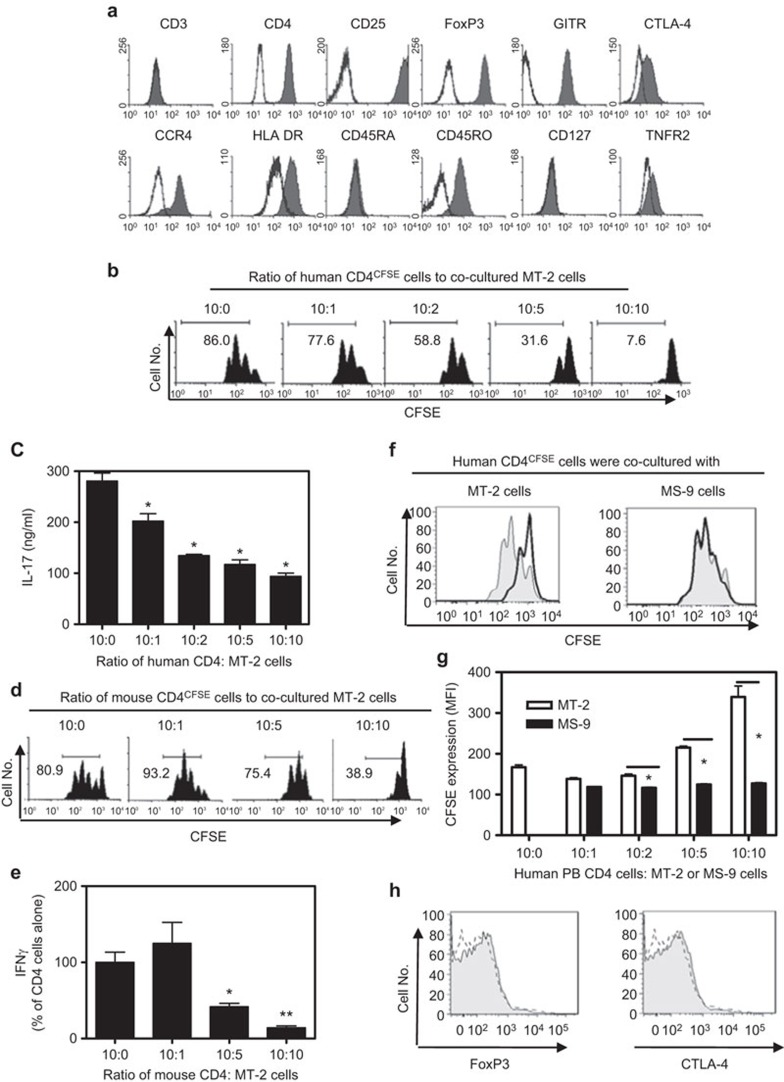
Human Treg-like phenotypic markers expressed by MT-2 cells
First, we examined the expression of Treg phenotypic markers on MT-2 cells. As shown in Figure 1a, MT-2 cells expressed CD4; however, unlike human PB CD4 T cells, MT-2 cells did not express CD3. CD25 was expressed by human PB CD4 cells at low levels compared with mouse CD4 cells, and only highly expressing CD25 (CD25high) human cells had consistent suppressive function.3 By contrast, all MT-2 cells expressed high levels of CD25. We confirmed the previous observation2 that MT-2 cells expressed FoxP3, a specific marker for Tregs. Thus, MT-2 cells expressed the characteristic markers of the CD4+CD25hiFoxP3+ human Tregs.
Figure 1.
(a) FACS analysis of MT-2 cells. MT-2 cells were stained with the indicated anti-human Abs. CTLA-4 and FoxP3 were used to stain intracellularly, whereas the other antibodies stained the cell surface. Gray histogram shows antibody staining. Isotype controls are shown by line histogram. (b–e) Suppressive effect of MT-2 cells on primary human PB CD4 cells and primary mouse CD4 cells. MACS-purified PB human CD4 cells or mouse CD4 cells were stained with CFSE and were cocultured with MT-2 cells at the indicated ratios. The cells were stimulated with APCs and anti-CD3 Ab for 3 days. The proliferation of PB human CD4 cells (b) or mouse CD4 cells (d) was measured by CFSE dilution with FACS. The percentage of CFSE-diluted cells is shown in the histograms. Production of IL-17 by human CD4 cells (c) and IFN-γ by mouse CD4 cells (e) in the supernatant was determined and the data shown (mean±s.d., N=3–5) represent the summary from three separate experiments. Compared with CD4 cells alone: *P<0.05, **P<0.01. (f–h) The phenotypic and functional properties of HTLV-1-infected MS-9 cells. CFSE-labeled human PB CD4 cells were cocultured with MT-2 cells or MS-9 cells at the desired ratio. The cells were stimulated with APCs and anti-CD3 Ab for 3 days. The proliferation of human PB CD4 cells was measured by CFSE dilution with FACS. (f) Typical FACS analysis. Gray histogram: human PB CD4 cells cultured alone (without MT-2 or MS-9 cells); solid line histogram: human PB CD4 cells cultured with MT-2 cells (left) or MS-9 cells (right) at a ratio of 10∶10. (g) CFSE expression (MFI) by human PB CD4 cells when cultured alone, cocultured with MT-2 cells (open bar), or with MS-9 cells (black bar). *P<0.05 (mean±s.d., N=3). (h) Intracellular expression of FoxP3 and CTLA-4 by MS-9 cells was analyzed by FACS. Gray histogram: anti FoxP3 Ab or anti CTLA-4 Ab staining. Dashed line histogram: isotype control staining. The data shown are representative of at least three separate experiments with similar results.
Similar to human Tregs, MT-2 cells did not express CD127.4 We and others reported that TNFR2 was preferentially expressed by ∼40% of mouse Tregs5 and by a majority of human PB CD4+FoxP3+ Tregs.6 Only a small fraction of MT-2 cells expressed low levels of TNFR2, which is similar to the TNFR2 expression pattern of mouse Tregs but not human Tregs. All MT-2 cells expressed high levels of GITR on the surface, and the majority of them expressed CTLA-4, a potential effector molecule of Tregs. It has been shown that approximately one-third of human Tregs expressed HLA-DR; HLA-DR+ Tregs represented a distinct functional subset of Tregs responsible for contact-dependent suppression.7 Interestingly, more than half of the MT-2 cells expressed HLA-DR. In addition, MT-2 cells expressed high levels of CCR4, a chemokine receptor recently found to enhance viability and maintain a high frequency of Tregs in patients with HTLV-1 infection, as well as directing Tregs to the tumor environment. MT-2 cells were negative for CD45RA and expressed high levels of CD45RO, suggesting that they may be an ‘effector/memory' type human Treg-like cells.
Suppressive activity of MT-2 cells on primary human PB CD4 cells and mouse CD4 cells
We next examined the number of MT-2 cells required to inhibit cocultured human PB CD4 cells. As shown in Figure 1b, the proliferative responses of PB CD4 cells to TCR stimulation, as measured by CFSE dilution, were suppressed by 9.7%, 31.6%, 63.3% and 91.2% after the addition of MT-2 cells at ratios of 10∶1, 10∶2, 10∶5 and 10∶10 (PB CD4 cells/MT-2 cells), respectively. Because the MT-2 cells alone produced high levels of IFN-γ and TNF, but not IL-17A (data not shown), we used IL-17A as an indicator to examine the suppressive effect of MT-2 cells on the cytokine production of cocultured human primary CD4 cells. As shown in Figure 1c, MT-2 cells potently inhibited IL-17A production over a ratio of 10∶1 to 10∶10 (CD4/MT-2 cells, P<0.05).
We then investigated whether the suppressive function of MT-2 cells was allogeneic in manner. We used mouse CD4 cells as responder cells to examine the suppressive effect of MT-2 cells. As shown in Figure 1d, at a ratio of 10∶5 to 10∶10 (CD4/MT-2 cells), MT-2 cells inhibited the proliferation of cocultured mouse CD4 cells by 8% and 51.9%, respectively. However, at a 10∶1 ratio, MT-2 cells stimulated, to a limited extent, the proliferation of cocultured mouse CD4 cells (P>0.05); this was probably caused by an allostimulatory effect of MT-2 cells. Similarly, IFN-γ production by mouse CD4 cells was markedly inhibited by MT-2 cells when cocultured at a ratio of 10∶5 and 10∶10 (CD4/MT-2, P<0.05–0.01), but not at a ratio of 10/1 (P>0.05, Figure 1e). Although MT-2 cells also suppressed TNF and IL-17 production by mouse CD4 cells (data not shown), they did not suppress IL-2 production (data not shown).
HTLV-1 infection is not sufficient to confer the Treg phenotype and function of MT-2 cells
To exclude the possibility that the suppressive activity of MT-2 cells was exerted by transferring HTLV-1 virus to responder cells, we compared MT-2 cells with HTLV-1-infected MS-9 cells because both of them can infect or transform other T cells in vitro.8,9 As shown in Figure 1f and g, MT-2 cells consistently showed suppressive activity, whereas MS-9 cells did not suppress the proliferation of cocultured primary human CD4 cells. Furthermore, unlike MT-2 cells, MS-9 cells did not express the characteristic Treg molecules such as FoxP3 and CTLA-4 (Figure 1h), although they expressed CD25 (data not shown). These data indicate that infection by HTLV-1 is not sufficient to confer the phenotypic and functional attributes of Tregs, and the suppressive activity of MT-2 cells is not based on its release of infectious HTLV-1. CTLA-4 is expressed by MT-2 cells but not by MS-9 cells, suggesting a role of CTLA-4 in mediating the suppressive activity of MT-2 cells. Nevertheless, MT-2 cells have the potential to produce TGF-β10 this immunosuppressive cytokine may also contribute to the suppressive activity of MT-2 cells.
Taken together, our data suggest that MT-2 cells have the phenotypic and functional characteristics of human Tregs and therefore may be used as a human Treg-like cell line for Treg studies. Similar to human Tregs, MT-2 cells were CD4+CD25hiFoxP3+ and expressed CTLA-4, GITR, CCR4, HLA-DR, CD45RO and TNFR2 but not CD127. The expression levels of these molecules on MT-2 cells are readily manipulated by molecular biological techniques, which may help reveal the roles of these molecules on the biological function of Tregs. The suppressive activity of MT-2 cells can be conveniently monitored with a CFSE dilution-based proliferation assay or with cytokine levels by using normal human PB CD4 cells or normal mouse CD4 cells as responders. Our data also revealed some differences between MT-2 cells and primary human Tregs. These differences should be taken into account when using MT-2 cells as a cellular model to study human Tregs. Nevertheless, MT-2 cells can be used as a positive control for human Treg studies and for biochemical studies of Tregs, which require larger numbers of cells.
Acknowledgments
The authors thank Drs Christine Lai, Kathryn S Jones, O M Zack Howard, Francis W Ruscetti and Ji Ming Wang for their help in this study.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organization imply endorsement by the U.S. Government.
This Research was supported (in part) by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research and (in part) by the University of Macau (SRG2014-00024-ICMS-QRCM).
Footnotes
Supplementary information accompanies the paper on Cellular & Molecular Immunology's website. (http://www.nature.com/cmi).
Supplementary Information
References
- 1Miyoshi I, Kubonishi I, Yoshimoto S, Akagi T, Ohtsuki Y, Shiraishi Y et al. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature 1981; 294: 770–771. [DOI] [PubMed] [Google Scholar]
- 2Chen S, Ishii N, Ine S, Ikeda S, Fujimura T, Ndhlovu LC et al. Regulatory T cell-like activity of Foxp3+ adult T cell leukemia cells. Int Immunol 2006; 18: 269–277. [DOI] [PubMed] [Google Scholar]
- 3Chen X, Oppenheim JJ. Resolving the identity myth: key markers of functional CD4+FoxP3+ regulatory T cells. Int Immunopharmacol 2011; 11: 1489–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ Treg cells. J Exp Med 2006; 203: 1701–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5Chen X, Baumel M, Mannel DN, Howard OM, Oppenheim JJ. Interaction of TNF with TNF receptor type 2 promotes expansion and function of mouse CD4+CD25+ T regulatory cells. J Immunol 2007; 179: 154–161. [DOI] [PubMed] [Google Scholar]
- 6Chen X, Subleski JJ, Hamano R, Howard OM, Wiltrout RH, Oppenheim JJ. Co-expression of TNFR2 and CD25 identifies more of the functional CD4+FOXP3+ regulatory T cells in human peripheral blood. Eur J Immunol 2010; 40: 1099–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7Costantino CM, Baecher-Allan CM, Hafler DA. Human regulatory T cells and autoimmunity. Eur J Immunol 2008; 38: 921–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8Jones KS, Petrow-Sadowski C, Huang YK, Bertolette DC, Ruscetti FW. Cell-free HTLV-1 infects dendritic cells leading to transmission and transformation of CD4+ T cells. Nat Med 2008; 14: 429–436. [DOI] [PubMed] [Google Scholar]
- 9Hill SA, Shuh M, Derse D. Comparisons of defective HTLV-I proviruses predict the mode of origin and coding potential of internally deleted genomes. Virology 1999; 263: 273–281. [DOI] [PubMed] [Google Scholar]
- 10Maeda M, Chen Y, Hayashi H, Kumagai-Takei N, Matsuzaki H, Lee S et al. Chronic exposure to asbestos enhances TGF-beta1 production in the human adult T cell leukemia virus-immortalized T cell line MT-2. Int J Oncol 2014; 45: 2522–2532. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.