Abstract
Decidual natural killer (dNK) cells actively participate in the establishment and maintenance of maternal–fetal immune tolerance and act as local guardians against infection. However, how dNK cells maintain the immune balance between tolerance and anti-infection immune responses during pregnancy remains unknown. Here, we demonstrated that the inhibitory molecule T-cell immunoglobulin domain and mucin domain-containing molecule-3 (Tim-3) are expressed on over 60% of dNK cells. Tim-3+ dNK cells display higher interleukin (IL)-4 and lower tumor necrosis factor (TNF)-α and perforin production. Human trophoblast cells can induce the transformation of peripheral NK cells into a dNK-like phenotype via the secretion of galectin-9 (Gal-9) and the interaction between Gal-9 and Tim-3. In addition, trophoblasts inhibit lipopolysaccharide (LPS)-induced pro-inflammatory cytokine and perforin production by dNK cells, which can be attenuated by Tim-3 neutralizing antibodies. Interestingly, a decreased percentage of Tim-3-expressing dNK cells were observed in human miscarriages and murine abortion-prone models. Moreover, T helper (Th)2-type cytokines were decreased and Th1-type cytokines were increased in Tim-3+ but not Tim-3− dNK cells from human and mouse miscarriages. Therefore, our results suggest that the Gal-9/Tim-3 signal is important for the regulation of dNK cell function, which is beneficial for the maintenance of a normal pregnancy.
Keywords: cytotoxicity, galectin-9/Tim-3, NK cells, pregnancy, Th2
Introduction
In normal pregnancies, maternal immune cells are in direct contact with fetal trophoblast cells, which express paternal antigens. Therefore, a successful pregnancy requires the maternal immune system to tolerate the semi-allogeneic fetus.1,2,3 Multiple mechanisms are believed to exist to promote maternal–fetal immune tolerance. For example, T helper (Th)2 cytokine polarization,4 the expression of non-classical major histocompatibility complex molecules on trophoblast cells,5,6 the inhibition of complement activation,7,8 and a delicate balance between costimulatory (CD80 and CD86) and co-inhibitory (CTLA-4 and PD-1) signals have been observed in normal pregnancies.9 In addition, immunoregulatory molecules, including indoleamine 2,3-dioxygenase, interleukin (IL)-10 and transforming growth factor-β, are expressed at high levels at the maternal–fetal interface.10,11
Decidual natural killer (dNK) cells, the dominant lymphocyte population that accumulates in the decidua, play a pivotal role in successful pregnancies. It is generally accepted that dNK cells are recruited from the peripheral blood and then are educated in the microenvironment at the maternal–fetal interface.12,13,14 dNK cells display unique phenotypic properties and functional profiles. By expressing multiple cell surface molecules, secreting a variety of soluble factors, and interacting with other cell subsets in decidua, dNK cells participate in maintaining maternal–fetal tolerance, trophoblast invasion and vascular remodeling.15,16 Crosstalk between decidual NK cells and CD14+ myelomonocytic cells was reported to result in induction of regulatory T cells and immunosuppression.17 Recent studies have found that dNK cells promote immune tolerance and successful pregnancies by dampening inflammatory TH17 cells via interferon (IFN)-γ secreted by the CD56brightCD27+ NK subset.18 Consequently, dNK cells have been shown to be a key regulatory subset that facilitates maternal-fetal immune tolerance. Abnormal changes in dNK cell number and function are found to be closely related with adverse pregnancy outcomes, such as recurrent spontaneous abortion.
As a major contributor to innate immunity, NK cells also provide competent responses to infections, in addition to its immune regulatory actions during pregnancy. Maternal infections with bacterial or viral agents during pregnancy are associated with an increased incidence of miscarriage. Moderate inflammation is necessary to eliminate the outside invaders, but uncontrolled or exaggerated infection-triggered inflammation may be an important cause of pregnancy loss. Lipopolysaccharide (LPS) exposure resulting from microbial invasion of the endometrium has been linked to the risk of idiopathic miscarriage in a range of human and animal studies.19 Upon binding with its ligand Toll-like receptor (TLR)4, LPS initiates a robust inflammatory response, which is characterized by the production of pro-inflammatory cytokines, such as tumor necrosis factor (TNF)-α and IL-1, which disturb the Th1/Th2 balance at the fetomaternal interface.20 dNK cells have also been reported to be targets of LPS, which can induce dNK cytotoxic activation.21 Therefore, as an active defender against microbial invasion, maintenance of a proper dNK cell inflammatory response is critical for a successful pregnancy during pathogen infection.
T-cell immunoglobulin domain and mucin domain-containing molecule-3 (Tim-3), a newly defined regulatory factor, downregulates Th1 responses through transduction of apoptosis signaling by galectin-9 (Gal-9) engagement, suggesting that Tim-3 may modulate the Th1/Th2 balance.22,23 In addition to being expressed on activated T cells, Tim-3 is also constitutively expressed on cells of the innate immune system in both mice and humans. Increasing numbers of studies have shown that abnormal expression of Tim-3 is an important cause of autoimmune diseases, infections, transplantation problems and cancers.24 Recent data have shown that NK cells can also be regulated by Tim-3. Tim-3 was found to act as a marker of activation or maturation of NK cells and suppress NK cell cytotoxicity.25 In contrast, other reports have provided evidence that increased Tim-3 expression on NK cells leads to NK cell dysfunction in chronic virus infections, such as hepatitis B and HIV infection.26,27 Therefore, we propose that the regulatory effects of Tim-3 on NK cells are distinct in different immune microenvironments. However, Gal-9/Tim-3 signaling has not yet been found to regulate the function of NK cells at the maternal–fetal interface.
In the present study, we first detected the expression of Tim-3 in dNK cells and analyzed the cytokine profile and cytotoxicity of Tim-3+ and Tim-3− dNK cells. Then, we investigated the role of Gal-9/Tim-3 signaling in the shift from pNK cells to a dNK cell-like phenotype, as instructed by trophoblasts. Moreover, we observed the role of Gal-9/Tim-3 signaling in the cytokine production and cytotoxicity of dNK cells after LPS stimulation. Finally, the number of Tim-3+ dNK cells and the cytokine profile of Tim-3+ and Tim-3− dNK cells in normal pregnancies and miscarriages were compared. Our data provide evidence that Gal-9/Tim-3 signaling plays an important physiological and pathological role in the regulation of dNK cell function during early pregnancy, which is also helpful for developing novel strategies to target Gal-9/Tim-3 signaling to promote maternal–fetal tolerance and prevent pregnancy loss.
Materials and methods
Human sample collection
This study was approved by the Human Research Ethics Committee of Obstetrics and Gynecology Hospital, Fudan University, Shanghai, China. All subjects gave informed written consent for the collection and study of tissue samples. First-trimester villous tissues were obtained from the placentas of healthy pregnant women (age: 27.50±3.42 years; gestational age at sampling: 8.28±1.25 weeks; mean± tandard deviation), and peripheral blood was also obtained for peripheral blood mononuclear cell isolation. First-trimester decidual tissues were obtained from healthy pregnant women (age: 29.15±5.27 years; gestational age at sampling: 8.35±1.12 weeks). Decidual tissues were also obtained from women with unexplained spontaneous abortions that occurred during the first trimester of pregnancy (age: 29.1±6.8 years; gestational age at sampling: 7.45±1.44 weeks). All normal pregnancies were terminated for non-medical reasons, and miscarriages were classified as unexplained after the exclusion of endocrine, anatomic, genetic abnormalities, infection, etc. All tissues were immediately collected into ice-cold Dulbecco's modified Eagle's medium (DMEM) with high D-glucose or DMEM F12 (Gibco, Grand Island, NY, USA), transported to the laboratory within 30 min after surgery and washed in calcium- and magnesium-free Hank's balanced salt solution for cell isolation.
Isolation and culture of human trophoblasts
Trophoblasts were isolated by trypsin-DNase I digestion and discontinuous Percoll gradient centrifugation from pooled villi obtained from four to six different pregnancies, as previously described.28 Cells were then seeded into tissue culture plates for further purification based on differential adherent velocity to eliminate adherent fibroblast cells and unattached leukocytes. This produced a 95% pure culture of trophoblast cells, as assessed by immunocytochemistry and flow cytometry (FCM) for cytokeratin 7 positivity, HLA-G positivity and vimentin negativity (data not shown). The purified trophoblasts were seeded into a 24-well plate at 5×105 cells per milliliter.
Isolation and culture of dNK cells
Decidual tissues (4–6 g) were cut and digested in DMEM F12 supplemented with type IV collagenase (1.0 mg/ml; Sigma-Aldrich Corp. St. Louis, MO, USA) in 1% FBS for 30 min at 37 °C with gentle agitation. The suspension was filtered and enriched by discontinuous Percoll gradient centrifugation, as previously described.29 dNK cells were isolated using NK Cell Isolation Kits (Miltenyi Biotec, Auburn, CA, USA) following the manufacturer's protocol. The purity of the CD56+CD3− dNK cells was more than 95%, as measured by FCM with CD56-PE-Cy7 and CD3-FITC. The purified dNK were cultured with the human trophoblasts and were subjected to LPS (100 ng/ml; PeproTech, Rocky Hill, NJ, USA), rhGal-9 (1000 ng/ml; R&D Systems, Minneapolis, MN, USA), anti-Tim-3 neutralizing antibodies (Abs) (10 µg/ml; R&D Systems, Minneapolis, MN, USA) or control treatments. The cells were harvested for FCM after 48 h of incubation.
NK and human primary trophoblast coculture
Peripheral NK cells were isolated from peripheral blood mononuclear cells using NK Cell Isolation Kits (Miltenyi Biotec). Human primary trophoblasts were cultured with purified pNK cells at a ratio of 1∶3 (trophoblast/NK). Anti-Tim-3 neutralizing Abs were added to the coculture system. The pNK cells were harvested for FCM after 72 h of coculture.
FCM
Cells were washed and incubated with the appropriate fluorochrome-conjugated Abs for 30 min at 4 °C for cell surface staining. The following human monoclonal antibodies (mAbs) were used: FITC-conjugated anti-CD3 (clone: HIT3a) and anti-CD11c (clone: 3.9) mAbs; PE-Cy7-conjugated anti-CD56 (clone: HCD56) and anti-CD4 (clone: RPA-T4) mAbs; APC-conjugated anti-CD8 (clone: SK1), anti-CD25 (clone: BC96) and anti-CD14 (clone: M5E2) mAbs. Cells were fixed and permeabilized according to the manufacturer's protocol. Permeabilized cells were stained for intracellular cytokines and Tim-3 (a transmembrane molecule). PE-conjugated anti-Tim-3 (clone: F38-2E2), anti-IL-4 (clone: 8D4-8), anti-Gal-9 (clone: 9M1-3) and anti-IL-10 (clone: JES3-9D7); FITC-conjugated (clone: Dg9) or APC-conjugated anti-perforin (clone: dG9); PE-Cy7-conjugated anti-TNF-α (clone: MAb11) and anti-IFN-γ (clone: 4S.B3); and APC-conjugated anti-IL-4 (clone: 8D4-8), anti-IL-10 (clone: JES3-9D7), anti-TNF-α (clone: MAb11) and anti-IFN-γ (clone: 4S.B3) were used. The following murine mAbs were also used: 488-conjugated anti-CD3 (clone: 17A2) mAb; PE-conjugated anti-Tim-3 (clone: RMT3-23) mAb; PE-Cy7-conjugated anti-Nkp46 (clone: 29A1.4) mAb; BV421-conjugated anti-IL-4 (clone; 11B11), anti-IL-10 (clone: JES5-16E3) and anti-transforming growth factor-β (clone: TW7-16B4) mAbs; BV510-conjugated anti-IFN-γ (clone: XMG1.2) and anti-TNF-α (clone: MP6-XT22) mAbs; and APC-conjugated anti-granzyme A (clone: GzA-3G8.5) mAb. All antibodies were purchased from Biolegend or eBioscience (eBioscience, San Diego, CA, USA). FCM analysis was performed on a Beckman-Coulter CyAN ADP Analyzer (Beckman Coulter, Inc. Kraemer Boulevard Brea, CA, USA). Data were analyzed with FlowJo Version 6.1 software (TreeStar, Asland, OR, USA).
ELISA
The purified trophoblasts were seeded into a six-well plate at a density of 1×106 cells/ml. The culture plates were pre-coated with Matrigel. The trophoblast supernatants were collected after 12, 24, 36, 48 and 60 h of culture. Each supernatant was centrifuged and stored at −20 °C. The human Galectin-9 ELISA kit (Cusabio, WuHan, HuBei Province, China) was used to measure Gal-9 production in each supernatant according to the manufacturer's instructions.
Murine models of pregnancy
Male DBA/2 and female CBA/J mice (8–10 weeks old) were purchased from Beijing HFK Bioscience Co, BeiJing, China. Male Balb/c mice (8–10 weeks old) were purchased from the Department of Laboratory Animal Science, Fudan University (Shanghai, China). All animals were kept under specific pathogen-free conditions. All of the experimental procedures involving animals were conducted in accordance with the National Guidelines for Animal Use in Research (China), and permission was given by the Human Research Ethics Committee of Obstetrics and Gynecology Hospital of Fudan University. Female CBA/J mice were mated in natural cycling with male Balb/c mice, and allogeneic CBA/J×DBA/2 mating combinations were established as follows: female CBA/J mice were mated in natural cycling with male DBA/2 mice. Detection of a vaginal plug was chosen to indicate day 0.5 of gestation. Both groups were euthanized at 14.5 days of gestation to examine the fetal resorption rate and cytokine expression. Uteri from pregnant mice were dissected free from the mesometrium and removed by cuts at the ovaries and cervix. The uteri were washed twice in ice-cold PBS and were cut into approximately 1-mm3 pieces. Minced uteri were enzymatically digested in DMEM F12 containing 1 mg/ml type IV collagenase and 0.2 mg/ml DNase I for 40 min at 37 °C with gentle agitation. The suspension was filtered through a 74-mm cell strainer.
Statistical analysis
All data are presented as the mean±s.e.m. The significance of the difference between two groups was determined using the two-tailed t-test. Multiple groups were analyzed with GraphPad Prism version 5 by one-way or two-way ANOVA with Bonferroni post-tests. For all statistical tests, P values <0.05 were considered statistically significant.
Results
Decidual Tim-3-expressing NK cells display features of immune tolerance
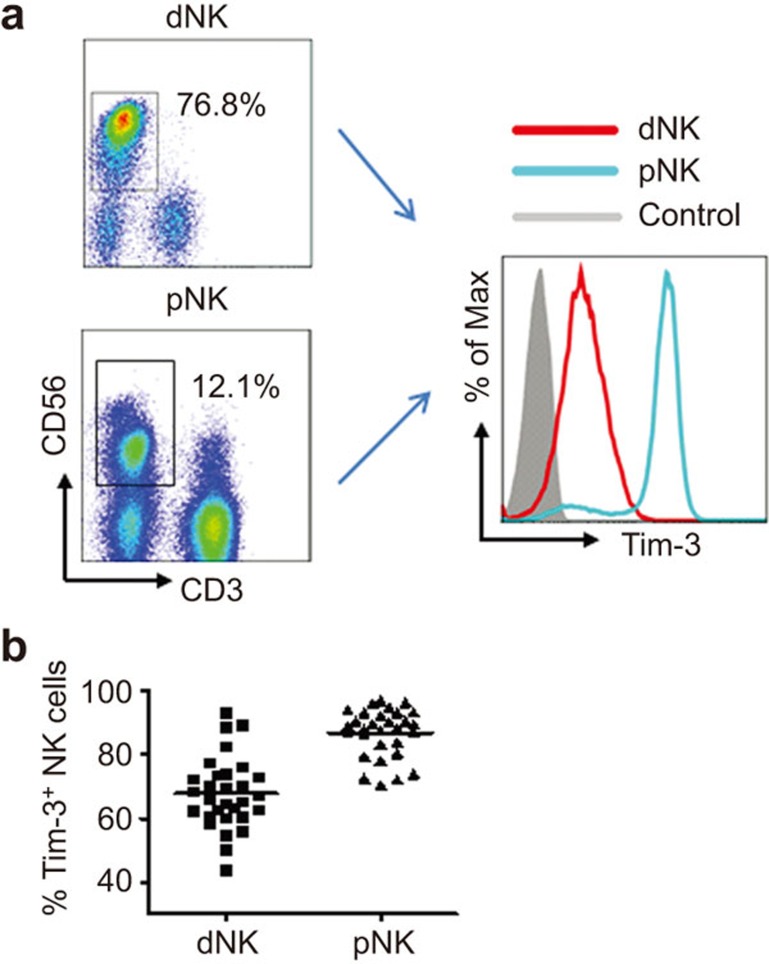
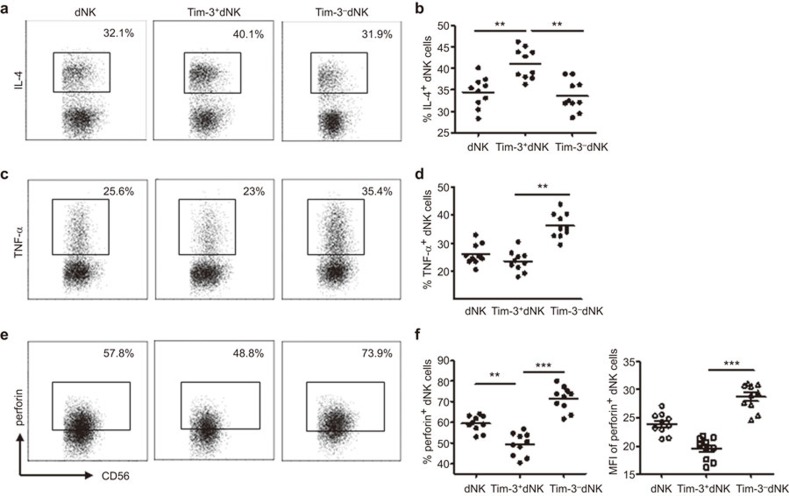
To investigate the regulation of Gal-9/Tim-3 signaling on decidual NK cells, we first analyzed the expression of Tim-3 in these cells. The results in Figure 1 show that more than 60% of decidual NK cells express Tim-3. We also detected the expression of Tim-3 on peripheral blood NK cells (pNK cells) and found that approximately 90% of pNK cells are Tim-3-positive (Figure 1a and b). To explore whether there is a correlation between Tim-3 expression and the functional status of dNK cells, we assessed their cytokine production and cytotoxicity. A higher amount of Th2-type cytokine IL-4 and a lower amount of Th1-type cytokine TNF-α were found to be expressed in Tim-3+ dNK cells than in Tim-3− dNK cells (Figure 2a–d). Thus, dNK cells expressing Tim-3 were more conducive to Th2 bias at the maternal-fetal interface than Tim-3− dNK cells. Cytotoxicity is another important function of NK cells. For this reason, perforin expression was assessed in Tim-3+ and Tim-3− dNK cells. Tim-3+ dNK cells consistently produced less perforin than Tim-3− dNK cells (Figure 2e and f). These data suggest that dNK cells can be divided into two different functional phenotypes based on Tim-3 expression and that Tim-3+ dNK cells show characteristics of immune tolerance.
Figure 1.
Tim-3+ NK cells are present in human deciduas during the first trimester. (a) Representative density plots showing the analysis of Tim-3 expression in decidual and peripheral blood CD3−CD56+ NK cells during the first trimester (Anti–Tim-3: open graph; isotype control: filled graph). (b) Relative number of Tim-3+ NK cells in gated CD3−CD56+ dNK and pNK cells (n=30). The data in b are presented as the mean±s.e.m. dNK, decidual NK cells; pNK, peripheral blood NK cells.
Figure 2.
Decidual Tim-3+ NK cells display a Th2 shift with low cytotoxicity. After treatment of the purified dNK cells with PMA, ionomycin and BFA for 4 h, cells were harvested and subjected to FCM analysis. The relative amount of Th2-type cytokines (a and b), Th1-type cytokines (c, d) and perforin (e, f) in gated CD3−CD56+ dNK cells, Tim-3+CD3−CD56+ dNK cells and Tim-3−CD3−CD56+ dNK cells, respectively, are shown. Data in b, d and f are presented as the mean±s.e.m. of 10 different samples. Representative density plots are shown in a, c and e. **P<0.01; ***P<0.001. dNK, decidual NK cells; FCM, flow cytometry.
The Gal-9/Tim-3 pathway is involved in the instruction of pNK cells
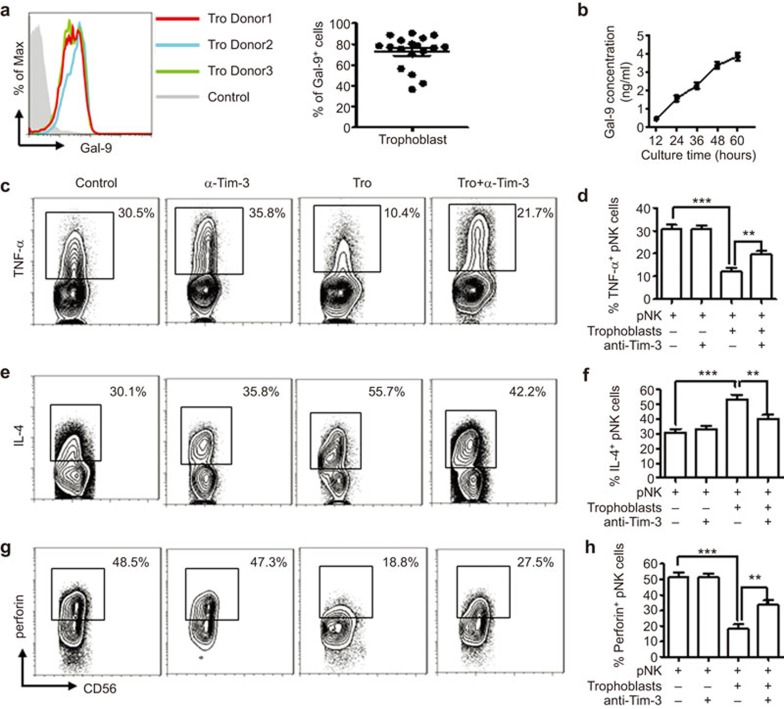
dNK cells have previously been reported to be recruited from peripheral blood by trophoblasts via CXCL12/CXCR4 interaction.30 These NK cells experience modulation by trophoblasts after enrichment at the maternal–fetal interface.37 Because Tim-3 was detected on peripheral NK cells and dNK cells in early pregnancy, we speculated here that the Gal-9/Tim-3 pathway may participate in the instruction of pNK cells by trophoblasts. We first determined the expression of Gal-9 in placental trophoblasts. As shown in Figure 3a, approximately 75% of trophoblast cells expressed Gal-9. The secretion of Gal-9 from human primary trophoblasts was then measured. As shown in Figure 3b, primary-cultured trophoblast cells secreted Gal-9 constitutively. The accumulated concentration of Gal-9 was 3.86±0.416 ng/ml after culture for 60 h seeded at 1×106 cells/ml. Then, a coculture system of pNK cells and trophoblasts was set up. After 72 h, the cytokine production and cytotoxicity of pNK cells were determined by FCM. As shown in Figure 3c–h, pNK cells produced more IL-4 but less TNF-α after coculture with trophoblasts. The cytotoxicity of pNK cells cocultured with trophoblasts decreased accordingly. Blocking Tim-3 signaling with anti-Tim-3 neutralizing Abs abrogated the regulation of pNK cells by trophoblasts. All these data suggest that trophoblasts induce transformation of pNK cells into a more immune-tolerant phenotype via secretion of Gal-9 and the interaction between Gal-9 and Tim-3.
Figure 3.
Trophoblast cells instruct pNKs to exhibit dNK cell-like phenotypes via interactions between Tim-3 and Gal-9. (a) FCM was performed to analyze intracellular expression of Gal-9 in trophoblasts. (b) An ELISA assay was used to assess the secretion of Gal-9 from human primary trophoblasts cultured for different periods of time. (c–h) Purified pNK cells from the first trimester were cultured with trophoblasts in the presence or absence of anti-Tim-3 neutralizing Abs for 72 h. FCM analysis was used to determine the levels of (c, d) Th1-type cytokines, (e, f) Th2-type cytokines and (g, h) cytotoxicity of pNK cells. The results are representative of 9 independent experiments. Data are shown as the mean±s.e.m. *P<0.05; **P<0.01; ***P<0.001. FCM, flow cytometry; pNK, peripheral NK cells; Tro, trophoblast; α-Tim-3, anti-Tim-3 neutralizing antibodies.
Tim-3 signaling negatively regulates the inflammatory response of dNK cells to LPS stimulation
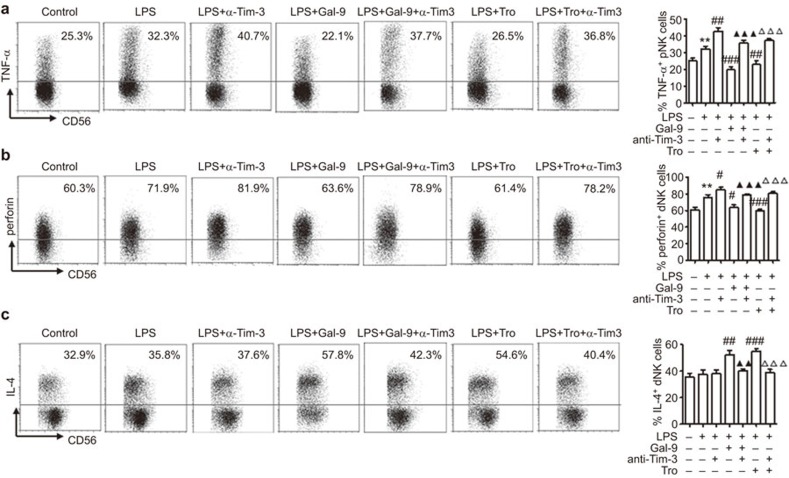
To investigate the involvement of Tim-3 in dNK cell function under pathological conditions, the effects of Tim-3 signaling on an LPS-triggered inflammatory response of dNK cells were examined. The dNK cells were obtained and treated with LPS. Tim-3 or rh-Gal-9 neutralizing Abs were pre-added to the media of some wells. As shown in Figure 4a, LPS treatment stimulated TNF-α production by dNK cells. This effect was abrogated by the administration of rhGal-9, the Tim-3 ligand. Because trophoblasts produce a large amount of Gal-9, we also observed the effect of trophoblasts on the production of TNF-α by dNK cells after LPS stimulation. Interestingly, human primary trophoblast cells significantly inhibited LPS-triggered TNF-α production by dNK cells. However, the administration of anti-Tim-3 neutralizing Abs significantly enhanced the TNF-α production of dNK cells in response to LPS alone or in the presence of rhGal-9 or trophoblasts. A similar regulation pattern of perforin expression in dNK cells by rh-Gal and Tim-3 antibodies was observed (Figure 4b). Although LPS had no obvious effect on the production of IL-4 by dNK cells, rhGal-9 and human trophoblasts significantly increased IL-4 production by dNK cells under LPS stimulation (Figure 4c), which was abrogated by anti-Tim-3 neutralizing Abs. These data indicate that trophoblasts may exert important regulatory effects on dNK cells via the secretion of Gal-9 to control excess inflammatory response to a pathogenic invader.
Figure 4.
Tim-3 signaling inhibits inflammatory cytokine production by LPS-induced dNK cells. Purified dNK cells alone or cocultured with trophoblasts were treated with control or LPS for 48 h. Some cells were pretreated with rhGalectin-9 or anti-Tim-3 neutralizing Abs. The percentages of cells producing the inflammatory cytokine TNF-α (a), cytotoxic perforin (b) and IL-4 (c) in gated CD3−CD56+ dNK cells were determined using FCM after pre-stimulation with PMA, ionomycin and BFA for 4 h. The results are representative of nine independent experiments. Data are shown as the mean±s.e.m. **P<0.01, compared with dNK cells cultured alone; # P<0.05, ## P<0.01, ### P<0.001 compared with dNK cells treated with LPS; ▴▴ P<0.01, ▴▴▴ P<0.001 compared with dNK cells treated with LPS and rhGal-9; ▵▵▵ P<0.001 compared with dNK cells cocultured with trophoblasts and treated with LPS; dNK, decidual NK cells; FCM, flow cytometry; LPS, Lipopolysaccharide; Tro, trophoblast; α-Tim-3, anti-Tim-3 neutralizing antibodies.
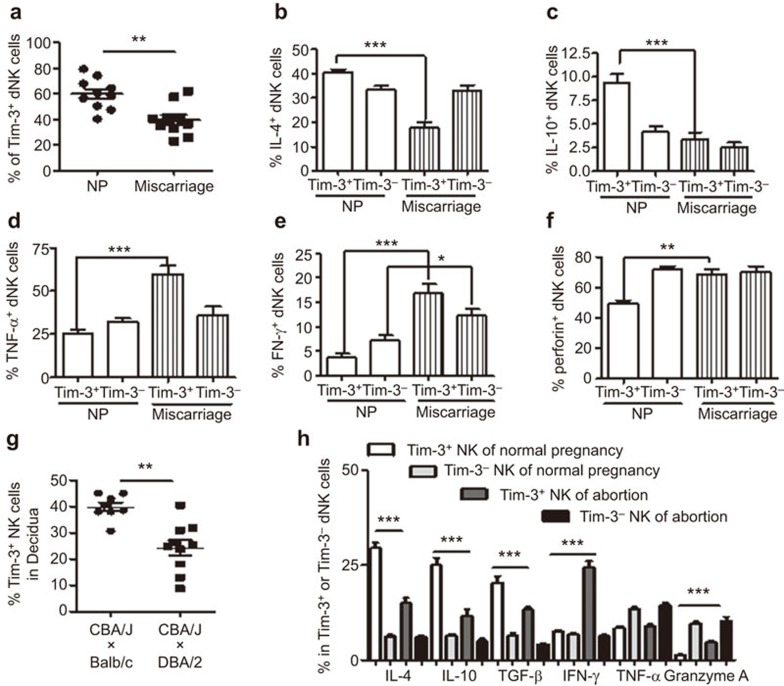
A decreased percentage of Tim-3+ dNK cells are accompanied by disturbed Th2 predominance and higher cytotoxicity in miscarriages
To obtain further evidence for the role of Tim-3 in dNK cell function during early pregnancy, Tim-3 expression by dNK cells was detected in normal pregnancies and miscarriages. Compared with normal pregnancies, the percentage of Tim-3+ dNK cells was significantly decreased in miscarriages (Figure 5a). Furthermore, the production of Th2-type cytokines, such as IL-4 and IL-10, was significantly decreased in Tim-3+ dNK cells from miscarriage compared with that from normal pregnancies. However, no distinct difference in IL-4 or IL-10 production was observed in Tim-3− dNK cells from normal pregnancies and miscarriages (Figure 5b and c). The expression of the Th1-type cytokine TNF-α was significantly upregulated in Tim-3+ dNK cells but not in Tim-3− dNK cells in miscarriages (Figure 5d). Although IFN-γ production was higher in both subsets in miscarriages (Figure 5e), the increase in levels of IFN-γ in Tim-3+ dNK cells was more obvious than that in Tim-3− dNK cells from miscarriages. A significant elevation in perforin expression was also observed in Tim-3+ cells but not in Tim-3− dNK cells from miscarriages (Figure 5f). A similar phenomenon was also observed in abortion-prone mouse models (Figure 5g and h). These results show that not only the number of Tim-3-expressing dNK cells, but also the function of these Tim-3+ dNK cells are critical for the maintenance of normal pregnancy. The dysfunction of Tim-3+ dNK may be an important cause of miscarriage in early pregnancy.
Figure 5.
Decreased numbers of Tim-3+ dNK cells is accompanied by dysregulated Th2 predominance and higher cytotoxicity in human miscarriages. (a) FCM was used to analyze the relative number of Tim-3+ dNK cells in normal pregnancies and miscarriages. (b–f) dNK cells from normal pregnancies and miscarriages were treated with PMA, ionomycin and BFA for 4 h, and intracellular cytokines and perforin were detected using FCM. Histograms show Th2 cytokine (IL-4 and IL-10) (b, c), Th1 cytokine (TNF-α and IFN-γ) (d, e) and perforin (f) production by Tim-3+ and Tim-3− dNK cells from normal pregnancies and miscarriages. (g) Tim-3 expression on NK cells from decidual tissues of normal pregnant mice and abortion-prone mice during the first trimester. (h) FCM analysis of cytokine production by NK cells from decidua of normal pregnant mice and abortion-prone mice. Data are shown as the mean±s.e.m. of 10 different samples. *P<0.05; **P<0.01; ***P<0.001. NP, normal pregnancy. CBA/J×Balb/c, normal pregnancy mouse model; CBA/J×DBA/2, abortion-prone mouse model; dNK, decidual NK cells; FCM, flow cytometry.
Discussion
In this study, we demonstrated that over 60% of dNK cells express Tim-3. Tim-3+ dNK cells serve as an immune-tolerant subset, which displays high levels of Th2-type cytokine production but low levels of Th1-type cytokine production and poor cytotoxicity. In addition, Tim-3 can also suppress LPS-triggered inflammatory responses by dNK cells, which is enhanced by rhGal-9 and trophoblasts and abolished by anti-Tim-3 neutralizing Abs. However, trophoblast cells induce pNK cells to be Th2 predominant with reduced perforin expression through Gal-9/Tim-3 signaling. Because dNK cells are believed to be recruited from peripheral blood, Gal-9/Tim-3 may be an important mediator in the education of pNK cells to a dNK cell-like phenotype. Interestingly, Tim-3 expression in dNK cells from miscarriages declines, and the function of Tim3+ dNK cells is impaired accordingly, which was also observed in abortion-prone mouse models. Our data have demonstrated that the Gal-9/Tim-3 pathway is essential for the maintenance of normal pregnancies via the regulation of dNK function in both physiological and pathological situations.
During successful gestation, Th2 dominance has been demonstrated to be present at the maternal–fetal interface.31 Furthermore, maternal administration of the immunoregulatory cytokine IL-10 or blockade of TNF-α activity was shown to prevent pregnancy loss induced by lipopolysaccharide.19,32 Thus, Th2 bias has been a common hypothesis to explain pregnancy tolerance and has been regarded as an important marker of successful pregnancies. Gal-9/Tim-3 plays a vital role in the regulation of the Th1/Th2 balance by inducing the apoptosis of Th1 cells and subsequently suppressing the Th1-type immune response.33 Tim-3 has been shown to be a negative mediator of not only adaptive immunity but also of innate immunity.34,35 The current study showed Tim-3 expression on more than 60% of dNK cells. Thus, dNK cells can be divided into two different populations based on Tim-3 expression. Interestingly, Tim-3+ dNK cells produce more of the Th2-type cytokine IL-4 and less of the Th1-type cytokine TNF-α and perforin than Tim-3− dNK cells, suggesting that dNK cells may be conducive to a Th2-predominant environment and tolerance instead of attacking trophoblast cells via the expression of Tim-3. Tim-3 could be used as an important marker indicating the tolerant state of dNK cells at the maternal–fetal interface.
It is generally accepted that dNK cells migrate from peripheral blood via interactions with chemokine–chemokine receptors.30 However, there are huge differences between pNK and dNK cell types. Most pNK cells are CD56dimCD16+ with relatively high cytotoxic activation, but dNK cells are mainly CD56brightCD16− with poor cytotoxic activation. The functions of these two NK-cell subsets also differ. pNK cells are mainly responsible for fighting pathogen infections via secretion of perforin and granzymes, but dNK cells participate in fetomaternal immune tolerance, trophoblast invasion and vascular remodeling through secretion of various types of cytokines.15,36 The environment at the maternal-fetal interface is assumed to modify recruited pNK cells, instructing them to be dNK-like cells in phenotype and function.28,37 Here, we found that the coculture of pNK cells with trophoblasts significantly increased Th2-type cytokine production and decreased Th1-type cytokine and perforin expression by pNK cells. This makes pNK cells more adaptable to a dNK cell phenotype. Because Tim-3 expression was also detected on pNK cells and trophoblast cells secreted high levels of Gal-9, we speculated that trophoblast-derived Gal-9 interacting with Tim-3 on pNK cells may mediate this modulatory process. Blocking Tim-3 signaling weakened this regulatory effect, indicating that the Tim-3 and Gal-9 interaction is indeed involved in the conversion of pNK cells to a dNK cell-like phenotype. Interestingly, our unpublished data from another project also showed higher expression of Tim-3 in CD56dim pNK cells (which account for 90% of total pNK cells) than in CD56bright pNK cells. Moreover, trophoblasts preferentially affect the production of Th1/Th2 cytokines by CD56dim but not CD56bright pNK cells, an effect that can be abrogated by Tim-3 neutralizing Abs.
During normal pregnancies, dNK cells serve as a regulatory cell subset to help establish and maintain immune tolerance. However, once pathogens invade, dNK cells transform into effective defenders against infection.38,39,40 Mild inflammation either systemically or locally at the maternal-fetal interface does not lead to fetal demise due to these protective effects. However, uncontrolled inflammation mediated by dNK cells may result in pregnancy failure.41
LPS is a characteristic component of the cell wall of Gram-negative bacteria. LPS is a potent inducer of pro-inflammatory cytokines that trigger abortions via binding to TLR4. Several studies have demonstrated the action of LPS on NK cells. The treatment of isolated human peripheral blood mononuclear cells with LPS results in the proliferation and enhanced cytotoxic activation of NK cells.42,43 The depletion of NK cells can restore pregnancy in IL-10−/− mice treated with LPS, which suggests that dNK cells are the targets of LPS stimulation resulting in fetal demise.21 Furthermore, TLR4 can be detected on NK cells.44,45 All this suggests that LPS may act on NK cells directly. In this context, we demonstrated that LPS stimulation caused a significant increase in the production of TNF-α by dNK cells. This was further augmented by using anti-Tim-3 neutralizing Abs to block Tim-3 signaling. However, the activation of Tim-3 signaling by the addition of rhGal-9 or coculture with trophoblasts suppressed the inflammatory response induced by LPS. Similar effects were observed on perforin production. These results indicate that Gal-9/Tim-3 signaling plays a vital role in controlling LPS-triggered inflammatory reactions and dNK cell cytotoxicity.
A large body of evidence suggests that spontaneous miscarriages are associated with a disturbed Th1/Th2 cytokine profile and defective dNK cell function. Here, we determined that the number of Tim-3-expressing dNK cells was significantly decreased in miscarriages. Moreover, the production of Th2 and Th1 cytokines was dysregulated in Tim-3+ dNK but not in Tim-3− dNK cells both in human miscarriages and abortion-prone mouse models. These data indicate that both the number and the function of Tim-3+ dNK cells are abnormal in miscarriage.
Collectively, our study demonstrates that Tim-3 signaling is critical to dNK function in physiological and pathological situations involving the immune balance between maternal–fetal tolerance and inflammatory responses against pathogens during pregnancy. The dysregulation of Tim-3 signaling in dNK cells may be the main cause of miscarriages during early pregnancy. Further studies using conditional knockout mice will focus on the mechanism of Tim-3+ dNK cell maintenance of normal pregnancies.
Acknowledgments
This work was supported by the National Basic Research Program of China (2015CB943300); the Key Project of Shanghai Basic Research from the Shanghai Municipal Science and Technology Commission (STCSM) (12JC1401600 to DJ Li); the Key Project of Shanghai Municipal Education Commission (MECSM) (14ZZ013 to MR Du); the Extension Project of the Shanghai Health System (2013SY034 to MR Du); the Personnel Training Plan of the Health Care System (2013-3-021 to WH Zhou) and the Nature Science Foundation of the National Nature Science Foundation of China (NSFC) (NSFC31270969 to DJ Li; NSFC81070537, NSFC31171437 and NSFC81370770 to MR Du; NSFC81270753 to WH Zhou; NSFC31300751 to HL Piao; NSFC81370730 to Q Fu).
References
- 1Zenclussen AC, Schumacher A, Zenclussen ML, Wafula P, Volk HD. Immunology of pregnancy: cellular mechanisms allowing fetal survival within the maternal uterus. Expert Rev Mol Med 2007; 9: 1–14. [DOI] [PubMed] [Google Scholar]
- 2Erlebacher A. Immunology of the maternal–fetal interface. Annu Rev Immunol 2013; 31: 387–411. [DOI] [PubMed] [Google Scholar]
- 3Sharma S. Natural killer cells and regulatory T cells in early pregnancy loss. Int J Dev Biol 2014; 58: 219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4Krasnow JS, Tollerud DJ, Naus G, DeLoia JA. Endometrial Th2 cytokine expression throughout the menstrual cycle and early pregnancy. Hum Reprod 1996; 11: 1747–1754. [DOI] [PubMed] [Google Scholar]
- 5Tilburgs T, Scherjon SA, Claas FH. Major histocompatibility complex (MHC)-mediated immune regulation of decidual leukocytes at the fetal–maternal interface. J Reprod Immunol 2010; 85: 58–62. [DOI] [PubMed] [Google Scholar]
- 6Hviid TV. HLA-G in human reproduction: aspects of genetics, function and pregnancy complications. Hum Reprod Update 2006; 12: 209–232. [DOI] [PubMed] [Google Scholar]
- 7Mellor AL, Sivakumar J, Chandler P, Smith K, Molina H, Mao D et al. Prevention of T cell-driven complement activation and inflammation by tryptophan catabolism during pregnancy. Nat Immunol 2001; 2: 64–68. [DOI] [PubMed] [Google Scholar]
- 8Girardi G, Yarilin D, Thurman JM, Holers VM, Salmon JE. Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. J Exp Med 2006; 203; 2165–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9Zhou WH, Dong L, Du MR, Zhu XY, Li DJ. Cyclosporin A improves murine pregnancy outcome in abortion-prone matings: involvement of CD80/86 and CD28/CTLA-4. Reproduction 2008; 135: 385–395. [DOI] [PubMed] [Google Scholar]
- 10Curti A, Trabanelli S, Salvestrini V, Baccarani M, Lemoli RM. The role of indoleamine 2,3-dioxygenase in the induction of immune tolerance: focus on hematology. Blood 2009; 113: 2394–2401. [DOI] [PubMed] [Google Scholar]
- 11Santner-Nanan B, Straubinger K, Hsu P, Parnell G, Tang B, Xu B et al. Fetal–maternal alignment of regulatory T cells correlates with IL-10 and Bcl-2 upregulation in pregnancy. J Immunol 2013; 191: 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12Cerdeira AS, Rajakumar A, Royle CM, Lo L, Husain Z, Thadhani RI et al. Conversion of peripheral blood NK cells to a decidual NK-like phenotype by a cocktail of defined factors. J Immunol 2013; 190; 3939–3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13Keskin DB, Allan DS, Rybalov B, Andzelm MM, Stern JN, Kopcow HD et al. TGFbeta promotes conversion of CD16+ peripheral blood NK cells into CD16− NK cells with similarities to decidual NK cells. Proc Natl Acad Sci USA 2007; 104: 3378–3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Carlino C, Stabile H, Morrone S, Bulla R, Soriani A, Agostinis C et al. Recruitment of circulating NK cells through decidual tissues: a possible mechanism controlling NK cell accumulation in the uterus during early pregnancy. Blood 2008; 111: 3108–3115. [DOI] [PubMed] [Google Scholar]
- 15Vacca P, Moretta L, Moretta A, Mingari MC. Origin, phenotype and function of human natural killer cells in pregnancy. Trends Immunol 2011; 32; 517–523. [DOI] [PubMed] [Google Scholar]
- 16Tabiasco J, Rabot M, Aguerre-Girr M, El Costa H, Berrebi A, Parant O et al. Human decidual NK cells: unique phenotype and functional properties—a review. Placenta 2006; 27 Suppl A: S34–S39. [DOI] [PubMed] [Google Scholar]
- 17Vacca P, Cantoni C, Vitale M, Prato C, Canegallo F, Fenoglio D et al. Crosstalk between decidual NK and CD14+ myelomonocytic cells results in induction of Tregs and immunosuppression. Proc Natl Acad Sci USA 2010; 107: 11918–11923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18Fu B, Li X, Sun R, Tong X, Ling B, Tian Z et al. Natural killer cells promote immune tolerance by regulating inflammatory TH17 cells at the human maternal–fetal interface. Proc Natl Acad Sci USA 2013; 110: E231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19Renaud SJ, Cotechini T, Quirt JS, Macdonald-Goodfellow SK, Othman M, Graham CH. Spontaneous pregnancy loss mediated by abnormal maternal inflammation in rats is linked to deficient uteroplacental perfusion. J Immunol 2011; 186: 1799–1808. [DOI] [PubMed] [Google Scholar]
- 20Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. The structural basis of lipopolysaccharide recognition by the TLR4–MD-2 complex. Nature 2009; 458: 1191–1195. [DOI] [PubMed] [Google Scholar]
- 21Murphy SP, Fast LD, Hanna NN, Sharma S. Uterine NK cells mediate inflammation-induced fetal demise in IL-10-null mice. J Immunol 2005; 175: 4084–4090. [DOI] [PubMed] [Google Scholar]
- 22Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol 2005; 6: 1245–1252. [DOI] [PubMed] [Google Scholar]
- 23Sanchez-Fueyo A, Tian J, Picarella D, Domenig C, Zheng XX, Sabatos CA et al. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat Immunol 2003; 4: 1093–1101. [DOI] [PubMed] [Google Scholar]
- 24Han G, Chen G, Shen B, Li Y. Tim-3: an activation marker and activation limiter of innate immune cells. Front Immunol 2013; 4: 449–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25Ndhlovu LC, Lopez-Verges S, Barbour JD, Jones RB, Jha AR, Long BR et al. Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood 2012; 119: 3734–3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26Jost S, Moreno-Nieves UY, Garcia-Beltran WF, Rands K, Reardon J, Toth I et al. Dysregulated Tim-3 expression on natural killer cells is associated with increased Galectin-9 levels in HIV-1 infection. Retrovirology 2013; 10: 74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27Ju Y, Hou N, Meng J, Wang X, Zhang X, Zhao D et al. T cell immunoglobulin- and mucin-domain-containing molecule-3 (Tim-3) mediates natural killer cell suppression in chronic hepatitis B. J Hepatol 2010; 52: 322–329. [DOI] [PubMed] [Google Scholar]
- 28Du MR, Guo PF, Piao HL, Wang SC, Sun C, Jin LP et al. Embryonic trophoblasts induce decidual regulatory T cell differentiation and maternal–fetal tolerance through thymic stromal lymphopoietin instructing dendritic cells. J Immunol 2014; 192: 1502–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29Wu X, Jin LP, Yuan MM, Zhu Y, Wang MY, Li DJ. Human first-trimester trophoblast cells recruit CD56brightCD16− NK cells into decidua by way of expressing and secreting of CXCL12/stromal cell-derived factor 1. J Immunol 2005; 175: 61–68. [DOI] [PubMed] [Google Scholar]
- 30Guo PF, Du MR, Wu HX, Lin Y, Jin LP, Li DJ. Thymic stromal lymphopoietin from trophoblasts induces dendritic cell-mediated regulatory TH2 bias in the decidua during early gestation in humans. Blood 2010; 116: 2061–2069. [DOI] [PubMed] [Google Scholar]
- 31Matthiesen L, Kalkunte S, Sharma S. Multiple pregnancy failures: an immunological paradigm. Am J Reprod Immunol 2012; 67: 334–340. [DOI] [PubMed] [Google Scholar]
- 32Sabatos CA, Chakravarti S, Cha E, Schubart A, Sanchez-Fueyo A, Zheng XX et al. Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nat Immunol 2003; 4: 1102–1110. [DOI] [PubMed] [Google Scholar]
- 33Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 2002; 415: 536–541. [DOI] [PubMed] [Google Scholar]
- 34Nagahara K, Arikawa T, Oomizu S, Kontani K, Nobumoto A, Tateno H et al. Galectin-9 increases Tim-3+ dendritic cells and CD8+ T cells and enhances antitumor immunity via galectin-9-Tim-3 interactions. J Immunol 2008; 181: 7660–7669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35Hanna J, Wald O, Goldman-Wohl D, Prus D, Markel G, Gazit R et al. CXCL12 expression by invasive trophoblasts induces the specific migration of CD16− human natural killer cells. Blood 2003; 102: 1569–1577. [DOI] [PubMed] [Google Scholar]
- 36Winger EE, Reed JL. The multiple faces of the decidual natural killer cell. Am J ReprodImmunol 2013; 70: 1–9. [DOI] [PubMed] [Google Scholar]
- 37Tao Y, Li YH, Piao HL, Zhou WJ, Zhang D, Fu Q et al. CD56CD25 NK cells are preferentially recruited to the maternal/fetal interface in early human pregnancy. Cell Mol Immunol 2014; in press. [DOI] [PMC free article] [PubMed]
- 38Mselle TF, Howell AL, Ghosh M, Wira CR, Sentman CL. Human uterine natural killer cells but not blood natural killer cells inhibit human immunodeficiency virus type 1 infection by secretion of CXCL12. J Virol 2009; 83: 11188–11195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39Marlin R, Nugeyre MT, Duriez M, Cannou C, Le Breton A, Berkane N et al. Decidual soluble factors participate in the control of HIV-1 infection at the maternofetal interface. Retrovirology 2011; 8: 58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40Siewiera J, El Costa H, Tabiasco J, Berrebi A, Cartron G, Le Bouteiller P et al. Human cytomegalovirus infection elicits new decidual natural killer cell effector functions. PLoS Pathog 2013; 9: e1003257–1003273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41Kwak-Kim J, Bao S, Lee SK, Kim JW, Gilman-Sachs A. Immunological modes of pregnancy loss: inflammation, immune effectors, and stress. Am J Reprod Immunol 2014; 72: 129–140. [DOI] [PubMed] [Google Scholar]
- 42Goodier MR, Londei M. Lipopolysaccharide stimulates the proliferation of human CD56+CD3− NK cells: a regulatory role of monocytes and IL-10. J Immunol 2000; 165: 139–147. [DOI] [PubMed] [Google Scholar]
- 43Miranda D, Puente J, Blanco L, Wolf ME, Mosnaim AD. In vitro effect of bacterial lipopolysaccharide on the cytotoxicity of human natural killer cells. Res Commun Mol Pathol Pharmacol 1998; 100: 3–14. [PubMed] [Google Scholar]
- 44Mian MF, Lauzon NM, Andrews DW, Lichty BD, Ashkar AA. FimH can directly activate human and murine natural killer cells via TLR4. Mol Ther 2010; 18: 1379–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45Duriez M, Quillay H, Madec Y, El Costa H, Cannou C, Marlin R et al. Human decidual macrophages and NK cells differentially express Toll-like receptors and display distinct cytokine profiles upon TLR stimulation. Front Microbiol 2014; 5: 316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]