Abstract
Complement 5a (C5a) has been implicated in the pathogenesis of sepsis by inducing the functional impairment of neutrophils; however, the utility of C5a receptors (C5aRs; C5aR and C5L2) as biomarkers for the management of sepsis is uncertain. This study investigated the dynamic expression of C5aR and C5L2 on neutrophils and their effects on neutrophil function. We found that sepsis patients displayed low expression levels of C5aR and C5L2 on neutrophils compared to healthy and systemic inflammatory response syndrome (SIRS) subjects, and this expression pattern was correlated with disease severity. Additionally, the expression levels of C5aR and C5L2 were associated with the survival of sepsis patients. In vitro, the addition of C5a significantly reduced C5aR and C5L2 expression levels and IL-8 production in neutrophils from sepsis patients. Those findings suggest that the reduced expression of C5aRs was associated with the functional impairment of neutrophils and a poor prognosis for sepsis patients. Overall, these findings may help establish C5aRs expression levels as early markers to predict the severity of sepsis.
Keywords: complement 5a, complement 5a receptor, neutrophil, prognosis, sepsis
Introduction
Sepsis, defined as a systemic inflammatory response to infection, is generally associated with evidence of organ dysfunction, including tissue hypoperfusion and hypoxia, lactic acidosis, oliguria or altered cerebral function.1,2 Despite prompt treatment with antibiotics, the provision of adequate fluid resuscitation and technological support of organ function, approximately 20%–50% of patients with sepsis die in intensive care units (ICU).3 The high mortality and lack of effective therapies can be traced to an incomplete understanding of the inflammatory pathogenesis of sepsis and organ dysfunction. Thus, studies of the cellular pathogenesis of sepsis could provide a better understanding of this complex syndrome and aid in identifying more effective therapies.
The innate immune system is the first line of defense against initial environmental challenges and injury. However, over-activation of the innate immune response and the complement system are generally associated with the excessive inflammatory response that characterizes sepsis. Regardless of the initial site of infection, both animal models and human sepsis show activated neutrophil sequestration in the expansive capillary networks of the internal organs, such as the liver and lung, where they mediate damage and organ dysfunction.4,5 Notably, the complex interactions between the neutrophils and complement system result in a poor outcome in sepsis patients. Complement activation causes harm by generating complement protein split products. Complement 5a (C5a), one of the most potent inflammatory peptides, functions by binding to the high-affinity C5a receptors (C5aRs; C5aR and C5L2). The interaction of C5a with C5aRs leads to pleiotropic effects, including the release of cytokines and chemokines, and the recruitment of inflammatory cells. Generally, C5aR and C5L2 are independent, and the putative ‘default' receptor, C5L2, has an important role in balancing the biological effects of C5a.6 However, the role of C5L2 in inflammatory responses is controversial,7,8 its elucidation will require further investigation. In sepsis, it has been reported that excessive systemic C5a levels were associated with exhaustion of the granulocytic response.9 Additionally, the blockade of C5a or C5aR has been associated with attenuated coagulopathy, preservation of thymic function, rescue of lymphocytes from apoptosis and reduced levels of bacteremia in experimental sepsis.10 This compelling evidence has highlighted the C5a and C5aRs interaction as a pivotal factor in the worsening of sepsis. However, whether and how dynamic changes of C5aR and C5L2 expression occur in neutrophils, and their correlation with disease outcome in sepsis patients remains to be elucidated.
This study focused on the dynamics of C5aRs expression in sepsis patients and the crosstalk between C5a and C5aRs on neutrophil function. These results highlight the role of C5a and C5aRs in predicting the outcome of sepsis.
Materials and methods
Patients and study design
Health controls and patients with systemic inflammatory response syndrome (SIRS) or sepsis were consecutively enrolled from 2012 to 2013. The study protocol was approved by the ethics committee of 302 Hospital and written informed consent was obtained from each subject. We enrolled 34 patients with sepsis, 19 patients with SIRS and 18 healthy subjects as controls in the study. The criteria for SIRS, severe sepsis and septic shock were defined by the criteria of the American College of Chest Physicians and the Society of Critical Care Medicine (ACCP/SCCM).11 In brief, SIRS was diagnosed if two or more of the following criteria were met: a temperature greater than 38 °C or less than 36 °C, a heart rate greater than 90 beats/min in the absence of a pacemaker, a respiratory rate greater than 20 times per minute or a PaCO2 less than 4.3 kPa (32 mmHg), a white blood cell count of greater than 12×109/l or less than 4×109/l, or >10% immature band forms. The SIRS patients enrolled in our study had no signs of infection from the first day after elective surgery, and SIRS patients who developed into sepsis were excluded in the follow-up period. Severe sepsis was defined as sepsis with at least one organ failure. Septic shock was defined as sepsis with a blood pressure less than 90 mmHg, despite fluid resuscitation and requiring vasopressor therapy. Both severe sepsis and septic shock were recorded as sepsis, as described elsewhere.12 The exclusion criteria included age less than 18 years, pregnancy, malignancy, infection with HIV and the receipt of immunosuppressive therapy. Blood samples were obtained within 12 hours upon admitted to the ICU, the following data were recorded for each patient: age, sex, severity of the underlying medical condition, a sepsis-related organ failure assessment (SOFA) score, reasons for admission into the ICU, principal diagnosis, vital signs, respiratory parameters, routine blood tests and microbiological culture results. Survival or death was assessed during a follow-up period of up to 28 days. Blood was obtained at day 0, day 5 and when a patient left the ICU (death or survival).
Flow cytometry
All of the fluorescein-conjugated antibodies were provided by BD Bioscience (San Jose, CA, USA), except for FITC-conjugated anti-human CD66b antibody (Abcam, Cambridge, UK), APC-conjugated anti-human C5aR (CD88) antibody (R&D Systems, Minneapolis, MN, USA) and PE-conjugated anti-C5L2 (Biolegend, San Diego CA, USA). For C5aR and C5L2 staining, fresh heparinized peripheral blood (100 µl) was incubated with C5aR and C5L2 antibodies, corresponding isotype controls were added according to the manufacturer's instructions, and CD66b positivity defined granulocytes in the whole blood. For detection the C5aR and C5L2 expression on neutrophils in response to C5a in vitro, fresh heparinized peripheral blood (100 µl) was incubated with C5a (10–100 nM; Sigma-Aldrich, St Louis, MO, USA) for 15–30 min. Antibodies or isotype control antibodies were added to the 100 µl whole blood for 15 min at room temperature. The blood was lysed and analyzed by a Calibur flow cytometer. For intracellular IL-8 staining, fresh heparinized peripheral blood (1 ml) was incubated with C5a (100 nM) for 4.5 h, golgistop (BD PharMingen, San Diego, CA, USA) was added at the same time of incubation. The blood was then lysed, permeabilized and stained with APC-conjugated anti-human IL-8 antibody (eBioscience, San Diego, CA, USA), or an isotype control. The cells were analyzed using a Calibur flow cytometer and CELLQuest software.
Statistical analyses
Data analysis was performed with SPSS version 13.0 software (SPAA Inc., Chicago, IL, USA) and data were expressed as means±standard deviation. Statistically significant differences between two groups were determined by the Mann–Whitney non-parametric U test. Comparisons of data from the same individual were performed using the Wilcoxon matched-pairs t-test. Correlation analyses were determined by the Spearman rank correlation test. Values of P<0.05 were considered as statistically significant differences. Receiver-operating characteristic curve analysis provides a standardized appreciation of the accuracy of a marker for predicting an event at the ICU during follow-up. This statistic allows for the comparison of the accuracy of different prognostic scores within a population. A receiver-operating characteristic curve represents a plot of sensitivity against ‘1–specificity'.
Results
Population characteristics
The clinical characteristics of the subjects are shown in Table 1. Eighteen healthy subjects, nineteen patients with SIRS and thirty-four patients with sepsis were enrolled in our study. The gender and age of both the SIRS and sepsis patients were comparable to the healthy control (HC) subjects. Notably, patients with sepsis had higher SOFA scores and more infections than the SIRS patients, including lung (20 patients, 58.8%), abdomen (11 patients, 32.3%) and urinary tract (3 patients, 8.8%) infections. Pathogen culture included Gram-negative bacteria (18 patients, 52.9%), Gram-positive bacteria (7 patients, 20.5%), fungi (2 patients, 5.9%) and negative culture (7 patients, 20.7%). The 28-day mortality rate of the sepsis patients was 52.9%.
Table 1. Clinical characteristics of enrolled subjects.
| HC | SIRS | Sepsis | P | |
|---|---|---|---|---|
| Numbers | 18 | 19 | 34 | |
| Age (years) | 35±10 | 53±17 | 49±14 | 0.12 |
| Sex (male/female) | 10/8 | 11/8 | 18/16 | 0.93 |
| SOFA score | — | 3.5±1.9 | 10.7±4.1 | <0.01 |
| Site of infection | ||||
| Lung | — | — | 20 (58.8%) | — |
| Abdomen | — | — | 11 (32.3%) | — |
| Urinary tract | — | — | 3 (8.9%) | — |
| Pathogen culture | — | |||
| Gram− bacterial | — | — | 18 (52.9%) | — |
| Gram+ bacterial | — | — | 7 (20.5%) | — |
| Fungi | — | — | 2 (5.9%) | — |
| Negative culture | — | — | 7 (20.7%) | — |
| WBC (×109/l) | — | 14.4±3.2 | 15.9±5.9 | 0.57 |
| Mechanical ventilation (n) | 3 | 12 | 0.13 | |
| Renal replacement therapy (n) | — | 1 | 8 | 0.17 |
| Mortality (survival/non-survival) | — | 0% (0/19) | 52.9% (16/18) | <0.01 |
Abbreviations: HC, healthy control; SIRS, systemic inflammatory response syndrome; SOFA, sepsis-related organ failure assessment; WBC, white blood cell.
Data are shown as number (%) or mean±s.d.
Decreased C5aR and C5L2 expression levels on neutrophils are associated with the severity of sepsis
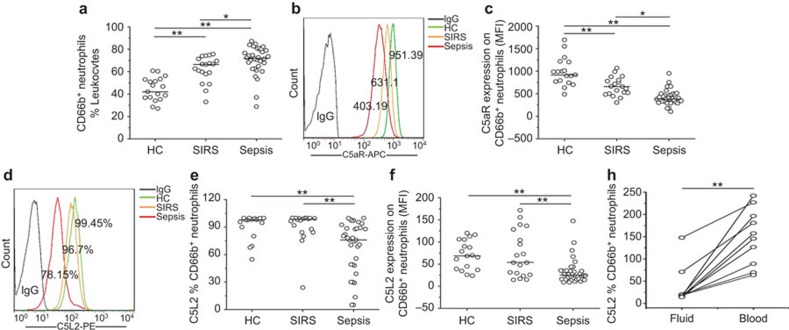
We first quantitatively measured the C5aR and C5L2 expression levels on blood neutrophils in the subjects. The percentage of neutrophils at admission was significantly higher in SIRS and sepsis patients compared to HC subjects. Additionally, sepsis patients displayed higher percentages of neutrophils than SIRS patients (Figure 1a). Further analysis indicated that the mean fluorescence intensity (MFI) of C5aR expression on neutrophils was higher in SIRS patients compared to that in sepsis patients (Figure 1b and c). No difference in the percentage of C5aR expression was found between the HC controls and sepsis patients (data not shown). Meanwhile, the percentage of C5L2-expressing neutrophils (Figure 1d and e) and the MFI of C5L2 (Figure 1f) were both significantly reduced in sepsis patients. We also found that C5L2 expression on neutrophils from the fluid of pleural effusion and ascites was lower than on peripheral neutrophils (n=10, Figure 1g).
Figure 1.
The expression of C5aR and C5L2 on neutrophils in sepsis patients. (a) The percentages of neutrophils, defined as CD66b-positive leukocytes in HC, SIRS and sepsis patients. (b) Representative dotplots show the expression of C5aR on CD66b-positive cells in patients and health controls. (c) The pooled data of the MFI of C5aR expression on CD66b-positive cells in HC, SIRS and sepsis patients. (d) Representative dotplots show the expression of C5L2 on CD66b-positive cells in patients and health controls. (e) The pooled data of the percentage of C5L2 expression in HC, SIRS and sepsis patients. (f) The pooled data of the MFI of C5L2 expression in HC, SIRS and sepsis patients. (g) Differential expression of C5L2 on CD66b-positive cells in blood and fluid from sepsis patients. Each dot represents an individual. *P<0.05; **P<0.01. C5aR, complement 5a receptor; HC, healthy control; MFI, mean fluorescence intensity; SIRS, systemic inflammatory response syndrome.
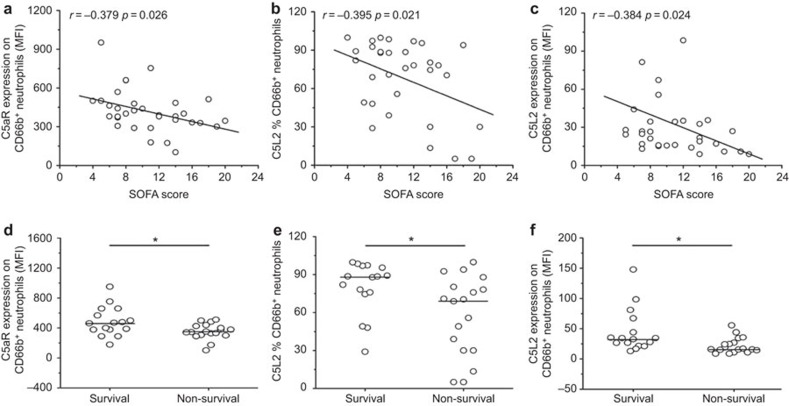
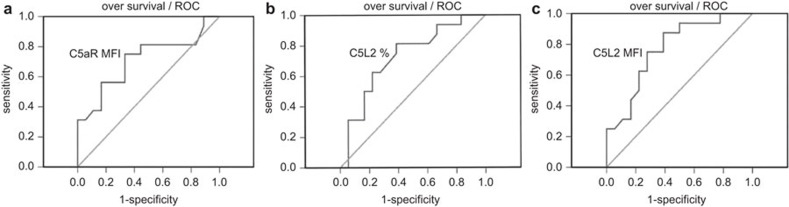
C5aR and C5L2 expression on neutrophils were significantly negatively correlated with SOFA scores at the day of sepsis diagnosis (Figure 2a–c). Furthermore, C5aR and C5L2 expression levels on neutrophils at admission significantly stratified sepsis patients into survival and non-survival groups (Figure 2d and e). In receiver-operating characteristic curve analysis, we found the expression of C5aR and C5L2 can be served as biomarkers for predicting survival of septic patients. The AUC of C5aR MFI was 0.710 (95% confidence intervals: 0.528–0.892), the specificity and sensitivity were 75.1% and 67.7%, respectively, when using 379.8 as a cutoff point (Figure 3a). The AUC of C5L2 percentage was 0.724 (95% confidence intervals: 0.55–0.898), the specificity and sensitivity were 81.3% and 61.1% respectively, when using 72.7 as a cutoff point (Figure 3b). The AUC of C5L2 MFI was 0.771 (95% confidence intervals: 0.612–0.93), the specificity and sensitivity were 87.5% and 61.1% respectively, when using 379.8 as a cutoff point (Figure 3c).
Figure 2.
Reductions in C5aR and C5L2 expression are positively associated with the severity of sepsis. (a–c) The C5aR and C5L2 levels were correlated with the SOFA score of septic patients. (d and e) The C5aR and C5L2 levels were associated with mortality in septic patients. Each circle represents an individual. *P<0.05; **P<0.01. C5aR, complement 5a receptor; SOFA, sepsis-related organ failure assessment.
Figure 3.
C5aR levels are prognostic markers for survival in sepsis patients. (a) Receiver-operating characteristic curve analysis of C5aR MFI to predict overall survival in sepsis patients. (b) Receiver-operating characteristic curve analysis of C5L2 percentage to predict overall survival in sepsis patients. (c) Receiver-operating characteristic curve analysis of C5L2 MFI to predict overall survival in sepsis patients. C5aR, complement 5a receptor; MFI, mean fluorescence intensity.
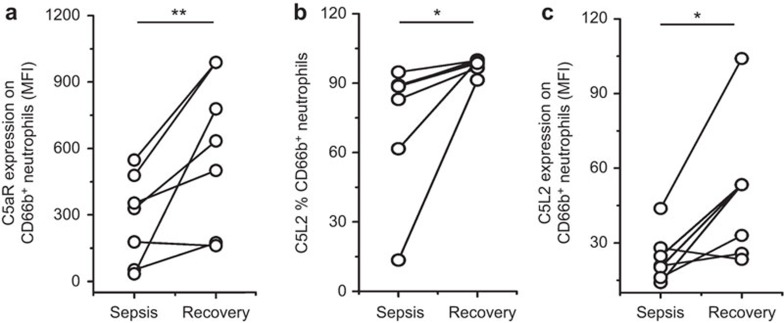
We also longitudinally measured C5aR and C5L2 expression on neutrophils at admission and on the recovery day when sepsis had improved to SIRS or better. These sepsis patients showed significant increases in C5aR and C5L2 expression levels as they recovered from the disease (Figure 4). These data indicated that the C5aR and C5L2 expression levels on neutrophils were significantly decreased in sepsis; the reduction was further associated with the severity of sepsis.
Figure 4.
The dynamics of C5aR and C5L2 expression on neutrophils in patients at various phases of disease. (a) The increased C5aR expression on CD66b-positive cells was correlated with improvement in sepsis patients. (b and c) The increased C5L2 expression on CD66b-positive cells was correlated with improvement in sepsis patients. Each circle represents an individual. *P<0.05; **P<0.01. C5aR, complement 5a receptor.
Exposure of neutrophils to C5a reduced C5aR and C5L2 expression levels in vitro
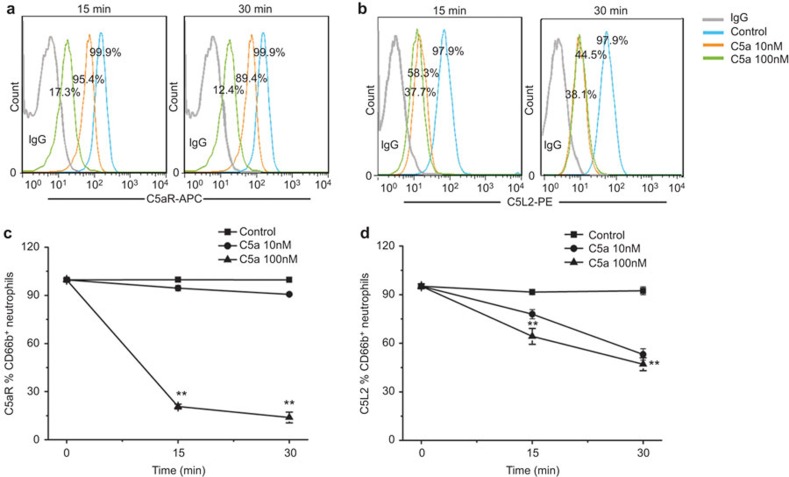
To further explore the mechanism whereby the expression levels of C5aR and C5L2 on neutrophils were reduced, we evaluated the effect of C5a on C5aR and C5L2 expression on neutrophils. Peripheral neutrophils were exposed to 10 or 100 nM C5a, and then cells were evaluated for their expression of C5aRs. As shown in Figure 5, there was a reduction in C5aR and C5L2 expression levels on neutrophils when exposed to C5a compared to the controls. Notably, 100 nM C5a stimulation induced a more significant reduction in C5aR and C5L2 expression levels than 10 nM C5a.
Figure 5.
Changes in C5aR and C5L2 expression on neutrophils in response to C5a in vitro. (a) Representative dotplots show the expression of C5aR on CD66b-positive cells. (b) Representative dotplots show the expression of C5L2 on CD66b-positive cells. (c) The pooled data of the C5aR expression on CD66b-positive cells when exposed to C5a in vitro. (d) The pooled data of the C5L2 expression on CD66b-positive cells when exposed to C5a in vitro. Data indicate the means and standard deviations. **P<0.01. C5aR, complement 5a receptor.
C5a induced less IL-8 production by neutrophils in sepsis patients
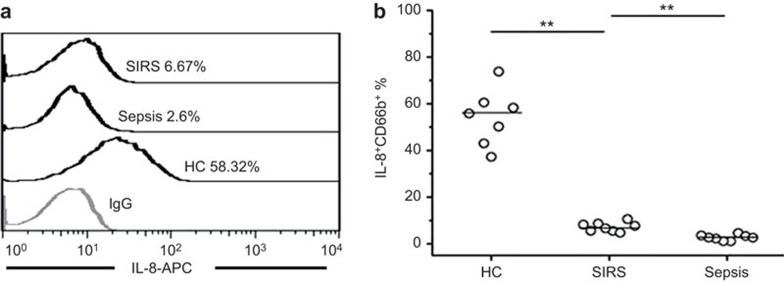
Although the total levels of pro-inflammatory mediators, including IL-8, increased during sepsis (data not shown), the cytokine secretion ability of neutrophils from different patient groups was not known. As shown in Figure 6a, neutrophils displayed differential capacities to produce IL-8 upon stimulation by C5a. Further analysis indicated that neutrophils from sepsis patients exhibited a relatively poor ability to secrete IL-8 in response to C5a in vitro, when compared to healthy controls or SIRS patients. This finding indicated that C5a-mediated IL-8 production by neutrophils was impaired in sepsis patients.
Figure 6.
Neutrophils from septic patients exhibit a reduced ability to produce IL-8 when exposed to C5a. (a) Representative dot plots show the co-expression of CD66b and IL-8 in neutrophils in vitro. (b) The pooled data of IL-8 expression on CD66b-positive cells in HC, SIRS and sepsis patients when exposed to C5a. **P<0.01. C5a, complement 5a.
Discussion
Neutrophil dysfunction has been previously reported in patients with severe sepsis.13 This finding indicated the global impairment of immune functions and that it was correlated with poor outcomes in these patients. However, the mechanisms underlying this phenomenon in sepsis and whether it is only restricted to sepsis or is related to disease severity had remained unknown. Here, our findings indicated that C5a might mediate neutrophil dysfunction through interactions with C5aRs, which was also found to be associated with a poor outcome in severe sepsis.
Previously, changes in C5aR expression levels were associated with a poor prognosis during sepsis,14,15 which indicated the C5aR expression on neutrophils and the presence of circulating form of C5aR in serum were important markers for sepsis patients. However, the coordinated expression of C5aR and C5L2 and their association with disease progression were not detected in patients with sepsis. Our data provided five pieces of evidence to support an association between the reduction of C5aRs levels and sepsis progression. First, the expression levels of C5aR and C5L2 were obviously reduced in sepsis patients when compared to SIRS patients and HC subjects; notably, the reduced expression of C5L2 in the fluid of pleural effusions and ascites from sepsis patients was more obvious than in peripheral blood, which might indicate that in the locus of infection the change of C5L2 was more significant. Second, there was a significant negative correlation between the expression of C5aRs on neutrophils and SOFA score. Third, high levels of C5aR and C5L2 expression on neutrophils were associated with the survival of sepsis patients, whereas low levels of C5aR and C5L2 on neutrophils were found to predict high mortality in sepsis patients. Fourth, the expression levels of C5aR and C5L2 were significantly increased as sepsis improved in a long-term follow-up. Fifth, the MFI of C5aR, the percentage of C5L2 and the MFI of C5L2 showed similar sensitivities and specificities to predict survival of sepsis. These data suggest that the expression of C5aRs on neutrophils may serve as prognostic markers for predicting the outcome of sepsis.
The mechanisms underlying C5aRs downregulation remain unclear. Previous in vitro experiments suggested that the binding of C5a could lead to the reduced surface expression of C5aR, which rendering cells resistant to subsequent challenges with C5a.16 Here, we showed that C5a rapidly induced the internalization of C5aR and C5L2 within human neutrophils. Interestingly, the plasma concentration of C5a was not significantly changed in patients with sepsis in our study (data not shown), which most likely reflects increased C5a consumption during the inflammatory response.
C5a functions by binding to its receptors, C5aR and C5L2. The reduction in C5aRs expression levels might correlate with the loss of innate immune function in neutrophils. However, in different animal models, the functions of C5aR and C5L2 are controversial.17,18 Thus, changes in the expression levels of C5aRs need to be linked with disease status. Under physiological conditions, relatively low levels of C5a may accelerate the priming of neutrophils and monocytes, and also activate endothelial cells, supporting efficient natural immune responses in the case of infections.19 Excessive C5a production, however, may induce neutrophil dysfunction, including abnormal chemotaxis, reactive oxygen species production and phagocytosis.20,21 Our study supported a model in which C5a induces impaired IL-8 production by neutrophils. This phenomenon might be associated with ‘sepsis-like' immune paralysis.22 In sepsis, the downregulation of HLA-DR expression and the reduction of TNF-α levels have been identified,23and dysfunction of neutrophils was also reported to be associated with acquisition of nosocomial infection,24 which further indicated the immune status of sepsis patients. Indeed, future studies should aim to elucidate the mechanism underlying C5a-induced immune paralysis.
Conclusions
In summary, our results show that sepsis is associated with the dysfunction of neutrophils, which is mediated by C5a. Persistent reductions of C5aR and C5L2 levels on neutrophils were associated with a poor outcome in sepsis. Interventions to alter the levels either C5a or C5aRs might improve survival during sepsis.
Acknowledgments
We appreciate all septic individuals, SIRS individuals and healthy participants in this study. The study was approved and has been reviewed by the ethics committee of our 302 Hospital. This work was supported by grants from the National Science Fund for Outstanding Young Scholars (No. 81222024), the National Science Fund for Distinguished Young Scholars (No. 81400626) and the Beijing Natural Science Foundation (No. 714428).
The authors declare no interests of competing.
References
- 1Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D et al. SCCM/ESICM/ACCP/ATS/SI:2001SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003; 31: 1250–1256. [DOI] [PubMed] [Google Scholar]
- 2Balk RA. Severe sepsis and septic shock. Crit Care Clin 2000; 16: 179–192. [DOI] [PubMed] [Google Scholar]
- 3Angus DC, Wax RS. Epidemiology of sepsis: an update. Crit Care Med 2001; 29: S109–S116. [DOI] [PubMed] [Google Scholar]
- 4Andonegui G, Zhou H, Bullard D, Kelly MM, Mullaly SC, McDonald B et al. Mice that exclusively express TLR4 on endothelial cells can efficiently clear a lethal systemic Gram-negative bacterial infection. J Clin Invest 2009; 119: 1921–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5Brown KA, Treacher DF. Neutrophils as potential therapeutic targets in sepsis. Discov Med 2006; 6: 118–122. [PubMed] [Google Scholar]
- 6Okinaga S, Slattery D, Humbles A, Zsengeller Z, Morteau O, Kinrade MB et al. C5L2, a nonsignaling C5A binding protein. Biochemistry 2003; 42: 9406–9415. [DOI] [PubMed] [Google Scholar]
- 7Li R, Coulthard LG, Wu MC, Taylor SM, Woodruff TM. C5L2: a controversial receptor of complement anaphylatoxin, C5a. FASEB J 2013; 27: 855–864. [DOI] [PubMed] [Google Scholar]
- 8Huber-Lang M, Sarma JV, Rittirsch D, Schreiber H, Weiss M, Flierl M et al. Changes in the novel orphan, C5a receptor (C5L2), during experimental sepsis and sepsis in humans. J Immunol 2005; 1174: 1104–1110. [DOI] [PubMed] [Google Scholar]
- 9Gressner OA, Koch A, Sanson E, Trautwein C, Tacke F. High C5a levels are associated with increased mortality in sepsis patients—no enhancing effect by actin-free Gc-globulin. Clin Biochem 2008; 41:974–980. [DOI] [PubMed] [Google Scholar]
- 10Guo RF, Huber-Lang M, Wang X, Sarma V, Padgaonkar VA, Craig RA et al. Protective effects of anti-C5a in sepsis-induced thymocyte apoptosis. J Clin Invest 2000; 106: 1271–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11Bone RC, Balk RA, Cerra FB. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis: the ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992; 101: 1644–1655. [DOI] [PubMed] [Google Scholar]
- 12Poehlmann H, Schefold JC, Zuckermann-Becker H, Volk HD, Meisel C. Phenotype changes and impaired function of dendritic cell subsets in patients with sepsis: a prospective observational analysis. Crit Care 2009; 13: R119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13Solomkin JS, Jenkins MK, Nelson RD, Chenoweth D, Simmons RL. Neutrophil dysfunction in sepsis. II. Evidence for the role of complement activation products in cellular deactivation. Surgery 1981; 90: 319–327. [PubMed] [Google Scholar]
- 14Guo RF, Riedemann NC, Bernacki KD, Sarma VJ, Laudes IJ, Reuben JS et al. Neutrophil C5a receptor and the outcome in a rat model of sepsis. FASEB J 2003; 17: 1889–1891. [DOI] [PubMed] [Google Scholar]
- 15Unnewehr H, Rittirsch D, Sarma JV, Zetoune F, Flierl MA, Perl M et al. Changes and regulation of the C5a receptor on neutrophils during septic shock in humans. J Immunol 2013; 1908: 4215–4225. [DOI] [PubMed] [Google Scholar]
- 16Huber-Lang MS, Younkin EM, Sarma JV, McGuire SR, Lu KT, Guo RF et al. Complement-induced impairment of innate immunity during sepsis. J Immunol 2002; 169: 3223–3231. [DOI] [PubMed] [Google Scholar]
- 17Kim H, Erdman LK, Lu Z, Serghides L, Zhong K, Dhabangi A et al. Functional roles for C5a and C5aR, but not C5L2, in the pathogenesis of human and experimental cerebral malaria. Infect Immun 2014; 82: 371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18Wang R, Lu B, Gerard C, Gerard NP. Disruption of the complement anaphylatoxin receptor C5L2 exacerbates inflammation in allergic contact dermatitis. J Immunol 2013; 191: 4001–4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19Guo RF, Riedemann NC, Laudes IJ, Ward PA. Altered neutrophil trafficking during sepsis. J Immunol 2002; 169: 307–314. [DOI] [PubMed] [Google Scholar]
- 20Riedemann NC, Guo RF, Ward PA. A key role of C5a/C5aR activation for the development of sepsis. J Leukoc Biol 2003; 74: 966–970. [DOI] [PubMed] [Google Scholar]
- 21Conway MA, Kefala K, Wilkinson TS, Dhaliwal K, Farreii L, Walsh T et al. C5a mediates peripheral blood neutrophil dysfunction in critically ill patients. Am J Respir Crit Care Med 2009; 180: 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22Wasmuth HE, Kunz D, Yagmur E, Timmer-Stranghöner A, Vidacek D, Siewert E et al. Patients with acute on chronic liver failure display “sepsis-like” immune paralysis. J Hepatol 2005; 42: 195–201. [DOI] [PubMed] [Google Scholar]
- 23Döcke WD, Randow F, Syrbe U, Krausch D, Asadullah K, Reinke P et al. Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nat Med 1997; 3: 678–681. [DOI] [PubMed] [Google Scholar]
- 24Conway MA, Anderson N, Brittan M, Wilkinson TS, McAuley DF, Antonelli J et al. Combined dysfunctions of immune cells predict nosocomial infection in critically ill patients. Br J Anaesth 2013; 111:778–787. [DOI] [PubMed] [Google Scholar]