Abstract
All currently efficacious antipsychotic drugs have as part of their mechanism the ability to attenuate some or all of their signaling through the dopamine D2 receptor. More recently, the dopamine D1 receptor has been hypothesized to be a promising target for the treatment of negative and/or cognitive aspects of schizophrenia that are not improved by current antipsychotics. Although cAMP has been presumed to be the primary messenger for signaling through the dopamine receptors, the last decade has unveiled a complexity that has provided exciting avenues for the future discovery of antipsychotic drugs (APDs). We review the signaling mechanisms of currently approved APDs at dopamine D2 receptors, and note that aripiprazole is a compound that is clearly differentiated from other approved drugs. Although aripiprazole has been postulated to cause dopamine stabilization due to its partial D2 agonist properties, a body of literature suggests that an alternate mechanism, functional selectivity, is of primary importance. Finally, we review the signaling at dopamine D1 receptors, and the idea that drugs that activate D1 receptors may have use as APDs for improving negative and cognitive symptoms. We address the current state of drug discovery in the D1 area, and its relationship to novel signaling mechanisms. Our conclusion is that although the first APD targeting dopamine receptors was discovered more than a half-century ago, recent research advances offer the possibility that novel and/or improved drugs will emerge in the next decade.
Keywords: Dopamine, schizophrenia, antipsychotic drug, functional selectivity, drug discovery
1. Introduction
Dopamine plays a role in numerous biological processes, making it unsurprising that many drugs target dopamine receptors. Although dopamine neurons are relatively scarce in the brain, their importance is highlighted by the number of normal brain processes (e.g., memory, motor behavior, and reward) and diseases that are modulated by dopamine receptor signaling. For example, the loss of dopamine neurons in the substantia nigra results in Parkinson’s disease, whereas hyperactive dopaminergic signaling is believed to be a major factor in the positive symptoms of schizophrenia. Since the serendipitous discovery of the antipsychotic drug (APD) chlorpromazine over 50 years ago, many advances have been made in the understanding of dopamine structure and signaling. After 50 years of schizophrenia drug development, however, the efficacy of APDs has not significantly improved, although therapeutic indices are somewhat better because of a reduction in many side effects. Although positive symptoms of schizophrenia can be treated effectively by current drugs, negative symptoms and cognitive defects are much less responsive to current drugs. Thus, there is clearly a need for novel therapies. Drugs that activate D1-mediated signaling pathways may alleviate the negative symptoms and cognitive deficits and decrease some of the side effects of current APDs. Because the mechanisms of many APDs are not completely understood, understanding how different dopamine signaling pathways play a role in the actions of APDs will be one critical direction for the design of novel treatments. This chapter will provide an overview of the current state of research in these areas.
1.1. Background on Dopamine Receptors
Dopamine receptors are members of the G protein-coupled receptor (GPCR) superfamily. There are five dopamine receptor genes that produce six distinct dopamine receptors in humans. Dopamine receptors were initially divided into two pharmacological families based on their ligand recognition properties and their effects on cAMP production: the D1-like receptors and the D2-like receptors (Garau et al., 1978; Kebabian and Calne, 1979). The D1-like receptors consist of the D1 and D5 dopamine subtypes, whereas the D2-like receptors include two major splice variants of the D2 receptor [D2L (long), and D2S (short)] plus the D3 and D4 receptors. There are also splice variants for the D3 (Giros et al., 1989; Sokoloff et al., 1990) and D4 (O’Malley et al., 1992; van Tol et al., 1991) receptors, but the effects of these variants on behavioral phenotypes have been controversial (Jonsson et al., 2001; Jonsson et al., 2002; Kotler et al., 2000; Mill et al., 2001; Schmidt et al., 2001; Swanson et al., 1998). No splice variants exist for the D1-like receptors because their genes are intron-less. The D1-like receptors activate Gαs/olf and stimulate cAMP production, whereas the D2-like receptors activate Gαi/o to inhibit adenylate cyclase activity and cAMP production. There are also differences in the localization of the dopamine receptors. The D1-like receptors are predominately found postsynaptically (Levey et al., 1993), whereas the D2-like receptors are found postsynaptically on dopaminergic target neurons (Levey et al., 1993; Sesack et al., 1994) and as presynaptic and autoreceptors on dopamine neurons (L’hirondel et al., 1998; Mercuri et al., 1997). Although several subtypes of dopamine receptors may co-localize on some cells (Surmeier et al., 1996), the receptors are often largely segregated (Gerfen et al., 1990; Le Moine and Bloch, 1995).
There are three major brain dopamine pathways that are involved in brain actions: the nigrostriatal (from cells in the A9 region), the mesolimbic-cortical (from cells in the A10 or ventral tegmentum), and the tuberoinfundibular (hypothalamic) system (Ungerstedt, 1971). In addition, peripheral dopamine neurons are involved in important physiological functions, such as renal and cardiovascular functions and immune regulation. Given that dopamine is involved in both central nervous system (CNS) and peripheral functions, it is not surprising that alterations in dopaminergic signaling are related to numerous diseases and disorders, such as Parkinson’s disease, schizophrenia, depression, dyskinesias, and hypertension.
1.2. Dopamine Receptors and Antipsychotic Drug Action
Dopaminergic signaling is critical for a variety of functions, and abnormal dopaminergic signaling is involved in a variety of CNS disorders. Early studies demonstrated that dopamine and its receptors play a role in the therapy or etiology of psychiatric and neurological disorders (Carlsson, 1964; Carlsson and Lindqvist, 1963; Carlsson et al., 1957; Creese et al., 1976; Ehringer and Hornykiewicz, 1960). For motor function, the localization of dopamine receptors in the two principal striatal outflow pathways was shown to affect their role in neurological diseases (Albin et al., 1989; DeLong, 1990; Gerfen, 2000a; Gerfen, 2000b; Graybiel, 1990; Robertson et al., 1992; Starr, 1995). D1 receptors are expressed on striatal GABAergic medium spiny neurons (MSNs) that project to the medial globus pallidus and the substantia nigra pars reticulata (i.e., the direct nigrostriatal pathway), whereas D2 receptors are expressed on MSNs that project to the lateral globus pallidus (i.e., the indirect pathway).
In schizophrenia, antidopaminergic drugs have been used clinically for more than 50 years, yet many mechanisms of dopaminergic drug actions remain unknown. The classical dopamine hypothesis postulated that the positive symptoms of schizophrenia were a response to hyperactive dopaminergic signaling, a hypothesis that was supported by the ability of APDs to block D2 receptors and the psychotic effects induced by dopaminergic drugs (Carlsson and Lindqvist, 1963; Creese et al., 1976; Seeman et al., 1975; Seeman and Lee, 1975). Based on this early data, much of the effort in APD development has been focused on antagonizing D2 receptors. Although D1 and D2 receptors are generally not found on the same neurons, the activation of D1 receptors can enhance the stereotyped and motor D2–mediated behavioral responses (LaHoste et al., 2000; Mailman et al., 1984; Martin-Iverson and Yamada, 1992; White et al., 1988). In addition, both D1 and D2 receptors are involved in learning and memory (Goldman-Rakic et al., 2004). Dopamine D1 and D2 receptors signal through Gα subunits with opposing effects; however, they often signal through the same βγ subunits, suggesting that βγ signaling may mediate some of the interactions between these receptors. The interactions between D1 and D2 receptors may play an important role in schizophrenia and the design of novel APDs to treat all of the symptoms of schizophrenia. Selective D1 receptor antagonists have not shown efficacy in treating schizophrenia, and may actually exacerbate extrapyramidal side effects and tardive dyskinesia (Den Boer et al., 1995; Karle et al., 1995; Karlsson et al., 1995). Conversely, all currently approved antipsychotics have actions at dopamine D2 receptors, suggesting this is an important, if not obligatory, aspect of efficacy against positive symptoms (Miyamoto et al., 2000). As will be discussed below, the combination of a D1 agonist and D2 antagonist/functionally selective drug may be one route to next generation therapy.
1.3. Dopamine Receptors and Functional Selectivity
The first evidence for a biochemical mechanism of dopamine systems was the observation that dopamine could dose-dependently stimulate the synthesis of the second messenger cAMP (Kebabian et al., 1972), and this activation of cAMP was antagonized by antipsychotic drugs (Clement-Cormier et al., 1974) in proportion to the clinical potency of the drugs (Clement-Cormier et al., 1974; Iversen, 1975). For many years, the primary focus of drug discovery efforts was on the regional and cellular localization of different dopamine receptors and how subtype-selective drugs might be useful in schizophrenia and other CNS disorders. The common assumption was that drug effects on dopamine receptor-mediated cAMP signaling would be predictive of clinical action (see reviews, e.g., Beaulieu and Gainetdinov, 2011; Huang et al., 2001; Mailman, 2007). Thus, the development of novel antipsychotic drugs focused on several areas: 1) non-dopamine receptor targets; 2) dopamine isoform-selective antagonists; 3) multi-targeted ligands (“magic shotguns”); and/or 4) selective presynaptic dopamine agonists (Roth et al., 2004). A drug that worked via any of these mechanisms usually would be assessed preclinically by its biochemical actions on receptor-mediated cAMP synthesis. The accepted concept in all such discovery research was that a drug would have a property called ‘intrinsic efficacy” that could be assessed by its action at any signaling pathway of the targeted receptor. Thus, classical pharmacology posited that a ligand either acts as an agonist, partial agonist, antagonist, or inverse agonist (i.e., its intrinsic efficacy); and a drug would always cause the same type of functional change, although this could be quantitatively affected by factors such as receptor reserve and strength of signaling.
Although dopamine receptors were originally classified based on their effects on cAMP synthesis, it became clear that signaling through modulation of adenylate cyclases is only a fraction of how dopamine receptors modulate cellular function. Many recent reviews have covered the signaling mechanisms of dopamine receptors (Beaulieu and Gainetdinov, 2011; Mailman and Huang, 2007; Neve et al., 2004). Dopamine receptor signaling is now widely appreciated to be more complex than simply changes in cAMP levels. Recent studies have shown that dopamine receptors may even signal through G protein-independent pathways such as those involving β-arrestin (Beaulieu et al., 2007a). With this awareness of the complexity of mechanisms of signaling, there is also mounting evidence that some drugs can cause markedly different responses at the signaling cascades mediated by a single receptor isoform. This remarkable departure from classical pharmacological dogma has been referred to by many names, but the most commonly used term is “functional selectivity” (Urban et al., 2007a).
The term “functional selectivity” was first used to describe findings with the antipsychotic drug aripiprazole (Lawler et al., 1994) and with experimental dopamine agonists (Mailman et al., 1998). Essentially, functional selectivity means that a drug may have different actions at different signaling pathways mediated by the same receptor (e.g., a ligand could be an agonist at one pathway and an antagonist at another), and these differences are not explicable by classical concepts, such as receptor reserve or strength of signaling. A consequence of this idea is that assigning a specific type of intrinsic efficacy (vide supra, a constant inherent property of a drug) to a drug may not be valid. Thus, drugs that are functionally selective will have different intrinsic activity (agonist, partial agonist, antagonist, or inverse agonist) in two or more different assay systems after accounting for factors such as receptor reserve. This places particular importance on the milieu in which a drug is characterized (e.g., the cell or tissue being studied, the signaling pathway being assessed, and/or the degree of receptor expression). We recently provided an overview of the molecular mechanisms that may influence the functional selectivity of a variety of GPCRs (Urban et al., 2007a); many of the mechanisms are relevant to dopamine receptors.
Upon reflection, it should not be surprising that functional selectivity exists. GPCRs can couple with multiple G proteins and signal through G protein-independent mechanisms, such as β-arrestins. Moreover, the presence of various kinases, phosphatases, and other signaling molecules [e.g., G protein-coupled receptor kinases (GRKs) and regulators of G protein signaling (RGS) proteins] are dependent on cellular localization and can influence signaling. In addition, dopamine receptor signaling can vary in different brain regions depending on the expression patterns of GRKs, β-arrestins, and other signaling molecules. For example, RGS proteins can affect ligand actions and signaling by negatively modulating G protein signaling, and RGS proteins may be particularly important for D2 signaling because they enhance GTP hydrolysis through Gαi/o but not Gαs. As if the signaling mechanisms are not already complicated enough, dopamine receptors and other GPCRs can form homodimers as well as heterodimers with receptors from other superfamilies, and these receptor complexes may result in significant pharmacological changes. For functional considerations, we must consider the interaction of drugs with receptor-based signaling complexes, sometimes called the “signalsome” (Mercurio et al., 1997; Wang and Malbon, 2011), rather than the receptor alone (Urban et al., 2007a) because different ligands induce or stabilize different receptor conformational states that influence which signaling pathways are activated by the receptor. Understanding the signaling molecules that are important for specific functions will be critical for the development of functionally selective drugs.
Rather than simply targeting specific receptor isoforms, taking advantage of the functionally selective nature of novel ligands could, in theory, allow for greater control over the effects of drugs. For example, drugs could be designed that selectively activate the signaling pathways that are responsible for the therapeutic effects of a drug without stimulating pathways that are involved in side effects. With the exciting possibilities of functionally selective ligands, however, comes a greater importance for understanding receptor structure, dynamics, and signal transduction. Using APDs as an example, understanding all of the signaling mechanisms of clinically used APDs and the signaling pathways responsible for drug effects and disease manifestation will provide invaluable knowledge for the development of novel therapeutics.
1.4. Allosteric Targeting of Dopamine Receptors
Recently, the idea of allosteric ligands for GPCRs has been an important arena of investigation because of the potential of allosteric ligands to have actions (Conn et al., 2009; Jakubik et al., 1996). Although no allosteric ligands have not yet been conclusively demonstrated for dopamine receptors, this is likely to become important in the future. The conservation in the ligand binding domains of the dopamine receptor family sometimes has created difficulties in discovering ligands with adequate pharmacological selectivity (e.g., D1 vs. D5). This problem could be overcome if less conserved aspects of the receptor could be targeted. Second, allosteric ligands can theoretically have actions far more diverse than orthosteric ligands. Indeed, allosteric ligands could cause conformational changes that directly activate the receptor (allosteric agonist) or conformational changes that block the actions of orthosteric ligands (allosteric antagonist). In addition to these direct actions on receptor function, allosteric ligands could have modulatory functions via interactions with orthosteric ligands, either synergizing (positive allosteric modulation) or attenuating (negative allosteric modulation) the actions of the endogenous ligand. In theory, allosteric ligands offer the potential for fine-tuning cellular signaling, especially in situations where the discovery of orthosteric ligands has been unsuccessful, and there are now examples that allosteric targeting can lead to an approved drug for a GPCR (e.g., cinacalcet; Block et al., 2004). These possibilities may be useful for both D1 and D2 receptors as it relates to aspects of the treatment of schizophrenia. As noted earlier, there is no ligand yet identified for any of the dopamine receptors that is a clear allosteric ligand, but there has been some suggestion that this may be possible (Agnati et al., 2006; Huber et al., 2012).
2. D2-like signaling
A major mediator of D2-like signaling (i.e., D2, D3, and D4 receptors) is the Gαi/o class of G proteins, which are inactivated by pertussis toxin-catalyzed ADP-ribosylation (Bokoch et al., 1983; Kurose et al., 1983). The Gαi/o class of G proteins inhibits adenylate cyclase and cAMP accumulation (De Camilli et al., 1979; Stoof and Kebabian, 1981). Interestingly, studies have shown that D2-like inhibition of adenylate cyclase can lead to presynaptic/autoreceptor decreases in tyrosine hydroxylase activity and decreased firing of nigrostriatal dopamine neurons. The two isoforms of D2 receptors [i.e., D2Long (D2L) and D2Short (D2S)] appear to activate multiple Gαi/o subtypes, but the Gαo subtype is the subtype that is most robustly activated by both D2L and D2S (Jiang et al., 2001). Both D2 and D4 receptors inhibit adenylate cyclase in most clonal and in situ cells (Huff, 1997; Oak et al., 2000); however, inhibition of adenylate cyclase by D3 receptors is usually undetectable without molecular manipulation. Interestingly, D3 dopamine receptors tend to bind agonists with higher affinity than the other D2-like receptors. In addition, D3 dopamine receptors bind agonists in a GTP-insensitive manner (Sokoloff et al., 1992), which has been hypothesized to be because of a receptor conformation with high affinity for agonists regardless of interactions with G proteins (Vanhauwe et al., 2000).
Although D2 receptors are a target of all APDs, differences in the therapeutic profiles of APDs are influenced by interactions with other receptors and differential activation of D2-mediated signaling pathways. In addition to D2-like receptor signaling through adenylate cyclase, D2-like receptors modulate many other signaling pathways, including phospholipases, ion channels, mitogen activated protein (MAP) kinases, and the Na+/H+ exchanger (Huff et al., 1998). Moreover, the dissociation of Gβγ subunits following D2 receptor activation has significant effects on K+ currents, and D2 receptor-mediated increases in K+ currents decrease cell excitability. D2-like receptors activate a G protein-regulated inwardly rectifying potassium channel (GIRK or Kir3), which modulates several potassium currents in various tissues (Greif et al., 1995; Lacey et al., 1987; Liu et al., 1994; Oxford and Wagoner, 1989). In addition, dopamine release-regulating autoreceptors are coupled to potassium channels (Cass and Zahniser, 1991) rather than inhibition of adenylate cyclase (Memo et al., 1986), and there is robust regulation of GIRK currents by D2 receptors in substantia nigra dopamine neurons (Davila et al., 2003).
D2-like receptors can also regulate Na+ channels and L, N, and P/Q-type Ca2+ channels, and the regulation of these ion channels may involve Gβγ actions (Kuzhikandathil and Oxford, 1999; Lledo et al., 1992; Okada et al., 2003; Seabrook et al., 1994a; Seabrook et al., 1994b; Surmeier and Kitai, 1993; Yan et al., 1997; Zamponi and Snutch, 1998). Interestingly, D2-like receptor stimulation can either increase or decrease Na+ currents depending on the subtype of D2-like receptors that are expressed by a given cell (Surmeier et al., 1992; Surmeier and Kitai, 1993). In most D2 agonist-responsive neurons, however, D2-like receptor stimulation decreases Na+ currents. In addition, D2-like receptor signaling tends to inhibit Ca2+ channel activity. Moreover, D2-like receptor signaling can regulate neurotransmitter release via N-type Ca2+ channels (Dunlap et al., 1995; Koga and Momiyama, 2000; Svensson et al., 2003). Agonist effects at heterologously expressed D2, D3, or D4 receptors can also activate the widely expressed Na+/H+ exchanger NHE1 (Neve et al., 2004).
D2 receptor activation has also been shown to stimulate MAP kinases, including the two isozymes of extracellular signal-regulated kinase (ERK) (Choi et al., 1999; Ghahremani et al., 2000; Huff, 1996; Kim et al., 2004; Luo et al., 1998; Oak et al., 2001; Voyno-Yasenetskaya et al., 1994; Welsh et al., 1998) and stress-activated protein kinase/Jun amino-terminal kinase (SAPK/JNK) (Luo et al., 1998). In addition, D2 and D4 receptors can potentiate arachidonic acid release induced by calcium-mobilizing receptors both directly and through cytosolic phospholipase A2 (Chio et al., 1994; Kanterman et al., 1991; Piomelli et al., 1991; Vial and Piomelli, 1995). Arachidonic acid and its lipoxygenase and cyclooxygenase metabolites have numerous effects on cellular function, including feedback regulation of D2-like signaling and dopamine transport (DiMarzo and Piomelli, 1992; L’hirondel et al., 1995; Piomelli and Greengard, 1990; Zhang and Reith, 1996). Interestingly, D2 receptors can also stimulate phospholipase D, which catalyzes the hydrolysis of phosphatidylcholine to form choline and phosphatidic acid (Mitchell et al., 1998; Senogles, 2000).
Dopamine D2 receptor activation can also result in G protein-independent signaling, such as direct interactions with ion channels (e.g., NMDA) and signaling through GRKs and arrestins. Importantly, the GRK/arrestin pathway can both suppress G protein signaling and promote G protein-independent signaling. Although differential effects can occur based on the cellular expression of GRKs and arrestins, most evidence of G protein-independent signaling relates to the role of ß-arrestin 2. ß-arrestin 2 is a key member of the GPCR desensitization machinery that is involved in dopamine signaling through protein kinase B (Akt) and glycogen synthase kinase 3 (GSK3) (Beaulieu et al., 2008; Beaulieu et al., 2007b). Indeed, GRK phosphorylation of dopamine receptors leads to the recruitment and binding of ß-arrestins, which prevents G-protein-dependent signaling even if an agonist is still present. The majority of the evidence is based on studies using a variety of genetically altered mice that were deficient in major constituents of GPCR signaling, including GRKs, ß-arrestins, dopamine transporter (DAT), and Akt. Mechanistically, the time course of ß-arrestin/Akt signaling at the D2 receptor takes much longer to develop (hours versus minutes) compared with the G protein-mediated cAMP/PKA response (Beaulieu et al., 2005; Beaulieu et al., 2004; Beaulieu et al., 2007b). Thus, drugs that alter D2 receptor signaling (e.g., APDs) may result in opposing responses following drug administration. For example, the initial response would be a rapid G protein (i.e., cAMP) mediated response that could be desensitized, whereas delayed responses (e.g., β-arrestin-mediated) might last for a longer period of time and may not be able to be desensitized. The slow response of the ß-arrestin/Akt pathway may be indicative of its downstream effects on gene transcription, which can alter target protein transcriptional profiles and chromatin remodeling (Alimohamad et al., 2005; Beaulieu and Caron, 2005; Kang et al., 2005; Li et al., 2007). Although it is tempting to speculate that the ß-arrestin/Akt pathway is the one primarily involved in clinical response to antipsychotic drugs, it is unlikely that the answer will be so simple.
2.1. Evidence for Functional Selectivity at D2-Like Receptors
2.1.1. Hypothesized Presynaptic/Autoreceptor Selective Ligands and Functional Selectivity
Dopamine D2 receptors have been studied as a drug target for more than 50 years, and D2 ligands have been used for schizophrenia, Parkinson’s disease, and a variety of other psychiatric and neurological conditions. One of the major driving forces for the search for D2 drugs was the “dopamine hypothesis of schizophrenia”, which posits that excess dopamine release and/or excess sensitivity of dopamine-receptive neurons was the cause of what are now called positive symptoms. Interestingly, this hypothesis is highly relevant to the discovery and validation of the functional selectivity of dopamine receptor ligands. The classical APDs were D2 antagonists, and antagonism of D2 receptors has been accepted as a way of controlling the positive symptoms of schizophrenia (Creese et al., 1976). Interestingly, activation of dopamine autoreceptors, which are primarily D2 receptors and some D3 receptors, causes a decrease in both the synthesis and the release of dopamine and a decrease in the firing of dopamine neurons. Interestingly, both dopamine and D2 agonists tend to have higher potency at the effects mediated by the autoreceptors compared with the postsynaptic D2 functions (Feenstra et al., 1983). These important findings explain why the behavioral response to a full D2 agonist is typically biphasic with respect to dose: the inhibition seen at low doses is the result of autoreceptor stimulation, and the stimulation at higher doses is the result of direct postsynaptic activation. One of the major mechanisms explaining this biphasic effect is greater presynaptic D2-like receptor reserve (Meller et al., 1987).
Pharmacological theory suggests that high receptor reserve in the D2 presynaptic receptors would make partial agonists much more efficacious (Kenakin, 1997); thus, researchers suggested the use of D2 partial agonists as APDs. Data from in vitro and animal studies suggested that the partial agonist (−)3-PPP (preclamol) might be an excellent candidate. Because studies had shown that low doses of agonists selectively activate D2 autoreceptors and activation of D2 autoreceptors inhibits dopamine release and postsynaptic activation, another treatment strategy was to use a low dose of a full agonist. Although both mechanisms have a good theoretical basis, the early clinical data were disappointing (Corsini et al., 1981; Lahti et al., 1998; Smith et al., 1977; Tamminga et al., 1992; Tamminga et al., 1986; Tamminga et al., 1978). In retrospect, this was probably not unexpected because of issues in achieving the “right” presynaptic relative receptor occupancy without temporal fluctuations or finding a partial agonist with just the “right’ intrinsic activity. Nevertheless, the “partial agonist hypothesis” was revived when aripiprazole was shown to have clinical efficacy. The difference between aripiprazole and several earlier drugs, however, is that aripiprazole is a functionally selective drug.
2.1.2. Early Evidence for the Functional Selectivity of Dopaminergic Compounds
To the best of our knowledge, the first clear example of dopamine receptor functional selectivity resulted from serendipitous findings with dihydrexidine (DHX). Dihydrexidine was designed to be a selective D1 agonist, but studies showed that it also had D2 affinity (Brewster et al., 1990; Mottola et al., 1992). An examination of the functional characteristics of DHX and a more D2-selective analog (N-n-propyldihydrexidine; PrDHX) showed that both compounds competed for D2 receptors in heterologous systems and in brain tissue with shallow, guanine-nucleotide-sensitive curves that were similar to typical agonists (Kilts et al., 2002; Mottola et al., 1992; Mottola et al., 2002). We also examined the functional effects of these compounds in many in vitro systems, and both compounds had full intrinsic activity at inhibiting adenylate cyclase activity, which could be blocked by D2 antagonists (Kilts et al., 2002; Mottola et al., 2002). The compounds also inhibited prolactin secretion in vivo. Although the functional studies predicted that both DHX and PrDHX were full agonists, neither ligand activated D2-like presynaptic autoreceptors, which mediate inhibition of dopamine neuron firing, dopamine release, and dopamine synthesis (Kilts et al., 2002; Mottola et al., 2002). In addition, in vivo experiments showed that DHX was an antagonist of the physiological actions of the potent D2 agonist apomorphine (Mottola et al., 2002). Although the in vitro functional characterization suggested that DHX had both D1 and D2 agonist properties, its behavioral effects were different from apomorphine or amphetamine. Interestingly, the behavioral effects of DHX appeared to be primarily D1-like, even at very high doses (Darney, Jr. et al., 1991).
These early in vitro/in situ data suggested that DHX and PrDHX had high intrinsic activity at postsynaptic D2-like receptors but low intrinsic activity at presynaptic receptors (Mottola et al., 1991). Off-site actions were largely excluded by both receptor screening and competitive pharmacological studies (Mottola et al., 1992). Although a D4 contribution could be ruled out based on localization, it was possible that DHX and PrDHX were agonists at one dopamine receptor isoform (e.g., D2L) but antagonists at another (e.g., D2S or D3). Interestingly, studies showed that DHX and PrDHX were agonists at D2L receptors for the cAMP pathway, whereas they were antagonists at presynaptic autoreceptor functions. Taken together, these studies led to the hypothesis that DHX and PrDHX were D2 functionally selective (Mottola et al., 1991).
Following the discovery and initial characterization of DHX, its functional selectivity was demonstrated in several model systems. For example, DHX was a full agonist at inhibiting adenylate cyclase in pituitary lactotrophs, and this activity was blocked by D2 antagonists but not D1 antagonists. In addition, DHX had little intrinsic activity at D2 receptors coupled to GIRK and was an antagonist at D2 autoreceptors (Mottola et al., 2002). One advantage of the lactotrophs was that they only express products of the D2 gene (DRD2; Bouthenet et al., 1991). We also examined the effects of DHX and PrDHX in mesencephalic-derived MN9D cells, where the transfection of the D2L receptor has been shown to cause inhibition of adenylate cyclase and inhibition of dopamine release that is similar to dopamine neurons (O’Hara et al., 1996; Tang et al., 1994). Neither DHX nor PrDHX had effects in untransfected MN9D cells, but after D2L transfection, both compounds (as well as the reference D2 agonist quinpirole) were full agonists at inhibition of adenylate cyclase activity. Importantly, these agonist effects were blocked by D2, but not D1, antagonists. Although quinpirole caused a concentration-dependent, reversible depolarization-induced release of dopamine, neither DHX nor PrDHX inhibited dopamine release. Moreover, PrDHX actually antagonized the effects of quinpirole (Kilts et al., 2002). Taken together, these studies convinced us that functional selectivity was real.
The concept of functional selectivity was initially met with resistance because classical pharmacological theory did not allow for a molecule to be both a full agonist and pure antagonist at the same receptor (i.e., not having consistent intrinsic efficacy) (Stephenson, 1956); however, studies had shown that GPCRs could have effects via different signaling partners (Kenakin, 1990). Although the common theoretical conceptualizations of receptors was in the form of discrete active states (De Lean et al., 1980; Leff et al., 1997), our conceptualization was of a dynamic system in which ligands could induce an essentially limitless number of conformational states of a target receptor, which could cause markedly different effects on receptor signaling partners (Mailman and Gay, 2004; Mailman et al., 1997). Indeed, functional selectivity has sometimes been explained as the stabilization of novel discrete active states of a receptor. Our concept was that functionally selective ligands induce unique conformational states of a receptor that are distinct from those caused by either the endogenous ligand or by antagonists. In a sense, binding of the ligand provides the driving force needed for the receptor to assume conformations that would otherwise not be energetically favorable. Understanding the molecular mechanisms that mediate functional selectivity and the impacts on drug action in vivo are critical.
2.1.3. Functional Selectivity in Vitro Affects Pharmacological Effects in Vivo
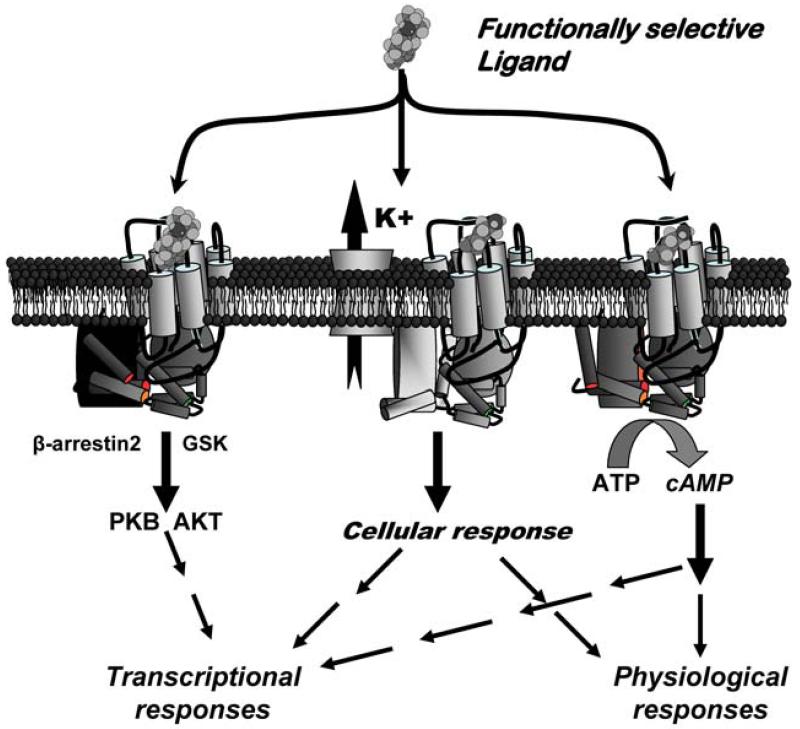
Because the concept of functional selectivity was initially met with such resistance, studies demonstrating the relevance of functional selectivity to drug actions in vivo were necessary. Studies with dihydrexidine did not show the typical behavioral actions of a D1:D2 agonist (Darney, Jr. et al., 1991), but this may be because the D1 selectivity masked the D2 effects. To avoid this issue, studies also examined PrDHX, which is much more D2:D1 selective (similar to apomorphine). Importantly, PrDHX showed full intrinsic activity at D2-mediated inhibition of adenylate cyclase. Although classic pharmacology would have predicted that a compound with full intrinsic activity at D2-mediated adenylate cyclase would have behavioral effects similar to apomorphine or the prototypical D2 agonist quinpirole (i.e., locomotor inhibition at low doses and locomotor stimulation at higher doses) (Eden et al., 1991), PrDHX only caused modest locomotor inhibition across a wide range of doses, and there were no competing behaviors that might have interfered with locomotion (Smith et al., 1997). This provided the first evidence that functionally selective compounds would have novel pharmacological actions. The potential impact of a functionally selective ligand is illustrated in Figure 1. In theory, a ligand should be able to discriminate between canonical functions mediated by different G protein heterotrimers and differentially affect long-term drug responses.
Figure 1.
The implications of functional selectivity at the dopamine D2 receptor are illustrated in this cartoon. Functionally selective drugs, unlike dopamine, might differentially affect both canonical and non-canonical signaling pathways. This could result in differential acute effects, which is illustrated by the center and right hand aspects of the figure. For example, a ligand would cause differential effects on mediators of acute action (e.g., the dopamine-induced hyperpolarization of a cell mediated via inward-rectifying potassium channels and acute actions of cAMP) as well as the longer term effects of drugs. Thus, changes in transcription initiated by cAMP on mechanisms like CREB could be altered by some drugs in a different manner than receptor-initiated changes in transcription initiated by β-arrestin2/Akt.
2.1.4. D2 Functionally Selective Drugs and Schizophrenia
Chlorpromazine was serendipitously discovered approximately 60 years ago (Delay et al., 1952), and scientists soon realized that the antipsychotic effects were due to antidopaminergic actions (Carlsson and Lindqvist, 1963), which were later shown to result from blockade of D2 dopamine receptors (Creese et al., 1976). Although non-dopaminergic drugs are being tested, all current APDs have some degree of D2 antagonism as part of their pharmacological profile. Classical APDs, however, elicit numerous side effects, and researchers are constantly searching for improved APDs. One of the novel hypotheses of interest to the field was that drugs with dopamine agonist properties might decrease dopamine neurotransmission and have dopamine antagonist-like effects (Tamminga and Carlsson, 2002). Indeed, a high-affinity partial dopamine agonist that activated presynaptic D2 autoreceptors and had low intrinsic activity at postsynaptic D2 receptors could reduce dopamine neurotransmission. One of the most recently approved APDs, aripiprazole, was touted as the first high affinity, low intrinsic activity partial D2 agonist. Although the compound has effects on several other receptors, many of the leading researchers/clinicians in schizophrenia biology have taken to calling aripiprazole the first “dopamine stabilizer” because of its D2 partial agonist properties (Lieberman, 2004; Stahl, 2001; Tamminga, 2002). It should be noted that aripiprazole does not fit the characteristics of a selective presynaptic D2-like agonist as was originally proposed (vide supra).
The idea of a partial agonist is intriguing because all partial agonists are also partial antagonists. The positive symptoms of schizophrenia are mediated by excess dopaminergic transmission in mesolimbic areas, and the partial agonist properties of aripiprazole compete with dopamine and cause partial antagonism, which provides clinical benefit. Conversely, in situations where extracellular dopamine concentrations are low (e.g., in dopamine circuits involved in working memory), partial agonists (e.g., aripiprazole) can occupy additional receptors and cause partial activation. Although aripiprazole looks like a low-to-moderate intrinsic activity partial agonist in many model systems (Burris et al., 2002; Lawler et al., 1999; Shapiro et al., 2003), other available data suggest that aripiprazole is a functionally selective D2 ligand rather than a simple partial agonist. For example, the intrinsic activity and potency of aripiprazole for the D2-mediated inhibition of cAMP accumulation is dependent on the cell line being studied: the drug demonstrates weak partial agonist activity in the CHO-D2L cell line but strong partial agonist activity in HEK-D2L cells (Burris et al., 2002; Lawler et al., 1999; Shapiro et al., 2003). Moreover, aripiprazole has been shown to have markedly different potencies at two D2L-mediated functions within the same cell line (Urban et al., 2007b). Furthermore, aripiprazole completely antagonizes both D2 agonist-mediated GTPγS binding and GIRK channel activity in some cell systems (Shapiro et al., 2003), whereas it is a full agonist in situ for D2-mediated inhibition of tyrosine hydroxylase (Kikuchi et al., 1995).
The wide array of D2-mediated intrinsic activities and/or potencies observed for aripiprazole in different systems cannot be explained by classic pharmacology and suggests that aripiprazole is functionally selective (Urban et al., 2007a). These in vitro findings can also be correlated with the actions of aripiprazole in vivo, which are irreconcilable with the partial agonist hypothesis. One of the clearest examples is the effect of aripiprazole in the unilaterally lesioned 6-hydroxydopamine (6-OHDA)-treated rat. Both full and partial dopamine agonists cause the 6-OHDA-lesioned rats to turn with high frequency in a tight contralateral fashion. The robust rotation in this model is a result of relative receptor/cellular hypersensitivity of the target receptors on the lesioned, dopamine-depleted side. As a partial agonist, aripiprazole should also cause contralateral rotation; however, this effect was not observed (Kikuchi et al., 1995). Another less direct example relates to Parkinson’s disease (PD). Many PD patients develop psychotic side effects as a result of their use of levodopa and/or dopamine agonists (the latter largely working via D2 receptors). Similar to the reasoning espoused for schizophrenia, aripiprazole (as a partial agonist) should be very useful in treating these psychotic symptoms when added to the dopaminergic regimen of a PD patient; however, aripiprazole not only lacks effectiveness in treating the psychoses, it tends to worsen motor function (Friedman et al., 2006).
Although aripiprazole is a functionally selective D2 ligand, aripiprazole also has actions at other receptors. Indeed, aripiprazole has high affinity for several receptors, including 5-HT1A, and modest affinity for several others (Shapiro et al., 2003). Nonetheless, the interest in this compound revolves around its D2 action. Because its intrinsic activity varies markedly depending on the signaling environment of the D2 receptor (Lawler et al., 1999; Shapiro et al., 2003; Urban et al., 2007a), we believe the logical explanation to integrate the in vitro and in vivo data is functional selectivity at the D2 receptor. If this hypothesis is true, a corollary is that compounds with similar D2 partial agonist effects at adenylate cyclase might have very different clinical effects in treating psychoses. This latter hypothesis may be testable as other “partial agonists” (e.g., bifeprunox) are brought into clinical trials.
3. D1-like signaling
D1-like receptors couple to Gαs/olf and stimulate adenylate cyclase activity, which subsequently activates PKA and other signaling molecules. Although Gαs appears to mediate D1-like receptor signaling, Gαolf also stimulates adenylate cyclase activity and is highly expressed in dopaminergic regions, such as the neostriatum, where there is little Gαs expression (Zhuang et al., 2000). Studies have also suggested that D1 receptors couple to other heterotrimeric G proteins, such as Gαo and Gαq (Jin et al., 2001; Kimura et al., 1995; Wang et al., 1995). In addition, D1-like receptors can mediate signaling at a variety of voltage-gated ion channels as well as NMDA and GABAA receptors, either directly or indirectly, through actions on DARPP-32, the MAPK pathway, and other kinases and phosphatases. Moreover, there is evidence that dopamine receptors (and G proteins) can have other protein-protein interactions, such as receptor oligomerization or interactions with scaffolding or other regulatory proteins, which can also affect dopamine receptor signaling (Neve et al., 2004). D1 receptor signaling can also occur through the βγ subunits that form heterotrimers with Gαs or Gαolf, but less is known about these signaling mechanisms. For example, βγ subunits can play a selective role in D1-like signaling as D1-mediated, but not D5-mediated, adenylate cyclase activity is attenuated by depletion of the γ7 subunit (Wang et al., 2001). Finally, as is typical for GPCRs, ligands can induce dopamine receptor internalization (Ryman-Rasmussen et al., 2007; Ryman-Rasmussen et al., 2005), and a recent study demonstrated a new role of endocytosis and endocytic machinery in dopamine D1 receptor signaling (Kotowski et al., 2011). It is too soon to know if this will impact the use of D1 agonists in treatment of negative and cognitive aspects of schizophrenia.
The signaling cascades that are activated by D1 receptors can also have long-term effects on cellular function by regulating transcription. Indeed, D1 agonists increase cAMP, and cAMP phosphorylates cAMP response element binding protein (CREB) at Ser133, which regulates the transcription of many genes that are important to a variety of drug responses (Andrisani, 1999; Hyman et al., 1995; Minowa et al., 1996; Sands and Palmer, 2008). In addition, D1 receptor activation can affect proteins in the MAPK signaling pathway, such as ERK (Brami-Cherrier et al., 2002). Interestingly, transcriptional changes in MAPK signaling molecules may be able to improve the therapeutic profile of D1 agonists. Developing a functionally selective drug that selectively activates signaling molecules and pathways associated with the desired effects can improve a drug’s therapeutic profile. For example, D1 agonists may have the potential to improve prefrontal cortical functions and working memory in schizophrenic patients. Indeed, SKF81297 has been shown to improve recognition and memory by increasing the phosphorylation of CREB and DARPP32 in rat prefrontal cortex (Bateup et al., 2008; Hotte et al., 2006). Thus, developing functionally selective drugs that differentially activate aspects of CREB and DARPP32 signaling could have marked therapeutic utility.
Certain effects of novel drugs and potential D1 ligands that have already been studied may not have been discovered because researchers may not have been examining the right pathway or using the right in vitro system to be able to observe effects. The concept of functional selectivity opens up exciting new opportunities for the development of many drugs, including APDs. Two decades ago, we found that D1 binding sites in the amygdala were not associated with an appreciable stimulation of adenylate cyclase, which led us to hypothesize a novel species of D1 receptor (Kilts et al., 1988; Mailman et al., 1986a; Mailman et al., 1986b). Molecular data over the next decade clearly demonstrated that these binding sites were actually the same D1 receptor that is found in striatum, and the receptor activity was determined to represent a non-traditional mechanism(s) of signaling (Leonard et al., 2003a; Leonard et al., 2003b). Today, functional selectivity promises to provide more exciting findings in the drug discovery and development realm, and schizophrenia is one area where a functionally selective D1 ligand could have a tremendous impact.
3.1. Evidence for Functional Selectivity at D1-Like Receptors
3.1.1. Possible Mechanisms of D1-Receptor Signaling that Could Evoke Functional Selectivity
Despite promising therapeutic potential for D1 receptor agonists, few efforts have been made to investigate functional selectivity at D1-like receptors. In regards to schizophrenia, D1 agonists may have therapeutic potential for negative symptoms and cognitive deficits. In addition, D1 agonists may even reduce the side effects associated with current APDs. A clearer understanding of D1-like signaling pathways is critical for the identification and design of functionally selective D1 compounds. This is complicated, however, because the signaling pathways activated by dopamine D1 receptors are dependent on several factors: the cellular machinery in a given cell or experimental system (e.g., G proteins, β-arrestins, and GRKs), differences in membrane dynamics (e.g., highly lipophilic neuronal cells vs. renal proximal tubular cells), and/or attenuation/potentiation of input signaling pathways (Mailman, 2007; Mailman and Huang, 2007; Neve et al., 2004). The most studied D1 signaling cascade is the cAMP/PKA pathway. One signaling molecule in this pathway, DARPP32, can both inhibit and promote D1 signaling, which provides an important mechanism for regulating dopaminergic signaling. Indeed, D1-like receptor activation of PKA leads to a phosphorylation/dephosphorylation cycle of DARPP32 at Thr34 and Thr35, which results in inhibition of protein phosphatase 1 (PP1) (Greengard et al., 1999), whereas phosphorylation at Thr75 by Cdk5 results in DARPP32 inhibition of PKA. D1-activated PKA can lead to the phosphorylation of other receptors in the cell (e.g., L-Ca2+ channels and NMDA) and inhibition of PP1, which prevents the dephosphorylation of many substrates, including the same receptors that are phosphorylated by PKA (Snyder et al., 1998). Although other pathways have been shown to be activated by D1 receptors, many pathways are dependent on cAMP/PKA signaling.
The MAP kinase pathway, which plays important roles in growth and cell cycle control, may be a G protein-independent D1 signaling pathway. Although few studies have investigated its role in D1-like receptor signaling, arrestins have been shown to act as adaptor proteins for MAP kinase. For example, p-ERK has been found to form stable heterotrimeric complexes with the D1 receptor and β-arrestin 2 (Chen et al., 2004). In addition, Nagai et al. (2007) revealed dose-dependent D1 activation of ERK1/2 in the mouse prefrontal cortex that was blocked by a D1 antagonist (but not a D2 antagonist). Furthermore, β-arrestin 2 has been suggested to play a role in D1-mediated ERK signaling (Urs et al., 2011). The mechanism(s) responsible for D1-MAP kinase activation is unclear, but evidence suggests a dependence on the β-arrestin scaffolding protein. In addition, D1 effects on MAP kinases may link D1 signaling with other neurotransmitters, such as glutamate. Studies have clearly shown that D1 receptors activate ERK in striatal MSNs, but further studies must be carried out to gain a clearer understanding of the mechanisms that are involved.
D1 dopamine receptors are also involved in calcium signaling. Calcium is a dynamic but tightly regulated second messenger pathway that is differentially controlled across non-nervous and nervous tissue. The slow IP3-mediated pathway via phospholipase C predominates in non-excitable cells. In excitable cells, however, voltage-dependent Ca2+ channels (L, N, and P/Q type) play a greater role and balance Ca2+ intake/output from the cell. D1-like receptors are positively coupled to L-type channels and negatively coupled to N and P/Q-type Ca2+ channels (Surmeier et al., 1995). Interestingly, studies have shown that D1 receptors can directly interact with N-type calcium channels, and D1 activation has been shown to inhibit N-type calcium channels (Kisilevsky et al., 2008). We previously mentioned that many D1-mediated pathways are dependent on cAMP/PKA signaling, and this also appears to be the case for D1-mediated inhibition of voltage-gated calcium channels (Fienberg et al., 1998). For example, D1-stimulated L-type Ca2+ channels have been shown to lead to PKA-dependent potentiation and PKC-dependent suppression of currents in rat prefrontal cortical neurons (Young and Yang, 2004).
3.1.2. Phospholipase C as a D1 Signaling Mechanism
Phospholipase C (PLC) hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) to produce 1,2-diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3). The latter binds to the IP receptor and stimulates the release of Ca2+ from intracellular stores within the endoplasmic reticulum. Diacylglycerol recruits protein kinase C (PKC) to the membrane, which leads to a variety of effects, including NF-κB activation and actin reorganization. Phospholipase C may be activated by both Gαq and Gβγ, and all four members of the Gαq/11 family (αq, α11, α14, α16) have been shown to activate PLC-β isoforms (Hepler et al., 1993; Kozasa et al., 1993; Lee et al., 1992). In addition, Gβγ subunits can activate PLC-β, and the composition of specific Gβγ subunits affects the potency for PLC-β (Boyer et al., 1994). Both Gαq and Gβγ play a role in PLC-β activation (either on their own or synergistically), and the finding that PLC-β has distinct sites for Gαq and Gβγ activation (Runnels and Scarlata, 1999) has important consequences for functional selectivity. Moreover, if both cAMP/PKA and PLC signaling occur via D1 receptors, then drug discovery efforts could potentially exploit specific signaling properties of ligands.
To truly test the functional selectivity of compounds, researchers must identify independent signaling pathways because signaling pathways that are dependent on other pathways can confound the results. In addition, various in vitro systems are needed to properly test functional selectivity because the signaling machinery may be different in different cell lines (i.e., not all cell lines are appropriate for testing certain signaling pathways). For example, one study showed that D1-linked PLC was dependent on PKA activation in LTK− cells (Yu et al., 1996); however, the predominant PLC isoform that was believed to be involved (i.e., PLC-β2) is not expressed in LTK− cells. The study concluded that PLC-γ was responsible for the observed activity, but different results may have been obtained if the model system contained PLC-β2. Although a recent study of D1 effects on intracellular Ca2+ currents supported the idea of Gαq-mediated PLC activation, this mechanism was found to be co-dependent on a cAMP/PKA signal (Dai et al., 2008). In addition, measurements of intracellular Ca2+ currents may be influenced by additional sources other than PLC stimulation. Indeed, similar data have been observed in other studies, and there is a strong possibility that changes in Ca2+ can result from a D1-mediated non-PLC Ca2+ mechanism (Lin et al., 1995).
3.1.3. Implications and Complications of Purported D1-mediated PLC Signaling
Non-cyclase D1–mediated signaling clearly occurs (Leonard et al., 2003a; Leonard et al., 2003b; Mailman et al., 1986a), and evidence for a cAMP/PKA-independent signaling pathway has been shown in studies of adenylate cyclase V deficient mice (Iwamoto et al., 2003). Although 85-90% of cyclase activity was abrogated in these mice, D1-mediated locomotion was enhanced. Such findings are direct evidence for the possibility of discovering D1 functionally selective compounds. Unlike the D1 actions on cAMP/PKA, the importance of PLC in direct D1 signaling is controversial. Indeed, several studies have suggested concurrent Gαq/11 and Gαs/olf coupling to the D1 receptor (Mannoury la et al., 2007; Panchalingam and Undie, 2000; Wang et al., 1995), and a recent report indicated differential coupling by SKF83959 (Rashid et al., 2007b).
Our group originally (and mistakenly) hypothesized that these non-adenylate cyclase-linked effects might represent a D1-like receptor from a different superfamily that had similar pharmacology to the known D1-like receptors (Mailman et al., 1986a). We quickly determined that the non-adenylate cyclase effects were more likely to be generated by the same gene product utilizing different signaling machinery (Kilts et al., 1988; Leonard et al., 2003a; Leonard et al., 2003b). Interestingly, another group subsequently resurrected the hypothesis of a novel “D1-like” receptor (Friedman et al., 1997; Undie et al., 1994), and those data are very relevant to the issue of D1 functional selectivity.
The data in support of the hypothesis for a novel D1-like receptor came from D1 ligand activation of PLC; however, limitations in the designs of the studies that have supported this hypothesis have raised doubts about whether the signaling is really D1 mediated. For reasons that are unclear, the concentrations of the drugs used in the published experiments have always been suprapharmacological (10 μM or greater, orders of magnitude higher than the affinities of the compounds). Moreover, there are good correlations between the binding affinities of D1 ligands and their potencies at activating adenylate cyclase, but these correlations were not observed between binding and PLC activation. Although functional selectivity can be expressed as large changes in potency and/or intrinsic activity (Gay et al., 2004; Mailman, 2007; Mailman and Gay, 2004; Urban et al., 2007a; Urban et al., 2007b), the combination of a requirement for high drug concentrations and SAR discordance in studies demonstrating D1-mediated PLC signaling may be a red flag.
To further investigate whether D1 receptors can couple to Gαq and signal through PLC, similar studies to the previously published reports that showed D1-mediated PLC signaling were repeated in D1 knockout mice. Importantly, the D1-mediated adenylate cyclase activity was abolished in the D1 knockout mice (Friedman et al., 1997), but PLC activation was similar to the wild-type controls. The conclusion from this study was that this was evidence for a novel D1 receptor (Friedman et al., 1997); however, an equally plausible explanation is that the PLC signaling was not D1 mediated. In support of the latter explanation, none of the known or orphan GPCRs have been matched to this proposed new D1-like receptor. Moreover, in D1 knockout mice, there is a profound decrease in phenylbenzazepine binding sites (Montague et al., 2001), which makes it highly unlikely that the pharmacological properties of benzazepines would be unaffected if this was a D1 effect. Furthermore, the purported D1-like Gαq-coupled receptor does not react with a D1 receptor antibody (Jin et al., 2001). Taken together, the evidence may favor the hypothesis that these PLC effects are mediated by a non-dopamine receptor binding site that can be activated with low potency by one class of dopamine ligands (i.e., phenylbenzazepines) (Leonard et al., 2003a). Interestingly, a more recent study demonstrated that the PLC activity induced by benzazepines was abolished in D5 knockout mice (Sahu et al., 2009).
In addition, several recent studies have suggested that phenylbenzazepine–based D1 ligands may actually signal through the PLC pathway in a functionally selective fashion. For example, a functional D1/D2 dimer has been reported to be activated by one phenylbenzazepine (SKF83959) but not another (SKF83822). Interestingly, this correlates with the suggestion that SKF83959 selectively activates D1-linked Gαq, whereas SKF83822 selectively affects Gαs (Lee et al., 2004; Rashid et al., 2007b), and these studies support the importance of PLC/Gαq signaling through D1 receptor heterodimers (e.g., with D2) (Rashid et al., 2007a). The signaling of D1/D2 heterodimers have recently been reviewed (Hasbi et al., 2011), but the physiological relevance of D1/D2 heterodimer signaling is controversial because studies have shown that most striatal neurons do not co-express D1 and D2 receptors (Valjent et al., 2009). Further studies are needed to clarify whether D1 receptors can directly activate Gαq and signal through the PLC pathway. If the PLC pathway is important for D1 signaling, then certain phenylbenzazepines (and possibly D1 ligands of other chemical classes) may be highly functionally selective (Ryman-Rasmussen et al., 2007; Ryman-Rasmussen et al., 2005), and the PLC signaling pathway may provide a route to the development of novel drugs.
3.1.4. Direct Evidence for Functional Selectivity at the D1-Like Receptors
Functional selectivity has been difficult to demonstrate for D1-like dopamine receptors because of the lack of clear effectors coupled to the receptor. Nevertheless, one study investigated the adenylate cyclase activation and receptor internalization induced by a variety of structurally different D1 agonists with various affinities for D1 receptors (Ryman-Rasmussen et al., 2005). Interestingly, the Ryman-Rasmussen et al. study identified several D1 agonists that activate adenylate cyclase with great efficacy but fail to cause receptor internalization. Importantly, internalization efficacy was found to be independent of agonist structural class and agonist affinity, suggesting that functional selectivity at D1 receptors cannot be predicted by simple structural examination of D1 agonists.
A subsequent study by Ryman-Rasmussen et al. (2007) compared the ability of dopamine and two structurally dissimilar agonists, A77636 (chemically an isochroman) and dinapsoline (DNS; chemically an isoquinoline), to regulate receptor internalization and trafficking. Both A77636 and DNS are full agonists at activating adenylate cyclase, and DNS exhibited a similar efficacy to dopamine in causing internalization, whereas A77636 caused significantly greater internalization. An investigation of the post-endocytic agonist effects on receptor trafficking revealed significant differences in agonist regulation of receptor trafficking. Dopamine caused the D1 receptor to recycle back to the cell surface within 1 h, whereas the D1 receptor persisted intracellularly for up to 48 h after the removal of A77636. Surprisingly, DNS caused the receptor to recycle back to the membrane after 48 h. Pulse-chase experiments and the use of actinomycin D to inhibit new protein biosynthesis demonstrated that cell surface recovery was not due to the synthesis of new proteins. Taken together, these data indicate that agonists target D1 receptors to different intracellular trafficking pathways.
3.1.5. Potential Utility of D1 Functionally Selective Drugs
The D1-like receptors have been implicated as therapeutic targets for numerous CNS disorders, such as Parkinson’s disease (Mailman et al., 2001; Taylor et al., 1991), schizophrenia, learning and memory dysfunctions (Arnsten et al., 1994; Goldman-Rakic et al., 2004), and attention deficit hyperactivity disorder (Heijtz et al., 2007). Importantly, D1 agonists may play a critical role in the treatment of the negative and cognitive symptoms of schizophrenia, which are not very responsive to current drugs. One of the issues that has been associated with the development of a selective full D1 agonist is side effects (e.g., hypotension and tachycardia) (Huang et al., 2001). Utilizing the concept of functional selectivity, however, we can potentially design a D1 agonist that interacts with specific signaling pathways to enhance the beneficial effects while avoiding unwanted side effects. For example, sodium levels play a critical role in regulating blood pressure, and the hypotension and tachycardia that are observed with D1 agonists result from excessive stimulation of peripheral D1 receptors, which can influence sodium concentrations. Although the D1-linked signaling cascade(s) regulating sodium transport are not entirely clear, evidence suggests that D1 receptors primarily modulate sodium resorption via the Na+/H+-exchanger and Na+-K+-ATPase (Hussain and Lokhandwala, 1998). Thus, the design of a D1 agonist that is less efficacious at these or other currently unidentified transduction pathway(s) could permit the use of high doses of a D1 agonist in patients.
Although functional selectivity has a lot of promise for the discovery and development of D1 ligands, a lot more knowledge is required to take full advantage of functional selectivity. For example, instead of just determining the binding characteristics and whether a drug is an agonist or antagonist at D1 receptors, researchers must understand which signaling pathways are activated by a given drug and which signaling pathways are critical for the desired effects of the drug. A thorough understanding of these issues will allow scientists to manipulate the chemical structures of novel D1 ligands to selectively activate signaling pathways. Interestingly, studies have shown that major ligand structural differences cannot explain differences in functional selectivity at the D1 receptor; thus, functional selectivity could be due to more subtle structural changes or to the G proteins and other signaling molecules that are coupled to D1 receptors in various brain regions. With the acceptance of functional selectivity and the ability to selectively modulate cellular functions in a much more specific manner than previously imagined, researchers have questioned whether diseases could be treated by modulating signaling pathways rather than directly targeting receptors. This strategy has flaws, however, because many of the signaling pathways that are mediated by dopamine receptors have critical functions throughout the body; thus, just targeting the signaling pathway would likely result in many serious side effects. Indeed, functionally selective ligands that target dopamine receptors could be designed to affect a specific signaling pathway, but the effects could be localized to dopaminergic diseases by activating or inhibiting a specific pathway through dopamine receptors.
Superficially, a D1 agonist seems counterintuitive for the treatment of schizophrenia; however, the differential localization and functional characteristics belie this. In addition, functionally selective D1 agonists might further improve the therapeutic index of drugs used to treat cognitive and/or negative domains of schizophrenia and related disorders. Interestingly, the primary function of almost all clinically used dopamine antagonists is the treatment of schizophrenia and other psychotic or manic disorders. All clinically used dopamine agonists (approved for a variety of disorders other than schizophrenia) were designed as D2 agonists (i.e., no clinically used dopaminergic drugs were designed as D1 agonists). Because so much of the focus of dopaminergic diseases, especially schizophrenia, has been aimed at D2 receptors, full D1 agonists have gone largely unnoticed. Whereas several studies have demonstrated that D1 antagonists would not be effective APDs (Den Boer et al., 1995; Karlsson et al., 1995), there is a large body of experimental research showing that D1 agonists can improve working memory processes in the prefrontal cortex (Arnsten et al., 1994; Cai and Arnsten, 1997; Castner et al., 2000; Goldman-Rakic et al., 2004; Hersi et al., 1995; Lidow et al., 1991; Schneider et al., 1994; Steele et al., 1997) as well as other functions (Han et al., 1997; Shohamy and Adcock, 2010). Importantly, the large body of data supporting the potential beneficial effects of D1 stimulation in cognition and memory also shows a clear dose-dependency for these effects. Indeed, higher doses of a D1 agonist have been demonstrated to impair memory performance in aged monkeys (Castner et al., 2000). The involved mechanisms by which D1 receptors affect memory and cognition are not fully understood, but a functionally selective D1 ligand might be able to avoid the biphasic dose-response relationships and only activate the signaling pathway(s) that enhance cognition. Such examples illustrate how the elucidation of mechanisms of functional selectivity of dopamine receptor ligands may have clinical relevance as well as intrinsic heuristic value.
Although numerous D2-like ligands have been the subject of clinical studies, the only reports of studies in humans for selective D1 agonists are for DHX (Brewster et al., 1990; Lovenberg et al., 1989; Mottola et al., 1992) and ABT-431 (nee A83959) (Michaelides et al., 1995; Shiosaki et al., 1996). Both compounds have similar pharmacology and have been shown to have profound antiparkinson activity (Giardina and Williams, 2001; Haney et al., 1999; Okumu et al., 2002; Rascol et al., 1999; Rascol et al., 2001; Self et al., 2000). Both compounds have little oral bioavailability, and only DHX remains available for clinical testing (George et al., 2007; Mu et al., 2007) with a phase IIa study as a cognitive enhancer in schizophrenia currently ongoing (Slifstein et al., 2011). If positive results come from such studies, it will spur efforts to discover compounds with better pharmacokinetics and, possibly, functionally selective signaling properties.
4. Conclusion
Current APDs are relatively effective at treating the positive symptoms of schizophrenia, but unwanted side effects and a lack of efficacy in treating negative and cognitive symptoms have highlighted the need for new and improved APDs. Although newer generation antipsychotics may have important therapeutic effects at receptors other than the dopamine receptor, all currently approved APDs share actions at D2 receptors. With the growing acceptance of functional selectivity and in-depth mechanistic studies into the signaling pathways mediated by dopamine receptors, this is an exciting time for the discovery of APDs. Current high-throughput screening assays for novel APDs that only examine effects on the cAMP pathway may be missing potential therapeutic compounds. Drug discovery efforts should also examine other signaling pathways that are activated by D1 and D2 receptors and search for functionally selective compounds. The primary target for APDs is the dopamine receptor system, but a thorough understanding of the dopamine-mediated signaling pathways that are responsible for the specific effects of schizophrenia will help to focus drug discovery and development efforts. Although current APDs are effective at treating the positive symptoms of schizophrenia, they are not very effective at treating negative symptoms and cognitive deficits. In addition, current APDs have significant side effects. The improvements that have been made from typical antipsychotics to atypical antipsychotics and newer functionally selective drugs, such as aripiprazole, highlight the possibility of improving APDs, and the design of novel functionally selective drugs holds promise for the treatment of schizophrenia.
Acknowledgments
This work was supported, in part, by Public Health Service research grants MH082441, MH40537, NS39036, ES007126, and GM007040.
Footnotes
Declaration of Conflict of Interest: Richard Mailman has a potential conflict of interest based on intellectual property in the dopamine drug arena that has been assigned to the University of North Carolina or Effipharma Inc.
Reference List
- 1.Agnati LF, Ferre S, Genedani S, Leo G, Guidolin D, Filaferro M, Carriba P, Casado V, Lluis C, Franco R, Woods AS, Fuxe K. Allosteric modulation of dopamine D2 receptors by homocysteine. J.Proteome.Res. 2006;5:3077–3083. doi: 10.1021/pr0601382. [DOI] [PubMed] [Google Scholar]
- 2.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 3.Alimohamad H, Rajakumar N, Seah YH, Rushlow W. Antipsychotics alter the protein expression levels of beta-catenin and GSK-3 in the rat medial prefrontal cortex and striatum. Biol Psychiatry. 2005;57:533–542. doi: 10.1016/j.biopsych.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 4.Andrisani OM. CREB-mediated transcriptional control. Crit Rev.Eukaryot.Gene Expr. 1999;9:19–32. [PubMed] [Google Scholar]
- 5.Arnsten AF, Cai JX, Murphy BL, Goldman-Rakic PS. Dopamine D1 receptor mechanisms in the cognitive performance of young adult and aged monkeys. Psychopharmacol. 1994;116:143–151. doi: 10.1007/BF02245056. [DOI] [PubMed] [Google Scholar]
- 6.Bateup HS, Svenningsson P, Kuroiwa M, Gong S, Nishi A, Heintz N, Greengard P. Cell type-specific regulation of DARPP-32 phosphorylation by psychostimulant and antipsychotic drugs. Nat.Neurosci. 2008;11:932–939. doi: 10.1038/nn.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beaulieu JM, Caron MG. Beta-arrestin goes nuclear. Cell. 2005;123:755–757. doi: 10.1016/j.cell.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol.Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- 9.Beaulieu JM, Gainetdinov RR, Caron MG. The Akt-GSK-3 signaling cascade in the actions of dopamine. Trends Pharmacol.Sci. 2007a;28:166–172. doi: 10.1016/j.tips.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Beaulieu JM, Marion S, Rodriguiz RM, Medvedev IO, Sotnikova TD, Ghisi V, Wetsel WC, Lefkowitz RJ, Gainetdinov RR, Caron MG. A beta-arrestin 2 signaling complex mediates lithium action on behavior. Cell. 2008;132:125–136. doi: 10.1016/j.cell.2007.11.041. [DOI] [PubMed] [Google Scholar]
- 11.Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 12.Beaulieu JM, Sotnikova TD, Yao WD, Kockeritz L, Woodgett JR, Gainetdinov RR, Caron MG. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proc.Natl.Acad.Sci.USA. 2004;101:5099–5104. doi: 10.1073/pnas.0307921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beaulieu JM, Tirotta E, Sotnikova TD, Masri B, Salahpour A, Gainetdinov RR, Borrelli E, Caron MG. Regulation of Akt signaling by D2 and D3 dopamine receptors in vivo. J.Neurosci. 2007b;27:881–885. doi: 10.1523/JNEUROSCI.5074-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Block GA, Martin KJ, de Francisco AL, Turner SA, Avram MM, Suranyi MG, Hercz G, Cunningham J, Abu-Alfa AK, Messa P, Coyne DW, Locatelli F, Cohen RM, Evenepoel P, Moe SM, Fournier A, Braun J, McCary LC, Zani VJ, Olson KA, Drueke TB, Goodman WG. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N.Engl.J.Med. 2004;350:1516–1525. doi: 10.1056/NEJMoa031633. [DOI] [PubMed] [Google Scholar]
- 15.Bokoch GM, Katada T, Northup JK, Hewlett EL, Gilman AG. Identification of the predominant substrate for ADP-ribosylation by islet activating protein. J.Biol.Chem. 1983;258:2072–2075. [PubMed] [Google Scholar]
- 16.Bouthenet ML, Souil E, Martres MP, Sokoloff P, Giros B, Schwartz JC. Localization of dopamine D3 receptor mRNA in the rat brain using in situ hybridization histochemistry: comparison with dopamine D2 receptor mRNA. Brain Res. 1991;564:203–219. doi: 10.1016/0006-8993(91)91456-b. [DOI] [PubMed] [Google Scholar]
- 17.Boyer JL, Graber SG, Waldo GL, Harden TK, Garrison JC. Selective activation of phospholipase C by recombinant G-protein alpha- and beta gamma-subunits. J.Biol.Chem. 1994;269:2814–2819. [PubMed] [Google Scholar]
- 18.Brami-Cherrier K, Valjent E, Garcia M, Pages C, Hipskind RA, Caboche J. Dopamine induces a PI3-kinase-independent activation of Akt in striatal neurons: a new route to cAMP response element-binding protein phosphorylation. J.Neurosci. 2002;22:8911–8921. doi: 10.1523/JNEUROSCI.22-20-08911.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brewster WK, Nichols DE, Riggs RM, Mottola DM, Lovenberg TW, Lewis MH, Mailman RB. trans-10,11-dihydroxy-5,6,6a,7,8,12b-hexahydrobenzo[a]phenanthridine: a highly potent selective dopamine D1 full agonist. J.Med.Chem. 1990;33:1756–1764. doi: 10.1021/jm00168a034. [DOI] [PubMed] [Google Scholar]
- 20.Burris KD, Molski TF, Xu C, Ryan E, Tottori K, Kikuchi T, Yocca FD, Molinoff PB. Aripiprazole, a novel antipsychotic, is a high-affinity partial agonist at human dopamine D2 receptors. J.Pharmacol.Exp.Ther. 2002;302:381–389. doi: 10.1124/jpet.102.033175. [DOI] [PubMed] [Google Scholar]
- 21.Cai JX, Arnsten AF. Dose-dependent effects of the dopamine D1 receptor agonists A77636 or SKF81297 on spatial working memory in aged monkeys. J.Pharmacol.Exp.Ther. 1997;283:183–189. [PubMed] [Google Scholar]
- 22.Carlsson A. Evidence for a role of dopamine in extrapyramidal functions. Acta Neuroveg.(Wien.) 1964;26:484–493. doi: 10.1007/BF01252144. [DOI] [PubMed] [Google Scholar]
- 23.Carlsson A, Lindqvist M. Effect of chlorpromazine and haloperidol on formation of 3-methoxytyramine and normetanephrine in mouse brain. Acta Pharmacol.Toxicol.(Copenh) 1963;20:140–144. doi: 10.1111/j.1600-0773.1963.tb01730.x. [DOI] [PubMed] [Google Scholar]
- 24.Carlsson A, Lindqvist M, Magnusson T. 3,4-Dihydroxyphenylalanine and 5-hydroxytryptophan as reserpine antagonists. Nature. 1957:1200. doi: 10.1038/1801200a0. [DOI] [PubMed] [Google Scholar]
- 25.Cass WA, Zahniser NR. Potassium channel blockers inhibit D2 dopamine, but not A1 adenosine, receptor-mediated inhibition of striatal dopamine release. J.Neurochem. 1991;57:147–152. doi: 10.1111/j.1471-4159.1991.tb02109.x. [DOI] [PubMed] [Google Scholar]
- 26.Castner SA, Williams GV, Goldman-Rakic PS. Reversal of antipsychotic-induced working memory deficits by short-term dopamine D1 receptor stimulation. Science. 2000;287:2020–2022. doi: 10.1126/science.287.5460.2020. [DOI] [PubMed] [Google Scholar]
- 27.Chen J, Rusnak M, Luedtke RR, Sidhu A. D1 dopamine receptor mediates dopamine-induced cytotoxicity via the ERK signal cascade. J.Biol.Chem. 2004;279:39317–39330. doi: 10.1074/jbc.M403891200. [DOI] [PubMed] [Google Scholar]
- 28.Chio CL, Drong RF, Riley DT, Gill GS, Slightom JL, Huff RM. D4 dopamine receptor-mediated signaling events determined in transfected Chinese hamster ovary cells. J.Biol.Chem. 1994;269:11813–11819. [PubMed] [Google Scholar]
- 29.Choi EY, Jeong D, Park KW, Baik JH. G protein-mediated mitogen-activated protein kinase activation by two dopamine D2 receptors. Biochem.Biophys.Res.Commun. 1999;256:33–40. doi: 10.1006/bbrc.1999.0286. [DOI] [PubMed] [Google Scholar]
- 30.Clement-Cormier YC, Kebabian JW, Petzold GL, Greengard P. Dopamine-sensitive adenylate cyclase in mammalian brain: a possible site of action of antipsychotic drugs. Proc.Natl.Acad.Sci.USA. 1974;71:1113–1117. doi: 10.1073/pnas.71.4.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conn PJ, Christopoulos A, Lindsley CW. Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat.Rev.Drug Discov. 2009;8:41–54. doi: 10.1038/nrd2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corsini GU, Pitzalis GF, Bernardi F, Bocchetta A, Del ZM. The use of dopamine agonists in the treatment of schizophrenia. Neuropharmacology. 1981;20:1309–1313. [PubMed] [Google Scholar]
- 33.Creese I, Burt DR, Snyder SH. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science. 1976;192:481–483. doi: 10.1126/science.3854. [DOI] [PubMed] [Google Scholar]
- 34.Dai R, Ali MK, Lezcano N, Bergson C. A crucial role for cAMP and protein kinase A in D1 dopamine receptor regulated intracellular calcium transients. Neurosignals. 2008;16:112–123. doi: 10.1159/000111557. [DOI] [PubMed] [Google Scholar]
- 35.Darney KJ, Jr., Lewis MH, Brewster WK, Nichols DE, Mailman RB. Behavioral effects in the rat of dihydrexidine, a high-potency, full-efficacy D1 dopamine receptor agonist. Neuropsychopharmacology. 1991;5:187–195. [PubMed] [Google Scholar]
- 36.Davila V, Yan Z, Craciun LC, Logothetis D, Sulzer D. D3 dopamine autoreceptors do not activate G-protein-gated inwardly rectifying potassium channel currents in substantia nigra dopamine neurons. J.Neurosci. 2003;23:5693–5697. doi: 10.1523/JNEUROSCI.23-13-05693.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Camilli P, Macconi D, Spada A. Dopamine inhibits adenylate cyclase in human prolactin-secreting pituitary adenomas. Nature. 1979;278:252–254. doi: 10.1038/278252a0. [DOI] [PubMed] [Google Scholar]
- 38.De Lean A, Stadel JM, Lefkowitz RJ. A ternary complex model explains the agonist-specific binding properties of the adenylate cyclase-coupled beta-adrenergic receptor. J Biol Chem. 1980;255:7108–7117. [PubMed] [Google Scholar]
- 39.Delay J, Deniker P, Harl JM. Therapeutic method derived from hiberno-therapy in excitation and agitation states. Ann.Med Psychol.(Paris) 1952;110:267–273. [PubMed] [Google Scholar]
- 40.DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- 41.Den Boer JA, van Megen HJ, Fleischhacker WW, Louwerens JW, Slaap BR, Westenberg HG, Burrows GD, Srivastava ON. Differential effects of the D1-DA receptor antagonist SCH39166 on positive and negative symptoms of schizophrenia. Psychopharmacology (Berlin) 1995;121:317–322. doi: 10.1007/BF02246069. [DOI] [PubMed] [Google Scholar]
- 42.DiMarzo V, Piomelli D. Participation of prostaglandin E2 in dopamine D2 receptor-dependent potentiation of arachidonic acid release. J.Neurochem. 1992;59:379–382. doi: 10.1111/j.1471-4159.1992.tb08915.x. [DOI] [PubMed] [Google Scholar]
- 43.Dunlap K, Luebke JI, Turner TJ. Exocytotic Ca2+ channels in mammalian central neurons. Trends Neurosci. 1995;18:89–98. [PubMed] [Google Scholar]
- 44.Eden RJ, Costall B, Domeney AM, Gerrard PA, Harvey CA, Kelly ME, Naylor RJ, Owen DA, Wright A. Preclinical pharmacology of ropinirole (SK&F 101468-A) a novel dopamine D2 agonist. Pharmacol.Biochem.Behav. 1991;38:147–154. doi: 10.1016/0091-3057(91)90603-y. [DOI] [PubMed] [Google Scholar]
- 45.Ehringer H, Hornykiewicz O. Verteilung vn Noradrenalin und Dopamin (3-hydroxytyramine) in Gehirn des Menshen und ihr Verhalten bei Evkrankungen des extrapyramidalen systems. Klin. Wschr. Klin.Wochenschr. 1960;38:1236–1239. doi: 10.1007/BF01485901. [DOI] [PubMed] [Google Scholar]
- 46.Feenstra MG, Sumners C, Goedemoed JH, de Vries JB, Rollema H, Horn AS. A comparison of the potencies of various dopamine receptor agonists in models for pre- and postsynaptic receptor activity. Naunyn Schmiedebergs Arch.Pharmacol. 1983;324:108–115. doi: 10.1007/BF00497015. [DOI] [PubMed] [Google Scholar]
- 47.Fienberg AA, Hiroi N, Mermelstein PG, Song W, Snyder GL, Nishi A, Cheramy A, O’Callaghan JP, Miller DB, Cole DG, Corbett R, Haile CN, Cooper DC, Onn SP, Grace AA, Ouimet CC, White FJ, Hyman SE, Surmeier DJ, Girault J, Nestler EJ, Greengard P. DARPP-32: regulator of the efficacy of dopaminergic neurotransmission. Science. 1998;281:838–842. doi: 10.1126/science.281.5378.838. [DOI] [PubMed] [Google Scholar]
- 48.Friedman E, Jin LQ, Cai GP, Hollon TR, Drago J, Sibley DR, Wang HY. D1-like dopaminergic activation of phosphoinositide hydrolysis is independent of D1A dopamine receptors: evidence from D1A knockout mice. Mol Pharmacol. 1997;51:6–11. doi: 10.1124/mol.51.1.6. [DOI] [PubMed] [Google Scholar]
- 49.Friedman JH, Berman RM, Goetz CG, Factor SA, Ondo WG, Wojcieszek J, Carson WH, Marcus RN. Open-label flexible-dose pilot study to evaluate the safety and tolerability of aripiprazole in patients with psychosis associated with Parkinson’s disease. Mov Disord. 2006;21:2078–2081. doi: 10.1002/mds.21091. [DOI] [PubMed] [Google Scholar]
- 50.Garau L, Govoni S, Stefanini E, Trabucchi M, Spano PF. Dopamine receptors: pharmacological and anatomical evidences indicate that two distinct dopamine receptor populations are present in rat striatum. Life Sci. 1978;23:1745–1750. doi: 10.1016/0024-3205(78)90102-9. [DOI] [PubMed] [Google Scholar]
- 51.Gay EA, Urban JD, Nichols DE, Oxford GS, Mailman RB. Functional selectivity of D2 receptor ligands in a Chinese hamster ovary hD2L cell line: evidence for induction of ligand-specific receptor states. Mol Pharmacol. 2004;66:97–105. doi: 10.1124/mol.66.1.97. [DOI] [PubMed] [Google Scholar]
- 52.George MS, Molnar CE, Grenesko EL, Anderson B, Mu Q, Johnson K, Nahas Z, Knable M, Fernandes P, Juncos J, Huang X, Nichols DE, Mailman RB. A single 20 mg dose of dihydrexidine (DAR-0100), a full dopamine D(1) agonist, is safe and tolerated in patients with schizophrenia. Schizophrenia Research. 2007;93:42–50. doi: 10.1016/j.schres.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 53.Gerfen CR. Dopamine-mediated gene regulation in models of Parkinson’s disease. Ann.Neurol. 2000a;47:S42–S50. [PubMed] [Google Scholar]
- 54.Gerfen CR. Molecular effects of dopamine on striatal-projection pathways. Trends Neurosci. 2000b;23:S64–S70. doi: 10.1016/s1471-1931(00)00019-7. [DOI] [PubMed] [Google Scholar]
- 55.Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJJ, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- 56.Ghahremani MH, Forget C, Albert PR. Distinct roles for Galphai2 and Gbetagamma in signaling to DNA synthesis and Galpha(i)3 in cellular transformation by dopamine D2S receptor activation in BALB/c 3T3 cells. Mol Cell Biol. 2000;20:1497–1506. doi: 10.1128/mcb.20.5.1497-1506.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Giardina WJ, Williams M. Adrogolide hcl (abt-431; das-431), a prodrug of the dopamine d(1) receptor agonist, a-86929: preclinical pharmacology and clinical data. CNS.Drug Rev. 2001;7:305–316. doi: 10.1111/j.1527-3458.2001.tb00201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Giros B, Sokoloff P, Martres MP, Riou JF, Emorine LJ, Schwartz JC. Alternative splicing directs the expression of two D2 dopamine receptor isoforms. Nature. 1989;342:923–926. doi: 10.1038/342923a0. [DOI] [PubMed] [Google Scholar]
- 59.Goldman-Rakic PS, Castner SA, Svensson TH, Siever LJ, Williams GV. Targeting the dopamine D1 receptor in schizophrenia: insights for cognitive dysfunction. Psychopharmacology (Berl) 2004;174:3–16. doi: 10.1007/s00213-004-1793-y. [DOI] [PubMed] [Google Scholar]
- 60.Graybiel AM. Neurotransmitters and neuromodulators in the basal ganglia. Trends Neurosci. 1990;13:244–254. doi: 10.1016/0166-2236(90)90104-i. [DOI] [PubMed] [Google Scholar]
- 61.Greengard P, Allen PB, Nairn AC. Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron. 1999;23:435–447. doi: 10.1016/s0896-6273(00)80798-9. [DOI] [PubMed] [Google Scholar]
- 62.Greif GJ, Lin YJ, Liu JC, Freedman JE. Dopamine-modulated potassium channels on rat striatal neurons: specific activation and cellular expression. J.Neurosci. 1995;15:4533–4544. doi: 10.1523/JNEUROSCI.15-06-04533.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Han JS, McMahan RW, Holland P, Gallagher M. The role of an amygdalo-nigrostriatal pathway in associative learning. J.Neurosci. 1997;17:3913–3919. doi: 10.1523/JNEUROSCI.17-10-03913.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haney M, Collins ED, Ward AS, Foltin RW, Fischman MW. Effect of a selective dopamine D1 agonist (ABT-431) on smoked cocaine self-administration in humans. Psychopharmacology (Berl) 1999;143:102–110. doi: 10.1007/s002130050925. [DOI] [PubMed] [Google Scholar]
- 65.Hasbi A, O’Dowd BF, George SR. Dopamine D1-D2 receptor heteromer signaling pathway in the brain: emerging physiological relevance. Mol.Brain. 2011;4:26. doi: 10.1186/1756-6606-4-26. PMC3138392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Heijtz RD, Kolb B, Forssberg H. Motor inhibitory role of dopamine D1 receptors: implications for ADHD. Physiol Behav. 2007;92:155–160. doi: 10.1016/j.physbeh.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 67.Hepler JR, Kozasa T, Smrcka AV, Simon MI, Rhee SG, Sternweis PC, Gilman AG. Purification from Sf9 cells and characterization of recombinant Gq alpha and G11 alpha. Activation of purified phospholipase C isozymes by G alpha subunits. J.Biol.Chem. 1993;268:14367–14375. [PubMed] [Google Scholar]
- 68.Hersi AI, Rowe W, Gaudreau P, Quirion R. Dopamine D1 receptor ligands modulate cognitive performance and hippocampal acetylcholine release in memory-impaired aged rats. Neuroscience. 1995;69:1067–1074. doi: 10.1016/0306-4522(95)00319-e. [DOI] [PubMed] [Google Scholar]
- 69.Hotte M, Thuault S, Lachaise F, Dineley KT, Hemmings HC, Nairn AC, Jay TM. D1 receptor modulation of memory retrieval performance is associated with changes in pCREB and pDARPP-32 in rat prefrontal cortex. Behav.Brain Res. 2006;171:127–133. doi: 10.1016/j.bbr.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 70.Huang X, Lawler CP, Lewis MM, Nichols DE, Mailman RB. D1 dopamine receptors. Int.Rev.Neurobiol. 2001;48:65–139. doi: 10.1016/s0074-7742(01)48014-7. [DOI] [PubMed] [Google Scholar]
- 71.Huber D, Lober S, Hubner H, Gmeiner P. Bivalent molecular probes for dopamine D(2)-like receptors. Bioorg.Med.Chem. 2012;20:455–466. doi: 10.1016/j.bmc.2011.10.063. [DOI] [PubMed] [Google Scholar]
- 72.Huff RM. Signal transduction pathways modulated by the D2 subfamily of dopamine receptors. Cell Signal. 1996;8:453–459. doi: 10.1016/s0898-6568(96)00074-5. [DOI] [PubMed] [Google Scholar]
- 73.Huff RM. Signaling pathways modulated by dopamine receptors. In: Neve KA, Neve RL, editors. The Dopamine Receptors. Humana Press; Totowa, NJ: 1997. pp. 167–192. [Google Scholar]
- 74.Huff RM, Chio CL, Lajiness ME, Goodman LV. Signal transduction pathways modulated by D2-like dopamine receptors. Adv.Pharmacol. 1998;42:454–457. doi: 10.1016/s1054-3589(08)60786-3. [DOI] [PubMed] [Google Scholar]
- 75.Hussain T, Lokhandwala MF. Renal dopamine receptor function in hypertension. Hypertension. 1998;32:187–197. doi: 10.1161/01.hyp.32.2.187. [DOI] [PubMed] [Google Scholar]
- 76.Hyman SE, Cole RL, Konradi C, Kosofsky BE. Dopamine regulation of transcription factor-target interactions in rat striatum. Chem.Senses. 1995;20:257–260. doi: 10.1093/chemse/20.2.257. [DOI] [PubMed] [Google Scholar]
- 77.Iversen LL. Dopamine receptors in the brain. Science. 1975;188:1084–1089. doi: 10.1126/science.2976. [DOI] [PubMed] [Google Scholar]
- 78.Iwamoto T, Okumura S, Iwatsubo K, Kawabe J, Ohtsu K, Sakai I, Hashimoto Y, Izumitani A, Sango K, Ajiki K, Toya Y, Umemura S, Goshima Y, Arai N, Vatner SF, Ishikawa Y. Motor dysfunction in type 5 adenylyl cyclase-null mice. J.Biol.Chem. 2003;278:16936–16940. doi: 10.1074/jbc.C300075200. [DOI] [PubMed] [Google Scholar]
- 79.Jakubik J, Bacakova L, Lisa V, el-Fakahany EE, Tucek S. Activation of muscarinic acetylcholine receptors via their allosteric binding sites. Proc.Natl.Acad.Sci.USA. 1996;93:8705–8709. doi: 10.1073/pnas.93.16.8705. PMC38737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jiang M, Spicher K, Boulay G, Wang Y, Birnbaumer L. Most central nervous system D2 dopamine receptors are coupled to their effectors by Go. Proc.Natl.Acad.Sci.U.S.A. 2001;98:3577–3582. doi: 10.1073/pnas.051632598. PMC30695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jin LQ, Wang HY, Friedman E. Stimulated D1 dopamine receptors couple to multiple Galpha proteins in different brain regions. J Neurochem. 2001;78:981–990. doi: 10.1046/j.1471-4159.2001.00470.x. [DOI] [PubMed] [Google Scholar]
- 82.Jonsson EG, Ivo R, Forslund K, Mattila-Evenden M, Rylander G, Cichon S, Propping P, Nothen MM, Asberg M, Sedvall GC. No association between a promoter dopamine D(4) receptor gene variant and schizophrenia. Am.J.Med.Genet. 2001;105:525–528. doi: 10.1002/ajmg.1478. [DOI] [PubMed] [Google Scholar]
- 83.Jonsson EG, Ivo R, Gustavsson JP, Geijer T, Forslund K, Mattila-Evenden M, Rylander G, Cichon S, Propping P, Bergman H, sberg M, Nothen MM. No association between dopamine D4 receptor gene variants and novelty seeking. Mol.Psychiatr. 2002;7:18–20. doi: 10.1038/sj.mp.4000950. [DOI] [PubMed] [Google Scholar]
- 84.Kang J, Shi Y, Xiang B, Qu B, Su W, Zhu M, Zhang M, Bao G, Wang F, Zhang X, Yang R, Fan F, Chen X, Pei G, Ma L. A nuclear function of beta-arrestin1 in GPCR signaling: regulation of histone acetylation and gene transcription. Cell. 2005;123:833–847. doi: 10.1016/j.cell.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 85.Kanterman RY, Mahan LC, Briley EM, Monsma FJ, Jr., Sibley DR, Axelrod J, Felder CC. Transfected D2 dopamine receptors mediate the potentiation of arachidonic acid release in Chinese hamster ovary cells. Mol Pharmacol. 1991;39:364–369. [PubMed] [Google Scholar]
- 86.Karle J, Clemmesen L, Hansen L, Andersen M, Andersen J, Fensbo C, Sloth-Nielsen M, Skrumsager BK, Lublin H, Gerlach J. NNC 01-0687, a selective dopamine D1 receptor antagonist, in the treatment of schizophrenia. Psychopharmacology (Berl) 1995;121:328–329. doi: 10.1007/BF02246071. [DOI] [PubMed] [Google Scholar]
- 87.Karlsson P, Smith L, Farde L, Harnryd C, Sedvall G, Wiesel FA. Lack of apparent antipsychotic effect of the D1-dopamine receptor antagonist SCH39166 in acutely ill schizophrenic patients. Psychopharmacology (Berlin) 1995;121:309–316. doi: 10.1007/BF02246068. [DOI] [PubMed] [Google Scholar]
- 88.Kebabian JW, Calne DB. Multiple receptors for dopamine. Nature. 1979;277:93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- 89.Kebabian JW, Petzold GL, Greengard P. Dopamine-sensitive adenylate cyclase in caudate nucleus of rat brain, and its similarity to the “dopamine receptor”. Proc.Natl.Acad.Sci.USA. 1972;69:2145–2149. doi: 10.1073/pnas.69.8.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kenakin T. Drugs and receptors. An overview of the current state of knowledge. Drugs. 1990;40:666–687. doi: 10.2165/00003495-199040050-00003. [DOI] [PubMed] [Google Scholar]
- 91.Kenakin TP. Pharmacologic analysis of drug-receptor interaction. Lippincott-Raven Publishers; Philadelphia: 1997. ISSN/ISBN 0397518153 (hc) [Google Scholar]
- 92.Kikuchi T, Tottori K, Uwahodo Y, Hirose T, Miwa T, Oshiro Y, Morita S. 7-(4-[4-(2,3-Dichlorophenyl)-1-piperazinyl]butyloxy)-3,4-dihydro-2(1H)-qui nolinone (OPC-14597), a new putative antipsychotic drug with both presynaptic dopamine autoreceptor agonistic activity and postsynaptic D2 receptor antagonistic activity. J.Pharmacol.Exp.Ther. 1995;274:329–336. [PubMed] [Google Scholar]
- 93.Kilts CD, Anderson CM, Ely TD, Mailman RB. The biochemistry and pharmacology of mesoamygdaloid dopamine neurons. Ann.N.Y.Acad.Sci. 1988;537:173–187. doi: 10.1111/j.1749-6632.1988.tb42105.x. [DOI] [PubMed] [Google Scholar]
- 94.Kilts JD, Connery HS, Arrington EG, Lewis MM, Lawler CP, Oxford GS, O’Malley KL, Todd RD, Blake BL, Nichols DE, Mailman RB. Functional selectivity of dopamine receptor agonists. II. Actions of dihydrexidine in D2L receptor-transfected MN9D cells and pituitary lactotrophs. J.Pharmacol.Exp.Ther. 2002;301:1179–1189. doi: 10.1124/jpet.301.3.1179. [DOI] [PubMed] [Google Scholar]
- 95.Kim SJ, Kim MY, Lee EJ, Ahn YS, Baik JH. Distinct regulation of internalization and mitogen-activated protein kinase activation by two isoforms of the dopamine D2 receptor. Mol Endocrinol. 2004;18:640–652. doi: 10.1210/me.2003-0066. [DOI] [PubMed] [Google Scholar]
- 96.Kimura K, White BH, Sidhu A. Coupling of human D-1 dopamine receptors to different guanine nucleotide binding proteins. Evidence that D-1 dopamine receptors can couple to both Gs and G(o) J.Biol.Chem. 1995;270:14672–14678. doi: 10.1074/jbc.270.24.14672. [DOI] [PubMed] [Google Scholar]
- 97.Kisilevsky AE, Mulligan SJ, Altier C, Iftinca MC, Varela D, Tai C, Chen L, Hameed S, Hamid J, Macvicar BA, Zamponi GW. D1 receptors physically interact with N-type calcium channels to regulate channel distribution and dendritic calcium entry. Neuron. 2008;58:557–570. doi: 10.1016/j.neuron.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 98.Koga E, Momiyama T. Presynaptic dopamine D2-like receptors inhibit excitatory transmission onto rat ventral tegmental dopaminergic neurones. J.Physiol. 2000;523(Pt 1):163–173. doi: 10.1111/j.1469-7793.2000.t01-2-00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kotler M, Manor I, Sever Y, Eisenberg J, Cohen H, Ebstein RP, Tyano S. Failure to replicate an excess of the long dopamine D4 exon III repeat polymorphism in ADHD in a family-based study. Am.J.Med.Genet. 2000;96:278–281. doi: 10.1002/1096-8628(20000612)96:3<278::aid-ajmg8>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 100.Kotowski SJ, Hopf FW, Seif T, Bonci A, von ZM. Endocytosis promotes rapid dopaminergic signaling. Neuron. 2011;71:278–290. doi: 10.1016/j.neuron.2011.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kozasa T, Hepler JR, Smrcka AV, Simon MI, Rhee SG, Sternweis PC, Gilman AG. Purification and characterization of recombinant G16 alpha from Sf9 cells: activation of purified phospholipase C isozymes by G-protein alpha subunits. Proc.Natl.Acad.Sci.USA. 1993;90:9176–9180. doi: 10.1073/pnas.90.19.9176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kurose H, Katada T, Amano T, Ui M. Specific uncoupling by islet-activating protein, pertussis toxin, of negative signal transduction via alpha-adrenergic, cholinergic, and opiate receptors in neuroblastoma × glioma hybrid cells. J.Biol.Chem. 1983;258:4870–4875. [PubMed] [Google Scholar]
- 103.Kuzhikandathil EV, Oxford GS. Activation of human D3 dopamine receptor inhibits P/Q-type calcium channels and secretory activity in AtT-20 cells. J.Neurosci. 1999;19:1698–1707. doi: 10.1523/JNEUROSCI.19-05-01698.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.L’hirondel M, Cheramy A, Godeheu G, Artaud F, Saiardi A, Borrelli E, Glowinski J. Lack of autoreceptor-mediated inhibitory control of dopamine release in striatal synaptosomes of D2 receptor-deficient mice. Brain Res. 1998;792:253–262. doi: 10.1016/s0006-8993(98)00146-2. [DOI] [PubMed] [Google Scholar]
- 105.L’hirondel M, Cheramy A, Godeheu G, Glowinski J. Effects of arachidonic acid on dopamine synthesis, spontaneous release, and uptake in striatal synaptosomes from the rat. J.Neurochem. 1995;64:1406–1409. doi: 10.1046/j.1471-4159.1995.64031406.x. [DOI] [PubMed] [Google Scholar]
- 106.Lacey MG, Mercuri NB, North RA. Dopamine acts on D2 receptors to increase potassium conductance in neurones of the rat substantia nigra zona compacta. J.Physiol (Lond) 1987;392:397–416. doi: 10.1113/jphysiol.1987.sp016787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.LaHoste GJ, Henry BL, Marshall JF. Dopamine D1 receptors synergize with D2, but not D3 or D4, receptors in the striatum without the involvement of action potentials. J.Neurosci. 2000;20:6666–6671. doi: 10.1523/JNEUROSCI.20-17-06666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lahti AC, Weiler MA, Corey PK, Lahti RA, Carlsson A, Tamminga CA. Antipsychotic properties of the partial dopamine agonist (−)-3-(3-hydroxyphenyl)-N-n-propylpiperidine(preclamol) in schizophrenia. Biol Psychiatry. 1998;43:2–11. doi: 10.1016/s0006-3223(97)00030-9. [DOI] [PubMed] [Google Scholar]
- 109.Lawler CP, Prioleau C, Lewis MM, Mak C, Jiang D, Schetz JA, Gonzalez AM, Sibley DR, Mailman RB. Interactions of the novel antipsychotic aripiprazole (OPC-14597) with dopamine and serotonin receptor subtypes. Neuropsychopharmacology. 1999;20:612–627. doi: 10.1016/S0893-133X(98)00099-2. [DOI] [PubMed] [Google Scholar]
- 110.Lawler CP, Watts VJ, Booth RG, Southerland SB, Mailman RB. Discrete functional selectivity of drugs: OPC-14597. a selective antagonist for post-synaptic dopamine D2 receptors. Society for Neuroscience Abstracts. 1994;20:525. [Google Scholar]
- 111.Le Moine C, Bloch B. D1 and D2 dopamine receptor gene expression in the rat striatum: sensitive cRNA probes demonstrate prominent segregation of D1 and D2 mRNAs in distinct neuronal populations of the dorsal and ventral striatum. J.Comp.Neurol. 1995;355:418–426. doi: 10.1002/cne.903550308. [DOI] [PubMed] [Google Scholar]
- 112.Lee CH, Park D, Wu D, Rhee SG, Simon MI. Members of the Gq alpha subunit gene family activate phospholipase C beta isozymes. J.Biol.Chem. 1992;267:16044–16047. [PubMed] [Google Scholar]
- 113.Lee SP, So CH, Rashid AJ, Varghese G, Cheng R, Lanca AJ, O’Dowd BF, George SR. Dopamine D1 and D2 receptor Co-activation generates a novel phospholipase C-mediated calcium signal. J Biol Chem. 2004;279:35671–35678. doi: 10.1074/jbc.M401923200. [DOI] [PubMed] [Google Scholar]
- 114.Leff P, Scaramellini C, Law C, McKechnie K. A three-state receptor model of agonist action. Trends Pharmacol.Sci. 1997:355–362. doi: 10.1016/s0165-6147(97)01105-x. [DOI] [PubMed] [Google Scholar]
- 115.Leonard SK, Anderson CM, Lachowicz JE, Schulz DW, Kilts CD, Mailman RB. Amygdaloid D1 receptors are not linked to stimulation of adenylate cyclase. Synapse. 2003a;50:320–333. doi: 10.1002/syn.10272. [DOI] [PubMed] [Google Scholar]
- 116.Leonard SK, Petitto JM, Anderson CM, Mooney DH, Lachowicz JE, Schulz DW, Kilts CD, Mailman RB. D1 dopamine receptors in the amygdala exhibit unique properties. Ann.N.Y.Acad.Sci. 2003b;985:536–539. [Google Scholar]
- 117.Levey AI, Hersch SM, Rye DB, Sunahara RK, Niznik HB, Kitt CA, Price DL, Maggio R, Brann MR, Ciliax BJ. Localization of D1 and D2 dopamine receptors in brain with subtype-specific antibodies. Proc.Natl.Acad.Sci.USA. 1993;90:8861–8865. doi: 10.1073/pnas.90.19.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li X, Rosborough KM, Friedman AB, Zhu W, Roth KA. Regulation of mouse brain glycogen synthase kinase-3 by atypical antipsychotics. Int.J Neuropsychopharmacol. 2007;10:7–19. doi: 10.1017/S1461145706006547. [DOI] [PubMed] [Google Scholar]
- 119.Lidow MS, Goldman-Rakic PS, Gallager DW, Rakic P. Distribution of dopaminergic receptors in the primate cerebral cortex: quantitative autoradiographic analysis using [3H]raclopride, [3H]spiperone and [3H]SCH23390. Neuroscience. 1991;40:657–671. doi: 10.1016/0306-4522(91)90003-7. [DOI] [PubMed] [Google Scholar]
- 120.Lieberman JA. Dopamine partial agonists: a new class of antipsychotic. CNS.Drugs. 2004;18:251–267. doi: 10.2165/00023210-200418040-00005. [DOI] [PubMed] [Google Scholar]
- 121.Lin CW, Miller TR, Witte DG, Bianchi BR, Stashko M, Manelli AM, Frail DE. Characterization of cloned human dopamine D1 receptor-mediated calcium release in 293 cells. Mol.Pharmacol. 1995;47:131–139. [PubMed] [Google Scholar]
- 122.Liu L, Shen RY, Kapatos G, Chiodo LA. Dopamine neuron membrane physiology: characterization of the transient outward current (IA) and demonstration of a common signal transduction pathway for IA and IK. Synapse. 1994;17:230–240. doi: 10.1002/syn.890170404. [DOI] [PubMed] [Google Scholar]
- 123.Lledo PM, Homburger V, Bockaert J, Vincent JD. Differential G protein-mediated coupling of D2 dopamine receptors to K+ and Ca2+ currents in rat anterior pituitary cells. Neuron. 1992;8:455–463. doi: 10.1016/0896-6273(92)90273-g. [DOI] [PubMed] [Google Scholar]
- 124.Lovenberg TW, Brewster WK, Mottola DM, Lee RC, Riggs RM, Nichols DE, Lewis MH, Mailman RB. Dihydrexidine, a novel selective high potency full dopamine D-1 receptor agonist. Eur.J.Pharmacol. 1989;166:111–113. doi: 10.1016/0014-2999(89)90690-0. [DOI] [PubMed] [Google Scholar]
- 125.Luo Y, Kokkonen GC, Wang X, Neve KA, Roth GS. D2 dopamine receptors stimulate mitogenesis through pertussis toxin-sensitive G proteins and Ras-involved ERK and SAP/JNK pathways in rat C6-D2L glioma cells. J.Neurochem. 1998;71:980–990. doi: 10.1046/j.1471-4159.1998.71030980.x. [DOI] [PubMed] [Google Scholar]
- 126.Mailman R, Huang X, Nichols DE. Parkinson’s disease and D1 dopamine receptors. Curr.Opin.Inves.Drugs. 2001;2:1582–1591. [PubMed] [Google Scholar]
- 127.Mailman RB. GPCR functional selectivity has therapeutic impact. Trends Pharmacol.Sci. 2007;28:390–396. doi: 10.1016/j.tips.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mailman RB, Gay EA. Novel mechanisms of drug action: functional selectivity at D2 dopamine receptors (a lesson for drug discovery) Med.Chem.Res. 2004;13:115–126. [Google Scholar]
- 129.Mailman RB, Huang X. In: Dopamine receptor pharmacology, in Parkinson’s disease and related disorders, Part 1. Koller WC, Melamed E, editors. Elsevier; 2007. pp. 77–105. [Google Scholar]
- 130.Mailman RB, Nichols DE, Lewis MM, Blake BL, Lawler CP. Functional Effects of Novel Dopamine Ligands: Dihydrexidine and Parkinson’s Disease as a First Step. In: Jenner P, Demirdemar R, editors. Dopamine Receptor Subtypes: From Basic Science to Clinical Application. IOS Stockton Press; 1998. pp. 64–83. ISSN/ISBN 90-5199-291-2. [Google Scholar]
- 131.Mailman RB, Nichols DE, Tropsha A. Molecular Drug Design and Dopamine Receptors. In: Neve KA, Neve RL, editors. The Dopamine Receptors. Humana Press; Totowa, NJ: 1997. pp. 105–133. [Google Scholar]
- 132.Mailman RB, Schulz DW, Kilts CD, Lewis MH, Rollema H, Wyrick S. Multiple forms of the D1 dopamine receptor: its linkage to adenylate cyclase and psychopharmacological effects. Psychopharmacol.Bull. 1986a;22:593–598. [PubMed] [Google Scholar]
- 133.Mailman RB, Schulz DW, Kilts CD, Lewis MH, Rollema H, Wyrick S. The multiplicity of the D1 dopamine receptor. Advances.in Experimental Medicine & Biology. 1986b;204:53–72. doi: 10.1007/978-1-4684-5191-7_4. [DOI] [PubMed] [Google Scholar]
- 134.Mailman RB, Schulz DW, Lewis MH, Staples L, Rollema H, DeHaven DL. SCH-23390: a selective D1 dopamine antagonist with potent D2 behavioral actions. Eur.J.Pharmacol. 1984;101:159–160. doi: 10.1016/0014-2999(84)90044-x. [DOI] [PubMed] [Google Scholar]
- 135.Mannoury la CC, Vidal S, Pasteau V, Cussac D, Millan MJ. Dopamine D1 receptor coupling to Gs/olf and Gq in rat striatum and cortex: a scintillation proximity assay (SPA)/antibody-capture characterization of benzazepine agonists. Neuropharmacology. 2007;52:1003–1014. doi: 10.1016/j.neuropharm.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 136.Martin-Iverson MT, Yamada N. Synergistic behavioural effects of dopamine D1 and D2 receptor agonists are determined by circadian rhythms. Eur.J.Pharmacol. 1992;215:119–125. doi: 10.1016/0014-2999(92)90616-c. [DOI] [PubMed] [Google Scholar]
- 137.Meller E, Bohmaker K, Namba Y, Friedhoff AJ, Goldstein M. Relationship between receptor occupancy and response at striatal dopamine autoreceptors. Mol.Pharmacol. 1987;31:592–598. [PubMed] [Google Scholar]
- 138.Memo M, Missale C, Carruba MO, Spano PF. D2 dopamine receptors associated with inhibition of dopamine release from rat neostriatum are independent of cyclic AMP. Neurosci.Lett. 1986;71:192–196. doi: 10.1016/0304-3940(86)90557-4. [DOI] [PubMed] [Google Scholar]
- 139.Mercuri NB, Saiardi A, Bonci A, Picetti R, Calabresi P, Bernardi G, Borrelli E. Loss of autoreceptor function in dopaminergic neurons from dopamine D2 receptor deficient mice. Neuroscience. 1997;79:323–327. doi: 10.1016/s0306-4522(97)00135-8. [DOI] [PubMed] [Google Scholar]
- 140.Mercurio F, Zhu H, Murray BW, Shevchenko A, Bennett BL, Li J, Young DB, Barbosa M, Mann M, Manning A, Rao A. IKK-1 and IKK-2: cytokine-activated IkappaB kinases essential for NF-kappaB activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 141.Michaelides MR, Hong Y, DiDomenico SJ, Asin KE, Britton DR, Lin CW, Williams M, Shiosaki K. (5aR,11bS)-4,5,5a,6,7,11b-hexahydro-2-propyl-3-thia-5-azacyclopent-1- ena[c]-phenanthrene-9,10-diol (A-86929): a potent and selective dopamine D1 agonist that maintains behavioral efficacy following repeated administration and characterization of its diacetyl prodrug (ABT-431) Journal of Medicinal Chemistry. 1995;38:3445–3447. doi: 10.1021/jm00018a002. [DOI] [PubMed] [Google Scholar]
- 142.Mill J, Curran S, Kent L, Richards S, Gould A, Virdee V, Huckett L, Sharp J, Batten C, Fernando S, Simanoff E, Thompson M, Zhao J, Sham P, Taylor E, Asherson P. Attention deficit hyperactivity disorder (ADHD) and the dopamine D4 receptor gene: evidence of association but no linkage in a UK sample. Mol.Psychiatr. 2001;6:440–444. doi: 10.1038/sj.mp.4000881. [DOI] [PubMed] [Google Scholar]
- 143.Minowa MT, Lee SH, Mouradian MM. Autoregulation of the human D1A dopamine receptor gene by cAMP. DNA Cell Biol. 1996;15:759–767. doi: 10.1089/dna.1996.15.759. [DOI] [PubMed] [Google Scholar]
- 144.Mitchell R, McCulloch D, Lutz E, Johnson M, MacKenzie C, Fennell M, Fink G, Zhou W, Sealfon SC. Rhodopsin-family receptors associate with small G proteins to activate phospholipase D. Nature. 1998;392:411–414. doi: 10.1038/32937. [DOI] [PubMed] [Google Scholar]
- 145.Miyamoto S, Duncan GE, Mailman RB, Lieberman JA. Developing novel antipsychotic drugs: strategies and goals. Curr.Opin.CPNS Invest.Drugs. 2000;2:25–39. [Google Scholar]
- 146.Montague DM, Striplin CD, Overcash JS, Drago F, Lawler CP, Mailman RB. Quantification of D1B (D5) receptors in dopamine D1A receptor-deficient mice. Synapse. 2001;39:319–322. doi: 10.1002/1098-2396(20010315)39:4<319::AID-SYN1015>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 147.Mottola DM, Brewster WK, Cook LL, Nichols DE, Mailman RB. Dihydrexidine, a novel full efficacy D1 dopamine receptor agonist. J.Pharmacol.Exp.Ther. 1992;262:383–393. [PubMed] [Google Scholar]
- 148.Mottola DM, Cook LL, Jones SR, Booth RG, Nichols DE, Mailman RB. Dihydrexidine, a selective dopamine receptor agonist that may discriminate postsynaptic D2 receptors. Soc.Neurosci.Abstr. 1991;17:818. [Google Scholar]
- 149.Mottola DM, Kilts JD, Lewis MM, Connery HS, Walker QD, Jones SR, Booth RG, Hyslop DK, Piercey M, Wightman RM, Lawler CP, Nichols DE, Mailman RB. Functional selectivity of dopamine receptor agonists. I. Selective activation of postsynaptic dopamine D2 receptors linked to adenylate cyclase. J.Pharmacol.Exp.Ther. 2002;301:1166–1178. doi: 10.1124/jpet.301.3.1166. [DOI] [PubMed] [Google Scholar]
- 150.Mu Q, Johnson K, Morgan PS, Grenesko EL, Molnar CE, Anderson B, Nahas Z, Kozel FA, Kose S, Knable M, Fernandes P, Nichols DE, Mailman RB, George MS. A single 20 mg dose of the full D(1) dopamine agonist dihydrexidine (DAR-0100) increases prefrontal perfusion in schizophrenia. Schizophrenia Research. 2007;94:332–341. doi: 10.1016/j.schres.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 151.Nagai T, Takuma K, Kamei H, Ito Y, Nakamichi N, Ibi D, Nakanishi Y, Murai M, Mizoguchi H, Nabeshima T, Yamada K. Dopamine D1 receptors regulate protein synthesis-dependent long-term recognition memory via extracellular signal-regulated kinase 1/2 in the prefrontal cortex. Learn.Mem. 2007;14:117–125. doi: 10.1101/lm.461407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Neve KA, Seamans JK, Trantham-Davidson H. Dopamine receptor signaling. J.Rec.Sign.Transd.Res. 2004;24:165–205. doi: 10.1081/rrs-200029981. [DOI] [PubMed] [Google Scholar]
- 153.O’Hara CM, Uhland-Smith A, O’Malley KL, Todd RD. Inhibition of dopamine synthesis by dopamine D2 and D3 but not D4 receptors. J.Pharmacol.Exp.Ther. 1996;277:186–192. [PubMed] [Google Scholar]
- 154.O’Malley KL, Harmon S, Tang L, Todd RD. The rat dopamine D4 receptor: sequence, gene structure, and demonstration of expression in the cardiovascular system. New Biol. 1992;4:137–146. [PubMed] [Google Scholar]
- 155.Oak JN, Lavine N, van Tol HH. Dopamine D4 and D2L Receptor Stimulation of the Mitogen-Activated Protein Kinase Pathway Is Dependent on trans-Activation of the Platelet-Derived Growth Factor Receptor. Mol.Pharmacol. 2001;60:92–103. doi: 10.1124/mol.60.1.92. [DOI] [PubMed] [Google Scholar]
- 156.Oak JN, Oldenhof J, van Tol HH. The dopamine D4 receptor: one decade of research. Eur.J.Pharmacol. 2000;405:303–327. doi: 10.1016/s0014-2999(00)00562-8. [DOI] [PubMed] [Google Scholar]
- 157.Okada Y, Miyamoto T, Toda K. Dopamine modulates a voltage-gated calcium channel in rat olfactory receptor neurons. Brain Res. 2003;968:248–255. doi: 10.1016/s0006-8993(03)02267-4. [DOI] [PubMed] [Google Scholar]
- 158.Okumu FW, Lee RY, Blanchard JD, Queirolo A, Woods CM, Lloyd PM, Okikawa J, Gonda I, Farr SJ, Rubsamen R, Adjei AL, Bertz RJ. Evaluation of the AERx pulmonary delivery system for systemic delivery of a poorly soluble selective D-1 agonist, ABT-431. Pharm.Res. 2002;19:1009–1012. doi: 10.1023/a:1016559707084. [DOI] [PubMed] [Google Scholar]
- 159.Oxford GS, Wagoner PK. The inactivating K+ current in GH3 pituitary cells and its modification by chemical reagents. J.Physiol (Lond) 1989;410:587–612. doi: 10.1113/jphysiol.1989.sp017550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Panchalingam S, Undie AS. Optimized binding of [35S]GTPgammaS to Gq-like proteins stimulated with dopamine D1-like receptor agonists. Neurochem.Res. 2000;25:759–767. doi: 10.1023/a:1007553004615. [DOI] [PubMed] [Google Scholar]
- 161.Piomelli D, Greengard P. Lipoxygenase metabolites of arachidonic acid in neuronal transmembrane signalling. Trends Pharmacol.Sci. 1990;11:367–373. doi: 10.1016/0165-6147(90)90182-8. [DOI] [PubMed] [Google Scholar]
- 162.Piomelli D, Pilon C, Giros B, Sokoloff P, Martres MP, Schwartz JC. Dopamine activation of the arachidonic acid cascade as a basis for D1/D2 receptor synergism. Nature. 1991;353:164–167. doi: 10.1038/353164a0. [DOI] [PubMed] [Google Scholar]
- 163.Rascol O, Blin O, Thalamas C, Descombes S, Soubrouillard C, Azulay P, Fabre N, Viallet F, Lafnitzegger K, Wright S, Carter JH, Nutt JG. ABT-431, a D1 receptor agonist prodrug, has efficacy in Parkinson’s disease. Annals of Neurology. 1999;45:736–741. doi: 10.1002/1531-8249(199906)45:6<736::aid-ana7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 164.Rascol O, Nutt JG, Blin O, Goetz CG, Trugman JM, Soubrouillard C, Carter JH, Currie LJ, Fabre N, Thalamas C, Giardina WJ, Wright S. Induction by dopamine D1 receptor agonist ABT-431 of dyskinesia similar to levodopa in patients with Parkinson’s disease. Arch.Neurol. 2001;58:249–254. doi: 10.1001/archneur.58.2.249. [DOI] [PubMed] [Google Scholar]
- 165.Rashid AJ, O’Dowd BF, Verma V, George SR. Neuronal Gq/11-coupled dopamine receptors: an uncharted role for dopamine. Trends Pharmacol.Sci. 2007a;28:551–555. doi: 10.1016/j.tips.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 166.Rashid AJ, So CH, Kong MM, Furtak T, El-Ghundi M, Cheng R, O’Dowd BF, George SR. D1-D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proc.Natl.Acad.Sci.USA. 2007b;104:654–659. doi: 10.1073/pnas.0604049104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Robertson GS, Vincent SR, Fibiger HC. D1 and D2 dopamine receptors differentially regulate c-fos expression in striatonigral and striatopallidal neurons. Neuroscience. 1992;49:285–296. doi: 10.1016/0306-4522(92)90096-k. [DOI] [PubMed] [Google Scholar]
- 168.Roth BL, Sheffler DJ, Kroeze WK. Magic shotguns versus magic bullets: selectively non-selective drugs for mood disorders and schizophrenia. Nat.Rev.Drug Discov. 2004;3:353–359. doi: 10.1038/nrd1346. [DOI] [PubMed] [Google Scholar]
- 169.Runnels LW, Scarlata SF. Determination of the affinities between heterotrimeric G protein subunits and their phospholipase C-beta effectors. Biochemistry. 1999;38:1488–1496. doi: 10.1021/bi9821519. [DOI] [PubMed] [Google Scholar]
- 170.Ryman-Rasmussen JP, Griffith A, Oloff S, Vaidehi N, Brown JT, Goddard WA, III, Mailman RB. Functional selectivity of dopamine D(1) receptor agonists in regulating the fate of internalized receptors. Neuropharmacology. 2007;52:562–575. doi: 10.1016/j.neuropharm.2006.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ryman-Rasmussen JP, Nichols DE, Mailman RB. Differential activation of adenylate cyclase and receptor internalization by novel dopamine D1 receptor agonists. Mol Pharmacol. 2005;68:1039–1048. doi: 10.1124/mol.105.012153. [DOI] [PubMed] [Google Scholar]
- 172.Sahu A, Tyeryar KR, Vongtau HO, Sibley DR, Undieh AS. D5 dopamine receptors are required for dopaminergic activation of phospholipase C. Mol.Pharmacol. 2009;75:447–453. doi: 10.1124/mol.108.053017. PMC2684903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sands WA, Palmer TM. Regulating gene transcription in response to cyclic AMP elevation. Cell Signal. 2008;20:460–466. doi: 10.1016/j.cellsig.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 174.Schmidt LA, Fox NA, Perez-Edgar K, Hu S, Hamer DH. Association of DRD4 with attention problems in normal childhood development. Psychiatr.Genet. 2001;11:25–29. doi: 10.1097/00041444-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 175.Schneider JS, Sun ZQ, Roeltgen DP. Effects of dihydrexidine, a full dopamine D-1 receptor agonist, on delayed response performance in chronic low dose MPTP-treated monkeys. Brain Res. 1994;663:140–144. doi: 10.1016/0006-8993(94)90471-5. [DOI] [PubMed] [Google Scholar]
- 176.Seabrook GR, Knowles M, Brown N, Myers J, Sinclair H, Patel S, Freedman SB, Mcallister G. Pharmacology of high-threshold calcium currents in GH4C1 pituitary cells and their regulation by activation of human D2 and D4 dopamine receptors. Br.J Pharmacol. 1994a;112:728–734. doi: 10.1111/j.1476-5381.1994.tb13138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Seabrook GR, Mcallister G, Knowles MR, Myers J, Sinclair H, Patel S, Freedman SB, Kemp JA. Depression of high-threshold calcium currents by activation of human D2 (short) dopamine receptors expressed in differentiated NG108-15 cells. Br.J Pharmacol. 1994b;111:1061–1066. doi: 10.1111/j.1476-5381.1994.tb14852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Seeman P, Chau-Wong M, Tedesco J, Wong K. Brain receptors for antipsychotic drugs and dopamine: direct binding assays. Proc.Natl.Acad.Sci.USA. 1975;72:4376–4380. doi: 10.1073/pnas.72.11.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Seeman P, Lee T. Antipsychotic drugs: direct correlation between clinical potency and presynaptic action on dopamine neurons. Science. 1975;188:1217–1219. doi: 10.1126/science.1145194. [DOI] [PubMed] [Google Scholar]
- 180.Self DW, Karanian DA, Spencer JJ. Effects of the novel D1 dopamine receptor agonist ABT-431 on cocaine self-administration and reinstatement. Annals of the New York Academy of Sciences. 2000;909:133–144. doi: 10.1111/j.1749-6632.2000.tb06679.x. [DOI] [PubMed] [Google Scholar]
- 181.Senogles SE. The D2s dopamine receptor stimulates phospholipase D activity: A novel signaling pathway for dopamine. Mol.Pharmacol. 2000;58:455–462. doi: 10.1124/mol.58.2.455. [DOI] [PubMed] [Google Scholar]
- 182.Sesack SR, Aoki C, Pickel VM. Ultrastructural localization of D2 receptor-like immunoreactivity in midbrain dopamine neurons and their striatal targets. J.Neurosci. 1994;14:88–106. doi: 10.1523/JNEUROSCI.14-01-00088.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Shapiro DA, Renock S, Arrington E, Sibley DR, Chiodo LA, Roth BL, Mailman RB. Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology. 2003;28:1400–1411. doi: 10.1038/sj.npp.1300203. [DOI] [PubMed] [Google Scholar]
- 184.Shiosaki K, Jenner P, Asin KE, Britton DR, Lin CW, Michaelides M, Smith L, Bianchi B, Didomenico S, Hodges L, Hong Y, Mahan L, Mikusa J, Miller T, Nikkel A, Stashko M, Witte D, Williams M. ABT-431: the diacetyl prodrug of A-86929, a potent and selective dopamine D1 receptor agonist: in vitro characterization and effects in animal models of Parkinson’s disease. J.Pharmacol.Exp.Ther. 1996;276:150–160. [PubMed] [Google Scholar]
- 185.Shohamy D, Adcock RA. Dopamine and adaptive memory. Trends Cogn Sci. 2010;14:464–472. doi: 10.1016/j.tics.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 186.Slifstein M, Suckow RF, Javitch JA, Cooper T, Lieberman J, Abi-Dargham A. Characterization of in vivo pharmacokinetic properties of the dopamine D1 receptor agonist DAR-0100A in nonhuman primates using PET with [11C] NNC112 and [11C] raclopride. J.Cereb.Blood Flow Metab. 2011;31:293–304. doi: 10.1038/jcbfm.2010.91. PMC3049493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Smith HP, Nichols DE, Mailman RB, Lawler CP. Locomotor inhibition, yawning and vacuous chewing induced by a novel dopamine D2 post-synaptic receptor agonist. Eur.J.Pharmacol. 1997;323:27–36. doi: 10.1016/s0014-2999(97)00026-5. [DOI] [PubMed] [Google Scholar]
- 188.Smith RC, Tamminga C, Davis JM. Effect of apomorphine on schizophrenic symptoms. J Neural Transm. 1977;40:171–176. doi: 10.1007/BF01250567. [DOI] [PubMed] [Google Scholar]
- 189.Snyder GL, Fienberg AA, Huganir RL, Greengard P. A dopamine/D1 receptor/protein kinase A/dopamine- and cAMP-regulated phosphoprotein (Mr 32 kDa)/protein phosphatase-1 pathway regulates dephosphorylation of the NMDA receptor. J.Neurosci. 1998;18:10297–10303. doi: 10.1523/JNEUROSCI.18-24-10297.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sokoloff P, Andrieux M, Besancon R, Pilon C, Martres MP, Giros B, Schwartz JC. Pharmacology of human dopamine D3 receptor expressed in a mammalian cell line: comparison with D2 receptor. Eur.J.Pharmacol. 1992;225:331–337. doi: 10.1016/0922-4106(92)90107-7. [DOI] [PubMed] [Google Scholar]
- 191.Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347:146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- 192.Stahl SM. Dopamine system stabilizers, aripiprazole, and the next generation of antipsychotics, part 1, “Goldilocks” actions at dopamine receptors. J.Clin.Psychiatry. 2001;62:841–842. doi: 10.4088/jcp.v62n1101. [DOI] [PubMed] [Google Scholar]
- 193.Starr MS. Glutamate/dopamine D1/D2 balance in the basal ganglia and its relevance to Parkinson’s disease. Synapse. 1995;19:264–293. doi: 10.1002/syn.890190405. [DOI] [PubMed] [Google Scholar]
- 194.Steele TD, Hodges DB, Jr., Levesque TR, Locke KW. D1 agonist dihydrexidine releases acetylcholine and improves cognitive performance in rats. Pharmacol.Biochem.Behav. 1997;58:477–483. doi: 10.1016/s0091-3057(97)00290-6. [DOI] [PubMed] [Google Scholar]
- 195.Stephenson RP. A modification of receptor theory. Br.J Pharmacol. 1956;11:379–393. doi: 10.1111/j.1476-5381.1956.tb00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Stoof JC, Kebabian JW. Opposing roles for D-1 and D-2 dopamine receptors in efflux of cyclic AMP from rat neostriatum. Nature. 1981;294:366–368. doi: 10.1038/294366a0. [DOI] [PubMed] [Google Scholar]
- 197.Surmeier DJ, Bargas J, Hemmings HC, Jr., Nairn AC, Greengard P. Modulation of calcium currents by a D1 dopaminergic protein kinase/phosphatase cascade in rat neostriatal neurons. Neuron. 1995;14:385–397. doi: 10.1016/0896-6273(95)90294-5. [DOI] [PubMed] [Google Scholar]
- 198.Surmeier DJ, Eberwine J, Wilson CJ, Cao Y, Stefani A, Kitai ST. Dopamine receptor subtypes colocalize in rat striatonigral neurons. Proc.Natl.Acad.Sci.USA. 1992;89:10178–10182. doi: 10.1073/pnas.89.21.10178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Surmeier DJ, Kitai ST. D1 and D2 dopamine receptor modulation of sodium and potassium currents in rat neostriatal neurons. Prog.Brain Res. 1993;99:309–324. doi: 10.1016/s0079-6123(08)61354-0. [DOI] [PubMed] [Google Scholar]
- 200.Surmeier DJ, Song WJ, Yan Z. Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. J.Neurosci. 1996;16:6579–6591. doi: 10.1523/JNEUROSCI.16-20-06579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Svensson E, Wikstrom MA, Hill RH, Grillner S. Endogenous and exogenous dopamine presynaptically inhibits glutamatergic reticulospinal transmission via an action of D2-receptors on N-type Ca2+ channels. Eur.J.Neurosci. 2003;17:447–454. doi: 10.1046/j.1460-9568.2003.02466.x. [DOI] [PubMed] [Google Scholar]
- 202.Swanson JM, Sunohara GA, Kennedy JL, Regino R, Fineberg E, Wigal T, Lerner M, Williams L, LaHoste GJ, Wigal S. Association of the dopamine receptor D4 (DRD4) gene with a refined phenotype of attention deficit hyperactivity disorder (ADHD): a family-based approach. Mol.Psychiatr. 1998;3:38–41. doi: 10.1038/sj.mp.4000354. [DOI] [PubMed] [Google Scholar]
- 203.Tamminga CA. Partial dopamine agonists in the treatment of psychosis. J.Neural Transm. 2002;109:411–420. doi: 10.1007/s007020200033. [DOI] [PubMed] [Google Scholar]
- 204.Tamminga CA, Carlsson A. Partial dopamine agonists and dopaminergic stabilizers, in the treatment of psychosis. Curr.Drug Targets.CNS.Neurol.Disord. 2002;1:141–147. doi: 10.2174/1568007024606195. [DOI] [PubMed] [Google Scholar]
- 205.Tamminga CA, Cascella NG, Lahti RA, Lindberg M, Carlsson A. Pharmacologic properties of (−)-3PPP (preclamol) in man. J.Neural Transm.Gen.Sect. 1992;88:165–175. doi: 10.1007/BF01244730. [DOI] [PubMed] [Google Scholar]
- 206.Tamminga CA, Gotts MD, Thaker GK, Alphs LD, Foster NL. Dopamine agonist treatment of schizophrenia with N-propylnorapomorphine. Arch.Gen.Psychiatry. 1986;43:398–402. doi: 10.1001/archpsyc.1986.01800040108015. [DOI] [PubMed] [Google Scholar]
- 207.Tamminga CA, Schaffer MH, Smith RC, Davis JM. Schizophrenic symptoms improve with apomorphine. Science. 1978;200:567–568. doi: 10.1126/science.347574. [DOI] [PubMed] [Google Scholar]
- 208.Tang L, Todd RD, O’Malley KL. Dopamine D2 and D3 receptors inhibit dopamine release. J.Pharmacol.Exp.Ther. 1994;270:475–479. [PubMed] [Google Scholar]
- 209.Taylor JR, Lawrence MS, Redmond DE, Jr., Elsworth JD, Roth RH, Nichols DE, Mailman RB. Dihydrexidine, a full dopamine D1 agonist, reduces MPTP-induced parkinsonism in monkeys. Eur.J.Pharmacol. 1991;199:389–391. doi: 10.1016/0014-2999(91)90508-n. [DOI] [PubMed] [Google Scholar]
- 210.Undie AS, Weinstock J, Sarau HM, Friedman E. Evidence for a distinct D1-like dopamine receptor that couples to activation of phosphoinositide metabolism in brain. J.Neurochem. 1994;62:2045–2048. doi: 10.1046/j.1471-4159.1994.62052045.x. [DOI] [PubMed] [Google Scholar]
- 211.Ungerstedt U. Stereotaxic mapping of the monoamine pathways in the rat brain. Acta Physiol Scand.Suppl. 1971;367:1–48. doi: 10.1111/j.1365-201x.1971.tb10998.x. [DOI] [PubMed] [Google Scholar]
- 212.Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, Miller KJ, Spedding M, Mailman RB. Functional selectivity and classical concepts of quantitative pharmacology. J.Pharmacol.Exp.Ther. 2007a;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- 213.Urban JD, Vargas GA, von Zastrow M, Mailman RB. Aripiprazole has Functionally Selective Actions at Dopamine D(2) Receptor-Mediated Signaling Pathways. Neuropsychopharmacology. 2007b;32:67–77. doi: 10.1038/sj.npp.1301071. [DOI] [PubMed] [Google Scholar]
- 214.Urs NM, Daigle TL, Caron MG. A dopamine D1 receptor-dependent beta-arrestin signaling complex potentially regulates morphine-induced psychomotor activation but not reward in mice. Neuropsychopharmacology. 2011;36:551–558. doi: 10.1038/npp.2010.186. PMC3021093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Valjent E, Bertran-Gonzalez J, Herve D, Fisone G, Girault JA. Looking BAC at striatal signaling: cell-specific analysis in new transgenic mice. Trends Neurosci. 2009;32:538–547. doi: 10.1016/j.tins.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 216.van Tol HH, Bunzow JR, Guan HC, Sunahara RK, Seeman P, Niznik HB, Civelli O. Cloning of the gene for a human dopamine D4 receptor with high affinity for the antipsychotic clozapine. Nature. 1991;350:610–614. doi: 10.1038/350610a0. [DOI] [PubMed] [Google Scholar]
- 217.Vanhauwe JF, Josson K, Luyten WH, Driessen AJ, Leysen JE. G-protein sensitivity of ligand binding to human dopamine D2 and D3 receptors expressed in Escherichia coli: clues for a constrained D(3) receptor structure. J.Pharmacol.Exp.Ther. 2000;295:274–283. [PubMed] [Google Scholar]
- 218.Vial D, Piomelli D. Dopamine D2 receptors potentiate arachidonate release via activation of cytosolic, arachidonate-specific phospholipase A2. J.Neurochem. 1995;64:2765–2772. doi: 10.1046/j.1471-4159.1995.64062765.x. [DOI] [PubMed] [Google Scholar]
- 219.Voyno-Yasenetskaya T, Conklin BR, Gilbert RL, Hooley R, Bourne HR, Barber DL. G alpha 13 stimulates Na-H exchange. J.Biol.Chem. 1994;269:4721–4724. [PubMed] [Google Scholar]
- 220.Wang HY, Malbon CC. Probing the physical nature and composition of signalsomes. J.Mol.Signal. 2011;6:1. doi: 10.1186/1750-2187-6-1. PMC3027200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wang HY, Undie AS, Friedman E. Evidence for the coupling of Gq protein to D1-like dopamine sites in rat striatum: possible role in dopamine-mediated inositol phosphate formation. Mol Pharmacol. 1995;48:988–994. [PubMed] [Google Scholar]
- 222.Wang Q, Jolly JP, Surmeier JD, Mullah BM, Lidow MS, Bergson CM, Robishaw JD. Differential dependence of the D1 and D5 dopamine receptors on the G protein gamma 7 subunit for activation of adenylylcyclase. J.Biol.Chem. 2001;276:39386–39393. doi: 10.1074/jbc.M104981200. [DOI] [PubMed] [Google Scholar]
- 223.Welsh GI, Hall DA, Warnes A, Strange PG, Proud CG. Activation of microtubule-associated protein kinase (Erk) and p70 S6 kinase by D2 dopamine receptors. J Neurochem. 1998;70:2139–2146. doi: 10.1046/j.1471-4159.1998.70052139.x. [DOI] [PubMed] [Google Scholar]
- 224.White FJ, Bednarz LM, Wachtel SR, Hjorth S, Brooderson RJ. Is stimulation of both D1 and D2 receptors necessary for the expression of dopamine-mediated behaviors? Pharmacology Biochemistry and Behavior. 1988;30:189–193. doi: 10.1016/0091-3057(88)90442-x. [DOI] [PubMed] [Google Scholar]
- 225.Yan Z, Song WJ, Surmeier J. D2 dopamine receptors reduce N-type Ca2+ currents in rat neostriatal cholinergic interneurons through a membrane-delimited, protein-kinase-C-insensitive pathway. J.Neurophysiol. 1997;77:1003–1015. doi: 10.1152/jn.1997.77.2.1003. [DOI] [PubMed] [Google Scholar]
- 226.Young CE, Yang CR. Dopamine D1/D5 receptor modulates state-dependent switching of soma-dendritic Ca2+ potentials via differential protein kinase A and C activation in rat prefrontal cortical neurons. J.Neurosci. 2004;24:8–23. doi: 10.1523/JNEUROSCI.1650-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Yu PY, Eisner GM, Yamaguchi I, Mouradian MM, Felder RA, Jose PA. Dopamine D1A receptor regulation of phospholipase C isoform. J.Biol.Chem. 1996;271:19503–19508. doi: 10.1074/jbc.271.32.19503. [DOI] [PubMed] [Google Scholar]
- 228.Zamponi GW, Snutch TP. Decay of prepulse facilitation of N type calcium channels during G protein inhibition is consistent with binding of a single Gbeta subunit. Proc.Natl.Acad.Sci.USA. 1998;95:4035–4039. doi: 10.1073/pnas.95.7.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Zhang L, Reith ME. Regulation of the functional activity of the human dopamine transporter by the arachidonic acid pathway. Eur.J.Pharmacol. 1996;315:345–354. doi: 10.1016/s0014-2999(96)00646-2. [DOI] [PubMed] [Google Scholar]
- 230.Zhuang X, Belluscio L, Hen R. G(olf)alpha mediates dopamine D1 receptor signaling. J.Neurosci. 2000;20:RC91. doi: 10.1523/JNEUROSCI.20-16-j0001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]