Significance
Hepatitis C virus (HCV) utilizes various host factors to enter host cells. During the process of HCV entry, cell surface-residing lipoprotein receptors such as scavenger receptor class B member 1 (SR-BI) play important roles through interactions with virus envelope protein E2 and virus-associated apolipoproteins such as apolipoprotein E (ApoE). CD81 plays a crucial role during this process in association with HCV. Here, we identified another pathway for HCV entry that does not use CD81. This pathway involves an association with the very-low-density lipoprotein receptor (VLDLR) and does not require previously reported host factors such as claudin, occludin, or CD81. This finding may shed new light on the process of HCV entry.
Keywords: virus entry, VLDLR, CD81, hypoxia, hepatitis C virus
Abstract
Various host factors are involved in the cellular entry of hepatitis C virus (HCV). In addition to the factors previously reported, we discovered that the very-low-density lipoprotein receptor (VLDLR) mediates HCV entry independent of CD81. Culturing Huh7.5 cells under hypoxic conditions significantly increased HCV entry as a result of the expression of VLDLR, which was not expressed under normoxic conditions in this cell line. Ectopic VLDLR expression conferred susceptibility to HCV entry of CD81-deficient Huh7.5 cells. Additionally, VLDLR-mediated HCV entry was not affected by the knockdown of cellular factors known to act as HCV receptors or HCV entry factors. Because VLDLR is expressed in primary human hepatocytes, our results suggest that VLDLR functions in vivo as an HCV receptor independent of canonical CD81-mediated HCV entry.
Hepatitis C virus (HCV) infects more than 170 million people worldwide and is a major cause of chronic liver disease. The virus persists in 80% of infected individuals and can lead to chronic liver diseases including fibrosis, cirrhosis, steatosis, and hepatocellular carcinoma. HCV, an enveloped positive-stranded virus, enters host cells by using various host factors that function as receptors and mediate endocytosis. Several host factors, including CD81 (1), claudin-1 (CLDN1) (2), occludin (OCLN) (3), and scavenger receptor class B member I (SR-BI) (4), have been identified as receptors. Heparan sulfate glycosaminoglycan represents the first attachment site (5) before the interaction of the virus with these factors. Because all the entry factors are required for productive HCV infection, HCV entry seems to be the result of an orchestrated process involving these factors. Additionally, low-density lipoprotein receptor (LDLR) (6), Niemann-Pic C1-like 1 (NPC1L1) (7), transferrin receptor 1 (TfR1) (8), and epidermal growth factor receptor (EGFR) (9) have been shown to play a role in HCV entry. CD81 was the first factor to be identified as an HCV receptor, and it plays an important role in this process by binding with the HCV envelope glycoprotein E2 (10, 11). Indeed, CD81-deficient cell lines such as HepG2 do not permit the entry of HCV (2, 3).
Recent studies have demonstrated that HCV RNA replication in Huh7.5 cells is enhanced under hypoxic conditions (12). Because the oxygen content in liver tissue in vivo is estimated to be lower (with a gradient of 9–3%) than the oxygen content under in vitro culture conditions (13), the HCV life cycle may differ significantly from that observed using in vitro culture systems. The very-low-density lipoprotein receptor (VLDLR) is induced under hypoxic conditions. In turn, this receptor enhances the uptake of low-density lipoproteins (LDLs) and very-low-density lipoproteins (VLDLs) (14), possibly through the recognition of ligands (such as apolipoprotein) that associate with the lipoproteins (15). VLDLR is a type I transmembrane lipoprotein receptor belonging to the LDLR family (16). The expression of VLDLR increased 4.2-fold and 3.5-fold in HCV cirrhotic and HCV-HCC patients, respectively, as compared with normal controls without liver disease (17). In vitro analysis has shown that during the early stage of infection HCV recognizes lipoprotein receptors such as SR-BI and LDLR on target cells via the association of the virus with apolipoprotein E (ApoE) or other related ligands (18). However, the cell lines that have been widely used for the analysis of HCV infection/replication (i.e., Huh7 and its derivatives) do not express VLDLR under conventional culture conditions (12), thereby preventing analysis of the role of VLDLR in HCV infection.
The HCV particle is a lipo-viro-particle (LPV) that contains lipoprotein components such as triglycerides, apolipoprotein B-100 (ApoB), and ApoE (19, 20). Because hypoxia affects the uptake of lipoproteins and therefore might influence HCV entry and replication, we hypothesized that the HCV life cycle might be influenced by oxygen levels.
Here, we elucidate the presence of a novel HCV entry pathway that uses VLDLR. Under hypoxic conditions, HCV entry into an in vitro cell-culture system was increased by up-regulating VLDLR expression. Moreover, VLDLR-mediated HCV entry was independent of the CD81-mediated HCV entry pathway.
Results
Increase in HCV Infection Under Hypoxic Conditions.
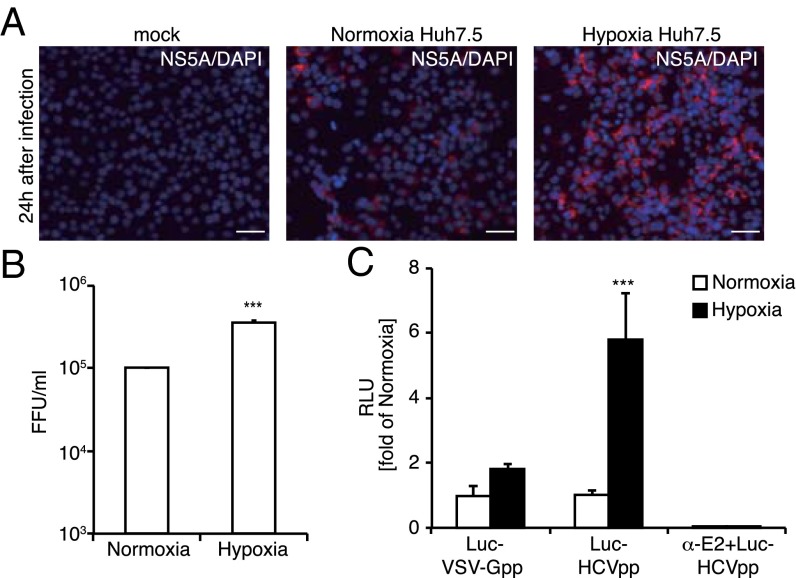
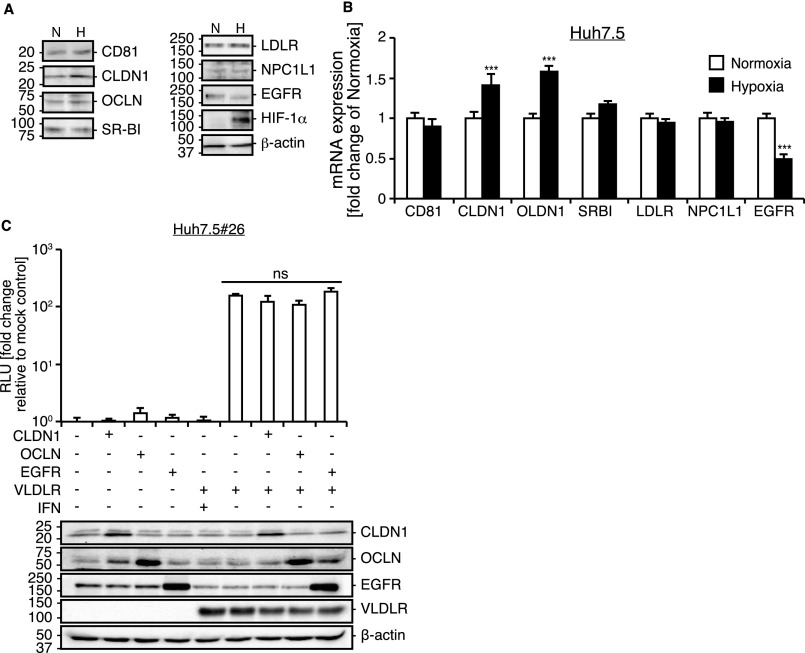
It has been shown that hypoxic conditions enhance HCV replication (12). We analyzed HCV infection in Huh7.5 cells under hypoxic conditions and observed increased infectivity of JFH1 (HCVccJFH1), an infectious HCV clone (Fig. 1A). The HCVccJFH1 titer was approximately threefold higher under hypoxic conditions (Fig. 1B). To analyze whether the increased infection by HCVccJFH1 under hypoxic conditions is dependent not only on postinfection events but also on virus entry events, an HCV entry analysis was performed with luciferase-encoded HCV genotype 2a enveloped pseudoparticles (Luc-HCVpp) constructed with a lentivirus vector system (Fig. 1C). Luc-HCVpp specifically monitor the effects of HCV entry. Compared with vesicular stomatitis virus G protein pseudoparticles (Luc-VSV-Gpp) infection levels, which were unaffected by O2 conditions, the luciferase activity in cells infected with Luc-HCVpp was approximately sixfold higher under hypoxic conditions. Then we analyzed the expression of various factors known to be involved in HCV entry to see if the enhanced virus entry was the consequence of an enhancement of the conventional entry mechanism. Protein and mRNA expression levels of CD81, SR-BI, LDLR, and NPC1L1 were unchanged under hypoxic or normoxic conditions, but expression of CLDN1 and OCLN were slightly increased under hypoxic conditions (Fig. S1 A and B), and EGFR expression was reduced under hypoxic conditions. Because ectopic expression of CLDN1, OCLN, and EGFR did not alter the level of HCV infection (Fig. S1C), it is unlikely that these factors are involved in increased HCV infection under hypoxic conditions. This evidence led us to hypothesize that a yet-to-be-identified receptor or entry factor is involved in HCV entry under hypoxic conditions.
Fig. 1.
Increase in HCV entry under hypoxic conditions. (A) Huh7.5 cells cultured under normoxic or hypoxic conditions (1% O2) were infected with HCVccJFH1 with a multiplicity of infection (MOI) of 1. At 24 h postinfection, the cells were stained with NS5A (red). (Scale bars, 50 μm.) (B) Analysis of HCV infectivity. Huh7.5 cells cultured under normoxic or hypoxic conditions were infected with serially diluted HCVccJFH1 for 24 h. Then HCV-infected cells stained with anti-HCV NS5A antibody were counted to obtain focus-forming units (FFU) (average ± SD; n = 3). (C) The effect of Huh7.5 cells cultured under normoxic or hypoxic conditions on HCV entry. Huh7.5 cells cultured under normoxic (white bar) or hypoxic (black bar) conditions were infected with Luc-VSV-Gpp and Luc-HCVpp (genotype 2a). At 24 h postinfection, luciferase activity was quantified (average ± SD; n = 3). Treatment with the E2 antibody (15 μg/mL) was included as a control. RLU, relative light units. ***P < 0.005 (Student’s t-test).
Fig. S1.
Expression of the HCV receptor genes and proteins under hypoxic conditions. (A) Analysis of protein production in Huh7.5 cells cultured under hypoxic and normoxic conditions. A cell lysate was prepared after culturing for 48 h under each condition. H, hypoxic conditions; N, normoxic conditions. (B) Total RNA was extracted from Huh7.5 cells cultured under normoxic or hypoxic condition for 48 h. Expression of mRNAs previously reported to function as HCV receptor genes was analyzed by qRT-PCR (average ± SD; n = 4). (C) Effect of CLDN1, OCLN, and EGFR on Luc-HCVccJFH1 infection of CD81-defective Huh7.5 cells. Huh7.5#26 cells that lack CD81 expression were transfected with CLDN-, OCLD-, and EGFR-expressing plasmids with or without VLDLR-expressing plasmids. After transfection, cells were infected with Luc-HCVccJFH1. At 48 h postinfection, luciferase activity was quantified (average ± SD; n = 3). Expression levels of each HCV entry factor were analyzed by immunoblotting. ***P < 0.005, ns, not significant (Student’s t-test).
HCV Entry Is Enhanced by the Induced Expression of VLDLR Under Hypoxic Conditions.
Infectious HCV constitutes a complex with lipid components such as triglycerides, ApoB, and ApoE, resulting in the formation of an LVP (19). The association of virus-associated ApoE with lipoprotein receptors on the cell surface is thought to be required for infectivity (21, 22). The uptake of LDL and VLDL is increased in hepatocytes under hypoxic conditions because of the induction of VLDLR expression and the association with ApoE (14, 15). These reports led us to analyze the role of VLDLR in HCV entry.
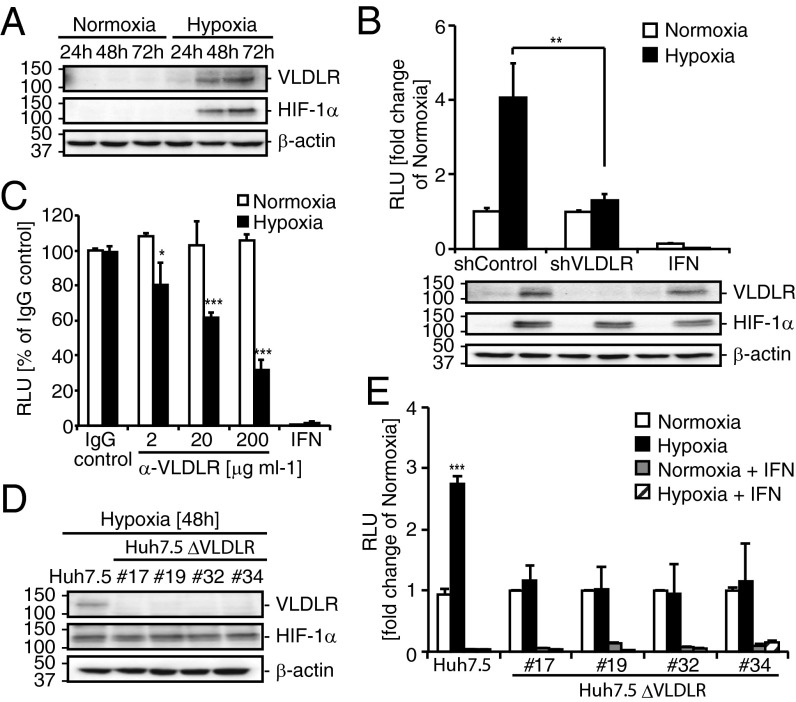
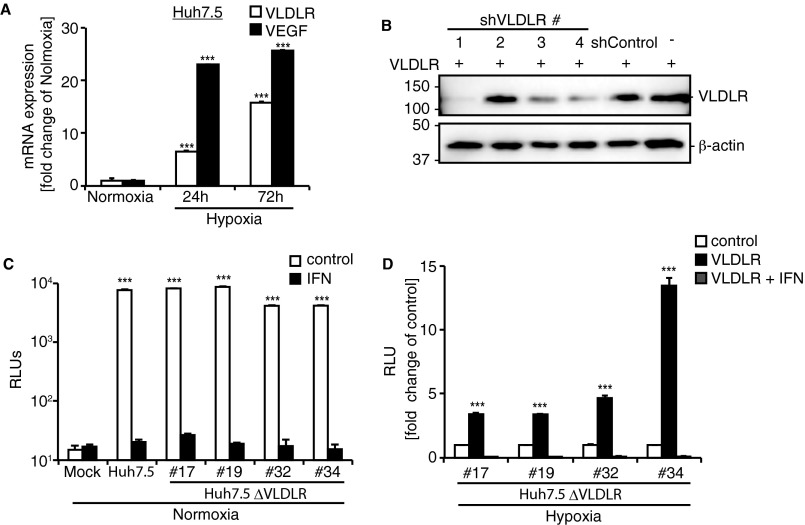
The expression of VLDLR in Huh7.5 cells was induced under hypoxic conditions at the protein (Fig. 2A) and mRNA (Fig. S2A) levels. To test whether VLDLR affects HCV entry, VLDLR was knocked down transiently in Huh7.5 cells using shRNA (Fig. S2B), and the infection of Luc-HCVccJFH1 was verified using siRNA#1 because this cell line had the best knockdown efficiency (Fig. 2B and Fig. S2B). Under hypoxic conditions an approximately threefold reduction in Luc-HCVccJFH1 infection was observed in shVLDLR#1-treated Huh7.5 cells as compared with shControl cells, even though the infection in shVLDLR#1-treated and shControl cells was unchanged under normoxic conditions (Fig. 2B). Moreover, we examined the effect of a VLDLR antibody on HCV infection. The inhibition of Luc-HCVccJFH1 entry was observed in a dose-dependent manner in Huh7.5 cells grown under hypoxic conditions, but no effect was observed in the cells grown under normoxic conditions (Fig. 2C).
Fig. 2.
HCV entry is enhanced by VLDLR under hypoxic conditions. (A) VLDLR, hypoxia-induced factor 1-alpha (HIF-1α), and β-actin levels were analyzed 24, 48, and 72 h after culturing under normoxic or hypoxic conditions. (B) shControl- or shVLDLR#1-transfected Huh7.5 cells were infected with Luc-HCVccJFH1 (MOI = 0.1). Luciferase activity was analyzed 24 h postinfection (average ± SD; n = 3). Treatment with IFN-β (100 IU/mL) was included as a control. The VLDLR knockdown effect was verified by immunoblotting. (C) Hypoxic or normoxic cultured Huh7.5 cells were preincubated with IgG as a control or with anti-VLDLR for 1 h at 37 °C. After treatment, the cells were infected with Luc-HCVccJFH1 (MOI = 0.1) for 24 h (average ± SD; n = 3). (D) Immunoblot analysis of VLDLR, HIF-1α, and β-actin levels 48 h after culture of Huh7.5 or Huh7.5 ΔVLDLR cells (#17, #19, #32, and #34) under hypoxic conditions. (E) Infection of Luc-HCVccJFH1 (MOI = 0.1) in Huh7.5 ΔVLDLR clones cultured under normoxic or hypoxic conditions. Cell lysates were analyzed 24 h after infection (average ± SD; n = 3). The data represent three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.005 (Student’s t-test).
Fig. S2.
Expression of VLDLR in Huh7.5 cells cultured under hypoxic conditions and the effect of VLDLR expression on HCV infection. (A) Total RNA was extracted from Huh7.5 cells cultured under normoxia or hypoxia. VLDLR and VEGF mRNAs were analyzed by qRT-PCR (average ± SD; n = 3). VEGF was used as a positive control for hypoxia. (B) Huh7.5 cells were treated with shControl or various shVLDLRs with different sequences (nos.1, 2, 3, and 4). VLDLR knockdown was analyzed by Western blot. (C) Huh7.5 and Huh7.5 ΔVLDLR clones were infected with Luc-HCVccJFH1 (MOI = 0.1) under normoxic culture conditions. Luciferase activity was analyzed 24 h postinfection (average ± SD; n = 3). (D) Rescue of HCV infection by expression of VLDLR plasmid. Expression plasmid for VLDLR variant 2 was transfected into Huh7.5 ΔVLDLR#17, #19, #32, and #34 cells, followed by infection with Luc-HCVccJFH1 (MOI = 0.1). Luciferase activity was analyzed 24 h postinfection (average ± SD; n = 3). The data shown represent three independent experiments. ***P < 0.005 (Student’s t-test).
To investigate further the role of VLDLR in HCV entry, we established a VLDLR-knockout Huh7.5 cell line (Huh7.5 ΔVLDLR) using the clustered, regularly interspaced short-palindromic-repeat (CRISPR)/Cas9 system targeting a consensus sequence of mRNAs for all VLDLR isoforms (Fig. 2D). All cell clones lacking expression of the VLDLR gene failed to induce VLDLR expression under hypoxic conditions. The ability of HCV to infect each clone was nearly unchanged under normoxic conditions (Fig. S2C). Although none of these cells exhibited increased Luc-HCVccJFH1 infection under hypoxia (Fig. 2E), Luc-HCVccJFH1 infection was rescued by the ectopic expression of VLDLR in all Huh7.5 ΔVLDLR clones, with the level of rescue varying from three- to 13-fold, depending on the clone (Fig. S2D). These results suggest that Luc-HCVccJFH1 infection was increased by the induced expression of VLDLR under hypoxic conditions.
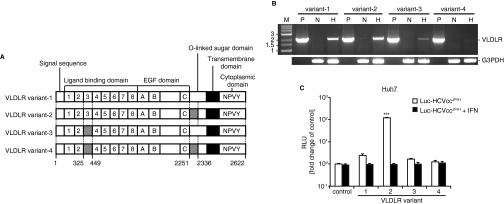
Ectopic Expression of VLDLR Variant 2 Showed the Greatest Entry of Luc-HCVccJFH1 Under Normoxic Conditions.
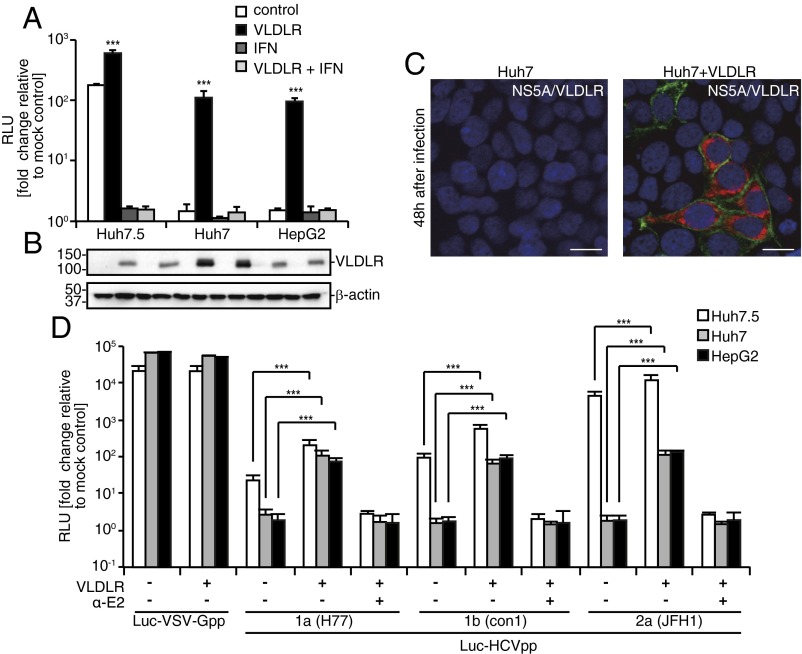
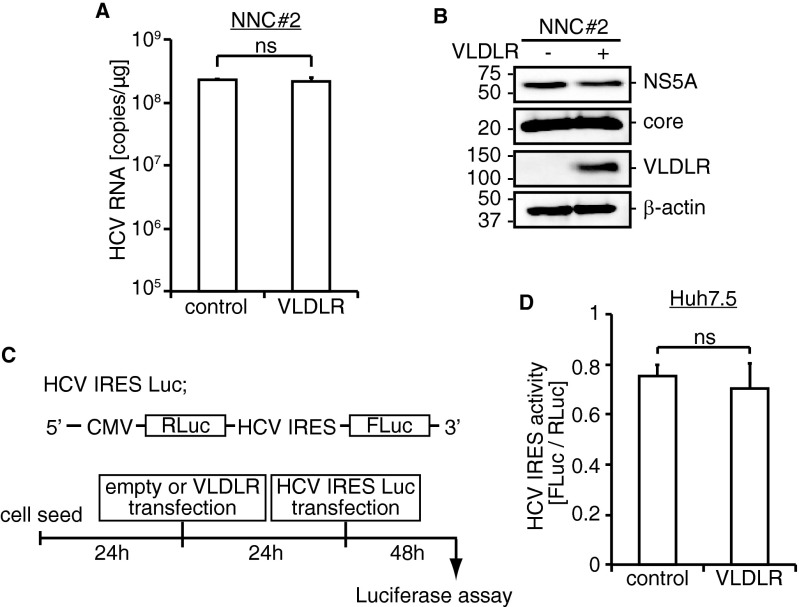
The VLDLR mRNA encodes four splicing variants (Fig. S3A) (23, 24). Variants 1 and 2 were the major variants induced in Huh7.5 cells under hypoxic conditions (Fig. S3B). To test whether ectopic expression of a variant of VLDLR influences HCV infection under normoxia, Huh7 cells were transfected with plasmids expressing VLDLR variants 1–4 followed by challenge with Luc-HCVccJFH1. Cells transfected with VLDLR variant 2 showed the highest Luc-HCVccJFH1 infection; cells transfected with the other variants showed only a marginal increase in Luc-HCVccJFH1 infection (Fig. S3C). To analyze the effect of VLDLR variant 2 on other cells under normoxic conditions, Huh7.5, HepG2, and Huh7 cells were transfected with a plasmid expressing VLDLR variant 2 followed by Luc-HCVccJFH1 infection (Fig. 3A). The VLDLR expression levels are shown in Fig. 3B. Luciferase activity was increased fivefold in Huh7.5 cells expressing VLDLR (Fig. 3A), and Huh7 and HepG2 cells expressing VLDLR showed a 100-fold and 95-fold increase, respectively. HCVccJFH1 infection was detected by immunostaining in Huh7 cells expressing ectopic VLDLR (Fig. 3C). To analyze the effect of VLDLR on HCV replication, we assessed the levels of HCV RNA and HCV proteins in HCV full-length RNA replicon cells, NNC#2, with or without the expression of VLDLR. We found no differences in the level of HCV RNA and proteins (Fig. S4 A and B). Moreover, the activity of the HCV internal ribosome entry site (IRES) examined by the HCV IRES-luc plasmid was not affected by VLDLR (Fig. S4 C and D). Subsequently, using the Luc-HCVpp lentivirus vector system, we analyzed whether VLDLR-dependent infection was affected by the HCV genotype. VLDLR did not affect infection by Luc-VSV-Gpp. However, Luc-HCVpp infection was increased irrespective of HCV genotype in VLDLR-expressing cells (Fig. 3D). Therefore, we think that the increase in HCV infection was caused by the enhanced entry of HCV resulting from VLDLR expression.
Fig. S3.
Effect of VLDLR variants on HCV infection. (A) Schematic drawing of the VLDLR splice variants. (B) The expression level of each variant was analyzed in Huh7.5 cells by RT-PCR. H, cells cultured under hypoxic conditions; N, cells cultured under normoxic conditions; P, plasmid control. G3PDH was used as an internal control. (C) Effect of the ectopic expression of VLDLR variants on HCV infection. Huh7 cells were transfected with each VLDLR-expressing plasmid (variants 1–4). After transfection, the cells were infected with Luc-HCVccJFH1 (MOI = 0.1) for 48 h (average ± SD; n = 4). The data shown represent three independent experiments. ***P < 0.005 (Student’s t-test).
Fig. 3.
Ectopic expression of VLDLR increased HCV infection even under normoxic conditions. (A) Cells transfected with a VLDLR-expressing or empty plasmid were infected with Luc-HCVccJFH1 (MOI = 0.1). Luciferase activity was analyzed 48 h postinfection (average ± SD; n = 3). (B) Immunoblot of VLDLR and β-actin. (C) Huh7 cells transfected with VLDLR-expressing or empty plasmid were infected with HCVccJFH1 (MOI = 1). The cells were stained for NS5A (red) and VLDLR (green) 48 h postinfection. Images were analyzed by confocal microscopy. (Scale bars, 20 μm.) (D) Cells transfected with an empty or VLDLR-expressing plasmid were infected with luciferase-encoding pseudoparticles bearing the indicated envelopes. Luciferase activity was analyzed 72 h postinfection (average ± SD; n = 3). The data shown represent three independent experiments. ***P < 0.005 (Student’s t-test).
Fig. S4.
Effect of VLDLR on HCV replication. (A) NNC#2 cells were transfected with empty or VLDLR plasmids. Forty-eight hours posttransfection HCV RNA was quantified (average ± SD; n = 3). (B) The protein expression level was analyzed by Western blotting. (C) Schematic of HCV IRES Luc plasmid and experimental schedule. (D) VLDLR- or empty plasmid-transfected Huh7.5 cells were transfected with the HCV IRES Luc plasmid. At 48 h after transfection, HCV IRES activity was analyzed by a dual luciferase assay (average ± SD; n = 3); ns, not significant (Student’s t-test).
VLDLR-Mediated HCV Entry Requires HCV E2 and ApoE.
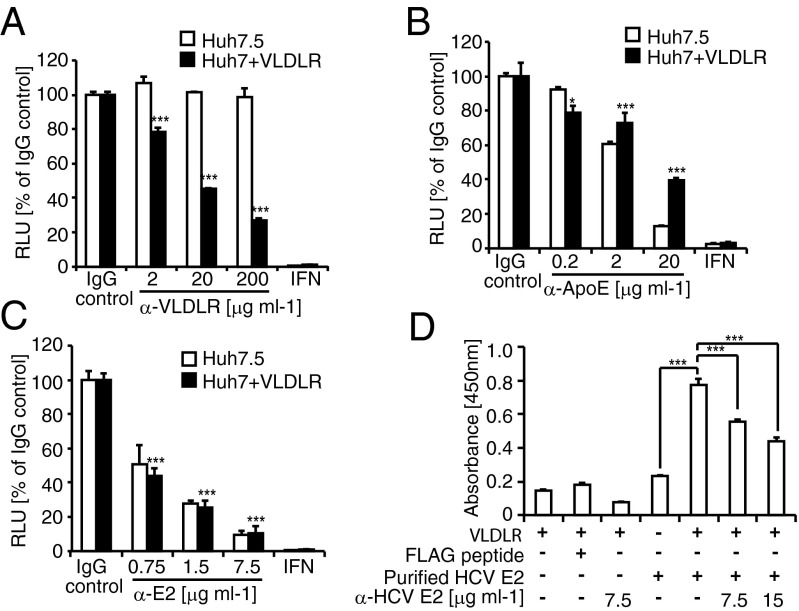
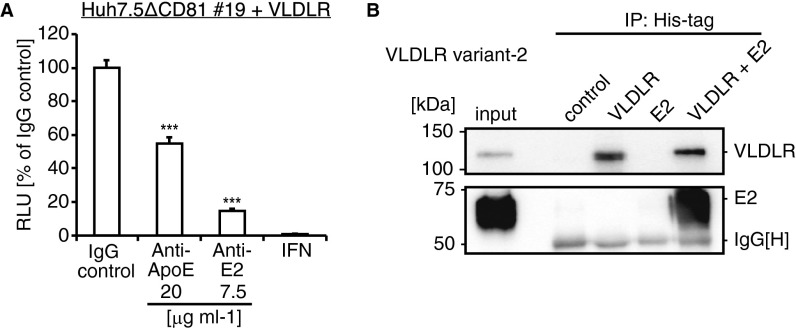
To clarify the role of VLDLR in HCV entry, we examined the effect of ApoE, a ligand for VLDLR, and HCV E2. The HCVccJFH1 infection of VLDLR-expressing Huh7 cells was inhibited by the dose dependence of the anti-VLDLR antibody, whereas no effect was observed in Huh7.5 cells (Fig. 4A). This finding confirms that VLDLR is an HCV entry factor. Next, we tested the inhibition of HCV infection by antibodies directed against ApoE and HCV E2. Both antibodies suppressed Luc-HCVccJFH1 luciferase activity in a dose-dependent manner in Huh7.5 cells and in Huh7 cells expressing VLDLR (Fig. 4 B and C). The effect of ApoE and HCV E2 on VLDLR-dependent HCV infection also was examined using CD81-knockout VLDLR-bearing Huh7.5 cells. HCV infection in the cells also was inhibited by the antibodies against ApoE and HCV E2 (Fig. S5A). The binding of VLDLR to HCV E2 was observed by ELISA using recombinant VLDLR and purified HCV E2 (Fig. 4D). This binding was specific, because it was competitively inhibited by the addition of an HCV E2 antibody (Fig. 4D). Additionally, VLDLR and HCV E2 interaction was confirmed by an immunoprecipitation experiment (Fig. S5B). These results suggest that ApoE and HCV E2 play roles in VLDLR-mediated HCV entry.
Fig. 4.
Effect of HCV E2 and ApoE on VLDLR-mediated HCV entry. (A) Huh7.5 or Huh7 cells transfected with a VLDLR-expressing plasmid were preincubated with IgG as a control or with anti-VLDLR for 1 h at 37 °C. (B and C) After treatment, the cells were infected with Luc-HCVccJFH1 (MOI = 0.1) for 48 h (average ± SD; n = 3). Luc-HCVccJFH1 was preincubated with an IgG control or anti-ApoE (B) and anti-HCV E2 (C). Huh7.5 or Huh7 cells transfected with a VLDLR-expressing plasmid were infected with antibody-treated Luc-HCVccJFH1 (MOI = 0.1) for 48 h (average ± SD; n = 3). (D) Recombinant VLDLR-coated plates were reacted with purified HCV E2 or with purified HCV E2 treated with anti-E2 antibody. The signal was detected using an anti-FLAG antibody and HRP-conjugated mouse IgG. Light absorbance was measured at 450 nm (average ± SD; n = 3). The data shown represent three independent experiments. *P < 0.05, ***P < 0.005 (Student’s t-test).
Fig. S5.
ApoE and HCV E2 are important for VLDLR-mediated HCV entry. (A) Luc-HCVccJFH1 (MOI = 0.1) was preincubated with IgG or anti-ApoE and anti-E2 for 1 h at 37 °C and then infected to CD81-knockout Huh7.5 #19 (Huh7.5ΔCD81 #19) cells bearing VLDLR for 48 h (average ± SD; n = 3). (B) 293FT cells were transfected with His-tagged VLDLR variant 2 and FLAG-tagged HCV E2. The cell lysates were immunoprecipitated with anti-His antibody–conjugated beads 48 h posttransfection. Immunoblots were performed with anti-VLDLR and anti-FLAG M2 antibodies. The data shown represent three independent experiments. ***P < 0.005 (Student’s t-test).
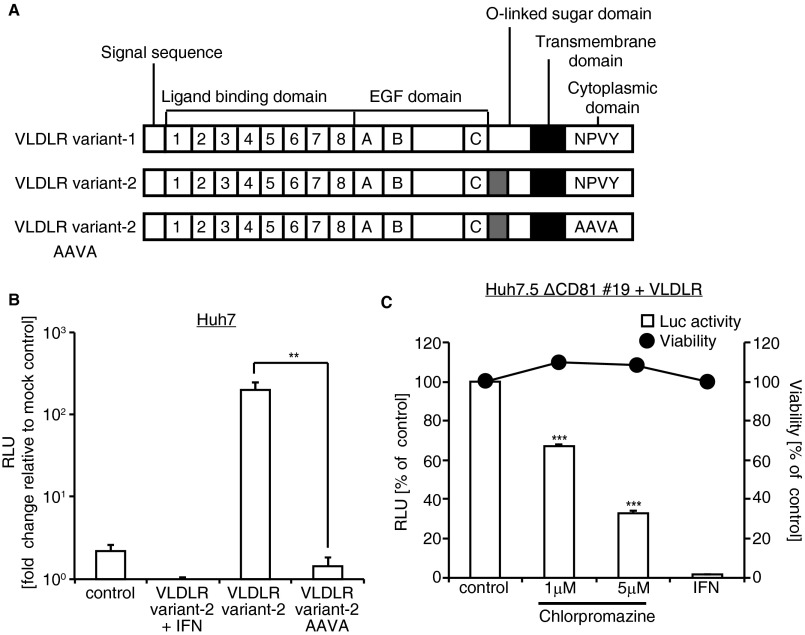
The NPVY domain of VLDLR is important for clathrin-dependent endocytosis (16). To ascertain the role of VLDLR-mediated endocytosis in HCV entry, we analyzed HCV entry in cells expressing a VLDLR with the NPVY motif in the VLDLR cytoplasmic domain mutated to AAVA (Fig. S6A). The mutated VLDLR did not allow the entry of Luc-HCVccJFH1 (Fig. S6B). Furthermore, treatment with chlorpromazine, an inhibitor of clathrin-dependent endocytosis, reduced Luc-HCVccJFH1 infection in VLDLR-expressing Huh7.5ΔCD81#19 cells (Fig. S6C). Thus, HCV uses clathrin-dependent endocytosis via VLDLR to enter the target cells.
Fig. S6.
VLDLR mediates HCV entry via clathrin-dependent endocytosis. (A) Schematic drawing of VLDLR variants 1 and 2 with amino acid mutations in the cytoplasmic domain. The NPVY amino acid residues were mutated to AAVA in VLDLR variant 2. (B) VLDLR variant 2 AAVA does not affect HCV infection. Huh7 cells were transfected with VLDLR variant 2 or VLDLR variant 2 AAVA. Twenty-four hours after transfection, the cells were infected with Luc-HCVccJFH1 for 48 h (average ± SD; n = 3). (C) VLDLR-transfected Huh7.5ΔCD81#19 cells were pretreated with chlorpromazine. After treatment, the cells were infected with Luc-HCVccJFH1 for 48 h (average ± SD; n = 3). Cell viability (filled circle) was analyzed using the CellTiter-Glo assay. The data shown represent three independent experiments. **P < 0.01, ***P < 0.005 (Student’s t-test).
VLDLR-Mediated HCV Entry Does Not Require Known HCV Receptors and Entry Factors.
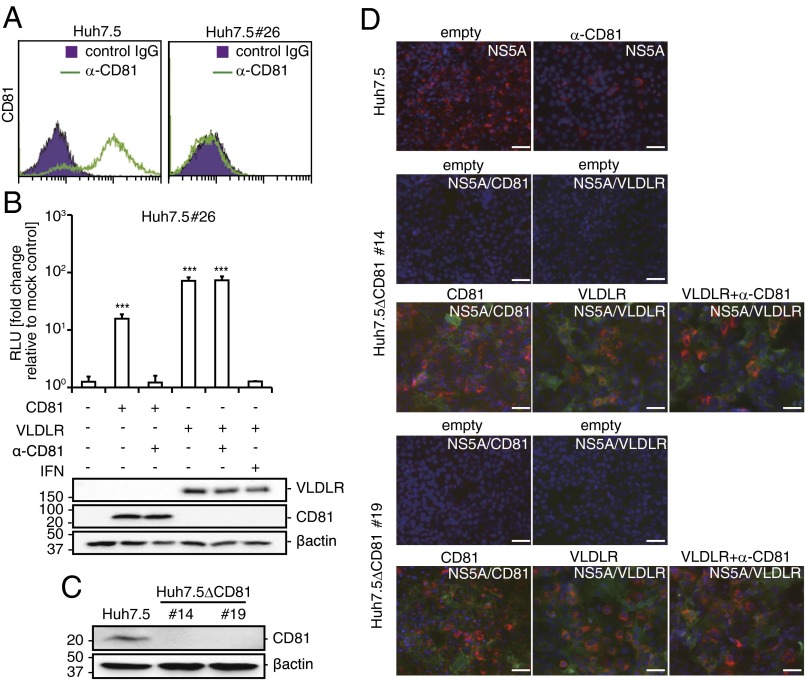
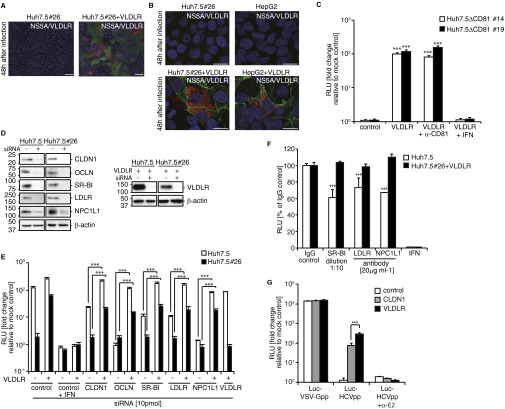
To determine whether VLDLR-mediated HCV entry is CD81 dependent, we examined HCV infection in CD81-deficient Huh7.5 (Huh7.5#26) cells ectopically expressing VLDLR. The CD81-deficient Huh7.5#26 cell line was established from an analysis of Huh7.5 cells that showed a resistant phenotype to HCV infection. The low level of CD81 expression in Huh7.5#26 cells was confirmed by flow cytometry (Fig. 5A). As expected, HCV infection of Huh7.5#26 cells was not observed using immunofluorescence (Fig. S7A). However, a 70-fold increase in Luc-HCVccJFH1 infection was evident in Huh7.5#26 cells expressing VLDLR (Fig. 5B). HCV infection was confirmed by immunostaining (Fig. S7 A and B). More importantly, similar results were observed in VLDLR-expressing HepG2 cells that were CD81 deficient (Fig. S7B). Moreover, we established Huh7.5ΔCD81 clones #14 and #19 using the CRISPR method (Fig. 5C). These clones do not express VLDLR when grown under normoxic conditions and are resistant to HCV infection. However, they became susceptible to HCV infection when transduced with CD81 or VLDLR (Fig. 5D). Importantly, HCV infection in VLDLR-expressing Huh7.5ΔCD81#14 and #19 cells was not affected by CD81 antibody treatment (Fig. S7C). These results clearly indicate that VLDLR-mediated HCV infection is CD81 independent. Next, the requirement for the previously identified HCV receptors and entry factors in VLDLR-mediated HCV entry was analyzed by knockdown of each factor by siRNA. The target siRNA sequences are shown in Table S1. The siRNA knockdown efficiency was confirmed by Western blotting (Fig. S7D). We observed that knockdown of the factors CLDN1, OCLN, SR-BI, LDLR, and NPC1L1 did not suppress Luc-HCVccJFH1 entry into VLDLR-expressing Huh7.5 and Huh7.5#26 cells (Fig. S7E). However, in the absence of exogenous VLDLR expression, as expected, the inhibition of Luc-HCVccJFH1 infection ranging from 20–40% was observed in Huh7.5 cells after treatment with antibodies against SR-BI, LDLR, and NPC1L1 (Fig. S7F). Luciferase activity in VLDLR-expressing Huh7.5#26 cells that lacked CD81 expression after infection by Luc-HCVccJFH1 was not suppressed by treatment with these antibodies. Moreover, VLDLR-mediated Luc-HCVpp entry was observed in CLDN1-deficient 293FT cells (Fig. S7G). These results suggest that SR-BI, LDLR, NPC1L1, and CLDN1 are not directly involved in VLDLR-mediated HCV infection.
Fig. 5.
VLDLR-mediated HCV entry using a CD81-independent pathway. (A) Huh7.5 or Huh7.5#26 cells were stained for CD81 (solid green line). The purple area indicates isotype-control staining. (B) Huh7.5#26 cells transfected with an empty, CD81-, or VLDLR-expressing plasmid were infected with Luc-HCVccJFH1 (MOI = 0.1). CD81 antibody (10 μg/mL) or IFN-β (100 IU/mL) was used to pretreat or treat cells, respectively. Luciferase activity was measured after 48 h (average ± SD; n = 3). VLDLR and CD81 expression levels were analyzed by immunoblotting. (C) Expression of CD81 in Huh7.5, Huh7.5ΔCD81#14, and Huh7.5ΔCD81#19 cells were verified by immunoblotting. (D) Huh7.5, Huh7.5ΔCD81#14, and Huh7.5ΔCD81#19 cells transfected with empty, CD81-, or VLDLR-lentiviral vector were infected with HCVccJFH1 (MOI = 1). The CD81 antibody (10 μg/mL) was pretreated for 1 h at 37 °C. The cells were stained for NS5A (red) and VLDLR (green) or CD81 (green) 48 h postinfection. Images were analyzed by fluorescent microscopy. (Scale bars, 50 μm.) The data shown represent three independent experiments. ***P < 0.005 (Student’s t-test).
Fig. S7.
VLDLR-mediated HCV infection is productive. (A) Huh7.5#26 cells transfected with an empty or VLDLR lentiviral vector were infected with HCVccJFH1 (MOI = 1). The cells were stained with NS5A (red) and VLDLR (green) antibodies 48 h postinfection. (Scale bars, 50 μm.) (B) Huh7.5#26 and HepG2 cells transfected with an empty or VLDLR lentiviral vector were infected with HCVccJFH1 (MOI = 1). The cells were stained with NS5A (red) and VLDLR (green) antibodies 48 h postinfection. The images were analyzed by confocal microscopy. (Scale bars, 20 μm.) (C) Huh7.5ΔCD81#14 and Huh7.5ΔCD81#19 cells transfected with an empty or VLDLR-expressing plasmid were infected with Luc-HCVccJFH1 (MOI = 0.1). CD81 antibody (10 μg/mL) was preincubated for 1 h at 37 °C. Luciferase activity was measured after 48 h (average ± SD; n = 3). (D) The effect of siRNA knockdown of each gene was assessed by immunoblotting. (E) Huh7.5 and Huh7.5#26 cells treated with siRNA for each gene were infected with Luc-HCVccJFH1 (MOI = 0.1) for 48 h. The cells were transfected with an empty vector or a VLDLR-expressing plasmid 24 h before infection (average ± SD; n = 3). (F) Suppression of Luc-HCVccJFH1 entry by antibodies against SR-BI, LDLR, and NPC1L1. Huh7.5 and VLDLR-transduced Huh7.5#26 cells were treated with the antibodies for 1 h. After treatment, the cells were infected with Luc-HCVccJFH1 for 48 h (average ± SD; n = 3). (G) 293FT cells were transfected with CLDN1- or VLDLR-expressing plasmid. At 24 h Luc-HCVpp or Luc-VSV-Gpp were infected for 72 h (average ± SD; n = 3). The data shown represent three independent experiments. ***P < 0.005 (Student’s t-test).
Table S1.
Primers and siRNA sequences
| Primer | Sequence | |
| Expression plasmid primers | ||
| hVLDLR | CACCATGGGCCTCCCCGAGCCGGGCCC | TCAAGCTAGATCATCATCTGTGCTTAC |
| mVLDLR | CACCATGGGCACGTCCGCGCGCTGGGCGCTGT | TCAAGCCAGATCATCATCTGTGCTTACAACTG |
| CRISPR VLDLR | CACCGGCTGCTGCTCGCGCTGTGC | AAACGCACAGCGCGAGCAGCAGCC |
| CRISPR CD81 1 | CACCATCTACATCCTCATCGCTGT | AAACACAGCGATGAGGATGTAGAT |
| CRISPR CD81 2 | CACCGGACGGTAAGGCAGGGAGGC | AAACGCCTCCCTGCCTTACCGTCC |
| RT-PCR primers | ||
| GAPDH | AGGTCGGTGTGAACGGATTTG | TGTAGACCATGTAGTTGAGGTCA |
| CD81 | ACCTCCTGTATCTGGAGCTGG | TTGGCGATCTGGTCCTTGTTG |
| SR-BI | TCGCAGGCATTGGACAAACT | CTCCTTATCCTTTGAGCCCTTTT |
| CLDN1 | GTGGAGGATTTACTCCTATGCCG | ATCAAGGCACGGGTTGCTT |
| OCLN | TCAAACCGAATCATTATGCACCA | AGATGGCAATGCACATCACAA |
| NPC1L1 | TATGGTCGCCCGAAGCA | TGCGGTTGTTCTGGAAATACTG |
| VLDLR | CGCCTCTATTGGCTTGATTCTAA | TCCTACGATCTTGGCCATTCA |
| VEGF | CTTGCCTTGCTGCTCTACC | CACACAGGATGGCTTGAAG |
| EGFR | GCGTCTCTTGCCGGAATG | GGCTCACCCTCCAGAAGGTT |
| VLDLR V1 | CCAGAACAGTGCCATATGAGAACA | |
| VLDLR V2 | AGTTGCAGTACTTTGACAGTCTCG | |
| VLDLR V3 | CACATTGATCCTTTGACAGTCTCG | |
| VLDLR V4 | CCAGAACAGTGCCGCAATATAACA | |
| Taq-Man PCR primer | ||
| HCV primer | CCCTCCCGGGAGAGCCATAGTG | GTCTCGCGGGGGCACGCCCAAAT |
| Taq-Man probe | 6-carboxyfluorescent(FAM)-TCTGCGGAACCGGTGAGTACAC-BHQ1 | |
| siRNA sequence | ||
| SR-BI | GCAGCAGGUCCUUAAGAAC | |
| LDLR | GGACAGAUAUCAUCAACGA | |
| CLDN1 | UAACAUUAGGACCUUAGAA | |
| OCLN | UAACGAGGCUGCCUGAAGUCAUCCA | |
| VLDLR | AUUCGUUUAUAUGACACUC | |
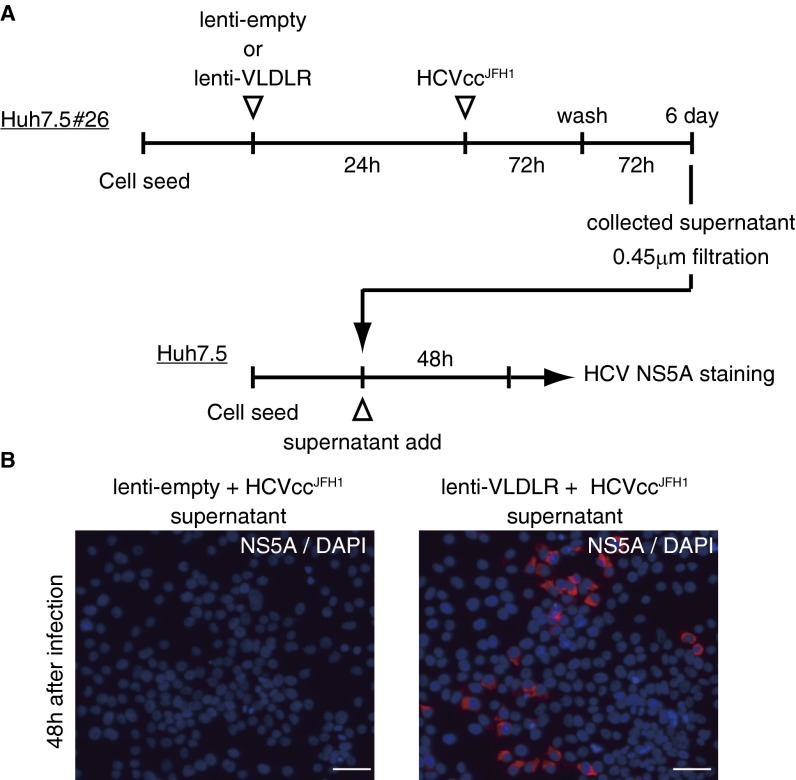
Finally, we investigated whether VLDLR-mediated HCV entry resulted in abortive or productive infection. Supernatants were recovered from VLDLR-expressing Huh7.5#26 cells infected with HCVccJFH1 and were applied to Huh7.5 cells to analyze infection (Fig. S8 A and B). Infected cells were observed by confocal microscopy, indicating that VLDLR-mediated HCV entry into the cells culminates in productive release.
Fig. S8.
VLDLR-mediated HCV infection culminates in productive release. (A) Schematic schedule of the productive infection assay. (B) Culture medium was harvested 6 d after Huh7.5 cells were infected with supernatants from HCVccJFH1-infected Huh7.5#26 cells. The cells were stained with NS5A (red) 48 h postinfection. (Scale bars, 50 μm.) The data shown represent three independent experiments.
Mouse VLDLR Is Capable of Mediating HCV Entry.
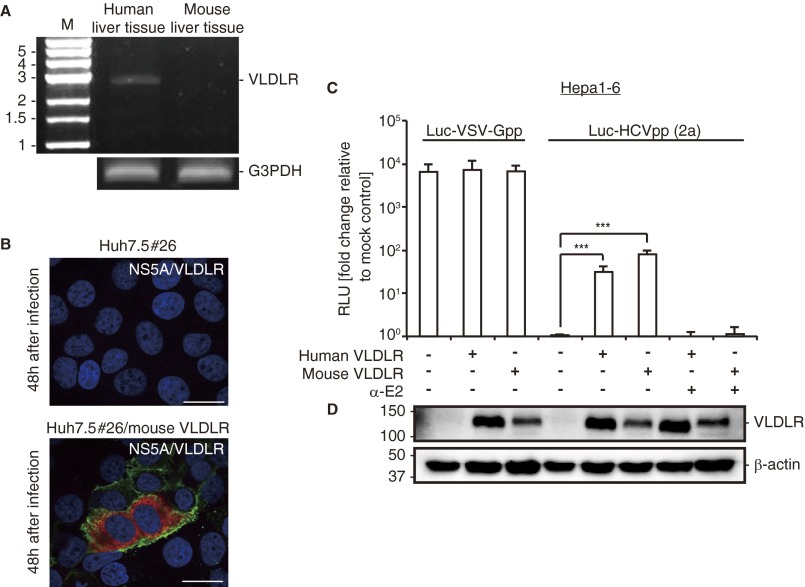
Mouse hepatocytes become permissive for HCV entry when human CD81 and OCLN are expressed (3). However, it is likely that other factors expressed in mouse hepatocytes can be substituted and can function cooperatively in HCV entry. Transgenic mice expressing human CD81 and OCLN also support HCV entry (25). The discovery of the involvement of mouse VLDLR in HCV entry in Huh7.5#26 cells raised the question of whether HCV entry into mouse hepatocytes occurs exclusively via the CD81-dependent pathway. VLDLR expression was not observed in the mouse liver (26), and we confirmed this result (Fig. S9A). Thus, it is possible that the mouse liver does not take in HCV via the VLDLR pathway. However, a potential role for mouse VLDLR as a HCV receptor cannot be ruled out completely. To analyze this issue further, we molecularly cloned the mouse ortholog of the VLDLR gene and analyzed its function in HCV infection by ectopic expression in Huh7.5#26 cells that lack expression of endogenous VLDLR (Fig. S9B). HCVcc infection was observed in mouse VLDLR-transfected Huh7.5#26 cells (Fig. S9B). VLDLR expression was not observed in the mouse Hepa1-6 cell line (Fig. S9D). However, expression of mouse VLDLR in Hepa1-6 cells enabled the entry of Luc-HCVpp without affecting the entry of the Luc-VSV-Gpp control (Fig. S9 C and D). Therefore, we propose that the lack of HCV infection in mouse cells in the absence of the human CD81 and OCLN genes results from a lack of sufficient expression of VLDLR and that HCV infection may occur through the VLDLR pathway if VLDLR expression is induced by environmental stimuli.
Fig. S9.
Mouse VLDLR promotes HCV entry. (A) VLDLR expression was analyzed by RT-PCR using cDNAs from human and mouse liver tissue. G3PDH was used as an internal control. (B) Huh7.5 #26 cells transfected with empty or mouse VLDLR-expressing plasmid were infected with HCVccJFH1 (MOI = 1). The cells were stained with NS5A (red) and VLDLR (6A6) (green) antibodies 48 h postinfection. The images were analyzed by confocal microscopy. (Scale bars, 20 μm.) (C) Hepa1-6 cells (mouse hepatocytes) transfected with an empty vector, a human VLDLR-expressing plasmid, or a mouse VLDLR-expressing plasmid were infected with Luc-VSV-Gpp or Luc-HCVpp (2a). The luciferase activity was analyzed 72 h postinfection (average ± SD; n = 3). (D) Human and mouse VLDLR expression in Hepa1-6 cells was assessed by immunoblotting. The data shown represent three independent experiments. ***P < 0.005 (Student’s t-test).
VLDLR-Mediated HCV Entry Occurs in Primary Human Hepatocytes.
The molecular mechanism of HCV entry was revealed using an in vitro HCV infection/replication system that is dependent primarily on the use of Huh7.5 and related cell lines. As described here, VLDLR is not expressed in Huh7.5 cells under normal culture conditions. Therefore, the role of VLDLR under physiological conditions has not been fully demonstrated.
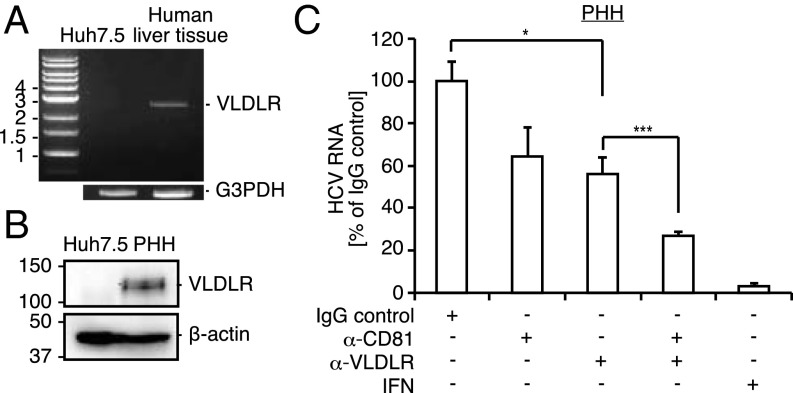
To investigate the significance of VLDLR-mediated HCV entry in vivo, we analyzed the expression of VLDLR in cDNA derived from human liver specimens (Fig. 6A). Additionally, VLDLR protein expression levels were analyzed in primary human hepatocytes (PHH) derived from urokinase-type plasminogen activator severe-combined immunodeficiency (uPA/SCID) mice bearing human hepatocytes (Fig. 6B). VLDLR mRNA and protein expression were not observed in Huh7.5 cells but were observed in human liver tissue and PHHs. Thus, VLDLR is expressed in the liver under physiological conditions. Next, we investigated whether VLDLR is used for HCV entry in PHHs by adding a VLDLR antibody during infection (Fig. 6C). The VLDLR antibody inhibited the entry of HCVccJFH1 by 45% (Fig. 6C). Moreover, cotreatment with CD81 and VLDLR antibodies blocked the entry of HCVccJFH1 by 75% (Fig. 6C). These results suggest the involvement of VLDLR-mediated HCV entry under physiological conditions.
Fig. 6.
VLDLR-mediated HCV entry occurs in PHHs. (A) Expression of VLDLR in human liver tissue cDNA and Huh7.5 mRNA was analyzed by PCR or RT-PCR, respectively. G3PDH was used as the internal control. (B) VLDLR expression in Huh7.5 cells and PHHs was assessed by immunoblotting. (C) PHHs was preincubated with anti-VLDLR (20 μg/mL), anti-CD81 (1 μg/mL), anti-VLDLR (20 μg/mL) + anti-CD81 (1 μg/mL), or an IgG control for 1 h at 37 °C before infection with HCVccJFH1 (MOI = 1). HCV RNA was quantified by quantitative RT-PCR (qRT-PCR) 72 h postinfection (average ± SD; n = 3). The data shown represent three independent experiments. *P < 0.05, ***P < 0.005 (Student’s t-test).
Discussion
The process of HCV entry into a target cell uses various host factors that seem to function via an orchestrated mechanism because infectivity is severely suppressed by the knockdown of any of these factors. The role of CD81 (to our knowledge the first identified HCV entry factor) in this process has been well characterized. CD81 interacts with the HCV E2 protein and SR-BI during an early stage of infection. Knockdown of CD81 or the use of CD81-deficient cells abolishes HCV infection, thereby demonstrating the importance of this molecule in HCV infection. During analysis of HCV entry using the Huh7.5 cell line (the cells most susceptible to HCV infection), we noticed that this cell line lacks expression of VLDLR. However, VLDLR expression was increased in Huh7.5 cells cultured under hypoxic conditions, leading us to analyze the role of this molecule in HCV infectivity.
The induced expression of VLDLR under hypoxic conditions increased HCV infectivity. Importantly, we found that the VLDLR-mediated HCV entry pathway was independent of CD81. In fact, HCV could enter Huh7.5 cells that lacked CD81 expression when VLDLR was ectopically expressed. HCV infection using VLDLR does not require CD81 and also does not require other factors that previously were demonstrated to function as host factors for HCV infection, because there was no reduction in infection following the knock down of CLDN1, OCLN, SR-BI, LDLR, and NPC1L1 in Huh7.5 or Huh7.5#26 cells transduced with VLDLR (Fig. S7E). This result suggests that the mechanism of VLDLR-mediated HCV infection is different from previously reported mechanisms (18).
There are several isoforms of VLDLR, variants 1–4. Variants 1, 2, and 3 were expressed under hypoxic but not normoxic conditions in Huh7.5 cells at different levels of expression, with the highest expression detected for variant 2 (Fig. S3B). Ectopic expression of VLDLR variant 2 induced the highest susceptibility to HCV infection (Fig. S3C). To determine whether VLDLR-dependent signaling plays a role in HCV infection, we generated mutants of VLDLR variant 2 (Fig. S6A). The conversion of the NPVY motif in the cytoplasmic domain of VLDLR to AAVA resulted in a striking reduction in HCV infection (Fig. S6B). Furthermore, chlorpromazine, an inhibitor for clathrin-mediated endocytosis, inhibited VLDLR-mediated HCV infection (Fig. S6C). These data strongly suggest that HCV entry uses VLDLR signaling and endocytosis.
Similar to SR-BI, VLDLR variant 2 interacted with the HCV E2 protein (Fig. S5B).
VLDLR-mediated HCV entry requires HCV E2 (Figs. 3D and 4C). Anti-ApoE suppressed VLDLR-mediated HCVcc entry (Fig. 4B). Because VLDLR binds to all types of ApoE isoforms, the interaction of ApoE with VLDLR may facilitate the entry of HCV. However, the precise role of ApoE in VLDLR-dependent HCV entry should be clarified further.
It is not known whether VLDLR-mediated HCV infection occurs along with CD81-dependent infection under physiological conditions in humans.
In addition to the mouse primary hepatocytes, we observed the expression of VLDLR in cDNA derived from normal human liver tissues and human hepatocytes derived from uPA/SCID mice expressing human hepatocytes (27). Furthermore, 55% of HCV entry into human hepatocytes derived from uPA/SCID mice was blocked following treatment with an anti-VLDLR antibody (Fig. 6C).
The expression of VLDLR mRNA in normal human liver specimens and the increased expression of VLDLR in HCV-infected individuals raise the possibility that VLDLR-mediated entry of HCV occurs under physiological conditions. Furthermore, because VLDLR expression is induced to variable degrees by environmental stimuli such as endoplasmic reticulum (ER) stress (28), the degree of VLDLR-dependent HCV entry compared with CD81-mediated entry may be affected by various factors in different individuals. A detailed analysis of this possibility warrants further investigation to obtain a conclusive result.
In summary, VLDLR is a novel HCV receptor and constitutes an HCV entry pathway that is distinct from the CD81-dependent pathway. VLDLR expression in hepatocytes is induced under hypoxic conditions. ER stress also induces VLDLR expression in hepatocytes in vivo (28). Thus, we can speculate that HCV infection of individuals is affected by environmental conditions that alter the hepatocyte physiology. In this regard, clarification of the mechanism of the VLDLR-dependent entry of HCV may be relevant to therapeutic approaches.
Materials and Methods
For details of antibodies and reagents, plasmids, RNAi, infection with HCVpp and HCVcc, assays of infectivity and HCV IRES activity, RT-PCR, ELISA, immunostaining, and statistical analyses, please see SI Materials and Methods.
Huh7.5, Huh7, HepG2, 293FT, NNC#2 (HCV full-length replicon genotype 1b) (29), Huh7.5#26, Huh7.5 ΔVLDLR (clones #17, #19, #32, and #34), and Hepa1-6 cells were used in this study. Huh7.5#26 cells (CD81-deficient Huh7.5 cell as to Huh7.5#26) were obtained by screening for an HCVccJFH1-resistant phenotype. VLDLR-knockout Huh7.5 cells and CD81-knockout Huh7.5 cells were isolated using the CRISPR/Cas9 knockout system. Hepa1-6, a mouse liver cell line, was provided by the RIKEN BRC through the National Bio-Resource Project of the Ministry of Education, Culture, Sports, Science and Technology, Japan. Primary human hepatocytes were purchased from PhoenixBio.
SI Materials and Methods
Antibodies and Reagents.
Mouse monoclonal antibodies against CD81 (JS81), HIF1-α, and the His tag were purchased from BD Biosciences. Mouse anti-CLDN1, anti-OCLN, Alexa Fluor 488-conjugated anti-mouse and anti-rabbit IgG, and Alexa Fluor 568-conjugated anti-rabbit IgG were purchased from Invitrogen. Rabbit anti-NPC1L1 (Cell Signaling), anti-EGFR (Abcam), and mouse anti-core (Institute of Immunology) were purchased also. Anti-mouse IgG (Santa Cruz Biotechnology), anti-CD81 (D-4) (Santa Cruz), anti-ApoE (Autogen Bioclear), anti-HCV E2 (AP33), anti-VLDLR (1H10) (Novus), anti-SR-BI (Novus), anti-LDLR (R&D), and anti-NPC1L1 (Santa Cruz) were used for analysis of antibody neutralization. Mouse anti-FLAG M2 and chlorpromazine were purchased from Sigma. Rabbit anti-NS5A (AP1-1), mouse anti-VLDLR (6A6; Santa Cruz Biotechnology) against mouse VLDLR, and mouse anti-VLDLR (1H10) against human VLDLR were used for immunostaining. Rabbit anti-NS5A AP1-1 was generated by immunizing a rabbit with a synthesized NS5A peptide (WARPDYNPPLVESC). Recombinant human VLDLR was purchased from Sino Biological.
Plasmids.
pcDNA3.1 TOPO VLDLR was generated by PCR amplification of the VLDLR ORF with oligos generated from Huh7.5 cDNA cultured under hypoxic conditions and subsequent cloning into pcDNA3.1D/V5His TOPO (Invitrogen). The mouse VLDLR expression vector was generated using a PCR-amplified VLDLR ORF with oligos from APOBEC1-knockout mouse liver cDNA. The VLDLR expression lentivirus vector was constructed using the CSII-CMV-MCS plasmid with the In-Fusion HD cloning kit (Clontech). The CRISPR/Cas9-based VLDLR- and CD81-knockout plasmids were generated using annealed oligonucleotide (Table S1) and were cloned into pX330 (Addgene: 42230). A consensus sequence in VLDLR variants 1–4 was used for constructing the knockout plasmid. pcDNA3.1 HCV E2 FLAG plasmid was generated using pJFH-1 (kindly provided by T. Wakita, National Institute of Infectious Disease, Tokyo) as a template and was cloned into the 3′ terminus of FLAG-tagged pcDNA3.1. All oligos are shown in Table S1.
RNAi.
siRNAs were transfected using the Lipofectamine RNAiMAX reagent (Invitrogen) according to the manufacturer’s protocol. The duplex nucleotides of siRNA specific for the mRNA of SR-B1, CLDN1, LDLR, and a control siRNA were purchased from Sigma. The siRNA against OCLN (stealth RNAi) was purchased from Invitrogen. The NPC1L1 siRNA (Silencer select s26632) was purchased from Ambion.
shRNAs were transfected using TransIT-LT1 (Mirus) according to the manufacturer’s protocol. Plasmids expressing shRNAs 1–4 targeting VLDLR (KH06222N) and a shRNA control were purchased from Qiagen.
Infection with HCVpp and HCVcc.
Cells were infected with pseudovirus-containing supernatant (diluted in fresh medium 1:5 for Luc-HCVpp and 1:5,000 for Luc-VSV-Gpp) (2). After 24 h, the medium was changed, and a luciferase assay was performed 72 h after infection. HCVcc infection in the hypoxia experiment was analyzed 24 h postinfection. HCVcc entry was analyzed 48 h postinfection. The cells were lysed in passive lysis buffer (PLB) (Promega), and luciferase activity was measured by the Luciferase Assay system (Promega) according to the manufacturer’s instructions (Promega), using a GloMax 96 Microplate Luminometer (Promega). As control experiment, treatment with IFN-β (100 IU/mL) or anti-E2 antibody (15 μg/mL) was included in each experiment when necessary.
Infectivity Assay.
The infectivity titer of Huh7.5 cells cultured under normoxic or hypoxic conditions was analyzed as follows: Serially diluted HCVccJFH1 was used to infect 1 × 104 cells per well of a 96-well plate for 24 h. Cells were stained with anti-NS5A, and the number of stained cells was counted. Infectivity of Huh7.5 cells cultured under the different conditions was expressed as focus-forming units per milliliter of the original virus solution (30).
HCV IRES Activity Assay.
HCV IRES activity was performed with the HCV IRES Luc plasmid. At 48 h posttransfection cells were lysed in PLB, and luciferase activity was measured using the Dual Luciferase Reporter assay system (Promega) according to the manufacturer’s instructions.
Hypoxia Experiment.
Huh7.5 cells were preincubated for 48 h in an hypoxia chamber filled with 1% O2 and 5% CO2 containing N2 gas (1% O2 mixed gas). The 1% O2 mixed gas was replaced every 24 h (4). After 48 h, the cells were treated with various agents, such as shRNA or antibodies, followed by HCV infection. Twenty-four hours postinfection the cells were fixed for staining or luciferase activity analyses were performed.
RT-PCR.
Total RNA was extracted from the cells using TRIzol reagent (Invitrogen), and cDNA was prepared with SuperScript III (Invitrogen) using oligo (dT) primers. qRT-PCR was performed with the Fast SYBR Green master mix (Applied Biosystems), and fluorescent signals were analyzed using the Fast RT-PCR System (Applied Biosystems). The PCR primers are provided in Table S1.
Quantification of HCV RNA was performed as previously described (30). The following primers and TaqMan probes were used (31). VLDLR variant expression was achieved using the V1, V2, V3, and V4 primers. The PCR primer pairs were as follows: VLDLR variant 1 (V1 and V2), VLDLR variant 2 (V1 and V3), VLDLR variant 3 (V4 and V2), and VLDLR variant 4 (V4 and V3).
VLDLR mRNA expression was analyzed using human liver QUICK-Clone cDNA (Clontech) and cDNA extracted from mouse liver tissue. The human and mouse G3PDH universal PCR primer was obtained from TOYOBO.
ELISA.
Recombinant human VLDLR was coated on 96-well plates. HCV E2 protein was purified from HCV E2 FLAG plasmid-transfected 293FT cell lysates by immunoprecipitation with FLAG antibody-conjugated Dynabeads (VERITAS), followed by elution with 3× FLAG peptide. Purified HCV E2 or HCV E2 depleted by anti-E2 (AP33) was reacted for 1 h on the VLDLR-coated 96-well plates. After treatment with HCV E2 and VLDLR, the plates were reacted with anti-Flag M2 followed by HRP-conjugated mouse IgG for 1 h. All ELISA reactions used BD OptEIA ELISA Sets (BD) according to the manufacturer’s protocol. Anti-HCV E2 (AP33) and 3× Flag peptide were used as controls.
Immunoprecipitation.
The His-tagged ectodomain expression VLDLR variant 1 or variant 2 plasmid was transfected together with the Flag-tagged HCV E2 plasmid into 293FT cells using the TransIT-LT1 transfection reagent. Forty-eight hours posttransfection, the cells were lysed and immunoprecipitated using anti-His antibody–conjugated beads. The precipitant was analyzed by immunoblotting using an anti-VLDLR antibody and Flag M2 antibody.
Immunostaining.
HCVccJFH1-infected cells were fixed with 4% paraformaldehyde and permeabilized with 0.05% Triton X-100/PBS. The permeabilized cells were stained with the HCV NS5A antibody (AP1-1) or human VLDLR antibody (1H10). Mouse VLDLR was detected using a mouse VLDLR (6A6) antibody and an anti-mouse or anti-rabbit Alexa Fluor 488 or 568. Cell imaging was performed by laser-scanning confocal microscopy using a FLUOVIEW fv1000 and fluorescent microscopy using an IX73 (OLYMPAS).
Statistical Analysis.
Data were expressed as the mean and SD. Statistical analyses were performed using Student’s t-test. P < 0.05 was considered statistically significant.
Acknowledgments
We thank H. Yamamoto and R. Suzuki for technical assistance; C. Rice for Huh7.5; R. Bartenschlager for pFK Con1 and pFK H77; I. S. Y. Chen for pNL4-3-lucΔenv; and H. Miyoshi for CSII-CMV-MCS, pCMV-VSV-G, pCAG-HIVgp, and pRSV-Rev. This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology and by Grants-in-Aid for research on hepatitis from the Ministry of Health, Labor, and Welfare of Japan.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. V.R. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1506524113/-/DCSupplemental.
References
- 1.Pileri P, et al. Binding of hepatitis C virus to CD81. Science. 1998;282(5390):938–941. doi: 10.1126/science.282.5390.938. [DOI] [PubMed] [Google Scholar]
- 2.Evans MJ, et al. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature. 2007;446(7137):801–805. doi: 10.1038/nature05654. [DOI] [PubMed] [Google Scholar]
- 3.Ploss A, et al. Human occludin is a hepatitis C virus entry factor required for infection of mouse cells. Nature. 2009;457(7231):882–886. doi: 10.1038/nature07684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scarselli E, et al. The human scavenger receptor class B type I is a novel candidate receptor for the hepatitis C virus. EMBO J. 2002;21(19):5017–5025. doi: 10.1093/emboj/cdf529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barth H, et al. Viral and cellular determinants of the hepatitis C virus envelope-heparan sulfate interaction. J Virol. 2006;80(21):10579–10590. doi: 10.1128/JVI.00941-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agnello V, Abel G, Elfahal M, Knight GB, Zhang QX. Hepatitis C virus and other flaviviridae viruses enter cells via low density lipoprotein receptor. Proc Natl Acad Sci USA. 1999;96(22):12766–12771. doi: 10.1073/pnas.96.22.12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sainz B, Jr, et al. Identification of the Niemann-Pick C1-like 1 cholesterol absorption receptor as a new hepatitis C virus entry factor. Nat Med. 2012;18(2):281–285. doi: 10.1038/nm.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin DN, Uprichard SL. Identification of transferrin receptor 1 as a hepatitis C virus entry factor. Proc Natl Acad Sci USA. 2013;110(26):10777–10782. doi: 10.1073/pnas.1301764110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lupberger J, et al. EGFR and EphA2 are host factors for hepatitis C virus entry and possible targets for antiviral therapy. Nat Med. 2011;17(5):589–595. doi: 10.1038/nm.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drummer HE, Boo I, Maerz AL, Poumbourios P. A conserved Gly436-Trp-Leu-Ala-Gly-Leu-Phe-Tyr motif in hepatitis C virus glycoprotein E2 is a determinant of CD81 binding and viral entry. J Virol. 2006;80(16):7844–7853. doi: 10.1128/JVI.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Owsianka AM, et al. Identification of conserved residues in the E2 envelope glycoprotein of the hepatitis C virus that are critical for CD81 binding. J Virol. 2006;80(17):8695–8704. doi: 10.1128/JVI.00271-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vassilaki N, et al. Low oxygen tension enhances hepatitis C virus replication. J Virol. 2013;87(5):2935–2948. doi: 10.1128/JVI.02534-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carreau A, El Hafny-Rahbi B, Matejuk A, Grillon C, Kieda C. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J Cell Mol Med. 2011;15(6):1239–1253. doi: 10.1111/j.1582-4934.2011.01258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen GM, et al. Hypoxia-inducible factor-1 (HIF-1) promotes LDL and VLDL uptake through inducing VLDLR under hypoxia. Biochem J. 2012;441(2):675–683. doi: 10.1042/BJ20111377. [DOI] [PubMed] [Google Scholar]
- 15.Ruiz J, et al. The apoE isoform binding properties of the VLDL receptor reveal marked differences from LRP and the LDL receptor. J Lipid Res. 2005;46(8):1721–1731. doi: 10.1194/jlr.M500114-JLR200. [DOI] [PubMed] [Google Scholar]
- 16.Reddy SS, Connor TE, Weeber EJ, Rebeck W. Similarities and differences in structure, expression, and functions of VLDLR and ApoER2. Mol Neurodegener. 2011;6(30):1–10. doi: 10.1186/1750-1326-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu JM, Skill NJ, Maluccio MA. Evidence of aberrant lipid metabolism in hepatitis C and hepatocellular carcinoma. HPB (Oxford) 2010;12(9):625–636. doi: 10.1111/j.1477-2574.2010.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ploss A, Rice CM. Towards a small animal model for hepatitis C. EMBO Rep. 2009;10(11):1220–1227. doi: 10.1038/embor.2009.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.André P, et al. Characterization of low- and very-low-density hepatitis C virus RNA-containing particles. J Virol. 2002;76(14):6919–6928. doi: 10.1128/JVI.76.14.6919-6928.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nielsen SU, et al. Association between hepatitis C virus and very-low-density lipoprotein (VLDL)/LDL analyzed in iodixanol density gradients. J Virol. 2006;80(5):2418–2428. doi: 10.1128/JVI.80.5.2418-2428.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang KS, Jiang J, Cai Z, Luo G. Human apolipoprotein e is required for infectivity and production of hepatitis C virus in cell culture. J Virol. 2007;81(24):13783–13793. doi: 10.1128/JVI.01091-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartosch B, et al. Cell entry of hepatitis C virus requires a set of co-receptors that include the CD81 tetraspanin and the SR-B1 scavenger receptor. J Biol Chem. 2003;278(43):41624–41630. doi: 10.1074/jbc.M305289200. [DOI] [PubMed] [Google Scholar]
- 23.Sakai J, et al. Structure, chromosome location, and expression of the human very low density lipoprotein receptor gene. J Biol Chem. 1994;269(3):2173–2182. [PubMed] [Google Scholar]
- 24.Rettenberger PM, et al. Ligand binding properties of the very low density lipoprotein receptor. Ligand binding properties of the very low density lipoprotein receptor. J Biol Chem. 1999;274(13):8973–8980. doi: 10.1074/jbc.274.13.8973. [DOI] [PubMed] [Google Scholar]
- 25.Dorner M, et al. Completion of the entire hepatitis C virus life cycle in genetically humanized mice. Nature. 2013;501(7466):237–241. doi: 10.1038/nature12427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oka K, et al. Mouse very-low-density-lipoprotein receptor (VLDLR) cDNA cloning, tissue-specific expression and evolutionary relationship with the low-density-lipoprotein receptor. Eur J Biochem. 1994;224(3):975–982. doi: 10.1111/j.1432-1033.1994.00975.x. [DOI] [PubMed] [Google Scholar]
- 27.Tateno C, et al. Near completely humanized liver in mice shows human-type metabolic responses to drugs. Am J Pathol. 2004;165(3):901–912. doi: 10.1016/S0002-9440(10)63352-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jo H, et al. Endoplasmic reticulum stress induces hepatic steatosis via increased expression of the hepatic very low-density lipoprotein receptor. Hepatology. 2013;57(4):1366–1377. doi: 10.1002/hep.26126. [DOI] [PubMed] [Google Scholar]
- 29.Ishii N, et al. Diverse effects of cyclosporine on hepatitis C virus strain replication. J Virol. 2006;80(9):4510–4520. doi: 10.1128/JVI.80.9.4510-4520.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hishiki T, et al. Infectivity of hepatitis C virus is influenced by association with apolipoprotein E isoforms. J Virol. 2010;84(22):12048–12057. doi: 10.1128/JVI.01063-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sugiyama K, et al. Genetic analysis of hepatitis C virus with defective genome and its infectivity in vitro. J Virol. 2009;83(13):6922–6928. doi: 10.1128/JVI.02674-08. [DOI] [PMC free article] [PubMed] [Google Scholar]