Significance
It has gradually been recognized that the vesicles secreted by cells are a major means by which cells communicate with each other. This recognition has stimulated interest in using vesicles to deliver therapeutic agents. The results presented here address several limitations to progress in the field by developing protocols to produce and isolate large numbers of extracellular vesicles (EVs) from stem-like cells found in the bone marrow. The isolated EVs were found to reduce the adverse effects of traumatic injury to the brain in mice.
Keywords: MSCs, neuroinflammation, exosomes, efficacy assay
Abstract
Extracellular vesicles (EVs) secreted by cells present an attractive strategy for developing new therapies, but progress in the field is limited by several issues: The quality of the EVs varies with the type and physiological status of the producer cells; protocols used to isolate the EVs are difficult to scale up; and assays for efficacy are difficult to develop. In the present report, we have addressed these issues by using human mesenchymal stem/stromal cells (MSCs) that produce EVs when incubated in a protein-free medium, preselecting the preparations of MSCs with a biomarker for their potency in modulating inflammation, incubating the cells in a chemically defined protein-free medium that provided a stable environment, isolating the EVs with a scalable chromatographic procedure, and developing an in vivo assay for efficacy of the cells in suppressing neuroinflammation after traumatic brain injury (TBI) in mice. In addition, we demonstrate that i.v. infusion of the isolated EVs shortly after induction of TBI rescued pattern separation and spatial learning impairments 1 mo later.
Traumatic brain injury (TBI) has devastating effects on the victims and creates a large burden on the healthcare system (1). TBI was originally considered an acute injury syndrome, but it is now recognized to have chronic effects similar to those found in neurodegenerative disorders (2–5). In the acute phase, the trauma destroys tissue, and it also triggers a cascade of events that include excessive neural excitability, oxidative stress, disruption of the blood–brain barrier, and inflammation. The cascade causes additional cell death that occurs through necrosis, apoptosis, and excessive autophagy. The cascade involves astrocytes and microglia, in addition to invading neutrophils, monocytes/macrophages, and T cells. The sequence of events is similar to the sequence seen with sterile injuries to other tissues. Initially, proinflammatory effects predominate and are useful in clearing tissue debris. Thereafter, there is a transition to an antiinflammatory phase, with the microglia and macrophages transiting from “classical” proinflammatory M1 phenotype to multiple alternative M2 phenotypes that suppress the M1 proinflammatory mediators and enhance tissue repair. The chronic effects of TBI occur because the inflammatory phase is not fully suppressed. Instead, the inflammatory responses persist, and they initiate a self-perpetuating cycle of tissue destruction, followed by further inflammation. A similar cycle is now recognized to contribute to the pathology of many chronic diseases.
Multiple strategies have been tested to modulate inflammation in TBI and other CNS disorders (2–4). Among these strategies is the use of mesenchymal stem/stromal cells (MSCs) from bone marrow and other tissues (6–19). The beneficial effects of the MSCs are probably explained by their normal roles as perivascular cells that are among the first responders to tissue injury. One of their responses is to act in concert with other cells as guardians of excessive inflammation because they are activated by proinflammatory cytokines such as TNF-α to secrete modulators of inflammation that include TNF-alpha stimulated gene/protein 6 (TSG-6), PGE-2, STC-1, IL-1 receptor antagonist, and TIMP3 (18, 20–25).
Recently, we have explored the hypothesis that extracellular vesicles (EVs) produced by MSCs may be an effective therapy for TBI because extensive recent reports indicate that EVs may provide a highly efficient means of delivering therapeutic factors to target cells (26–29). As noted by György et al. (29), there are several issues that currently limit therapeutic applications of EVs. In the present report, we have addressed most of these issues. In addition, we demonstrate the efficacy of EVs isolated from MSCs in a mouse model for TBI. As this work was in progress, Zhang et al. (30) reported that exosomes isolated from MSCs improved functional recovery in a rat model for TBI, but they did not characterize the exosomes.
Results
Selection of Optimal MSCs and Culture Conditions for Production of EVs.
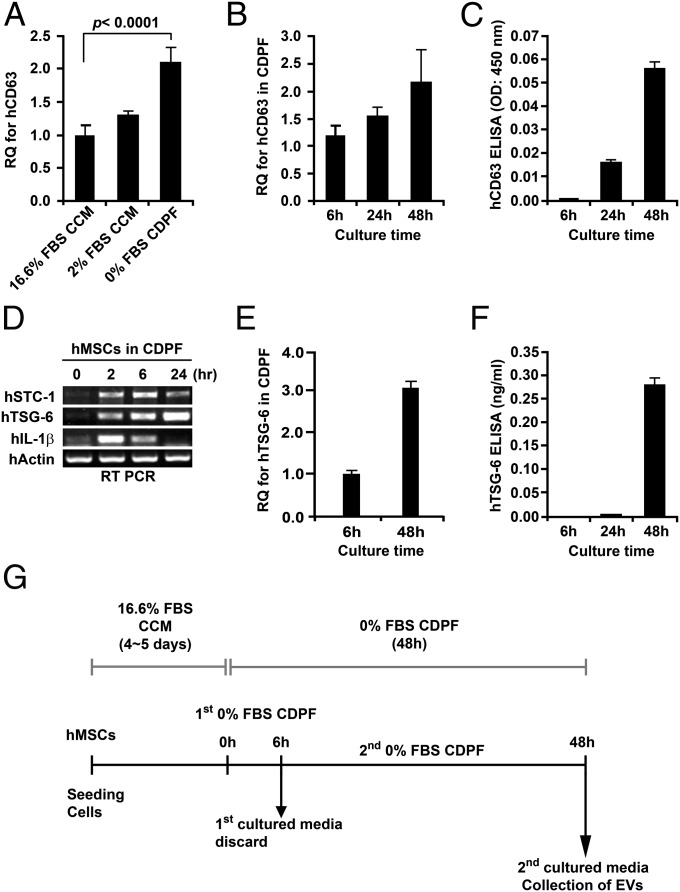
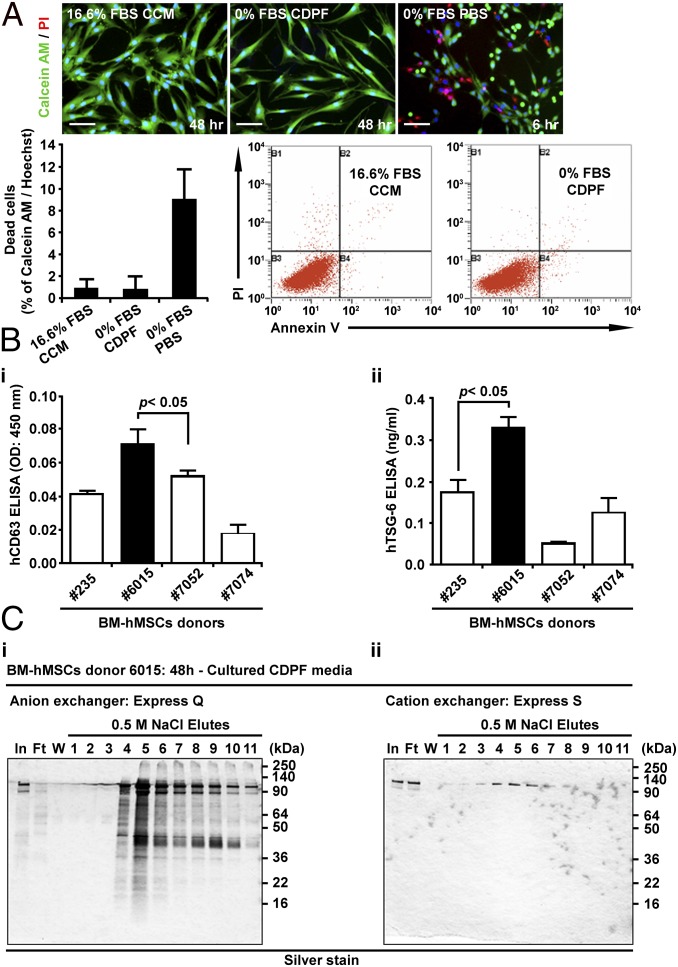
Preparations of tissue-derived MSCs vary in their characteristics dependent on undefined properties of the human donor of the tissue and the site from which the cells are obtained from the same donor (31–34). Therefore, we selected a preparation of bone marrow MSCs (defined as donor 6015), from our NIH-sponsored center for distribution of MSCs, that met the classical in vitro criteria for MSCs and that ranked among the top 3 of 13 MSC preparations in expression of the biomarker of mRNA for TSG-6 that was highly correlated with the efficacy of the cells in modulating inflammation in three murine models (34). MSCs also vary with culture conditions, such as cell densities, and the culture medium (6). To reduce the variability, we followed a protocol in which the MSCs were consistently plated at 500 cells per cm2 in a standardized medium (21–24) containing 17% of a pretested batch of FBS [defined as complete culture medium (CCM)]. The CCM was replaced after 2 or 3 d. After 5 d, the medium was changed to a proprietary chemically defined and protein medium (CDPF) that was initially optimized by a commercial supplier for production of recombinant proteins by Chinese hamster ovary cells (Invitrogen). We further supplemented the medium (Table S1) to minimize aggregation of cells secreting TSG-6 by cross-linking hyaluronan on the cell surface. As a convenient marker for EVs, we used assays for CD63 (Fig. S1), a tetraspan protein frequently found in EVs (26–29). Culture of MSCs in the CDPF medium increased the expression of mRNA for CD63 (Fig. 1A). The expression of the mRNA increased for at least 48 h and was accompanied by the accumulation of the CD63 protein in the medium (Fig. 1 B and C). However, the pattern of genes expressed differed during the time of incubation in the CDPF. At 2 h, there was a high level of expression of mRNA for IL-1β, a major proinflammatory cytokine. In contrast, expression of mRNA for the inflammation-modulating protein TSG-6 was low at 2 h and increased progressively at 6, 24, and 48 h (Fig. 1 D and E). The TSG-6 protein in medium did not increase until about 48 h (Fig. 1F). On the basis of these observations, we developed a standardized protocol for production on EVs that might have antiinflammatory properties (Fig. 1G). The MSCs did not expand, but there was little evidence of cell death (Fig. 2A). Comparison of preparations of MSCs demonstrated that the levels of CD63 protein in the harvested medium were higher in MSCs from donor 6015, the preparation initially selected here, than in three other preparations (Fig. 2 B, i). As expected, the level of TSG-6 in the harvested medium was the highest in donor 6015 (compare Fig. 2 B, ii, with figure 4A in ref. 34).
Table S1.
CDPF media for the preparation of hMSC-derived extracellular vesicles
| Components | Concentrations, per liter | Sources |
| CD-CHO protein-free medium | 925 mL | 10743-011; Invitrogen |
| HT supplements* | 10 mL | 11067-030; Invitrogen |
| 200 mM l-glutamine | 40 mL | 25030-081; Invitrogen |
| d-[+]-glucose | 2 g | G6152; Sigma |
| 100× nonessential amino acids | 10 mL | 11140-050; Invitrogen |
| 100× MEM vitamin solution | 10 mL | 11120-052; Invitrogen |
A mixture of hypoxanthine (10 mM) and thymidine (1.6 mM).
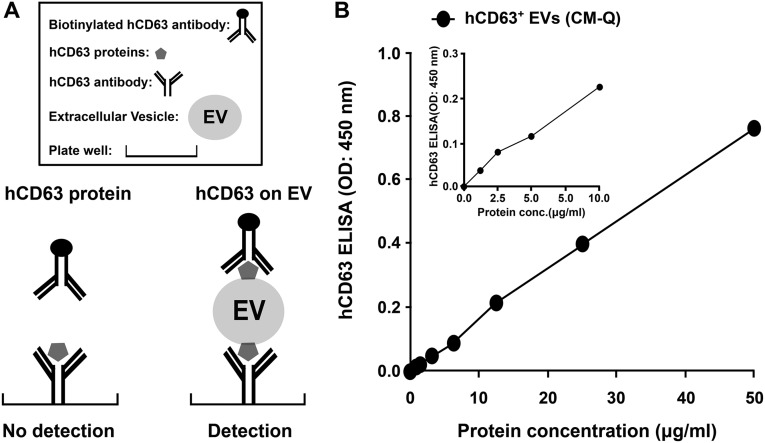
Fig. S1.
ELISA for CD63+ proteins on EVs. (A) Schematic of the assay. (B) Standard curve prepared with varying amounts of protein from pooled fractions of column (Fig. 3 A, ii). Assays by nanoparticle diffusion analysis indicated 1 µg = 0.51 × 109 EVs.
Fig. 1.
Defining conditions for production of EVs. Cultures of human MSCs (donor 6015) at 70–80% confluent were transferred to the media indicated and incubated for 6–48 h. (A) Expression of mRNA for CD63 was increased by culture for 48 h in CDPF medium compared with culture in CCM with standard concentration of FBS (16.6%) or reduced FBS (2%). Assay by RT-PCR. (B) Expression of mRNA for CD63 was increased with time of incubation in CDPF. (C) Secretion of CD63+ was increased with time in CDPF. Medium was assayed by ELISA for vesicle-bound protein (Fig. S1). (D) RT-PCR assays in MSCs incubated in CDPF indicated that the proinflammatory cytokine IL-1β was expressed for up to 6 h and that expression of the inflammation-modulating protein TSG-6 increased between 2 and 24 h. Expression of the antiapoptotic/calcium-phosphate metabolic protein STC-1 peaked at about 6 h. (E) Expression of mRNA for TSG-6 increased with time of incubation in CDPF. (F) Secretion of TSG-6 increased with time in CDPF. Assay by ELISA. (G) Schematic for protocol developed for production of EVs by MSCs.
Fig. 2.
Survival of the MSCs under the culture conditions, comparisons of four donors, and demonstration that most of the secreted proteins and EVs are anionic. (A) Survival of MSCs in CDPF. MSCs were expanded to about 70% confluence and then incubated an additional 48 h in CCM, CDPF, or PBS. (Top) Cultures labeled with Hoechst, Calcein AM, and propidium iodide (PI) demonstrate viable cells in CDPF but not PBS. (Bottom) Assays of the same cultures by flow cytometry after labeling with PI and Annexin V demonstrated survival in CCM or CDPF medium but not PBS. (B) Comparisons of four different preparations of MSCs (donors 235, 6015, 7052 and 7074) after incubation as in Fig. 1G. (i) CD63+ in medium assayed by ELISA. (ii) TSG-6 in medium assayed by ELISA. (C) Small-scale assays in SDS-electrophoretic gels demonstrated that most of the medium proteins bound to and were eluted from an anionic resin and not a cationic resin. Gels were stained with silver.
Isolation of EVs with a Scalable Protocol.
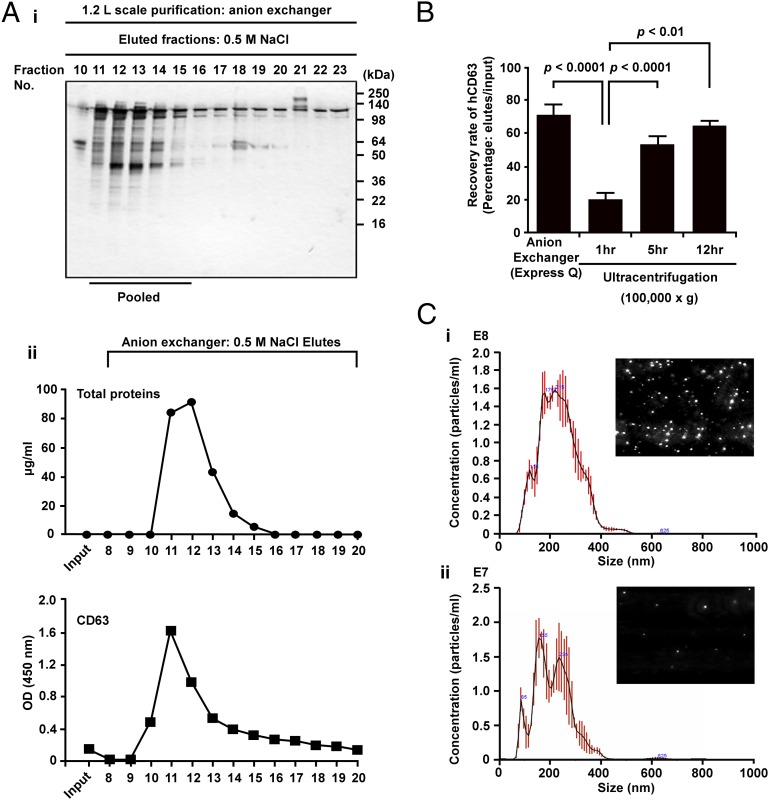
Most of the published protocols for isolation of EVs involve high-speed centrifugation or other procedures that cannot be readily scaled up for large-scale production (29). To develop a scalable protocol, we isolated EVs from the harvested medium by chromatography. In a small-scale test, we found that most of the protein in the harvested medium bound to an anion exchange resin but that little bound to a cation exchange resin (Fig. 2C). Therefore, we developed a protocol in which the harvested medium was centrifuged at 2,500 × g for 15 min and the supernatant was chromatographed on an anion exchange column. The protein eluted with 0.5 M NaCl was recovered as a single broad peak that contained CD63 (Fig. 3 A, i and ii). The recovery of CD63 in the peak ranged from 73% to 81% (n = 3) and was slightly higher than was obtained by centrifuging the harvested medium at 100,000 × g for 12 h (Fig. 3B). Assay of the peak fractions with a nanoparticle tracking system (Fig. 3C) demonstrated that they contained about 0.51 × 109 vesicles per microgram of protein. Assays at decreasing concentrations indicated that the mean size of the vesicles was 231 ± 3.2 nm (SEM), 216 ± 2.3 nm, and 207 ± 1.8 nm, respectively. Interestingly, the three peaks observed at the lowest concentration were 85, 165, and 236 nm, the expected sizes of EVs of 85 nm that were also recovered as dimers and trimers.
Fig. 3.
Chromatographic isolation and characterization of EVs from the medium. (A) Preparation and characterization of CD63+ EVs from medium of MSCs incubated as in Fig. 1G. (i) Assay by SDS-electrophoretic gel of medium eluted from anion exchange column with 0.5 M NaCl. Gel was silver-stained. (ii) Assays of eluted fractions for protein and CD63. (B) Recovery of CD63+ protein from the column was slightly greater than recovery by centrifuging the same samples at 100,000 × g for 12 h. (C) Assays of eluted fractions by nanoparticle diffusion analysis demonstrated that the mean size of the vesicles ranged from 209 ± 1.8 nm (SEM) to 231 ± 3.2 nm. The three peaks at the lower concentration (ii) were 85, 165, and 236 nm. (Insets) Photos of nanoparticles in the instrument.
Surface Epitopes of the Isolated EVs.
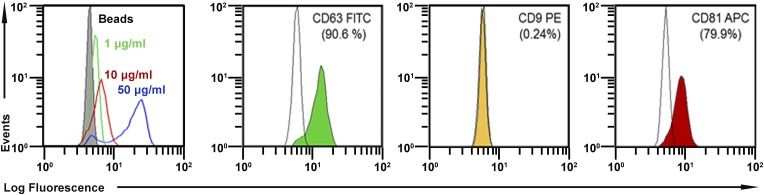
To map surface epitopes, we used a previously published method (35) whereby EVs are first trapped with a large bead linked to an antibody to CD63, and then additional epitopes on the trapped EVs are assayed with standard protocols for flow cytometry. As expected, the EVs captured with the protocol were positive for CD63 (Fig. S2). They were also about 80% positive for CD81, another epitope frequently found on EVs (26–29). However, they were negative for CD9, a third epitope frequently found on EVs. Also, they were also negative for 13 epitopes found on the surface of MSCs (Table 1). Therefore, they probably correspond to the EVs frequently referred to as exosomes (26–29).
Fig. S2.
Assays of epitopes on the isolated EVs. The EVs were trapped on a magnetic bead covalently linked to anti-CD63 and then assayed for additional epitopes by flow cytometry (35). Data were gated for single beads. (Left) Detection of CD63 as a function of the concentration of protein added. In the remaining panels, EVs were positive for CD63 and CD81 but not for CD9.
Table 1.
Surface epitopes in hMSCs and EVs
| Surface epitopes | hMSCs* | EVs* | |
| CCM | CDPF | ||
| hMSC markers | |||
| CD29 | >99 | >99 | <1 |
| CD44 | >99 | >99 | <2 |
| CD49c | >99 | >99 | <1 |
| CD49f | >99 | >99 | <1 |
| CD59 | >99 | >99 | 2.04 |
| CD73 | >99 | >99 | <2 |
| CD90 | >99 | >99 | <1 |
| CD105 | >99 | >99 | <1 |
| CD146 | >99 | >99 | <2 |
| CD147 | >99 | >99 | <1 |
| CD166 | >99 | >99 | <1 |
| HLA-a, b, c | >99 | >99 | <2 |
| PODXL | 95 | 91 | <2 |
| EV markers | |||
| CD9 | 93 | 99 | <1 |
| CD63 | 48 | 85 | 90.6 |
| CD81 | >99 | >99 | 79.9 |
hMSC, human mesenchymal stem/stromal cell.
Positively stained cells or EVs (% of total) with antibodies indicated in Table S2.
Assay for Efficacy of EVs in Suppressing of Neuroinflammation after TBI.
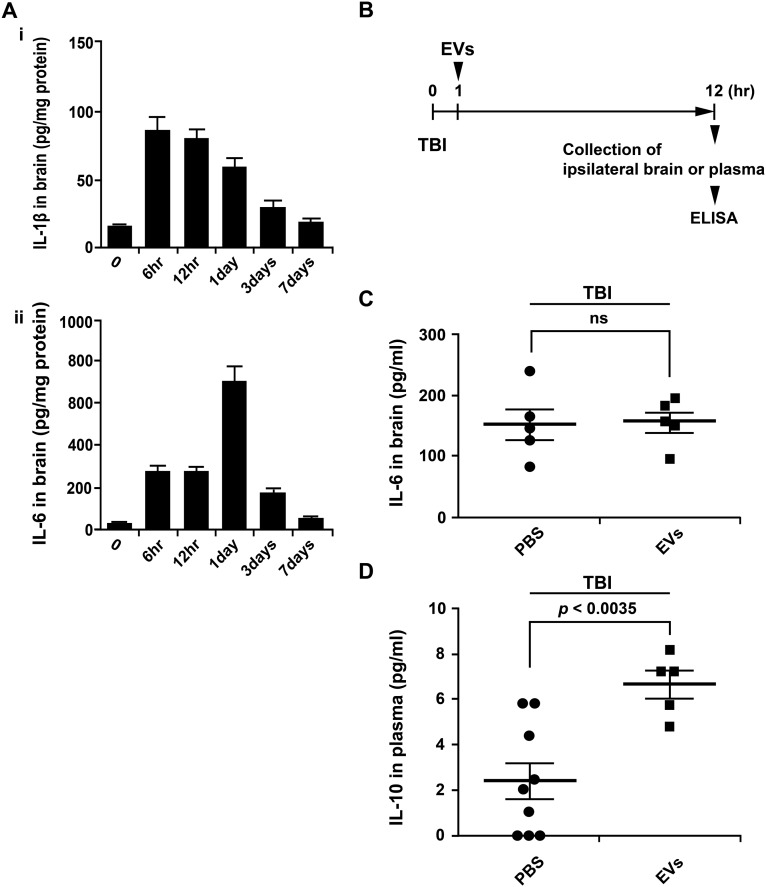
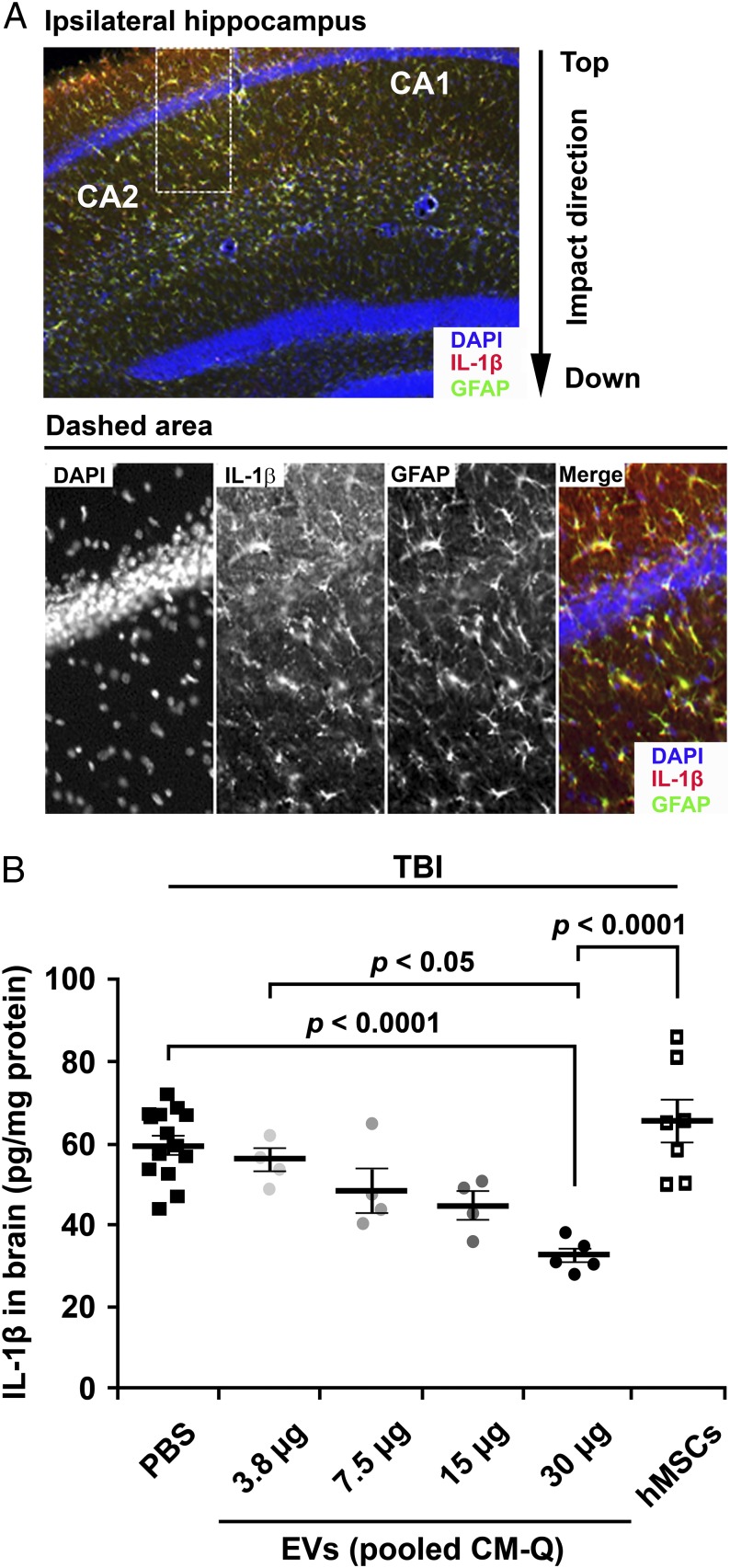
Quantitative assays for efficacy are critical for the development of most therapeutics (29). We elected to develop a quantitative assay for the efficacy of EVs in a model for TBI by ELISAs on brain homogenates. Initial experiments (Figs. S3 and S4) demonstrated that, after TBI, levels for the proinflammatory cytokine IL-1β peaked between 6 and 12 h and that the IL-1β colocalized with GFAP+ astrocytes (Fig. 4A). Therefore, we followed a protocol in which TBI was produced in mice and IL-1β levels in brain were assayed 12 h after the TBI. Administration of the EVs decreased the levels of IL-1β in a dose-dependent manner (Fig. 4B). The highest dose of EVs was more effective than i.v. infusion of 1 million of MSCs expanded in CCM, apparently because the brains were assayed 12 h after administration of the cells but i.v.-administered MSCs that are trapped in the lung do not express high levels of TSG-6 until 24 h after infusion (36). The dose of EVs that produced the largest effect (30 µg of protein and 15 × 109 EVs) was synthesized by about 1 million MSCs under the conditions used here (Fig. 1G), but the in vitro and in vivo data are obviously directly comparable. Of special interest was a result in which the dose of EVs that produced the largest effect (30 µg of protein and 15 × 109 EVs) contained only 4 ng of TSG-6 whereas administration of 50 µg of recombinant TSG-6 i.v. was required previously in four models of induced inflammation in mice (21–23).
Fig. S3.
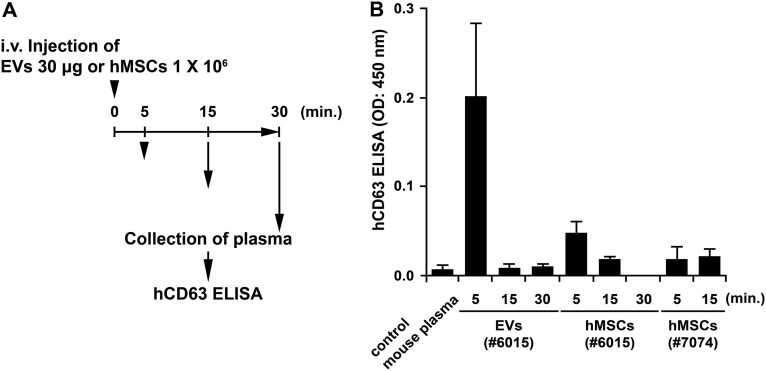
Plasma EVs after i.v. injection of EVs or MSCs from two donors into C57BL/6J mice. (A) Schematic of experiment. (B) ELISAs for CD63+ EVs in plasma. ELISAs were performed as in Fig. S1. n = 3 mice for each condition.
Fig. S4.
ELISAs for cytokines after TBI. (A) Brain levels of IL-1β and IL-6 in ipsilateral brain 6 h to 7 d after TBI. n = 4 mice for each time point. (B) Schematic for the experiment in C and D. (C) IL-6 in brain 12 h after TBI and i.v. PBS or 30 µg of protein from pooled fraction from Fig. 3 A, ii. (D) Plasma levels of IL-10.
Fig. 4.
Dose–response data for suppression of neuroinflammation by EVs after TBI. (A) Immunochemistry of brain sections demonstrated that TBI increased IL-1β in GFAP+ astrocytes. Sections from brain recovered 12 h after TBI and sections from region indicated were stained for DAPI, IL-1β, and GFAP. (B) Dose-dependent decrease in IL-1β after i.v. administration of PBS or EVs. Amounts varied from 3.5 to 30 µg of protein or 1.8–15.3 × 109 EVs. PBS or EVs were administered 1 h after TBI, and assays were by ELISA on homogenates of ipsilateral brain isolated 12 h after TBI. The i.v. administration of 1 million MSCs cultured in CCM had little effect, apparently because they were not fully activated in 12 h to express TSG-6 by embolization of the lung (21).
After i.v. administration of 30 μg of CD63+ EVs into naive mice, the EVs were detected by ELISAs of plasma after 5 min, but they were not detected after 15 min (Fig. S3). Therefore, they were apparently rapidly distributed to tissues. Low levels of CD63+ EVs were also detected in plasma 5 min after infusion of 1 × 106 MSCs from donor 6015.
Effects of EVs on Pattern Separation Function and Spatial Learning Ability After TBI.
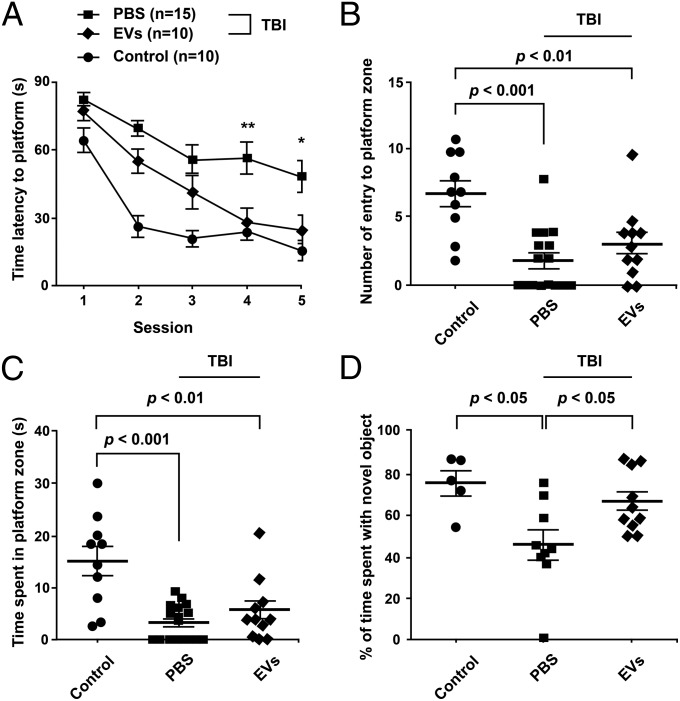
Because the isolated EVs decreased inflammation 12 h after TBI, we tested their effects on pattern separation ability and spatial learning and memory function a month after TBI (Fig. 5). Pattern separation is proficiency for discriminating analogous experiences through storage of similar representations in a nonoverlapping manner (37, 38), and the hippocampus neurogenesis plays a major role in maintaining this function. An object-based behavioral test demonstrated preservation of the ability for pattern separation in TBI mice treated with i.v. EVs, in comparison with TBI mice treated with vehicle exhibiting pattern separation deficits (Fig. 5D). Furthermore, in a water maze test (Fig. 5A), TBI mice treated with i.v. EVs learned to find the concealed platform after three trials as reflected in the decrease in latency time that became similar to the latency time in control mice that received sham operations, implying preservation of spatial learning ability in TBI mice receiving EVs. In contrast, mice that received vehicle after TBI improved only slightly. There were no significant differences in memory retrieval function in a probe test between TBI mice receiving EVs or vehicle because the number of times mice entered the platform zone and the time spent in the platform zone (Fig. 5 B and C) were similar between the two TBI groups, which contrasted with the behavior of control mice exhibiting greater dwell times in the platform zone.
Fig. 5.
Improved cognitive function after TBI and i.v. EVs. About 1 h after TBI, each mouse received i.v. PBS or 30 µg of protein (about 15.3 × 109 EVs) from the pooled peak from the anion exchange column (pooled CM-Q) (Fig. 3 A, ii). Behavior in the water maze was tested 28–33 d after TBI. The pattern separation test was performed 35 d after TBI. (A) In the water maze, treated mice with TBI learned to locate the hidden platform with the same latency as controls after four trials and better than TBI mice that received PBS. There was no significant effect of the therapy on the number of entries to platform zone (B) or time spent in the platform zone (C) in the probe test. (D) The treated mice performed better than TBI mice that received PBS in the pattern separation test.
Discussion
The role of EVs in cellular communication has stimulated renewed interest in using vesicles for the delivery of therapeutic agents (26–29). The strategies have included vesicles prepared from purified components (liposomes), a strategy initiated many years ago, as well as newer strategies with vesicles prepared by fragmentation of cellular membranes and EVs produced by cultured cells. Each strategy is likely to be ideal for different applications. EVs produced by cells have the advantage that they may contain naturally occurring therapeutic cargos and specific cell-targeting ligands. Also, EVs can be engineered to contain novel cargos and cell-targeting ligands.
The experiments presented here addressed several issues that currently limit therapeutic applications of EVs. Two of the issues are the choice of the producing cells and the culture conditions under which the cells produce EVs. MSCs are attractive choices of cells for several reasons. They can be obtained from multiple tissues, including fat, umbilical cord, and synovial membranes (6–8). The cells from human bone marrow have been the most extensively studied and, like MSCs from other human tissues, can readily be expanded in culture (39). However, they are not tumorigenic (40). Also, they do not undergo spontaneous transformation during expansion in culture to generate malignant lines, as is observed with mouse MSCs (41) and as has been overlooked in multiple publications with murine MSCs (6, 40, 41). Therefore, they are unlikely to transfer tumorigenic factors in the EVs they produce, a risk inherent with immortal cell lines. Instead, the EVs produced by MSCs are likely to transfer therapeutic components, because MSCs have been shown to have beneficial effects in a large number of disease models (6, 7) and in a few clinical trials in patients (42–44). Moreover, the beneficial effects are largely explained by MSCs being activated by signals from injured cells to transfer a large number of therapeutic factors, including cytokines, chemokines, microRNAs, and mitochondria (6, 7, 45, 46). Also, MSCs tend to home to injured tissues, and some of the EVs they produce may retain this homing ability. In addition, MSCs are an attractive source of EVs because of an unusual property of the cells: They can survive for weeks in culture with medium containing no protein or growth factors but continue to produce EVs (47–50). During incubation of MSCs from bone marrow in α-MEM, the cultures underwent selection with survival of a subpopulation that expressed genes characteristic of embryonic cells (47). Pochampally and coworkers subsequently demonstrated that the MSCs incubated in α-MEM produced EVs, and they extensively characterized the EVs (48). They also demonstrated that the EVs supported tumor growth. Phinney et al. (49) demonstrated that EVs produced by human MSCs under different culture conditions partially rescued silica-induced fibrosis of lung in mice.
One limitation in using MSCs for producing EVs is the number of cells that can be obtained (29) because MSCs senesce after extensive expansion in culture (39, 40). Another limitation is that different preparations vary. The present protocol in part overcame these limitations by preselecting an initial preparation of bone marrow MSCs that could be expanded to provide up to 1015 cells from a small bone marrow aspirate of 2–4 mL (39). The 1015 MSCs are about 106 times the amount required for the experiments described here, but it is likely that larger samples of MSCs from bone marrow or other tissues will be required for clinical therapies. The preparation was also preselected by the criteria that the cells expressed high levels of the biomarker TSG-6 mRNA that was highly correlated with the efficacy of the MSCs in suppressing inflammation in three mouse models (34). As indicated here, the preselected cells expressed high levels of mRNA for CD63, an observation that may provide a potency marker for the efficiency of MSCs is producing EVs. To minimize the variations introduced by culture conditions, the cells were expanded at low density with a standardized protocol under which the cells retain most of their progenitor features (39). Also, to recover EVs, the cells were incubated in a chemically defined protein-free medium that was optimized for growth and production of recombinant proteins by CHO cells on a commercial scale (Invitrogen). Apparently, because it is protein-free, the medium induced stress on the MSCs, but it minimized the cell death seen with culturing MSCs under other conditions used to produce EVs (28, 49). The differences in culture conditions may explain why the medium harvested as a 2,600 × g supernatant did not contain the large vesicles isolated by Phinney et al. (49).
The experiments were also designed to address two further issues: scalable protocols and quantitative assays for efficacy. To provide a scalable protocol, we used a chromatographic column to isolate the EVs. Use of the column provided a 500-fold concentration of the EVs and a protocol that can more readily be scaled up than protocols than use high-speed centrifugation and related techniques (29). We addressed the need for an efficacy assay with a protocol in which EVs were i.v. infused after TBI in mice and IL-1β levels in brain were measured by ELISA 12 h later. The EVs produced by human MSCs were effective in the WT mice, an observation consistent with the expression by human MSCs of undetectable levels of MHC class II and very low levels of MHC class I (6, 7). Also, it is consistent with the observation here that the EVs were HLA-a, -b, and -c negative. In addition, the observation that human EVs were effective is consistent with previous reports that i.v. administrations of human MSCs produced therapeutic effects in immune competent mice (6), including a model for TBI (36). Therefore, immune reactions to single administrations of human EVs in mouse models are unlikely to produce complicating immune reactions; models requiring repeated administrations may need to be examined more carefully.
Subsequently, we were able to demonstrate that infusion of the effective dose of the isolated EVs after TBI rescued pattern separation and spatial learning impairments 1 mo later. Therefore, the results suggested that, by modulating the initial inflammation produced by the TBI, the EVs interrupted the self-perpetuating cycle of tissue destruction and inflammation that largely explains the chronic effects of TBI (2–5).
At the same time, the results did not resolve several important issues. The CD63+CD81+ EVs seemed to account for most of the EVs secreted by the cells, but the data did not exclude the possibility that a small fraction of the EVs (less than 10% or 20%) were CD63-negative. The results are similar to the results reported by Vallabhaneni et al. (48), but the data are not directly comparable because of differences in the conditions used to produce MSCs and in many of the assays used. A second unresolved issue is the molecular mechanism whereby the i.v.-infused EVs reduced inflammation and rescue cognitive impairments in the TBI model. The EVs isolated here contained TSG-6, and previous results indicated that some of—but not necessarily all of—the antiinflammation effects of MSCs were explainable by the cells being activated to express TSG-6 (21, 22). However, the amount of TSG-6 in the effective dose of EVs was less than 1/10,000 the amount of recombinant TSG-6 required to suppress inflammation in several animal models. Therefore, the efficacy of the EVs observed here may well be explained by their containing many components other than TSG-6. Unfortunately, the technologies to define the active components of EVs and their effects of EVs on target cells are still challenging (26–29). For example, sequencing of the microRNAs and other RNAs in EVs provides data on millions of potential targets for the microRNAs (48), but it is difficult to identify those with significant effects on their target cells (48, 50).
Materials and Methods
Details are presented in SI Materials and Methods on all methods, including culture conditions, chromatographic isolation of EVs, PCR and ELISA assays, nanoparticle tracking analysis, controlled cortical impact injury, behavioral studies, and statistical tests. hMSCs were from the Center for Distribution (medicine.tamhsc.edu/irm/msc-distribution.html) and all animal protocols were approved by the Texas A&M Animal Care and Use Committee. Human MSCs were obtained from normal, healthy donors with informed consent under Scott & White and Texas A&M Institutional Review Boards approved procedures.
SI Materials and Methods
Culture Conditions for Producing EVs.
A frozen vial of passage 4 MSCs was thawed at 37 °C and plated directly at about 500 cells per cm2 in 150 × 20-mm-diameter tissue culture plates (cat. no. 430599; Corning) in complete culture medium (CCM). The CCM medium was replaced after 2–3 d. After the cells reached about 70% confluency in 4–6 d, the medium was replaced with a medium optimized for Chinese hamster ovary cells (CD-CHO Medium; cat. no. 10743–002; Invitrogen) that was further supplemented to prevent aggregation of cells synthesizing TSG-6 (Table S1). The medium was recovered after 6 h for assays and discarded. The medium replaced and the medium recovered between 6 and 48 h was either stored at −80 °C or used directly to isolate EVs.
Isolation of EVs by Chromatography.
Preliminary experiments were carried out with medium harvested from four or five 15-cm-diameter plates and tested for binding of the protein to a cation exchange resin (Express S; cat. no. 40792025; Whatman) or an anion exchange resin (Express Q; cat. no. 4079302; Whatman). For isolation of EVs, medium harvested from 40 to 45 plates (about 1.2 L) was used directly or after thawing. The medium was centrifuged at 2,565 × g for 15 min to remove cellular debris, and the supernatant was applied directly at room temperature to a column containing the anion exchange resin (100-mL bed volume) that had been equilibrated with 50 mM NaCl in 50 mM Tris buffer (pH 8.0). The medium was applied at a flow rate of 4 mL/min and at room temperature. The column resin was washed with 10 volumes of the equilibration buffer and then eluted with 25 volumes of 500 mM NaCl in 50 mM Tris buffer (pH 8.0). Fractions of 20–30 mL were collected and stored at either 4 °C or −20 °C.
Human MSCs from Bone Marrow.
Passage 1 WT MSCs (anonymously identified as from donors 235, 6015, 7052, and 7074) were isolated from bone marrow aspirates and cultured as previously described (38) For the experiments described here, a frozen vial of about 1 million passage 1 MSCs was thawed at 37 °C and plated in CCM consisting of α-minimum essential medium (α-MEM; Gibco), 17% (vol/vol) FBS (Atlanta Biologicals), 100 units/mL penicillin (Gibco), 100 µg/mL streptomycin (Gibco), and 2 mM l-glutamine (Gibco) on a 152-cm2 culture dish (Corning). After 15–24 h, the medium was removed, the cell layer was washed with PBS, and the adherent viable cells were harvested using 0.25% trypsin and 1 mM EDTA (Gibco) for 3–4 min at 37 °C, reseeded at 500 cells per cm2 in CCM, and incubated for 5–7 d (with medium change on day 3) until 70–80% confluency (from 6,000–10,000 cells per cm2). The medium was removed, the cell layer was washed with PBS, and the cells were lifted with trypsin/EDTA and frozen at a concentration of about 1 million cells per mL in α-MEM containing 30% (vol/vol) FBS and 5% (vol/vol) dimethyl sulfoxide (Sigma). For the experiments here, the cells were expanded under the same conditions, and passage 4 cells were used.
RT-PCR and Real-Time PCR Assays.
RNA extraction and cDNA synthesis were performed as previously described (34). The synthesized cDNA was amplified by Ex Taq DNA Polymerase (TaKaRa) and the following primers: human STC-1, sense ATGCTCCAAAACTCAGCAGTG, antisense TATGCACTCTCATGGGATGTGC; human TSG-6, sense TCACATTTCAGCCACTGCTC, antisense AGACCGTGCTTCTCTGTGGT; human IL-1β, sense GCACGATGCACCTGTACGAT, antisense CACCAAGCTTTTTTGCTGTGAGT; and human β-actin, sense AGGCACCAGGGCGTGAT, antisense GCCCACATAGGAATCCTTCTGAC. Real-time amplification was performed using a TaqMan Universal PCR Master Mix and primers [human CD63, Hs01041237; human TSG-6, Hs01113602; human GAPDH, Hs0275891 (Life Technologies)] and was conducted with the 7900HT fast real-time PCR system (Life Technologies). The mRNA levels were normalized to the level of GAPDH.
Live and Dead Cell Staining.
hMSCs (1 × 105) were incubated in CCM on a six-well plate. After 24 h, medium was changed to a different concentrated FBS. After incubation of hMSCs with 16.6% (vol/vol) FBS, 0% FBS CDPF for 48 h, or 0% FBS PBS for 4 h, cells were stained with Hoechst (Thermo) and a LIVE/DEAD Cell Imaging Kit (Molecular Probes) for 15 min at 25 °C, and then live fluorescence was visualized using a microscope (Nikon Eclipse 80i). The possibility that the CDPF induced apoptotic cell death of hMSCs was assessed by using the Annexin V-FITC apoptosis detection kit (Sigma) according to the manufacturer’s instructions. After culture with the CDPF, 1 × 106 cells per 300 µL were resuspended in Annexin V binding buffer and stained with Annexin V-FITC and PI for 10 min at room temperature. The cells were analyzed by a FC500 flow cytometer [Beckman-Coulter (https://www.beckmancoulter.com/wsrportal/wsr/index.htm)].
Isolation of EVs by Ultracentrifugation.
To compare the yield of EVs containing CD63, EVs were isolated by ultracentrifugation. The medium was centrifuged 2,565 × g for 15 min to remove cells and debris and centrifuged at 100,000 × g (Sorvall WX Floor Ultra Centrifuge and AH-629 36 mL swinging Bucket Rotor; Thermo) for 1, 5, and 12 h at 4 °C. EVs were stored in PBS at 4 °C or −20 °C. EV protein content was quantified by the Bradford method (Bio-Rad).
ELISA for CD63+ EVs.
A 96-well plate (cat. no. 441653; Nunc) was coated with monoclonal 5 μg/mL anti-CD63 antibody (clone H5C6, cat. no. 556019; Becton-Dickinson) in a volume of 50 μL per well of carbonate buffer (pH 9.4) and incubated overnight at 4 °C. After three washes with PBS containing 0.1% Tween 20, 100 μL per well of blocking solution (PBS containing 1.0% BSA) were added at room temperature for 1 h. After one wash in PBS containing 0.1% Tween 20, intact extracellular vesicles were captured in a 96-well at a final volume of 100 μL and incubated for 3 h at room temperature. After three washes with PBS containing 0.1% Tween 20, biotinylated anti-CD63 antibodies (clone H5C6, cat. no. 353018; BioLegend) diluted to 4 μg/mL were added and incubated for 1.5 h at room temperature. After three washes with PBS containing 0.1% Tween 20, the plate was incubated with 50 μL of streptavidin-horseradish peroxidase (R&D Systems) diluted 1:200 in PBS for 20 min at room temperature. After the final four washes with PBS containing 0.1% Tween 20, the detection substrate reagent (cat. no. DY999; R&D Systems) was added at 100 μL per well and incubated for 4 min. The response was blocked with 0.1 mL of 1 M H2SO4, and optical densities were recorded at 450 nm. The standard curve prepared with pooled peak from Fig. 3 A, ii, was linear over a range of about 1–50 µg of protein (Fig. S1).
Surface Markers of EVs.
The assay was performed as described by Oksvold et al. (35). Human anti-CD63–coated magnetic beads (40 μL, cat. no. 106–06D; Life Technologies) were washed with PBS and mixed with 100 μL of samples. The mixture was incubated at 4 °C overnight with rotation, after which unbound or excess extracellular vesicles were removed using a magnetic rack (DynaMag-Spin; Life Technologies) to capture the beads and wash the beads twice with 500 μL of PBS containing 0.1% BSA. The washed beads were released from the rack and resuspended in 300 μL of PBS containing 0.1% BSA. The samples were incubated with the conjugated antibodies at room temperature for 30 min with rotating in the dark. The stained samples were washed twice by using a magnetic rack with PBS containing 0.1% BSA. The samples were assayed on a FC500 flow cytometer (Beckman-Coulter; https://www.beckmancoulter.com/wsrportal/wsr/index.htm) with antibodies indicated in Table S2.
Table S2.
Antibodies used for flow cytometry analyses
| Antibody | Origin | Vendor | Catalog no. |
| hMSC markers | |||
| CD29 | mIgG-1 | BD Biosciences | 559882 |
| CD44 | mIgG-2b | BD Biosciences | 559942 |
| CD49c | mIgG-1 | BD Biosciences | 556025 |
| CD49f | rIgG-2a | BD Biosciences | 551129 |
| CD59 | mIgG-2a | Beckman | IM3457U |
| CD73 | mIgG-1 | BD Biosciences | 550257 |
| CD90 | mIgG-1 | Beckman | IM3703 |
| CD105 | mIgG-3 | Beckman | A07414 |
| CD146 | mIgG-2a | Beckman | A07483 |
| CD147 | mIgG-2 | BD Biosciences | 555962 |
| CD166 | mIgG-1 | Beckman | A22361 |
| HLA-a, b, c | mIgG-1 | BD Biosciences | 555552 |
| PODXL | mIgG-2a | MBL | M084-4 |
| EV Markers | |||
| CD9 | migG-1 | BD Biosciences | 341647 |
| CD63 | migG-1 | BD Biosciences | 557288 |
| CD81 | migG-1 | BD Biosciences | 551112 |
Assay of EVs with Nanoparticle Tracking Analysis.
The concentration and size distribution of particles were measured by nanoparticle tracking analysis (Nanosight LM10; Malvern). Temperature was monitored throughout the measurements. For all our recordings, we used a camera level of 13 or 14 and automatic functions for all postacquisition settings, except for the detection threshold, which was set at 5. The instrument was standardized with polystyrene latex microsphere [cat. no. NTA4088 (100 nm) and no. NTA4089 (200 nm)] beads. Samples were diluted to achieve a particle count of between 2 × 108 and 1 × 109 per milliliter. They were dispersed briefly before the assay to dissociate aggregates.
Controlled Cortical Impact Injury.
All animals were treated in accordance with a protocol approved by the Institutional Animal Care and Use Committee of Texas A&M Health Science Center College of Medicine. Male C57BL/6J mice were purchased from The Jackson Laboratory and were 7–8 wk old at the time of controlled cortical impact (CCI). A CCI device (eCCI Model 6.3; Custom Design and Fabrication at Virginia Commonwealth University Medical Center) was used to induce TBI. Mice were anesthetized with 3% (vol/vol) sevoflurane in O2 and mounted in a stereotactic frame. An ∼4-mm craniotomy was performed over the right parietal cortex between the bregma and the lambda sutures. The impact, with a velocity of 4.5 m/s and a dwell time of 250 ms and a deformation depth of 1.0 mm using a 3-mm-diameter impactor tip was applied. After the injury, the bone fragment was put back in place and a disk made from dental cement was adhered to the skull using Vetbond tissue adhesive (3M). The scalp was fastened with sutures. Body temperature was maintained at 37 °C using a heating pad. One hour after CCI, the mouse was placed in a tail vein injection restrainer with warming water bath (40 °C), which restrained the animal and gently warmed the tail while allowing access to the tail vein. The hMSCs (donor 6015, 1 × 106 cells per mouse) or chromatographically concentrated CCM (3.8, 7.5, 15, 30 μg per mouse) in 200 μL of PBS were injected using a 29G insulin syringe.
Immunohistochemistry.
Animals were perfused with 4% (vol/vol) paraformaldehyde in PBS, and the brains were postfixed in the same fixative for 24 h. Brains were then cryoprotected in 20% (vol/vol) sucrose and sectioned (40–50 µm), and subsequent immunostaining was performed by the free-floating method. Briefly, after an antigen retrieval process for 30 min in 10 mM sodium citrate buffer (pH 8.5) at 80 °C, the brain slices were blocked and permeabilized with PBS-T (0.2% Triton X-100 in PBS) containing 2% (vol/vol) horse serum for 1 h, incubated with IL-1β (ab9722; Abcam) and GFAP (SC6170; Santa Cruz) antibodies overnight, incubated with Alexa 594-conjugated anti-rabbit IgG, Alexa 488-conjugated anti-rabbit IgG, and DAPI (1 µg/mL) for 30 min, mounted onto slides, and observed under a microscope (Nikon Eclipse 80i).
ELISA for IL-1β and IL-6 in Brain and for IL-10 in Plasma.
Twelve hours after CCI, the mouse was anesthetized with ketamine/xylazine. Blood was recovered by heart puncture in heparin-coated capillary blood collection tubes (Terumo), and it was centrifuged at 3,000 × g for 10 min at 4 °C for measuring IL-10 in plasma. The centrifugation step was repeated twice to minimize platelet contamination, and the clear plasma fraction was stored at −80 °C. The levels of IL-10 were measured by ELISA kit (R&D Systems). After collection of blood, the mouse was transcardially perfused with PBS. The right side of the injured brain was immediately collected and frozen at −80 °C. For protein extraction, the brain was sonicated on ice in lysis buffer PBS containing 1% Triton X-100, protease inhibitor mixture (complete ULTRA Tablet; Roche), and centrifuged at 20,000 × g for 10 min at 4 °C. Total protein concentrations were measured using a BCA Protein Assay Kit (Thermo) and adjusted to 10 mg/mL. The levels of IL-1β and IL-6 were determined in 0.5 mg of total protein using an ELISA kit (R&D Systems).
Detection of EVs in Plasma.
About 30 μg of chromatographically concentrated CM or 1 × 106 hMSCs (donors 6015 or 7074) were injected into the tail vein of a C57BL/6J mouse. Blood was collected by cardiac puncture at 0 min, 5 min, 15 min, and 30 min after i.v. injection. The mice were anesthetized with ketamine/xylazine, and blood was recovered in heparin-coated capillary blood collection tubes (Terumo). To separate plasma, the sample was centrifuged at 3,000 × g for 10 min at 4 °C. The levels of intact extracellular vesicles containing CD63 in plasma were determined by ELISA described above.
Morris Water Maze Test.
Mice were tested for spatial learning and memory function in the daylight period on days 28–33 after CCI using the Morris water maze paradigm. The water maze tank, a circular plastic pool measuring 120 cm in diameter and 60 cm in height, was filled with water maintained at 25 °C and made opaque with white paint. The extra-maze visual cues were hung on the walls surrounding the pool, and a hidden platform was submerged 1 cm below the surface of the water. The mouse was first taught to locate a square platform submerged in water within one of the four quadrants, using spatial cues. The swim path of mice in the water maze tank was continuously video-tracked and recorded using the computerized ANY-maze video-tracking system. There were five learning sessions over 5 d with four acquisition trials (90 s per one trial) per session, with an intertrial interval of 120 s. For each trial, the mouse was released into the water facing the wall of the pool in a pseudorandom manner so that each trial commenced from a different start location. Once the mouse reached the platform, it was allowed to stay there for 10 s. When the mouse failed to find the platform within the ceiling period of 90 s, it was guided into the platform where it stayed for 10 s. The location of the platform remained constant across all learning sessions. After each trial, the mouse was wiped thoroughly with dry towels, air-dried, and placed in the home cage. Tracking software (ANY-maze; San Diego Instruments) was used to record latency to find the platform, swim speed, and swim path. From the data, mean values were calculated for each parameter for every learning session and compared between groups. The latency to reach the submerged platform was measured as an indicator of learning ability. On the sixth day of testing (day 33 after CCI), a 90-s retention (probe) test was conducted in which the platform was removed. The mouse was released from a quadrant that was opposite to the position of the platform during learning sessions. The number of entries into the platform area and time spent in the platform area were calculated from the ANY-maze program.
Pattern Separation Test.
Pattern separation function is the ability to distinguish between similar experiences that require maintenance of hippocampal neurogenesis (37, 38). Mice are naturally attracted by novel objects, and this behavior can be easily quantified and used to study simple recognition memory in rodents. Mice were tested for pattern separation function in the daylight period on day 35 after CCI. Before starting the test, the mouse was acclimatized to the open field apparatus (45 × 45 cm each). The test involved three trials. In each trial, the behavior of the mouse was examined for 5 min. The apparatus was cleaned with 70% (vol/vol) alcohol and air-dried before the commencement of each trial for every mouse. In the first trial, the mouse was placed with two identical objects (object type 1) in an open field apparatus with a floor pattern (pattern type 1). For each trial, the mouse was placed on the middle of the two objects. At the end of 5 min, the mouse was placed back in its home cage. Thirty minutes after the first trial, each mouse went to trial 2. In this trial, the floor pattern was changed (pattern type 2) and a different object pair (object type 2) was used. At the end of 5 min, the mouse was placed back in its home cage. After a delay of 2 h, each mouse went to trial 3. During this trial, the floor pattern remained the same as in trial 2 (pattern type 2), but an object from trial 1 (object type 1) replaced one of the objects used in trial 2, which had now become a novel object for trial 3. The mouse explored for 5 min. Exploration of the novel object was defined as the length of time a mouse’s nose was 1 cm away from the object. Data, such as times spent in exploring the novel object (object type 1 on pattern type 2) and the familiar object (object type 2 on pattern type 2) and the total time spent in object exploration in trial 3, were measured. Furthermore, a novel object discrimination index was calculated by using the following formula: the time spent with the novel object/the total object exploration time × 100.
Statistical Tests.
Data are represented as mean SEM. Comparison of three or more groups was performed using one-way ANOVA with Tukey's multiple comparison test. Two-way ANOVA with Bonferroni posttests was carried out for the water maze learning test. A P value of less than 0.05 was considered to be statistically significant.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1522297113/-/DCSupplemental.
References
- 1.Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7(8):728–741. doi: 10.1016/S1474-4422(08)70164-9. [DOI] [PubMed] [Google Scholar]
- 2.Heneka MT, Kummer MP, Latz E. Innate immune activation in neurodegenerative disease. Nat Rev Immunol. 2014;14(7):463–477. doi: 10.1038/nri3705. [DOI] [PubMed] [Google Scholar]
- 3.Lozano D, et al. Neuroinflammatory responses to traumatic brain injury: Etiology, clinical consequences, and therapeutic opportunities. Neuropsychiatr Dis Treat. 2015;11:97–106. doi: 10.2147/NDT.S65815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reis C, et al. What’s new in traumatic brain injury: Update on tracking, monitoring and treatment. Int J Mol Sci. 2015;16(6):11903–11965. doi: 10.3390/ijms160611903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loane DJ, Stoica BA, Faden AI. Neuroprotection for traumatic brain injury. Handb Clin Neurol. 2015;127:343–366. doi: 10.1016/B978-0-444-52892-6.00022-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prockop DJ, Kota DJ, Bazhanov N, Reger RL. Evolving paradigms for repair of tissues by adult stem/progenitor cells (MSCs) J Cell Mol Med. 2010;14(9):2190–2199. doi: 10.1111/j.1582-4934.2010.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keating A. Mesenchymal stromal cells: New directions. Cell Stem Cell. 2012;10(6):709–716. doi: 10.1016/j.stem.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 8.Kramann R, et al. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell. 2015;16(1):51–66. doi: 10.1016/j.stem.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwarz EJ, Alexander GM, Prockop DJ, Azizi SA. Multipotential marrow stromal cells transduced to produce L-DOPA: Engraftment in a rat model of Parkinson disease. Hum Gene Ther. 1999;10(15):2539–2549. doi: 10.1089/10430349950016870. [DOI] [PubMed] [Google Scholar]
- 10.Hofstetter CP, et al. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci USA. 2002;99(4):2199–2204. doi: 10.1073/pnas.042678299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohtaki H, et al. Stem/progenitor cells from bone marrow decrease neuronal death in global ischemia by modulation of inflammatory/immune responses. Proc Natl Acad Sci USA. 2008;105(38):14638–14643. doi: 10.1073/pnas.0803670105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qu C, et al. Treatment of traumatic brain injury in mice with marrow stromal cells. Brain Res. 2008;1208:234–239. doi: 10.1016/j.brainres.2008.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim PK, Patel SA, Gregory LA, Rameshwar P. Neurogenesis: Role for microRNAs and mesenchymal stem cells in pathological states. Curr Med Chem. 2010;17(20):2159–2167. doi: 10.2174/092986710791299894. [DOI] [PubMed] [Google Scholar]
- 14.Joyce N, et al. Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen Med. 2010;5(6):933–946. doi: 10.2217/rme.10.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uccelli A, Benvenuto F, Laroni A, Giunti D. Neuroprotective features of mesenchymal stem cells. Best Pract Res Clin Haematol. 2011;24(1):59–64. doi: 10.1016/j.beha.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Kocsis JD, Honmou O. Bone marrow stem cells in experimental stroke. Prog Brain Res. 2012;201:79–98. doi: 10.1016/B978-0-444-59544-7.00005-6. [DOI] [PubMed] [Google Scholar]
- 17.Forostyak S, Jendelova P, Sykova E. The role of mesenchymal stromal cells in spinal cord injury, regenerative medicine and possible clinical applications. Biochimie. 2013;95(12):2257–2270. doi: 10.1016/j.biochi.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Zhang R, et al. Anti-inflammatory and immunomodulatory mechanisms of mesenchymal stem cell transplantation in experimental traumatic brain injury. J Neuroinflammation. 2013;10:106. doi: 10.1186/1742-2094-10-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng W, et al. Systematic review and meta-analysis of efficacy of mesenchymal stem cells on locomotor recovery in animal models of traumatic brain injury. Stem Cell Res Ther. 2015;6:47. doi: 10.1186/s13287-015-0034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prockop DJ, Oh JY. Mesenchymal stem/stromal cells (MSCs): Role as guardians of inflammation. Mol Ther. 2012;20(1):14–20. doi: 10.1038/mt.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee RH, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5(1):54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oh JY, et al. Anti-inflammatory protein TSG-6 reduces inflammatory damage to the cornea following chemical and mechanical injury. Proc Natl Acad Sci USA. 2010;107(39):16875–16880. doi: 10.1073/pnas.1012451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi H, Lee RH, Bazhanov N, Oh JY, Prockop DJ. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-κB signaling in resident macrophages. Blood. 2011;118(2):330–338. doi: 10.1182/blood-2010-12-327353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oh JY, et al. Intravenous mesenchymal stem cells prevented rejection of allogeneic corneal transplants by aborting the early inflammatory response. Mol Ther. 2012;20(11):2143–2152. doi: 10.1038/mt.2012.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibb SL, et al. TIMP3 attenuates the loss of neural stem cells, mature neurons and neurocognitive dysfunction in traumatic brain injury. Stem Cells. 2015;33(12):3530–3544. doi: 10.1002/stem.2189. [DOI] [PubMed] [Google Scholar]
- 26.Yáñez-Mó M, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lo Cicero A, Stahl PD, Raposo G. Extracellular vesicles shuffling intercellular messages: For good or for bad. Curr Opin Cell Biol. 2015;35:69–77. doi: 10.1016/j.ceb.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 28.Heldring N, Mäger I, Wood MJ, Le Blanc K, Andaloussi SE. Therapeutic potential of multipotent mesenchymal stromal cells and their extracellular vesicles. Hum Gene Ther. 2015;26(8):506–517. doi: 10.1089/hum.2015.072. [DOI] [PubMed] [Google Scholar]
- 29.György B, Hung ME, Breakefield XO, Leonard JN. Therapeutic applications of extracellular vesicles: Clinical promise and open questions. Annu Rev Pharmacol Toxicol. 2015;55:439–464. doi: 10.1146/annurev-pharmtox-010814-124630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, et al. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J Neurosurg. 2015;122(4):856–867. doi: 10.3171/2014.11.JNS14770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phinney DG, et al. Donor variation in the growth properties and osteogenic potential of human marrow stromal cells. J Cell Biochem. 1999;75(3):424–436. [PubMed] [Google Scholar]
- 32.Montzka K, et al. Neural differentiation potential of human bone marrow-derived mesenchymal stromal cells: Misleading marker gene expression. BMC Neurosci. 2009;10:16. doi: 10.1186/1471-2202-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siddappa R, Licht R, van Blitterswijk C, de Boer J. Donor variation and loss of multipotency during in vitro expansion of human mesenchymal stem cells for bone tissue engineering. J Orthop Res. 2007;25(8):1029–1041. doi: 10.1002/jor.20402. [DOI] [PubMed] [Google Scholar]
- 34.Lee RH, et al. TSG-6 as a biomarker to predict efficacy of human mesenchymal stem/progenitor cells (hMSCs) in modulating sterile inflammation in vivo. Proc Natl Acad Sci USA. 2014;111(47):16766–16771. doi: 10.1073/pnas.1416121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oksvold MP, Neurauter A, Pedersen KW. Magnetic bead-based isolation of exosomes. Methods Mol Biol. 2015;1218:465–481. doi: 10.1007/978-1-4939-1538-5_27. [DOI] [PubMed] [Google Scholar]
- 36.Pati S, et al. Human mesenchymal stem cells inhibit vascular permeability by modulating vascular endothelial cadherin/β-catenin signaling. Stem Cells Dev. 2011;20(1):89–101. doi: 10.1089/scd.2010.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leutgeb S, Leutgeb JK. Pattern separation, pattern completion, and new neuronal codes within a continuous CA3 map. Learn Mem. 2007;14(11):745–757. doi: 10.1101/lm.703907. [DOI] [PubMed] [Google Scholar]
- 38.Yassa MA, Stark CE. Pattern separation in the hippocampus. Trends Neurosci. 2011;34(10):515–525. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sekiya I, et al. Expansion of human adult stem cells from bone marrow stroma: Conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells. 2002;20(6):530–541. doi: 10.1634/stemcells.20-6-530. [DOI] [PubMed] [Google Scholar]
- 40.Prockop DJ, Keating A. Relearning the lessons of genomic stability of human cells during expansion in culture: Implications for clinical research. Stem Cells. 2012;30(6):1051–1052. doi: 10.1002/stem.1103. [DOI] [PubMed] [Google Scholar]
- 41.Boregowda SV, et al. Atmospheric oxygen inhibits growth and differentiation of marrow-derived mouse mesenchymal stem cells via a p53-dependent mechanism: implications for long-term culture expansion. Stem Cells. 2012;30(5):975–987. doi: 10.1002/stem.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharma RR, Pollock K, Hubel A, McKenna D. Mesenchymal stem or stromal cells: A review of clinical applications and manufacturing practices. Transfusion. 2014;54(5):1418–1437. doi: 10.1111/trf.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu KD, et al. Design and implementation of the START (STem cells for ARDS Treatment) trial, a phase 1/2 trial of human mesenchymal stem/stromal cells for the treatment of moderate-severe acute respiratory distress syndrome. Ann Intensive Care. 2014;4:22. doi: 10.1186/s13613-014-0022-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sekiya I, Muneta T, Horie M, Koga H. Arthroscopic transplantation of synovial stem cells improves clinical outcomes in knees with cartilage defects. Clin Orthop Relat Res. 2015;473(7):2316–2326. doi: 10.1007/s11999-015-4324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spees JL, Olson SD, Whitney MJ, Prockop DJ. Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci USA. 2006;103(5):1283–1288. doi: 10.1073/pnas.0510511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Islam MN, et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18(5):759–765. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pochampally RR, Smith JR, Ylostalo J, Prockop DJ. Serum deprivation of human marrow stromal cells (hMSCs) selects for a subpopulation of early progenitor cells with enhanced expression of OCT-4 and other embryonic genes. Blood. 2004;103(5):1647–1652. doi: 10.1182/blood-2003-06-1967. [DOI] [PubMed] [Google Scholar]
- 48.Vallabhaneni KC, et al. Extracellular vesicles from bone marrow mesenchymal stem/stromal cells transport tumor regulatory microRNA, proteins, and metabolites. Oncotarget. 2015;6(7):4953–4967. doi: 10.18632/oncotarget.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Phinney DG, et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun. 2015;6:8472. doi: 10.1038/ncomms9472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cuiffo BG, et al. MSC-regulated microRNAs converge on the transcription factor FOXP2 and promote breast cancer metastasis. Cell Stem Cell. 2014;15(6):762–774. doi: 10.1016/j.stem.2014.10.001. [DOI] [PubMed] [Google Scholar]