Significance
This study describes hybrid single ion-conducting electrolytes based on inorganic sulfide glasses and perfluoropolyether polymers for lithium batteries. Herein, it is shown that hybrid electrolytes provide a compelling alternative to the traditional glass, ceramic, or polymer battery electrolytes. These electrolytes present high transference numbers, unprecedented ionic conductivities at room temperature, and excellent electrochemical stability, and they limit the dissolution of lithium polysulfides. The results in this work represent a significant step toward addressing the challenges of enabling the next generation cathodes, such as lithium nickel manganese cobalt oxide and sulfur.
Keywords: hybrid electrolytes, inorganic sulfide glasses, fluorinated polymers, lithium batteries, lithium-sulfur batteries
Abstract
Despite high ionic conductivities, current inorganic solid electrolytes cannot be used in lithium batteries because of a lack of compliance and adhesion to active particles in battery electrodes as they are discharged and charged. We have successfully developed a compliant, nonflammable, hybrid single ion-conducting electrolyte comprising inorganic sulfide glass particles covalently bonded to a perfluoropolyether polymer. The hybrid with 23 wt% perfluoropolyether exhibits low shear modulus relative to neat glass electrolytes, ionic conductivity of 10−4 S/cm at room temperature, a cation transference number close to unity, and an electrochemical stability window up to 5 V relative to Li+/Li. X-ray absorption spectroscopy indicates that the hybrid electrolyte limits lithium polysulfide dissolution and is, thus, ideally suited for Li-S cells. Our work opens a previously unidentified route for developing compliant solid electrolytes that will address the challenges of lithium batteries.
Electrolytes used in lithium ion batteries that power personal electronic devices and electric vehicles comprise lithium salts dissolved in flammable organic liquids. Catastrophic battery failure often begins with the electrolyte decomposition and combustion. In addition, side reactions between the electrolyte and anode particles result in steady capacity fade. Some of the byproducts of side reactions can dissolve in the electrolyte and migrate from one electrode to the other. This effect is minimized in the case of solid electrolytes because of limited solubility and slow diffusion (1). Mixtures of liquids and salts have additional limitations. The passage of current results in an accumulation of salt in the vicinity of one electrode and depletion close to the other electrode, because only the cation participates in the electrochemical reactions. Both overconcentrated and depleted electrolytes have lower conductivity, which accentuates cell polarization and reduces power capability. Concentration polarization is absent in single-ion conductors, wherein the anions are immobilized (2). Nonflammable, single ion-conducting solid electrolytes have the potential to dramatically improve safety and performance of lithium batteries (3–6).
Solid electrolytes, such as inorganic sulfide glasses (Li2S–P2S5), are single-ion conductors with high shear moduli (18–25 GPa) and high ionic conductivity (over 10−4 S/cm) at room temperature (7, 8). However, these materials, on their own, cannot serve as efficient electrolytes, because they cannot adhere to moving boundaries of the active particles in the battery electrode as they are charged and discharged. Hayashi et al. (9) prepared hybrid electrolytes by mixing sulfide glasses and poly(ethylene oxide) (PEO) polymers. Although the addition of PEO improves mechanical flexibility, there is a dramatic decrease in ionic conductivity because of the insulative nature of PEO. For example, the addition of 17 weight percent (wt%) of PEO [molecular weight (Mw) of 400 g/mol] results in a 100-fold decrease in the ionic conductivity. If the polymer weight fraction is reduced to 2 wt%, then there is no decrease in the measured ionic conductivity. It is, however, unlikely that the mechanical properties of such a composite would differ substantially from those of the pure glass electrolyte. The conductivity of the polymeric phase in the electrolyte may be improved by the addition of salt, but then, the hybrid is no longer a single-ion conductor. Recent studies indicate that interfaces between conventional electrolytes and single-ion conductors are characterized by prohibitively large interfacial impedances (10). In addition to losing the key advantage of single-ion conduction, hybrids of inorganic single-ion conductors and conventional electrolytes containing salt are likely to have low ionic conductivity because of this effect.
Recently, Wong et al. (11) reported on single ion-conducting polymer electrolytes based on perfluoropolyether (PFPE) random copolymers. The lithium transference number, t+, was between 0.91 and 1.0. In this paper, we describe the synthesis and characterization of flexible hybrid electrolytes comprising sulfide glasses with covalently grafted PFPE chains and added lithium salt. These hybrid solid electrolytes have high ionic conductivities (10−4 S/cm) at room temperature, transference numbers close to the unity, and a large electrochemical stability window.
Results and Discussion
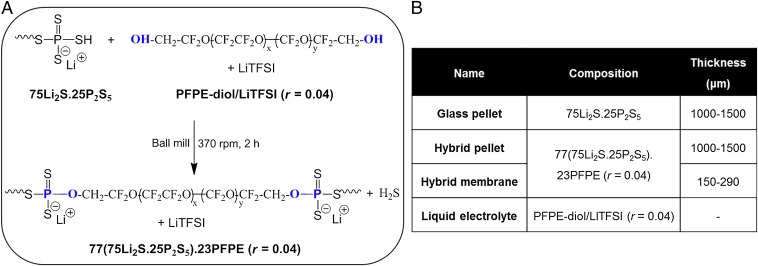
Following the work by Hayashi et al. (9), single ion-conducting hybrid solid electrolytes were prepared by conducting mechanochemical reactions between sulfide glass (75Li2S.25P2S5), hydroxy-terminated perfluoropolyether (PFPE-diol, average molecular weight 1 kg/mol), and lithium bis(trifluoromethane) sulfonimide (LiTFSI) in a planetary ball mill. We have replaced the hydroxyl-terminated PEO used by Hayashi et al. (9) with PFPE-diol. The molar ratio, r, of Li+ ions to perfluoroalkylene oxide moieties in the chain was fixed at 0.04. The mechanochemical reaction is presented in Fig. 1A. Reactions between lithiated phosphorus sulfide groups in the glass and hydroxyl groups on PFPE-diol result in a glass–PFPE composite with the elimination of H2S. The final product is a hybrid solid electrolyte made by combining 77 wt% (75Li2S.25P2S5) and 23 wt% PFPE-diol/LiTFSI (r = 0.04).
Fig. 1.
Hybrid single ion-conducting electrolytes. (A) Synthesis of the hybrid electrolyte 77(75Li2S.25P2S5).23PFPE (r = 0.04) by mechanochemical reaction. (B) Characteristics of all of the electrolytes used in this study.
We focus on three samples, which are named glass pellet, hybrid pellet, and hybrid membrane. In addition, we also studied a liquid electrolyte comprising a mixture of PFPE-diol and LiTFSI with r = 0.04 prepared using the method described in ref. 11. The compositions of all three electrolytes are given in Fig. 1B.
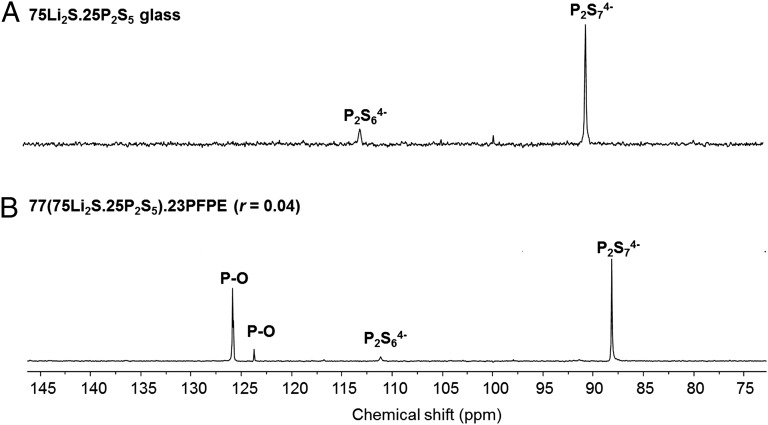
Fig. 2 shows 31P-NMR spectra of the pure glass and hybrid electrolyte obtained by ball milling. 31P-NMR spectrum of the glass shows two peaks at 90 and 113 ppm. These chemical shifts are in good agreement with those reported for P2S74− and P2S64−, respectively (12). In previous studies, this class of electrolyte exhibits either two or three NMR peaks. Although the results in Fig. 2A are qualitatively consistent with the literature, quantitative differences remain. It is not clear if these differences are because of differences in the sample preparation method or characterization methods (solution vs. solid NMR samples) or intrinsic differences in the nature of the glass prepared. The 31P-NMR spectrum of the hybrid electrolyte shows two new peaks at 124 and 126 ppm, which are not present in the pure glass. These peaks are attributed to P-O bonds. They confirm the reaction between the P-SH groups of the glass and the OH groups of PFPE-diol polymer. The two peaks in Fig. 2B indicate the presence of two chemical environments. Additional characterization is required to determine the origin of these environments.
Fig. 2.
31P-NMR characterization. 31P-NMR spectra of (A) the inorganic sulfide glass electrolyte (75Li2S.25P2S5) and (B) the hybrid electrolyte [77(75Li2S.25P2S5).23PFPE (r = 0.04)].
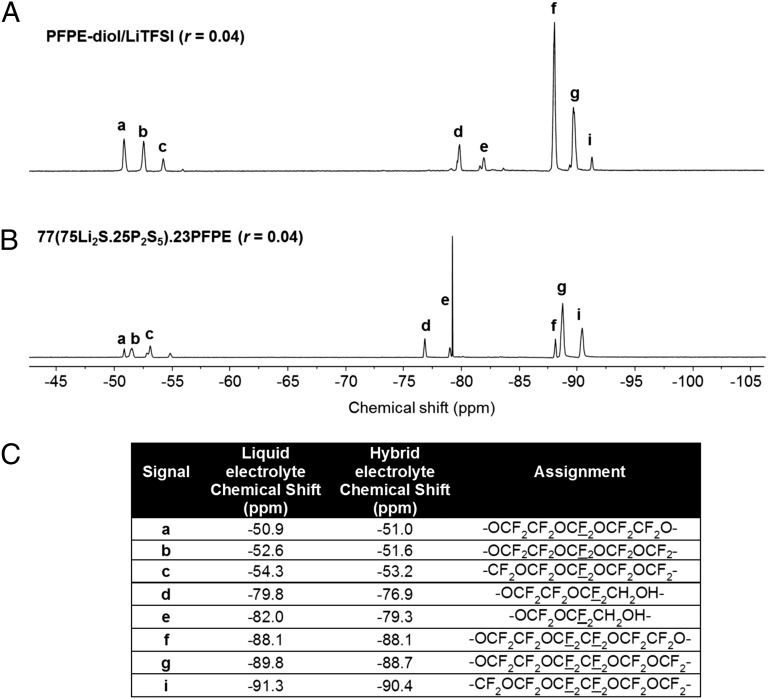
19F-NMR measurements were performed to ensure that ball milling did not degrade the PFPE polymer. Fig. 3A shows the NMR spectrum of the neat liquid electrolyte, whereas Fig. 3B shows the NMR spectrum of a suspension of the hybrid electrolyte in deuterated THF-d8. The spectra are similar, indicating that PFPE chains remain intact during the milling process. Fig. 3C shows the peak position obtained from these two systems. The biggest differences in the chemical shifts are seen in the fluorine atoms near the chain ends (signals d and e in Fig. 3). We attribute this to the presence of P-O bonds caused by the reaction with the glass. Although we have clear evidence for the reaction between PFPE chains and glass particles shown in Fig. 1A, we cannot assert that all of the chains in our hybrid are attached to the particles.
Fig. 3.
19F-NMR characterization.19F-NMR spectra of (A) the liquid electrolyte [PFPE-diol/LiTFSI (r = 0.04)] and (B) the hybrid electrolyte [77(75Li2S.25P2S5).23PFPE (r = 0.04)]. (C) Chemical shifts of 19F-NMR of the liquid electrolyte and the hybrid electrolyte.
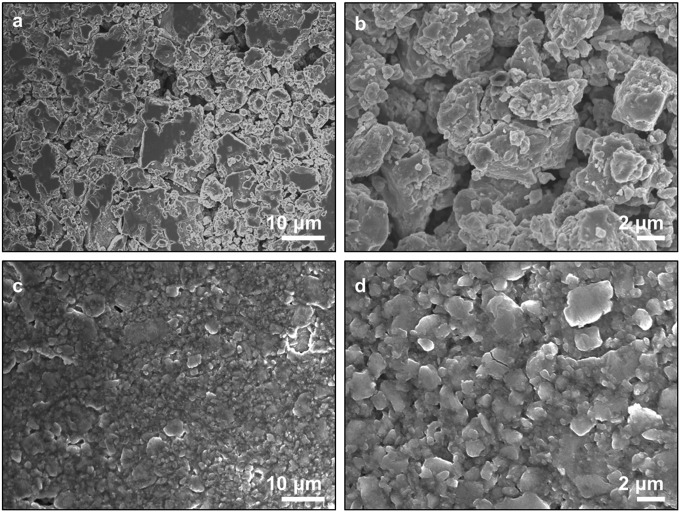
The morphologies of the surfaces of the sulfide glass pellet and the hybrid membrane were studied by SEM, and the results are shown in Fig. 4. The glass pellet (Fig. 4 A and B) shows 1- to 10-μm-sized particles, with voids between the particles. The hybrid membrane (Fig. 4 C and D) exhibits much smaller particles with relatively few voids. We expect significantly faster ion transport in the hybrid membrane because of the lower void fraction.
Fig. 4.
Morphology characterization. SEM images of (A and B) the sulfide glass pellet and (C and D) the hybrid electrolyte membrane.
SEM/energy-dispersive X-ray spectroscopy (EDS) images were obtained to determine the morphology and chemical composition of the hybrid (Methods). SI Appendix, Fig. S1A shows the SEM of the sample from which we determined elemental maps. The electron spectrum of our sample is shown in SI Appendix, Fig. S1E. As expected, the spectrum is dominated by sulfur (S), phosphorus (P), and fluorine (F). All three components are distributed more or less uniformly as shown in SI Appendix, Fig. S1 B–D, which show sulfur, phosphorus, and fluorine, respectively. The intensity of fluorine (SI Appendix, Fig. S1D) is lower than that of sulfur and phosphorous (SI Appendix, Fig. S1 B and C) because of the low volume fraction of PFPE in the hybrid. These results confirm the presence of PFPE chains in the hybrid and are consistent with 31P-NMR results. Additional work is needed to determine the geometry of inorganic and organic phases in our hybrid.
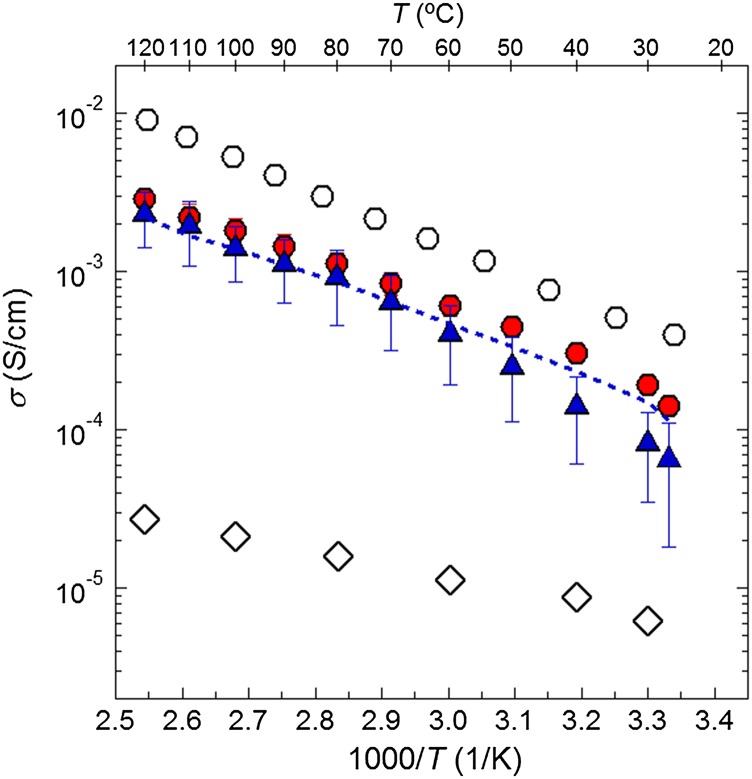
The temperature dependence of the ionic conductivity (σ) of the glass pellet, the hybrid membrane, and the liquid electrolyte are shown in Fig. 5. A typical ac impedance spectrum obtained from the hybrid electrolyte is shown in SI Appendix, Fig. S2. The spectrum is dominated by a single relaxation process. The conductivity of a sulfide glass reported by Minami et al. (13) is also shown in Fig. 5. The glass obtained by Minami et al. (13) presents a conductivity that is a factor of 2.5 larger than that of our sulfide glass pellet. It is likely that this is because of the differences in pressure used to densify the samples. The ionic conductivity of our sulfide glass is higher than that of the PFPE-diol/LiTFSI liquid electrolyte by a factor that ranges from 20 to 100. The conductivity of our hybrid membrane is only a factor of 0.4 to 0.8 lower than that of our sulfide glass. In an attempt to predict the ionic conductivity of the hybrid, the following equation was used (ignoring any tortuosity):
| [1] |
where ϕglass, the volume fraction of the sulfide glass, is 0.76, and ϕPFPE-diol/LiTFSI, the volume fraction of the PFPE-diol/LiTFSI electrolyte, is 0.24 (based on glass density, dglass = 1.9 g/cm3 and PFPE-diol/LiTFSI density, dPFPE-diol/LiTFSI = 1.8 g/cm3). As Fig. 5 shows, the values of ionic conductivity predicted are very similar to those obtained experimentally. It is evident that we have obtained a unique solid electrolyte that exhibits ionic conductivity of 10−4 S/cm at 30 °C, despite the fact that it contains 23 wt% polymer.
Fig. 5.
Ionic conductivity, σ, as a function of the inverse of the temperature. (red ○) Glass pellet, (blue △) hybrid membrane, (○) glass pellet described in ref. 13, and (♢) PFPE-diol/LiTFSI described in ref. 11. The dashed curve shows the calculated conductivity of the hybrid using Eq. 1.
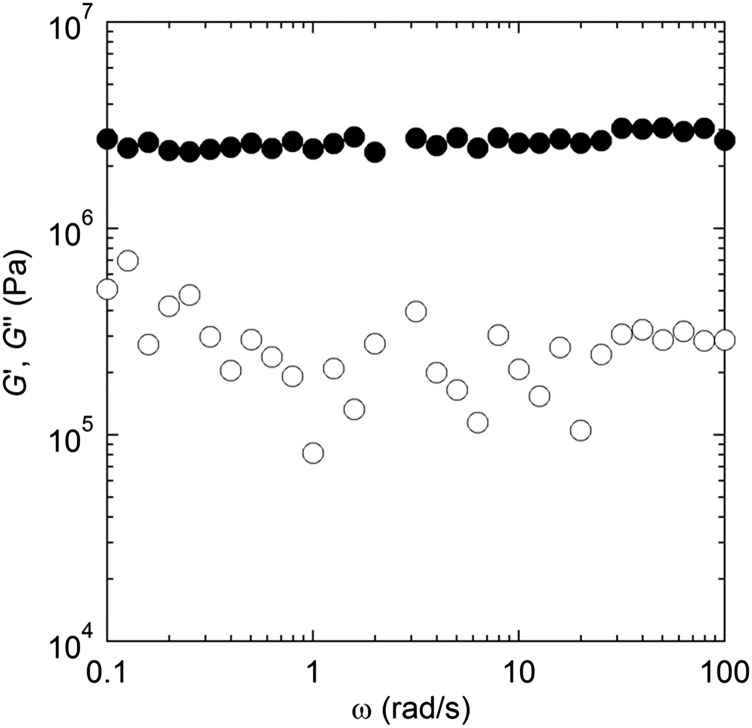
The mechanical properties of electrolytes affect the adhesion at the electrode–electrolyte interface during cycling. Adhesion is important in solid electrolytes, because intimate contact between the electrolyte and active particles is essential for a functioning battery. In Fig. 6, we show the frequency (ω) dependence of the storage (G′) and loss (G″) shear moduli of the hybrid electrolyte at 30 °C. Throughout our frequency window, G′ is much greater than G″, and both moduli are independent of the frequency. These characteristics are hallmarks of elastic solids. The noise in the G″ data is caused by the fact that G′ >> G″ (i.e., the out-of-phase stress signal is very weak compared with the in-phase stress signal). Interpretation of the measured value of G′ in terms of microstructure is nontrivial because of the fact that the strain is expected to be localized in the polymeric phase. PFPE-diol is a viscous liquid, with room temperature viscosity of 0.12 Pa.s. The shear modulus of our hybrid electrolyte is 2.6 MPa, three orders of magnitude lower than that of a glass sulfide reported by Sakuda et al. (8), which exhibited a shear modulus of 5.9 GPa (sample prepared with a molding pressure of 360 MPa). The composite electrolyte, thus, has drastically improved the adhesive properties of the electrolyte with a relatively minor effect on ionic conductivity.
Fig. 6.
Linear rheology measurements. Frequency (ω) dependency of (●) storage (G′) and (○) loss (G″) moduli for hybrid electrolyte measured at 30 °C.
It is important to establish that the introduction of PFPE-diol/LiTFSI does not reduce the single-ion conduction property of the composite electrolyte. This property is quantified by the cation transference number (the fraction of the total current carried by the cation). In single ion-conducting electrolytes, the cation transference number is unity because of the lack of mobility of the anion. Ohm’s law should be observed in the limit of small dc potentials in a single-ion conductor. However, in conventional electrolytes comprising two mobile charge carriers, concentration polarization will lead to large deviations from Ohm’s law. The hybrid membrane electrolyte exhibits behavior similar to that of a single-ion conductor. SI Appendix, Fig. S3 shows the time-dependence of current after applying a dc potential of 80 mV for 1 h, while the inset in Fig. S3 shows the ac impedance spectra obtained before applying the potential and after the completion of the dc experiment. The measured total resistance after completing the experiment was 5,215 Ω⋅cm2, and the measured current density, im, was 1.91 × 10−1 mA⋅cm−2. The current density expected from Ohm’s law, i0, is 1.94 × 10−1 mA⋅cm−2, which gives im/i0 = 0.99. We, thus, estimate that the lithium transference number, t+, of our hybrid electrolyte is 0.99 (i.e., most of the current in the hybrid membrane electrolyte is carried by Li+).
The electrochemical stability of the hybrid membrane was investigated by cyclic voltammetry as shown in SI Appendix, Fig. S4. The measurements were performed at 30 °C in the potential range between −0.5 and 5.0 V (vs. Li+/Li) at a scan rate of 1 mV/s. The low potential current corresponds to the reduction and oxidation of the Li+/Li0 couple, where lithium ions are reduced to Li metal at negative potentials and then stripped from the lithium metal electrode during the subsequent oxidation at 0.3 V (14). Throughout the 1.5- to 5-V potential range, the current density remains low, indicating that our hybrid electrolyte is stable up to 5 V. Thus, we expect our hybrid electrolyte to be suitable for use in a lithium battery comprising a high-potential positive active material, such as lithium nickel manganese cobalt oxide (NMC).
One potential application of these electrolytes is for lithium-sulfur (Li-S) batteries, which are known to suffer from the issue of lithium polysulfide dissolution. Lithium polysulfide reaction intermediates (Li2Sx; 2 ≤ x ≤ 8) formed during the Li-S charge/discharge reaction processes are highly soluble in many battery electrolytes. Thus, after their formation, polysulfides may diffuse out of the cathode and into the electrolyte separator, causing capacity to fade and leading to degradation reactions at the lithium anode. Solid inorganic electrolytes have become an increasingly popular approach to resolve this issue, because they prevent polysulfide dissolution while allowing for the passage of lithium ions (15).
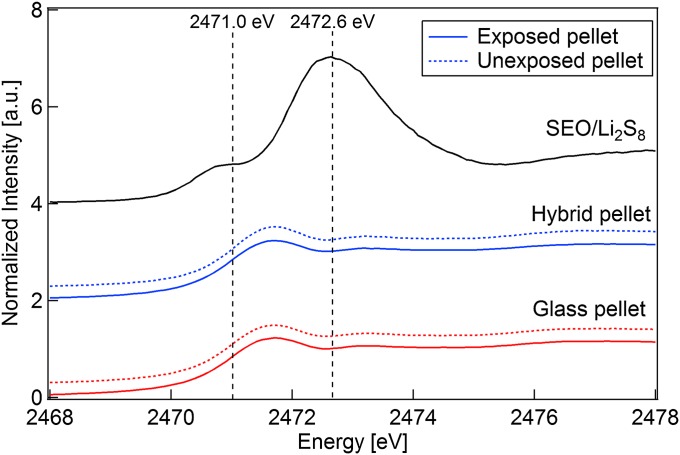
In recent studies, X-ray absorption spectroscopy (XAS) at the sulfur K edge has been used to detect the presence of lithium polysulfide intermediates in Li-S battery electrolytes (16, 17). The benefit of XAS is that it is an element-specific spectroscopic probe of both the electronic structure and the local environment surrounding sulfur atoms. The spectra for lithium polysulfide dianions are characterized by two spectral features: a main edge peak at 2,472.6 eV attributed to internal, neutrally charged sulfur and a pre-edge peak at 2,471.0 eV caused by the charged terminal sulfur atoms (17). These distinguishing features allow one to use XAS to determine whether polysulfides are present in the medium being probed.
To test whether polysulfide species are soluble in the hybrid electrolyte, a hybrid pellet was pressed against a solid polymer electrolyte loaded with Li2S8, a polysulfide molecule with solubility and diffusivity that are representative of all other lithium polysulfide species. The polymer electrolyte was a polystyrene-b-poly(ethylene oxide) (SEO) copolymer, and solubility of Li2S8 in this electrolyte was studied by Wujcik et al. (16). The two solids were contacted by hand-pressing at 75 °C for 3 d. A small piece of the hybrid pellet was taken, and XAS was performed on the side of the pellet that was in direct contact with the SEO/Li2S8 membrane. Similarly, sulfide glass pellets were also exposed to SEO/Li2S8. We present data obtained after 3 d of exposure time. XAS at the sulfur K-edge was performed on glass and hybrid pellets after exposure to Li2S8. XAS spectra were also taken of glass and hybrid pellets that were not exposed to Li2S8. These spectra serve as backgrounds; note that both glass and hybrid samples contain sulfur.
In Fig. 7, we show the sulfur K-edge spectra of Li2S8 obtained by performing XAS on a film of SEO that contained Li2S8. If Li2S8 was present in the pellets, the resulting spectrum for each exposed pellet would be a linear combination of the Li2S8 and the unexposed pellet spectra. The spectra for the unexposed and exposed pellets are identical for both the hybrid and glass pellets. This result indicates that neither the hybrid nor the glass pellets contain Li2S8. The exclusion of Li2S8 from hybrid electrolyte may, at first, seem counterintuitive because of the presence of PFPE. Note, however, that solubility of lithium-containing salts in PFPE is driven by the fluorinated anions that are absent in lithium polysulfides (11). According to the data in Fig. 7, our hybrid electrolyte would be ideally suited for lithium-sulfur cells because of the insolubility of the lithium polysulfide intermediates.
Fig. 7.
Normalized sulfur K-edge XAS of SEO/Li2S8, glass pellet, and hybrid pellet exposed to lithium polysulfides. For pellet spectra, lines denote unexposed pellets, and dashed lines denote pellets that were pressed to the SEO/Li2S8 membrane for 3 d at 75 °C.
Conclusion
We have successfully developed nonflammable hybrid single ion-conducting electrolytes comprising inorganic sulfide glass particles covalently bonded to a PFPE polymer. The hybrid with 23 wt% PFPE exhibits low shear modulus relative to neat glass electrolyte, unprecedented ionic conductivity (about 10−4 S/cm at room temperature), cation transference number close to the unity, and a wide electrochemical stability window (up to 5 V). Furthermore, XAS measurements indicate that this hybrid electrolyte limits the dissolution of lithium polysulfides and thus, is ideally suited for Li-S cells. Although we have shown that 150-μm-thick membranes can be made in a simple press, we anticipate that thinner membranes can be made by more sophisticated processing techniques. High-voltage cathodes can be prepared by ball milling the active particles with our electrolyte. Although much work remains to be done, we believe that our work opens a previously unidentified route for developing hybrid solid electrolytes that will address the current challenges of lithium batteries.
Methods
Hybrid Electrolyte Preparation.
Predetermined amounts of crystalline Li2S and P2S5 powders were used as starting materials to get 75Li2S·25P2S5 mol% by ball milling. The powders were placed in a zirconia jar (volume of 50 mL) with eight zirconia balls (10 mm in diameter). A glassy powder was obtained after mechanical milling for 15 h at 510 rpm and room temperature under argon. Based on the work described in ref. 9, lithium ion-conducting hybrid solid electrolytes were prepared by ball milling the obtained sulfide glass, PFPE-diol, and LiTFSI. The same zirconia jar and balls were used, and the mixtures were milled for 2 h at 370 rpm and room temperature under argon. The final product is a hybrid solid electrolyte 77(75Li2S·25P2S5).23PFPE (r = 0.04).
Characterization of Glass and Hybrid Electrolytes.
19F and 31P NMR spectra were obtained on a Bruker Advance 400 and 600 Spectrometer in CDCl3 or deuterated THF-d8. The morphological characterizations of the sulfide glass pellets and hybrid membranes were accomplished by SEM/EDS. All of the samples were prepared and measured under argon. SEM experiments were performed on a JEOL-7500F Field Emission Microscope, with accelerating voltages of 5 kV for SEM measurements and 10 kV for EDS measurements. For linear rheology measurements, a hybrid pellet of 8-mm diameter and 1.5-mm thickness was prepared using the same method as the hybrid pellets for conductivity measurements. The rheology measurements were performed in a Rheometric Scientific ARES Rheostat. The rheometer plattens were cleaned and heated to 30 °C under nitrogen. The platen gap position was zeroed, and then, the sample was placed between the plattens. The plattens were then heated to 30 °C, and the sample was left to equilibrate for 1 h. At each measurement temperature, a dynamic strain test was performed at a frequency of 10 rad/s to ensure measurement in the linear regime. Then, a dynamic frequency test was performed at a low strain in the linear regime.
AC Impedance Measurements.
Aluminum symmetric cells were assembled in the glovebox using the inorganic sulfide glass or the hybrid as electrolyte. Glass and hybrid pellets were obtained in pneumatic cold (57 and 23 MPa, respectively). After ball milling, the glass powder was placed in a pellet die between two mirror-polished aluminum electrodes. The diameter and thickness of the pellets were 13 mm and about 1 mm, respectively. The aluminum current collector tabs are placed on each electrode. Hybrid electrolyte membranes (thickness about 250 μm) were obtained using a manual press. The hybrid electrolyte powder from the ball mill was placed at the center of an insulating spacer in the press with a 3.17-mm diameter central hole and two mirror-polished aluminum electrodes to ensure good contact between the electrodes and the electrolyte. The press was heated to 90 °C for 5 s. Aluminum current collector tabs are placed on each electrode. Finally, both pellets and hybrid membranes were vacuum-sealed in a pouch bag to isolate them from air. Impedance spectroscopy measurements were performed using a VMP3 (Bio-Logic) with an ac amplitude of 50 mV in the frequency range 1 MHz to 1 Hz. Impedance spectra were recorded at 10 °C intervals during heating and cooling scans in the temperature, T, range from 27 °C to 120 °C. The ionic conductivity, σ, of the conducting phase in the glass electrolyte or hybrid electrolyte is calculated from the measured sample thickness, l, the cross-sectional area S of the spacer, and electrolyte resistance Rel; σ(Τ) is given by σ(T) = l/(S × Rel (T)).
XAS Measurements.
XAS was performed at beamline 4–3 at the Stanford Synchrotron Radiation Lightsource. Preliminary work was performed at beamline 9.3.1 of the Advanced Light Source. Samples were transferred from an argon-filled glovebox to the beamline in an air-tight sample holder with a 3-μm-thick Mylar film window that enables X-rays to access the sample. Samples were measured in fluorescence mode using a four-element silicon drift Vortex detector. The beamline energy was calibrated using sodium thiosulfate, setting the first peak’s maximum intensity to 2,472.02 eV. Spectra were taken over the range of 2,440–2,575 eV, with an energy resolution as low as 0.08 eV in the area of the absorption edge. Three consecutive scans were taken for each sample without any movement of the beam spot location between scans and then averaged for additional data analysis. X-ray spectra were normalized and background-subtracted using SIXPACK.
Supplementary Material
Acknowledgments
We would like to thank Dunyang Wang for the preparation of SEO/Li2S8 membranes and Nicole S. Schauser for help with rheology measurements. This work was supported as part of the Joint Center for Energy Storage Research, an Energy Innovation Hub funded by the US Department of Energy (DOE), Office of Science, Basic Energy Sciences (BES). Use of the Stanford Synchrotron Radiation Lightsource, Stanford Linear Accelerator Center (SLAC) is supported by the US DOE, Office of Science, Office of Basic Energy Sciences under Contract no. DE-AC02-76SF00515. The Advanced Light Source is supported by the Director, Office of Science, Office of BES, of the US DOE under Contract no. DE-AC02-05CH11231.
Footnotes
Conflict of interest statement: J.M.D. and N.P.B. have personal financial interests in the early-stage battery materials company, Blue Current, which they cofounded based on prior published research which the research described herein builds upon; a new patent application has been filed on this work.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1520394112/-/DCSupplemental.
References
- 1.Tarascon JM, Armand M. Issues and challenges facing rechargeable lithium batteries. Nature. 2001;414(6861):359–367. doi: 10.1038/35104644. [DOI] [PubMed] [Google Scholar]
- 2.Doyle M, Fuller TF, Newman J. The importance of the lithium ion transference number in lithium/polymer cells. Electrochimica Acta. 1994;39(13):2073–2081. [Google Scholar]
- 3.Bouchet R, et al. Single-ion BAB triblock copolymers as highly efficient electrolytes for lithium-metal batteries. Nat Mater. 2013;12(5):452–457. doi: 10.1038/nmat3602. [DOI] [PubMed] [Google Scholar]
- 4.Xie H, et al. Li6La3SnMO12 (M = Sb, Nb, Ta), a family of lithium garnets with high Li-ion conductivity. J Electrochem Soc. 2012;159(8):A1148–A1151. [Google Scholar]
- 5.Kamaya N, et al. A lithium superionic conductor. Nat Mater. 2011;10(9):682–686. doi: 10.1038/nmat3066. [DOI] [PubMed] [Google Scholar]
- 6.Sun X-G, Kerr JB. Synthesis and characterization of network single ion conductors based on comb-branched polyepoxide ethers and lithium bis(allylmalonato)borate. Macromolecules. 2006;39(1):362–372. [Google Scholar]
- 7.Liu Z, et al. Anomalous high ionic conductivity of nanoporous β-Li3PS4. J Am Chem Soc. 2013;135(3):975–978. doi: 10.1021/ja3110895. [DOI] [PubMed] [Google Scholar]
- 8.Sakuda A, Hayashi A, Takigawa Y, Higashi K, Tatsumisago M. Evaluation of elastic modulus of Li2S-P2S5 glassy solid electrolyte by ultrasonic sound velocity measurement and compression test. J Ceram Soc Jpn. 2013;121(1419):946–949. [Google Scholar]
- 9.Hayashi A, Harayama T, Mizuno F, Tatsumisago M. Mechanochemical synthesis of hybrid electrolytes from the Li2S-P2S5 glasses and polyethers. J Power Sources. 2006;163(1):289–293. [Google Scholar]
- 10.Mehrotra A, Ross PN, Srinivasan V. Quantifying polarization losses in an organic liquid electrolyte/single ion conductor interface. J Electrochem Soc. 2014;161(10):A1681–A1690. [Google Scholar]
- 11.Wong DH, et al. Nonflammable perfluoropolyether-based electrolytes for lithium batteries. Proc Natl Acad Sci USA. 2014;111(9):3327–3331. doi: 10.1073/pnas.1314615111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seino Y, et al. Analysis of the structure and degree of crystallisation of 70Li2S-30P2S5 glass ceramic. J Mater Chem A Mater Energy Sustain. 2015;3:2756–2761. [Google Scholar]
- 13.Minami K, Hayashi A, Tatsumisago M. Characterization of solid electrolytes prepared from Li2S-P2S5 glass and ionic liquids. J Electrochem Soc. 2010;157(12):A1296–A1301. [Google Scholar]
- 14.Sylla S, Sanchez JY, Armand M. Electrochemical study of linear and crosslinked POE-based polymer electrolytes. Electrochim Acta. 1992;37(9):1699–1701. [Google Scholar]
- 15.Lin Z, Liu Z, Fu W, Dudney NJ, Liang C. Lithium polysulfidophosphates: A family of lithium-conducting sulfur-rich compounds for lithium-sulfur batteries. Angew Chem Int Ed Engl. 2013;52(29):7460–7463. doi: 10.1002/anie.201300680. [DOI] [PubMed] [Google Scholar]
- 16.Wujcik KH, et al. Fingerprinting lithium-sulfur battery reaction products by X-ray absorption spectroscopy. J Electrochem Soc. 2014;161(6):A1100–A1106. [Google Scholar]
- 17.Pascal TA, et al. X-ray absorption spectra of dissolved polysulfides in lithium-sulfur batteries from first-principles. J Phys Chem Lett. 2014;5(9):1547–1551. doi: 10.1021/jz500260s. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.