Significance
Many of the world’s crops are pollinated by insects, and bees are often assumed to be the most important pollinators. To our knowledge, our study is the first quantitative evaluation of the relative contribution of non-bee pollinators to global pollinator-dependent crops. Across 39 studies we show that insects other than bees are efficient pollinators providing 39% of visits to crop flowers. A shift in perspective from a bee-only focus is needed for assessments of crop pollinator biodiversity and the economic value of pollination. These studies should also consider the services provided by other types of insects, such as flies, wasps, beetles, and butterflies—important pollinators that are currently overlooked.
Keywords: unmanaged pollinator, insect pollinator, fly, bee, beetle
Abstract
Wild and managed bees are well documented as effective pollinators of global crops of economic importance. However, the contributions by pollinators other than bees have been little explored despite their potential to contribute to crop production and stability in the face of environmental change. Non-bee pollinators include flies, beetles, moths, butterflies, wasps, ants, birds, and bats, among others. Here we focus on non-bee insects and synthesize 39 field studies from five continents that directly measured the crop pollination services provided by non-bees, honey bees, and other bees to compare the relative contributions of these taxa. Non-bees performed 25–50% of the total number of flower visits. Although non-bees were less effective pollinators than bees per flower visit, they made more visits; thus these two factors compensated for each other, resulting in pollination services rendered by non-bees that were similar to those provided by bees. In the subset of studies that measured fruit set, fruit set increased with non-bee insect visits independently of bee visitation rates, indicating that non-bee insects provide a unique benefit that is not provided by bees. We also show that non-bee insects are not as reliant as bees on the presence of remnant natural or seminatural habitat in the surrounding landscape. These results strongly suggest that non-bee insect pollinators play a significant role in global crop production and respond differently than bees to landscape structure, probably making their crop pollination services more robust to changes in land use. Non-bee insects provide a valuable service and provide potential insurance against bee population declines.
Pollinator-dependent crops are increasingly grown to provide food, fiber, and fuel as well as micronutrients essential to human health (1–5). The yield and quality of these crops benefit to varying degrees from flower visitation by animals. The honey bee, Apis mellifera L. (Hymenoptera: Apidae), is the most versatile, ubiquitous, and commonly used managed pollinator (6), but the global reliance on this single pollinator species is a risky strategy, especially given major threats to the health of managed honey bee colonies because of poor nutrition, the ectoparasitic mite Varroa destructor Anderson and Trueman (Mesostigmata: Varroidae), and a number of other pests and diseases (7–10).
However, honey bees are not the only insects that pollinate crops. Apart from a few managed bee taxa, the great majority of other pollinators are free-living or wild, providing an ecosystem service to crops. Wild pollinators other than honey bees recently have been recognized for their role in increasing and stabilizing crop-pollination services (11, 12). Wild bees are known to improve seed set, quality, shelf life, and commercial value of a variety of crops (13–17). Increasingly, studies indicate that insect pollinators other than bees, such as flies, beetles, moths, and butterflies, are equally if not more important for the production of some crops (18–24). Nonetheless, the contribution to crop pollination by non-bee insects has been largely unnoticed, with most global syntheses focusing on bees (25–28) or grouping together all bee and non-bee wild-insect pollinators (11).
Diverse pollinator assemblages have been shown to increase pollination services as a result of complementary resource use arising from variations in morphology and behavior among pollinator taxa (29, 30). For example, pollinator species may visit different parts within a flower or inflorescence or different flowers within a plant (high versus low flowers), improving the quality or quantity of pollination services overall (13, 31–33). Non-bee taxa, in particular, often have broader temporal activity ranges (34–36) and can provide pollination services at different times of the day compared with bees and in weather conditions when bees are unable to forage (37–40). In addition, non-bee taxa may be more efficient in transferring pollen for some crops under certain conditions (18, 19, 38) and/or carry pollen further distances than some bees (41). It has been suggested that this long-distance pollen transfer could have important genetic consequences for wild plants (42, 43). However, there is little information on the overall importance of the diverse group of non-bee wild pollinators (but see refs. 39 and 44) and their importance to global crop production.
Anthropogenic land use change and intensification are considered to be among the main drivers of bee declines (45, 46). One of the mechanisms underlying observed declines is thought to be the loss of habitat that supports host plants (47) and nesting sites (48). However, different pollinator taxa respond differently to disturbances (49, 50). The proximity and area of natural habitat are often associated with higher crop flower visitation and bee diversity (25, 46, 51). Yet, although several studies have investigated the habitat requirements of non-bee taxa (52–55), little is known about how habitat availability affects crop-pollination services from non-bee taxa (but see ref. 44). Thus, differential responses to habitat proximity by bees and non-bees, if such exists, could provide an additional stabilizing effect on crop-pollination services.
In summary, non-bees are often neglected as potential providers of crop ecosystem services by the scientific community and by growers. In the data collection for the present synthesis, for example, 33% of the original 58 pollination studies we obtained did not record or distinguish non-bee pollinators from bee pollinators and thus had to be excluded.
In this study we address the knowledge gap about non-bee crop pollination and ask:
-
i)
How does the crop pollination provided by non-bee insects compare with that provided by honey bees and other bees?
-
ii)
How does the crop pollination provided by non-bees, honey bees, and other bees translate into fruit/seed set?
-
iii)
Do non-bee crop pollinators respond similarly to bees with regard to isolation from natural and semi/natural habitats?
To answer these questions, we compiled a dataset comprising 39 studies of crop pollinators around the world and the pollination services they provide (Table S1).
Results
Pollination Services Provided by Honey Bees, Other Bees, and Non-Bees.
Flower-visitor assemblages were diverse, with representatives from the orders Hymenoptera, Diptera, Lepidoptera, and Coleoptera. Non-bee taxa included flies (Diptera: mainly dominated by Syrphidae, Calliphoridae, Tachinidae, Empididae, and Muscidae), butterflies and moths (Lepidoptera), and various beetle families (Coleoptera) and hymenopterans including ants (Formicidae) and wasps (Fig. S1). Bees observed in the studies included Apidae (e.g., Meliponini, Bombus spp., Xylocopini, and Ceratinini), Halictidae, Colletidae, Megachilidae, and Andrenidae.
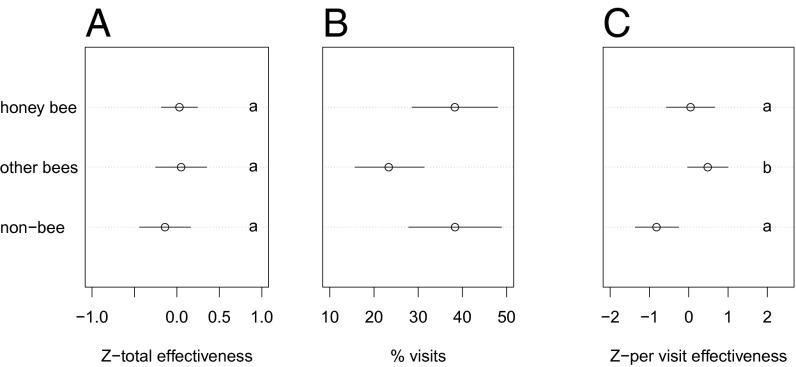
The total pollination services provided, which we calculated as the product of visitation frequency and pollen deposition or fruit set per visit (n = 9 studies) (56) did not differ significantly among honey bees, other bees, and non-bees (Fig. 1A). On average, non-bees accounted for 38% [confidence interval (CI): 29–49%], honey bees for 39% (CI: 29–50%), and other bees for 23% (CI: 15–33%) of the visits to crop flowers (n = 37 studies) (Fig. 1B). Visitation rates of other bees and non-bees were very weakly correlated (Pearson's product–moment correlation: 0.22), and the visitation rates of non-bees and honey bees and of other bees and honey bees were not correlated (0.02 and 0.04, respectively). In contrast, the per-visit pollen deposition or fruit set (n = 11 studies) was significantly lower for non-bees than for either type of bee (Fig. 1C and Fig. S2). Thus, non-bees’ higher visitation frequency and lower per visit effectiveness were compensatory, resulting in levels of pollination-service delivery similar to that provided by bees (Fig. 1A).
Fig. 1.
The contribution by honey bees, other bees, and non-bees to crop pollination. Data from individual crop studies were standardized by z-scores before analysis. (A) Pollination considered as a function of visits*single-visit effectiveness among guilds for the nine studies with effectiveness and visitation data. Note that per capita effectiveness in each guild is measured only in a subset of dominant species in each study. (B) The contributions of different insect groups to visitation (i.e., percentage of visits). (C) The relative effectiveness of honey bees, other bees, and non-bees as measured by pollen deposition or fruit set per visit, combined across the 11 crop studies for which data were available. Letters depict post hoc test differences (at P < 0.05) among pollinator groups.
Spatial Variation in Pollinator Community Composition.
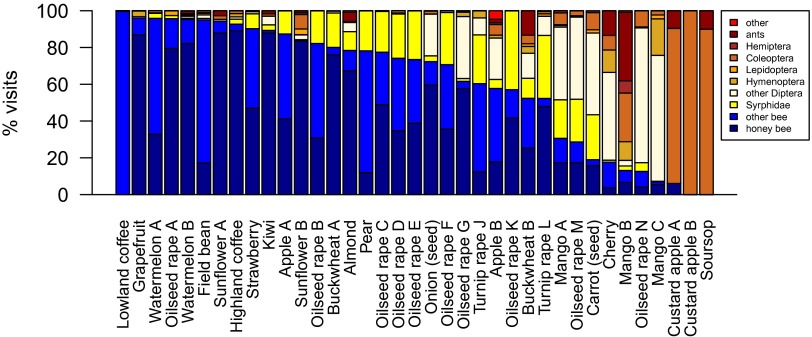
Observations of insect visitation rates revealed that assemblage composition varied across crop type and region (Fig. 2). Across the 37 crop studies, 31 recorded visits by all three groups of taxa, i.e., honey bees, other bees (all species other than Apis mellifera), and non-bees (Fig. 2). Two custard apple crops in Australia and Brazil (Annona sp.) were visited exclusively by non-bee taxa. Spatial variation in composition of the pollinator community resulted in some crops being visited by a more diverse group of insects than others, even within the same crop type. For example, pollinators of oilseed rape (Brassica napus) were surveyed in Sweden, Germany, the United Kingdom, the Netherlands, Ireland, and Australia, and the contribution to visitation by non-bees differed markedly (5–80%) among these surveys. Even within the three studies in Sweden (oilseed rape A, G, and M), visitation by non-bees ranged from 5–60%, demonstrating that location can have a strong influence, as can crop type, in determining assemblage composition (Fig. 2).
Fig. 2.
The contribution of different insect groups to flower visitation across the 37 crop studies for which visitation data were available. Crops are ordered, left to right, from mostly bee-dominated to mostly non-bee–dominated.
Fruit/Seed Set.
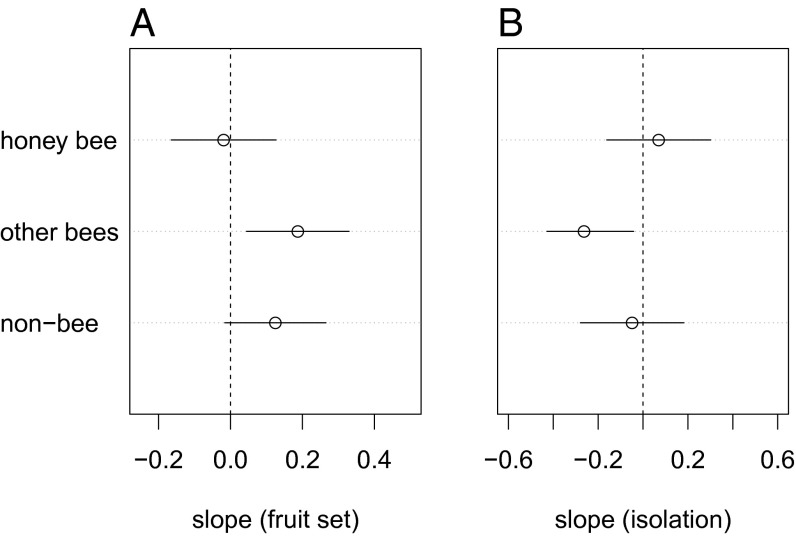
Higher visitation rates by non-bees and other bees each enhanced crop fruit and seed set more so than similar increases in visitation by honey bees (n = 19 studies) (Fig. 3A). In fact, honey bee visitation was not correlated with fruit set, with the average slope of this relationship centered on zero (β = −0.019, 2.5% CI = −0.164, 97.5% CI = 0.126), whereas non-bees show a positive slope (β = 0.12) minimally overlapping with zero (2.5% CI = −0.016, 97.5% CI = 0.265). The strongest relationship was between other bee visitation and fruit set (β = 0.187, 2.5% CI = 0.044, 97.5% CI = 0.330). Importantly, fruit set increased with non-bee visits independently of bee visitation rates, indicating that non-bee pollinators supplement rather than substitute for bee visitation. Therefore both groups are required for optimal pollination services.
Fig. 3.
Regression coefficients (i.e., slopes ßi ± 95% CI) representing honey bee, other bees, and non-bee contributions to overall fruit set and distance from natural/seminatural habitat. (A) Overall fruit set measured by seed set across 19 crop studies, estimated from the relationship between visitation and fruit set variation. Visitation by other bees increased fruit set (i.e., the average slope is positive, and CIs for regression coefficients did not include zero). The average regression coefficients across crops for non-bees increased fruit set (i.e., positive mean), but CIs minimally overlapped zero. (B) Distance from natural/seminatural habitat was measured across 23 studies. Visitation by other bees was negatively related to distance from natural/seminatural habitat (i.e., the average slope is negative, CIs for regression coefficients did not include zero). Visitation by honey bees and non-bees was not related to distance from natural/seminatural habitat (i.e., the average slope is negative, but confidence intervals overlapped zero for both taxa). Data from individual crop studies were standardized by z-scores before analysis to permit direct comparison of slopes.
Response to Changes in Land Use.
To test whether non-bees and bees respond differently to isolation from natural or seminatural vegetation, we investigated the relationship between the proximity to these features and the visitation rate of honey bees, other bees, and non-bee taxa across 23 studies. When data across all crop studies are considered, other bee visits declined sharply with increasing isolation from natural/seminatural vegetation (β = −0.263, 2.5% CI = −0.484, 97.5% CI = −0.042) (Fig. 3B). In contrast, non-bee declines are moderate, and the CIs include zero (β = −0.049, 2.5% CI = −0.270, 97.5% CI = 0.182), whereas honey bee visits show no response to proximity to natural/seminatural vegetation (β = 0.070, 2.5% CI = −0.161, 97.5% CI = 0.301).
Discussion
The clear importance of non-bees as global crop pollinators, as shown in this study, illustrates how important the omission of non-bees from crop pollination studies is to our understanding of crop-pollination services by wild insects. This crop pollination role is in addition to the well-established contributions that non-bees make to the reproduction of wild, native plant species (44, 57). Although on average the amount of pollen deposited per visit to crop flowers is lower for non-bees than for bees, the high visitation frequency of non-bees to crop flowers compensates for the deficit in per-visit effectiveness and results in high pollination services overall (Fig. 1). Thus, our results are consistent with other studies that have found that visitation frequency drives the overall function provided by a species, because the variance across species in their flower visitation is much larger than the variance in per-visit function (28, 58). One outcome is that taxa with less efficient pollen deposition may be the most important pollinators in certain years or seasons when they are at high abundance relative to other taxa (28, 59, 60).
Increased visitation by other bees and by non-bees each enhanced crop fruit and seed set more than increased visitation by honey bees (Fig. 3A). Measuring this downstream outcome variable is important, because pollen deposition does not necessarily lead to fruit set (61) [e.g., if pollinator visits are at saturating levels and result in flower damage or the transfer of poor quality/incompatible pollen (62, 63)]. For example, in our study, honey bees were good at depositing pollen in many crops, but increased honey bee visitation did not increase fruit set, a result that other researchers (11, 64) also have found. In contrast, increased visits from other bees, as from non-bees, were associated with increased fruit set. As argued by Garibaldi et al. (11), these patterns suggest that the effect of other bees and non-bees is additive to the effect of honey bees in the datasets examined.
A final benefit of non-bees documented here is that they respond less negatively than bees to changes in land use (Fig. 3B). Thus, where non-bees and bees pollinate the same crop, the presence of non-bees could help stabilize crop-pollination services against changes in land use through a mechanism known as “response diversity” (49). Hence differences in responses among bee and non-bee taxa potentially could provide pollination “insurance” in the event of bee declines (33). Although other bees responded positively to natural habitat, non-bees and honey bees did not show a clear pattern, perhaps because most other bees are central place foragers, some of which require untilled ground and sparsely vegetated ground for nesting. Other bees also require reliable, long-term pollen and nectar resources, and these habitat features are associated with seminatural or natural vegetation (46). In contrast, many non-bee taxa have diverse nesting habits; e.g., many flies lack central nest locations, and others are dependent on floral resources only during adult life stages (65). For this diverse group of insects the agricultural matrix may be more permeable than it is for many bees (66).
The diversity of life history strategies exhibited by non-bees necessitates an approach to habitat management different from that used for bees to ensure that a wide range of foraging and nesting resources are available. For example, within the hoverfly family (Diptera: Syrphidae) the larvae of some species feed on pollen (67), or aphids (65), or plant matter (68), or dung, among other resources (69), but the adults usually are generalist flower visitors. Furthermore, at least some hoverfly species appear to be less affected by changes in land use than bees, because many hoverfly species are able to use resources from highly modified habitats, including agricultural fields (44, 46, 66). The variability among life histories may explain why some non-bee pollinator populations are known to benefit from the same pollinator-enhancement practices as bees but others do not (54, 70, 71).
There are several reasons why non-bees generally have been overlooked in crop pollination studies until now. The diversity of families and the taxonomy of non-bee taxa are often poorly resolved (72, 73). Some non-bee taxa (such as flies and small wasps) move quickly and are difficult to follow in visual observations (e.g., transects). Further, many researchers have made the erroneous assumption that non-bee taxa are unimportant to pollination, as demonstrated by the 33% of studies reviewed that did not collect data on non-bee taxa as an a priori decision.
With the growing economic importance of crops that require animal-mediated pollination (74), wild insect pollinators are increasingly being recognized for their role in improving and stabilizing crop-pollination services (75). Here, we show that wild pollinators other than bees also make substantial contributions to global crop-pollination services. This study demonstrates the importance of including non-bee pollinators in future crop-pollination surveys, pollination estimates, and pollinator-management practices to ensure that we ascertain the relative contributions from all crop-pollinating taxa, over and above the well-known bee taxa.
Materials and Methods
We analyzed data from 480 fields for 17 crops examined in 39 studies on five continents. Fields ranged from extensive monocultures to small, diversified systems (Tables S1 and S2). All crop studies that were included benefit in some way from insect pollination. The protocols and identity of studies used to investigate the visitation rate, effectiveness, contribution to yield, and response to natural or seminatural vegetation in each study are provided in Tables S1 and S2. Across all the studies, 37 provided data on visitation frequency; 11 studies provided data on pollen transfer or fruit set per-visit effectiveness; 19 studies provided data on seed or fruit set; and 23 provided data on distance to natural/seminatural vegetation. Thirteen of the 39 crop studies have not been included in any previous synthesis on wild pollinator contributions to crop pollination.
Flower Visitation Frequency.
To investigate the frequency with which non-bees visit crop flowers in comparison with bees across our studies, we observed flower visitors within standardized quadrats and transects and measured flower visitation per unit of time for each insect species/group (37 studies). Pollinator observations were carried out during peak flowering. In several studies, visitation was standardized with respect to a unit area or branch (because some crops have hundreds of small flowers per plant, visits per flower could not be accurately assessed). We analyzed visitation by three different groups: honey bees, other bees, and non-bees (i.e., all other insects). In this synthesis across all studies, we considered Apis mellifera as the only species within the honey bee group for consistency across all datasets. Other Apis bees (e.g., Apis cerana indica) were pooled into the other-bee category. We analyzed all feral and managed honey bees as a single group because they cannot be distinguished during field observations. Feral honey bees were uncommon in most studies except for those in South Africa and Argentina. The exact methods and numbers of sampling points surveyed in each study are published elsewhere or are provided in the supporting information (Table S1).
Effectiveness per Flower Visit.
To investigate differences in per-visit effectiveness among bee and non-bee taxa (11 studies) (Table S2), pollen deposition on stigmatic surfaces (76) or fruit set after a single visit was estimated in fine weather conditions from pollination-effectiveness experiments in which virgin inflorescences were bagged with a fine mesh to exclude pollinators. When bagged flowers opened, the bag was removed, and the flowers were observed until an insect visited the flower and contacted the stigma. The stigma then was removed by carefully severing it from the style using finely pointed forceps, and the pollen grains or pollen tubes were counted after one visit by each insect. A variation of this method was used for several crops (i.e., radish, kiwi, avocado, carrot, and watermelon), which involved removing the virgin flower and positioning it to allow visitation by particular taxa (Tables S1 and S2). Single-visit pollen-deposition values generally were available only for the dominant taxa; hence this analysis does not necessarily represent the effectiveness of entire communities.
Calculating Total Pollination per Species.
Total pollination is often considered to be a function of both visitation frequency and per-visit effectiveness (56). We estimated total pollination for the nine studies in which these data were available. We used species-level visitation records and multiplied total visitation of each group (i.e., honey bee, other bees, and non-bees) by the mean per-visit pollen deposition of each group (Fig. 1).
Fruit/Seed Set.
To investigate differences in fruit set or seed set in relation to visitation by bee and non-bee taxa (19 studies) (Table S2), we recorded the proportion of flowers that set fruit or the total number of fruits or seeds as a measure of pollination success.
Isolation from Natural/Seminatural Habitat.
Finally, to investigate the response of bees and non-bees to isolation from natural/seminatural vegetation, we calculated the linear distance (in kilometers) from each field site to the nearest patch of natural or seminatural vegetation (23 studies) (Table S2). For two crops, almond and oilseed rape E, we transformed the percentage of seminatural vegetation within a 1-km area to linear distances following ref. 12.
Study Selection.
We initially contacted 58 data holders with the following criteria for inclusion of datasets in the synthesis: field studies must have set out to record all groups of pollinators (i.e., both bee and non-bee groups). Studies were excluded that did not set out to record non-bees (n = 14) or that did not set out to record honey bees (n = 1). If a researcher stated that a systematic survey was performed with the aim of sampling all pollinators (even though an entire group of pollinators was absent), we included that study. Finally, studies that included either bees or non-bees on an ad hoc basis (rather than in a systematic survey) were excluded (n = 4). Although the present study is limited to crop studies in which data were available for non-bee taxa, we do include several crops for which bees are assumed to be the primary visitors, such as almond and watermelon (77, 78). Furthermore, the ratio of bee- to non-bee–visited crops in the FAOSTAT crop database (6) is comparable to the ratio investigated in this synthesis (Table S3). Nonetheless, we acknowledge that the study represents a limited number of crops, and a greater range of datasets is required to obtain a fuller picture of the relative importance of these different groups of pollinators.
Data Analysis.
Data on visitation rates, pollination effectiveness, fruit or seed set, and isolation from natural/seminatural vegetation were standardized for cross-study analysis with the calculation of z-scores within each study (Datasets S1–S4). Z-scores do not modify the form (e.g., linear or nonlinear) of the relationship between response and predictor variables and allow direct comparison of the values collected in different studies (79).
We analyzed all data using general linear mixed-effects models using R software version 3.0.2, nlme package, lme function, with Gaussian error distribution (80). By including crop study as a random variable, our models estimated different intercepts (αj) for each study (j), accounting for the hierarchical structure of the data, i.e., different fields are nested within each study (79, 81). The overall intercept (μα) reflects a weighted average over crop studies (αj), in which the relative influence of each crop study increases with the precision of its local model fit and its sample size (79, 82).
To answer the first question regarding differences in crop-pollination services provided to crop flowers by non-bee and bee taxa, we ran a different model for each group (honey bees, wild bees, and non-bees) with no predictor. This model enabled calculation of the overall intercept (i.e., mean percent visitation) and CIs for each of the three groups, taking into account the hierarchical structure of the data. Per capita effectiveness values were regressed against pollinator group (categorical: honey bee, other bee, non-bee). Post hoc Tukey tests were used to disentangle the differences in effectiveness among the three groups using the multcomp package (83) with a Hochberg correction for multiple comparisons. To answer the second question, we built three sets of models to examine the relationship between fruit set and the visitation rates of the different insect groups. To determine whether increased visitation rate by each of the three groups was associated with increased fruit set, the first model consisted of fruit set regressed against total visitation of honey bees, other bees, and non-bees, with random intercepts for crop study. The second set of models included both random intercepts and random slopes. A third set of models was run including pairwise interactions among the three groups and only random intercepts. The three models were compared using the Akaike information criterion (AIC) (84). The first model had the greatest support (AIC = 555) followed by both the interaction model (ΔAIC = 5) and the random slopes model (ΔAIC = 4); hence only the random intercept models are presented. Finally, to answer the third question, visitation rate by each group was regressed against isolation from natural habitats in a separate model with random intercepts as described above. We present estimated slopes and CIs for all analyses (Table S4). To meet the assumptions of homoscedasticity, we used a constant variance function when necessary. Variance inflation factors of the predictors were always below 1.5, indicating no multicollinearity (85).
Supplementary Material
Acknowledgments
Data collection was funded by a University of New England seed grant (to R.R.). I.B. was supported by European Union Project BeeFun PCIG14-GA-2013-631653; L.A.G. was supported by Universidad Nacional de Río Negro Grant PI 40-B-399 and Consejo Nacional de Investigaciones Científicas y Técnicas Resolución 3260/14, Expediente 3207/14; A.-M.K. and C.B. were supported by the German Science Foundation; D.K., M. Reemer, and J.S. were supported by the Dutch Ministry of Economic Affairs Grants BO-11-011.01-011 and KB-14-003-006; L.G.C. D.K., J.S., R.B., H.S., M.W., M. Rundlöf, and S.G.P. were supported by the European Community’s Seventh Framework Programme FP7/2007–2013 under Grant Agreement 244090, Status and Trends of European Pollinators; H.S. and M.W. were supported by European Community’s Sixth Framework Programme under Grant Agreement GOCE-CT-2003-506675, Assessing Large Scale Risks for Biodiversity with Tested Methods Project; S.A.M.L. was supported the Swedish Farmers’ Foundation for Agricultural Research and the Swedish Board of Agriculture; M.P.D.G. and S.G.P. were supported by a grant from Biotechnology and Biological Sciences Research Council, Defra, the Natural Environment Research Council, the Scottish Government, and the Wellcome Trust under the UK Insect Pollinators Initiative; H.G.S. and R.B. were supported by the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning; C.S. was supported by the Swiss National Science Foundation under Grant 3100A0-127632 (FRAGMENT) to F.H. and M.H.E.; J.C.S. and D.A.S. were supported by Irish Environmental Protection Agency Grant EPA 2007-B-CD-1-S1 under the Sectoral Impacts on Biodiversity and Ecosystems Services (SIMBIOSYS) project; B.M.F. and L.G.C. were supported by National Council for Scientific and Technological Development-Brasília Research Grants 05126/2013-0 and 300005/2015-6, respectively; Y.M. and G.P. were supported by The Israel Science Foundation; S.K. was supported by The North-South Centre, Swiss Federal Institute of Technology, Zurich; B.F.V. and J.H. were supported by the Ministry of the Environment and the Brazilian Research Council; and the study on Highland coffee was supported by Grant SEMARNAT-CONACyT 2002-C01-0194 from Mexico’s Environmental Ministry (to C.H.V.). H.T. was supported by the Global Environment Research Fund (E-0801 and S-9) of the Ministry of the Environment, Japan. Funding for kiwi in New Zealand provided by the Thomas J. Watson Foundation (to M.M.M.). B.G.H. and D.E.P. were supported by Ministry for Business Innovation and Employment (C11X1309).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1517092112/-/DCSupplemental.
References
- 1.Chaplin-Kramer R, et al. Global malnutrition overlaps with pollinator-dependent micronutrient production. Proc Biol Sci. 2014;281(1794):20141799. doi: 10.1098/rspb.2014.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eilers EJ, Kremen C, Smith Greenleaf S, Garber AK, Klein AM. Contribution of pollinator-mediated crops to nutrients in the human food supply. PLoS One. 2011;6(6):e21363. doi: 10.1371/journal.pone.0021363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foley JA, et al. Solutions for a cultivated planet. Nature. 2011;478(7369):337–342. doi: 10.1038/nature10452. [DOI] [PubMed] [Google Scholar]
- 4.Tilman D, Balzer C, Hill J, Befort BL. Global food demand and the sustainable intensification of agriculture. Proc Natl Acad Sci USA. 2011;108(50):20260–20264. doi: 10.1073/pnas.1116437108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smith MR, Singh GM, Mozaffarian D, Myers SS (2015) Effects of decreases of animal pollinators on human nutrition and global health: A modelling analysis. The Lancet, 10.1016/S0140-6736(15)61085. [DOI] [PubMed]
- 6.Klein AM, et al. Importance of pollinators in changing landscapes for world crops. Proc Biol Sci. 2007;274(1608):303–313. doi: 10.1098/rspb.2006.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Engelsdorp D, Hayes J, Jr, Underwood RM, Pettis J. A survey of honey bee colony losses in the U.S., fall 2007 to spring 2008. PLoS One. 2008;3(12):e4071. doi: 10.1371/journal.pone.0004071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Potts SG, et al. Declines of managed honeybees and beekeepers in Europe? J Apic Res. 2010;49(1):15–22. [Google Scholar]
- 9.Kraus B, Page RE. Effect of Varroa jacobsoni (Mesostigmata: Varroidae) on feral Apis mellifera (Hymenoptera: Apidae) in California. Environ Entomol. 1995;24(6):1473–1480. [Google Scholar]
- 10.Winfree R. Pollinator-dependent crops: An increasingly risky business. Curr Biol. 2008;18(20):R968–R969. doi: 10.1016/j.cub.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Garibaldi LA, et al. Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science. 2013;339(6127):1608–1611. doi: 10.1126/science.1230200. [DOI] [PubMed] [Google Scholar]
- 12.Garibaldi LA, et al. Stability of pollination services decreases with isolation from natural areas despite honey bee visits. Ecol Lett. 2011;14(10):1062–1072. doi: 10.1111/j.1461-0248.2011.01669.x. [DOI] [PubMed] [Google Scholar]
- 13.Hoehn P, Tscharntke T, Tylianakis JM, Steffan-Dewenter I. Functional group diversity of bee pollinators increases crop yield. Proc Biol Sci. 2008;275(1648):2283–2291. doi: 10.1098/rspb.2008.0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mallinger RE, Gratton C. Species richness of wild bees, but not the use of managed honey bees, increases fruit set of a pollinator-dependent crop. J Appl Ecol. 2015;52(2):323–330. [Google Scholar]
- 15.Bartomeus I, et al. Contribution of insect pollinators to crop yield and quality varies with agricultural intensification. PeerJ. 2014;2:e328. doi: 10.7717/peerj.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klatt BK, et al. Bee pollination improves crop quality, shelf life and commercial value. Proc Biol Sci. 2014;281(1775):20132440. doi: 10.1098/rspb.2013.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winfree R, Gross BJ, Kremen C. Valuing pollination services to agriculture. Ecol Econ. 2011;71:80–88. [Google Scholar]
- 18.Rader R, et al. Alternative pollinator taxa are equally efficient but not as effective as the honeybee in a mass flowering crop. J Appl Ecol. 2009;46(5):1080–1087. [Google Scholar]
- 19.Jauker F, Wolters V. Hover flies are efficient pollinators of oilseed rape. Oecologia. 2008;156(4):819–823. doi: 10.1007/s00442-008-1034-x. [DOI] [PubMed] [Google Scholar]
- 20.Blanche R, Cunningham SA. Rain forest provides pollinating beetles for atemoya crops. J Econ Entomol. 2005;98(4):1193–1201. doi: 10.1603/0022-0493-98.4.1193. [DOI] [PubMed] [Google Scholar]
- 21.Jarlan A, De Oliveiha D, Gingras J. Effects of Eristalis tenax (Diptera: Syrphidae) pollination on characteristics of greenhouse sweet pepper fruits. J Econ Entomol. 1997;90(6):1650–1654. [Google Scholar]
- 22.Jarlan A, De Oliveiha D, Gingras J. Pollination by Eristalis tenax (Diptera: Syrphidae) and seed set of greenhouse sweet pepper. J Econ Entomol. 1997;90(6):1646–1649. [Google Scholar]
- 23.Kendall DA, Solomon ME. Quantities of pollen on the bodies of insects visiting apple blossom. J Appl Ecol. 1973;10(2):627–634. [Google Scholar]
- 24.Larson BMH, Kevan PG, Inouye DW. Flies and flowers: Taxonomic diversity of anthophiles and pollinators. Can Entomol. 2001;133(4):439–465. [Google Scholar]
- 25.Kennedy CM, et al. A global quantitative synthesis of local and landscape effects on wild bee pollinators in agroecosystems. Ecol Lett. 2013;16(5):584–599. doi: 10.1111/ele.12082. [DOI] [PubMed] [Google Scholar]
- 26.Williams NM, et al. Ecological and life history traits predict bee species responses to environmental disturbances. Biol Conserv. 2010;143(10):2280–2291. [Google Scholar]
- 27.Winfree R, Aguilar R, Vázquez DP, LeBuhn G, Aizen MA. A meta-analysis of bees’ responses to anthropogenic disturbance. Ecology. 2009;90(8):2068–2076. doi: 10.1890/08-1245.1. [DOI] [PubMed] [Google Scholar]
- 28.Kleijn D, et al. Delivery of crop pollination services is an insufficient argument for wild pollinator conservation. Nat Commun. 2015;6(7414):1–8. doi: 10.1038/ncomms8414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bluthgen N, Klein AM. Functional complementarity and specialisation: The role of biodiversity in plant-pollinator interactions. Basic Appl Ecol. 2011;12(4):282–291. [Google Scholar]
- 30.Albrecht M, Schmid B, Hautier Y, Müller CB. Diverse pollinator communities enhance plant reproductive success. Proc Roy Soc B: Biol Sci. 2012;279(1748):4845–4852. doi: 10.1098/rspb.2012.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chagnon M, Gingras J, Deoliveira D. Complemenatary aspects of strawberry pollination by honey and indigenous bees (Hymenoptera) J Econ Entomol. 1993;86(2):416–420. [Google Scholar]
- 32.Winfree R, Kremen C. Are ecosystem services stabilized by differences among species? A test using crop pollination. Proc Biol Sci. 2009;276(1655):229–237. doi: 10.1098/rspb.2008.0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brittain C, Kremen C, Klein A-M. Biodiversity buffers pollination from changes in environmental conditions. Glob Change Biol. 2013;19(2):540–547. doi: 10.1111/gcb.12043. [DOI] [PubMed] [Google Scholar]
- 34.Primack RB. Variability in New Zealand montane and alpine pollinator assemblages. N Z J Ecol. 1978;1:66–73. [Google Scholar]
- 35.Owen J, Gilbert FS. On the abundance of hoverflies (Syrphidae) Oikos. 1989;55(2):183–193. [Google Scholar]
- 36.McCall C, Primack RB. Influence of flower characteristics, weather, time of day, and season on insect visitation rates in three plant communities. Am J Bot. 1992;79(4):434–442. [Google Scholar]
- 37.Rader R, Edwards W, Westcott DA, Cunningham SA, Howlett BG. Diurnal effectiveness of pollination by bees and flies in agricultural Brassica rapa: Implications for ecosystem resilience. Basic Appl Ecol. 2013;14(1):20–27. [Google Scholar]
- 38.Howlett BG. Hybrid carrot seed crop pollination by the fly Calliphora vicina (Diptera: Calliphoridae) J Appl Entomol. 2012;136(6):421–430. [Google Scholar]
- 39.Gupta JK. Wild Pollinators and Pesticides on Apples in Himachal Pradesh, India: Community Learning and Innovation. Pollinator Safety in Agriculture. United Nations Food and Agriculture Organization; Rome: 2014. [Google Scholar]
- 40.Cutler GC, Reeh KW, Sproule JM, Ramanaidu K. Berry unexpected: Nocturnal pollination of lowbush blueberry. Can J Plant Sci. 2012;92(4):707–711. [Google Scholar]
- 41.Rader R, Edwards W, Westcott DA, Cunningham SA, Howlett BG. Pollen transport differs among bees and flies in a human-modified landscape. Divers Distrib. 2011;17(3):519–529. [Google Scholar]
- 42.Herrera CM. Components of pollinator “quality”: Comparative analysis of a diverse insect assemblage. Oikos. 1987;50(1):79–90. [Google Scholar]
- 43.Jha S, Dick CW. Native bees mediate long-distance pollen dispersal in a shade coffee landscape mosaic. Proc Natl Acad Sci USA. 2010;107(31):13760–13764. doi: 10.1073/pnas.1002490107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Orford KA, Vaughan IP, Memmott J. The forgotten flies: The importance of non-syrphid Diptera as pollinators. Proc Biol Sci. 2015;282(1805):20142934. doi: 10.1098/rspb.2014.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Potts SG, et al. Global pollinator declines: Trends, impacts and drivers. Trends Ecol Evol. 2010;25(6):345–353. doi: 10.1016/j.tree.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 46.Winfree R, Bartomeus I, Cariveau DP. Native pollinators in anthropogenic habitats. Annu Rev Ecol Evol Syst. 2011;42(1):1–22. [Google Scholar]
- 47.Scheper J, et al. Museum specimens reveal loss of pollen host plants as key factor driving wild bee decline in The Netherlands. Proc Natl Acad Sci USA. 2014;111(49):17552–17557. doi: 10.1073/pnas.1412973111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Winfree R. The conservation and restoration of wild bees. Ann N Y Acad Sci. 2010;1195(1):169–197. doi: 10.1111/j.1749-6632.2010.05449.x. [DOI] [PubMed] [Google Scholar]
- 49.Cariveau DP, Williams NM, Benjamin FE, Winfree R. Response diversity to land use occurs but does not consistently stabilise ecosystem services provided by native pollinators. Ecol Lett. 2013;16(7):903–911. doi: 10.1111/ele.12126. [DOI] [PubMed] [Google Scholar]
- 50.Rader R, Bartomeus I, Tylianakis JM, Laliberté E. The winners and losers of land use intensification: Pollinator community disassembly is non-random and alters functional diversity. Divers Distrib. 2014;20(8):908–917. [Google Scholar]
- 51.Kremen C, Williams NM, Thorp RW. Crop pollination from native bees at risk from agricultural intensification. Proc Natl Acad Sci USA. 2002;99(26):16812–16816. doi: 10.1073/pnas.262413599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kleijn D, van Langevelde F. Interacting effects of landscape context and habitat quality on flower visiting insects in agricultural landscapes. Basic Appl Ecol. 2006;7(3):201–214. [Google Scholar]
- 53.Kohler F, Verhulst J, Van Klink R, Kleijn D. At what spatial scale do high-quality habitats enhance the diversity of forbs and pollinators in intensively farmed landscapes? J Appl Ecol. 2008;45(3):753–762. [Google Scholar]
- 54.Jauker F, Diekötter T, Schwarzbach F, Wolters V. Pollinator dispersal in an agricultural matrix: Opposing responses of wild bees and hoverflies to landscape structure and distance from main habitat. Landscape Ecol. 2009;24(4):547–555. [Google Scholar]
- 55.Jönsson AM, et al. Sown flower strips in southern Sweden increase abundances of wild bees and hoverflies in the wider landscape. Biol Conserv. 2015;184(0):51–58. [Google Scholar]
- 56.Ne’eman G, Jürgens A, Newstrom-Lloyd L, Potts SG, Dafni A. A framework for comparing pollinator performance: Effectiveness and efficiency. Biol Rev Camb Philos Soc. 2010;85(3):435–451. doi: 10.1111/j.1469-185X.2009.00108.x. [DOI] [PubMed] [Google Scholar]
- 57.Woodcock TS, Larson BMH, Kevan PJ, Inouye DW, Lunau K. Flies and flowers II: Floral attractants and rewards. Journal of Pollination Ecology. 2014;12(8):63–94. [Google Scholar]
- 58.Vazquez DP, Morris WF, Jordano P. Interaction frequency as a surrogate for the total effect of animal mutualists on plants. Ecol Lett. 2005;8(10):1088–1094. [Google Scholar]
- 59.Mayfield MM, Waser NM, Price MV. Exploring the ‘most effective pollinator principle’ with complex flowers: Bumblebees and Ipomopsis aggregata. Ann Bot (Lond) 2001;88(4):591–596. [Google Scholar]
- 60.Madjidian JA, Morales CL, Smith HG. Displacement of a native by an alien bumblebee: Lower pollinator efficiency overcome by overwhelmingly higher visitation frequency. Oecologia. 2008;156(4):835–845. doi: 10.1007/s00442-008-1039-5. [DOI] [PubMed] [Google Scholar]
- 61.Dogterom MH, Winston ML, Mukai A. Effect of pollen load size and source (self, outcross) on seed and fruit production in highbush blueberry cv. ‘Bluecrop’ (VACCINIUM CORYMBOSUM; Ericaceae) Am J Bot. 2000;87(11):1584–1591. [PubMed] [Google Scholar]
- 62.Morris WF, Vázquez DP, Chacoff NP. Benefit and cost curves for typical pollination mutualisms. Ecology. 2010;91(5):1276–1285. doi: 10.1890/08-2278.1. [DOI] [PubMed] [Google Scholar]
- 63.Aizen MA, et al. When mutualism goes bad: Density-dependent impacts of introduced bees on plant reproduction. New Phytol. 2014;204(2):322–328. [Google Scholar]
- 64.Chacoff NP, Aizen MA, Aschero V. Proximity to forest edge does not affect crop production despite pollen limitation. Proc Biol Sci. 2008;275(1637):907–913. doi: 10.1098/rspb.2007.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mizuno M, Itioka T, Tatematsu Y, Itô Y. Food utilization of aphidophagous hoverfly larvae (Diptera: Syrphidae, Chamaemyiidae) on herbaceous plants in an urban habitat. Ecol Res. 1997;12(3):239–248. [Google Scholar]
- 66.Raymond L, et al. Immature hoverflies overwinter in cultivated fields and may significantly control aphid populations in autumn. Agric Ecosyst Environ. 2014;185:99–105. [Google Scholar]
- 67.Nunes-Silva P, et al. Pollenivory in larval and adult flower flies: Pollen availability and visitation rate by Toxomerus politus SAY (Diptera: Syrphidae) on sorghum Sorghum bicolor (L.) MOENCH (Poaceae) Stud Dipterologica. 2010;17:177–185. [Google Scholar]
- 68.Hövemeyer K. Trophic links, nutrient fluxes, and natural history in the Allium ursinum food web, with particular reference to life history traits of two hoverfly herbivores (Diptera: Syrphidae) Oecologia. 1995;102(1):86–94. doi: 10.1007/BF00333314. [DOI] [PubMed] [Google Scholar]
- 69.Owen J. Trophic variety and abundance of hoverflies (Diptera, Syrphidae) in an english suburban garden. Holarct Ecol. 1981;4(3):221–228. [Google Scholar]
- 70.Biesmeijer JC, et al. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science. 2006;313(5785):351–354. doi: 10.1126/science.1127863. [DOI] [PubMed] [Google Scholar]
- 71.Meyer B, Jauker F, Steffan-Dewenter I. Contrasting resource-dependent responses of hoverfly richness and density to landscape structure. Basic Appl Ecol. 2009;10(2):178–186. [Google Scholar]
- 72.Meier R, Shiyang K, Vaidya G, Ng PKL. DNA barcoding and taxonomy in Diptera: A tale of high intraspecific variability and low identification success. Syst Biol. 2006;55(5):715–728. doi: 10.1080/10635150600969864. [DOI] [PubMed] [Google Scholar]
- 73.Pape T, Bickel D, Meier R. Diptera Diversity: Status, Challenges and Tools. Brill; Leiden, The Netherlands: 2009. [Google Scholar]
- 74.Aizen MA, Garibaldi LA, Cunningham SA, Klein AM. Long-term global trends in crop yield and production reveal no current pollination shortage but increasing pollinator dependency. Curr Biol. 2008;18(20):1572–1575. doi: 10.1016/j.cub.2008.08.066. [DOI] [PubMed] [Google Scholar]
- 75.Garibaldi LA, et al. From research to action: Enhancing crop yield through wild pollinators. Front Ecol Environ. 2014;12(8):439–447. [Google Scholar]
- 76.Inouye DW, Gill DE, Dudash MR, Fenster CB. A model and lexicon for pollen fate. Am J Bot. 1994;81(12):1517–1530. [Google Scholar]
- 77.Thomson JD, Goodell K. Pollen removal and deposition by honeybee and bumblebee visitors to apple and almond flowers. J Appl Ecol. 2001;38(5):1032–1044. [Google Scholar]
- 78.Brittain C, Williams N, Kremen C, Klein A-M. Synergistic effects of non-Apis bees and honey bees for pollination services. Proc Biol Sci. 2013;280(1754):20122767. doi: 10.1098/rspb.2012.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gelman A, Hill J. Data Analysis Using Regression and Multilevel/Hierarchical Models. Cambridge Univ Press; Cambridge, UK: 2007. [Google Scholar]
- 80. R Development Core and Team, eds (2013) R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna)
- 81.Bates D, Maechler M, Bolker B. 2011 lme4: Linear mixed-effects models using S4 classes. R package version 0.999375-32. Available at CRAN.R-project.org/package=lme4. Accessed August 31, 2015.
- 82.Qian SS, Cuffney TF, Alameddine I, McMahon G, Reckhow KH. On the application of multilevel modeling in environmental and ecological studies. Ecology. 2010;91(2):355–361. doi: 10.1890/09-1043.1. [DOI] [PubMed] [Google Scholar]
- 83.Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biom J. 2008;50(3):346–363. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- 84.Burnham KP, Anderson DR. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. Springer Science and Business Media; New York: 2002. [Google Scholar]
- 85.Zuur AF, Ieno EN, Elphick CS. A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol. 2010;1(1):3–14. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.