Significance
Microglia (brain macrophages) become rapidly activated in most neuropsychiatric disorders. A popular concept is that a single pathogenic stimulus, such as bacterial lipopolysaccharide (LPS) through Toll-like receptor 4 (TLR4), is sufficient to induce a reactive proinflammatory phenotype in microglia that exerts neurotoxicity. This concept is biologically risky, however. Here we provide evidence that chronic activation with either LPS or the leukocyte cytokine IFN-γ induces different reactive phenotypes in microglia of postnatal hippocampal tissue. Notably, these phenotypes only moderately alter diverse neuronal functions. In contrast, coactivation of TLR4 and IFN-γ receptors results in massive neural dysfunction and death. Thus, activation of TLR4 in microglia in situ requires concomitant IFN-γ signaling from other host immune cells to induce neurodegeneration.
Keywords: hippocampus, microglia, neuronal activity, slice culture, Toll-like receptor
Abstract
Microglia (tissue-resident macrophages) represent the main cell type of the innate immune system in the CNS; however, the mechanisms that control the activation of microglia are widely unknown. We systematically explored microglial activation and functional microglia–neuron interactions in organotypic hippocampal slice cultures, i.e., postnatal cortical tissue that lacks adaptive immunity. We applied electrophysiological recordings of local field potential and extracellular K+ concentration, immunohistochemistry, design-based stereology, morphometry, Sholl analysis, and biochemical analyses. We show that chronic activation with either bacterial lipopolysaccharide through Toll-like receptor 4 (TLR4) or leukocyte cytokine IFN-γ induces reactive phenotypes in microglia associated with morphological changes, population expansion, CD11b and CD68 up-regulation, and proinflammatory cytokine (IL-1β, TNF-α, IL-6) and nitric oxide (NO) release. Notably, these reactive phenotypes only moderately alter intrinsic neuronal excitability and gamma oscillations (30–100 Hz), which emerge from precise synaptic communication of glutamatergic pyramidal cells and fast-spiking, parvalbumin-positive GABAergic interneurons, in local hippocampal networks. Short-term synaptic plasticity and extracellular potassium homeostasis during neural excitation, also reflecting astrocyte function, are unaffected. In contrast, the coactivation of TLR4 and IFN-γ receptors results in neuronal dysfunction and death, caused mainly by enhanced microglial inducible nitric oxide synthase (iNOS) expression and NO release, because iNOS inhibition is neuroprotective. Thus, activation of TLR4 in microglia in situ requires concomitant IFN-γ receptor signaling from peripheral immune cells, such as T helper type 1 and natural killer cells, to unleash neurotoxicity and inflammation-induced neurodegeneration. Our findings provide crucial mechanistic insight into the complex process of microglia activation, with relevance to several neurologic and psychiatric disorders.
Microglia are tissue-resident macrophages in the CNS that become activated in most brain disorders, such as bacterial meningoencephalitis, multiple sclerosis, and Alzheimer’s disease (1, 2). Activation of microglia features changes in morphology and receptor expression, antigen presentation, cytokine release, migration, and phagocytosis, and it ranges from proinflammatory and potentially neurotoxic to anti-inflammatory and neuroprotective phenotypes (1, 3, 4). The mechanisms that control the transition of microglia to reactive phenotypes, including the impact on neuronal function, are mostly unknown, however (5–7).
Sensing of microbial or modified endogenous ligands by microglia is mediated by innate pattern recognition receptors, such as scavenger receptors and Toll-like receptors (TLRs). A prime example is TLR4, which acts with CD14, MD-2, and lipopolysaccharide (LPS)-binding protein in recognizing LPS, a cell wall component of Gram-negative bacteria (8, 9). TLR4 is also central to microglial recognition of amyloid-β peptide, which is thought to be part of the inflammatory response in Alzheimer’s disease (7, 10).
LPS has been widely used to study the molecular mechanisms of microglial activation in inflammatory neurodegeneration (1–3). In primary monocultures and microglia-neuron cultures, LPS exposure alone or in combination with IFN-γ for a “booster” triggers the massive release of proinflammatory and cytotoxic factors, such as TNF-α, IL-6, and nitric oxide (NO), finally resulting in neuronal death (8, 11–18). Similar effects were observed in vivo after intracerebral administration of LPS (19–21). These and other studies have contributed to the concept that microglial TLR4 activation with LPS (i.e., with a single pathogenic stimulus) is sufficient to induce neurodegeneration (22, 23); however, this concept is biologically risky, and has been questioned in some experimental works and reviews (2–4, 11, 24, 25).
Most previous studies focused on two aspects of microglial TLR4 activation with LPS: (i) the properties of the reactive microglial phenotype(s) and (ii) the degree of neurodegeneration. For this purpose, either simple culture systems or in vivo models, in which interactions with leukocytes infiltrating from the blood are inevitable, have been used (1, 4). Thus, it is widely unknown how TLR4 and IFN-γ receptor signaling in microglia individually contribute to neurotoxicity and neurodegeneration in situ. This aspect is highly relevant for several neurologic and psychiatric disorders. Moreover, concomitant alterations in neuronal information processing (i.e., dysfunction in excitatory pyramidal cells and inhibitory GABAergic interneurons, including astrocytes) have been little explored (25–27).
We rigorously addressed these fundamental questions in postnatal neuronal tissue (1, 4). To mimic microglial confrontation with LPS in situ and, notably, in the absence of infiltrating leukocytes, we used organotypic hippocampal slice cultures that feature highly preserved cytoarchitectures and complex neuronal network functions (5, 28). Microglial interaction with infiltrating T helper type 1 (Th1) cells and/or natural killer (NK) cells was mimicked by recombinant IFN-γ administration.
Results
LPS or IFN-γ Alone Induces Reactive Phenotypes in Microglia with Moderate Effects on Neuronal Function.
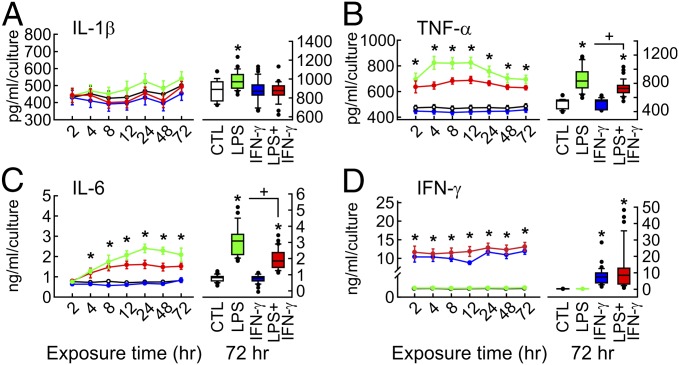
We exposed slice cultures to LPS at high concentration (10 µg/mL) for 72 h (“chronic”) to achieve sufficient tissue penetration and full stimulatory potential of TLR4 activation in microglia (Figs. 1 and 2 and SI Appendix, Fig. S1). The culture medium contained serum to provide accessory factors that are usually recruited during bacterial infection owing to blood–brain barrier impairment (5, 11, 15). LPS exposure induced quite stable release of proinflammatory cytokines IL-1β, TNF-α, and IL-6 (Fig. 1 A–C), as well as NO through up-regulated inducible nitric oxide synthase (iNOS) expression (Fig. 3 A, C, and D), indicative of a clearly chronic proinflammatory condition. LPS exposure with daily medium exchange revealed that major cytokine release occurred in the first 24 h (SI Appendix, SI Materials and Methods and Fig. S2 G–J). LPS exposure did not induce the release of the anti-inflammatory cytokine IL-10 (SI Appendix, Fig. S3). Notably, IFN-γ was not detected in either the control group or the LPS group (Fig. 1D), confirming that IFN-γ release from brain cells is negligible under physiological and most pathophysiological conditions (7, 29). We note that astrocytes also might contribute to cytokine release (2, 14, 30).
Fig. 1.
Cytokine release. Slice cultures were exposed to LPS (10 µg/mL), IFN-γ (100 ng/mL), or both (LPS+IFN-γ) at day in vitro 7 (SI Appendix, Fig. S1). (A–D) (Left) Profile of cytokine accumulation in the medium, normalized to the number of slice cultures per membrane. Dots and error bars represent mean ± SEM. *P < 0.001 of LPS and LPS+IFN-γ vs. CTL and IFN-γ, one-way ANOVA or ANOVA on ranks. For n/N membranes/preparations: CTL, 15/7; LPS, 19/7; IFN-γ, 21/7; LPS+IFN-γ, 19/7. (Right) Histograms of cytokine levels after 72 h of exposure. Boxplots: *P < 0.05, ANOVA on ranks vs. CTL; +P < 0.05, rank-sum test. CTL, 37/14; LPS, 34/12; IFN-γ, 33/11; LPS+IFN-γ, 37/12. IFN-γ levels most likely derive from the added protein. Control experiments are shown in SI Appendix, Fig. S2.
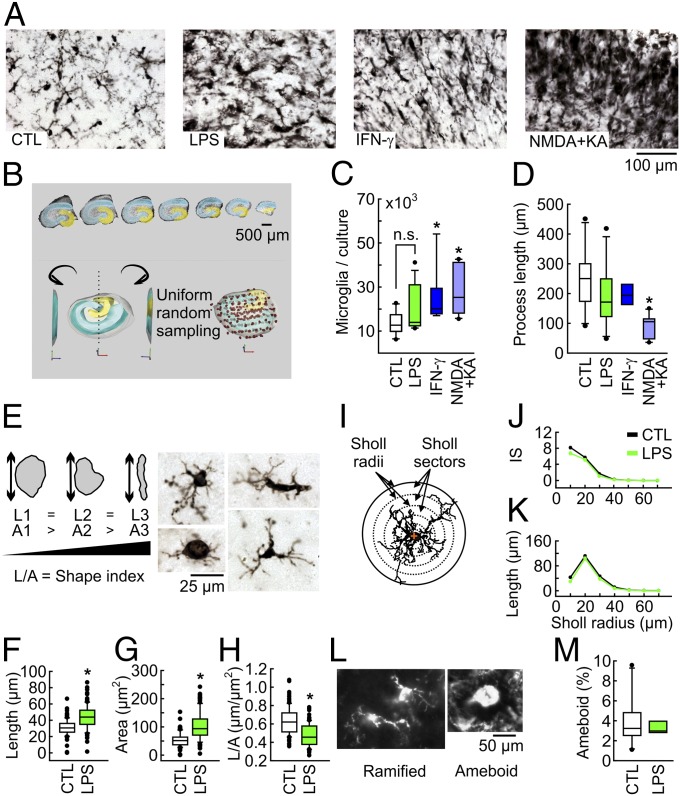
Fig. 2.
Morphological correlates of microglial activation as revealed by Iba1 immunohistochemistry. (A) CA3 region of control (CTL) and slice cultures exposed to LPS (10 µg/mL), IFN-γ (100 ng/mL), or NMDA+KA (5 µM) for 48 h to induce excitotoxic death. (B) Design-based stereology in cryosections (25 µm) of individual slice cultures. The dentate gyrus is in yellow, and CA3-CA1 is in blue. (Inset) Interpolated 3D reconstruction of sampling contours with randomly sampled microglial cells (dots). Estimates refer to the total hippocampus. (C) Stereological estimation of Iba1-positive cells per slice culture with the optical fractionator probe. *P < 0.05 vs. control, ANOVA on ranks. n/N as in D. (D) Stereological estimation of total process length per cell with the spaceballs probe. *P < 0.05 vs. control, one-way ANOVA. For n/N cultures/preparations: CTL, 10/5; LPS, 13/5; IFN-γ, 9/4; NMDA+KA, 10/4. (E) Parameterization of 2D somatic projections based on maximum length (L) and projection area (A). The somatic shape index (L/A) increases in rod-shaped somata. Images, spectrum of microglial shapes. (F–H) Microglial somatic L, A, and L/A. *P < 0.05, rank-sum test. CTL, 114 cells/9/7; LPS, 102 cells/10/5. (I) Three-dimensional Sholl grid of concentric spheres describing microglial branch length and number as a function of distance from the geometric center of the cell somata (red cross). Branch intersections with the spherical grid quantify the number, whereas the branch length between consecutive spheres estimates the degree of convolution. (J and K) Sholl analysis of microglial processes. IS, intersections. CTL, 116 cells/9/7; LPS, 102 cells/10/5. (L) Fluorescent images of ramified and ameboid microglia. (M) Stereological quantification of ameboid cells with enlarged, round somata (cell bodies) showing no more than a single process and a few filopodia, with the optical fractionator probe. P > 0.05, rank-sum test. CTL, 11/11; LPS, 7/7.
Fig. 3.
iNOS-mediated neurodegeneration. (A) Immunohistochemistry of MAP2 (Left), parvalbumin in CA3 (Middle), and iNOS in cryosections (25 µm) (Right). Note the complex morphology of parvalbumin-positive GABAergic interneurons (fine meshwork, black) contacting the perisomatic region of pyramidal cells. (B) Cell death revealed by LDH activity in the medium after 72 h of exposure. For n/N membranes/preparations: CTL, 36/11; LPS, 34/12; IFN-γ, 33/11; LPS+IFN-γ, 37/12; NMDA+KA, 21/7. (C) Griess reaction for nitrite to detect NO release. For n/N membranes/preparations: CTL, 8/3; LPS, 8/3; IFN-γ, 8/3; LPS+IFN-γ, 8/3; 1400W, 9/3; 1400W+LPS+IFN-γ, 9/3. *P < 0.05 vs. control, ANOVA on ranks with Dunn´s post hoc pairwise test; +P < 0.001, rank-sum or t test (B and C). (D) iNOS staining in stratum pyramidale of CA3.
LPS exposure induced the up-regulation of microglial activation markers, ionized calcium-binding adaptor molecule 1 (Iba1), CD11b, and CD68 (Fig. 2A and SI Appendix, Fig. S4). Design-based stereology and Sholl analysis (31) revealed that the length and area of microglial somata (cell bodies) increased disproportionally, indicative of somatic enlargement (Fig. 2 E–H). In contrast, microglial population size, process length, ramification degree, and, notably, the fraction of ameboid cells were unaffected (Fig. 2 B–D and I–M). Exposure to recombinant IFN-γ at a concentration of 100 ng/mL, which is known to induce maximal effects in microglia (15), was associated with the significant increase in the number of microglial cells, i.e., population expansion (Fig. 2C), as well as NO release via iNOS (Fig. 3 A and C), similar to TLR4 activation. In contrast, the levels of IL-1β, TNF-α, IL-6, and IL-10 were unaffected (Fig. 1 A–C and SI Appendix, Fig. S3).
Despite inducing diverse reactive phenotypes of microglia, neither LPS nor IFN-γ resulted in significant tissue damage, which was determined by lactate dehydrogenase (LDH) activity as a general biochemical marker of cell death and neuron-specific immunohistochemistry (Fig. 3 A and B and SI Appendix, Fig. S5). The morphology of dendrites (MAP2 staining) and GABAergic interneurons (parvalbumin staining) that are exquisitely susceptible to metabolic and oxidative stress (27) was well preserved. As a positive control, slice cultures were exposed to glutamate receptor agonists N-methyl-d-aspartate (NMDA; 5 µM) and kainic acid (KA; 5 µM) to induce excitotoxicity (32, 33). NMDA+KA resulted in massive neuronal death (Fig. 3 A and B and SI Appendix, Fig. S5) and another microglial phenotype, i.e., large population expansion and a significant decrease in process length, reflecting ameboid microglia that phagocytose cellular debris (Fig. 2 C and D). These experiments demonstrate that slice cultures are a reliable model for effective tissue exposure and induction of diverse reactive microglial phenotypes (33, 34).
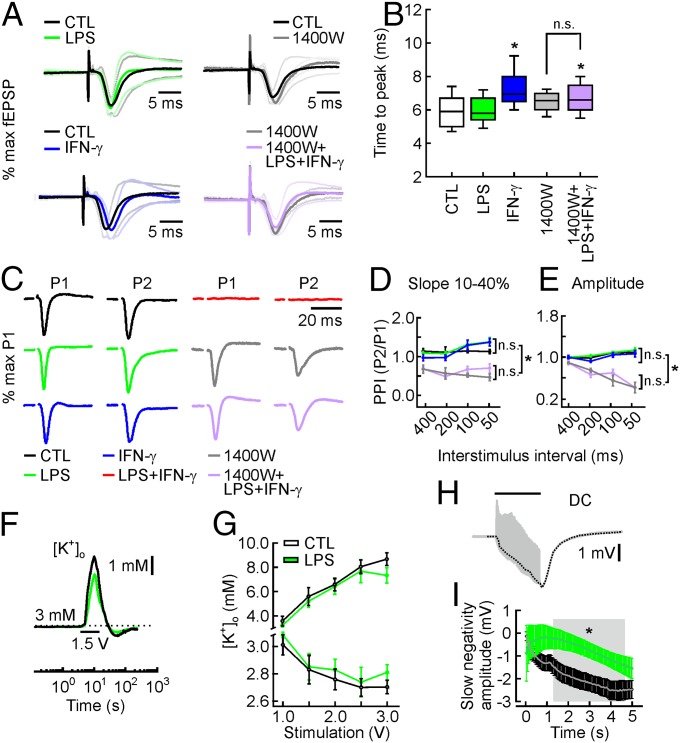
Alterations of LDH activity and neuronal morphology indicate only massive structural tissue damage. Thus, we investigated in detail the integrity of neuronal function with electrophysiological methods in local networks of hippocampal regions CA3 and CA1. Quantification of electrical stimulus-evoked field excitatory postsynaptic potential (fEPSP) in stratum radiatum and field population spike (fPopS) in stratum pyramidale (SI Appendix, Fig. S6) revealed that LPS exposure shifted the stimulation–response curve of fEPSPs to the left and altered the fEPSP–fPopS coupling, indicative of slight changes in postsynaptic responses and intrinsic neuronal excitability (SI Appendix, Fig. S7 A, B, and D). Although it showed a trend, IFN-γ exposure had no significant impact on these parameters. In contrast, only IFN-γ affected the fEPSP kinetics, which depend on both the number and synchrony of activated synapses (Fig. 4 A and B). Neither LPS nor IFN-γ altered the paired-pulse facilitation index at several interstimulus intervals, reflecting intact short-term synaptic plasticity (Fig. 4 C–E).
Fig. 4.
fEPSP kinetics, short-term synaptic plasticity, and K+ homeostasis. (A) fEPSP responses in CA3 plotted as normalized to their maximum amplitude to better appreciate the fEPSP kinetics. Solid lines represent grand averages of normalized fEPSP traces ± 1σ (dotted lines). (B) The time to peak between electrical stimulation and maximum fEPSP amplitude, describing the fEPSP kinetics. It is slower for IFN-γ and 1400W+LPS+IFN-γ, but unchanged when comparing 1400W and 1400W+LPS+IFN-γ. For n/N cultures/preparations: CTL, 78/9; LPS, 65/7; IFN-γ, 48/6; 1400W, 12/2; 1400W+LPS+IFN-γ, 80/3. *P < 0.05, ANOVA on ranks with Dunn´s post hoc pairwise test. n.s., nonsignificant. (C) fEPSP responses in CA3 evoked by paired-pulse stimulation at 50% of maximum response (stimulation artifacts truncated). Grand averages of paired-pulse fEPSP responses with an interstimulus interval (ISI) of 100 ms, normalized to the maximum amplitude as evoked by the first pulse. P1, first pulse; P2, second pulse. The paired-pulse index (PPI) is defined as the ratio P2/P1. (D and E) The PPI of 1400W+LPS+IFN-γ and 1400W is significantly lower compared with that of CTL, LPS, and IFN-γ for all ISIs. P < 0.05, ANOVA on ranks. There are no differences among CTL, LPS, and IFN-γ, or between 1400W+LPS+IFN-γ and 1400W. For n/N cultures/preparations: CTL, 23/9; LPS, 12/7; IFN-γ, 15/6; 1400W, 3/2; 1400W+LPS+IFN-γ, 14/3. P > 0.05, ANOVA on ranks. (F) Extracellular K+ homeostasis during electrical stimulation (5 s, 20 Hz, 1.5 V; black bar) of fiber tracts projecting to CA1. The double-barreled microelectrode ([K+]o and LFP) was positioned in stratum pyramidale of CA1. Increase, decay, and undershoot of [K+]o transients reflect K+ release from neurons, K+ (re)uptake from neurons and astrocytes, and K+ redistribution within the gap junction-coupled astrocytic syncytium, respectively. The traces illustrate average responses for control (black) and LPS exposure (10 µg/mL, for 72 h) (green). (G) Increase and undershoot of stimulus-induced [K+]o transients were similar for control and LPS. P > 0.05, rank-sum test. For n/N cultures/preparations: CTL, 15/10; LPS, 22/10. (H) Original LFP trace (gray) during stimulation (5 s, 20 Hz, 1.5 V; black bar) with filtered slow negativity (black dotted line). (I) Average slow DC-negativity during stimulation (5 s, 20 Hz). Gray area indicates period with significant differences. *P < 0.05, rank-sum test. n/N as in G.
We further tested the functional integrity of neurons and astrocytes by quantifying transient changes in the extracellular K+ concentration ([K+]o) and the local field potential (LFP) during robust neural excitation induced by electrical stimulation. The amplitude and kinetics of such stimulus-induced [K+]o transients were unchanged after LPS exposure (Fig. 4 F and G and SI Appendix, Fig. S8), indicating that Na+/K+ ATPases and ion channels involved in K+ reuptake and astrocytic K+ redistribution were intact (28, 35). The maximal amplitude of the slow negative direct current (DC) component in the LFP, which is known to correlate with local K+ uptake by astrocytes, was also unaffected, albeit with somewhat slower kinetics during the stimulation (Fig. 4 H and I). This is in line with slight changes in astrocytic staining, glial fibrillary acidic protein (GFAP), and S100b (SI Appendix, Fig. S9).
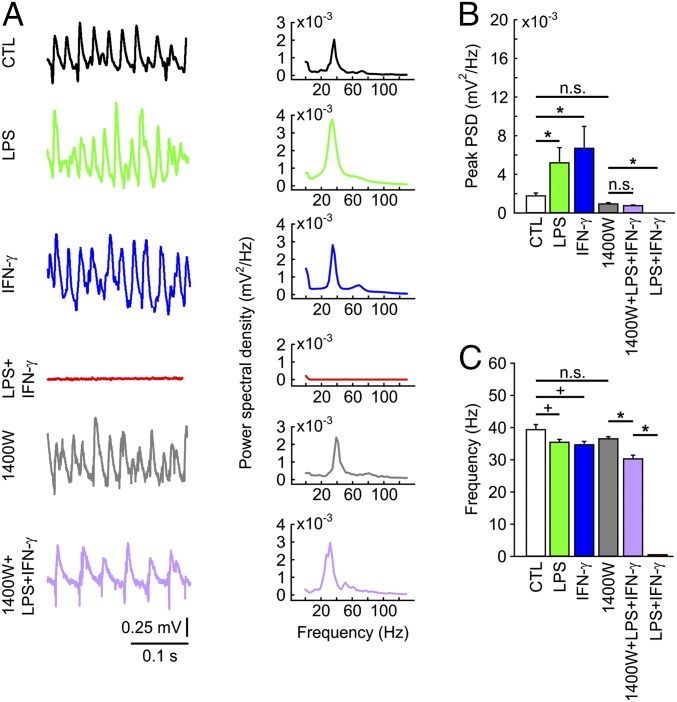
Hippocampal slice cultures show spontaneous neuronal activity and reliably express fast network oscillations in the gamma frequency band (30–100 Hz) in the presence of acetylcholine (28, 36). Gamma oscillations are a common mode of complex cortical information processing in vivo and require precise synaptic communication of excitatory pyramidal cells and inhibitory interneurons (27). Gamma oscillations also provide a sensitive readout for local network (dys)function because they are exquisitely susceptible to structural and metabolic alterations, particularly in fast-spiking, parvalbumin-positive GABAergic interneurons that exert fast rhythmic inhibition on pyramidal cells (27). Notably, persistent gamma oscillations were still present in slice cultures exposed to LPS or IFN-γ (Fig. 5 A–C). We even observed a significant increase in the oscillation power, but with a slightly decreased frequency within the gamma band.
Fig. 5.
Fast network oscillations. (A) Gamma oscillations in stratum pyramidale of CA3 in the presence of acetylcholine (2 µM) and physostigmine (400 nM). LFP traces (Left) with corresponding power spectrograms for 10–130 Hz (Right). (B) Peak power spectral density derived from power spectrograms (A, Right). *P < 0.05, ANOVA on ranks. n.s., nonsignificant. (C) Peak frequency derived from power spectrograms (A, Right). *P < 0.05, ANOVA on ranks; +P < 0.05, rank-sum test. For n/N cultures/preparations: CTL, 12/5; LPS, 9/3; IFN-γ, 8/3; 1400W, 40/5; 1400W+LPS+IFN-γ, 33/5; LPS+IFN-γ, 10/3.
The foregoing findings suggest that chronic activation of TLR4 or IFN-γ receptor in situ induces diverse reactive proinflammatory phenotypes in microglia, with only moderate effects on the function of neuronal subtypes and astrocytes.
The Combination of LPS and IFN-γ Induces Inflammatory Neurodegeneration Mainly by iNOS-Mediated Microglial NO Release.
The aforementioned phenotypes avoided substantial neuronal dysfunction and damage. We next applied LPS (10 µg/mL) and IFN-γ (100 ng/mL) in combination for 72 h (11, 15, 37). LPS+IFN-γ strongly increased iNOS expression and NO release from microglia (Fig. 3 A, C, and D). Notably, LPS+IFN-γ resulted in complete disintegration of slice cultures, even exceeding the severity of the damage caused by NMDA+KA. In particular, toluidine blue, MAP2, and parvalbumin staining showed severe alterations in neuronal morphology and slice architecture (Fig. 3A and SI Appendix, Fig. S5), whereas microglia strongly positive for CD11b and CD68 were still present (SI Appendix, Fig. S4). Massive cell death was also reflected by high LDH activity (Fig. 3B). Accordingly, neuronal activity was completely absent in slice cultures (Figs. 4C and 5 A–C and SI Appendix, Fig. S7). Similar results were obtained with lower concentrations of LPS+IFN-γ and exposure for 24 h (SI Appendix, Fig. S10). Interestingly, LPS+IFN-γ exposure was associated with lower levels of TNF-α and IL-6 release compared with LPS exposure alone (Fig. 1 B and C).
Studies in primary neuron-glia cultures have shown that enhanced iNOS expression and concomitant NO release play major roles in inflammatory neuronal death during exposure to LPS+IFN-γ (11, 12, 14). We tested this mechanism in situ using N-3-aminomethyl-benzyl-acetamidine (1400W), a slowly reversible inhibitor of NO synthases that is highly selective for the iNOS isoform (14, 34). We coapplied 1400W in an effective but nontoxic concentration (400 µM) that still permitted some NO release (Fig. 3C and SI Appendix, SI Materials and Methods). Coapplication of 1400W during LPS+IFN-γ exposure significantly inhibited NO release to levels similar to those obtained with LPS or IFN-γ alone and was associated with reduced iNOS expression compared with LPS+IFN-γ (Fig. 3 A and C and SI Appendix, Fig. S11), presumably owing to alterations in positive feedback mechanisms on the transcriptional level (38). Effective inhibition of NO release was reflected by widely preserved neuronal morphology (Fig. 3A) and by the integrity of neuronal function.
The stimulation–response curves of electrical stimulus-evoked fEPSPs in stratum radiatum and the fEPSP–fPopS coupling were rescued by 1400W (SI Appendix, Fig. S7). Similar rescue effects were obtained for the kinetics of fEPSP and paired-pulse facilitation at several interstimulus intervals (Fig. 4). Strikingly, 1400W also widely recovered gamma oscillations that are exquisitely susceptible to structural and metabolic alterations (Fig. 5 A–C). Although there were no differences in the power of gamma oscillations, 1400W+LPS+IFN-γ was associated with a significant decrease in the frequency of ∼6 Hz. Comparing 1400W with controls (naive slice cultures) revealed no significant differences in fEPSP kinetics or gamma oscillations, but a significant reduction in paired-pulse facilitation (Figs. 4 and 5 and SI Appendix, Fig. S7). This might indicate that a partial blockade of NO release interferes with presynaptic short-term plasticity without affecting action potential generation and network oscillations (39). Chemical depletion of microglia from slice cultures using liposome-encapsulated clodronate confirmed that microglial iNOS expression was essential to trigger neurodegeneration (SI Appendix, SI Materials and Methods and Fig. S12). Neurodegeneration likely was not the result of depletion of l-arginine, the sole substrate of iNOS (40) (SI Appendix, Fig. S13).
Discussion
We have shown that TLR4 activation with LPS induces a reactive phenotype in microglia in situ. Similar phenotypes were observed in primary microglia cultures, neuron-glia cultures, and slice cultures exposed to LPS alone (5, 9, 12, 13, 15, 34, 41). Our exposure protocol permitted the accumulation of proinflammatory cytokines and potential neurotoxins, such as TNF-α and NO (12, 14). Strikingly, we did not observe neurotoxic effects at either structural or functional levels. This expands previous data from slice cultures (5, 25, 34), but contrasts with studies of neuron-glia cultures and in vivo, in which LPS exposure alone was neurotoxic (8, 12, 13, 17, 18, 20, 21). Several factors might account for these differences, including (i) insufficient NO levels to amplify inflammatory responses of glial cells and/or inhibit mitochondrial respiration in neurons (14, 32); (ii) the neural subtype investigated (42); (iii) suppressive effects on microglia (“off” signals) from active neurons and astrocytes through cell membrane contacts, neurotransmitters, and cytokines (1, 4, 7); and (iv) the presence of antioxidative extracellular matrix components that protect neurons (43).
Notably, TLR4-activated microglia altered intrinsic neuronal excitability and gamma oscillations only slightly, whereas short-term synaptic plasticity was unaffected. Similarly, no or only moderate changes in intrinsic membrane properties and postsynaptic potentials were described for CA1 pyramidal cells, both during acute LPS application in hippocampal slices (26, 44) and after LPS exposure for days in slice cultures (25). We also found a regular K+ homeostasis after LPS exposure. This further suggests that (i) there was no significant metabolic impairment of neuronal Na+/K+ ATPase activity, and (ii) astrocytes were functionally widely intact. These findings are of central importance because they show that during microglial TLR4 activation, the functional integrity of excitatory pyramidal cells, inhibitory interneurons, and astrocytes is well preserved in situ. This is supported by the fact that the slight increase in the ratio of excitation and inhibition was not associated with any pathological neuronal activity in our experiments, even in the presence of exogenous acetylcholine or robust electrical stimulation. The increased neuronal excitability in hippocampal slices might be caused by enhanced trafficking of postsynaptic AMPA receptors as mediated by TNF-α (45) and/or the higher incidence of excitatory postsynaptic currents in pyramidal cells as mediated by complex neuron–glia interactions during LPS application (26). Alternatively, enhanced NO release as induced by LPS or IFN-γ might primarily interfere with the specialized function of inhibitory interneurons (27, 39).
In contrast to TLR4, IFN-γ receptors are more abundant and functionally present in microglia, astrocytes, and perhaps in neurons (11, 46, 47). IFN-γ is a dimerized soluble cytokine and the only member of the type II class of IFNs. It is released predominantly by leukocyte subtypes, such as NK cells, Th1 cells, and cytotoxic T lymphocytes (3, 37). Exposure to IFN-γ alone resulted in different characteristics of microglia, such as higher cell numbers, in line with previously reported findings (15); however, the moderate effects on neuronal function were similar to those of LPS. We did not observe direct toxic effects in situ as has been described for primary cortical neuron cultures (46). The decreased frequency of gamma oscillations might contribute to cognitive deficits and sickness behavior during systemic infections, multiple sclerosis, and Alzheimer’s disease featuring increased IFN-γ levels (7, 48–50).
We have shown that for neuronal tissue lacking adaptive immunity, TLR4-activated microglia require concomitant IFN-γ signaling to induce massive inflammatory neurodegeneration, which is caused mainly by iNOS-mediated microglial NO release. This finding supports previous reports on LPS+IFN-γ exposure in primary neuron-glia cultures and slice cultures (11, 12, 14, 34). Notably, in vivo models using LPS also show inflammatory neurodegeneration and likely feature the release of IFN-γ from infiltrating leukocytes (7, 19–21). Activation of IFN-γ receptors triggers various responses in macrophages and microglia (37, 51). Moreover, there is cross-talk between TLR4 and the IFN-γ receptor via autocrine and paracrine loops at various intracellular downstream cascades (37, 52). The promoter of iNOS gene (Inos) is operated by both STAT1 and NFκΒ transcription factors, activated by IFN-γ receptor and TLR4, respectively (37, 53); thus, coincident STAT1/NFκB signaling up-regulates iNOS transcription and subsequent NO release to the critical neurotoxic level (11, 32, 34). TLR4 and/or IFN-γ receptor activation also up-regulate NADPH oxidase, which generates superoxide anions (20, 52). Superoxide anions can rapidly oxidize NO, resulting in highly toxic peroxynitrite (27). Thus, iNOS and NADPH oxidase in microglia are strong candidates for the mechanism that induces inflammatory neurodegeneration (14, 34, 44, 52).
In pathology, T and NK cells infiltrate the brain parenchyma and become stimulated by activated microglia. Th1 and NK cells, representing adaptive and innate immunity, respectively, are the main sources of IFN-γ in inflamed neuronal and various other tissues (1, 7, 37). Interestingly, different T-cell subtypes express TLR4, which may accelerate the interaction with resident macrophages during infection (54). Our findings support the hypothesis that chronic peripheral infections and/or transient impairment of the blood–brain barrier promote microglial priming and favor microglial neurotoxicity on exposure to secondary inflammatory stimuli, such as bacterial cell wall components during meningitis (49). In Alzheimer’s disease, even moderate infiltration of Th1 and NK cells may exacerbate the inflammatory response of microglia activated by amyloid-β peptide via cell surface receptors, such as TLR4, TLR6, CD36, and CD14 (1, 7, 10).
We provide in situ evidence for the general concept of microglial activation, i.e., the requirement of a foreign or modified endogenous pathogenic stimulus and at least a second “security” signal from peripheral immune cells (two-step activation process) to unleash inflammation-induced neurodegeneration and host attack. Our study might contribute to the development of new pharmacologic strategies for treating several neurologic and psychiatric disorders.
Materials and Methods
More detailed information is provided in SI Appendix, SI Materials and Methods.
Slice Cultures and Exposures.
Care and killing of Wistar rats (Charles River Laboratories) and experimental procedures were approved by the authorities of Baden-Württemberg (T56/11). Hippocampal slice cultures were prepared from 7- to 9-d-old pups in sterile conditions and maintained on biopore membranes at the interface between serum-containing culture medium and humidified atmosphere [5% (vol/vol) CO2, 36.5 °C] (28, 36). Cell culture materials were certified free of endotoxin and IFN-γ. Exposures to LPS from Escherichia coli (Enzo Life Sciences), recombinant IFN-γ (PeproTech), 1400W, NMDA, and KA (Sigma-Aldrich) were done in the dark.
Biochemical Analyses.
Culture medium was sampled (SI Appendix, Fig. S1) and rapidly frozen to −80 °C. Calibrations and biochemical analyses were performed in accordance with the manufacturer’s instructions. Samples were analyzed with ELISA kits (R&D Systems). NO release was derived from the concentration of its degradation product, nitrite, with a Griess reaction-based assay (Merck Chemicals). LDH activity was measured with a standard kit (Sigma-Aldrich).
Immunohistochemistry and Quantitative Morphology.
Slice cultures were fixed in 4% (vol/vol) paraformaldehyde and cut into thin (<25 µm) sections with a cryostat (CM1850; Leica Microsystems). Neurons were stained with toluidine blue (Sigma-Aldrich), anti-MAP2 (Abcam), anti-parvalbumin (Abcam), microglia with isolectin Β4 (Sigma-Aldrich), anti-Iba1 (WAKO Chemicals), anti-CD11b (Serotec), anti-CD68 (Abcam), astrocytes with anti-GFAP (Abcam), anti-S100b (Vector Laboratories), and iNOS with anti-iNOS (Merck Chemicals). Primary antibodies were visualized with fluorescent or biotin-conjugated secondary antibodies (31). Stereological analyses were performed with Stereo Investigator (MBF Bioscience). Volume-corrected cell counting was done using an optical fractionator probe (31). Morphometric quantification was done with Neurolucida for cell tracing and Neuroexplorer (MBF Bioscience) for analysis of vectorized cell reconstructions, according to the Sholl analysis model (31).
LFP and Extracellular K+ Concentration.
Slice cultures were kept in a recording chamber [95% (vol/vol) O2, 5% (vol/vol) CO2; 34 ± 1 °C]. The recording solution contained 129 mM NaCl, 3 mM KCl, 1.25 mM NaH2PO4, 1.8 mM MgSO4, 1.6 mM CaCl2, 26 mM NaHCO3, and 10 mM glucose (pH 7.3). LFP was recorded with microelectrodes connected to an amplifier (EXT 10-2F; npi electronic), AC-filtered, and digitized at 10 kHz (CED 1401; Cambridge Electronic Design). Electrical stimulation was done using a platinum electrode and an ISO-Flex stimulus isolator (AMPI). Stimulation–response and fEPSP–fPopS coupling curves were obtained by applying repetitive pulses (100 µs, 0.1 Hz) at various intensities. The stimulation intensity of paired pulses was adjusted to elicit 50% of the maximum response. Gamma oscillations were induced by bath application of acetylcholine (Sigma-Aldrich) and physostigmine (Tocris Bioscience) (36). Changes in [K+]o were determined with double-barreled microelectrodes (28, 55). The reference barrel was filled with 154 mM NaCl, and the ion-sensitive barrel was filled with K+ ionophore I cocktail A (Sigma-Aldrich) and 100 mM KCl. K+-related changes in voltage were digitized at 1 kHz (0.3-kHz low-pass filter).
Calculations and Statistics.
Data are reported as mean ± SEM from cultures or membranes (n) and independent preparations (N) unless stated otherwise. Data distribution was tested for normality with the Shapiro–Wilk test. Statistical tests are specified in the figure legends. For analysis of gamma oscillations, data segments >13 min were low-pass filtered at 200 Hz and processed with Welch’s algorithm at a Nyquist frequency of 2,048. Post hoc analysis was done using MatLab 11.0 (MathWorks). A modified Nernst equation (28) was used to translate K+-related changes in voltage (mV) in [K+]o (mM).
Supplementary Material
Acknowledgments
We thank Elke Pralle and Susanne Kieke for technical support, and Prof. Dr. Uwe Heinemann and Dr. Denise van Rossum for helpful discussions. This work was supported by grants from the German Research Foundation (SFB/TRR43 and FOR1336, to U.-K.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. H.L.W. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1513853113/-/DCSupplemental.
References
- 1.Ransohoff RM, Perry VH. Microglial physiology: Unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- 2.Rivest S. Regulation of innate immune responses in the brain. Nat Rev Immunol. 2009;9(6):429–439. doi: 10.1038/nri2565. [DOI] [PubMed] [Google Scholar]
- 3.Hanisch U-K, Kettenmann H. Microglia: Active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10(11):1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 4.Biber K, Owens T, Boddeke E. What is microglia neurotoxicity (Not)? Glia. 2014;62(6):841–854. doi: 10.1002/glia.22654. [DOI] [PubMed] [Google Scholar]
- 5.Ajmone-Cat MA, Mancini M, De Simone R, Cilli P, Minghetti L. Microglial polarization and plasticity: Evidence from organotypic hippocampal slice cultures. Glia. 2013;61(10):1698–1711. doi: 10.1002/glia.22550. [DOI] [PubMed] [Google Scholar]
- 6.Kettenmann H, Kirchhoff F, Verkhratsky A. Microglia: New roles for the synaptic stripper. Neuron. 2013;77(1):10–18. doi: 10.1016/j.neuron.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 7.Lynch MA. The impact of neuroimmune changes on development of amyloid pathology: Relevance to Alzheimer’s disease. Immunology. 2014;141(3):292–301. doi: 10.1111/imm.12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lehnardt S, et al. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4–dependent pathway. Proc Natl Acad Sci USA. 2003;100(14):8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Regen T, et al. CD14 and TRIF govern distinct responsiveness and responses in mouse microglial TLR4 challenges by structural variants of LPS. Brain Behav Immun. 2011;25(5):957–970. doi: 10.1016/j.bbi.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Stewart CR, et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11(2):155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chao CC, Hu S, Molitor TW, Shaskan EG, Peterson PK. Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. J Immunol. 1992;149(8):2736–2741. [PubMed] [Google Scholar]
- 12.Dawson VL, Brahmbhatt HP, Mong JA, Dawson TM. Expression of inducible nitric oxide synthase causes delayed neurotoxicity in primary mixed neuronal-glial cortical cultures. Neuropharmacology. 1994;33(11):1425–1430. doi: 10.1016/0028-3908(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 13.Araki E, Forster C, Dubinsky JM, Ross ME, Iadecola C. Cyclooxygenase-2 inhibitor ns-398 protects neuronal cultures from lipopolysaccharide-induced neurotoxicity. Stroke. 2001;32(10):2370–2375. doi: 10.1161/hs1001.096057. [DOI] [PubMed] [Google Scholar]
- 14.Bal-Price A, Brown GC. Inflammatory neurodegeneration mediated by nitric oxide from activated glia-inhibiting neuronal respiration, causing glutamate release and excitotoxicity. J Neurosci. 2001;21(17):6480–6491. doi: 10.1523/JNEUROSCI.21-17-06480.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Häusler KG, et al. Interferon-γ differentially modulates the release of cytokines and chemokines in lipopolysaccharide- and pneumococcal cell wall-stimulated mouse microglia and macrophages. Eur J Neurosci. 2002;16(11):2113–2122. doi: 10.1046/j.1460-9568.2002.02287.x. [DOI] [PubMed] [Google Scholar]
- 16.Taylor DL, Diemel LT, Pocock JM. Activation of microglial group III metabotropic glutamate receptors protects neurons against microglial neurotoxicity. J Neurosci. 2003;23(6):2150–2160. doi: 10.1523/JNEUROSCI.23-06-02150.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burguillos MA, et al. Caspase signalling controls microglia activation and neurotoxicity. Nature. 2011;472(7343):319–324. doi: 10.1038/nature09788. [DOI] [PubMed] [Google Scholar]
- 18.Starossom SC, et al. Galectin-1 deactivates classically activated microglia and protects from inflammation-induced neurodegeneration. Immunity. 2012;37(2):249–263. doi: 10.1016/j.immuni.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamada K, et al. Brain dysfunction associated with an induction of nitric oxide synthase following an intracerebral injection of lipopolysaccharide in rats. Neuroscience. 1999;88(1):281–294. doi: 10.1016/s0306-4522(98)00237-1. [DOI] [PubMed] [Google Scholar]
- 20.Qin L, et al. NADPH oxidase mediates lipopolysaccharide-induced neurotoxicity and proinflammatory gene expression in activated microglia. J Biol Chem. 2004;279(2):1415–1421. doi: 10.1074/jbc.M307657200. [DOI] [PubMed] [Google Scholar]
- 21.Ambrosini A, Louin G, Croci N, Plotkine M, Jafarian-Tehrani M. Characterization of a rat model to study acute neuroinflammation on histopathological, biochemical and functional outcomes. J Neurosci Methods. 2005;144(2):183–191. doi: 10.1016/j.jneumeth.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Block ML, Zecca L, Hong J-S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8(1):57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 23.Cunningham C. Microglia and neurodegeneration: The role of systemic inflammation. Glia. 2013;61(1):71–90. doi: 10.1002/glia.22350. [DOI] [PubMed] [Google Scholar]
- 24.Nadeau S, Rivest S. Glucocorticoids play a fundamental role in protecting the brain during innate immune response. J Neurosci. 2003;23(13):5536–5544. doi: 10.1523/JNEUROSCI.23-13-05536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hellstrom IC, Danik M, Luheshi GN, Williams S. Chronic LPS exposure produces changes in intrinsic membrane properties and a sustained IL-β–dependent increase in GABAergic inhibition in hippocampal CA1 pyramidal neurons. Hippocampus. 2005;15(5):656–664. doi: 10.1002/hipo.20086. [DOI] [PubMed] [Google Scholar]
- 26.Pascual O, Ben Achour S, Rostaing P, Triller A, Bessis A. Microglia activation triggers astrocyte-mediated modulation of excitatory neurotransmission. Proc Natl Acad Sci USA. 2012;109(4):E197–E205. doi: 10.1073/pnas.1111098109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kann O, Papageorgiou IE, Draguhn A. Highly energized inhibitory interneurons are a central element for information processing in cortical networks. J Cereb Blood Flow Metab. 2014;34(8):1270–1282. doi: 10.1038/jcbfm.2014.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kann O, Huchzermeyer C, Kovács R, Wirtz S, Schuelke M. Gamma oscillations in the hippocampus require high complex I gene expression and strong functional performance of mitochondria. Brain. 2011;134(Pt 2):345–358. doi: 10.1093/brain/awq333. [DOI] [PubMed] [Google Scholar]
- 29.Fu X, et al. Central administration of lipopolysaccharide induces depressive-like behavior in vivo and activates brain indoleamine 2,3 dioxygenase in murine organotypic hippocampal slice cultures. J Neuroinflammation. 2010;7:43. doi: 10.1186/1742-2094-7-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carpentier PA, et al. Differential activation of astrocytes by innate and adaptive immune stimuli. Glia. 2005;49(3):360–374. doi: 10.1002/glia.20117. [DOI] [PubMed] [Google Scholar]
- 31.Papageorgiou IE, Fetani AF, Lewen A, Heinemann U, Kann O. Widespread activation of microglial cells in the hippocampus of chronic epileptic rats correlates only partially with neurodegeneration. Brain Struct Funct. 2015;220(4):2423–2439. doi: 10.1007/s00429-014-0802-0. [DOI] [PubMed] [Google Scholar]
- 32.Keynes RG, Duport S, Garthwaite J. Hippocampal neurons in organotypic slice culture are highly resistant to damage by endogenous and exogenous nitric oxide. Eur J Neurosci. 2004;19(5):1163–1173. doi: 10.1111/j.1460-9568.2004.03217.x. [DOI] [PubMed] [Google Scholar]
- 33.Vinet J, et al. Neuroprotective function for ramified microglia in hippocampal excitotoxicity. J Neuroinflammation. 2012;9:27. doi: 10.1186/1742-2094-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duport S, Garthwaite J. Pathological consequences of inducible nitric oxide synthase expression in hippocampal slice cultures. Neuroscience. 2005;135(4):1155–1166. doi: 10.1016/j.neuroscience.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 35.Wallraff A, et al. The impact of astrocytic gap junctional coupling on potassium buffering in the hippocampus. J Neurosci. 2006;26(20):5438–5447. doi: 10.1523/JNEUROSCI.0037-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schneider J, et al. A reliable model for gamma oscillations in hippocampal tissue. J Neurosci Res. 2015;93(7):1067–1078. doi: 10.1002/jnr.23590. [DOI] [PubMed] [Google Scholar]
- 37.Schroder K, Sweet MJ, Hume DA. Signal integration between IFNgamma and TLR signalling pathways in macrophages. Immunobiology. 2006;211(6-8):511–524. doi: 10.1016/j.imbio.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 38.Lee M, Choy JC. Positive feedback regulation of human inducible nitric oxide synthase expression by Ras protein S-nitrosylation. J Biol Chem. 2013;288(22):15677–15686. doi: 10.1074/jbc.M113.475319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amitai Y. Physiologic role for “inducible” nitric oxide synthase: A new form of astrocytic-neuronal interface. Glia. 2010;58(15):1775–1781. doi: 10.1002/glia.21057. [DOI] [PubMed] [Google Scholar]
- 40.Kan MJ, et al. Arginine deprivation and immune suppression in a mouse model of Alzheimer’s disease. J Neurosci. 2015;35(15):5969–5982. doi: 10.1523/JNEUROSCI.4668-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huuskonen J, Suuronen T, Miettinen R, van Groen T, Salminen A. A refined in vitro model to study inflammatory responses in organotypic membrane culture of postnatal rat hippocampal slices. J Neuroinflammation. 2005;2:25. doi: 10.1186/1742-2094-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim W-G, et al. Regional difference in susceptibility to lipopolysaccharide-induced neurotoxicity in the rat brain: Role of microglia. J Neurosci. 2000;20(16):6309–6316. doi: 10.1523/JNEUROSCI.20-16-06309.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cabungcal J-H, et al. Perineuronal nets protect fast-spiking interneurons against oxidative stress. Proc Natl Acad Sci USA. 2013;110(22):9130–9135. doi: 10.1073/pnas.1300454110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang J, et al. Microglial CR3 activation triggers long-term synaptic depression in the hippocampus via NADPH oxidase. Neuron. 2014;82(1):195–207. doi: 10.1016/j.neuron.2014.01.043. [DOI] [PubMed] [Google Scholar]
- 45.Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-α. Nature. 2006;440(7087):1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- 46.Mizuno T, et al. Interferon-γ directly induces neurotoxicity through a neuron specific, calcium-permeable complex of IFN-γ receptor and AMPA GluR1 receptor. FASEB J. 2008;22(6):1797–1806. doi: 10.1096/fj.07-099499. [DOI] [PubMed] [Google Scholar]
- 47.Hashioka S, Klegeris A, Schwab C, Yu S, McGeer PL. Differential expression of interferon-γ receptor on human glial cells in vivo and in vitro. J Neuroimmunol. 2010;225(1-2):91–99. doi: 10.1016/j.jneuroim.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 48.Heesen C, et al. Fatigue in multiple sclerosis: An example of cytokine mediated sickness behaviour? J Neurol Neurosurg Psychiatry. 2006;77(1):34–39. doi: 10.1136/jnnp.2005.065805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perry VH, Holmes C. Microglial priming in neurodegenerative disease. Nat Rev Neurol. 2014;10(4):217–224. doi: 10.1038/nrneurol.2014.38. [DOI] [PubMed] [Google Scholar]
- 50.Vasconcelos AR, et al. Intermittent fasting attenuates lipopolysaccharide-induced neuroinflammation and memory impairment. J Neuroinflammation. 2014;11:85. doi: 10.1186/1742-2094-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stohwasser R, et al. Biochemical analysis of proteasomes from mouse microglia: Induction of immunoproteasomes by interferon-γ and lipopolysaccharide. Glia. 2000;29(4):355–365. [PubMed] [Google Scholar]
- 52.Chéret C, et al. Neurotoxic activation of microglia is promoted by a nox1-dependent NADPH oxidase. J Neurosci. 2008;28(46):12039–12051. doi: 10.1523/JNEUROSCI.3568-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taylor BS, Geller DA. Molecular regulation of the human inducible nitric oxide synthase (iNOS) gene. Shock. 2000;13(6):413–424. doi: 10.1097/00024382-200006000-00001. [DOI] [PubMed] [Google Scholar]
- 54.Reynolds JM, Dong C. Toll-like receptor regulation of effector T lymphocyte function. Trends Immunol. 2013;34(10):511–519. doi: 10.1016/j.it.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 55.Heinemann U, Lux HD. Undershoots following stimulus-induced rises of extracellular potassium concentration in cerebral cortex of cat. Brain Res. 1975;93(1):63–76. doi: 10.1016/0006-8993(75)90286-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.