Abstract
Accurate estimates of host-vector contact rates are required for precise determination of arbovirus transmission intensity. We designed and tested a novel mosquito collection device, the Nest Mosquito Trap (NMT), to collect mosquitoes as they attempt to feed on unrestrained nesting birds in artificial nest boxes. In the laboratory, the NMT collected nearly one-third of the mosquitoes introduced to the nest boxes. We then used these laboratory data to estimate our capture efficiency of field-collected bird-seeking mosquitoes collected over 66 trap nights. We estimated that 7.5 mosquitoes per trap night attempted to feed on nesting birds in artificial nest boxes. Presence of the NMT did not have a negative effect on avian nest success when compared to occupied nest boxes that were not sampled with the trap. Future studies using the NMT may elucidate the role of nestlings in arbovirus transmission and further refine estimates of nesting bird and vector contact rates.
Keywords: Nest Mosquito Trap, West Nile virus, host-seeking rates, nestling
INTRODUCTION
Avian arboviral pathogens, such as West Nile virus (WNV), are transferred via the interaction of feeding mosquito vectors and their avian hosts. The frequency of these interactions (i.e., host-feeding rates) is a central parameter of the basic reproductive rate, R0, of vector-borne pathogens (Ross 1910, Macdonald 1952). As such, the determination of precise estimates of per capita host-feeding rates in natural settings is of paramount importance to accurately describing the intensity of avian arbovirus transmission (Hasibeder and Dye 1988, Kilpatrick et al. 2006a).
A variety of trap designs employ avian bait to approximate mosquito host-seeking rates (reviewed by Silver 2008). Traditionally, these have included the small animal-baited trap (Davies 1973), the Trinidad No. 10 (Service 1969) and No. 17 (Davies 1971), and lard can traps (Bellamy and Reeves 1952). More recently, Darbro and Harrington (2006) evaluated the use of bird-baited traps for WNV surveillance. Griffing et al. (2007) summarized the literature regarding the use of caged birds to determine avian-host seeking rates for calculating arboviral transmission intensity. Though all of the above studies succeed in quantifying feeding and landing rates, it is unclear how these numbers correspond to natural exposures of unrestrained hosts to host-seeking mosquitoes. In their recent study, Griffing et al. (2007) established a mosquito landing rate by directly observing mosquitoes attempting to feed on nesting American Robins (Turdus migratorius L. (Passeriformes: Turdidae)) using infrared video. While direct quantification of landing rates by video provides an unobtrusive method to observe the natural interactions of vectors and nesting hosts, mosquitoes are not retained for species identification and pathogen detection and host-feeding rates may be overestimated due to double counting of individual mosquitoes. Noting this limitation, Loss et al. (2009) attempted to collect nesting bird-seeking mosquitoes by modifying a CDC light trap to collect mosquitoes on nest boxes. Their modified trap collected less than one mosquito per trap night and not significantly more than nearby control traps without avian bait (Loss et al. 2009).
We designed and implemented a novel collection device, the Nest Mosquito Trap (NMT) to optimize the capture of live, bird-seeking mosquitoes as they attempted to feed on nesting birds in artificial nest boxes. Nest boxes have been used in research on a variety of avian species for decades. To test the utility of the device we: a) determined the capture efficiency of the device in a controlled laboratory setting, b) assessed whether the trap affects avian nesting success, and c) used the device in a field setting to collect nesting bird-seeking mosquitoes to estimate the number of mosquitoes seeking avian blood meals.
MATERIALS AND METHODS
Trap design and construction
The Nest Mosquito Trap, a continuously operated suction and collection device was designed to aspirate mosquitoes from within the enclosure of a nest box (Figure 1). Mosquitoes entering, and presumably seeking to feed on the nest box occupant(s), were collected intact in a mesh reservoir (four holes per cm2). The mesh collection bag was located between the interior of the nest box and the suction device so that mosquitoes did not pass through a fan. The body of the NMT was composed of an opaque, polypropylene box (17.8 × 12.7 cm) with a circular, threaded portal at one end (diameter = 10.8 cm) for attachment to the nest box and for insertion of a drawstring mesh collection bag (13.5 × 11.5 cm). A 1-cm mesh (hole-size) plastic hardware cloth screen was placed between the interior of the nest box and the mesh mosquito collection bag to prevent the birds from consuming the trapped mosquitoes while allowing the mosquitoes to pass through to the collection bag. The polypropylene box was painted black to prevent sunlight from potentially disturbing the nest occupants and also from desiccating and damaging the specimens collected. The trap's suction was supplied by a 12.0 cm 12 v (5.1 w) direct current fan (Sunon Inc. product number: MEC0381V2-0000-A99) rated for 2600 RPM and 116 CFM mounted on the opposite side of the polypropylene box to the collection bag. A sealed gel 12-volt 12 Ah rechargeable battery (Tempest Inc.) powered the fan.
Figure 1.
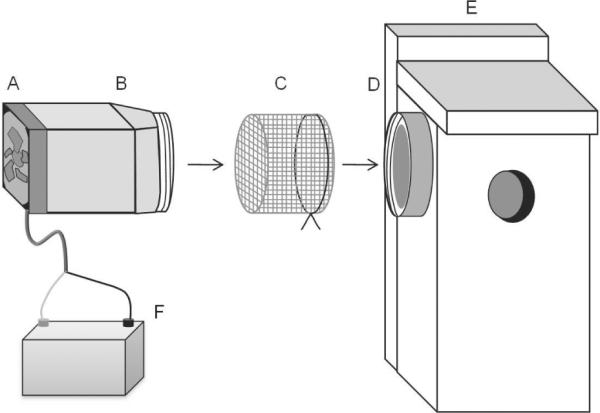
Design schematic of the Nest Mosquito Trap (NMT), collection bag, and means of attachment to a bird nest box. A) 12 volt 120mm fan (Sunon Inc. product number: MEC0381V2-0000-A99) provides suction to the trap. B) Plastic trap body with threaded opening. C) Four holes per cm2 mesh collection bag with drawstring. D) Threaded cap with center cut out is screwed to the nest box. E). Bird nest box with 3.18 cm diameter entrance hole. F). 12 volt sealed gel rechargeable battery powers the trap. A 1 cm diameter mesh plastic hardware cloth screen (not pictured) between the mesh collection bag (C) and the attachment ring (D) prevents the bird occupants from consuming the captured mosquitoes.
Laboratory assessment of Nest Mosquito Trap efficiency
Culex pipiens pipiens L. (Diptera: Culicidae) egg rafts were collected from stormwater drop inlets at various locations around Richmond, VA. Single egg rafts were placed in 1 gallon of ultrapure filtered (Millipore) water until hatching. First instar larval density was limited to 100 larvae per gallon water. The larvae were fed a solution of three parts bovine liver powder (Sigma) and two parts Brewer's Yeast (Twinlab); adults were fed 10% sucrose solution (Vrzal 2010). Mosquitoes were held at 37° C for all stages of development.
In order to determine the overall capture efficiency of the NMT and the effect of nest box size on capture efficiency, a laboratory test was performed using first generation (F0) lab-reared mosquitoes. Female mosquitoes were used within 24–72 h of adult emergence. Capture efficiency was compared for two nest box sizes typically used by Prothonotary Warblers (Protonotaria citrea, Boddaert (Passeriformes: Parulidae) and Eastern Bluebirds (Sialia sialis, L. (Passeriformes: Turdidae)). The small nest box measured 8 cm × 15 cm × 26 cm and was designed for occupancy by Prothonotary Warblers. The large nest box, measuring 11 cm × 15 cm × 26 cm, was designed for use by Eastern Bluebirds. Both rectangular boxes were constructed with 1.91 cm thick wood and have a single 3.18 cm bird entrance hole. For each testing replicate, ten female mosquitoes were manually aspirated into a sealed funnel affixed to the entrance of the nest box and allowed to recover for 1 min before the trap power supply was engaged. A barrier placed to prevent the mosquitoes from prematurely entering the nest box was then removed and the trap was allowed to run for 5 min. Mosquitoes were allowed to enter naturally (via walking or flying) rather than being blown, in order to reflect the natural movement of the vectors in a field setting. The numbers of mosquitoes entering and the number captured were recorded. Test results were not included unless all ten mosquitoes could be accounted for at the end of the test. The test was repeated 38 times for each box size.
The efficiency of the trap was calculated by dividing the number of mosquitoes captured by the number of mosquitoes that entered the bird box. The proportions of captured mosquitoes were compared between the two box sizes (α=0.05) using logistic regression analysis (SAS 9.2, 2009).
Site descriptions
Three sites located along the James River in Charles City County and Henrico County, VA, were used in this study. The first is the VCU Rice Center, Virginia Commonwealth University's ecological field station (N37.325558°, W-77.204117°). The two other sites were private residences. Field sites were located at least 5 km from each other. An additional site, the Presquile National Wildlife Refuge (N37.368619°, W77.242173°), was used as a control site for the comparison of avian nest success. All field sites border river tributaries (James River and Chickahominy River) of the lower Chesapeake Bay. Site elevations range from 1 m (at river level) to 14 m above sea level. Each site has a mixture of manicured lawns, unmaintained vegetated meadows, and mixed hardwood forests dominated by White Oak (Quercus alba, L. (Fagales: Fagaceae)) with occasional Loblolly Pine trees (Pinus taeda, L. (Pinales: Pinaceae).
Nest box placement
Eighty-one small nest boxes designed for Prothonotary Warblers were placed on 1.91 cm diameter metal conduit poles at each site at least 20 m apart and 1.5–1.8 m above ground or water level at the time of installation. Forty-two of these boxes were placed over water within 2 m of the land edge at the VCU Rice Center. Thirty-nine more small boxes were placed in upland habitats at the VCU Rice Center and at the two private residences. In addition to these small nest boxes designed for Prothonotary Warblers, 15 large nest boxes designed to accommodate Eastern Bluebirds were installed using the same distancing criteria as the small Prothonotary Warbler boxes across the three sites. All 15 large boxes were placed away from water in habitats where Eastern Bluebirds were frequently observed. From 19 March to 22 July 2010, all of the nest boxes were surveyed at least once each week for occupancy. At each visit, we recorded the avian species, developmental stage, age, and number of offspring (hereafter: nestlings) occupying the nest box.
Nest Mosquito Trap deployment and retrieval
During the mid-to-late avian nesting season (from 27 May to 22 July 2010) NMTs were were deployed on occupied nest boxes without regard for life stage (egg or nestling) or age of nestlings. Traps were operated continuously overnight for 19–21 h. Nest Mosquito Traps were retrieved in the same order they were deployed to ensure equal running time between the subjects. In the laboratory, female mosquitoes were categorized using a description of the blood meal taken, i.e., unfed/empty, engorged, half engorged/half gravid, or gravid. Mosquitoes were then enumerated and identified to species using regional identification keys (Slaff and Apperson 1989).
Effect of Nest Mosquito Trap on nest success
To assess the impact of NMT operation on avian nest success, Mayfield (1961, 1975) survival rates and 95% confidence intervals were compared between Prothonotary Warbler nests at the VCU Rice Center that had been fitted with NMTs and those at a nearby site, Presquile National Wildlife Refuge, where no nests had been sampled with the NMTs. Mayfield daily and stage survival rate maximum-likelihood estimates account for bias in calculating nest success (Johnson 1979). We defined successful nests as those in which the nestlings survived at least five days post-hatching. Nestlings that survived five days were considered successes, having reached the midpoint of their physiological development and the period of highest rate of growth (Podlesak and Blem 2002). Nestlings were aged based on their feather coverage, ability to hold their heads up, and nest survey record as described by Podlesak and Blem (2002). Nests were considered to have failed if one of the following situations was observed: (1) a female was never documented incubating the full clutch of eggs and the eggs were cool to the touch, (2) none of the eggs hatched within the typical incubation period (12 days), or (3) boxes with nestlings younger than five days old that were subsequently found empty or dead. Nests that were visited for the final time before fledging during the first four days after hatching or nests for which insufficient data exists were not included in the analysis. The parameters used to assess nestling success were based on the well-documented breeding cycle of the Prothonotary Warbler described by Podlesak and Blem (2002). Prothonotary Warblers lay one or two clutches of four to six eggs over a nesting season. Nestlings hatch after approximately 12 days of incubation by the female and typically fledge ten days later (Podlesak and Blem 2002).
Field comparison of Nest Mosquito Trap to existing surveillance methods
We compared the number of mosquitoes collected by the NMT to traditional mosquito collection devices. One CO2-baited (1.3 kg dry ice) CDC light and one CDC Gravid trap were set at each of the three sites on a weekly basis on different sampling nights from the NMT collections so as to not directly compete with each other. The CDC light trap was operated 1.5 m above the ground while the CDC gravid trap was operated on the ground at a location central to the monitored nest boxes at each of the three sites. Both traps were operated continuously overnight for 19–21 h. The traps were retrieved in the same order they were deployed. The CDC Gravid trap was baited with a mixture of 20 liters of water, 250 g of hay, 250 g of grass clippings, 30 g of chicken manure, and 5 g of Brewer's yeast that had been allowed to ferment in a sealed bucket over no less than 24 h (Cooperband et al. 2008, White et al. 2009). Bait proportions were modified to optimize local Culex spp. collections by the Henrico County Standing Water Initiative. Captured mosquitoes were killed by freezing at −20° C, identified to species, and enumerated in the laboratory (Slaff and Apperson 1989).
We calculated an estimate of the total number of mosquitoes that entered the nest boxes, a, by multiplying the reciprocal of the laboratory capture efficiency, e, by the number of mosquitoes collected in the field, m. This calculation is summarized by the formula, a=(1/e)m.
RESULTS
Laboratory assessment of Nest Mosquito Trap efficiency
In a controlled setting, the Nest Mosquito Trap collected a mean of 38.3% (15.7 SE) of mosquitoes that entered the small nest box and 32.1% (16.2 SE) of mosquitoes entering the large nest box. Though the collection efficiency of the NMT on the larger nest box was lower than on the smaller box, this difference was not statistically significant (t= 2.75, DF = 1, p > 0.05).
Effect of Nest Mosquito Trap on avian nesting success
Eastern Bluebirds, Carolina Chickadees (Poecile carolinensis Audubon (Passeriformes: Paridae)) Prothonotary Warblers, Tree Swallows (Tachycineta bicolor Vieillot (Passeriformes: Hirundinidae)), and Wren spp. (Passeriformes: Troglodytidae)) occupied approximately half of the 96 nest boxes. The majority of nest boxes were occupied by Prothonotary Warblers. We utilized nest success data from Prothonotary Warbler nest boxes at the nearby Presquile National Wildlife Refuge to compare daily and stage survival rates observed from nests that had been sampled with a NMT. We found no significant differences in daily survival rate estimates between the NMT sampled or control nest boxes (Table 1). Though the incubation stage survival rate for nests sampled with the NMT (0.93, 95% CI: 0.79–1.07) exceeded that of the control nests (0.71, 95% CI: 0.60–0.81), this difference was not statistically significant. We found similar stage survival rates between NMT (0.92, 95% CI: 0.78–1.07) and control nests (0.87, 95% CI: 0.79–0.96) during nestling periods.
Table 1.
Comparison of nest success between nests sampled with the Nest Mosquito Trap (NMT) and control nests that were not sampled with the NMT.
| Control | Nest Mosquito Trap | ||||
|---|---|---|---|---|---|
|
|
|||||
| Nest stage | Parameter | Exposure days† | Survival rate (95% CI) | Exposure days† | Survival rate (95% CI) |
| Incubation period | 725 | 162 | |||
| Daily survival probability | 0.97 (0.96–0.98) | 0.99 (0.98–1.01) | |||
| Stage survival probability | 0.71 (0.60–0.81) | 0.93 (0.79–1.07) | |||
| Nestling period | 299 | 63 | |||
| Daily survival probability | 0.97 (0.95–0.99) | 0.98 (0.95–1.02) | |||
| Stage survival probability | 0.87 (0.79–0.96) | 0.92 (0.78–1.07) | |||
Exposure days = # of nests observed
number of days observed.
Field comparison of Nest Mosquito Trap to existing surveillance methods
Over 66 trap nights the NMT collected a total of 154 mosquitoes (2.3 mosquitoes per trap night ± 1.4 SD). No mosquitoes were collected from nest boxes with eggs during 12 NMT trap nights. Therefore all NMT collected mosquitoes were collected in nest boxes with nestlings over 54 NMT trap nights. Most of the mosquitoes collected (71.4%, 110/154) were unfed Culex salinarius Coquillett (Diptera: Culicidae) (Table 2). Twenty-one percent (33/154) of the mosquitoes collected were Cx. pipiens/restuans L. (Diptera: Culicidae). The majority (66.7%, 22/33) of Cx. pipiens/restuans collected were visibily engorged with blood (Table 2). Culex erraticus Dyar and Knab (Diptera: Culicidae) (5.8%, 9/154) and Aedes albopictus Skuse (Diptera: Culicidae) (1.3%, 2/154) were also collected. On 16 trap nights over the same time period, CO2-baited (dry ice) CDC light and CDC gravid traps collected 1,700 mosquitoes (212.5 mosquitoes per trap night ±237.5). Multiplying the field-observed number of mosquitoes entering nest boxes (154) by the reciprocal of the laboratory-determined capture efficiency (2.61) results in an estimated total of 402.1 mosquitoes, or 7.45 per trap night, entering nest boxes with nestlings.
Table 2.
Status of mosquito species collected by NMTs over 54 trap nights of sampling nest boxes occupied by nestlings (percentage, number collected).
| Species | Empty | Engorged | Half engorged/Half gravid | Gravid | Total (No.) |
|---|---|---|---|---|---|
| Aedes albopictus | 100.0 (2) | 0 (0) | 0 (0) | 0 (0) | 2 |
| Culex erraticus | 100.0 (9) | 0 (0) | 0 (0) | 0 (0) | 9 |
| Cx. pipiens/restuans | 27.3 (9) | 66.7 (22) | 0 (0) | 6.1 (2) | 33 |
| Cx. salinarius | 99.1 (109) | 0 (0) | 0 (0) | 0.10 (1) | 110 |
DISCUSSION
The abundance and infection status of mosquitoes seeking blood meals from nesting birds are primary factors that affect the transmission intensity of arboviruses (Anderson and May 1991). Prior to the design of the Nest Mosquito Trap, these metrics of avian arbovirus transmission intensity were difficult to accurately measure for unrestrained avian hosts. The evidence provided in this study documents the efficacy of the NMT as a means of monitoring mosquito host-seeking rates on nesting birds.
The primary limitation of the NMT is that it has been developed only for collecting mosquitoes attempting to feed on nesting birds in nest boxes. The Nest Mosquito Trap cannot be operated on natural tree cavity nests occupied by cavity nesting birds such as those in this study or others (e.g., White-breasted nuthatches, Sitta carolinensis and Tufted Titmice, Baeolophus bicolor). Also, mosquitoes attempting to feed on egg-incubating adults and nestlings in open cup nests are not represented in our NMT collections. Additionally, it is not possible to identify whether blood-fed mosquitoes collected by the NMT have fed on brooding adult or nestling birds. The NMT may be adapted for use on a variety of nest structures not evaluated in this study, including nest platforms (occasionally used by American Robins), and larger nest boxes such as those used by Barred Owls. We would expect a loss of suction and therefore mosquito capture efficiency to accompany the use of the NMT in its current configuration to more open-air or larger nest structures. An assessment of NMT capture efficiency when traps are operated adjacent to open-cup nests is warranted.
Host-feeding shifts from birds to mammals (Kilpatrick et al. 2006b, Kent et al. 2009) or from certain bird species to others (Molaei et al. 2006, Hamer et al. 2009) have been widely documented to occur in concert with the end of the avian nesting season. This host shift has been associated with the beginning of the annual occurrence of human WNV cases (Kilpatrick et al. 2006b). Despite this temporal association, few studies have attempted to quantitatively explain the mechanistic role of avian nesting and the nestling maturation period in initiating annual occurrences of human WNV and Saint Louis encephalitis virus activity (Day and Stark 1999, Shaman et al. 2002, Griffing et al. 2007, Loss et al. 2009). To our knowledge only one study to date (Loss et al. 2009) has attempted to collect mosquitoes attracted to nesting birds. Loss et al. (2009) attached a modified CDC light trap to the outside of nest boxes but did not collect significantly more mosquitoes than nearby control traps lacking nesting birds. Since the Loss et al. (2009) trap did not employ suction from the interior of the nest box, it is likely that this study significantly underestimated the number of nesting bird-seeking mosquitoes. By explicitly designing a trap for the purpose of collecting mosquitoes entering nest boxes, we were able to observe more mosquitoes entering nest boxes than had previously been documented. As such, the NMT may provide a mechanism to gain insights into avian arbovirus transmission during bird nesting periods. Additionally, the NMT can be used to compare host-seeking rates of a variety of cavity-nesting bird species to document species-specific vector-host contact rates.
Controlled experiments indicate that the NMT captures roughly one-third of unfed Cx. pipiens pipiens mosquitoes entering bird nest boxes. Experiments that document the mosquito capture efficiency or escape rate of various trap designs are rare but are important to understanding potential collection biases. Darbro and Harrington (2006) presented evidence of significant numbers of mosquitoes escaping passive “funnel” bird baited traps where feeding access to caged birds was prevented. Since the NMT is an active suction method where mosquitoes succumbing to the trap's suction are unable to escape, we present the trap's capture efficiency. A high proportion of Cx. pipiens/restuans collected by the NMT were visibly engorged compared to nearly none of the three other species. This difference suggests that there likely exists a species-specific and life stage-specific susceptibility to the NMT's suction. These mosquitoes were likely able to avoid collection upon entry into the nest boxes and may have succumbed to the suction of the NMT after feeding upon the nest box occupants. The other NMT-collected mosquito species either did not feed on the nesting birds or were collected before having an opportunity to feed. Future modifications to the NMT, such as increasing the trap's suction power, may increase the trap's collection efficiency. Future studies evaluating this and other mosquito collection devices should determine capture efficiencies on multiple mosquito species at multiple life stages.
In our study, the presence of the NMT did not increase the rate of nest failure in Prothonotary Warblers. We observed no significant differences in nest success, as measured by daily or stage survival rates, among NMT-sampled nests and control (unsampled) nests. Our sample of only 14 NMT sampled nest boxes compared to the 145 unsampled nest boxes may have contributed to an artificially high rate of nest success for NMT-sampled nests. Though we did not observe any negative effects of the NMT on nest abandonment or failure, our study was limited to one avian species. Future studies should evaluate the effect of sampling with the NMT on nest success in a variety of bird species and field settings.
Though the field observations using the NMT demonstrate only a fraction of the mosquitoes collected by the CDC light and gravid traps overall, the NMT targets a specific subset of the general mosquito population that traditional collection methods may under-represent. Specifically, traditional collection methods cannot estimate avian landing rates. The estimated mean of 7.5 mosquitoes per trap night entering the nest box is substantially lower than 37.3, the average number of landings on nestlings reported by Griffing et al. (2007), but much higher than the mean of <1 mosquito per trap night reported by Loss et al. (2009). In the study by Griffing et al. (2007), landings were observed on American Robins in their open cup nests over a 24-h period. These rates may be overestimating the actual biting rates as single mosquitoes may be counted multiple times due to repeated feeding attempts. Also, further research is needed to investigate differences in landing rates in open nests vs the cavity nests typical of Prothonotary Warblers and Eastern Bluebirds. It is possible that our much lower estimates of landing rates are a result of some degree of protection from mosquito biting that cavity nesting birds enjoy over open nesting birds. We hypothesize that host attraction cues may be attenuated in natural cavities or nest boxes. Further, mosquito attraction to different bird species likely accounts for differences across studies.
In conclusion, the Nest Mosquito Trap may be a useful tool to answer important ecological questions pertaining to avian host selection by mosquitoes that may drive avian arbovirus transmission. In particular, the NMT may refine spatial and temporal estimates of arbovirus transmission intensity by providing previously unattainable evidence of vector-host contact rates.
Acknowledgments
We thank Cathy Viverette and Brian Rhodes for Prothonotary Warbler data collection and Dr. Roy T. Sabo for assistance with statistical analyses. Many volunteers assisted with the construction of the nest boxes. Mr. George Green and Lynn and Irv Wilson generously granted access to their properties. This paper is in partial fulfillment of the M.S. degree of A.E.R. The project was supported by NIEHS T32 Training Grant # 2T32ES007334 to K.A.C. and a VCU Rice Center Research Grant to A.E.R. This is VCU Rice Center publication #21.
REFERENCES CITED
- Anderson RM, May RM. Infectious Diseases of Humans. Dynamics and Control. Oxford University Press; London: 1991. p. 768. [Google Scholar]
- Bellamy RE, Reeves WC. A portable mosquito bait-trap. Mosq. News. 1952;12:256–258. [Google Scholar]
- Cooperband MF, McElfresh JS, Millar JG, Carde RT. Attraction of female Culex quinquefasciatus Say (Diptera: Culicidae) to odors from chicken feces. J. Insect Physiol. 2008;54:1184–1192. doi: 10.1016/j.jinsphys.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Darbro JM, Harrington LC. Bird-baited traps for surveillance of West Nile mosquito vectors: effect of bird species, trap height, and mosquito escape rates. J. Med. Entomol. 2006;43:83–92. doi: 10.1093/jmedent/43.1.83. [DOI] [PubMed] [Google Scholar]
- Davies JB. A small mosquito trap for use with animal or carbon dioxide baits. Mosq. News. 1971;31:441–443. [Google Scholar]
- Davies JB. A simple battery operated suction trap for insects attracted to animal, light or chemical bait. Mosq. News. 1973;33:102–104. [Google Scholar]
- Day JF, Stark LM. Avian serology in a St. Louis encephalitis epicenter before, during, and after a widespread epidemic in south Florida, USA. J. Med. Entomol. 1999;36:614–624. doi: 10.1093/jmedent/36.5.614. [DOI] [PubMed] [Google Scholar]
- Griffing SM, Kilpatrick AM, Clark L, Marra PP. Mosquito landing rates on nesting American robins (Turdus migratorius) Vector Borne Zoonotic Dis. 2007;7:437–443. doi: 10.1089/vbz.2006.0560. [DOI] [PubMed] [Google Scholar]
- Hamer GL, Kitron UD, Goldberg TL, Brawn JD, Loss SR, Ruiz MO, Hayes DB, Walker ED. Host selection by Culex pipiens mosquitoes and West Nile virus amplification. Am. J. Trop. Med. Hyg. 2009;80:268–278. [PubMed] [Google Scholar]
- Hasibeder G, Dye C. Population dynamics of mosquito-borne disease: persistence in a completely heterogeneous environment. Theor. Popul. Biol. 1988;33:31–53. doi: 10.1016/0040-5809(88)90003-2. [DOI] [PubMed] [Google Scholar]
- Johnson DH. Estimating nest success: the Mayfield method and an alternative. The Auk. 1979;96:651–661. [Google Scholar]
- Kent R, Juliusson L, Weissmann M, Evans S, Komar N. Seasonal blood-feeding behavior of Culex tarsalis (Diptera: Culicidae) in Weld County, Colorado, 2007. J. Med. Entomol. 2009;46:380–390. doi: 10.1603/033.046.0226. [DOI] [PubMed] [Google Scholar]
- Kilpatrick AM, Daszak P, Jones MJ, Marra PP, Kramer LD. Host heterogeneity dominates West Nile virus transmission. Proc. Biol. Sci. 2006a;273:2327–2333. doi: 10.1098/rspb.2006.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick AM, Kramer LD, Jones MJ, Marra PP, Daszak P. West Nile virus epidemics in North America are driven by shifts in mosquito feeding behavior. PLoS Biol. 2006b;4:e82. doi: 10.1371/journal.pbio.0040082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loss SR, Hamer GL, Goldberg TL, Ruiz MO, Kitron UD, Walker ED, Brawn JD. Nestling passerines are not important hosts for amplification of West Nile virus in Chicago, Illinois. Vector Borne Zoonot. Dis. 2009;9:13–18. doi: 10.1089/vbz.2008.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald G. The analysis of equilibrium in malaria. Trop. Dis. Bull. 1952;49:813–829. [PubMed] [Google Scholar]
- Mayfield H. Nesting success calculated from exposure. Wilson Bull. 1961;73:255–261. [Google Scholar]
- Mayfield H. Suggestions for calculating nest success. Wilson Bull. 1975;87:456–466. [Google Scholar]
- Molaei G, Andreadis TG, Armstrong PM, Anderson JF, Vossbrinck CR. Host feeding patterns of Culex mosquitoes and West Nile virus transmission, northeastern United States. Emerg. Infect. Dis. 2006;12:468–474. doi: 10.3201/eid1203.051004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podlesak DW, Blem CR. Determination of age of nestling Prothonotary Warblers. J. Field Ornithol. 2002;73:33–37. [Google Scholar]
- Ross R. The Prevention of Malaria. E.P. Dutton; New York: 1910. p. 772. [Google Scholar]
- Service MW. Use of traps in sampling mosquito populations. Entomol. Exp. Appl. 1969;12:403–412. [Google Scholar]
- Shaman J, Day JF, Stieglitz M. Drought-induced amplification of Saint Louis encephalitis virus, Florida. Emerg. Infect. Dis. 2002;8:575–580. doi: 10.3201/eid0806.010417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver JB. Mosquito Ecology. 3rd ed Springer; NY: 2008. p. 1498. [Google Scholar]
- Slaff ME, Apperson CS. A key to the mosquitoes of North Carolina and the mid-Atlantic states. Agric. Ext. Ser. N. Carolina State Univ. publication no. AG. 1989:412. [Google Scholar]
- White SL, Ward MP, Budke CM, Cyr T, Bueno R., Jr A comparison of gravid and under-house CO2-baited CDC light traps for mosquito species of public health importance in Houston, Texas. J. Med. Entomol. 2009;46:1494–1497. doi: 10.1603/033.046.0637. [DOI] [PubMed] [Google Scholar]


