Abstract
Increases in vector-host contact rates can enhance arbovirus transmission intensity. We investigated weekly fluctuations in contact rates between mosquitoes and nesting birds using the recently described Nest Mosquito Trap (NMT). The number of mosquitoes per nestling increased from < 1 mosquito per trap night to 36.2 in the final 2 wk of the nesting season. Our evidence suggests the coincidence of the end of the avian nesting season and increasing mosquito abundances may have caused a “host funnel,” concentrating host-seeking mosquitoes to the few remaining nestlings. The relative abundance of mosquitoes collected by the NMT suggests that significantly more Aedes albopictus (Skuse) and Culex pipiens (L.)/restuans (Theobald) sought nesting bird bloodmeals than were predicted by their relative abundances in CO2-baited Centers for Disease Control and Prevention light and gravid traps. Culex salinarius (Coquillett) and Culex erraticus Dyar and Knab were collected in NMTs in proportion to their relative abundances in the generic traps. Temporal host funnels and nesting bird host specificity may enhance arbovirus amplification and explain observed West Nile virus and St. Louis encephalitis virus amplification periods.
Keywords: host-seeking rate, Nest Mosquito Trap, West Nile virus
In many areas of North America the annual transmission dynamics of St. Louis encephalitis virus (family Flaviviridae, genus Flavivirus, SLEV) and West Nile virus (family Flaviviridae, genus Flavivirus, WNV) are quite similar excepting often more intense and perennial WNV transmission (Shaman et al. 2005, USGS 2011). Both pathogens exhibit enzootic cycling, primarily between avian hosts and Culex spp. mosquitoes; both also undergo a phase of amplification that coincides with the end of the avian nesting season (Day and Stark 1999, USGS 2011). WNV vector species shift hosts at the time of avian dispersal from nests, and this shift in hosts is associated with increased human WNV incidence (Kilpatrick et al. 2006a, Hamer et al. 2009, Kent et al. 2009). A similar bird-to-mammal host shift occurs for Culex nigripalpus Theobald, a vector of SLEV in Florida (Edman and Taylor 1968).
Nestlings may be particularly important to WNV and SLEV amplification. Nestlings represent a large proportion of the immunologically naïve avian population, are constrained to a fixed location, lack the protective mature feather coverage of adults, exhibit weak defensive behavior (Scott 1991, Day and Stark 1999, Shaman et al. 2002), and exhibit greater duration and intensity of SLEV viremia than adult birds (McLean 1980, Mahmood et al. 2004). Despite this, there is little known about the interactions between nestlings and vector mosquitoes (Griffing et al. 2007). Both the capacity for an avian community to harbor arboviruses and the host preferences of mosquito populations vary greatly across time and space (Kilpatrick et al. 2006a, b; Hamer et al. 2011). This makes temporal and species-specific knowledge of vector-host contact rates critical to understanding arbovirus ecology (Kilpatrick et al. 2006b). To determine temporal trends of vector-nestling contact we quantified contact rates for several mosquito species using the recently developed Nest Mosquito Trap (NMT) (Caillouet et al. 2012).
We collected mosquitoes seeking to feed on cavity nesting eastern bluebirds (EABLs; Sialia sialis, L. (Passeriformes: Turdidae)), prothonotary warblers (PROWs; (Protonotaria citrea, Boddaert (Passeriformes: Parulidae)), and wrens (Passeriformes: Troglodytidae) at three sites in rural central Virginia using the NMT. The NMT is a continuously operated suction device that attaches to the side of a specially designed bird nest box. For a more detailed description of the NMT see Caillouet et al. (2012). Field sites that were at least 5 km from each other included the Virginia Commonwealth University Rice Center, an ecological research station, and two private residences. Each site is bordered by river tributaries of the lower Chesapeake Bay.
In total, 120 large (n = 39) and small nest boxes (n = 81) that were spaced at least 20 m apart and 1.5–1.8 m above ground or water level were surveyed at least once each week for occupancy from 19 March to 22 July, 2010. NMTs were deployed only on nest boxes that contained nestlings. Fifty-one trap exposures (or trap nights) were sampled with NMTs from 19 March to 22 July, 2010. Traps were operated continuously for 19–21 h between 1230 and 1730 hours and retrieved the following day between 0930 and 1230 hours. Traps were retrieved in the same order they were deployed to provide equal sampling time for the traps.
In addition to trapping mosquitoes at nest boxes, we also collected mosquitoes using CO2-baited (1.3 kg dry ice) Centers for Disease Control and Prevention (CDC) light and CDC Gravid traps. These traps were set at the three sites on a weekly basis on different sampling nights from the NMT collections so as to not directly compete with each other. Captured mosquitoes were killed by freezing at −20°C, identified to species using regional identification keys (Slaff 1989), and enumerated in the laboratory.
We estimated the per nestling host-seeking rate (hereafter estimated HSR) by adjusting the number of mosquitoes collected per nest box per trap night by a previously determined (Caillouet et al. 2012) laboratory capture efficiency ratio of (38.2%) and dividing the estimated nest box total by the number of nestlings within each nest box.
We compared the mosquito species abundances collected by the NMT to abundances of mosquitoes in the CDC light and gravid traps. An assessment of the mosquito species relative abundance ratio was performed by determining 95% CIs for each species. Ratios in excess of one indicate that these species were observed disproportionately more often in NMTs. Likewise, relative abundance ratios that are < 1 indicate that these species were not collected as often by the NMT relative to CDC light and gravid collections.
We used Pearson correlation coefficients to determine whether the bi-weekly estimated HSR was associated with ambient mosquito abundance, the total number of nestlings occupying nest boxes at our study sites, or the ratio of ambient mosquito abundance to nestling abundance. All statistical analyses were performed using SAS 9.0 (SAS Institute, Cary, NC).
Over a 10 week period we collected 149 mosquitoes with the NMT during 51 trap nights (2.9 ± 1.81; mean/trap night ± SEM). Adjusting for the laboratory efficiency rating of the NMT (38.2%) we estimated a mean number of mosquitoes seeking nesting-bird bloodmeals of 2.0 per nestling per trap night (± 1.19). The majority of these mosquitoes were Culex salinarius Coquillett (2.0 ± 1.46). Culex pipiens (L.)/restuans (Theobald) (0.6 ± 0.33), Culex erraticus Dyar and Knab (0.2 ± 0.09), and Aedes albopictus Skuse (0 ± 0.03) were also collected in NMTs. The relative abundance ratios (RAR) for both Cx. erraticus (RAR: 1.45; 95% CI: 0.90–2.00) and Cx. salinarius (0.78; 0.27–1.29) suggest that these species were collected in proportion to the abundances approximated by CDC light and gravid traps, whereas, Cx. pipiens/restuans (8.58; 8.04–9.12) and Ae. albopictus (3.67; 3.03–4.31) were represented more in NMT collections (Table 1).
Table 1.
Mosquito species abundance ratios (mean per trap night (%)) captured with Nest Mosquito Traps and with CDC light and gravid traps near nesting birds
| Mosquito species | Nest mosquito trap | CDC light and gravid trap | Abundance ratioa | 95% CI |
|---|---|---|---|---|
| Aedes albopictus | 0.0 (1.3) | 0.3 (0.4) | 3.67 | 3.03, 4.31 |
| Culex erraticus | 0.2 (5.4) | 3.0 (3.7) | 1.45 | 0.90, 2.00 |
| Cx. pipiens/restuans | 0.6 (20.8) | 2.0 (2.4) | 8.58 | 8.04, 9.12 |
| Cx. salinarius | 2.1 (72.5) | 75.7 (93.5) | 0.78 | 0.27, 1.29 |
Abundance ratio = NMT (%)/CDC (%).
The mean estimated HSR remained < 1 mosquito per trap night for the first 6 wk (weeks 21–26) until weeks 27 and 28 when it increased to 1.2 (± 0.62) (Fig. 1). In weeks 29 and 30 estimated HSR increased to 36.3 (± 21.92). Week 30 marked the end of the nesting season with no more occupied nest boxes. During this same period, nestling abundance in our study linearly declined from apeak of 46.3 in weeks 21 and 22–0 in week 30. Meanwhile mosquito abundance approximated by CDC light and gravid traps exhibited pulses in abundance typical of pulsed-hatching of Cx. salinarius, the most abundant species collected. The peak of mosquito abundance from CDC light and gravid traps (197.3 ± 174.84) was observed at the end of our study coinciding with the end of avian nesting. Neither the bi-weekly total number of nestlings (r = −0.81; P value = 0.099) nor the bi-weekly abundance of mosquitoes collected by CDC light and gravid traps (r = 0.80; P value = 0.106) was associated with a temporal increase in the estimated HSR. However, the ratio of mosquitoes collected by CDC light and gravid traps to the total number of nestlings was significantly and positively associated with estimated HSR (r = 0.99; P value = 0.0002).
Fig. 1.
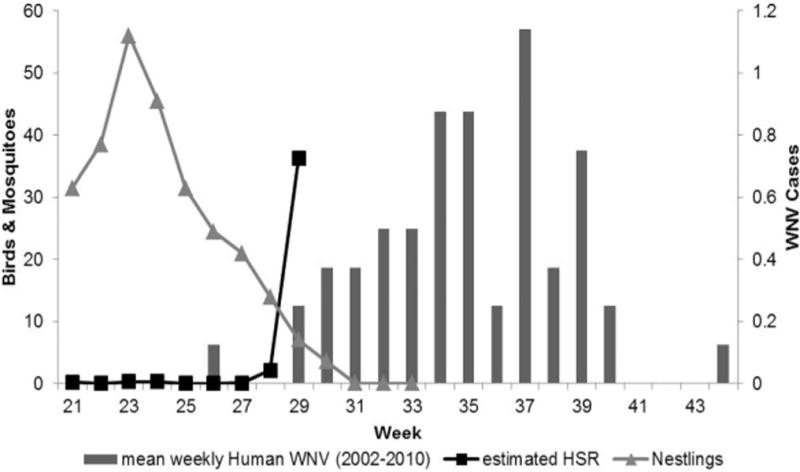
Synchrony of the end of avian nesting with increased per capita vector feeding rate (HSR) and the onset of human WNV cases in Virginia.
To examine the relationship between changes in nestling HSR and human disease, we compared temporal trends in our data to the historic temporal trends for human WNV cases, calculated as the mean number of WNV human cases by week for the years 2003–2010 in the state of Virginia. WNV human case data included all reports of WNV fever and WNV neuroinvasive disease (CDC Arbonet). The spike in nestling HSR at week 29 coincides with the historical onset of human cases of WNV in Virginia (Fig. 1).
In his review of SLEV epidemics, Day (2001) stated that SLEV transmission “requires an intricate synchronization of vector and amplification host population dynamic.” We propose that such an “intricate synchronization” may exist in SLEV and WNV amplification specifically because of the decline of available nestling hosts at the end of the avian nesting season.
Several field studies have demonstrated shifts in mosquito bloodmeal preference from birds to mammals including humans that are temporally associated with postbreeding avian dispersal and human arbovirus risk (Kilpatrick et al. 2006a, Hamer et al. 2009, Kent et al. 2009). Similar timing of increases in mosquito WNV infection rates have been noted in 2005 and 2006 in Chicago (Hamer et al. 2008) and in many regions of the United States. Recently, Burkett–Cadena et al. (2012), demonstrated that winter harshness explains variation in the timing of host feeding shifts ostensibly because of delays in avian nesting caused by severe winters.
In addition to studies documenting temporal associations of increased WNV transmission with avian dispersal, theoretical models of WNV and SLEV amplification have demonstrated that changes in the spatial distribution of hosts and vectors can influence arbovirus amplification (Shaman 2007, 2011). Shaman et al. (2002) observed that springtime drought-induced congregations of Cx. nigripalpus vectors and nesting birds facilitated SLEV amplification. This study suggested that SLEV was amplified by the spatial clustering of abundant vectors and hosts leading to increased avian and subsequent human SLEV infections (Shaman et al. 2002). Though spatial clustering of abundant hosts and vectors is likely necessary to sustain arbovirus transmission, a temporal reduction in host abundance without a reduction in vector abundance would increase HSR and likely enhance arbovirus amplification for short time periods; a phenomenon observed in a theoretical time-continuous model by Wonham et al. (2004). In an experimental setting, Foppa et al. (2011) demonstrated that spatial clustering in flocks of 10 chickens reduced per capita HSR; lone chickens were fed on at a per capita rate 4.5 times greater than chickens in flocks.
Though our evidence is limited because of a small sample from 1 yr, we suggest that the decrease in nestling hosts because of the culmination of the nesting season (avian dispersal) and the seasonal increase in mosquito populations may cause a “host funnel” in which the last birds remaining on nests experience significantly greater mosquito burden. Our conclusions support the model proposed by Wonham et al. (2004) that demonstrated that low densities of birds exacerbate disease transmission because of increased per capita biting rates. A model by Lord and Day (2001) also found that interactions between seasonal vector abundance and wild bird reproduction were important to the timing and likelihood of epizootic transmission of SLEV. Though obvious differences exist in the periodicity of SLEV (rare) and WNV (annual) epidemics, increases in the vector-host contact ratio associated with the culmination of the nesting season may enhance the probability of amplification for either pathogen.
In the only other study to observe mosquito contact rates through time on nesting birds, Griffing et al. (2007) found that the abundance of mosquito landings on American robins peaked in early June; well before the end of the nesting season. This study documented much greater abundances of mosquitoes landing on nesting birds than we observed. It may be that differences in mosquito exposure exist between open cup and cavity nesting birds. In addition, Loss et al. (2009) provided evidence that nestlings do not play an important role in WNV transmission in Chicago, IL. Hatch-year birds (first year birds that have fledged the nest) have been documented to play a major role in the amplification of both SLEV (Day and Stark 1999) and WNV (Hamer et al. 2008). Conflicting evidence regarding the role of nestlings in the amplification of arboviruses may be the result of geographic and meteorological differences that modulate HSR including: vector abundance, host heterogeneity, host reproductive timing, and host spatial distribution. Such site-specific and inter-annual differences may influence the consequences of host funnels and play a role in determining outbreaks. Spatio-temporal heterogeneity in the amplification of arboviruses underscores the need for longer term, spatially explicit investigations of HSR at finely resolved time-scales to inform transmission models and ultimately prevent human infections.
Cx. salinarius, the most numerous mosquito species collected in this study, has been documented to feed primarily on mammals (Edman 1974, Apperson et al. 2004). Apperson et al. (2002) found a 1:4 ratio of avian to mammalian-fed Cx. salinarius mosquitoes. In our study, Cx. salinarius was collected seeking nesting-bird bloodmeals in proportion to its ambient abundance, evidence that it was not selectively seeking nesting bird bloodmeals. Though our study confirms that this primarily mammalophagic species does feed on birds in significant numbers, its role as a viral amplifying vector is likely limited because of its propensity to feed on less-competent host taxa. In the same study, Apperson et al. (2002) reported a 23:1 avian/mammalian bloodmeal ratio for Cx. pipiens and 6:1 for Cx. restuans. Compared with Cx. salinarius we collected relatively few Cx. pipiens/restuans mosquitoes in the NMT, but those that were collected exceeded by eight-fold the abundance that would have been predicted if they were caught in proportion to their abundance in generic traps.
The concentration of mosquitoes onto immunologically naïve nestling birds that occurs at the end of the nesting season may result in the “intricate synchronization” necessary to explain the marked spatial synchrony of annual WNV amplification and sporadic SLEV amplification. Because of the limitations of this study, more field data are needed to validate our host funnel hypothesis as a mechanism to explain WNV and SLEV amplification. Future studies will incorporate more extensive temporal sampling on multiple avian species in disparate geographic regions.
Acknowledgments
We thank Jayne Deichmeister for retrieval of WNV human case data. Many volunteers assisted with the construction of the nest boxes. Thanks to Mark Rider and Nick Komar for their discussions regarding this project. George Green and Lynn and Irv Wilson generously granted access to their properties. This manuscript benefitted greatly from the comments of two anonymous reviewers. This article is in partial fulfillment of the M.S. degree of A.E.R. The project was supported by NIEHS T32 Training Grant 2T32ES007334 to K.A.C. and a VCU Rice Center Research Grant to A.E.R. This is VCU Rice Center publication number 28.
References Cited
- Apperson CS, Harrison BA, Unnasch TR, Hassan HK, Irby WS, Savage HM, Aspen SE, Watson DW, Rueda LM, Engber BR, Nasci RS. Host-feeding habits of Culex and other mosquitoes (Diptera: Culicidae) in the Borough of Queens in New York City, with characters and techniques for identification of Culex mosquitoes. J Med Entomol. 2002;39:777–785. doi: 10.1603/0022-2585-39.5.777. [DOI] [PubMed] [Google Scholar]
- Apperson CS, Hassan HK, Harrison BA, Savage HM, Aspen SE, Farajollahi A, Crans W, Daniels TJ, Falco RC, Benedict M, Anderson M, McMillen L, Unnasch TR. Host feeding patterns of established and potential mosquito vectors of West Nile virus in the eastern United States. Vector Borne Zoon Dis. 2004;4:71–82. doi: 10.1089/153036604773083013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkett–Cadena ND, Hassan HK, Eubanks MD, Cupp EW, Unnasch TR. Winter severity predicts the timing of host shifts in the mosquito Culex erraticus. Biol Lett. 2012;8:567–569. doi: 10.1098/rsbl.2012.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillouet KA, Riggan AE, Rider M, Bulluck LP. Nest Mosquito Trap quantifies contact rates between nesting birds and mosquitoes. J Vector Ecol. 2012;37:210–215. doi: 10.1111/j.1948-7134.2012.00218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JF. Predicting St. Louis encephalitis virus epidemics: lessons from recent, and not so recent, outbreaks. Annu Rev Entomol. 2001;46:111–138. doi: 10.1146/annurev.ento.46.1.111. [DOI] [PubMed] [Google Scholar]
- Day JF, Stark LM. Avian serology in a St. Louis encephalitis epicenter before, during, and after a widespread epidemic in south Florida, USA. J Med Entomol. 1999;36:614–624. doi: 10.1093/jmedent/36.5.614. [DOI] [PubMed] [Google Scholar]
- Edman JD. Host-feeding patterns of Florida mosquitoes. 3. Culex (Culex) and Culex (Neoculex) J Med Entomol. 1974;11:95–104. doi: 10.1093/jmedent/11.1.95. [DOI] [PubMed] [Google Scholar]
- Edman JD, Taylor DJ. Culex nigripalpus: seasonal shift in the bird-mammal feeding ratio in a mosquito vector of human encephalitis. Science. 1968;161:67–68. doi: 10.1126/science.161.3836.67. [DOI] [PubMed] [Google Scholar]
- Foppa IM, Moore J, Caillouet KA, Wesson DM. Disproportionate mosquito feeding on aggregated hosts. J Med Entomol. 2011;48:1210–1213. doi: 10.1603/me11007. [DOI] [PubMed] [Google Scholar]
- Griffing SM, Kilpatrick AM, Clark L, Marra PP. Mosquito landing rates on nesting American robins (Turdus migratorius) Vector Borne Zoon Dis. 2007;7:437–443. doi: 10.1089/vbz.2006.0560. [DOI] [PubMed] [Google Scholar]
- Hamer GL, Kitron UD, Goldberg TL, Brawn JD, Loss SR, Ruiz MO, Hayes DB, Walker ED. Host selection by Culex pipiens mosquitoes and West Nile virus amplification. Am J Trop Med Hyg. 2009;80:268–278. [PubMed] [Google Scholar]
- Hamer GL, Chaves LF, Anderson TK, Kitron UD, Brawn JD, Ruiz MO, Loss SR, Walker ED, Goldberg TL. Fine-scale variation in vector host use and force of infection drive localized patterns of West Nile virus transmission. PLoS ONE. 2011;6:e23767. doi: 10.1371/journal.pone.0023767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer GL, Walker ED, Brawn JD, Loss SR, Ruiz MO, Goldberg TL, Schotthoefer AM, Brown WM, Wheeler E, Kitron UD. Rapid amplification of West Nile virus: the role of hatch-year birds. Vector Borne Zoon Dis. 2008;8:57–67. doi: 10.1089/vbz.2007.0123. [DOI] [PubMed] [Google Scholar]
- Kent R, Juliusson L, Weissmann M, Evans S, Komar N. Seasonal blood-feeding behavior of Culex tarsalis (Diptera: Culicidae) in Weld County, Colorado, 2007. J Med Entomol. 2009;46:380–390. doi: 10.1603/033.046.0226. [DOI] [PubMed] [Google Scholar]
- Kilpatrick AM, Kramer LD, Jones MJ, Marra PP, Daszak P. West Nile virus epidemics in North America are driven by shifts in mosquito feeding behavior. PLoS Biol. 2006a;4:e82. doi: 10.1371/journal.pbio.0040082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick AM, Daszak P, Jones MJ, Marra PP, Kramer LD. Host heterogeneity dominates West Nile virus transmission. Proc Biol Sci. 2006b;273:2327–2333. doi: 10.1098/rspb.2006.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord CC, Day JF. Simulation studies of St. Louis encephalitis virus in south Florida. Vector Borne Zoon Dis. 2001;1:299–315. doi: 10.1089/15303660160025921. [DOI] [PubMed] [Google Scholar]
- Loss SR, Hamer GL, Goldberg TL, Ruiz MO, Kitron UD, Walker ED, Brawn JD. Nestling passerines are not important hosts for amplification of West Nile virus in Chicago, Illinois. Vector Borne Zoon Dis. 2009;9:13–18. doi: 10.1089/vbz.2008.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood F, Chiles RE, Fang Y, Barker CM, Reisen WK. Role of nestling mourning doves and house finches as amplifying hosts of St. Louis encephalitis virus. J Med Entomol. 2004;41:965–972. doi: 10.1603/0022-2585-41.5.965. [DOI] [PubMed] [Google Scholar]
- McLean RG, Bowen GS. Vertebrate hosts. In: Monath TP, editor. St Louis Encephalitis 1980. American Public Health Association; Washington, DC: 1980. pp. 381–450. [Google Scholar]
- Scott TW, Edman JD. Effects of avian host age and arbovirus infection on mosquito attraction and blood-feeding success. In: Loye JE, Zuk M, editors. Bird-Parasite Interactions: Ecology, Evolution, and Behavior. Oxford University Press; New York, NY: 1991. pp. 179–204. [Google Scholar]
- Shaman J. Amplification due to spatial clustering in an individual-based model of mosquito-avian arbovirus transmission. Trans R Soc Trop Med Hyg. 2007;101:469–483. doi: 10.1016/j.trstmh.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Shaman J. Strategies for controlling the epizootic amplification of arboviruses. J Med Entomol. 2011;48:1189–1196. doi: 10.1603/me11085. [DOI] [PubMed] [Google Scholar]
- Shaman J, Day JF, Stieglitz M. Drought-induced amplification of Saint Louis encephalitis virus, Florida. Emerg Infect Dis. 2002;8:575–580. doi: 10.3201/eid0806.010417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaman J, Day JF, Stieglitz M. Drought-induced amplification and epidemic transmission of West Nile virus in southern Florida. J Med Entomol. 2005;42:134–141. doi: 10.1093/jmedent/42.2.134. [DOI] [PubMed] [Google Scholar]
- Slaff ME, Apperson CS, Rogers ML. A key to the mosquitoes of North Carolina and the mid-Atlantic states. Agric Ext Ser North Carolina State University; 1989. Raleigh Publ No AG-412. [Google Scholar]
- Wonham MJ, de Camino–Beck T, Lewis MA. An epidemiological model for West-Nile virus: invasion analysis and control application. Proc R Soc Lond B. 2004;271:501–507. doi: 10.1098/rspb.2003.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]


