Abstract
The nuclear receptor steroidogenic factor 1 (SF-1, AD4BP, NR5A1) is a key regulator of the endocrine axes and is essential for adrenal and gonad development. Partial rescue of Nr5a1−/− mice with an SF-1-expressing transgene caused a hypomorphic phenotype that revealed its roles in Leydig cell development. In contrast to controls, all male rescue mice (Nr5a1−/−;tg+/0) showed varying signs of androgen deficiency, including spermatogenic arrest, cryptorchidism, and poor virilization. Expression of various Leydig cell markers measured by immunohistochemistry, Western blot analysis, and RT-PCR indicated fetal and adult Leydig cell development were differentially impaired. Whereas fetal Leydig cell development was delayed in Nr5a1−/−;tg+/0 embryos, it recovered to control levels by birth. In contrast, Sult1e1, Vcam1, and Hsd3b6 transcript levels in adult rescue testes indicated complete blockage in adult Leydig cell development. In addition, between Postnatal Days 8 and 12, peritubular cells expressing PTCH1, SF-1, and CYP11A1 were observed in control testes but not in rescue testes, indicating SF-1 is needed for either survival or differentiation of adult Leydig cell progenitors. Cultured prepubertal rat peritubular cells also expressed SF-1 and PTCH1, but Cyp11a1 was expressed only after treatment with cAMP and retinoic acid. Together, data show SF-1 is needed for proper development of fetal and adult Leydig cells but with distinct primary functions; in fetal Leydig cells, it regulates differentiation, whereas in adult Leydig cells it regulates progenitor cell formation and/or survival.
Keywords: AD4BP, adult Leydig cell, development, fetal Leydig cell, NR5A1, SF-1, steroidogenic factor 1
INTRODUCTION
Steroidogenic factor 1 (SF-1, AD4BP, NR5A1) is a member of the nuclear receptor family that is essential for development and function of the adrenal and gonadal endocrine systems [1–4]. Studies in both mice and humans link SF-1 mutations to XY sex reversal, ovarian failure, and adrenal insufficiency and have revealed its importance to organ formation and endocrine regulation [5–11]. SF-1 expression and function are primarily restricted to the adrenal gland and the hypothalamic-pituitary-gonadal axis, where it acts in support of steroidogenic cell lineages of the gonads, adrenocortical cells, pituitary gonadotropes, and the ventromedial hypothalamus and to regulate tissue-specific transcription of genes required for hormone production and development [11–15]. In mice lacking SF-1, adrenal and gonad development failed in utero, and all animals, regardless of genetic sex, were born female and died shortly after birth from severe corticosterone deficiency [12, 13]. With respect to the gonads, their early developmental arrest suggested SF-1 functions in pre-Sertoli/granulosa cells to direct formation of the indifferent gonad, whereas its identified target genes indicated it subsequently regulates hormone production within the steroidogenic cells (i.e., Leydig and theca cells) [3, 16–18]. Because of the integrated influences of the endocrine axis and the lack of adrenal glands and gonads, evaluation of SF-1 null mice provide limited information on its specific cellular roles in these organs. Consequently, other models of SF-1 deficiency, including conditional deletions, compound mutations, and haploinsufficiency, were developed and used to gain deeper insight into its functions [19–25]. Although some of these implicated SF-1 in Leydig cell development, direct evidence and details are still missing.
Leydig cells, located within the interstitial region of the testis, are the major androgen-producing cells and, therefore, are responsible for establishing the endocrine environment required for normal male development, physiology, and reproductive function [26, 27]. In many species, including humans and rodents, distinct Leydig cell populations are present during fetal and postnatal life [28–31]. Prior to birth, fetal Leydig cells (FLCs) produce androgens and other molecules required for development of male internal and external genitalia and testis descent [32, 33]. After birth, androgens produced by adult Leydig cells (ALCs) are required for production of sperm and acquisition and maintenance of male characteristics [34]. In mice, FLCs appear in the interstitium at approximately 12.5 days post coitum (dpc) and persist in the testis until approximately 1 wk after birth when ALCs begin to develop [29, 32]. The fate of FLCs remains controversial as there is evidence that favors both their absence and their presence (in small numbers) in adult testes [31, 35–37]. Importantly, ALCs do not emerge from FLCs but are derived from undifferentiated precursors that differentiate through intermediate stages of progenitors and immature Leydig cells before becoming fully mature ALCs [30, 34, 36, 38–42]. However, although it is clear that ALCs and FLCs develop independently, it is still uncertain whether cells from a common progenitor pool contribute to both lineages.
SF-1 is expressed in both fetal and adult Leydig cells, but it is unknown whether its actions are mirrored or divergent in the two lineages [12, 43]. The current study used a hypomorphic mouse model of SF-1, revealing important and distinct functions in ALC and FLC development. These mice were developed by a rescue strategy that used a yeast artificial chromosome (YAC) transgene harboring the rat Nr5a1 locus, and their initial characterization is reported elsewhere [44, 45]. Relevant to the current study is that several independent transgenic mouse lines were created and shown to accurately express Nr5a1 mRNA from the YAC transgene. The rescue strategy used two of the lines, 7 and 14, which were crossed into the Nr5a1−/− background generating rescue (Nr5a1−/−;tg+/0) mice to test the transgenes' functional capacity. Histological analysis confirmed correct cellular expression of the transgenes in both of the lines, but differences were observed in their ability to rescue testis and ovarian function [44, 45]. Gonad histology and fertility of line 14 rescue mice was indistinguishable from that of wild type (wt) mice, but despite the transgene's correct tissue and cellular expression, line 7 Nr5a1−/−;tg+/0 mice had gonad and fertility defects. Structural analysis of the transgenes showed line 14 had 2 integrated copies of the Nr5a1 YAC, whereas line 7 had only 1 [44]. However, gonads of Nr5a1+/− mice were normal (postnatally), suggesting that SF-1 activity in line 7 Nr5a1−/−;tg+/0 mice was below that of Nr5a1 heterozygosity and that further investigation of the hypomorphic rescue mice would provide insight into the role of SF-1 in gonad development [13, 15, 44]. The goal of the current study was to reveal novel testicular functions of SF-1 through further evaluation of the Nr5a1−/−;tg+/0 line 7 mice.
MATERIALS AND METHODS
Mice
Generation, genotyping, and initial characterization of transgenic and rescue mice are described elsewhere [44, 45]. The hypomorphic mice described herein were all derived from line 7. All animals were cared for in accordance with U.S. National Institutes of Health guidelines, and the Laboratory Animal Research Committee at the University of Kansas Medical Center approved all experimental procedures. Mice were maintained on a 12L:12D cycle and given food and water ad libitum.
Preparation of Primary Peritubular Cells
Primary peritubular myoid cells were prepared from 14-day-old rats, as previously described, and cultured in 100-mm dishes for 4 days in the presence of Ham F12 medium modified with l-glutamine medium supplemented with 10% fetal bovine serum, 1.5 mM HEPES, and 1% penicillin-streptomycin solution [46]. At confluency, cells were removed from the dishes, diluted 1:2 in the above-described medium, and cultured in 150-mm dishes. After 48 h in culture, cells were treated with vehicle or 1 mM 8-bromo-cAMP (product no. B7880; Sigma-Aldrich) plus 0.5 μM all-trans retinoic acid (product no. R2625; Sigma-Aldrich) and harvested 48 h later.
Semiquantitative RT-PCR
Total RNA was isolated using TRIzol reagent (Life Technologies), according to the manufacturer's recommendations. RNA was prepared from testes of 2-mo-old mice by using three mice in each represented group: line 7 Nr5a1−/−;tg+/0 mice with descended testes (Rd), line 7 Nr5a1−/−;tg+/0 mice with cryptorchid testes (Rc), and wt mice. Testis RNA isolated from two 5-day-old (Nr5a1+/−) mice was used as a negative control for the expression of ALC markers. RT-PCR was performed as previously described, and all primers are listed in Table 1 [47]. For semiquantitative analysis, amplification was terminated during the logarithmic phase of amplification, after 16, 18, and 20 cycles. The amplified products were separated by agarose gel (1% w/v) electrophoresis and evaluated by Southern blot analysis using internal 32P-end–labeled oligodeoxynucleotide probes, and expression levels were quantified by phosphor imaging (Phosphoimager SI; GE Healthcare Bio-Sciences Corp.) and ImageQuant TL software (GE Healthcare Bio-Sciences Corp.). The volume integration signal of each individual band was normalized to the signal intensity of either Rpl7 (L7) or Cyp11a1 (P450scc). Signals of different genotypes are reported as relative to that of wt testes. All PCR products were verified by sequence analysis.
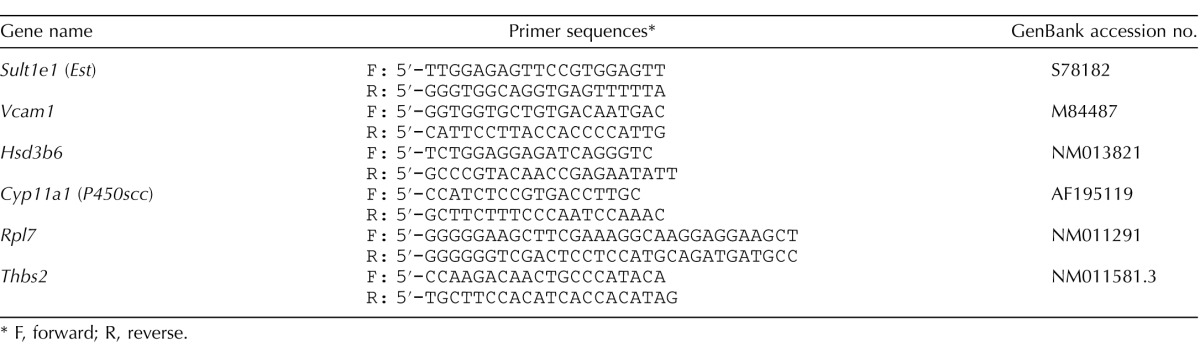
TABLE 1.
RT-PCR primers.
F, forward; R, reverse.
PCR analysis of Thbs2 was performed using cDNA generated from testis RNA isolated from Rc (Postnatal Day 55 [P55] and P260) and Rd (ages P55, P75, P195, P251, and P251) mice, using CloneAmp HiFi PCR Premix (Clonetech Laboratories) according to the manufacturer's suggested procedure. Expression of Rpl7 (L7) was similarly analyzed to confirm cDNA integrity. Cycle number for Rpl7 (L7) was 21 and that for Thbs2 was 34.
Histology
Embryos (14.5 dpc) and testes were dissected from newborn, prepubertal (8 or 12 days old), and adult (2, 4, or 8 mo) Nr5a1−/−;tg+/0, Nr5a1+/− and Nr5a1+/+ wt mice and immersion fixed at +4°C for 2 to 12 h (based on tissue size) in freshly prepared 4% paraformaldehyde buffered with phosphate-buffered saline (PBS; pH 7.4). Tissue samples were serially dehydrated and embedded in paraffin according to standard procedures. Five-micrometer sections were treated to remove paraffin by serial washes in Clear-rite 3 solution (Richard-Allan Scientific, VWR) and ethanol and either stained with hematoxylin (Sigma-Aldrich) and eosin (H&E) or used for immunohistochemistry (IHC). To perform antigen retrieval for IHC, serial sections were boiled using a microwave oven for 12 min in 0.01 M sodium citrate solution (pH 6.0). Samples were incubated with primary antibody overnight at +4°C and then with secondary antibody for 1 h at room temperature. Sections were washed and mounted with Fluoromount-G (SouthernBiotech). Primary antibodies, sources, and dilutions were as follow: polyclonal rabbit SF-1 antibody (1:1000 dilution; a generous gift from Dr. K. Morohashi, Department of Molecular Biology, Graduate School of Medical Sciences, Kyushu University, Japan), polyclonal rabbit cytochrome P450, family 11, subfamily A, polypeptide 1 (CYP11A1) antibody (1:500 dilution; a generous gift from Dr. M. Soares, Department of Pathology and Laboratory Medicine, University of Kansas Medical Center, Kansas City, KS), polyclonal goat ghrelin (C-18) antibody (1:700 dilution; product no. sc-10368, Santa Cruz Biotechnology, Inc.), and goat polyclonal Mullerian inhibiting substance antibody (MIS) (C-20; 1:1000 dilution; product no. sc-6886; Santa Cruz Biotechnology, Inc.) for detection of anti-Mullerian hormone (AMH). For secondary antibodies, tetramethylrhodamine (TRITC)-conjugated goat anti-rabbit antibody (1:200 dilution; product no. 111-075-144; Jackson ImmunoResearch Laboratories, Inc.) was used to visualize SF-1 and CYP11A1 and Cy2-conjugated donkey anti-goat antibody (1:200 dilution; product no. 705-225-147; Jackson ImmunoResearch Laboratories, Inc.) to visualize ghrelin and AMH. Transversely sectioned 14.5-dpc embryos were fixed and stained as above.
For co-localization of SF-1 and PTCH1, 12- and 16-days-old mouse testes were immersed in Sainte Marie's fixative (99:1, 95% ethyl alcohol: glacial acetic acid) for 1 hour, dehydrated, paraffin embedded and five-micron sections prepared for immunofluorescence. Anti-SF-1 antibody (described above) and polyclonal goat anti-patched antibody (G-19), (1:500 dilution; product no. sc-6149, Santa Cruz Biotechnology, Inc.) were incubated together, followed by TRITC-conjugated goat anti-rabbit secondary antibody and the Cy2–conjugated donkey anti-goat secondary antibody, as above. Immunofluorescent staining of testis sections was analyzed using an Eclipse 80i model microscope (Nikon Inc. Instrument Group). and digital images were captured using the Retiga 2000R model fast camera and QCapture software (QIMAGING). Images were overlaid for colocalization of different immunofluorescence signals using Photoshop software (Adobe).
Hormone Levels
Hormone levels for luteinizing hormone (LH), follicle-stimulating hormone (FSH), and testosterone (T) were measured in adult male mice ranging in age from 2.5 to 8 mo old. Samples from Nr5a1−/−;tg+/0 mice included both phenotypes, that is, ones with cryptorchid testes (Rc) and ones with Rd, and controls included wt mice (LH and FSH) and wt plus Nr5a1+/− mice (T). LH and FSH levels were measured in serum prepared from blood obtained from mice anesthetized by intraperitoneal injection of 2.5% avertin (0.015 ml/g per body weight). Blood was collected through the retro-orbital sinus, using heparinized Natelson collection tubes (Fisher Scientific), and the serum was separated by centrifugation at 2000 × g for 15 min and stored at −20°C. Intratesticular T levels were measured in a single testis from each adult mouse used in the study. Testes were homogenized in 500 μl of PBS, using 10 strokes in a Dounce tissue homogenizer (Kontes Glass Co.), followed by sonication for 60 sec with a Vibracell VC130 ultrasonic processor (Sonics & Materials, Newtown, CT). Tissue homogenates were centrifuged, and supernatants were collected and stored at −20°C until assayed for testosterone. Testosterone and FSH levels were measured by radioimmunoassay and LH levels by a mouse LH sandwich assay (performed by Ligand Assay and Analysis Core, Center for Research in Reproduction, University of Virginia, Charlottesville, VA [supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health Specialized Cooperative Centers Program in Reproduction and Infertility Research grant U54-HD28934]). A two-tailed Student t-test assuming equal variance was used for statistical analysis.
Western Blotting
Cultured peritubular cells (described above) and testes and adrenal glands from 2-mo-old wt mice were homogenized in cell lysis buffer (50 mM Hepes, pH 7.6, 250 mM NaCl, 0.5 mM EDTA, 0.1% Triton-X 100, 5 mM β mercaptoethanol) containing complete protease inhibitor cocktail (Roche Diagnostics). Cellular and tissue lysates were analyzed for PTCH1, SF-1, and CYP11A1 by Western blot analysis, as described elsewhere [47, 48]. Antibodies for SF-1, CYP11A1, and PTCH1 (described above) were used at dilutions of 1:2000, 1:1000, and 1:400, respectively, with either anti-goat peroxidase-conjugated donkey antibody (1:10 000 dilution; product no. 705-035-003; Jackson ImmunoResearch Laboratories Inc.) or anti-rabbit peroxidase-conjugated donkey antibody (1:20 000 dilution; product no. 711-035-152; Jackson ImmunoResearch Laboratories Inc.).
Quantification of Seminiferous Tubules
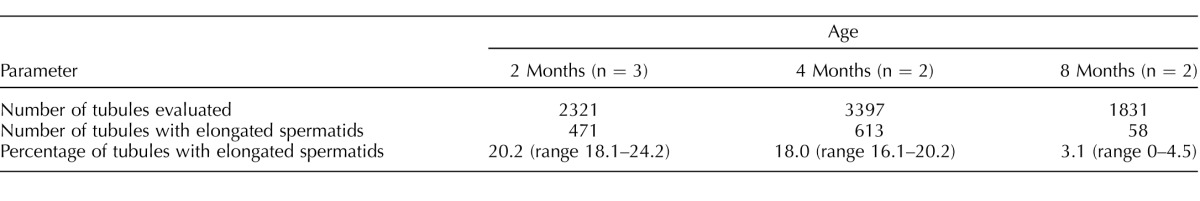
The age-related decline of spermatogenesis was determined in seven Nr5a1−/−;tg+/0 mice at 2, 4, and 8 mo of age by counting the number of seminiferous tubule cross-sections containing elongated spermatids, which was selected as the most advanced spermatogenic cell. Counting was performed on paraffin-embedded sections, which were mounted onto a microscope slide and processed for H&E staining. Cross-sections were observed under brightfield, using an Eclipse 80i microscope (Nikon) with magnifications of 100× and 200×. A minimum of 5 randomly selected cross-sections of each evaluated testis were used for quantification. Results of statistical analyses are means, and range is indicated as a measure of dispersion.
RESULTS
Defective Testis Development Resulting From Incomplete Transgenic Rescue of Nr5a1−/− Mice
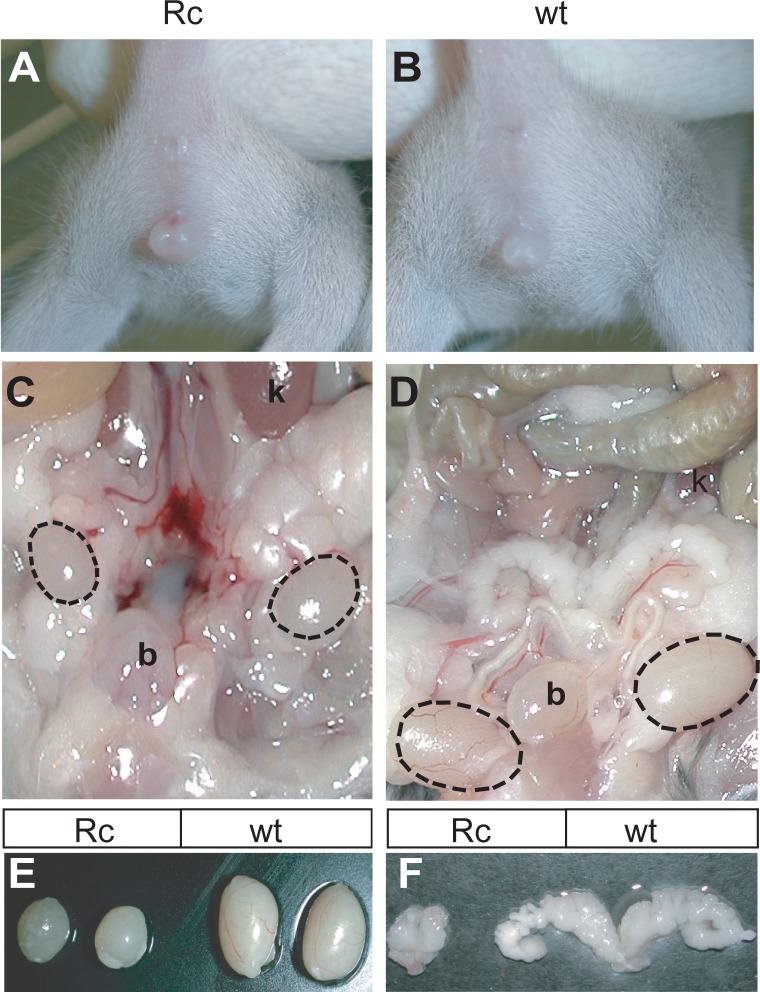
Because line 7 Nr5a1−/−;tg+/0 mice had distinct gonadal defects, they were further investigated to bring insight to the role of SF-1 in testis development and male fertility [44]. All genotypic male (Sry-positive) Nr5a1−/−;tg+/0 mice (n = 28) were infertile, and approximately half (54%) presented with signs of feminization, including areola/nipple retention, reduced anogenital distance, and indications of hypospadias (Fig.1, A and B, and data not shown). All feminized Nr5a1−/−;tg+/0 male mice also had cryptorchid testes positioned abdominally between the kidney and bladder and are referred to herein as rescue c (Rc) mice (Fig. 1C, dotted ovals). Masculinized Nr5a1−/−;tg+/0 male mice, with respect to external genitalia and testis descent, were indistinguishable from wt control males and are referred to herein as rescue d (Rd) mice (Fig. 1D and data not shown). The phenotype of these mice indicated that about half of them (i.e., the Rc mice) had experienced androgen deficiency in utero. However, all adult Nr5a1−/−;tg+/0 mice (Rc and Rd) had signs of androgen deficiency, as revealed by abnormal seminal vesicles, which were either absent (16%) or underdeveloped (Fig. 1F). These observations indicated there were defects in both fetal and adult Leydig cell populations. In addition, testis sizes and weights were significantly reduced in all rescue mice compared to that of controls (mean Rc weight = 0.024 ± 0.009 g; mean Rd weight = 0.044 ± 0.011 g; control weight = 0.117 ± 0.014 g) (Fig. 1E), indicating spermatogenesis was impaired. Notably, no differences in testis weights, testis descent, spermatogenesis, and virilization were observed between wt and Nr5a1+/− mice or between wt and transgenic mice on either Nr5a1+/− or wt backgrounds (data not shown), which demonstrated that the phenotypes of Rc and Rd mice were caused by the transgene's inability to support normal testis development and function and suggested that further evaluation of the hypomorphic mice would provide new information for the role of SF-1 in Leydig cells.
FIG. 1.
Male reproductive development in Nr5a1−/−;tg+/0 mice. A and B) Fifty-four percent of Nr5a1−/−;tg+/0 male mice presented with signs of feminization (referred to as Rc mice). Anogenital distances in feminized Nr5a1−/−;tg+/0 male mice (A) were reduced compared to that of wild type (wt) mice (B) or masculinized Nr5a1−/-;tg7/0 males (not shown). C and D) Testis descent is shown in adult Nr5a1−/−;tg+/0 feminized and wt male mice. Testes (dotted ovals) in feminized Nr5a1−/−;tg+/0 male mice (C) were located above the bladder, whereas testes in wt (D) and masculinized Nr5a1−/-;tg7/0 male mice (not shown) were descended below the level of the bladder. E–F). Seminal vesicle and testis size are compared in Nr5a1−/−;tg+/0 and wt mice. Seminal vesicles from feminized Nr5a1−/−;tg+/0 (left) and wt (right) mice (F). Testes form feminized Nr5a1−/−;tg+/0 (left) and wt (right) mice (E). b = bladder; k = kidney.
Delayed Fetal Leydig Cell Development in Nr5a1−/−;tg+/0 Mice
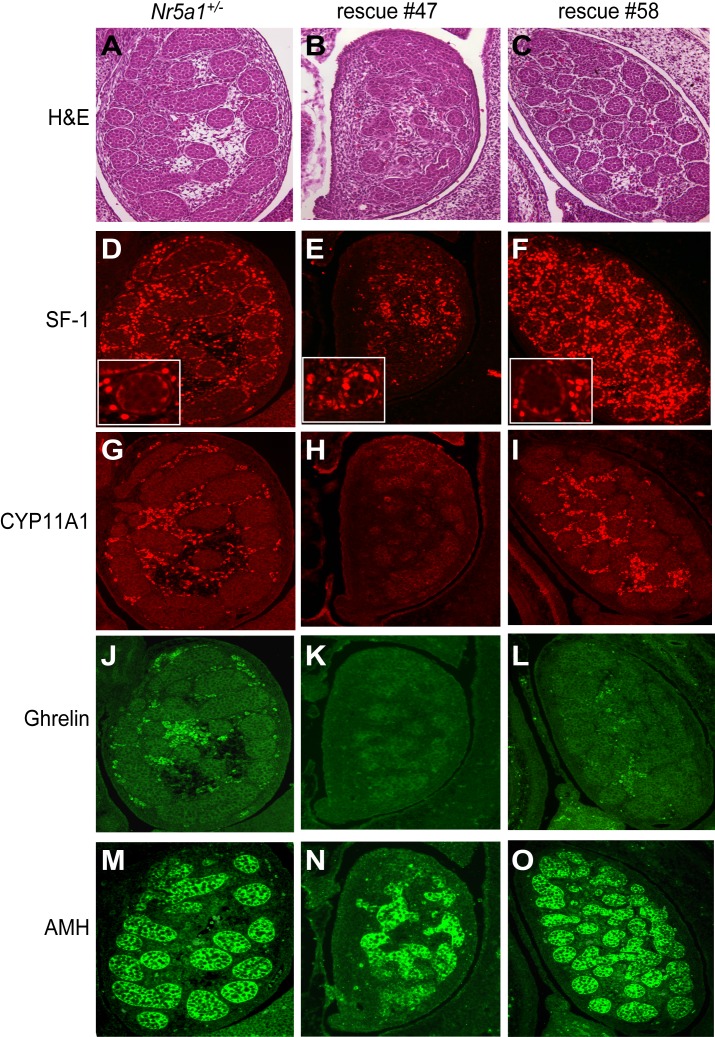
H&E staining and IHC were used to evaluate morphology and protein expression, respectively, in 14.5-dpc testes from Nr5a1−/−;tg+/0 and control (Nr5a1+/−) embryos. Protein expression was evaluated for SF-1, which is present in both Sertoli and Leydig cells and proteins restricted to, and thus distinguished, Sertoli cells and Leydig cells. Proteins included anti-Mullerian hormone (AMH), a Sertoli cell protein, cholesterol side-chain cleavage enzyme (CYP11A1), a Leydig cell protein, and ghrelin, a Leydig cell protein shown previously to be restricted to fully differentiated fetal and adult Leydig cells [49, 50]. Similar to the mixed phenotypes at birth, testes of Nr5a1−/−;tg+/0 embryos presented with two distinct phenotypes, as seen in representative embryos rescue 47 and rescue 58 testes (Fig. 2). Testes from rescue 58, with the exception of ghrelin staining, had similar morphology and gene expression profiles as that of control (Nr5a1+/−) testes; both having clearly defined tubules by H&E staining, and by IHC, robust interstitial staining (Leydig cells) of SF-1 and CYP11A1 and intratubular staining (Sertoli cells) of AMH, whereas signals for SF-1 in Sertoli cells were less intense (Fig. 2, A and C, D and F and insets, G and I, and M and O). Ghrelin staining, on the other hand, appeared significantly reduced in rescue 58 compared to that in control (Fig. 2, J and L).
FIG. 2.
Histological evaluation of 14.5 dpc testes from Nr5a1+/- and Nr5a1−/−;tg+/0 embryos. Histology of testes from Nr5a1+/− (A, D, G, J, and M) and Nr5a1−/−;tg+/0 embryos (rescue 47 and rescue 58) at 14.5 dpc. Rescue 47 (B, E, H, K, and N) and rescue 58 (C, F, I, L, and O) represent the two characteristic phenotypes of Nr5a1−/−;tg+/0 testes at 14.5 dpc. A–C) H&E-stained 14.5 dpc testis sections. D-F) SF-1 immunofluorescence of 14.5 dpc testis sections. Insets show enlarged area around a testis cord to emphasize staining intensity between cells in the cords and interstitium. G–I) CYP11A1 immunofluorescence of 14.5-dpc testis sections. J–L) ghrelin immunofluorescence of 14.5 dpc testis sections. M–O) AMH immunofluorescence in 14.5-dpc testis sections. Original magnification ×200 (A–O) and ×400 (D–F insets).
In contrast to rescue 58, rescue 47 testes differed significantly from those of the control. In particular, cord structures were poorly developed, and SF-1-positive cells were greatly reduced and lacked the distinct compartmentalized differences in signal intensities observed in the control and rescue 58 (Fig. 2, A–F and D–F insets). In addition, expression levels of CYP11A1 and ghrelin were not detected in rescue 47 testis, whereas AMH was readily observed within the dysmorphic cord-like structures (Figs. 2, H, K, and N). The presence of AMH in Sertoli cells of Nr5a1−/−;tg+/0 embryos together with the mixed phenotypes in cord structure and gene expression suggested Sertoli cell function was largely normal in the rescue embryos but that reduced SF-1 activity caused either a blockage or delay in development that predominantly influenced FLC differentiation [51–54]. Because androgens produced by FLCs are required for virilization, the finding of significant FLC deficiency in some Nr5a1−/−;tg+/0 embryos, as shown by rescue 47, and more mild defects in others (e.g., rescue 58) is consistent with the feminized and masculinized phenotypes observed at birth.
To determine whether the FLC defects observed at 14.5 dpc represented blocked or delayed development, we evaluated newborn (P0) testes from Nr5a1+/− and Nr5a1−/−;tg+/0 (both Rc and Rd) mice. Interestingly, testis morphology (data not shown) and IHC results from SF-1, CYP11A1, and ghrelin assays were similar among all mice (Fig. 3). As only FLCs are present at birth, the results indicated that reduced SF-1 activity from the transgene delayed rather than arrested FLC development [38, 55, 56].
FIG. 3.
SF-1, CYP11A1, and ghrelin immunofluorescence in newborn testes from Nr5a1+/− and Nr5a1−/−;tg+/0 mice. Immunofluorescence of SF-1 (A–C), CYP11A1 (D–F), and ghrelin (G–I) in testes sections from newborn Nr5a1+/− mice (A, D, G) and from Nr5a1−/−;tg+/0 mice with either Rc (B, E, H) or Rd testes (C, F, I). Original magnification ×200.
Spermatogenic Arrest and Hypergonadotropic Hypogonadism in Adult Nr5a1−/−;tg+/0 Mice
Changes in adult Nr5a1−/−;tg+/0 mice (i.e., reduced testicular size and underdeveloped seminal vesicles) led to further investigation of adult animals. Morphological evaluation of testis cross-sections revealed impaired spermatogenesis in both Rc and Rd mice but with slightly different outcomes (Figs. 4, A–C). In contrast to the full compliment of germ cells observed in wt mice (Fig. 4A), the most mature germ cells in Rc and Rd mice were pachytene spermatocytes and elongating spermatids, respectively (Figs. 4, B and C, insets). In Rd mice, there was also an age-dependent decline in the number of spermatid-containing tubules, which dropped from approximately 20% at 2 mo to only 3% at 8 mo (Table 2). Given that the phenotype of all Nr5a1−/−;tg+/0 mice implicated androgen deficiency, intratesticular testosterone levels were measured and compared between Nr5a1−/−;tg+/0 and control (wt and Nr5a1+/−) mice. This comparison revealed significantly lower (P < 0.02) intratesticular testosterone in Nr5a1−/−;tg+/0 male mice (mean T concentration = 505 ± 287 ng/dl; n = 6 mice) than in controls (mean T = 2915 ± 781 ng/dl; n = 4 mice). Because SF-1 is also important for pituitary gonadotropin production and LH is the major stimulus of adult testosterone biosynthesis, compromised LH production was considered the potential cause for lower testosterone levels [57]. Hence, serum LH and FSH concentrations were measured in adult Nr5a1−/−;tg+/0 and wt mice, which identified significantly (P < 0.01) higher levels of LH and FSH in Nr5a1−/−;tg+/0 mice than in controls (LH in Nr5a1−/−;tg+/0 mice = 1.01 ± 0.73 ng/ml [n = 12]; and 0.26 ± 0.31 ng/ml in controls [n = 9]; FSH in Nr5a1−/−;tg+/0 mice = 83.12 ± 29.04 ng/ml (n = 8); and 36.97 ± 18.54 ng/ml in controls (n = 9]). Elevated gonadotropin levels were interpreted as the appropriate response to reduced negative feedback, suggesting normal pituitary function and the fact that androgen deficiency in Nr5a1−/−;tg+/0 mice was caused by a defect in the adult Leydig cells.
FIG. 4.
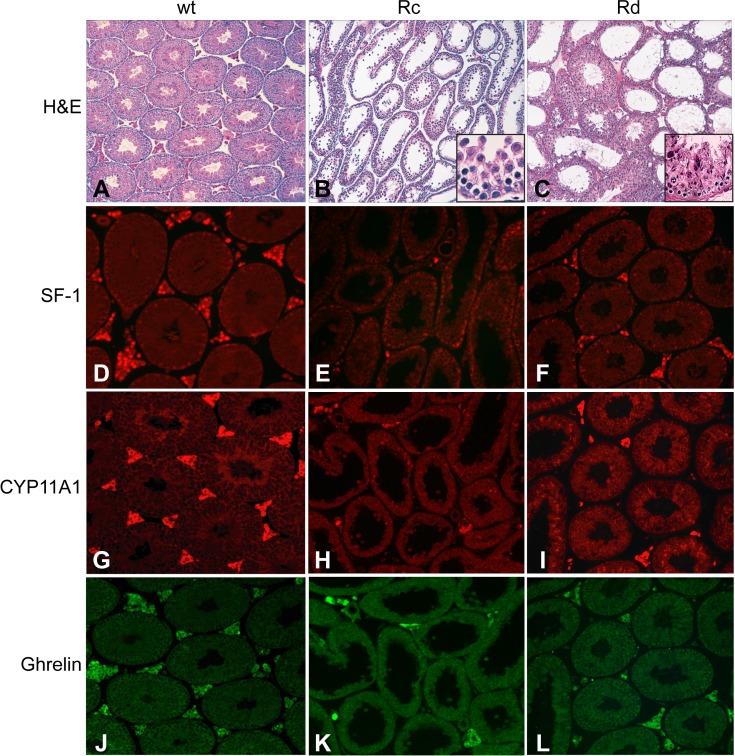
Histological evaluation of adult testes from wild type and Nr5a1−/−;tg+/0 mice. Adult testis sections from wild type (wt) mice (A, D, G, J) and Nr5a1−/−;tg+/0 mice with either Rc (B, E, H, K) or Rd testes (C, F, I, L) were examined by staining with H&E (A–C) or by immunofluorescence using antibodies against SF-1 (D–F), CYP11A1 (G–I), and ghrelin (J–L). Original magnification ×200 (A–L) and ×400 (B–C insets).
TABLE 2.
Degeneration of spermatogenesis in Nr5a1−/−;tg+/0 Rd mice.
Adult Leydig Cell Deficiency in Nr5a1−/−;tg+/0 Mice
To assess the status of Leydig cells in adult testes, IHC assay was performed to evaluate SF-1, CYP11A1, and ghrelin expression. In wt testes, the SF-1 antibody provided a robust nuclear signal in Leydig cells of the interstitium and a less intense signal from Sertoli nuclei at the periphery of seminiferous tubules (Fig. 4D). In testes from both Rc and Rd Nr5a1−/−;tg+/0 mice, there were considerably fewer SF-1–positive Leydig cells, whereas SF-1–positive Sertoli cells appeared comparable to those of controls (Figs. 4, E and F). In all animals, CYP11A1 (Fig. 4, G–I) and ghrelin-positive cells (Fig. 4, J–L) were restricted to the interstitial compartment and similar to those observed for SF-1. In all, data showed greatly reduced Leydig cell numbers in adult Nr5a1−/−;tg+/0 testes (both descended and cryptorchid), which, based on ghrelin expression, were fully differentiated.
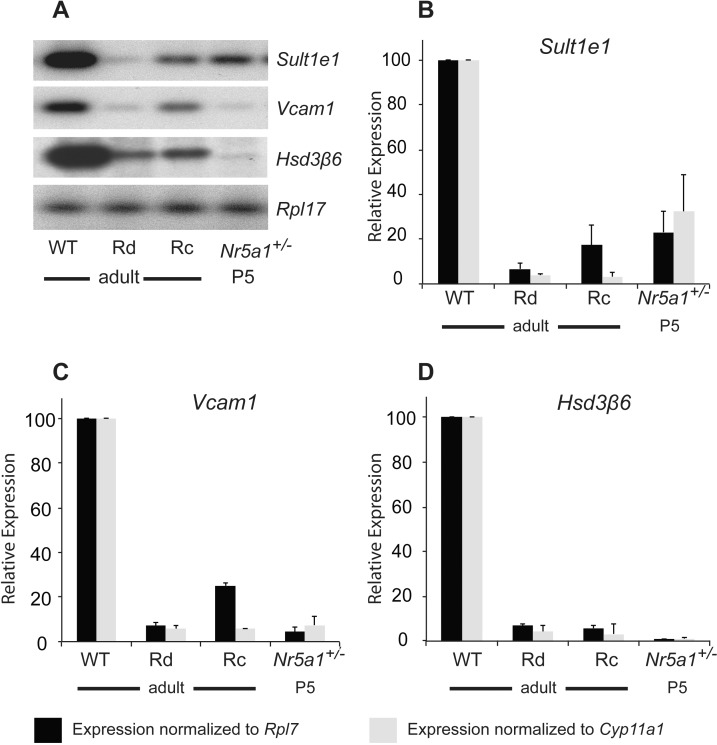
Although mature Leydig cells were evident in adult Nr5a1−/−;tg+/0 testes, it was uncertain whether they represented persistent FLCs or poorly expanded ALCs. To address this question, semiquantitative RT-PCR followed by Southern blot analysis, was used to measure mRNA levels of the selective ALC markers estrogen sulfotransferase (Sult1e1), vascular cell adhesion molecule I (Vcam1), and hydroxy-delta-5-steroid dehydrogenase, 3 beta- and steroid delta-isomerase 6 (Hsd3b6) in testis RNA extracted from adult wt and Nr5a1−/−;tg+/0 (Rd and Rc) mice and P5 Nr5a1+/− mice (Fig. 5A, and data not shown) [58, 59]. Control mRNAs included ribosomal protein L7 (Rpl7), a ubiquitously expressed gene, and Cyp11a1, a gene expressed in fetal and adult Leydig cells. Amplified DNA signal intensities (quantified by densitometry) for Sult1e1, Vcam1, and Hsd3b6 were normalized to those of either Rpl7 or Cyp11a1, and the values of Rc, Rd, and P5 samples were normalized relative to the normalized values from adult wt testes and were represented graphically (Fig. 5, B–D). Data showed that levels of Sult1e1, Vcam1, and Hsd3b6 mRNA in adult Rd and Rc testes were significantly lower than those in adult wt testes (Fig. 5, B–D). This was true regardless of whether the samples were normalized to those of Rpl7 or Cyp11a1. Notably, the levels of Sult1e1, Vcam1, and Hsd3b6 mRNA in Rd and Rc testes closely resembled those found in P5 testes when only FLCs were present (Figs. 5, B–D). These data, together with those showing Leydig cells in adult Rc and Rd testes, expressed ghrelin (Fig. 4), which is present only in fully differentiated cells, indicated that the few remaining Leydig cells in Nr5a1−/−;tg+/0 testes were persistent FLCs and not partially differentiated ALCs [49]. This was further supported by RT-PCR analysis results for thrombospondin 2 (Thbs2), a marker of FLCs [59–62]. This showed Thbs2 expressed in all adult rescue testes examined (Rc: n = 2; Rd: n = 5; data not shown).
FIG. 5.
Expression of adult Leydig cell markers in Nr5a1−/−;tg+/0 mice. Semiquantitative RT-PCR was used to measure mRNA levels for the ALC-specific genes Sult1e1, Vcam1, and Hsd3b6 (Hsd3β6) and the control genes Cyp11a1 and Rpl7. Template was generated using total RNA isolated from wild-type (wt), Nr5a1−/−;tg+/0 cryptorchid (Rc), and Nr5a1−/−;tg+/0 descended (Rd) adult (2-mo-old) testes and Nr5a1+/− P5 testes. A) Representative Southern blot of amplified signals for Sult1e1, Vcam1, Hsd3b6 (Hsd3β6), and Rpl7. B–D) Graphical representation of Rpl7 (black bars) and Cyp11a1 (gray bars) normalized Sult1e1 (B), Vcam1 (C), and Hsd3b6 (Hsd3β6) (D) signals from Rc, Rd, and P5 testes relative to those of wt testes. Autoradiographic signals were quantified by densitometry and values for ALC-specific mRNAs were normalized to values for either Rpl7 or Cyp11a1. Normalized values from Rd, Rc, and P5 testes were then made relative to the normalized values from wt testes.
Loss of ALC Progenitors in Postnatal Nr5a1−/−;tg+/0 Testes
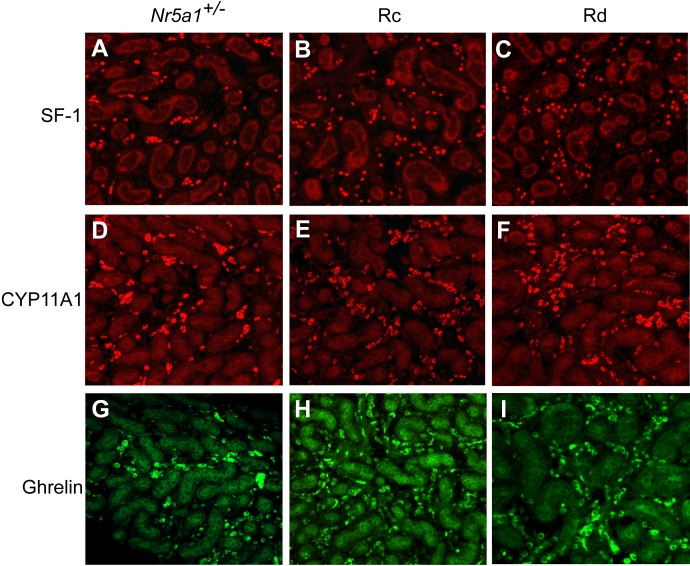
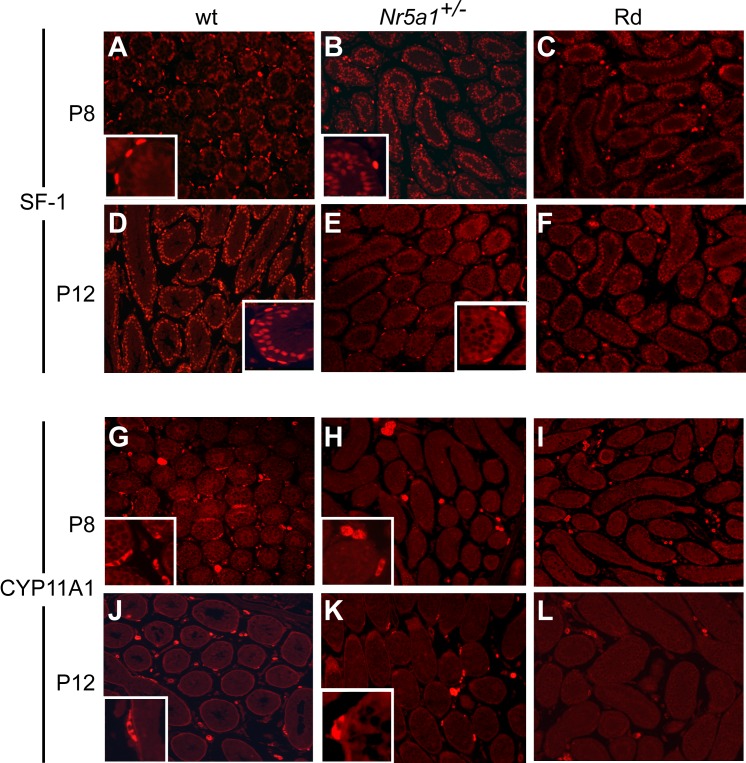
The absence of ALCs in Nr5a1−/−;tg+/0 testes suggested a defect in either cell survival or development. To determine whether ALC development initiated properly, IHC testing was used to observe ALC progenitors, which arise from peritubular cells within the first 2 wk of birth [34, 56, 63, 64]. Testes from wt, Nr5a1+/−, and Nr5a1−/−;tg+/0 mice at P8 and P12 were examined for SF-1 and CYP11A1 expression (Fig. 6). Small patches of SF-1–stained interstitial cells were observed in all genotypes, and in wt and Nr5a1+/− testes, SF-1 was observed in peritubular cells, but the number of positive cells was higher in wt mice than in Nr5a1+/− mice at both ages (Fig. 6, A–F). In contrast, SF-1-positive peritubular cells were not observed in Rd and Rc Nr5a1−/−;tg+/0 testes (Fig. 6, C and F and data not shown). Like SF-1, patches of CYP11A1-positive cells were evident in the interstitium of P8 and P12 testes from all genotypes, but peritubular staining was seen only in wt and Nr5a1+/− testes (Figs. 6, G-L). Together, data indicated that the ALC progenitor population was severely compromised in Nr5a1−/−;tg+/0 mice and that the SF-1- and CYP11A1-positive cells observed in prepubertal and adult Rc and Rd testes were residual FLCs.
FIG. 6.
SF-1 and CYP11A1 immunofluorescence in P8 and P12 testes from wild-type (wt), Nr5a1+/− and Nr5a1−/−;tg+/0 Rd mice. A–F) SF-1 immunofluorescence in tissue sections of P8 (A–C) and P12 (D–F) testes from wt (A and D), Nr5a1+/− (B and E), and Nr5a1−/−;tg+/0 Rd (C and F) mice. G–L) CYP11A1 immunofluorescence in tissue sections of P8 (G–I) and P12 (J–L) testes from wt (G and J), Nr5a1+/− (H and K), and Nr5a1−/−;tg+/0 Rd (I and L) mice. Original magnification ×200. Inset are shown at higher magnification (×5) to better reveal positive-staining of peritubular cells.
ALC Progenitors Express Both SF-1 and Patched
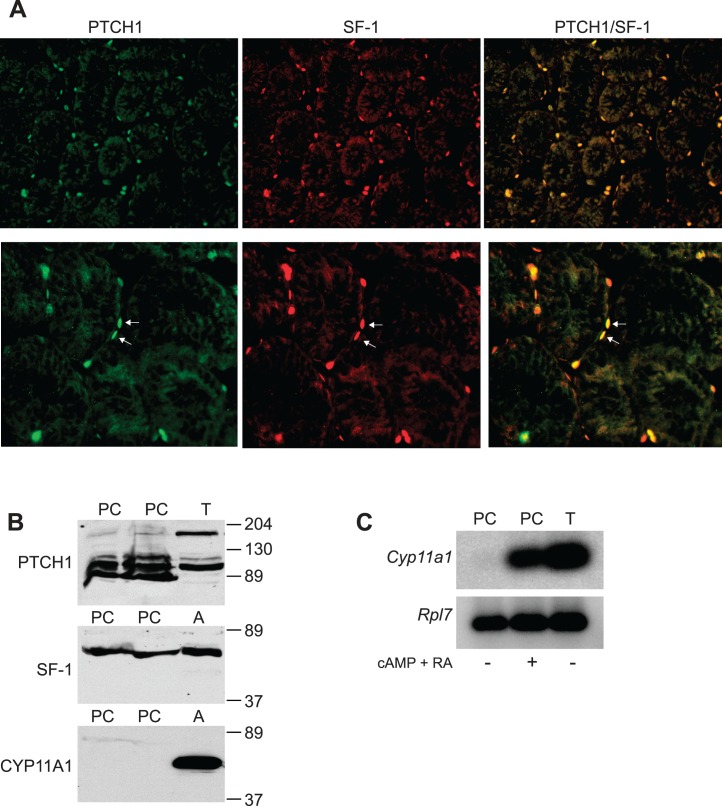
Desert hedgehog (DHH) is required for development of both fetal and adult Leydig cells and, similar to Nr5a1−/−;tg+/0 mice, genotypic male mice lacking DHH presented with masculinized and feminized phenotypes, and peritubular cell defects were observed and associated with abnormal Leydig cell differentiation [65, 66]. Similarities between the findings suggested a connection between DHH produced by Sertoli cells and SF-1-positive peritubular cells in ALC development. To determine whether these cells potentially respond to DHH, IHC assay was used to identify SF-1 and patched homolog 1 (PTCH1)-expressing cells in P12 wt testes. This experiment identified cells that expressed both SF-1 and PTCH1, some of which were peritubular (Fig. 7A, arrows in bottom panels). Similar results were obtained from P16 control testes, whereas testes from Nr5a1−/−;tg+/0 mice lacked PTCH1/SF-1–positive peritubular cells (Supplemental Fig. S1; all supplemental data are available online at www.biolreprod.org). Considered together with the earlier DHH studies, the data suggest DHH regulates SF-1–positive peritubular cells and that both are important for ALC development [65, 66].
FIG. 7.
Peritubular cells as ALC precursors. A) Immunofluorescent images of PTCH1 (green [left]), SF-1 (red [middle]) and PTCH1/SF-1 (merged [right]) in testis section from 12-day-old wt mice. White arrowheads (bottom right panel) indicate peritubular cells positive for both PTCH1 and SF-1. Original magnification ×200 (top) and ×400 (bottom). B) Western blot analysis of PTCH1 (top), SF-1 (middle), and CYP11A1 (bottom) using protein lysates from P14 rat peritubular cell cultures (PC) and adult mouse testes (T) and adrenal glands (A). C) Southern blot analysis of Cyp11a1 (top) and Rpl7 (bottom) mRNAs amplified by RT-PCR, using RNA isolated from rat peritubular cells (PC) cultured in the absence (−) or presence (+) of retinoic acid (RA) and 8-bromo-cAMP (cAMP) and testis RNA (T) as positive control.
To further investigate peritubular cells as ALC precursors, lysates of cultured peritubular cells prepared from P14 rat testes were examined by Western blot analysis for expression of SF-1, PTCH1, and CYP11A1. In mouse testis lysates, the anti-PTCH1 antibody detected a protein of ∼180 kDa, the size of full-length patched, and a more prominent ∼100-kDa band (Fig. 7B, top). In peritubular cell lysates, anti-PTCH1 antibody detected a faint band at 180 kDa, whereas the ∼ 100-kDa band was readily observed together with other bands, which were observed as fainter bands in testis lysates (Fig. 7B, top). A prevalent ∼100-kDa protein was previously observed for HA-tagged PTCH1, suggesting the smaller form is predominant in cultured peritubular cells [67]. Western blot analysis for SF-1 identified a single band at ∼ 46 kDa, its expected size, in lysates from peritubular cells and adrenal glands (Fig. 7B, middle). In contrast, CYP11A1, detected as a 49-kDa band, was observed only in lysates from adrenal glands, indicating it is not normally expressed in cultured peritubular cells (Fig. 7B, bottom). Similarly, RT-PCR followed by Southern blot analysis showed Cyp11a1 mRNA was absent from peritubular cells (Fig. 7C, left lane). The presence of SF-1 and absence of CYP11A1 suggested the cultured peritubular cells represented a precursor lineage prior to gaining steroidogenic capacity [34, 68]. To determine whether the cells could be induced toward a steroidogenic lineage, they were cultured in differentiation medium containing the cAMP analog 8-bromo-cAMP and retinoic acid (RA), and Cyp11a1 mRNA expression was measured as described above [69]. This resulted in a marked induction of Cyp11a1 mRNA. Together, the data suggest that SF-1 positive, CYP11A1-negative peritubular cells represent ALC precursors prior to their full commitment to the steroidogenic lineage (Fig. 7C).
DISCUSSION
In the current study, a unique transgenic rescue model of SF-1 hypomorphism was used to uncover new roles of SF-1 in testis development. Analysis of Nr5a1−/−;tg+/0 mice revealed that SF-1 differentially regulates fetal and adult Leydig cell development and suggested the two lineages derive from distinct progenitor pools. In utero, Nr5a1−/−;tg+/0 FLCs were slower to differentiate, but by birth, their maturation and numbers matched that of control testes (Figs. 2 and 3). Importantly, FLCs eventually developed in the Nr5a1−/−;tg+/0 embryos, confirming existence of FLC progenitors and indicating that SF-1 is either not required for progenitor formation or that its activity was sufficient for their derivation. This differed significantly from the ALC population in these mice, where the lack of ALCs (Figs. 4 and 5) and ALC progenitors (Fig. 6) indicated a blockage in lineage development. Hence, SF-1 is important for both lineages but contributes differently to their development.
Studies of SF-1 transcriptional activity, expression, and function have implicated it in Leydig cell development and function. Its transcriptional activity is known to regulate a number of genes important to steroid hormone production and, therefore, to Leydig cell function (reviewed in [11] and [16]). It is also expressed in Leydig cells at all stages of FLC and ALC development, and in both lineages, there is evidence suggesting SF-1 expression precedes steroidogenic capacity [12, 68, 70–75]. However, studies using animal models to help elucidate functional SF-1 contributions to Leydig cells have been less informative, largely because interpretation was either limited by a poorly resolved phenotype or confounded by the inclusion of additional gene mutations [13, 19–25]. Notably, previous studies using mice with SF-1 haploinsufficiency (i.e., Nr5a1+/− mice) did show delayed Sertoli and Leydig cell differentiation in the embryos, but in contrast to Nr5a1−/−;tg+/0 animals, FLCs recovered prior to 14.5 dpc and genotypic males were fully masculinized and fertile and had ALCs ([13, 15, 76] and data not shown). Two other models of note had, in combination with global SF-1 haploinsufficiency, either a gonad-specific deletion of Nr5a1 or a deletion for Dhh [21, 23]. Using the first of those models, Jeyasuria et al. [21] deleted Nr5a1 in testicular somatic cells by using the Amhr2-cre transgene. Testes of embryos carrying the deletion were developmentally delayed, significantly dysmorphic and lacked FLCs, as shown by the loss of key steroidogenic enzymes [21]. Data suggested significant effects on both Sertoli cells and Leydig cells, making it difficult to ascertain the primary reason for FLC loss. Postnatal testes from these mice were also significantly disrupted, but no evaluation of ALCs was provided. However, the authors did suggest Leydig cells were present in the adult testes and, thus, that SF-1 was not required for Leydig cell survival. The second study evaluated Nr5a1+/−;Dhh−/− mice, which had testes that lacked both Leydig cell lineages [23]. Although the data implicated SF-1 in the establishment of both Leydig cell lineages, the potential influence of the Dhh−/− mutation, which is strongly influenced by genetic background, confounded the interpretation with respect to contributions of SF-1 [23, 65, 66, 77]. In addition, testes of Nr5a1+/−;Dhh−/− mice were severely dysmorphic, with only tiny rudimentary structures present in prepubertal and adult animals. Overall, one can conclude that Leydig cells were absent from testes of Nr5a1+/− compound mutants, but the severe loss of structure and cellular composition, as well as potential contributions from the Dhh−/− mutation, obscured the ability to assign any direct role of SF-1 in Leydig cell differentiation.
For the most part, distinct regulatory signals control fetal and adult Leydig cell differentiation, which is monitored through the activation of genes involved in steroidogenesis [31, 38, 78, 79]. One major exception to this is the Sertoli cell-derived signaling molecule DHH, which is necessary for both ALC and FLC differentiation [65, 66, 75, 77]. Comparison of the Nr5a1−/−;tg+/0 phenotype to that reported for Dhh−/− mice revealed some interesting similarities and differences that suggest SF-1 and DHH act along a common pathway to support FLC development but diverge in their support of ALC development [65, 66, 75, 77]. The concept that SF-1 and DHH participate in the same pathway regulating FLC development was proposed earlier in a study of Dhh−/− mice, which indicated that DHH specifies the FLC lineage by up-regulating Nr5a1 and Cyp11a1 expression within Ptch1-positive progenitor cells located in the interstitium [75].
Comparison of the in utero results of Dhh−/− and Nr5a1−/−;tg+/0 mice suggested that neither phenotype indicated a disruption in the formation, proliferation, or survival of FLC progenitors but, rather, both of them showed evidence of a transient disruption in FLC differentiation, as indicated by temporal delays in Cyp11a1 expression [65, 75]. However, in Dhh−/− testes at 14.5 dpc, Cyp11a1 expression was still below that of controls, and later time points were not evaluated [75]. Therefore, although Nr5a1−/−;tg+/0 FLCs were shown to recover by P0, the final status of Dhh−/− FLCs remains uncertain. In addition, both Dhh−/− and Nr5a1−/−;tg+/0 genotypic male mice presented with a mixture of sexual phenotypes, indicating that fetal androgen levels and, thus, FLC function differed among developing embryos [23, 65]. However, the Dhh−/− mouse data for sexual phenotype and that for FLC development were reported in separate studies, and no associations were made between FLC defects and feminization. This contrasts with the current study in which the percentage of Nr5a1−/−;tg+/0 feminized male pups closely matched the percentage of 14.5-dpc embryos (∼50%) without recognizable FLCs. In addition, to changes in FLCs, Nr5a1−/−;tg+/0 and Dhh−/− fetal testes both had abnormal cords structures [23, 66]. Whereas the structural abnormalities in Nr5a1−/−;tg+/0 mice were resolved by P0, this does not appear to be the case for newborn Dhh−/− mice, which were reported to have irregularities in the seminiferous tubules, albeit to a lesser extend than those observed in adult animals [65].
Comparison between the results from postnatal Dhh−/− and Nr5a1−/−;tg+/0 mice also revealed similarities and differences with respect to the ALC population. The most prominent distinction was noted in testes from masculinized males, in which ALCs were present in Dhh−/− mice and absent in Nr5a1−/−;tg+/0 mice [23, 65]. In the feminized males, ALCs were missing in the testes of both Dhh−/− mice and Nr5a1−/−;tg+/0 mice [65]. Thus, Dhh−/− mice with signs of FLC deficiency (i.e., feminization) also lacked ALCs, whereas those without signs of FLC deficiency (i.e., masculinized) did not. Likewise, all genotypic male Nr5a1+/−;Dhh−/− mice were feminized and lacked ALCs [23]. Therefore, a strong association exists between loss of FLCs and loss of ALCs in the Dhh−/− and Nr5a1+/−;Dhh−/− mice. In contrast, this association was not observed in Nr5a1−/−;tg+/0 mice, as FLCs were present at birth (Fig. 4) and ALCs failed regardless of sexual status. Hence, only residual FLCs were present in adult testes of Nr5a1−/−;tg+/0 mice (Fig. 5), indicating that ALCs either failed to develop or survived. IHC results from P8 and P10 testes implicated developmental failure, as the peritubular progenitor cells, which expressed SF-1 and CYP11A1, were absent or severely deficient in Nr5a1−/−;tg+/0 testes. Interestingly, Clark et al. [65] also observed abnormal peritubular cells in Dhh−/− mice and, consistent with the findings herein, that the DHH receptor Ptch1 was present on peritubular cells.
The finding that SF-1-positive and CYP11A1-positive peritubular cells were absent from Nr5a1−/−;tg+/0 testes and accumulating evidence that peritubular cells are a major source of ALC progenitors (or stem cells) suggest SF-1 is important for the formation of ALC progenitors [34, 63, 64]. The lack of information about SF-1 in testicular peritubular cells or their relationship to ALC progenitors prompted further examination of SF-1 in these cells as well as their potential to differentiate into steroidogenic cells. Since previous studies suggested ALC progenitors express the DHH receptor, IHC assay was used to localize SF-1 and PTCH1 together in testis sections of wt mice, confirming the two proteins are coexpressed in the same cells (Fig. 7A), some of which localized to the peritubular region [65]. Additional support for SF-1–positive peritubular cells as ALC progenitors was provided by studies of cultured peritubular cells. These cells expressed both SF-1 and PTCH1 and induced Cyp11a1 when treated with cAMP and RA, indicating they differentiated toward a steroidogenic lineage (Fig. 7). Notably, the importance of SF-1 to steroidogenic lineage induction was previously indicated in a study that used embryonic stem (ES) cells stably expressing SF-1 [69]. In that study, the authors showed that treatment with cAMP and RA induced Cyp11a1 expression in SF-1–positive ES but not in SF-1–negative ES cells. Similar findings were obtained with mesenchymal stem cells stably expressing SF-1, which differentiated into steroidogenic cell lineages when treated with cAMP [80–82]. Finally, the importance of SF-1 to ALC progenitors is supported by transcriptome data derived from ALCs at different stages of development [68]. These data showed that Nr5a1 was expressed in isolated progenitors and/or stem Leydig cells and that its expression increased upon differentiation.
In humans, NR5A1 mutations are associated with both adrenal and reproductive disorders (reviewed in [7]). However, human phenotypes associated with NR5A1 mutations vary widely and indicate that loss of SF-1 activity has a greater impact on reproductive function than it does on adrenal function [7]. Accordingly, 46,XY individuals with NR5A1 haploinsufficiency typically present with reproductive disorders but not adrenal insufficiency [7, 83–88]. The clinical phenotypes of these patients range in severity from ambiguous genitalia at birth, partial gonadal dysgenesis, and absent or hypoplastic Mullerian structures to milder cases that include hypospadias and cryptorchidism. Interestingly, there are also reports of NR5A1 heterozygous mutations in 46, XY individuals who were born female or predominantly female and then, at puberty, were androgenized, indicating differences between fetal and adult Leydig cell functions [7, 89]. The phenotype of Nr5a1−/−;tg+/0 mice is similar in several ways to the reproductive abnormalities observed in humans with NR5A1 mutations, particularly with respect to the range of phenotypes associated with FLC dysfunction, suggesting the Nr5a1−/−;tg+/0 mice represent a unique model of SF-1 deficiency that can be used to advance our understanding of SF-1 and its relationship to human reproductive disorders.
In summary, evaluation of Nr5a1−/−;tg+/0 mice added to our understanding of SF-1 Leydig cell development by distinguishing its roles in the fetal and adult lineages. In Nr5a1−/−;tg+/0 embryos, reduced SF-1 activity delayed FLC development. Temporal differences in the delay and varying degrees of masculinization at birth implied that, like DHH, activity of SF-1 in utero was influenced by genetic variation between individual animals. In contrast, ALC progenitors were absent in Nr5a1−/−;tg+/0 mice, and therefore, the adult lineage never developed. To our knowledge, this is the first example of ALC loss without corresponding loss of FLCs. With that said, many previous studies did not evaluate both lineages, and therefore, similar unreported examples may exist. Regardless, the findings reported here suggest the existence of distinct progenitor pools for the fetal and adult Leydig cell lineages, a theory that is also supported in part by two previous studies [79, 90]. One study, although not including ALCs, established the existence of different Leydig progenitor cells that contributed to FLCs [90]. The second study revealed expression marker differences in the progenitors of ALCs and FLCs that suggested they are regulated by distinct signaling pathways [79]. A second interpretation of the present findings is that there is a shared common progenitor pool for ALCs and FLCs that was exhausted in the Nr5a1−/−;tg+/0 mice, after forming the FLC population. Further evaluation is required to distinguish the two possibilities.
ACKNOWLEDGMENT
We thank the staff of Laboratory Animal Resources of the University of Kansas Medical Center for assistance in animal husbandry, and we are grateful to Jing Huang in the Histology Core of Kansas Intellectual and Developmental Disabilities Research Center for technical assistance with tissue processing. We also thank Drs. K. Morohashi and M. Soares for providing the antibodies.
Footnotes
Supported by U.S. National Institutes of Health grants U54 HD033994 and R01 DK075807. This work was presented in part at the Second Annual Gilbert S. Greenwald Symposium on Reproduction, Kansas City, MO, September 23-25, 2005, the XVIII Annual North American Testis Workshop, Seattle, WA, March 30-April 2, 2005, and the Specialized Cooperative Centers Program in Reproduction and Infertility Research (U54) Meeting, Chicago, IL, April, 2005.
REFERENCES
- Ingraham HA, Lala DS, Ikeda Y, Luo X, Shen WH, Nachtigal MW, Abbud R, Nilson JH, Parker KL. The nuclear receptor steroidogenic factor 1 acts at multiple levels of the reproductive axis. Genes Dev. 1994;8:2302–2312. doi: 10.1101/gad.8.19.2302. [DOI] [PubMed] [Google Scholar]
- Morohashi KI, Omura T. Ad4BP/SF-1, a transcription factor essential for the transcription of steroidogenic cytochrome P450 genes and for the establishment of the reproductive function. FASEB J. 1996;10:1569–1577. doi: 10.1096/fasebj.10.14.9002548. [DOI] [PubMed] [Google Scholar]
- Parker KL, Rice DA, Lala DS, Ikeda Y, Luo X, Wong M, Bakke M, Zhao L, Frigeri C, Hanley NA, Stallings N, Schimmer BP. Steroidogenic factor 1: an essential mediator of endocrine development. Recent Prog Horm Res. 2002;57:19–36. doi: 10.1210/rp.57.1.19. [DOI] [PubMed] [Google Scholar]
- Val P, Lefrancois-Martinez AM, Veyssiere G, Martinez A. SF-1 a key player in the development and differentiation of steroidogenic tissues. Nucl Recept. 2003;1:8. doi: 10.1186/1478-1336-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashamboo A, Ferraz-de-Souza B, Lourenco D, Lin L, Sebire NJ, Montjean D, Bignon-Topalovic J, Mandelbaum J, Siffroi JP, Christin-Maitre S, Radhakrishna U, Rouba H, et al. Human male infertility associated with mutations in NR5A1 encoding steroidogenic factor 1. Am J Hum Genet. 2010;87:505–512. doi: 10.1016/j.ajhg.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camats N, Pandey AV, Fernandez-Cancio M, Andaluz P, Janner M, Toran N, Moreno F, Bereket A, Akcay T, Garcia-Garcia E, Munoz MT, Gracia R, et al. Ten novel mutations in the NR5A1 gene cause disordered sex development in 46,XY and ovarian insufficiency in 46,XX individuals. J Clin Endocrinol Metab. 2012;97:E1294–1306. doi: 10.1210/jc.2011-3169. [DOI] [PubMed] [Google Scholar]
- El-Khairi R, Achermann JC. Steroidogenic factor-1 and human disease. Semin Reprod Med. 2012;30:374–381. doi: 10.1055/s-0032-1324720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Khairi R, Martinez-Aguayo A, Ferraz-de-Souza B, Lin L, Achermann JC. Role of DAX-1 (NR0B1) and steroidogenic factor-1 (NR5A1) in human adrenal function. Endocr Dev. 2011;20:38–46. doi: 10.1159/000321213. [DOI] [PubMed] [Google Scholar]
- Kojima Y, Sasaki S, Hayashi Y, Umemoto Y, Morohashi K, Kohri K. Role of transcription factors Ad4bp/SF-1 and DAX-1 in steroidogenesis and spermatogenesis in human testicular development and idiopathic azoospermia. Int J Urol. 2006;13:785–793. doi: 10.1111/j.1442-2042.2006.01403.x. [DOI] [PubMed] [Google Scholar]
- Ozisik G, Achermann JC, Jameson JL. The role of SF1 in adrenal and reproductive function: insight from naturally occurring mutations in humans. Mol Genet Metab. 2002;76:85–91. doi: 10.1016/s1096-7192(02)00032-x. [DOI] [PubMed] [Google Scholar]
- Schimmer BP, White PC. Minireview: steroidogenic factor 1: its roles in differentiation, development, and disease. Mol Endocrinol. 2010;24:1322–1337. doi: 10.1210/me.2009-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatano O, Takayama K, Imai T, Waterman MR, Takakusu A, Omura T, Morohashi K. Sex-dependent expression of a transcription factor, Ad4BP, regulating steroidogenic P-450 genes in the gonads during prenatal and postnatal rat development. Development. 1994;120:2787–2797. doi: 10.1242/dev.120.10.2787. [DOI] [PubMed] [Google Scholar]
- Luo X, Ikeda Y, Parker KL. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994;77:481–490. doi: 10.1016/0092-8674(94)90211-9. [DOI] [PubMed] [Google Scholar]
- Luo X, Ikeda Y, Schlosser DA, Parker KL. Steroidogenic factor 1 is the essential transcript of the mouse Ftz-F1 gene. Mol Endocrinol. 1995;9:1233–1239. doi: 10.1210/mend.9.9.7491115. [DOI] [PubMed] [Google Scholar]
- Sadovsky Y, Crawford PA, Woodson KG, Polish JA, Clements MA, Tourtellotte LM, Simburger K, Milbrandt J. Mice deficient in the orphan receptor steroidogenic factor 1 lack adrenal glands and gonads but express P450 side-chain-cleavage enzyme in the placenta and have normal embryonic serum levels of corticosteroids. Proc Natl Acad Sci U S A. 1995;92:10939–10943. doi: 10.1073/pnas.92.24.10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KL, Schimmer BP. Steroidogenic factor 1: a key determinant of endocrine development and function. Endocr Rev. 1997;18:361–377. doi: 10.1210/edrv.18.3.0301. [DOI] [PubMed] [Google Scholar]
- Sekido R, Lovell-Badge R. Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature. 2008;453:930–934. doi: 10.1038/nature06944. [DOI] [PubMed] [Google Scholar]
- Wilhelm D, Englert C. The Wilms tumor suppressor WT1 regulates early gonad development by activation of Sf1. Genes Dev. 2002;16:1839–1851. doi: 10.1101/gad.220102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland ML, Jamieson CA, Akana SF, Bornstein SR, Eisenhofer G, Dallman MF, Ingraham HA. Haploinsufficiency of steroidogenic factor-1 in mice disrupts adrenal development leading to an impaired stress response. Proc Natl Acad Sci U S A. 2000;97:14488–14493. doi: 10.1073/pnas.97.26.14488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y, Luo X, Abbud R, Nilson JH, Parker KL. The nuclear receptor steroidogenic factor 1 is essential for the formation of the ventromedial hypothalamic nucleus. Mol Endocrinol. 1995;9:478–486. doi: 10.1210/mend.9.4.7659091. [DOI] [PubMed] [Google Scholar]
- Jeyasuria P, Ikeda Y, Jamin SP, Zhao L, De Rooij DG, Themmen AP, Behringer RR, Parker KL. Cell-specific knockout of steroidogenic factor 1 reveals its essential roles in gonadal function. Mol Endocrinol. 2004;18:1610–1619. doi: 10.1210/me.2003-0404. [DOI] [PubMed] [Google Scholar]
- Majdic G, Young M, Gomez-Sanchez E, Anderson P, Szczepaniak LS, Dobbins RL, McGarry JD, Parker KL. Knockout mice lacking steroidogenic factor 1 are a novel genetic model of hypothalamic obesity. Endocrinology. 2002;143:607–614. doi: 10.1210/endo.143.2.8652. [DOI] [PubMed] [Google Scholar]
- Park SY, Tong M, Jameson JL. Distinct roles for steroidogenic factor 1 and desert hedgehog pathways in fetal and adult Leydig cell development. Endocrinology. 2007;148:3704–3710. doi: 10.1210/en.2006-1731. [DOI] [PubMed] [Google Scholar]
- Zhao L, Bakke M, Hanley NA, Majdic G, Stallings NR, Jeyasuria P, Parker KL. Tissue-specific knockouts of steroidogenic factor 1. Mol Cell Endocrinol. 2004;215:89–94. doi: 10.1016/j.mce.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Zhao L, Bakke M, Krimkevich Y, Cushman LJ, Parlow AF, Camper SA, Parker KL. Hypomorphic phenotype in mice with pituitary-specific knockout of steroidogenic factor 1. Genesis. 2001;30:65–69. doi: 10.1002/gene.1034. [DOI] [PubMed] [Google Scholar]
- Brinkmann AO. Molecular mechanisms of androgen action–a historical perspective. Methods Mol Biol. 2011;776:3–24. doi: 10.1007/978-1-61779-243-4_1. [DOI] [PubMed] [Google Scholar]
- Forest MG. Role of androgens in fetal and pubertal development. Horm Res. 1983;18:69–83. doi: 10.1159/000179780. [DOI] [PubMed] [Google Scholar]
- Svechnikov K, Landreh L, Weisser J, Izzo G, Colon E, Svechnikova I, Soder O. Origin, development and regulation of human Leydig cells. Horm Res Paediatr. 2010;73:93–101. doi: 10.1159/000277141. [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy PJ, Baker PJ, Johnston H. The foetal Leydig cell– differentiation, function and regulation Int J Androl 2006. 29 90 95; discussion 105–108. [DOI] [PubMed] [Google Scholar]
- Haider SG. Cell biology of Leydig cells in the testis. Int Rev Cytol. 2004;233:181–241. doi: 10.1016/S0074-7696(04)33005-6. [DOI] [PubMed] [Google Scholar]
- Habert R, Lejeune H, Saez JM. Origin, differentiation and regulation of fetal and adult Leydig cells. Mol Cell Endocrinol. 2001;179:47–74. doi: 10.1016/s0303-7207(01)00461-0. [DOI] [PubMed] [Google Scholar]
- Huhtaniemi I, Pelliniemi LJ. Fetal Leydig cells: cellular origin, morphology, life span, and special functional features. Proc Soc Exp Biol Med. 1992;201:125–140. doi: 10.3181/00379727-201-43493. [DOI] [PubMed] [Google Scholar]
- Griswold SL, Behringer RR. Fetal Leydig cell origin and development. Sex Dev. 2009;3:1–15. doi: 10.1159/000200077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendis-Handagama SM, Ariyaratne HB. Differentiation of the adult Leydig cell population in the postnatal testis. Biol Reprod. 2001;65:660–671. doi: 10.1095/biolreprod65.3.660. [DOI] [PubMed] [Google Scholar]
- Kerr JB, Knell CM. The fate of fetal Leydig cells during the development of the fetal and postnatal rat testis. Development. 1988;103:535–544. doi: 10.1242/dev.103.3.535. [DOI] [PubMed] [Google Scholar]
- Russell LD, de Franca LR, Hess R, Cooke P. Characteristics of mitotic cells in developing and adult testes with observations on cell lineages. Tissue Cell. 1995;27:105–128. doi: 10.1016/s0040-8166(95)80015-8. [DOI] [PubMed] [Google Scholar]
- Siril Ariyaratne HB, Ian Mason J, Mendis-Handagama SM. Effects of thyroid and luteinizing hormones on the onset of precursor cell differentiation into leydig progenitor cells in the prepubertal rat testis. Biol Reprod. 2000;63:898–904. doi: 10.1095/biolreprod63.3.898. [DOI] [PubMed] [Google Scholar]
- Baker PJ, O'Shaughnessy PJ. Role of gonadotrophins in regulating numbers of Leydig and Sertoli cells during fetal and postnatal development in mice. Reproduction. 2001;122:227–234. doi: 10.1530/rep.0.1220227. [DOI] [PubMed] [Google Scholar]
- Haider SG, Laue D, Schwochau G, Hilscher B. Morphological studies on the origin of adult-type Leydig cells in rat testis Ital J Anat Embryol 1995. 100 (suppl 1): 535 541 [PubMed] [Google Scholar]
- Hardy MP, Kelce WR, Klinefelter GR, Ewing LL. Differentiation of Leydig cell precursors in vitro: a role for androgen. Endocrinology. 1990;127:488–490. doi: 10.1210/endo-127-1-488. [DOI] [PubMed] [Google Scholar]
- Lording DW, De Kretser DM. Interstitial cells in the developing rat testis. J Anat. 1970;106:191. [PubMed] [Google Scholar]
- Lording DW, De Kretser DM. Comparative ultrastructural and histochemical studies of the interstitial cells of the rat testis during fetal and postnatal development. J Reprod Fertil. 1972;29:261–269. doi: 10.1530/jrf.0.0290261. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Shen WH, Ingraham HA, Parker KL. Developmental expression of mouse steroidogenic factor-1, an essential regulator of the steroid hydroxylases. Mol Endocrinol. 1994;8:654–662. doi: 10.1210/mend.8.5.8058073. [DOI] [PubMed] [Google Scholar]
- Karpova T, Maran RR, Presley J, Scherrer SP, Tejada L, Heckert LL. Transgenic rescue of SF-1-null mice. Ann N Y Acad Sci. 2005;1061:55–64. doi: 10.1196/annals.1336.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpova T, Presley J, Manimaran RR, Scherrer SP, Tejada L, Peterson KR, Heckert LLA. FTZ-F1-containing yeast artificial chromosome recapitulates expression of steroidogenic factor 1 in vivo. Mol Endocrinol. 2005;19:2549–2563. doi: 10.1210/me.2004-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JK, Heckert LL. Dmrt1 expression is regulated by follicle-stimulating hormone and phorbol esters in postnatal Sertoli cells. Endocrinology. 2001;142:1167–1178. doi: 10.1210/endo.142.3.8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckert LL, Sawadogo M, Daggett MA, Chen JK. The USF proteins regulate transcription of the follicle-stimulating hormone receptor but are insufficient for cell-specific expression. Mol Endocrinol. 2000;14:1836–1848. doi: 10.1210/mend.14.11.0557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei N, Hornbaker KI, Rice DA, Karpova T, Agbor VA, Heckert LL. Sex-specific differences in mouse DMRT1 expression are both cell type- and stage-dependent during gonad development. Biol Reprod. 2007;77:466–475. doi: 10.1095/biolreprod.106.058784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreiro ML, Gaytan F, Caminos JE, Pinilla L, Casanueva FF, Aguilar E, Dieguez C, Tena-Sempere M. Cellular location and hormonal regulation of ghrelin expression in rat testis. Biol Reprod. 2002;67:1768–1776. doi: 10.1095/biolreprod.102.006965. [DOI] [PubMed] [Google Scholar]
- Tena-Sempere M, Barreiro ML, Gonzalez LC, Gaytan F, Zhang FP, Caminos JE, Pinilla L, Casanueva FF, Dieguez C, Aguilar E. Novel expression and functional role of ghrelin in rat testis. Endocrinology. 2002;143:717–725. doi: 10.1210/endo.143.2.8646. [DOI] [PubMed] [Google Scholar]
- Josso N. Anti-mullerian hormone and Sertoli cell function Horm Res 1992. 38 (suppl 2): 72 76 [DOI] [PubMed] [Google Scholar]
- Josso N, Vigier B, Tran D, Picard JY. Initiation of production of anti-mullerian hormone by the fetal gonad. Arch Anat Microsc Morphol Exp. 1985;74:96–100. [PubMed] [Google Scholar]
- Tran D, Muesy-Dessole N, Josso N. Anti-mullerian hormone is a functional marker of foetal Sertoli cells. Nature. 1977;269:411–412. doi: 10.1038/269411a0. [DOI] [PubMed] [Google Scholar]
- Tran D, Picard JY, Campargue J, Josso N. Immunocytochemical detection of anti-mullerian hormone in Sertoli cells of various mammalian species including human. J Histochem Cytochem. 1987;35:733–743. doi: 10.1177/35.7.3295030. [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy PJ, Johnston H, Willerton L, Baker PJ. Failure of normal adult Leydig cell development in androgen-receptor-deficient mice. J Cell Sci. 2002;115:3491–3496. doi: 10.1242/jcs.115.17.3491. [DOI] [PubMed] [Google Scholar]
- Siril Ariyaratne HB Chamindrani Mendis-Handagama S. Buchanan Hales D. Ian Mason J . Studies on the onset of Leydig precursor cell differentiation in the prepubertal rat testis. Biol Reprod. 2000;63:165–171. doi: 10.1095/biolreprod63.1.165. , , , [DOI] [PubMed] [Google Scholar]
- Zhao L, Bakke M, Parker KL. Pituitary-specific knockout of steroidogenic factor 1. Mol Cell Endocrinol. 2001;185:27–32. doi: 10.1016/s0303-7207(01)00621-9. [DOI] [PubMed] [Google Scholar]
- Baker PJ, Sha JA, McBride MW, Peng L, Payne AH, O'Shaughnessy PJ. Expression of 3beta-hydroxysteroid dehydrogenase type I and type VI isoforms in the mouse testis during development. Eur J Biochem. 1999;260:911–917. doi: 10.1046/j.1432-1327.1999.00245.x. [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy PJ, Willerton L, Baker PJ. Changes in Leydig cell gene expression during development in the mouse. Biol Reprod. 2002;66:966–975. doi: 10.1095/biolreprod66.4.966. [DOI] [PubMed] [Google Scholar]
- Ahtiainen P, Rulli SB, Shariatmadari R, Pelliniemi LJ, Toppari J, Poutanen M, Huhtaniemi IT. Fetal but not adult Leydig cells are susceptible to adenoma formation in response to persistently high hCG level: a study on hCG overexpressing transgenic mice. Oncogene. 2005;24:7301–7309. doi: 10.1038/sj.onc.1208893. [DOI] [PubMed] [Google Scholar]
- Zhang FP, Pakarainen T, Poutanen M, Toppari J, Huhtaniemi I. The low gonadotropin-independent constitutive production of testicular testosterone is sufficient to maintain spermatogenesis. Proc Natl Acad Sci U S A. 2003;100:13692–13697. doi: 10.1073/pnas.2232815100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang FP, Pakarainen T, Zhu F, Poutanen M, Huhtaniemi I. Molecular characterization of postnatal development of testicular steroidogenesis in luteinizing hormone receptor knockout mice. Endocrinology. 2004;145:1453–1463. doi: 10.1210/en.2003-1049. [DOI] [PubMed] [Google Scholar]
- Ge RS, Dong Q, Sottas CM, Papadopoulos V, Zirkin BR, Hardy MP. In search of rat stem Leydig cells: identification, isolation, and lineage-specific development. Proc Natl Acad Sci U S A. 2006;103:2719–2724. doi: 10.1073/pnas.0507692103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley E, Lin CY, Jin S, Liu J, Sottas CM, Ge R, Zirkin BR, Identification Chen H. proliferation, and differentiation of adult Leydig stem cells. Endocrinology. 2012;153:5002–5010. doi: 10.1210/en.2012-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AM, Garland KK, Russell LD. Desert hedgehog (Dhh) gene is required in the mouse testis for formation of adult-type Leydig cells and normal development of peritubular cells and seminiferous tubules. Biol Reprod. 2000;63:1825–1838. doi: 10.1095/biolreprod63.6.1825. [DOI] [PubMed] [Google Scholar]
- Pierucci-Alves F, Clark AM, Russell LD. A developmental study of the Desert hedgehog-null mouse testis. Biol Reprod. 2001;65:1392–1402. doi: 10.1095/biolreprod65.5.1392. [DOI] [PubMed] [Google Scholar]
- De Rivoyre M, Ruel L, Varjosalo M, Loubat A, Bidet M, Therond P, Mus-Veteau I. Human receptors patched and smoothened partially transduce hedgehog signal when expressed in Drosophila cells. J Biol Chem. 2006;281:28584–28595. doi: 10.1074/jbc.M512986200. [DOI] [PubMed] [Google Scholar]
- Stanley EL, Johnston DS, Fan J, Papadopoulos V, Chen H, Ge RS, Zirkin BR, Jelinsky SA. Stem leydig cell differentiation: gene expression during development of the adult rat population of leydig cells. Biol Reprod. 2011;85:1161–1166. doi: 10.1095/biolreprod.111.091850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford PA, Sadovsky Y, Milbrandt J. Nuclear receptor steroidogenic factor 1 directs embryonic stem cells toward the steroidogenic lineage. Mol Cell Biol. 1997;17:3997–4006. doi: 10.1128/mcb.17.7.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan J, Capel B. One tissue, two fates: molecular genetic events that underlie testis versus ovary development. Nat Rev Genet. 2004;5:509–521. doi: 10.1038/nrg1381. [DOI] [PubMed] [Google Scholar]
- Brennan J, Tilmann C, Capel B. Pdgfr-alpha mediates testis cord organization and fetal Leydig cell development in the XY gonad. Genes Dev. 2003;17:800–810. doi: 10.1101/gad.1052503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y, Takeda Y, Shikayama T, Mukai T, Hisano S, Morohashi KI. Comparative localization of Dax-1 and Ad4BP/SF-1 during development of the hypothalamic-pituitary-gonadal axis suggests their closely related and distinct functions. Dev Dyn. 2001;220:363–376. doi: 10.1002/dvdy.1116. [DOI] [PubMed] [Google Scholar]
- Miyabayashi K, Katoh-Fukui Y, Ogawa H, Baba T, Shima Y, Sugiyama N, Kitamura K, Morohashi K. Aristaless related homeobox gene, Arx, is implicated in mouse fetal Leydig cell differentiation possibly through expressing in the progenitor cells. PLoS One. 2013;8:e68050. doi: 10.1371/journal.pone.0068050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahl J, Eicher EM, Washburn LL, Capel B. Sry induces cell proliferation in the mouse gonad. Development. 2000;127:65–73. doi: 10.1242/dev.127.1.65. [DOI] [PubMed] [Google Scholar]
- Yao HH, Whoriskey W, Capel B. Desert Hedgehog/Patched 1 signaling specifies fetal Leydig cell fate in testis organogenesis. Genes Dev. 2002;16:1433–1440. doi: 10.1101/gad.981202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Meeks JJ, Raverot G, Pfaff LE, Weiss J, Hammer GD, Jameson JL. Nuclear receptors Sf1 and Dax1 function cooperatively to mediate somatic cell differentiation during testis development. Development. 2005;132:2415–2423. doi: 10.1242/dev.01826. [DOI] [PubMed] [Google Scholar]
- Bitgood MJ, Shen L, McMahon AP. Sertoli cell signaling by Desert hedgehog regulates the male germline. Curr Biol. 1996;6:298–304. doi: 10.1016/s0960-9822(02)00480-3. [DOI] [PubMed] [Google Scholar]
- Wu X, Wan S, Lee MM. Key factors in the regulation of fetal and postnatal Leydig cell development. J Cell Physiol. 2007;213:429–433. doi: 10.1002/jcp.21231. [DOI] [PubMed] [Google Scholar]
- Defalco T, Saraswathula A, Briot A, Iruela-Arispe ML, Capel B. Testosterone levels influence mouse fetal Leydig cell progenitors through notch signaling. Biol Reprod. 2013;88:91. doi: 10.1095/biolreprod.112.106138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazawa T, Mizutani T, Yamada K, Kawata H, Sekiguchi T, Yoshino M, Kajitani T, Shou Z, Umezawa A, Miyamoto K. Differentiation of adult stem cells derived from bone marrow stroma into Leydig or adrenocortical cells. Endocrinology. 2006;147:4104–4111. doi: 10.1210/en.2006-0162. [DOI] [PubMed] [Google Scholar]
- Yazawa T, Uesaka M, Inaoka Y, Mizutani T, Sekiguchi T, Kajitani T, Kitano T, Umezawa A, Miyamoto K. Cyp11b1 is induced in the murine gonad by luteinizing hormone/human chorionic gonadotropin and involved in the production of 11-ketotestosterone, a major fish androgen: conservation and evolution of the androgen metabolic pathway. Endocrinology. 2008;149:1786–1792. doi: 10.1210/en.2007-1015. [DOI] [PubMed] [Google Scholar]
- Yazawa T, Kawabe S, Inaoka Y, Okada R, Mizutani T, Imamichi Y, Ju Y, Yamazaki Y, Usami Y, Kuribayashi M, Umezawa A, Miyamoto K. Differentiation of mesenchymal stem cells and embryonic stem cells into steroidogenic cells using steroidogenic factor-1 and liver receptor homolog-1. Mol Cell Endocrinol. 2011;336:127–132. doi: 10.1016/j.mce.2010.11.025. [DOI] [PubMed] [Google Scholar]
- Kohler B, Achermann JC. Update–steroidogenic factor 1 (SF-1, NR5A1) Minerva Endocrinol. 2010;35:73–86. [PubMed] [Google Scholar]
- Kohler B, Lin L, Mazen I, Cetindag C, Biebermann H, Akkurt I, Rossi R, Hiort O, Gruters A, Achermann JC. The spectrum of phenotypes associated with mutations in steroidogenic factor 1 (SF-1, NR5A1, Ad4BP) includes severe penoscrotal hypospadias in 46,XY males without adrenal insufficiency. Eur J Endocrinol. 2009;161:237–242. doi: 10.1530/EJE-09-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantawy S, Mazen I, Soliman H, Anwar G, Atef A, El-Gammal M, El-Kotoury A, Mekkawy M, Torky A, Rudolf A, Schrumpf P, Gruters A, et al. Analysis of the gene coding for steroidogenic factor 1 (SF1, NR5A1) in a cohort of 50 Egyptian patients with 46,XY disorders of sex development. Eur J Endocrinol. 2014;170:759–767. doi: 10.1530/EJE-13-0965. [DOI] [PubMed] [Google Scholar]
- Nishina-Uchida N, Fukuzawa R, Numakura C, Suwanai AS, Hasegawa T, Hasegawa Y. Characteristic testicular histology is useful for the identification of NR5A1 gene mutations in prepubertal 46, XY patients. Horm Res Paediatr. 2013:119–128. doi: 10.1159/000353763. [DOI] [PubMed] [Google Scholar]
- Lin L, Achermann JC. Steroidogenic factor-1 (SF-1, Ad4BP, NR5A1) and disorders of testis development. Sex Dev. 2008;2:200–209. doi: 10.1159/000152036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Philibert P, Ferraz-de-Souza B, Kelberman D, Homfray T, Albanese A, Molini V, Sebire NJ, Einaudi S, Conway GS, Hughes IA, Jameson JL, et al. Heterozygous missense mutations in steroidogenic factor 1 (SF1/Ad4BP, NR5A1) are associated with 46,XY disorders of sex development with normal adrenal function. J Clin Endocrinol Metab. 2007;92:991–999. doi: 10.1210/jc.2006-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools M, Hoebeke P, Wolffenbuttel KP, Stoop H, Hersmus R, Barbaro M, Wedell A, Bruggenwirth H, Looijenga LH, Drop SL. Pubertal androgenization and gonadal histology in two 46,XY adolescents with NR5A1 mutations and predominantly female phenotype at birth. Eur J Endocrinol. 2012;166:341–349. doi: 10.1530/EJE-11-0392. [DOI] [PubMed] [Google Scholar]
- DeFalco T, Takahashi S, Capel B. Two distinct origins for Leydig cell progenitors in the fetal testis. Dev Biol. 2011;352:14–26. doi: 10.1016/j.ydbio.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]