Abstract
Aging is associated with performance reductions in executive function and episodic memory, although there is substantial individual variability in cognition among older adults. One factor that may be positively associated with cognition in aging is physical activity. To date, few studies have objectively assessed physical activity in young and older adults, and examined whether physical activity is differentially associated with cognition in aging. Young (n = 29, age 18–31 years) and older adults (n = 31, ages 55–82 years) completed standardized neuropsychological testing to assess executive function and episodic memory capacities. An experimental face-name relational memory task was administered to augment assessment of episodic memory. Physical activity (total step count and step rate) was objectively assessed using an accelerometer, and hierarchical regressions were used to evaluate relationships between cognition and physical activity. Older adults performed more poorly on tasks of executive function and episodic memory. Physical activity was positively associated with a composite measure of visual episodic memory and face-name memory accuracy in older adults. Physical activity associations with cognition were independent of sedentary behavior, which was negatively correlated with memory performance. Physical activity was not associated with cognitive performance in younger adults. Physical activity is positively associated with episodic memory performance in aging. The relationship appears to be strongest for face-name relational memory and visual episodic memory, likely attributable to the fact that these tasks make strong demands on the hippocampus. The results suggest that physical activity relates to cognition in older, but not younger adults.
Keywords: Executive function, Cognition, Exercise, Physical fitness, Actigraph, Accelerometer
INTRODUCTION
Aging has been associated with decreased executive function, including inhibition, task switching, maintenance, and manipulation of information in one’s mind (Goh, An, & Resnick, 2012), as well declines in episodic memory, that is, memory for events (Naveh-Benjamin, 2000). Yet, there is significant individual variability in cognitive performance among older adults, including evidence for preservation in domains typically affected by aging (Shimamura, Berry, Mangels, Rusting, & Jurica, 1995).
Physical activity is one individual difference factor that may positively impact executive function and episodic memory in older adults. Compelling evidence for the contribution of physical activity to cognitive function comes from exercise intervention studies. Exercise represents a distinct sub-category of physical activity that is “planned, structured, repetitive and purposive in the sense that improvement or maintenance of one or more components of physical fitness is an objective” (Caspersen, Powell, & Christenson, 1985, p. 128), whereas physical activity more broadly refers to “any bodily movement produced by skeletal muscle that results in energy expenditure” (Caspersen et al., 1985, p. 126), including leisure (e.g., watching TV), school/occupational (e.g., pacing while giving a lecture), household/domestic/self-care (e.g., rocking a baby, mowing the lawn), or transportation (e.g., driving a car) activities. A landmark meta-analysis of exercise studies in older adults reported that executive control processes benefit most from exercise-training intervention (Colcombe & Kramer, 2003), and a more recent meta-analysis of exercise intervention studies has highlighted the beneficial effects of aerobic exercise on episodic memory (Smith et al., 2010).
Unfortunately, over 50% of Americans do not meet the current recommended guidelines for regular aerobic physical activity (Haskell et al., 2007; Pescatello & American College of Sports Medicine, 2014): 150 min per week of moderate-intensity aerobic activity (e.g., brisk walking) or 75 min per week of vigorous aerobic activity (e.g., jogging or running), which is roughly equivalent to the aerobic exercise training prescribed in intervention studies. Furthermore, exercise intervention studies typically do not measure physical activity beyond those associated with the intervention. For instance, it is unknown whether those assigned to a walking intervention also increase physical activity in other aspects of their life (e.g., choosing to take the stairs rather than the elevator while at work). Thus, a critical question that arises is whether daily forms of physical activity, which could include exercise as well as other types of occupational and domestic physical activity performed throughout one’s day, are positively associated with cognitive performance.
Although previous work has examined the relationship between physical activity and cognitive function in older adults (see meta-analytic review by Sofi et al., 2011), our current knowledge of the relationship between daily physical activity and cognitive function in older adults is limited due to the majority of extant research relying on self-report measures of physical activity. Self-report of physical activity has been criticized because it may be inaccurate, as it is subject to omissions and bias, as well as misunderstanding questions (Prince et al., 2008; Rzewnicki, Vanden Auweele, & De Bourdeaudhuij, 2003; Shepard, 2003). Moreover, self-reported physical activity in older adults may be additionally compromised by age-related memory decline that could compound the unreliability of historical accounts.
Evolving technologies such as accelerometers can be worn on one’s hip or wrist to measure the direction and intensity of bodily movements as well as the number of steps taken (similar to a pedometer). These wearable devices provide objective measures of physical activity independent of self-report (Freedson, Bowles, Troiano, & Haskell, 2012; Freedson, Melanson, & Sirard, 1998). Objective measures of physical activity are more precise, eliminate response biases, and allow for quantification of daily movement that includes planned and purposeful exercise-related physical activity, as well as movement associated with leisure (e.g., shopping), occupational (e.g., working in a store), domestic (e.g., cleaning), and transportation (e.g., walking to the bus stop) activities.
To date, accelerometer-based measures of physical activity have been used in a limited number of studies of cognition in healthy older adults. Some have suggested that physical activity is associated with a global measure of cognition (Buchman, Wilson, & Bennett, 2008) and may protect against age-related cognitive decline (Buchman et al., 2012). Other studies assessing specific cognitive domains have shown a link between physical activity and executive function (Barnes et al., 2008; Brown et al., 2012; Wilbur et al., 2012), but a similar link between episodic memory and physical activity has been more elusive (Brown et al., 2012; Wilbur et al., 2012). This is surprising, given that the animal literature has consistently shown an association between wheel-running and performance on hippocampally-mediated, episodic-like, memory tasks (Cotman, Berchtold, & Christie, 2007; Nichol, Deeny, Seif, Camaclang, & Cotman, 2009; van Praag, Shubert, Zhao, & Gage, 2005). One reason for the discrepancy could be that episodic memory tasks used in human studies were not optimally sensitive to hippocampal function.
It is also noteworthy that few studies to date have examined the relationship between cognition and objective measures of physical activity in both younger and older adults, with a recent review highlighting the paucity of studies in younger adults (Prakash, Voss, Erickson, & Kramer, 2015). Thus, it is unknown whether objective measures of physical activity are associated with cognition in younger adults. Therefore, it is possible that a positive association between physical activity and cognition is present, regardless of age. Alternatively, physical activity may be differentially associated with cognition in aging. For instance, physical activity may have a weaker relationship with cognition in younger adults relative to older adults because younger adults are at their lifetime peak in terms of neural integrity, and, therefore, physical activity may have a more limited influence on cognition.
The current study examined the relationship between objectively measured physical activity and executive function and episodic memory in younger adults and older adults. Physical activity was assessed using an accelerometer, and step count was chosen as the primary independent variable of interest given that it represents a global objective indicator of physical activity and can be derived from a multitude of devices (cell phones, wearable fitness technologies) used to monitor physical activity. Physical activity intensity [step rate (i.e., how briskly one is walking)] and time spent sedentary were examined in follow-up analyses to provide additional perspective on the relationship between objective measures of physical activity and cognition. Executive function and episodic memory were assessed using standardized neuropsychological tests. To further evaluate episodic memory, an experimental relational memory task in which participants were asked to learn face-name associations was also administered. The task is known to rely on the integrity of the hippocampus (Giovanello, Verfaellie, & Keane, 2003; Hayes, Buchler, Stokes, Kragel, & Cabeza, 2011; Sperling et al., 2003), and, therefore, may more closely approximate hippocampally-mediated memory tasks implemented in animals. Furthermore, relational memory tasks elicit larger age-related memory deficits relative to memory for single items (e.g., remembering a name alone or face alone; (Old & Naveh-Benjamin, 2008), and, therefore, may be more sensitive to the effects of physical activity. We hypothesized that greater physical activity would be associated with better performance on measures of executive function and episodic memory in older adults, and, furthermore, that we would observe stronger associations between physical activity and cognition in older adults relative to younger adults.
METHODS
Participants
Thirty-four younger adults and 35 older adults were enrolled in the study. Data from five younger adults (four with invalid accelerometer wear time, one a statistical outlier in terms of physical fitness levels) and four older adults [with incidental Magnetic Resonance Imaging (MRI) findings; data not reported here] were excluded from analysis. The final sample consisted of 29 younger adults (age = 18 to 31 years) and 31 older adults (age = 55 to 82 years). Five older adults self-reported hypertension. Participant characteristics of the analysis sample are presented in Table 1. There were no significant differences between younger adults and older adults in terms of gender, χ2(1, N = 60) < 1, p = ns or depression, t(58) = 1.47, p = .15. Older adults had greater years of education, t(58) = 2.83, p = .01, as the majority of the younger adults sample consisted of college students in the process of earning their bachelor degree. Lower global cognitive status, as assessed by the Montreal Cognitive Assessment (MoCA; http://www.mocatest.org/), was observed in older adults relative to younger adults, t(58) = 2.02, p = .05. However, the cognitive status of both samples was within normal limits, suggesting this difference is not likely meaningful clinically. Although older adults wore the ActiGraph for more minutes than younger adults, t(58) = 2.32, p < .05, they took fewer steps than younger adults, but this difference did not reach statistical significance t(58) = 1.58, p = .12. A subset of participants, 27 younger adults and 23 older adults, also completed a face-name memory task. Older adults in this subset again exhibited more years of education, but there were no differences in gender, Center for Epidemiologic Studies Depression Scale (CES-D) scores, or MoCA scores.
Table 1.
Sample characteristics (mean and standard deviation)
| Younger adults | Older adults | |
|---|---|---|
| N | 29 | 31 |
| Age, years | 21.2 (3.2) | 64.5 (7.0) |
| Gender, n (%) female | 17 (56.4) | 18 (58.1) |
| Body mass indexa | 22.8 (2.9) | 25.6 (4.5) |
| Education, yearsa | 14.6 (1.8) | 16.2 (2.6) |
| CES-D score | 6.6 (4.1) | 5.0 (4.1) |
| MoCA scoreb | 28.5 (1.6) | 27.7 (1.8) |
| Total wear time (minutes)a | 4609 (1316) | 5532 (1721) |
| Total step count | 72,414 (31,781) | 58,800 (34,908) |
| Step counts per dayb | 12,736 (4524) | 9366 (5934) |
Older adults > younger adults.
Older adults < younger adults.
CES-D = Center for Epidemiologic Studies Depression Scale; MoCA = Montreal Cognitive Assessment.
Participants were recruited from established participant pools (Boston University for younger adults and the Boston University Memory Disorders Research Center at VA Boston, Boston University Alzheimer’s Disease Center, the Massachusetts Alzheimer’s Disease Research Center, and the Alzheimer’s Association TrialMatch). Recruitment flyers were posted at community track meets, YMCAs, and libraries, although no younger adults volunteered from these particular sources. Candidates completed a telephone-based comprehensive health questionnaire consisting of approximately 150 questions to screen for major medical, neurological, psychiatric or substance abuse issues that might affect cognition. Examples of exclusion criteria include: myocardial infarction, ischemic stroke, hemorrhagic stroke, transient ischemic attack, seizure disorders, head injury with loss of consciousness > 5 min, Alzheimer’s disease, Parkinson’s disease or any other neurodegenerative disorder, schizophrenia, bipolar disorder, or other psychiatric disorder. Additional exclusion criteria included education less than grade 12, and contraindications to cardiopulmonary exercise testing or MRI. Participants were screened for depression using a cut-off score of 16 on the CES-D (20-item version). Participants with MoCA scores ≤ 23 were excluded. Mobility was not formally assessed, although no participants used assistive walking devices during their visit to the lab, which required a roughly quarter mile walk from the parking garage (or nearest public transportation). Overall, the study sample was likely healthier and more educated than the general population.
Cardiorespiratory fitness data (peak VO2) and cognitive data from the sample are reported elsewhere (Hayes, Forman, & Verfaellie, 2014), as are diffusion tensor imaging data (Hayes, Salat, Forman, Sperling, & Verfaellie, 2015). All participants gave written informed consent and received financial compensation. The VA Boston Healthcare System institutional review board approved all experimental procedures.
Accelerometry
The ActiGraph GT3X-plus tri-axial accelerometer (Actigraph, Pensacola, FL) was used to assess physical activity. The device measures vertical, antero–posterior, and medio–lateral acceleration from which physical activity metrics such as step count (i.e., number of steps taken) can be derived. Participants were instructed how to wear the accelerometer, including placement of the accelerometer on the non-dominant hip centered over the kneecap, affixed to an elastic belt, and preferably worn under their waistbands. Participants were instructed to wear the accelerometer from the moment they awakened until they went to bed at night, excluding times when the accelerometer would come into contact with water (e.g., showering, swimming). Data were validated using a defined non-wear period of 90 min and spike tolerance of 2 min. That is, time points were classified as “non-wear time” if more than 90 consecutive min of zeroes were recorded on the device. After a non-wear period, time points were considered valid after 2 min of non-zero time points were recorded by the device. Data were considered valid if 10 or more hours per day of valid wear time were recorded. Analyses were restricted to participants with at least four days of valid wear time, and the mean number of valid days was 6.1 (SD = 1.6).
ActiGraph accelerometers were initialized and analyzed using ActiLife software version 6.11.5. The devices collected data over 10-s epochs, and data were converted to 60-s epochs for analysis. Total step counts were used as the primary physical activity variable of interest. Time spent sedentary was determined using established counts per min cutoffs (e.g., 0–99 counts per min).
Neurocognitive Testing
All participants completed neurocognitive testing before collection of physical activity data. The standardized battery included clinical neuropsychological tests of executive function and episodic memory, and an experimental face-name memory task, which are described below.
Clinical neuropsychological assessment
Executive function was assessed using the Trail Making and Verbal Fluency subtests from the Delis Kaplan Executive Function System (D-KEFS; (Delis, Kaplan, & Kramer, 2001), Mental Arithmetic and Digit Span (backwards) from the Wechsler Adult Intelligence Scale-Third Edition (WAIS-III; Wechsler, 1997), and the Wisconsin Card Sorting Test: Computer Version 4-Research Edition (WCST; Robert K. Heaton and PAR staff).
The Brief Visuospatial Memory Test-Revised (BVMT-R; Benedict, 1997) and the Faces subtest from the Wechsler Memory Scale-Third Edition (WMS-III; Wechsler, 1997) were used to assess visual memory. The California Verbal Learning Test-Second Edition (CVLT-II; Delis, Kramer, Kaplan, & Ober, 2000) and Logical Memory subtest from the WMS-III were used to assess verbal memory.
Neuropsychological test scores were transformed to Z-scores using the mean and standard deviation from the younger adults. For data reduction purposes and to reduce type II error, composite scores were calculated for executive function, visual episodic memory, and verbal episodic memory that consisted of the mean Z-scores of the neuropsychological measures that comprise each respective domain. Dependent variables included in the composite scores are listed in Table 2.
Table 2.
Neurocognitive performance (composite z-scores and raw test scores; mean and standard deviation) by age group
| Younger adults | Older adults | |
|---|---|---|
| Executive function Z-scorea | 0.00 (0.63) | −0.78 (1.01) |
| DKEFS Trails 4 (s) | 52.1 (10.8) | 78.1 (25.3) |
| WCST % Perseverative responses | 9.4 (3.7) | 10.94 (6.7) |
| Digit Span Backwards | 5.6 (1.5) | 5.6 (2.1) |
| Mental Arithmetic | 16.5 (1.7) | 15.2 (3.9) |
| Phonological Fluency (FAS) | 45.6 (12.0) | 48.2 (12.8) |
| Verbal memory Z-scorea | 0.00 (0.83) | −0.52 (0.95) |
| CVLT Total Recall (Trials 1–5) | 56.5 (8.2) | 52.2 (7.9) |
| CVLT Long Delay Free Recall | 13.1 (2.9) | 11.8 (3.0) |
| Logical Memory I Recall | 31.3 (5.2) | 30.5 (6.1) |
| Logical Memory II Recall | 34.1 (5.6) | 30.8 (6.9) |
| Visual memory Z-scorea | 0.00 (0.62) | −1.94 (1.41) |
| BVMT Total Recall | 30.9 (3.6) | 21.0 (6.0) |
| BVMT Delayed Recall | 11.2 (0.7) | 8.8 (2.3) |
| Faces I (WMS-III) | 40.2 (3.3) | 37.5 (4.7) |
| Faces II (WMS-III) | 40.3 (3.0) | 37.5 (4.9) |
| Face-name memory Z-score | 0.00 (1.00) | −2.48 (1.80) |
| Accuracy (%) | 81.5 (7.5) | 62.8 (14.5) |
Older adults < younger adults.
Experimental memory task
A face-name memory task (Figure 1) for 180 face-name pairs was administered (5 lists, each composed of 36 trials) during functional MRI (data not reported). During encoding, participants were instructed to remember each face-name pair and were asked to rate on a 4-point scale how well the name fit with the face. During retrieval for each list, participants were asked on a trial-by-trial basis to select the correct name out of two choices for a previously presented face, as well as to indicate their confidence in the selected choice (definite or probable). Between each encoding and retrieval block, participants completed a 20 s filler task. Presentation duration for each encoding and retrieval trial was 3.5 s, with an intertrial interval varying between 0.5 to 6.5 s. Overall accuracy for face-name trials was used as the dependent measure.
Fig. 1.
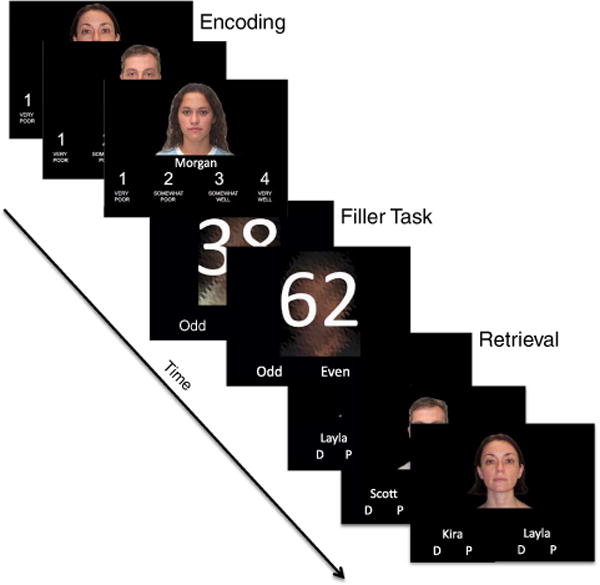
Example of experimental stimuli used during the face-name relational memory task.
Statistical Analyses
The alpha level was set at p < .05. Independent samples t-tests and chi-square tests were performed to examine differences between the younger adults and older adults in demographic variables (e.g., age, education, gender, etc.). To examine the relationship between step count and cognition, separate four-step hierarchical regression analyses were performed for the executive function composite score, verbal episodic memory composite score, visual episodic memory composite score, and face-name accuracy. In step 1 of each model, gender, education (years), depression (CES-D score), and accelerometer wear time (in minutes) were entered as covariates. In step 2, Group was entered to evaluate differences in cognitive performance between younger adults and older adults. In step 3, Step Count was entered to examine the association between physical activity and cognition in the overall sample. In step 4, the Group × Step Count interaction was entered into the model. Significant interactions were followed up with regression models in younger and older adults separately to examine the relationship between physical activity and cognitive performance. These follow-up models included covariates in step 1, and Step Count in step 2.
RESULTS
Association between Step Count and Cognition
Executive function
We first examined the association between Step Count and executive function. In a four-step hierarchical linear regression predicting executive function performance, Group emerged as the only significant variable, as younger adults had significantly higher executive function scores than older adults (Tables 2 and 3). Specifically, the covariates entered in step 1 (gender, education, depression, and wear time) were not significantly associated with executive function and accounted for only 4.4% of the variance. However, adding Group to the model in step 2 revealed a significant effect, accounting for an additional 14.3% of the variance. Adding Step Count and the Group × Step Count interaction did not improve the model (Table 3).
Table 3.
Results of hierarchical regression analyses examining the association between physical activity and cognitive performance
| Executive Function Composite | R2 | Δ R2 | F Δ (p) | Model F (p) |
|---|---|---|---|---|
| Model 1 | ||||
| Education, Gender, CES-D, Wear Time | 0.04 | — | — | .63 (0.65) |
| Model 2 | ||||
| Group | 0.19 | 0.14 | 9.52 (0.00) | 2.48 (0.04) |
| Model 3 | ||||
| Total Step Counts | 0.20 | 0.02 | 1.07 (0.31) | 2.25 (0.05) |
| Model 4 | ||||
| Group × Total Step | 0.24 | 0.04 | 2.45 (0.12) | 2.33 (0.04) |
| Counts | ||||
| Verbal Memory Composite | ||||
| Model 1 | ||||
| Education, Gender, CES-D, Wear Time | 0.04 | — | — | .55 (0.70) |
| Model 2 | ||||
| Group | 0.16 | 0.12 | 7.84 (0.01) | 2.06 (.09) |
| Model 3 | ||||
| Total Step Counts | 0.16 | 0.00 | 0.00 (0.97) | 1.68 (0.14) |
| Model 4 | ||||
| Group × Total Step Counts | 0.16 | 0.00 | 0.00 (0.99) | 1.42 (0.22) |
| Visual Memory Composite | ||||
| Model 1 | ||||
| Education, Gender, CES-D, Wear Time | 0.03 | — | — | .41 (0.80) |
| Model 2 | ||||
| Group | 0.56 | 0.53 | 64.96 (0.00) | 13.70 (0.00) |
| Model 3 | ||||
| Total Step Counts | 0.61 | 0.05 | 6.44 (0.01) | 13.64 (0.00) |
| Model 4 | ||||
| Group × Total Step Counts | 0.65 | 0.04 | 5.86 (0.02) | 13.60 (0.00) |
| Face-Name Memory | ||||
| Model 1 | ||||
| Education, Gender, CES-D, Wear Time | 0.10 | — | — | 1.25 (0.31) |
| Model 2 | ||||
| Group | 0.51 | 0.41 | 36.12 (0.00) | 9.00 (0.00) |
| Model 3 | ||||
| Total Step Counts | 0.60 | 0.10 | 10.29 (0.00) | 10.80 (0.00) |
| Model 4 | ||||
| Group × Total Step Counts | 0.62 | 0.02 | 2.44 (0.13) | 9.92 (0.00) |
In light of meta-analytic data showing a robust relationship between exercise and executive function in older adults (Colcombe & Kramer, 2003) and the paucity of data in younger adults, we completed regression analyses in the younger adult and older adult groups separately. Results revealed no significant associations between Step Count and executive function for either younger or older adults (Figure 2A). For younger adults, covariate accounted for 9.3% of the variance, F(4,28) < 1, p = .66. Step Count did not improve the model significantly, accounting for an additional 4.6% of the variance, F change = 1.24, overall model F(5,28) < 1, p = .28. In older adults, covariates accounted for 3.7% of the variance, F(4,30) < 1, p = .91. Step Count accounted for an additional 7.8% of variance, F change = 2.2, p = .15. The overall model was not significant, F(5,30) < 1, p = .66.
Fig. 2.
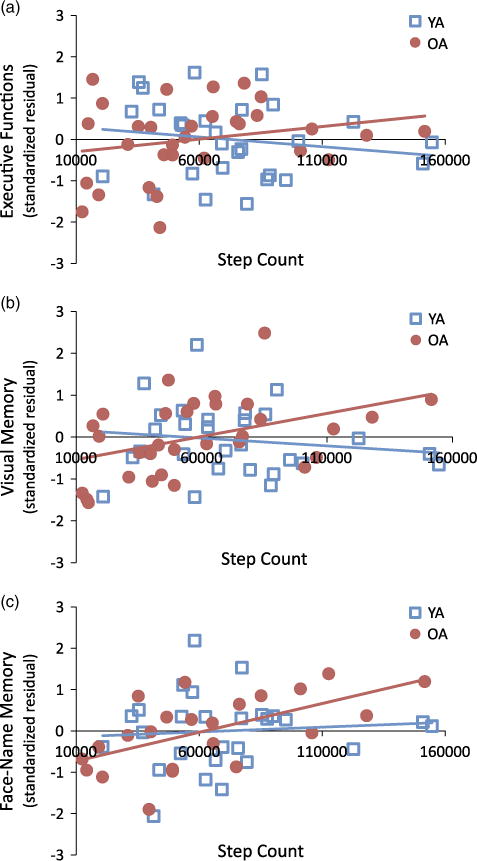
Scatter plot and best-fit line for younger adults and older adults showing the relationship between (a) Total step count and the executive function standardized residual (after accounting for age, gender, education, depression, and wear time) and (b) Total step count and the visual memory standardized residual; (c) Total step count and the face-name accuracy standardized residual. OA, older adults; YA, younger adults.
Episodic memory: verbal composite
Hierarchical linear regression showed a main effect of Group only in association with verbal memory, as younger adults had significantly greater composite verbal memory scores than older adults (Tables 2 and 3). In step 1, covariates accounted for 3.8% of the variance, which was not significant. Group accounted for an additional 12.2% of the variance, F change = 7.84, p < .01, with younger adults performing better than older adults. Adding Step Count and the Group × Step Count interaction did not improve the model (Table 3).
Episodic memory: visual composite
Hierarchical linear regression revealed that younger adults had higher visual memory composite scores than older adults (Tables 2 and 3). Step Count was positively associated with visual memory performance. However, this main effect was qualified by a Group × Step Count interaction, driven by a positive association between Step Count and visual memory in older adults, but not younger adults (Figure 2B; Table 3). Specifically, in step 1, covariates accounted for only 2.9% of the variance, which was not significant. The addition of Group accounted for an additional 53.0% of variance, which was significant. Step Count further improved the model, accounting for an additional 4.8% of the variance. The Group × Step Count interaction accounted for an additional 4.0% of variance in the model, and was significant (Table 3). In a follow-up regression analysis in younger adults, covariates, entered into the model first, accounted for 17% of the variance, F(4,28) = 1.23, p = .32. Step Count accounted for an additional 1.5% of the variance, F change < 1, p = .53, overall model F(5,28) = 1.04, p = .42. In contrast, in older adults there was a positive association between Step Count and visual memory. The covariates accounted for 26.6% of the variance, F(4,30) = 2.35, p = .08. Step Count accounted for an additional 16.9% of the variance, F change = 7.47, p < .05. The overall model was significant, F(5,30) = 3.84, p = .01.
Given the age-range of older adults in the current sample and the fact that some participants had hypertension, we sought to confirm that the observed association between step count and visual memory in older adults was related to physical activity and not attributable to age-related reductions in physical activity or to hypertension. Therefore, age (in years) and hypertension status were included with the covariates in step 1 of a regression model in older adults. These variables (age, gender, education, depression, hypertension, and wear time) accounted for 33.3% of the variance, F(6,30) = 2.00, p = .11. Adding Step Count to the model accounted for an additional 13.3% of variance, F change = 5.71, p < .05, and the overall model (covariates and Step Count) accounted for 46.6% of the variance and was significant, F(7,30) = 2.89, p < .05. Step Count was the only significant variable in the model, t = 2.39, p < .05; all other variables had t-values < 1.52, and p’s > .14.
Episodic memory: face-name task
The pattern for the face-name task was similar to that observed for the visual memory composite score. Hierarchical linear regression revealed that younger adults performed better on the face-name memory task than older adults (Tables 2 and 3). Step Count was positively associated with face-name memory performance. The Group × Step Count interaction did not reach statistical significance, but follow-up analyses revealed a positive association between Step Count and face-name memory in older, but not younger adults (Figure 2C). Specifically, for the face-name memory task, covariates accounted for a non-significant 10.0% of the variance. Group accounted for an additional 40.6% of variance in face-name accuracy, which was significant. Adding Step Count accounted for an additional 9.5% of the variance, which was significant. The interaction term was not significant (Table 3). Nevertheless, given the pattern observed with the standardized neuropsychological tests of visual memory, we further interrogated the face-name data with follow-up regressions, and found a similar pattern to that seen for the visual memory composite score. That is, a positive relationship between Step Count and face-name memory was observed in older, but not younger adults. Specifically, in younger adults, covariates (gender, education, depression, and wear time) accounted for 5.2% of the variance, F(4,26) < 1, p = .88. Step Count accounted for an additional 2.1% of the variance, F change < 1, p = .50, overall model, F(5,26) < 1, p = .89. In older adults, the covariates accounted for 25.4% of the variance, F(4,22) = 1.53, p = .24. Step Count accounted for an additional 29.6% of the variance, F change = 11.16, p < .005. The overall model was significant, F(5,22) = 4.15, p < .05.
To again confirm that the observed relationship for older adults was independent of age and hypertension, we performed an additional regression in the older group and included age and hypertension as additional covariates in step 1. In this model, covariates accounted for 26.9% of the variance, F(6,22) <1, p = .47. Step Count accounted for an additional 29.7% of the variance, F change = 10.27, p < .01, and the overall model accounted for 56.6% of the variance and was significant, F(7,22) = 2.80, p < .05.
Associations among Sedentary Behavior, Physical Activity Intensity, and Cognition
To determine the relationship between objective measures of sedentary behavior and cognition, and to examine whether physical activity intensity makes a unique contribution to cognitive performance after accounting for sedentary behavior, we completed hierarchical regressions focusing on those tasks that were positively associated with step counts in older adults: visual memory and face-name learning. We examined step rate (total steps taken/total min in light, moderate, and vigorous activities; older adults M = 31.6, SD = 14.0), based on the notion that this would provide an independent indicator of physical activity intensity (how briskly one is walking). Covariates (age, gender, education, depression, hypertension, and wear time) were entered in step 1. Sedentary Time (total minutes spent sedentary) was entered in step 2 (older adults M = 566 min per day, SD = 126). In step 3, Step Rate [steps per min (during light, moderate, and vigorous physical activity)] was entered.
For the visual memory composite score, the more sedentary older adults were, the lower their visual memory performance (pr = −.41). Although step rate was positively associated with visual memory performance after accounting for time spent sedentary (pr = .32), it did not significantly improve the model (Table 4). Specifically, covariates (age, education, gender, depression, hypertension, wear time) accounted for 33.3% of the variance, model F(6,30) = 2.00, p = .11. Sedentary Time accounted for an additional 11.2% of the variance, F change = 4.67, p < .05, overall model F(7,30) = 2.64, p < .05. Step Rate accounted for an additional 5.8% of the variance, F change = 2.57, p = .12, overall model F(8,30) = 2.79, p < .05.
Table 4.
Results of hierarchical regression analyses examining the association between sedentary behavior, physical activity intensity, and episodic memory in older adults
| Visual Memory Composite | R2 | Δ R2 | F Δ (p) | Model F (p) |
|---|---|---|---|---|
| Model 1 | ||||
| Age, Gender, Education, Hypertension, CES-D, Wear Time | 0.33 | — | — | 2.00 (0.11) |
| Model 2 | ||||
| Minutes Spent Sedentary | 0.45 | 0.11 | 4.67 (0.04) | 2.64 (0.04) |
| Model 3 | ||||
| Step Rate | 0.50 | 0.06 | 2.57 (0.12) | 2.79 (0.02) |
| Face-Name Memory | ||||
| Model 1 | ||||
| Age, Gender, Education, Hypertension, CES-D, Wear Time | 0.27 | — | — | 0.93 (0.47) |
| Model 2 | ||||
| Minutes Spent Sedentary | 0.48 | 0.21 | 5.98 (0.03) | 1.96 (0.13) |
| Model 3 | ||||
| Step Rate | 0.60 | 0.12 | 4.23 (0.06) | 2.61 (0.06) |
For the face-name memory task, both sedentary time and step rate were associated with task performance (Table 4). The more time that older adults were sedentary, the lower their face-name memory accuracy was (pr = −53). Step Rate was positively associated with face-name memory retrieval, after accounting for time spent sedentary (pr = .48). Specifically, Covariates accounted for 26.9% of the variance, F(6,22) < 1, p = .47. Sedentary Time accounted for an additional 20.8 % of the variance, F change = 5.98, p < .05, overall model F (7,22) = 1.96, p = .13. In step 3, Step Rate accounted for an additional 12.1% of the variance, F change = 4.23, p = .059, model F(8,22) = 2.61, p = .056.
DISCUSSION
The present study examined the relationship between physical activity and neurocognitive function in younger and older adults. The primary finding was that in older adults, physical activity was positively associated with performance on neuropsychological tests of visual episodic memory and an experimental face-name learning task. This relationship was not observed in the younger adult sample. Follow-up analyses of older adults revealed that sedentary time was negatively associated with visual episodic memory and face-name learning; furthermore, that sedentary time and step rate made independent contributions to episodic memory performance.
To our knowledge, this study is the first to demonstrate a positive association between an objective measure of physical activity and visual episodic memory in older adults, after adjusting for age, gender, education, depression, and hypertension. A previous study by Brown et al. (2012) also quantified physical activity based on accelerometry, although a relationship between visual memory and volume of physical activity, a measure closely related to the step count variable reported in our study, was not observed. The discrepancy in findings between our study and Brown et al. (2012) may be attributable to differences in data analysis, as in the latter study analyses of physical activity volume included covariates such as APOE ε4 status and did not adjust for wear time. Adjusting for wear time is critical because a less active participant could generate a higher activity count simply by wearing the accelerometer for a longer period of time than a participant who is more active but wore the accelerometer for less time. Importantly, our results demonstrate that the positive relationship between step count and visual episodic memory was maintained after adjusting for wear time, age, gender, education, depression, and hypertension, indicating that physical activity has a unique association with visual episodic memory performance in older adults.
A similar pattern of results was observed for the face-name learning task, where performance was also positively associated with step count. There is some indication that the face-name relational memory task may be more sensitive to physical activity in older adults, as step count accounted for 29.6% of the variance in the face-name memory task compared to 13.3% of the variance on the standardized neuropsychological tests of visual memory. Furthermore, physical activity intensity (step rate) accounted for additional variance on the face-name memory task even after taking into account the influence of sedentary behavior on face-name memory performance, although this finding was only at a trend level, and thus awaits replication. Nonetheless, these results extend the literature by showing that more physical activity is associated with better performance on a task that assesses the most common cognitive complaint among older adults, namely forgetting proper names (Reese, Cherry, & Norris, 1999).
The findings for visual memory and face-name learning stand in contrast to those in the verbal memory domain, where we found no evidence for an association between objective measures of physical activity and verbal memory performance (see also Brown et al., 2012; Wilbur et al., 2012). One possibility is that these contrasting outcomes reflect the differential reliance on the hippocampus. Specifically, the CVLT and Logical Memory subtest used to assess verbal memory in this study both have an inherent semantic structure, and as such, may be supported by frontally mediated strategic encoding and retrieval processes (Moscovitch, 1992). The BVMT and Faces subtest used to assess visual memory use non-nameable objects and thus may not be as easily supported by strategic memory processes. Alternatively, given that older adults performed more poorly on the face-name and visual memory (Z-scores = −2.48 and −1.94, respectively) than on the verbal memory (Z = −0.52) tasks, our findings may suggest that the effect of physical activity becomes apparent only when cognitive and neural resources are sufficiently challenged.
In light of this possibility, it is interesting to consider that older adults also were less impaired on tasks of executive function (Z = −.78) than on tasks of visual memory. The failure to obtain a significant relationship between physical activity and executive function was unexpected, but it is possible that in other studies that have demonstrated a relationship between objective measures of physical activity and executive function, older adults’ performance was more impaired. Although it is difficult to compare results across studies that used different tasks, our older adult sample had a mean Z-score of 0.87 (compared to age matched normative data) across our measures of executive function, which is in the high average range, whereas samples from other studies were estimated to perform in the average or low average range on tasks of executive function (Brown et al., 2012; Wilbur et al., 2012). Of interest, one pattern that emerges from our findings is that physical activity was most strongly associated with tasks on which the older adults exhibited the largest cognitive impairments.
The notion that physical activity may be most sensitive to difficult tasks may also account for the age-dependent associations observed in the current study. The lack of a relationship between physical activity and cognition in the younger adult sample could be due to the fact that younger adults are at their lifetime peak in terms of neural integrity and cognitive performance; therefore, physical activity exerts minimal impact on the brain structure and cognition in younger adults. In contrast, alterations in brain structure and reductions in cognitive performance are prevalent in older adults, and physical activity may be one factor that provides beneficial neural and cognitive effects. This pattern of age-dependence has been previously reported in a study of face-name learning task in middle-age and older adults that used a self-report measure of exercise activity (Hotting & Roder, 2013), as well as studies focused on cardiorespiratory fitness and cognition (Hayes et al., 2014) and white matter integrity (Hayes et al., 2015). More definitive evidence for the notion of age-dependence would be derived from longitudinal studies that directly compare changes in objective measures of physical activity and changes in cognition across the lifespan. Furthermore, futures studies could evaluate additional factors that may impact the relationship between physical activity and cognition, including, for example, aspects of vascular health (in addition to hypertension), medication use, and sleep.
Physical activity likely impacts cognition via multiple neurobiological mechanisms. In humans, physical activity has been linked to alterations of neural gray and white matter volume, gray matter density, white matter microstructure, cerebral blood volume, functional MRI activation, and functional MRI connectivity (for review, see Bherer, Erickson, & Liu-Ambrose, 2013; Hayes, Hayes, Cadden, & Verfaellie, 2013; Prakash et al., 2015). In animals, physical activity has been associated with increases in neurogenesis, synaptogenesis, and angiogenesis (formation of new neurons, synapses, and blood vessels, respectively), as well as growth factors that support these processes (e.g., brain derived neurotrophic factor), often attenuating age-related reductions (for review, see Cotman et al., 2007; Voss, Vivar, Kramer, & van Praag, 2013).
Although physical activity has been linked to positive neural and cognitive effects, it is also important to consider the relationship between sedentary behavior, the brain, and cognition. There is behavioral evidence that, despite higher levels of physical activity, one may remain predominately sedentary [e.g., folks who exercise vigorously for an hour but sit at a desk all day (Craft et al., 2012)]. Sedentary behavior has been associated with depression, diabetes, and cardiovascular risk factors, which are frequently associated with poor cognitive performance, and previous reviews have postulated that sedentary behavior, independent of physical activity, may negatively impact the brain and cognition (Vaynman & Gomez-Pinilla, 2006; Voss, Carr, Clark, & Weng, 2014). Thus, sedentary behavior could potentially negate the positive effects of physical activity. Our results demonstrating a negative association between sedentary behavior and episodic memory in older adults support the proposal that sedentary behavior is negatively associated with cognitive functions. Furthermore, our results suggest that physical activity intensity, even after accounting for the negative association with sedentary behavior, remains positively associated with face-name memory retrieval. To our knowledge, these data are the first to suggest independent contributions of sedentary behavior and physical activity to cognitive performance in older adults.
The current study reports associations, and does not necessarily represent a causal relationship between physical activity and cognition. Limitations of the current study include the cross-sectional design, and thus, other cohort factors such as genetics, diet, sleep, blood pressure could have influenced the results. The sample size was limited, and the sample was also more educated, healthier, and more active than the general population. Although the observed effect sizes in young participants were weak, it is possible that associations between physical activity and cognition might reach significance in a larger sample. Targeting a larger sample with a broader range of activity levels might also have yielded different results. For instance, inclusion of more sedentary older adults in the current sample may have revealed an association with executive function, as multiple studies showing a relationship between executive function and physical activity recruited sedentary individuals (Colcombe et al., 2004). It is also unknown whether our results would generalize to less educated samples. Future studies including participants with a broader range of education levels may provide additional insight to the relationship between physical activity and cognition. Future studies would also benefit from having subjects complete activity logs to confirm ActiGraph counts, and should take into account time spent in water-based activities such as swimming.
Despite the limitations of our study, the finding that step count and step rate were associated with episodic memory in older adults is compelling. Memory failure is the most common cognitive complaint in older adults, and the possibility that increasing one’s daily activity may improve memory performance has direct clinical relevance. Current technology allows for physical activity metrics to be easily monitored through a variety of devices, such as a pedometer, accelerometer (e.g., FitBit or Fuel band), or smart phone, and thereby can provide feedback regarding the intensity and duration of daily forms of physical activity (Tudor-Locke & Bassett, 2004; Tudor-Locke & Schuna, 2012).
Acknowledgments
This work was supported by the Department of Veterans Affairs, Rehabilitation Research & Development Service [Career Development Award e7822w awarded to SMH and F0834-R awarded to DEF], the Department of Veterans Affairs, Clinical Science Research & Development Service [MV], and the National Institute on Aging [K24-AG035007 awarded to RAS]. Assistance with participant recruitment was provided by the Massachusetts Alzheimer’s Disease Research Center (P50-AG005134) and the Boston University Alzheimer’s Disease Center (P30-AG13846). The authors thank Huiting Liu and Rebecca Lysiak for assistance with data collection and Korine Cabrera for assistance with data analysis. The contents of this article do not represent the views of the U.S. Department of Veterans Affairs, the National Institutes of Health, or the United States Government.
Footnotes
The authors have no conflicts of interest to report.
References
- Barnes DE, Blackwell T, Stone KL, Goldman SE, Hillier T, Yaffe K. Cognition in older women: The importance of daytime movement. Journal of the American Geriatric Society. 2008;56(9):1658–1664. doi: 10.1111/j.1532-5415.2008.01841.x. doi: JGS1841 [pii] 10.1111/j.1532-5415.2008. 01841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict R. Brief Visuospatial Memory Test-Revised: Professional Manual. Lutz, FL: Psychological Assessment Resources, Inc; 1997. [Google Scholar]
- Bherer L, Erickson KI, Liu-Ambrose T. A review of the effects of physical activity and exercise on cognitive and brain functions in older adults. Journal of Aging Research. 2013;2013:657508. doi: 10.1155/2013/657508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown BM, Peiffer JJ, Sohrabi HR, Mondal A, Gupta VB, Rainey-Smith SR. Intense physical activity is associated with cognitive performance in the elderly. Translational Psychiatry. 2012;2:e191. doi: 10.1038/tp.2012.118. doi: tp2012118 [pii]10.1038/tp.2012.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman AS, Boyle PA, Yu L, Shah RC, Wilson RS, Bennett DA. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology. 2012;78(17):1323–1329. doi: 10.1212/WNL.0b013e3182535d35. doi: WNL.0b013e3182535d35 [pii]10.1212/WNL.0b013e3182535d35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman AS, Wilson RS, Bennett DA. Total daily activity is associated with cognition in older persons. American Journal of Geriatric Psychiatry. 2008;16(8):697–701. doi: 10.1097/JGP.0b013e31817945f6. doi: 16/8/697 [pii] 10.1097/JGP.0b013e31817945f6. [DOI] [PubMed] [Google Scholar]
- Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Report. 1985;100(2):126–131. [PMC free article] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychological Science. 2003;14(2):125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ. Cardiovascular fitness, cortical plasticity, and aging. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(9):3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends in Neurosciences. 2007;30(9):464–472. doi: 10.1016/J.Tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Craft LL, Zderic TW, Gapstur SM, Vaniterson EH, Thomas DM, Siddique J. Evidence that women meeting physical activity guidelines do not sit less: An observational inclinometry study. The International Journal of Behavioral Nutrition and Physical Activity. 2012;9:122. doi: 10.1186/1479-5868-9-122. doi: 1479-5868-9-122 [pii]10.1186/1479-5868-9-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis D, Kaplan E, Kramer J. Delis-Kaplan Executive Function System. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- Delis D, Kramer J, Kaplan E, Ober B. California Verbal Learning Test-Second Edition: Adult Version. San Antonio, TX: The Psychological Coporation; 2000. [Google Scholar]
- Freedson P, Bowles HR, Troiano R, Haskell W. Assessment of physical activity using wearable monitors: Recommendations for monitor calibration and use in the field. Medicine and Science in Sports and Exercise. 2012;44(1 Suppl 1):S1–S4. doi: 10.1249/MSS.0b013e3182399b7e. doi: 10.1249/MSS.0b013e3182399b7e00005768-201201001-00001 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Medicine and Science in Sports and Exercise. 1998;30(5):777–781. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- Giovanello KS, Verfaellie M, Keane MM. Disproportionate deficit in associative recognition relative to item recognition in global amnesia. Cognitive, Affectice and Behavioral Neuroscience. 2003;3(3):186–194. doi: 10.3758/CABN.3.3.186. [DOI] [PubMed] [Google Scholar]
- Goh JO, An Y, Resnick SM. Differential trajectories of age-related changes in components of executive and memory processes. Psycholog and Aging. 2012;27(3):707–719. doi: 10.1037/a0026715. doi: 2011-30106-001 [pii]10.1037/a0026715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA. Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Medicine and Science in Sports and Exercise. 2007;39(8):1423–1434. doi: 10.1249/mss.0b013e3180616b27. doi:10.1249/mss.0b013e3180616b2700005768-200708000-00027 [pii] [DOI] [PubMed] [Google Scholar]
- Hayes SM, Buchler N, Stokes J, Kragel J, Cabeza R. Neural correlates of confidence during item recognition and source memory retrieval: Evidence for both dual-process and strength memory theories. Journal of Cognitive Neuroscience. 2011;23(12):3959–3971. doi: 10.1162/jocn_a_00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SM, Forman DE, Verfaellie M. Cardiorespiratory fitness is associated with cognitive performance in older but not younger adults. The Journals of Gerontology Series B Psychological Sciences and Social Sciences. 2014 doi: 10.1093/geronb/gbu167. doi:gbu167 [pii] 10.1093/geronb/gbu167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SM, Hayes JP, Cadden M, Verfaellie M. A review of cardiorespiratory fitness-related neuroplasticity in the aging brain. Frontiers in Aging Neuroscience. 2013;5:31. doi: 10.3389/fnagi.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SM, Salat DH, Forman DE, Sperling RA, Verfaellie M. Cardiorespiratory fitness is associated with white matter integrity in aging. Annals of Clinical and Translational Neurology. 2015;2(6):688–698. doi: 10.1002/acn3.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotting K, Roder B. Beneficial effects of physical exercise on neuroplasticity and cognition. Neuroscience and Biobehavioral Reviews. 2013;37(9 Pt B):2243–2257. doi: 10.1016/j.neubiorev.2013.04.005. doi: S0149-7634(13)00101-2 [pii]10.1016/j.neubiorev.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Moscovitch M. Memory and working-with-memory: A component process model based on modules and central systems. Journal of Cognitive Neuroscience. 1992;4(3):257–267. doi: 10.1162/jocn.1992.4.3.257. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M. Adult age differences in memory performance: Tests of an associative deficit hypothesis. Journal of Experimental Psychology. Learning, Memory, and Cognition. 2000;26(5):1170–1187. doi: 10.1037/0278-7393.26.5.1170. [DOI] [PubMed] [Google Scholar]
- Nichol K, Deeny SP, Seif J, Camaclang K, Cotman CW. Exercise improves cognition and hippocampal plasticity in APOE epsilon 4 mice. Alzheimers & Dementia. 2009;5(4):287–294. doi: 10.1016/J.Jalz.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old SR, Naveh-Benjamin M. Differential effects of age on item and associative measures of memory: A meta-analysis. Psychology and Aging. 2008;23(1):104–118. doi: 10.1037/0882-7974.23.1.104|issn0882-7974. [DOI] [PubMed] [Google Scholar]
- Pescatello LS, American College of Sports Medicine . ACSM’s guidelines for exercise testing and prescription. 9th. Wolters Kluwer/Lippincott Williams & Wilkins Health; 2014. [DOI] [PubMed] [Google Scholar]
- Prakash RS, Voss MW, Erickson KI, Kramer AF. Physical activity and cognitive vitality. Annual Review of Psychology. 2015;66:769–797. doi: 10.1146/annurev-psych-010814-015249. [DOI] [PubMed] [Google Scholar]
- Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: A systematic review. The International Journal of Behavioral Nutrition and Physical Activity. 2008;5:56. doi: 10.1186/1479-5868-5-56. doi: 1479-5868-5-56 [pii] 10.1186/1479-5868-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese CM, Cherry KE, Norris LE. Practical memory concerns of older adults. Journal of Clinical Geropsychology. 1999;5(4):231–244. doi: 10.1023/A:1022984622951. [DOI] [Google Scholar]
- Rzewnicki R, Vanden Auweele Y, De Bourdeaudhuij I. Addressing overreporting on the International Physical Activity Questionnaire (IPAQ) telephone survey with a population sample. Public Health Nutrition. 2003;6(3):299–305. doi: 10.1079/PHN2002427. doi:10.1079/PHN2002427 S1368980003000399 [pii] [DOI] [PubMed] [Google Scholar]
- Shepard RJ. Limits to the measurement of habitual physical activity by questionnaires. British Journal of Sports Medicine. 2003;37:197–206. doi: 10.1136/bjsm.37.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura AP, Berry JM, Mangels JA, Rusting CL, Jurica PJ. Memory and Cognitive-abilities in university professors – Evidence for successful aging. Psychological Science. 1995;6(5):271–277. [Google Scholar]
- Smith PJ, Blumenthal JA, Hoffman BM, Cooper H, Strauman TA, Welsh-Bohmer K. Aerobic exercise and neurocognitive performance: A meta-analytic review of randomized controlled trials. Psychosomatic Medicine. 2010;72(3):239–252. doi: 10.1097/Psy.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofi F, Valecchi D, Bacci D, Abbate R, Gensini GF, Casini A. Physical activity and risk of cognitive decline: A meta-analysis of prospective studies. Journal of Internal Medicine. 2011;269(1):107–117. doi: 10.1111/j.1365-2796.2010.02281.x. [DOI] [PubMed] [Google Scholar]
- Sperling R, Chua E, Cocchiarella A, Rand-Giovannetti E, Poldrack R, Schacter DL. Putting names to faces: Successful encoding of associative memories activates the anterior hippocampal formation. Neuroimage. 2003;20(2):1400–1410. doi: 10.1016/S1053-8119(03)00391-4. doi:10.1016/S1053-8119(03)00391-4S1053811903003914 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudor-Locke C, Bassett DR., Jr How many steps/day are enough? Preliminary pedometer indices for public health. Sports Medicine. 2004;34(1):1–8. doi: 10.2165/00007256-200434010-00001. doi:3411 [pii]. [DOI] [PubMed] [Google Scholar]
- Tudor-Locke C, Schuna JM., Jr Steps to preventing type 2 diabetes: Exercise, walk more, or sit less? Frontiers in Endocrinology (Lausanne) 2012;3:142. doi: 10.3389/fendo.2012.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao CM, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. Journal of Neuroscience. 2005;25(38):8680–8685. doi: 10.1523/Jneurosci.1731-05-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaynman S, Gomez-Pinilla F. Revenge of the “sit”: How lifestyle impacts neuronal and cognitive health through molecular systems that interface energy metabolism with neuronal plasticity. Journal of Neuroscience Research. 2006;84(4):699–715. doi: 10.1002/jnr.20979. [DOI] [PubMed] [Google Scholar]
- Voss MW, Carr LJ, Clark R, Weng T. Revenge of the “sit”: II: Does lifestyle impact neuronal and cognitive health through distinct mechanisms associated with sedentary behavior and physical activity? Mental Health and Physical Activity. 2014 doi: http://dx.doi.org/10.1016/j.mhpa.2014.01.001.
- Voss MW, Vivar C, Kramer AF, van Praag H. Bridging animal and human models of exercise-induced brain plasticity. Trends in Cognitive Science. 2013;17(10):525–544. doi: 10.1016/j.tics.2013.08.001. doi: S1364-6613(13)00166-6 [pii]10.1016/j.tics.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Third Edition. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Wechsler D. Wechsler Memory Scale-Third Edition. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Wilbur J, Marquez DX, Fogg L, Wilson RS, Staffileno BA, Hoyem RL. The relationship between physical activity and cognition in older Latinos. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences. 2012;67(5):525–534. doi: 10.1093/geronb/gbr137. doi: gbr137 [pii]10.1093/geronb/gbr137. [DOI] [PubMed] [Google Scholar]


