Abstract
Adrenocortical carcinoma (ACC) is a rare and aggressive endocrine malignancy with an estimated worldwide incidence of 0.5–2 per million/year.
Complete surgical removal of ACC represents the current treatment of choice for this tumor.
A disease-free resection margin (R0) is an important predictor of long-term survival: surgery is demanding and must be performed by a highly experienced surgical team.
We report the surgical strategy adopted in a patient with locally advanced ACC and virilization to obtain a R0 resection.
Keywords: Adrenal cancer, Endocrine, Laparotomy, Virilization
Introduction
Adrenocortical carcinoma (ACC) is a rare endocrine malignancy with an estimated worldwide incidence of 0.5–2 per million/year (1, 2).
We report the surgical strategy adopted in a patient with locally advanced ACC and virilization to obtain a R0 resection.
Case report
AM, a 66-year-old female patient referred to our hospital for 2-month onset of hypertension and hirsutism. Hormonal examinations revealed the presence of high levels of testosterone (252 ng/dl).
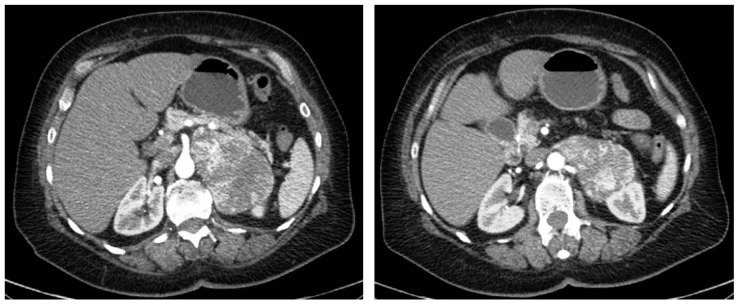
Abdominal CT revealed the presence of a large neoformation of 96×65 mm localized in the left adrenal space with regular borders and colliquation inside. This mass also compressed the left kidney with urostasis and the tail of pancreas and spleen. Medially the tumor was near the superior mesenteric artery, and posteriorly it was very close to the aorta and the diaphragm (Figure 1).
Fig. 1.
CT: large neoformation of 96×65 mm near the superior mesenteric artery, the tail of pancreas and spleen. Posteriorly it is very close to the aorta and the diaphragm with compression on the left kidney.
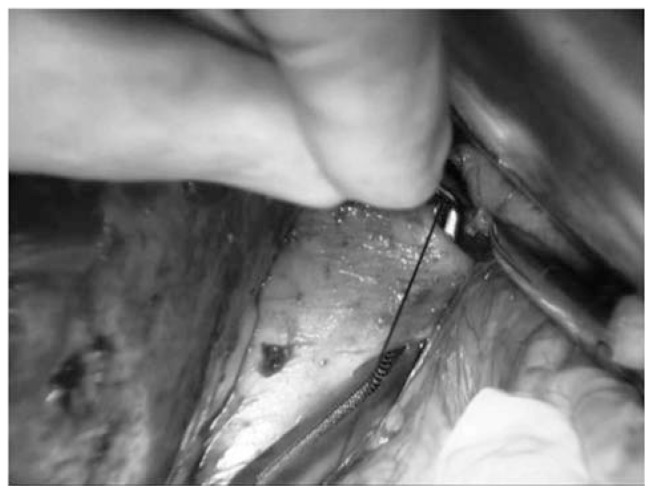
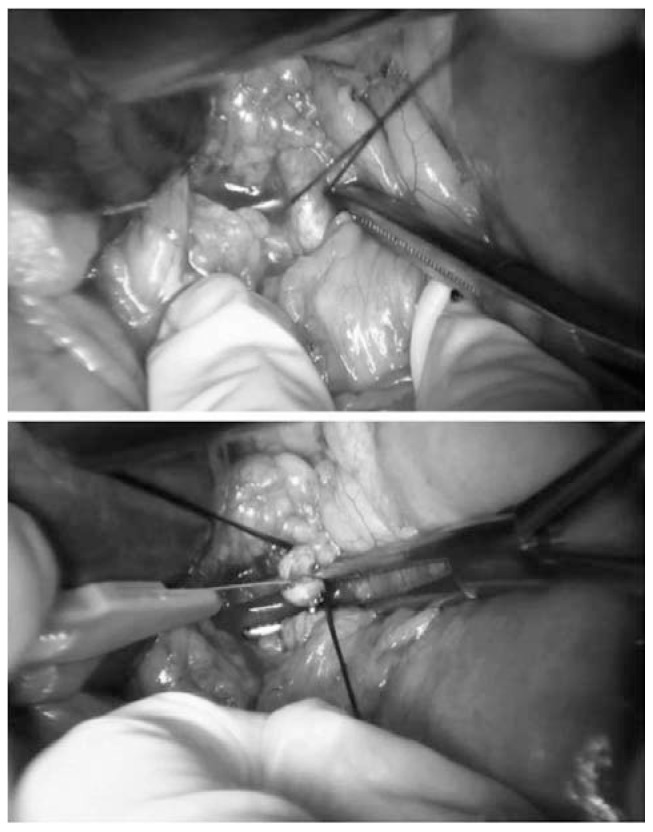
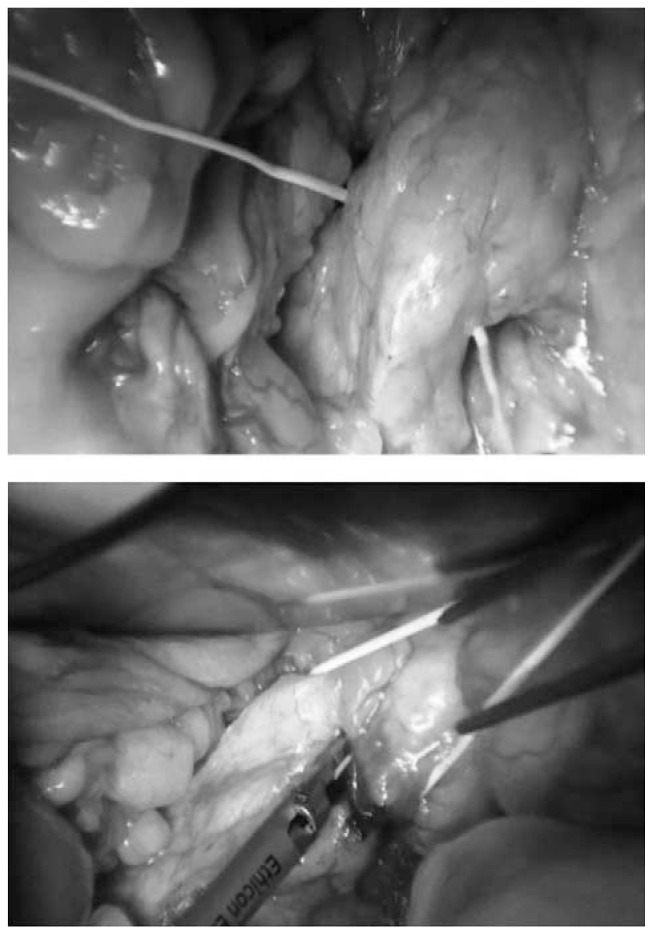
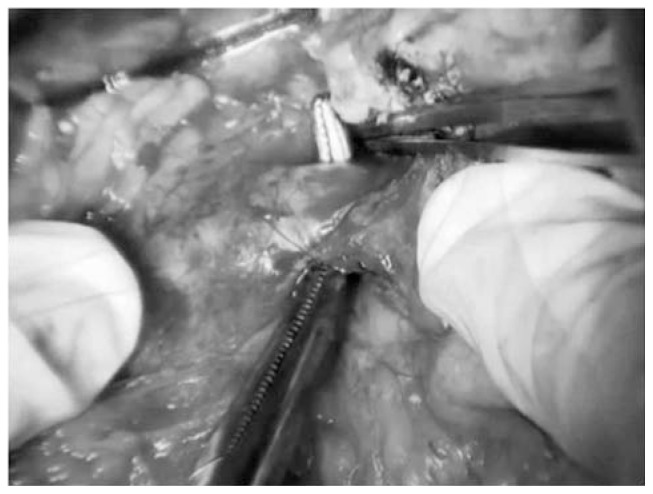
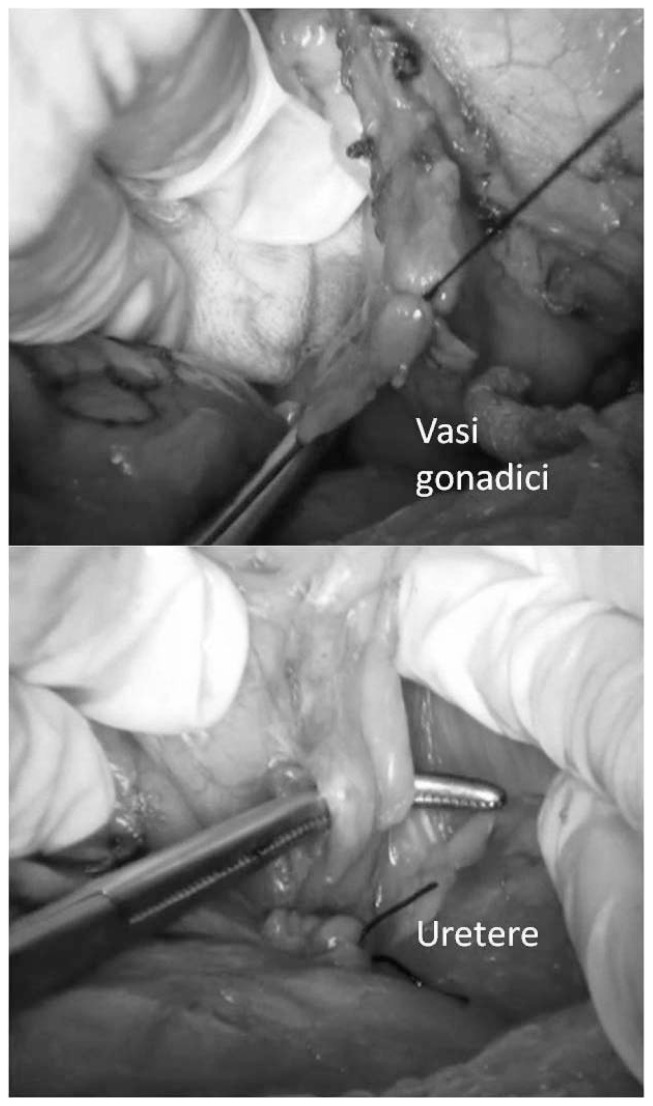
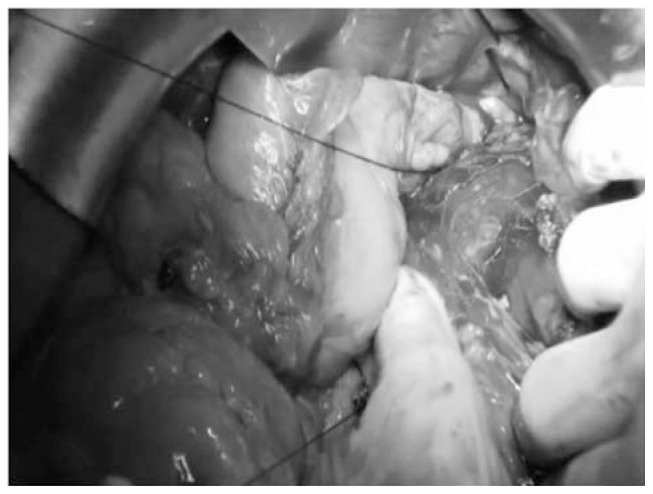
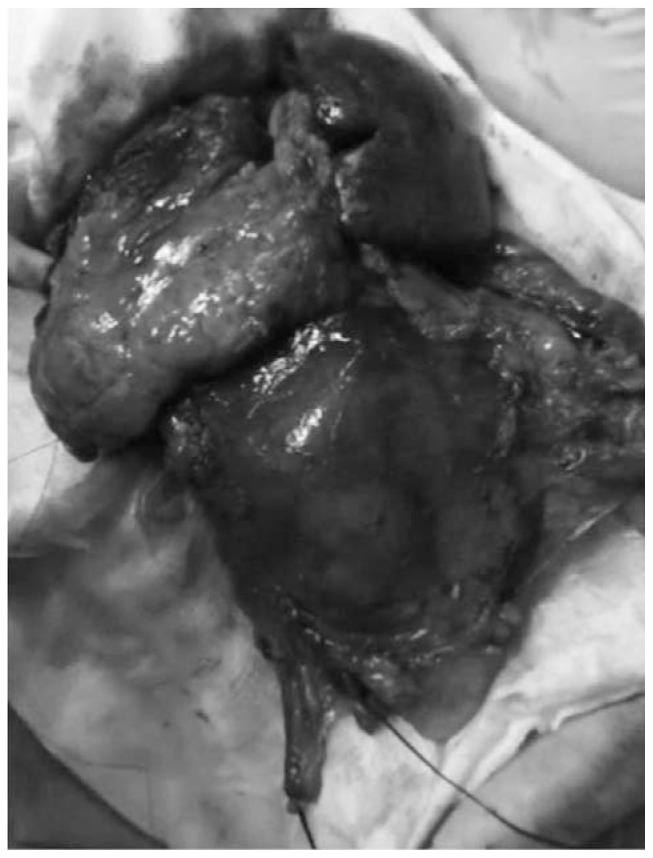
To obtain a complete resection of the tumor she underwent a laparotomic multi-organ resection. By a median laparotomy, we mobilized the right colon and we did a Kocher maneuver. Then we isolated from the right side the left renal vein (Figure 2). Then we proceed with the dissection of the splenic artery on the celiac trunk and its section (Figure 3). Dissection of the pancreatic isthmus from the portal vein and section with Harmonic Ace® (Figure 4). Section of the splenic vein (Figure 5) and of the inferior mesenteric vein with Endo - GIA. Splenic flexure mobilization and disinsertion of transverse mesocolon from the inferior border of the pancreas. Section of left gonadal vessels and of ureter (Figure 6). Full mobilization of the kidney and of the spleen and pancreas from the diaphragm. Separation of the mass from the superior mesenteric artery, the left border of aorta and the lumbar vertebral column. After complete control of the renal hilum (Figure 7), it was sectioned with mechanic suture (Echelon®): left nephroadrenalectomy en bloc with caudal splenopan-creasectomy (Figure 8).
Fig. 2.
Right approach to left renal vein.
Fig. 3.
Section by knots and clips of splenic artery.
Fig. 4.
Dissection of the pancreatic isthmus and section with Harmonic Ace®.
Fig. 5.
Splenic vein.
Fig. 6.
Dissection of left gonadal vessels and of ureter.
Fig. 7.
Complete control of the renal hilum.
Fig. 8.
Operative specimen: left nephroadrenalectomy en bloc with caudal splenopancreasectomy.
A suction drain was positioned in the subphrenic space and haemostasis was carefully controlled.
Postoperative course was uneventful and the drainage was removed in 8th. Oral intake was started from 2nd p.o. day with initial parenteral integration.
Histolgical examination revealed the presence of an aggressive ACC with microvascular invasion, numerous atypical mithosis and anaplasia. Immunoistochemical analysis showed: CK8-18+/−, vimentyn +, synaptofysin −, chromogranin −, S-100 −, alpha inhibin +, CD10 −, Cd56 −, CKpan −, calretinin −. Resection margins were not infiltrated (R0 resection).
Discussion
Adrenocortical carcinoma (ACC) is a rare and aggressive endocrine malignancy.
The age distribution is reported as bimodal with a first peak in childhood and a second higher peak in the fourth and fifth decade (3, 4). Moreover there is a slightly female predominance (4).
This neoplasm is characterized by a high risk of recurrence and a dismal prognosis owing to unsatisfactory overall 5-year survival that ranges between 23% and 60% (5).
Approximately 60% of patients present with symptoms of excess hormone secretion, most commonly in the form of cortisol hyperincretion (most commonly, hypercortisolism: Cushing’s syndrome), with or without virilization. Hormonal secretion patterns can vary according to size, differentiation, and stage of the tumor: androgen secreting ACCs in women induce hirsutism and virilization. On the contrary, estrogen-secreting adrenal tumors in males lead to gynecomastia and testicular atrophy (6). High level of DHEA-S is another marker suggesting ACC, whereas decreased serum DHEA-S concentrations are suggestive of a benign adenoma (6). ACC producing aldosterone is very rare and present with hypertension and pronounced hypokalemia (7).
In cases without clinical hormone overactivity, the most common presentation is related to tumor growth and encroachment on the surrounding viscera, with symptoms such as abdominal discomfort, back pain, and nausea or vomiting (8, 9).
The diagnosis of malignancy of adrenocortical tumors relies not only on careful clinical and biological investigations, but above all on radiological imaging such as computerized tomography (CT) or magnetic resonance imaging (MRI) and more recently the use of 18F-FDG PET to distinguish between benign and malignant lesions (10, 11).
Survival for patients with ACC is poor and related to stage at time of diagnosis: up to 70% of patients present with extra-adrenal disease (12).
Five-year survival for patients with disease confined to the adrenal gland is size-dependent and varies from 61 to 82%. Those with distant metastases at diagnosis have a five-year survival of only 18% (13).
The classification of ACC by the International Union against Cancer (UICC) and the World Health Organization (WHO) in 2004 is based on the tumor, lymph node and metastasis (TNM) criteria; in 2008, the European Network for the Study of Adrenal Tumors (EN-SAT) proposed a revision of this staging, in which stage III is defined by tumour infiltration in surrounding tissue or tumour thrombus in vena cava/renal vein or positive lymph nodes, and stage IV is defined only by the presence of distant metastases (14).
Unfortunately, ACC prognosis is very poor. It depends largely on the stage of tumor: the rate of survival at 5 years is estimated at 60% for patients with stage I cancer, 58% for stage II tumors, 24% for stage III tumors, and 0% for disease stage IV (15).
Patients with stage I and II of disease are amenable to potentially curative surgery. They have a prolonged survival compared to patients with stage III and IV (15).
Complete surgical removal of ACC represents the current treatment of choice for this tumor. The likelihood of achieving a healing is by radical surgery, especially in the lower stages of cancer (I and II). A disease-free resection margin (R0) is also an important predictor of long-term survival. However, locoregional recurrence or the appearance of distant metastases during the subsequent follow up is common (85%) even after complete resection of the tumor (16–18).
The probability of failure increases in advanced stage of disease. This happens when the lesion is greater than 12 centimeters in maximum diameter, with a high mitotic rate and intralesional hemorrhage (19).
Therefore surgery is demanding and must be performed by a highly experienced surgical team using a laparotomic approach.
Intraoperatively, maintaining tumor capsule integrity and preventing tumor spillage are key considerations (20). Notably, presence of tumor thrombi and vascular invasion are not contraindications to resection. In cases with extensive vascular involvement, usage of cardiopulmonary bypass can facilitate successful resection (21). A transabdominal, open surgical approach allows maximal exposure. This facilitates en bloc excision of tumor and other involved organs (usually kidney, spleen, pancreas or liver), maintenance of the tumor capsule, and effective vascular control when necessary.
Metastatic disease debulking surgery, which removes as much of the tumor as possible, helps to reduce the blockage caused by a mass, usually large as well as the hormonal excess produced by the tumor. However, there is no significant effect of this therapy, since the median survival of patients with incomplete resection of the primary tumor or inoperable metastatic disease not removable by surgery appears to be less than 12 months. Surgical resection of recurrent disease is accepted as a treatment option of choice, as it is associated in a different series with an increase in median overall survival (21, 22).
Radiotherapy treatment is indicated in patients with R1–R2 resections, stage III disease and metastatic disease (bones) (23, 24).
Medical therapy includes regimens with cytotoxic agents such as mitotane alone or in combinations with streptozocin, etoposide/doxorubicin/cisplatin (25–28).
Conclusion
Adrenocortical carcinoma (ACC) is a rare and aggressive tumor with poor survival.
Complete surgical removal of ACC represents the current treatment of choice for this tumor, and a disease-free resection margin (R0) is an important predictor of long-term survival.
Therefore surgery for ACC removal is demanding and must be performed by a highly experienced surgical team using a laparotomic approach.
Footnotes
Conflict of interests
The authors declare they have no conflict of interests.
References
- 1.National Cancer Institute. “Third national cancer survey: incidental data” DHEW publication no. (NIH) 75–787. NCI Monogr. 1975;41 [Google Scholar]
- 2.Dackiw APB, Lee JE, Gagel RF, Evans DB. Adrenal cortical carcinoma. World Journal of Surgery. 2001;25(7):914–926. doi: 10.1007/s00268-001-0030-7. [DOI] [PubMed] [Google Scholar]
- 3.Bielinska M, Parviainen H, Kiiveri S, Heikinheimo M, Wilson DB. Review paper: origin andmolecular pathology of adrenocortical neoplasms. Veterinary Pathology. 2009;46(2):194–210. doi: 10.1354/vp.46-2-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patalano A, Brancato V, Mantero F. Adrenocortical cancer treatment. Hormone Research. 2009;71(1):99–104. doi: 10.1159/000178049. [DOI] [PubMed] [Google Scholar]
- 5.Icard P, Goudet P, Charpenay C, Andreassian B, Carnaille B, Chapuis Y, Cougard P, Henry JF, Proye C. Adrenocortical carcinomas: surgical trends and results of a 253-patient series from the French Association of Endocrine Surgeons study group. World J Surg. 2001;25:891–897. doi: 10.1007/s00268-001-0047-y. [DOI] [PubMed] [Google Scholar]
- 6.Fassnacht M, Kenn W, Allolio B. Adrenal tumors: how to establish malignancy? Journal of Endocrinological Investigation. 2004;27(4):387–399. doi: 10.1007/BF03351068. [DOI] [PubMed] [Google Scholar]
- 7.Seccia TM, Fassina A, Nussdorfer GG, Pessina AC, Rossi GP. Aldosterone-producing adrenocortical carcinoma: an unusual cause of Conn’s syndrome with an ominous clinical course. Endocrine-Related Cancer. 2005;12(1):149–159. doi: 10.1677/erc.1.00867. [DOI] [PubMed] [Google Scholar]
- 8.Fassnacht M, Allolio B. Clinical management of adrenocortical carcinoma. Best Practice and Research. 2009;23(2):273–289. doi: 10.1016/j.beem.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 9.Zini L, Porpiglia F, Fassnacht M. Contemporary management of adrenocortical carcinoma. European Urology. 2011;60(5):1055–1065. doi: 10.1016/j.eururo.2011.07.062. [DOI] [PubMed] [Google Scholar]
- 10.Blake MA, Slattery JMA, Kalra MK, et al. Adrenal lesions: characterization with fused PET/CT image in patients with proved or suspected malignancy - initial experience. Radiology. 2006;238(3):970–977. doi: 10.1148/radiol.2383042164. [DOI] [PubMed] [Google Scholar]
- 11.Ilias I, Sahdev A, Reznek RH, Grossman AB, Pacak K. The optimal imaging of adrenal tumours: a comparison of different methods. Endocrine-Related Cancer. 2007;14(3):587–599. doi: 10.1677/ERC-07-0045. [DOI] [PubMed] [Google Scholar]
- 12.Allolio B, Fassnacht M. Clinical review: adrenocortical carcinoma: clinical update. Journal of Clinical Endocrinology and Metabolism. 2006;91(6):2027–2037. doi: 10.1210/jc.2005-2639. [DOI] [PubMed] [Google Scholar]
- 13.Fassnacht M, Johanssen S, Quinkler M, et al. Limited prognostic value of the 2004 International Union Against Cancer staging classification for adrenocortical carcinoma: proposal for a Revised TNM Classification. Cancer. 2009;115(2):243–250. doi: 10.1002/cncr.24030. [DOI] [PubMed] [Google Scholar]
- 14.Lughezzani G, Sun M, Perrotte P, et al. The European Network for the Study of Adrenal Tumors staging system is prognostically superior to the international union against cancer-staging system: a North American validation. European Journal of Cancer. 2010;46(4):713–719. doi: 10.1016/j.ejca.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Icard P, Goudet P, Charpenay C, et al. Adrenocortical carcinomas: surgical trends and results of a 253-patient series from the French Association of Endocrine Surgeons Study Group. World Journal of Surgery. 2001;25(7):891–897. doi: 10.1007/s00268-001-0047-y. [DOI] [PubMed] [Google Scholar]
- 16.Pommier RF, Brennan MF. An eleven-year experience with adrenocortical carcinoma. Surgery. 1992;112(6):963–971. [PubMed] [Google Scholar]
- 17.Hedican SP, Marshall FF. Adrenocortical carcinoma with in-tracaval extension. Journal of Urology. 1997;158(6):2056–2061. doi: 10.1016/s0022-5347(01)68152-7. [DOI] [PubMed] [Google Scholar]
- 18.Schulick RD, Brennan MF. Long-term survival after complete resection and repeat resection in patients with adrenocortical carcinoma. Annals of Surgical Oncology. 1999;6(8):719–726. doi: 10.1007/s10434-999-0719-7. [DOI] [PubMed] [Google Scholar]
- 19.Stojadinovic A, Ghossein RA, Hoos A, et al. Adrenocortical carcinoma: clinical, morphologic, and molecular characterization. Journal of Clinical Oncology. 2002;20(4):941–950. doi: 10.1200/JCO.2002.20.4.941. [DOI] [PubMed] [Google Scholar]
- 20.Chiche L, Dousset B, Kieffer E, Chapuis Y. Adrenocortical carcinoma extending into the inferior vena cava: presentation of a 15-patient series and review of the literature. Surgery. 2006;139(1):15–27. doi: 10.1016/j.surg.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 21.Reibetanz J, Jurowich C, Erdogan I, et al. Impact of lympha-denectomy on the oncologic outcome of patients with adreno-cortical carcinoma. Annals of Surgery. 2012;255(2):363–369. doi: 10.1097/SLA.0b013e3182367ac3. [DOI] [PubMed] [Google Scholar]
- 22.Crucitti F, Bellantone R, Ferrante A, et al. The italian registry for adrenal cortical carcinoma: analysis of a multiinstitutional series of 129 patients. Surgery. 1996;119(2):161–170. doi: 10.1016/s0039-6060(96)80164-4. [DOI] [PubMed] [Google Scholar]
- 23.Polat B, Fassnacht M, Pfreundner L, et al. Radiotherapy in adrenocortical carcinoma. Cancer. 2009;115(13):2816–2823. doi: 10.1002/cncr.24331. [DOI] [PubMed] [Google Scholar]
- 24.Milgrom SA, Goodman KA. The role of radiation therapy in the management of adrenal carcinoma and adrenal metastases. Journal of Surgical Oncology. 2012;106(5):647–650. doi: 10.1002/jso.23096. [DOI] [PubMed] [Google Scholar]
- 25.Khan TS, Imam H, Juhlin C, et al. Streptozocin and o,p_DDD in the treatment of adrenocortical cancer patients: long-term survival in its adjuvant use. Annals of Oncology. 2000;11(10):1281–1287. doi: 10.1023/a:1008377915129. [DOI] [PubMed] [Google Scholar]
- 26.Berruti A, Terzolo M, Sperone P, et al. Etoposide, doxorubicin and cisplatin plus mitotane in the treatment of advanced adrenocortical carcinoma: a large prospective phase II trial. Endocrine-Related Cancer. 2005;12(3):657–666. doi: 10.1677/erc.1.01025. [DOI] [PubMed] [Google Scholar]
- 27.Fassnacht M, Terzolo M, Allolio B, et al. Combination chemotherapy in advanced adrenocortical carcinoma. The New England Journal of Medicine. 2012;366(23):2189–2197. doi: 10.1056/NEJMoa1200966. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez RJ, Tamm EP, Ng C, et al. Response to mitotane predicts outcome in patients with recurrent adrenal cortical carcinoma. Surgery. 2007;142(6):867–875. doi: 10.1016/j.surg.2007.09.006. [DOI] [PubMed] [Google Scholar]