Abstract
There is little information on the function of epididymal basal cells. These cells secrete prostaglandins, can metabolize radical oxygen species, and have apical projections that are components of the blood-epididymis barrier. The objective of this study was to develop a reproducible protocol to isolate rat epididymal basal cells and to characterize their function by gene expression profiling. Integrin-alpha6 was used to isolate a highly purified population of basal cells. Microarray analysis indicated that expression levels of 552 genes were enriched in basal cells relative to other cell types. Among these genes, 45 were expressed at levels of 5-fold or greater. These highly expressed genes coded for proteins implicated in cell adhesion, cytoskeletal function, ion transport, cellular signaling, and epidermal function, and included proteases and antiproteases, signal transduction, and transcription factors. Several highly expressed genes have been reported in adult stem cells, suggesting that basal cells may represent an epididymal stem cell population. A basal cell culture was established that showed that these basal cells can differentiate in vitro from keratin (KRT) 5-positive cells to cells that express KRT8 and connexin 26, a marker of columnar cells. These data provide novel information on epididymal basal cell gene expression and suggest that these cells can act as adult stem cells.
Keywords: adult stem cells, cell isolation, cell signaling, epididymis, genomics, integrinα6
INTRODUCTION
In the adult rat epididymis, basal cells comprise approximately 20% of the total epithelial cell population, which also includes principal, clear, dendritic, apical, and narrow cells [1, 2]. As their name implies, these cells are lodged at the base of the epithelium, where they are in contact with the basement membrane (BM). Basal cells, with a low cytoplasm-to-nucleus ratio, have long processes that extend laterally along the BM, where they form an incomplete layer. Thin apical projections extend from some basal cells toward the lumen of the epididymis [3, 4], where they cross the tight junctions of the blood-epididymis barrier, thereby contributing to the barrier [4]. Basally and apically located projections are regulated by androgens and other testicular factors [5–7].
In the rat, the epithelium of the epididymis at birth is characterized by undifferentiated columnar cells [8]. At this age, the epithelium contains numerous mitotic figures and expresses the gap junction protein beta-2 (GJB2; connexin 26 [Cx26]) [8, 9]. The epithelium begins to differentiate around Postnatal Day 16 [1]. Basal cells first appear in the cauda epididymal region at Day 21 and are present throughout the epididymis at Day 28 [10]. Concomitant with the decline of GJB2 expression at this age is the beginning of GJB1 (Cx32) expression [9]. Sun and Flickinger [1] first proposed that basal cells were derived from columnar cells, in a manner similar to principal cell differentiation. Despite suggestions to the contrary, recent studies have supported the notion that basal cells are derived from columnar cells [11].
Murashima et al. [12] showed that the transcription factor tumor protein 63 (TP63) is required for the differentiation of basal cells in the epididymis. TP63 is detected at Postnatal Day 14 in the cauda region of the epididymis and by Day 18 in the caput region [13]. The expression of TP63 in the proximal regions of the epididymis is dependent on androgen receptor signaling, while, in the cauda region, this does not appear to be the case, as TP63 levels are unaltered in AR knockout mice [12].
Although the role of basal cells in the epididymis remains unclear, several studies have reported the presence of enzymes implicated in metabolizing free oxygen radicals. Nonogaki et al. [14] first reported the presence of Cu-Zn superoxide dismutase (SOD) 1 in epididymal basal cells. Several subunits of the glutathione-S-transferase family of proteins [15], as well as metallothionein (MTN) 1/2 [16] were also shown to be expressed in epididymal basal cells. It has also been proposed that basal cells are stationary, macrophage-like cells that protect spermatozoa from the immune system [17, 18]. However, the recent discovery of dendritic cells in the epididymal epithelium suggests that the immune functions originally attributed to basal cells may be due to dendritic cells localized in the same region at the base of the epithelium [19]. Basal cells have also been suggested to provide structural support to the epididymis [20].
Several studies have shown that basal cells interact directly with the other epithelial cells of the epididymis. Basal cells contain elevated levels of prostaglandin-endoperoxide synthase 1 (PTGS1; also known as cyclooxygenase-1) and secrete prostaglandin (PG) E2 and D, which can act on principal cells [21]. Cyr et al. [22] showed the presence of gap junction protein alpha 1 (GJA1; also known as Cx43), and gap junctions between basal cells and both adjacent principal and clear cells. Furthermore, Gregory et al. [23] showed that basal cells of the epididymis express the tight junction protein, claudin-1. Such interactions may be relevant to principal cell functions.
Basal cells are present in most stratified and pseudostratified epithelia, including the trachea, prostate, and mammary gland [24–26]. In these tissues, basal cells make up between 6% and 30% of epithelial cells. Several studies on the trachea indicate that basal cells drive homeostasis of the epithelium [27–29]. In tissues, such as the trachea and other organs, basal cells are multipotent stem cells that can be induced to differentiate into other cell types in order to regenerate the epithelium [30–34], but this has not yet been demonstrated for the epididymis. Alterations in basal cell proliferation and differentiation have been associated with the development of cancer in the trachea, prostate, and mammary glands [35–37]
One of the main difficulties in understanding the function of basal cells has been the isolation of a purified basal cell population. While previous studies have reported purification of basal cells using either density gradients or magnetic separation [38–40], the absence of specific basal cell markers has limited the validity of the claims of isolation of highly purified basal cells. The objective of this study was to isolate and characterize a highly purified basal cell population, in order to assess the nature of these cells within the epididymal epithelium.
MATERIALS AND METHODS
Animals and Tissues Collection
Male Sprague-Dawley rats (48 and 90 days of age) were purchased from Charles River Laboratories, Inc. Rats were acclimated for at least 1 wk under a constant photoperiod (12L:12D), and received food and water ad libitum. At the time of sampling, rats were anesthetized with CO2 and killed by cervical dislocation. Epididymides were dissected from the animals under aseptic conditions and placed in Dulbecco modified Eagle medium (DMEM)/nutrient F12 Ham culture media containing penicillin (50 U/ml) and streptomycin (50 μg/ml) (Sigma-Aldrich). For cryoblock preparation, epididymides were frozen in OCT compound (Fisher Scientific) on dry ice and stored at −86°C until sectioning. All the animal protocols used in this study were approved by the Institut National de la Recherche Scientifique University Animal Care Committee.
Immunofluorescence of Epididymal Sections
Frozen epididymal sections (10 μm) were fixed in ice-cold methanol for 20 min at −20°C. After rehydration in PBS-Tween (0.05%), sections were permeabilized in a solution of 0.3% Triton X-100 in PBS at room temperature for 20 min. Sections were incubated with blocking solution (5% bovine serum albumin [BSA] in PBS) for 30 min and then incubated with a mouse monoclonal anti-integrinα6 antibody (ITGA6; 1 μg/ml; Abd Serotec, Bio-Rad Laboratories) or a rabbit monoclonal anti-PTGS1 antibody (0.3 μg/ml; Abcam) diluted in blocking solution at room temperature for 1 hr. Sections were washed three times with PBS-Tween and subsequently incubated with an anti-mouse Alexa 594 (red; Life Technologies) or an anti-rabbit Alexa 488 (green; Life Technologies) -conjugated secondary antibody (2 μg/ml) at room temperature for 30 min in blocking buffer containing a Hoechst blue dye (1 μg/ml; Biotium). Finally, sections were washed twice with PBS-Tween and once with PBS and mounted with Fluoromount (Southern Biotech).
Colocalization experiments were done using cryosections prepared in the same fashion as described previously. Sections were incubated with anti-ITGA6 antibody (1 μg/ml) for 1 h at room temperature, rinsed in PBS, and subsequently incubated for 30 min at room temperature with Alexa 594-conjugated anti-mouse antisera (2 μg/ml). Sections were then rinsed three times in PBS-Tween and incubated with anti-PTGS1 antibody (0.30 μg/ml) for 1 h at room temperature. Sections were subsequently washed three times in PBS-Tween and incubated for 30 min at room temperature with an Alexa 488-conjugated anti-rabbit secondary antibody (2 μg/ml) containing a Hoechst blue dye (1 μg/ml) to stain the nuclei. Finally, sections were washed twice with PBS-Tween, once with PBS, and then mounted with Fluoromount.
Epithelial Cell Isolation
Epididymides from four rats (48 days of age) were used for each isolation. Tissues were cleared of fat and cut into small fragments (2–3 mm) with scissors. Tissue fragments were placed in DMEM/nutrient F12 Ham containing antibiotics and digested in two successive incubations (50 min) in collagenase (2 mg/ml; Life Technologies Inc.) at 37°C using a shaking water bath. Epididymal cells were dissociated between digestions by gently pipetting the small tissue fragments and allowing the cells to settle to the bottom of the tube. The supernatant was then replaced with fresh digestion medium. Following the second digestion, tissue fragments were placed in trypsin-ethylenediaminetetraacetic acid (EDTA; 0.25% W/V) for 15 min at 37°C in a shaking water bath. Trypsin activity was stopped by the addition of DMEM/HAM F12 medium containing antibiotics, L-glutamine (2 mM), insulin (10 μg/ml), transferrin (10 μg/ml), hydrocortisone (80 ng/ml), epidermal growth factor [EGF; 10 ng/ml]), cAMP (10 ng/ml) and fetal bovine serum (FBS; 5%; Fraction V; Sigma-Aldrich). Cells were collected by centrifugation (34 × g) for 3 min, resuspended in culture media and successively passed through three nylon filters of 160, 100, and 70 μm. An aliquot of cells was then counted with a hemocytometer.
Magnetic-Activated Cell Sorting
Cells were centrifuged at 1000 × g for 5 min and resuspended in filtered, cold, magnetic-activated cell sorting (MACS) buffer (2 mM EDTA, 0.5% BSA in PBS, pH 7.2). Cells were then incubated with a monoclonal antibody against ITGA6 (also known as CD49f; 1 μg/106 cells; Abd Serotec) on ice for 20 min. Cells were washed twice in 2 ml of MACS buffer and collected by centrifugation (1000 g for 10 min). Cells were then incubated for 15 min on ice with anti-mouse IgG microbeads (20 μl of beads in 80 μl of cold MACS buffer/107 cells; Miltenyi Biotec). Cells were washed twice, resuspended in 500 μl of cold MACS buffer, and placed on a MACS MS separation column (Miltenyi Biotec). The column was placed in a magnetic stand (Miltenyi Biotec) and cells that were first eluted through the column were considered as being the negative or nonbasal cell fraction. The column was then rinsed with MACS buffer and removed from the magnetic stand. MACS buffer (1 ml) was then used to elute the cells that were retained on the column. This was designated as the positive or basal cell fraction. The cells collected in the basal cell fraction were further purified by a second passage on a magnetic separation column.
Immunofluorescent Microscopy of Basal Cells
Following the magnetic separation, cells from each fraction were resuspended in PBS. The cell concentration was determined using a hemocytometer. An aliquot of 1 × 105 cells in 100 μl of PBS was placed on a glass microscope slide, dried for 40 min, and immersed in ice-cold methanol overnight. After rehydration in PBS, cells were permeabilized in a solution of 0.3% Triton X-100 in PBS at room temperature for 15 min. Cells were blocked with PBS containing 5% BSA (blocking solution) for 30 min and then incubated with a monoclonal anti-keratin (KRT) 5 antibody (1 μg/ml; Santa Cruz Biotechnologies, Dallas, TX) diluted in blocking solution at room temperature for 1 h. Cells were washed three times in PBS-Tween and subsequently incubated with an anti-mouse Alexa 594-conjugated secondary antibody (2 μg/ml; Life Technologies) at room temperature for 30 min. Slides were subsequently washed three times with PBS and mounted with Vectastain mounting medium containing 4′,6-diamidino-2-phenylindole (Vector Laboratories). Cells were examined under a Leica DMRE microscope (Leica Microsystems, Inc.).
Flow Cytometry
Cells (1 × 106) from the nonbasal and basal cell fractions were fixed in paraformaldehyde (1% in PBS) for 24 h. Cells were washed twice with FACS buffer (1 ml; 1% BSA in PBS) and recovered by centrifugation at 1000 × g for 10 min. The supernatant was removed and the cells were resuspended in a solution of Triton X-100 (0.3%) in PBS at room temperature for 15 min. Cells were then blocked in FACS buffer on ice for 30 min and incubated with an anti-PTGS1 monoclonal antibody conjugated with fluorescein isothiocyanate (FITC; 5 μg/ml; Cayman Chemical) for 1 h. Cells were washed three times with FACS buffer and resuspended in 500 μl of PBS containing 1% paraformaldehyde prior to analysis. Flow cytometric analyses were done using a FACSCalibur (Becton Dickinson) with an air-cooled argon laser providing an excitation at 488 nm and analyzed using the Cell Quest Pro software (BD Biosciences).
Electron Microscopy
Adult rats (n = 4; 90 days of age) were anesthetized by intraperitoneal injection of sodium pentobarbital (Somnitol; MTC Pharmaceuticals). The testes and epididymides were fixed by retrograde perfusion through the abdominal aorta with 2.5% glutaraldehyde buffered in sodium cacodylate (0.1 M) containing 0.05% calcium chloride (pH 7.4). After 10 min of perfusion, epididymides were removed and cut into four major regions (initial segment, caput, corpus, and cauda). Small 1-mm3 pieces were cut from each of the four regions and placed in the same fixative for an additional 2 h at 4°C. The tissue samples were subsequently rinsed three times in 0.1 M sodium cacodylate buffer containing 0.2 M sucrose and then left in this buffer overnight. The following day, the samples were postfixed in ferrocyanide-reduced osmium tetroxide for 1 h at 4°C, dehydrated in a graded series of ethanol and propylene oxide, and embedded in Epon. Semi-thin sections (0.5 mm) were cut with glass knives, stained with toluidine blue, and observed by light microscopy. Thin sections of selected regions of each block were cut with a diamond knife, placed on copper grids, and counterstained with uranyl acetate (5 min) and lead citrate (2 min). Sections were examined with either a Philips 400 or FEI Tecnai 12 120 kV Transmission Electron Microscope (FEI Company).
Cells of the basal cell fraction were collected by centrifugation (300 × g), washed, and resuspended in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer for 24 h. The cells were then washed in PBS and prepared for electron microscopy as describe above.
Polymerase Chain Reaction
Total cellular RNA was isolated from the cells using the RNeasy Plus Mini Kit (Qiagen) according to the manufacturer's instructions. Total RNA (500 ng) was reverse transcribed using an oligo (dT)16 primer. Specific transcripts were subsequently amplified by PCR. Primer sequences are listed in Supplemental Table S1 (Supplemental Data are available online at www.biolreprod.org). PCR amplifications were done at 94°C for 5 min, 25–35 cycles of 94°C for 30 sec, melting temperature for 30 sec, 72°C for 30 sec, and cooled at 4°C. PCR products were then separated on a 2% agarose gel and visualized using ethidium bromide (Bio-Rad Laboratories). PCR amplifications were also done on untranscribed RNA to confirm that the sample was not contaminated with genomic DNA. PCR analyses were done in triplicate. Each experiment was done in triplicate using three different isolated basal cell fractions.
Microarray Processing
Total cellular RNA was isolated from the nonbasal and basal cell fractions using the RNeasy Plus Mini Kit (Qiagen) according to the manufacturer's instructions. The RNA concentration was determined using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies), while the quality of the RNA was verified using an Agilent 2100 Bioanalyzer (Agilent Technologies). Gene expression profiling was performed with commercially available rat oligonucleotide microarrays, GeneChip Rat Gene 2.0 ST array, containing 23 586 genome-wide transcripts (Affymetrix). Hybridizations were performed by Genome Québec's Innovation Centre (McGill University). Target RNA was reverse transcribed into cDNA and in vitro transcription was performed to generate biotin-labeled cRNA. Hybridized target cRNA was labeled with streptavidin phycoerythrin and the arrays were scanned using a GeneChip Scanner (Affymetrix).
Microarray Analysis
The relative expression level of each transcript was obtained from three separate isolated fractions. Raw data were corrected for background using the Robust Multi-array Average algorithm [41] and Expression Console software (Affymetrix). Expression analysis was done according to Minimum Information about a Microarray Experiment standards [42] using GeneSpring 12X software (Agilent). Only genes showing a P value ≤ 0.05 were considered for data analysis. Significant gene expression differences were determined with moderated t-test (significance level ≤ 0.05). Fold-changes were expressed as the ratio between basal and nonbasal cell fractions. A 2-fold change cut-off value was used for the determination of differentially expressed genes. Genes with the same expression pattern were grouped in clusters using the K-means method with Pearson correlation and a maximum of 50 iterations. Enriched genes were imported into Database for Annotation, Visualization, and Integrated Discovery (DAVID) Bioinformatics Resources software (version 6.7; Database for Annotation, Visualization, and Integrated Discovery; National Institute of Allergy and Infectious Disease, National Institutes of Health) [43] to determine gene ontology. Calculations of enrichment scores were based on the Expression Analysis Systematic Explorer scores, which is a modified Fisher exact P value [44]. Functional analysis for pathways were done using DAVID linked to the Kyoto Encyclopedia of Genes and Genomes (KEGG) databank with a cut-off of 1.5-fold change.
Basal Cell Culture
Immediately after separation, isolated basal cells were resuspended in Prostacult medium (Stemcell technologies) containing EGF (10 ng/ml; (Sigma-Aldrich), basic fibroblast growth factor (bFGF; 10 ng/ml; ReproCELL), heparin (4 μg/ml; Life Technologies), and FBS charcoal-stripped (5%; Wisent), according to the manufacturers' instructions for stem cell culture. Experiments were done to determine whether or not bFGF and dihydrotestosterone (DHT) were necessary for maintaining basal cells in culture. In these experiments, cells were cultured for up to 7 days in the presence or absence of bFGF (10 ng/ml) and DHT (10 nM; Sigma Aldrich). In all subsequent experiments, DHT (10 nM) was added to the culture medium. Cells were seeded on Labtek Nunc II (Thermo Fisher Scientific) coated with a thin layer (40 μl/well) of phenol red-free high-concentration Matrigel (Corning) diluted 1:1 in medium. Cells were incubated at 32°C with 5% CO2. The medium (50%) was changed every 7 days.
Immunofluorescence of Cultured Cells
Cells were examined after 3, 7, 10, and 14 days of culture. Wells were rinsed twice with PBS and fixed with ice-cold methanol (KRT5) or paraformaldehyde (4%; for KRT8 and Cx26) for 10 min. Cells were then rinsed with Tween 0.05% in PBS for 5 min and permeabilized with 0.3% Triton-X-100 in PBS for KRT5. Immunolabeling was done as described previously using a monoclonal anti-KRT5 antibody (Santa Cruz Biotechnology), a polyclonal anti-KRT8 antibody (Santa Cruz Biotechnology), or an anti-Cx26 antibody (Life Technologies) diluted in blocking solution and incubated at room temperature for 1.5 h. Following washing, the cells were incubated at room temperature with an anti-mouse Alexa fluor 594-conjugated antibody (2 μg/ml; Life Technologies) or an anti-rabbit Alexa fluor 488-conjugated antibody (2 μg/ml, Life Technologies) containing Hoechst dye (1 μg/ml; Biotium). Slides were mounted in Fluoromount. Immunofluorescence was examined under a Nikon A1Plus confocal microscope, and images were analyzed with the NIS-Elements AR software (Nikon).
Statistics
Data are presented as the mean ± SEM. Statistical analyses were performed using ANOVA. Significance was established as P ≤ 0.05. Except for microarrays, all analyses were performed using GraphPad Prism software (GraphPad Software, Inc.).
RESULTS
Localization of ITGA6 in the Epididymis
Immunofluorescence experiments indicated that ITGA6 is localized to the base of the epithelium in all regions of both 48 day-old (Fig. 1, A–D) and adult (Supplemental Fig. S1) rat epididymis. To confirm that ITGA6 was localized to epididymal basal cells, colocalization experiments with PTGS1, a marker of basal cells [21], was done. The specificity of the PTGS1 antibody for basal cells was determined by immunofluorescence microscopy, which indicated that PTGS1 was localized to basal cells throughout the epididymis (Supplemental Fig. S2). The data showed that ITGA6 colocalized to the same cells as PTGS1, thus confirming that ITGA6 was indeed expressed by epididymal basal cells (Fig. 1E; Supplemental Fig. S3). Sections incubated in the absence of antibody were used as negative controls (Fig. 1F).
FIG. 1.
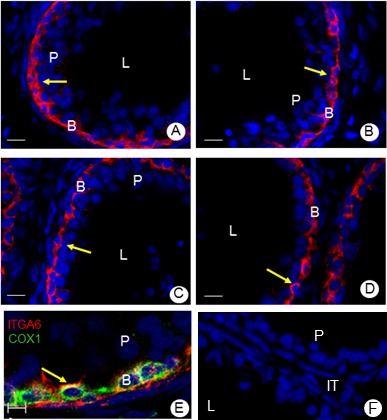
Immunolocalization of ITGA6 in the different regions of the epididymis in a rat of 48 days of age. ITGA6 (red; arrow) was localized to the basal compartment of the epithelium in all regions (A, initial segment; B, caput; C, corpus; D, cauda) of the epididymis. Bar = 10 μm. ITGA6 (red) and PTGS1 (green) in the basal cells of the caput epididymis (E). Merged image shows that ITGA6 and PTGS1 colocalize to basal cells (arrow). Similar results were observed in all other regions of the epididymis (Supplemental Fig. S3). A negative control, in which cells were incubated in the absence of primary antibody, is included (F). Bar = 5 μm. Nuclei are stained with Hoechst dye (blue). B, basal cells; L, lumen; P, principal cell.
Isolation of Epididymal Basal Cells
Using the ITGA6 antibody, basal cells were isolated from epididymal cells using magnetic separation. Immunofluorescent localization of KRT5, another basal cell marker [11], indicated that over 88.8 ± 1.7% of cells were positive for KRT5 (Fig. 2A). Only 2% of the cells isolated in the nonbasal cell fraction tested positive for KRT5. To further confirm that cells isolated by magnetic separation and ITGA6 antibody were basal cells, a flow cytometric analysis was done using PTGS1 as a marker of basal cells. Since the PTGS1 antibody used for flow cytometry was coupled to FITC, we confirmed its specificity by immunofluorescence microscopy, which indicated that PTGS1 was localized to basal cells throughout the epididymis, as expected (Supplemental Fig. S2). Merged flow cytometric graphs of PTGS1 immunofluorescent peaks showed a clear shift between curves for cells present in the nonbasal and basal cell fractions, demonstrating that there are two distinct cell fractions. Cell counts for immunostained cells indicated that 89.0 ± 1.5% of cells in the basal cell fraction were positive for PTGS1 (Fig. 2B). Thus, both immunofluorescence microscopy, using KRT5, and flow cytometry, using PTGS1, indicated similar levels of basal cell purity.
FIG. 2.
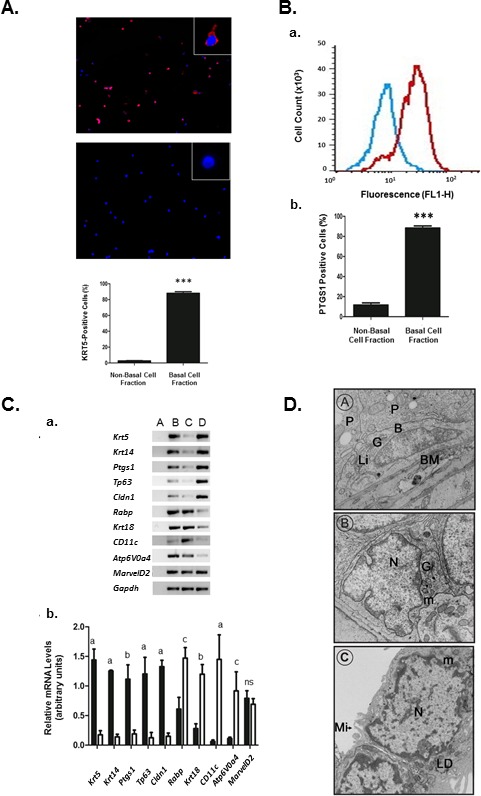
A) KRT5 immunostaining in cells from the isolated basal cell fraction and nonbasal cell fraction of the epididymis. Photomicrograph of KRT5 immunostaining (red) in the basal cell (upper panel) and nonbasal cell (lower panel) fractions. Insets show high magnification of a typical cell in both fractions. Nuclei are stained with Hoechst dye (blue). Original magnification ×100; insets, ×400. Cell counts of immunopositive cells indicate that 88.8 ± 1.7% of cells in the basal cell fraction and less than 2% of cells in the nonbasal cell fraction stained for KRT5 (n = 3); ***P ≤ 0.001 (ANOVA). B) Flow cytometric analysis of isolated cells immunostained with PTGS1. Comparison of PTGS1 immunostaining in the basal cell fraction and nonbasal cell fraction using flow cytometry. Blue curve represents the PTGS1 immunostaining of nonbasal cells, while red curve represents the immunostaining in the basal cell fraction. The data show a clear shift to the right in the basal cell fraction, indicating increased immunostaining. Cell counts indicate that there is a significant increase in PTGS1-positive cells in the basal cell fraction relative to the nonbasal cell fraction (n = 3). ***P ≤ 0.001 (ANOVA). C) Cellular mRNA levels (a) of genes expressed in epididymis (lane B), nonbasal cell fraction (lane C), and basal cell fraction (lane D). Lane A represents the negative control. RT-PCR of total cellular RNA indicate a significant increase (b) in mRNA levels in markers of basal cells (Krt5, Krt4, Ptgs1, Tp63, Cldn1) in the basal cell fraction versus the nonbasal cell fraction. Principal cell markers (Rabp, Krt18), dendritic cell (Cd11c), and clear cell (Atp6V0a4) markers were significantly elevated in the nonbasal cell fraction (white bars) as compared to the basal cell fraction (black bars). aP ≤ 0.05; bP ≤ 0.01; cP ≤ 0.001; ns, nonsignificant (ANOVA). D) Electron photomicrographs of basal cells in the epithelium of an adult rat epididymis and of isolated basal cells. D, A) An elongated basal cell contacts the BM and shows a flattened nucleus with a Golgi apparatus (G) and a lipid droplet (Li). B, basal cell; MY, myoid cell; N, nucleus; P, principal cell (original magnification ×15 200). D, B) A basal cell with an irregular nucleus. Note the low cytoplasm-to-nucleus ratio and the well-developed Golgi apparatus, including the presence of secretory granules (original magnification ×15 200). D, C) Two adjacent isolated basal cells showing a flattened nucleus; a low cytoplasm-to-nucleus ratio, and the presence of microvilli around the cell. Cell contact points between the cells suggest the presence of a junctional complex between the cells. B, basal cell; G, Golgi; LD, lipid droplet; Li, lipid; m, mitochondria; Mi, microvilli; My, myoid cell; N, nucleus; P, principal cell (original magnification ×29 000).
Expression of Basal Cell Markers
RT-PCR was used to verify the presence of transcripts for several epididymal cell markers. Krt5, Ptgs1, Krt14 [11, 40, 45], Cldn1, and Tp63 [13, 23] were used as markers for basal cells. All of these markers were present and were significantly enriched by 10- to 20-fold in the basal cell fraction (Fig. 2C).
Krt18 and retinoic acid-binding protein (Rabp) were used as markers for principal cells [35, 46]. H+ transporting lysosomal V0 subunit 4 (Atp6V0a4) was used as marker for clear cells and CD11c as a marker of dendritic cells [19, 47]. Total mRNA levels for each of the genes were significantly higher in the nonbasal cell fraction as compared to the basal cell fraction (Fig. 2C), confirming the high degree of purity in the basal cell fraction. Interestingly, there were no differences in the mRNA level for tight junction protein, tricellulin (MarvelD2), between basal and nonbasal cell fractions.
Analysis of Basal Cells Morphology
Ultrastructural analysis of basal cells in situ and in the basal cell fractions was done under the electron microscope (Fig. 2D). Two morphologically distinct basal cells appear to be present at the base of the epithelium. All basal cells are in contact with the BM. Some show a flattened nucleus (Fig. 2D, panel A), while others have an irregular nucleus and appear somewhat triangular in shape (Fig. 2D, panel B). The cells have a low cytoplasm-to-nucleus ratio and a well-developed Golgi apparatus, including the presence of secretory granules. In electron microsopy images of the isolated basal cell fraction, the cells assumed a rounded appearance with an irregular nucleus and few microvilli projecting from the periphery. These cells revealed a low cytoplasm-to-nuclear ratio, and the cytoplasm showed few organelles, as noted for in situ basal cells. In cells that remained in contact with one another, small areas in close proximity were apparent (Fig. 2D, panel C).
Gene Expression Profiling of Basal Cells
Microarray analyses were used to compare gene expression profiles for cells in the nonbasal and basal cell fractions. A rat oligonucleotide microarray containing 29 489 genes was used to assess gene expression levels. A total of 1470 genes with greater than 2-fold changes in steady-state mRNA levels were differentially expressed in basal cells as compared to cells in the negative fraction. Hierarchical clustering using the k-means method indicated that the basal and nonbasal cell fractions were distinct, with clearly identifiable groups of genes that were overexpressed in the basal cell fraction relative to the nonbasal cell fraction (Fig. 3A). A volcano plot of mRNA levels for each gene revealed a large number of genes that were significantly (P ≤ 0.05) enriched (≥2) or underrepresented (≤0.5) in the basal cell fraction as compared to the nonbasal cell fraction (Fig. 3B).
FIG. 3.
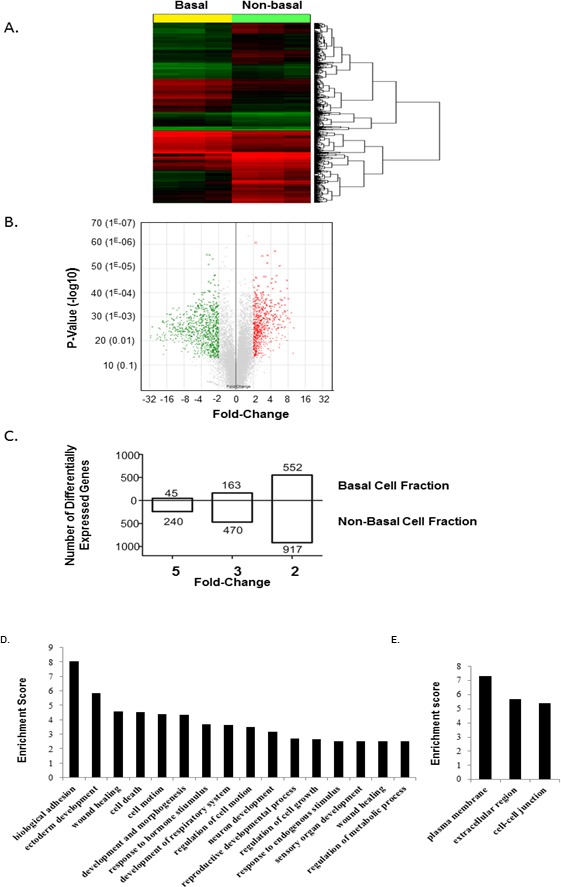
Identification of basal cell fraction-enriched transcripts. A) Heat map and hierarchical cluster analysis of genes expressed in the basal cell fraction (n = 3) compared to nonbasal cell fraction (n = 3). Genes expressed at levels above the average are represented in red, below average in green, and average in black. B) Volcano plot comparing the transcriptome of cells in the basal cell fraction (n = 3) and nonbasal cell fraction (n = 3). Red dots correspond to significantly up-regulated probe sets in basal cells (fold-change ≥ 2 with a p-value ≤ 0.05); green dots represent significantly down-regulated probe sets in basal cells (fold-change ≥ 2 with a P value ≤ 0.05); gray dots represent nonsignificant gene probe sets. C) Number of genes that are significantly differentially expressed by 2-, 4-, and 5-fold in the basal cell fraction compared to the nonbasal cell fraction. D) Differentially expressed genes in the isolated basal cell fraction and nonbasal cell fraction of the epididymis. Genes were grouped according to predicted biological function using the DAVID application software. Enrichment score calculations were based on Expression Analysis Systematic Explorer (EASE) score. Genes were considered differentially expressed when the difference was greater than 2-fold. D) Genes grouped according to their biological processes. E) Genes grouped according to the cellular components.
Of the 1470 differentially expressed genes, 552 genes were up-regulated (i.e., enriched in basal cells) and 917 genes were down-regulated (enriched in nonbasal cells). Among the enriched basal cell gene population, 163 genes were highly expressed, with mRNA levels 3-fold higher than in nonbasal cells, and 45 of these were expressed at levels of 5-fold or more (Fig. 3C). These highly expressed genes coded for proteins implicated in cell adhesion, cytoskeletal function, ion transport, proteases and antiproteases, signal transduction, transcription factors, cellular signaling, and epidermal function (Table 1). These data have been deposited in the National Center for Biotechnology Information's Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo; accession number GSE52693).
TABLE 1.
List of genes enriched in the basal cell fraction with a greater than 5 fold-change.
Specific Basal Cell Genes
Several genes previously defined as specific for basal cells were examined (Table 2). Cldn1, which we have shown to be present in basal and principal cells [23], was expressed at significantly higher levels in the basal cell fraction; two cytokeratins, Krt14 and Krt17, were also highly expressed in basal cells. Ptgs1, which was previously described in epididymal basal cells [21], Tp63 [48], and Cd44 [49] were all expressed at significantly higher levels in the basal cell fraction.
TABLE 2.
Expression level of previously described epididymal basal cell genes in the isolated basal cell fraction.
Certain genes previously suggested as being specific for epididymal basal cells were significantly lower in the basal cell fraction as compared to the nonbasal cell fraction. These included Agtr2 [4], Aqp3 [50], and Trpc3 [40].
Functional Classification of Genes Enriched in Epididymal Basal Cells
The genes that were differentially enriched in basal cells were classified into functional categories using gene ontology to predict biological processes, cellular components, and molecular functions of basal cell function (Fig. 3D). There were 16 clusters of biological processes that were predicted in basal cells (Fig. 3D), including biological adhesion, development and morphogenesis, cell motion, regulation of cell death, cell growth and cell proliferation, and response to endogenous stimuli. Highly expressed genes (≥4-fold) in basal cells could be grouped in to three specific categories of genes associated with the plasma membrane, cellular junctions, and interaction with the extracellular matrix (Fig. 3E).
Genes Implicated in Gap Junctions
Several genes coding for gap junction proteins were expressed at significantly higher levels in basal cells. These included gap junction protein alpha-1 (Gja1; Cx43), Gjb3 (Cx31), Gjb4 (Cx30.3), and Gjb5 (Cx31.1). These have all previously been shown to be expressed in the epididymis [9]. Interestingly, pannexin2 (Pnx2), which is expressed at high levels in the nervous system [51], was also present in basal cells (Fig. 4A). The large number of gap junction proteins suggests highly regulated intercellular communication between basal and other cell types of the epididymis.
FIG. 4.
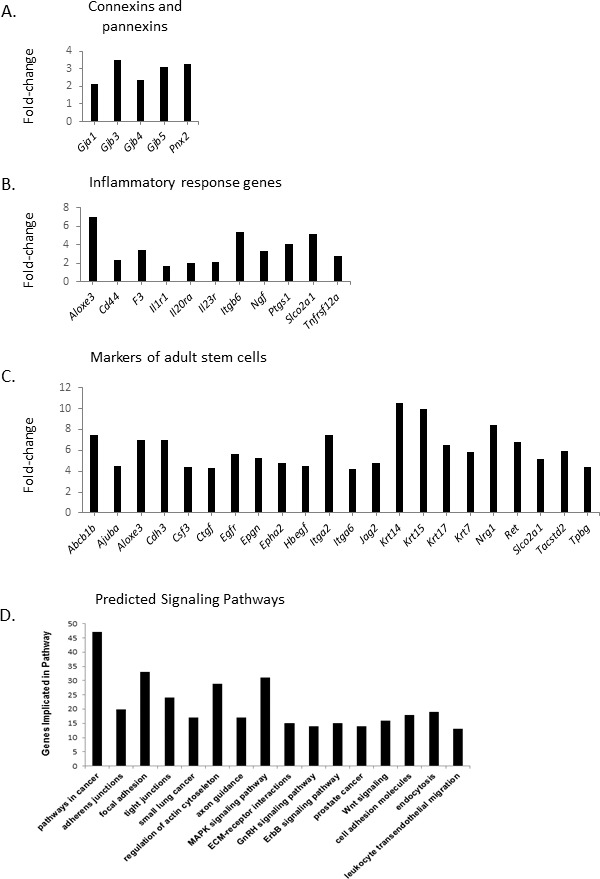
Differentially expressed genes implicated in gap junctions, prostaglandins synthesis, and multipotent stem cells in the basal cell fraction compared to the nonbasal cell fraction. A) Messenger RNA levels, enriched in the basal cell fraction, for genes coding for gap junction proteins and pannexins. B) Messenger RNA levels, enriched in the basal cell fraction, for genes implicated in inflammatory response. C) Messenger RNA levels, enriched in the basal cell fraction, for genes associated with multipotent adult stem cells. D) Predicted canonical pathways implicated in basal cell function. DAVID software was used to predict main pathways implicated in epididymal basal cells transcriptome. Genes are considered enriched with a differential regulation greater than 1.5-fold change with a P value ≤ 0.05.
Genes Implicated in the Inflammatory Response
Some genes involved in the biosynthesis and secretion of prostaglandins, known to be implicated in the inflammatory response, were expressed at significantly higher levels in basal cells. These included: Aloxe3, which mediates leukotriene degradation); Ptgs1, which converts arachidonic acid into prostaglandin; and solute carrier organic anion transporter family, member 2A1 (Slco2a1), which is the prostaglandin transporter. A number of anti-inflammatory genes were also expressed. Interleukin 6 (Il6) and Il23a, which are implicated in inflammation, were highly down-regulated, while Il1r2, which suppresses IL1 activity, was upregulated. Matrix metallopeptidase 2 (Mmp2), which degrades collagen IV and promotes inflammation, was also down-regulated. Annexin A1 (Anxa1) has a potential anti-inflammatory activity via an inhibition of eicosanoid synthesis (Fig. 4B).
Genes Implicated in Progenitor Cell Function
Some strongly expressed genes in basal cells are associated with adult multipotent stem cells in other tissues (Fig. 4C). Several ligands of known signaling pathways were present, including sonic hedgehog (Shh), the ligand of the Hedgehog pathway, required for embryonic stem cells, and jagged 2 (Jag2), a ligand of Notch signaling. Several cytokines were also specific to basal cells: Lif, KIT ligand (Kitlg), and colony-stimulating factor 3 (Csf3). Likewise, growth factors platelet-derived growth factor (Pdgf; Pdgfa; Pdgfb; Pdgfc), epithelial mitogen (Epgn), heparin-binding EGF-like growth factor (Hbegf), and neuregulin 1 (Nrg1) were all enriched in epididymal basal cells. Some receptors implicated in progenitor cell function were highly expressed in basal cells, including Cd44, Cd9, tumor-associated calcium signal transducer 2 (Tacstd2), ret proto-oncogene (Ret), epidermal growth factor receptor (Egfr), and EPH receptor A2 (Epha2). Finally, some transcription factors associated with gene expression in progenitor cells were also expressed in basal cells (GLI family zinc finger 3 [Gli3], Kruppel-like factor 5 [Klf5], sex-determining region Y-box 6 [Sox6], avian myelocytomatosis viral oncogene homolog [Myc], and ovo-like zinc finger 1 [Ovol1]).
TP63-Dependent Genes
Several highly expressed genes in epididymal basal cells have been shown to be regulated by the transcription factor TP63 in other cell types (Table 3). Several of these genes are implicated in cell adhesion (Itga3, Itga6, cadherin3 [Cdh3; P-Cadherin], and Cldn1). MDM2 proto-oncogene, E3 ubiquitin protein ligase (Mdm2), involved in the cell cycle, was also expressed at significantly higher levels in basal cells. v-ETS (avian erythroblastosis virus E26 oncogene homolog 1 [Ets1]) and tripartite motif containing 29 (Trim29), which are implicated in cellular differentiation, were expressed at significantly higher levels, as were ectodysplasin A receptor (Edar), bone morphogenetic protein 7 (Bmp7), interferon regulatory factor 6 (Irf6), and Jag2. Serpin peptidase inhibitor, clade B (ovalbumin), member 5 (Serpinb5), aldehyde dehydrogenase 1 family, member 3 (Aldh1a3), Serpine1, and glutathione peroxidase 2 (Gpx2) were all expressed at higher levels in basal cells. Finally, Krt14 was strongly expressed in epididymal basal cells. The large number of genes known to be regulated by TP63 suggests that it is a major regulator of basal cell function.
TABLE 3.
Differentially regulated genes expressed in the basal cell fraction and which are known to be regulated by TP63.
Intracellular Signaling Pathways
Several molecular pathways in epididymal basal cells were identified using DAVID software. Using KEGG, 16 different pathways were identified (Fig. 4D). Some of the KEGG pathways were related to structural components of the epithelium, and included pathways implicated in adherens and tight junctions, focal adhesion, extracellular matrix-receptor interactions, and cell adhesion molecules. Other pathways that were identified were related to signal transduction, such as those implicated in axon guidance, estrogen receptor β-2 (ErbB), WNT, and MAPK signaling. Surprisingly, signaling pathways implicated in cancer were strongly predicted in basal cells.
Cultured Epididymal Basal cells
Different methodologies were assessed to establish epididymal basal cell cultures. The cells did not adhere to either Cell + or collagen IV-coated plates. However, the cells adhered to Matrigel after 48 h of culture. The Matrigel was diluted 1:1 with culture medium, which has been used previously for culturing prostatic basal cells [52, 53]. Cell viability and maintenance of PTGS1 expression were assessed as indicators of basal cell health (Fig. 5A). Basic FGF was found to be critical for maintaining the expression of PTGS1. In the absence of bFGF, PTGS1 immunostaining disappeared after 3 days of culture. DHT was also required for maintaining the expression of PTGS1 in the cells. In cells incubated with levels of DHT (10 nM) equivalent to those present in the serum, the cells not only maintained the expression of PTGS1, but also formed spheroid acini (Fig. 5A). In the acini, KRT5-positive basal cells appeared to localize to the perimeter of the acini (Supplemental Movie S1). Cells grown in the presence of bFGF and DHT developed spheroid acinar structures (Fig. 5B). In the absence of bFGF and DHT, the acinar structures were not observed.
FIG. 5.
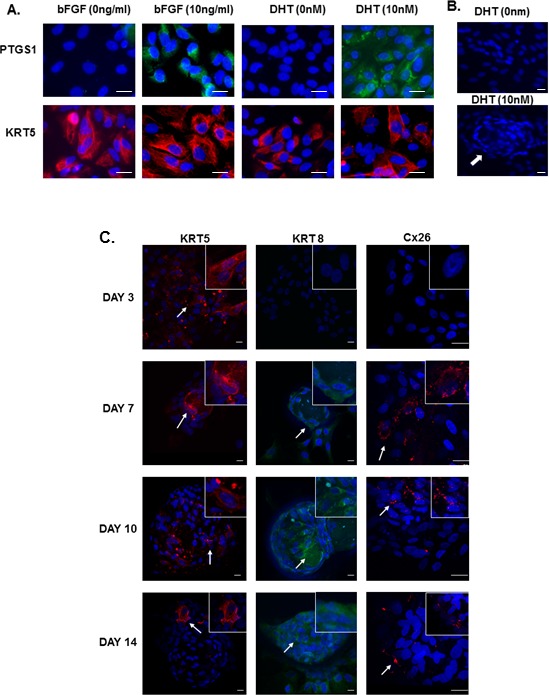
A) Immunolocalization of KRT5 and PTGS1 in basal cells cultured for 3 days in medium containing both DHT (10 nm) and bFGF (10 ng/ml). Without DHT or bFGF, cells were unable to maintain the expression of PTGS1. Bar = 20μm. B) After 7 days of culture, the cells cultured with DHT and bFGF formed acini (arrow). Cells cultured in the absence of these failed to form acini. Bar = 20 μm. C) Immunolocalization of KRT5 (red, left panel), KRT8 (green, middle panel), and GJB2 (red, right panel) in basal cells after 3, 7, 10, and 14 days of culture. Cells progressively formed spheroid structures, and KRT5 (arrows) disappeared from most of cells by Day 14. In contrast, both KRT8 (arrows) and GJB2 (arrows) immunostaining appear by Day 7 and remained expressed until Day 14. Nuclei were stained with Hoechst blue dye. Bar = 20 μm. Insets represent high-magnification representation of photomicrographs (see Supplemental Movies S1–S3.)
Basal Cell Differentiation
To determine if basal cells could differentiate in vitro, basal cells were cultured for up to 14 days. After 3 days of culture, cells remained in a flat, two-dimensional architecture. By 7 days, some spheroid acini began to appear. These spheroid acini became more prominent after 10 and 14 days of culture. While mitotic figures were observed outside the acini after 10 days of culture, these were not observed within the acinar structures. Immunostaining for the basal cell marker KRT5 indicated a progressive decrease in KRT5 immunostaining, which decreased from over 90% of the cells after 3 days of culture to as little as 5% of the cells by Day 14. This was particularly evident within the acini structures (Fig. 5C, Supplemental Movie S1). The decreased KRT5 immunostaining was accompanied by the appearance of KRT8 after 7 days of culture, which was not detected after 3 days of culture, but increased to almost 90% of the cells by 14 days (Fig. 5C, Supplemental Movie S2). Likewise, some of the cells also began expressing the gap junction protein Cx26 after 7 days of culture (Fig. 5C, Supplemental Movie S3).
DISCUSSION
The function of epididymal basal cells has remained an enigma. Studies have reported that the cells display endocrine properties with respect to the secretion of prostaglandins [21], and that they express a number of different proteins implicated in the metabolism of reactive oxygen species [10, 14]. However, there remains very limited information of the role of these cells in epididymal functions. While there have been several reports on the isolation of basal cells [38, 54, 55], many of these studies were done prior to the discovery of basal cell markers and the discovery of epididymal dendritic cells [56].
Based on studies examining basal cells in other epithelia, we examined the localization of ITGA6 in the epididymal epithelium in order to assess its potential utility in isolating epididymal basal cells [52, 57, 58]. ITGA6 was present at the base of the epithelium throughout the epididymis, and appeared to be localized to basal cells. In epithelia, such as the trachea, prostate, and mammary gland, basal cells act as adult stem cells and are immunopositive for ITGA6 [57, 59, 60]. These data support the notion that basal cells in the epididymis share certain similarities with basal cells from these other tissues.
Microarray analysis of isolated basal cells indicated clear differences in gene expression between basal cells and other cells of the epididymis. While several studies have examined gene expression profiles in the different regions of the epididymis in human, rat, and mice [61–68], few have examined gene expression profiles in specific cell types. Dubé et al. [61] examined gene expression profiles in an immortalized principal cell line of an azoospermic infertile patient versus a principal cell line derived from a fertile patient. The present study is the first to specifically compare gene expression in basal cells relative to other epididymal cells.
Gene expression profiles of epididymal basal cells indicated that most of the previously identified markers of basal cells were expressed in the isolated basal cells. Interestingly, some of the markers previously reported as expressed in basal cells were found to be expressed either at equivalent levels in basal and nonbasal cell fractions, or were, in fact, present at much higher levels in the nonbasal cell fraction. These differences suggest that the expression of certain genes, at least at the mRNA level, may be attributed to other cell types. Aquaporin 3 (AQP3) was previously reported in basal cells by immunohistochemistry [50], but not by immunofluorescence [69]. Studies have reported that, in other tissues, AQP3 is expressed in dendritic cells [70]. It is possible that, in the epididymis, both cell types express Aqp3, and, therefore, differences in mRNA did not show an enrichment of gene expression for Aqp3 in the basal cell fraction. Angiotensin II receptor, type 2 (AGTR2) has also been described as being specific to the basal cells in the epididymis [4]. However, in the present study, Agtr2 expression was much lower in the basal cell fraction versus the nonbasal cell fraction. Several studies have reported the presence of AGTR2 in dendritic cells [71, 72], and it is possible that the localization of this protein, as well as AQP3, may not be to basal cells, but rather to dendritic cells. Similarly, transient receptor potential cation channel, subfamily C, member 3 (Trpc3), previously suggested as being specific to epididymal basal cells [55], was not enriched in the basal cell population. MarvelD2, previously studied in epididymis, showed that basal cells do not form tripartite junctions with principal cells [73].
Previous studies have reported that gap junctions composed of GJA1 were present between basal cells and either principal or clear cells of the epididymis in multiple species [22, 74–76]. Gja1 mRNA levels were significantly higher in isolated basal cells, supporting previous reports of gap junctions in this tissue. Surprisingly, however, basal cells also contained significantly higher levels of Gjb3, Gjb4, and Gjb5. This is the first report of Gjb3, Gjb4, and Gjb5 expression in basal cells. We previously showed that these connexins were expressed in the epididymis, and that their mRNA levels increased as a function of postnatal development [9]. The contribution of basal cells to increasing expression levels of these connexins may be important. The fact that these four connexins are expressed in basal cells is suggestive of a close interaction or communication between basal cells and other epididymal cell types. Since gap junctions are known to be important sensors of epithelial health, it is tempting to speculate that these interactions play a similar role in the epididymis [77].
Several genes implicated in the inflammatory response were also enriched in epididymal basal cells, including genes implicated in prostaglandin synthesis and secretion. Numerous studies have shown that PTGS1 is present in basal cells, and that these cells can secrete prostaglandins [21, 40]. Inflammatory responses in other tissues have been shown to be important to stimulate differentiation of basal stem cells. In the prostate, inflammation enhances basal-to-luminal differentiation [78], while, in the trachea, basal cell differentiation into undifferentiated epithelial cells for epithelial repair is initiated by the inflammation following injury [29, 79].
TP63 is a specific marker of basal cells in several epithelia, including the epididymis [48]. In prostate, TP63 is essential for differentiation of basal cells [80]. In pseudostratified and stratified epithelia, TP63 was among the first genes proposed to have a function in the maintenance of stem cell populations [81], and loss of TP63 resulted in a dramatic epithelial phenotype during embryogenesis marked by the loss of stratification [82]. Furthermore, TP63 has been identified as a key determinant of the proliferative capacity of stem cells of stratified epithelia [83]. In the epididymis, Murashima et al. [12] showed that TP63 was essential for the formation of basal cells. In our study, TP63 was strongly expressed in basal cells, and several of the highly expressed genes found in our basal cell fraction were previously reported as being TP63-dependent genes (Table 3), reinforcing the importance of TP63 in regulating epididymal basal cell function.
Canonical Wnt signaling has been reported in the epididymis [84]. This pathway causes an accumulation of β-catenin in the cytoplasm and its eventual translocation into the nucleus, where it acts as a transcription factor. Previous studies by our lab have reported the presence of catenins in the epididymis and showed that they are regulated as a function of postnatal development [85]. WNT proteins have critical roles in embryo development and in tissue homeostasis in the adult, including cell proliferation, polarity, and cell differentiation [86]. In the present study, genes associated with the Wnt signaling pathway were found in basal cells, supporting our hypothesis that basal cells have a role in progenitor function.
Intracellular signaling pathway analyses indicated that cancer-related genes were among the most prevalent of the predicted pathways in isolated basal cells. Curiously, although there are very few cases of epididymal cancer [84], it would appear that epididymal basal cells have the capacity to promote cancer development. In other tissues, basal cells have been shown to play a significant role in the development of cancer, and, in the case of breast cancer, is often associated with poor prognosis [87, 88]. Clearly, however, the regulation of epididymal basal cells differs from that of basal cells in other tissues.
Several highly expressed genes in isolated basal cells are associated with multipotent stem cells in adult tissue (Fig. 4C). These data support our hypothesis that epididymal basal cells are, in fact, epididymal stem cells. The differentiation of stem cells is dependent on cell-specific activation, which is regulated by reciprocal signaling between basal cells and their niche [89]. Transcripts for various signaling ligands (Bmp7, Jag1, Jag2, Ccl20, Pdgf) and receptors (Egfr, Epha2, Trop2, Bcam, Edar) were all detected in the epididymal basal cell fraction, suggesting the potential for cellular differentiation.
In other epithelia, it is well established that basal cells act as multipotent stem cells [25, 30, 34, 90]. In the epididymis, Clermont and Flannery [91] reported that, in the adult, basal cells have a very low mitotic index (1.4% for 2.5-month-old animals, and below 0.6% after 4 months). Later studies suggested that basal cell development during the perinatal period was a determinant for the development of the cauda epididymidis and vas deferens [6]. In culture, primary human epididymal epithelial cells can form spheres [92]; this formation is a common characteristic of stem or progenitor cells, suggesting that one or several cell types in the epididymis have progenitor abilities. However, it has been proposed that basal cells are not required for differentiation of principal, clear, and narrow cells in the murine epididymis [12]. Shum et al. reported that epididymal basal cells are not progenitors of clear and principal cells during early development [11]. Based on the microarray analysis, we noted that epididymal basal cells have common characteristics with basal cells in other epithelia, and hypothesized that these included progenitor capacities. Our basal cell culture data show that basal cells can form spheroid structures, which are observed in embryonic stem cell cultures [93]. Our experiments also showed that basal cells differentiate in vitro from KRT5-positive cells to KRT8-positive cells. This is similar to basal cells from other tissues, such as the prostate, where basal cells give rise to cells that express KRT8 [94]. In the trachea, basal cells can differentiate into specialized ciliated and secretory cells during epithelium repair [30].
In epididymis, GJB2 is a marker of columnar cells. As columnar cells differentiate into principal and other cell types, GJB2 levels dramatically decrease [9]. In our basal cell cultures, no staining for GJB2 was seen after 3 days, but was observed in cells within acini after 7, 10, and 14 days of culture. This suggests that basal cells can differentiate into cells that share the phenotype of columnar cells. In the trachea, ablation of ciliated cells with SO2 activates basal cells to differentiate into undifferentiated progenitors, which then differentiate into ciliated and secretory cells, suggesting that differentiation occurs as a two-step process [95]. Our data would suggest a similar mechanism in the epididymis.
In conclusion, we have isolated epididymal basal cells and have shown, using gene expression profiling, that these cells are implicated in numerous epithelial functions, and may act as adult stem cells. Culture protocols support the notion that basal cells can differentiate in vitro, and appear to differentiate into columnar cells. This indicates that regeneration of the epididymal epithelium may be possible, and may explain the plasticity of this critical organ for male fertility.
ACKNOWLEDGMENT
The authors wish to thank Mary Gregory, Julie Dufresne, Marlene Fortier, Christophe Gonçalves (INRS), and Jeannie Mui (McGill University) for their helpful suggestions and assistance with this study.
Footnotes
Supported by Fonds de recherche du Québec en nature et technologies grant 148108 to D.G.C., Canadian Institutes of Health Research grant 84576, and Natural Sciences and Engineering Research Council of Canada grant 155065-06. M.M. was the recipient of a studentship from the Fondation Armand-Frappier. The data discussed in this publication have been deposited in the National Center for Biotechnology Information's Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo/), and are accessible under GEO accession number GSE52693.
REFERENCES
- Sun EL, Flickinger CJ. Development of cell types and of regional differences in the postnatal rat epididymis. Am J Anat. 1979;154:27–55. doi: 10.1002/aja.1001540104. [DOI] [PubMed] [Google Scholar]
- Hermo L, Robaire B. Epididymal cell types and their functions In: Robaire B, Hinton B. (eds.), The Epididymis: from Molecular to Clinical Practice New York: Plenum Press; 2002. 81 102 [Google Scholar]
- Veri JP, Hermo L, Robaire B. Immunocytochemical localization of the Yf subunit of glutathione S-transferase P shows regional variation in the staining of epithelial cells of the testis, efferent ducts, and epididymis of the male rat. J Androl. 1993;14:23–44. [PubMed] [Google Scholar]
- Shum WW, Da Silva N, McKee M, Smith PJ, Brown D, Breton S. Transepithelial projections from basal cells are luminal sensors in pseudostratified epithelia. Cell. 2008;135:1108–1117. doi: 10.1016/j.cell.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermo L, Papp S. Effects of ligation, orchidectomy, and hypophysectomy on expression of the Yf subunit of GST-P in principal and basal cells of the adult rat epididymis and on basal cell shape and overall arrangement. Anat Rec. 1996;244:59–69. doi: 10.1002/(SICI)1097-0185(199601)244:1<59::AID-AR6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Atanassova N, McKinnell C, Fisher J, Sharpe RM. Neonatal treatment of rats with diethylstilboestrol (DES) induces stromal-epithelial abnormalities of the vas deferens and cauda epididymis in adulthood following delayed basal cell development. Reproduction. 2005;129:589–601. doi: 10.1530/rep.1.00546. [DOI] [PubMed] [Google Scholar]
- Kim B, Roy J, Shum WW, Da Silva N, Breton S. Role of testicular luminal factors on basal cell elongation and proliferation in the mouse epididymis. Biol Reprod. 2015;92:9. doi: 10.1095/biolreprod.114.123943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun EL, Flickinger CJ. Proliferative activity in the rat epididymis during postnatal development. Anat Rec. 1982;203:273–284. doi: 10.1002/ar.1092030209. [DOI] [PubMed] [Google Scholar]
- Dufresne J, Finnson KW, Gregory M, Cyr DG. Expression of multiple connexins in the rat epididymis indicates a complex regulation of gap junctional communication. Am J Physiol Cell Physiol. 2003;284:C33–C43. doi: 10.1152/ajpcell.00111.2002. [DOI] [PubMed] [Google Scholar]
- Hermo L, Papp S, Robaire B. Developmental expression of the Yf subunit of glutathione S-transferase P in epithelial cells of the testis, efferent ducts, and epididymis of the rat. Anat Rec. 1994;239:421–440. doi: 10.1002/ar.1092390409. [DOI] [PubMed] [Google Scholar]
- Shum WW, Hill E, Brown D, Breton S. Plasticity of basal cells during postnatal development in the rat epididymis. Reproduction. 2013;146:455–469. doi: 10.1530/REP-12-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashima A, Miyagawa S, Ogino Y, Nishida-Fukuda H, Araki K, Matsumoto T, Kaneko T, Yoshinaga K, Yamamura K, Kurita T, Kato S, Moon AM, et al. Essential roles of androgen signaling in Wolffian duct stabilization and epididymal cell differentiation. Endocrinology. 2011;152:1640–1651. doi: 10.1210/en.2010-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Yoshinaga A, Ohno R, Ishii N, Kamata S, Yamada T. Expression of the p63 and Notch signaling systems in rat testes during postnatal development: comparison with their expression levels in the epididymis and vas deferens. J Androl. 2004;25:692–698. doi: 10.1002/j.1939-4640.2004.tb02843.x. [DOI] [PubMed] [Google Scholar]
- Nonogaki T, Noda Y, Narimoto K, Shiotani M, Mori T, Matsuda T, Yoshida O. Localization of CuZn-superoxide dismutase in the human male genital organs. Hum Reprod. 1992;7:81–85. doi: 10.1093/oxfordjournals.humrep.a137565. [DOI] [PubMed] [Google Scholar]
- Papp S, Robaire B, Hermo L. Immunocytochemical localization of the Ya, Yc, Yb1, and Yb2 subunits of glutathione S-transferases in the testis and epididymis of adult rats. Microsc Res Tech. 1995;30:1–23. doi: 10.1002/jemt.1070300102. [DOI] [PubMed] [Google Scholar]
- Cyr DG, Dufresne J, Pillet S, Alfieri TJ, Hermo L. Expression and regulation of metallothioneins in the rat epididymis. J Androl. 2001;22:124–135. [PubMed] [Google Scholar]
- Li Z, Sun ZJ, Liao CG, Ma L, Ma BF, Zhang YQ. Regulated upon activation normal T-cell expressed and secreted originating from the epididymis differentially associates with viable and defective spermatozoa. Fertil Steril. 2010;93:2661–2667. doi: 10.1016/j.fertnstert.2010.01.053. [DOI] [PubMed] [Google Scholar]
- Yeung CH, Nashan D, Sorg C, Oberpenning F, Schulze H, Nieschlag E, Cooper TG. Basal cells of the human epididymis–antigenic and ultrastructural similarities to tissue-fixed macrophages. Biol Reprod. 1994;50:917–926. doi: 10.1095/biolreprod50.4.917. [DOI] [PubMed] [Google Scholar]
- Shum WW, Smith TB, Cortez-Retamozo V, Grigoryeva LS, Roy JW, Hill E, Pittet MJ, Breton S, Da Silva N. Epithelial basal cells are distinct from dendritic cells and macrophages in the mouse epididymis. Biol Reprod. 2014;90:90. doi: 10.1095/biolreprod.113.116681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrighi S. Are the basal cells of the mammalian epididymis still an enigma? Reprod Fertil Dev. 2014;26:1061–1071. doi: 10.1071/RD13301. [DOI] [PubMed] [Google Scholar]
- Wong PY, Chan HC, Leung PS, Chung YW, Wong YL, Lee WM, Ng V, Dun NJ. Regulation of anion secretion by cyclo-oxygenase and prostanoids in cultured epididymal epithelia from the rat. J Physiol. 1999;514(pt 3):809–820. doi: 10.1111/j.1469-7793.1999.809ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyr DG, Hermo L, Laird DW. Immunocytochemical localization and regulation of connexin43 in the adult rat epididymis. Endocrinology. 1996;137:1474–1484. doi: 10.1210/endo.137.4.8625926. [DOI] [PubMed] [Google Scholar]
- Gregory M, Dufresne J, Hermo L, Cyr D. Claudin-1 is not restricted to tight junctions in the rat epididymis. Endocrinology. 2001;142:854–863. doi: 10.1210/endo.142.2.7975. [DOI] [PubMed] [Google Scholar]
- Jones C, Mackay A, Grigoriadis A, Cossu A, Reis-Filho JS, Fulford L, Dexter T, Davies S, Bulmer K, Ford E, Parry S, Budroni M, et al. Expression profiling of purified normal human luminal and myoepithelial breast cells: identification of novel prognostic markers for breast cancer. Cancer Res. 2004;64:3037–3045. doi: 10.1158/0008-5472.can-03-2028. [DOI] [PubMed] [Google Scholar]
- Robinson EJ, Neal DE, Collins AT. Basal cells are progenitors of luminal cells in primary cultures of differentiating human prostatic epithelium. Prostate. 1998;37:149–160. doi: 10.1002/(sici)1097-0045(19981101)37:3<149::aid-pros4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Breeze RG, Wheeldon EB. The cells of the pulmonary airways. Am Rev Respir Dis. 1977;116:705–777. doi: 10.1164/arrd.1977.116.4.705. [DOI] [PubMed] [Google Scholar]
- Gomperts BN, Belperio JA, Fishbein MC, Keane MP, Burdick MD, Strieter RM. Keratinocyte growth factor improves repair in the injured tracheal epithelium. Am J Respir Cell Mol Biol. 2007;37:48–56. doi: 10.1165/rcmb.2006-0384OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erjefalt JS, Sundler F, Persson CG. Epithelial barrier formation by airway basal cells. Thorax. 1997;52:213–217. doi: 10.1136/thx.52.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musah S, Chen J, Hoyle GW. Repair of tracheal epithelium by basal cells after chlorine-induced injury. Respir Res. 2012;13:107. doi: 10.1186/1465-9921-13-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, Hogan BL. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci U S A. 2009;106:12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Liu H, Zhang BH, Cadaneanu RM, Mayle AM, Garraway IP. Epcam, CD44, and CD49f distinguish sphere-forming human prostate basal cells from a subpopulation with predominant tubule initiation capability. PLoS One. 2012;7:e34219. doi: 10.1371/journal.pone.0034219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prater MD, Petit V. AlasdairRussell I, Giraddi RR, Shehata M, Menon S, Schulte R, Kalajzic I, Rath N, Olson MF, Metzger D, Faraldo MM, et al., editors. Mammary stem cells have myoepithelial cell properties. Nat Cell Biol. 2014;16:942–950. doi: 10.1038/ncb3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay WW, Axanova LS, Chen W, Romero L, Maund SL, Soker S, Lees CJ, Cramer SD. Characterization of adult prostatic progenitor/stem cells exhibiting self-renewal and multilineage differentiation. Stem Cells. 2008;26:600–610. doi: 10.1634/stemcells.2007-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole BB, Smith RW, Jenkins KM, Graham BB, Reynolds PR, Reynolds SD. Tracheal Basal cells: a facultative progenitor cell pool. Am J Pathol. 2010;177:362–376. doi: 10.2353/ajpath.2010.090870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZA, Mitrofanova A, Bergren SK, Abate-Shen C, Cardiff RD, Califano A, Shen MM. Lineage analysis of basal epithelial cells reveals their unexpected plasticity and supports a cell-of-origin model for prostate cancer heterogeneity. Nat Cell Biol. 2013;15:274–283. doi: 10.1038/ncb2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro-Silva A, Ramalho LN, Garcia SB, Brandao DF, Chahud F, Zucoloto S. p63 correlates with both BRCA1 and cytokeratin 5 in invasive breast carcinomas: further evidence for the pathogenesis of the basal phenotype of breast cancer. Histopathology. 2005;47:458–466. doi: 10.1111/j.1365-2559.2005.02249.x. [DOI] [PubMed] [Google Scholar]
- Rock JR, Randell SH, Hogan BL. Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Dis Model Mech. 2010;3:545–556. doi: 10.1242/dmm.006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killian GJ, Amann RP, Snyder J. Isolation of principal and basal cells from the epithelium of the hamster caput epididymidis by unit gravity sedimentation. Biol Reprod. 1976;15:266–279. doi: 10.1095/biolreprod15.2.266. [DOI] [PubMed] [Google Scholar]
- Finaz C, Boue F, Meduri G, Lefevre A. Characterization of rat epithelial epididymal cells purified on a discontinuous Percoll gradient. J Reprod Fertil. 1991;91:617–625. doi: 10.1530/jrf.0.0910617. [DOI] [PubMed] [Google Scholar]
- Leung GP, Cheung KH, Leung CT, Tsang MW, Wong PY. Regulation of epididymal principal cell functions by basal cells: role of transient receptor potential (Trp) proteins and cyclooxygenase-1 (COX-1) Mol Cell Endocrinol. 2004;216:5–13. doi: 10.1016/j.mce.2003.10.077. [DOI] [PubMed] [Google Scholar]
- Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- Brazma A, Hingamp P, Quackenbush J, Sherlock G, Spellman P, Stoeckert C, Aach J, Ansorge W, Ball CA, Causton HC, Gaasterland T, Glenisson P, et al. Minimum information about a microarray experiment (MIAME)—toward standards for microarray data. Nat Genet. 2001;29:365–371. doi: 10.1038/ng1201-365. [DOI] [PubMed] [Google Scholar]
- Dennis G, Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003;4:P3. [PubMed] [Google Scholar]
- Hosack DA, Dennis G, Jr., Sherman BT, Lane HC, Lempicki RA. Identifying biological themes within lists of genes with EASE. Genome Biol. 2003;4:R70. doi: 10.1186/gb-2003-4-10-r70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leenders GJ, Schalken JA. Epithelial cell differentiation in the human prostate epithelium: implications for the pathogenesis and therapy of prostate cancer. Crit Rev Oncol Hematol. 2003;46(suppl):S3–10. doi: 10.1016/s1040-8428(03)00059-3. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Yu X, Chaurand P, Araki Y, Lareyre JJ, Caprioli RM, Orgebin-Crist MC, Matusik RJ. Epididymis-specific lipocalin promoters. Asian J Androl. 2007;9:515–521. doi: 10.1111/j.1745-7262.2007.00300.x. [DOI] [PubMed] [Google Scholar]
- Brown D, Smith PJ, Breton S. Role of V-ATPase-rich cells in acidification of the male reproductive tract. J Exp Biol. 1997;200:257–262. doi: 10.1242/jeb.200.2.257. [DOI] [PubMed] [Google Scholar]
- Saito K, Kawakami S, Okada Y, Takazawa R, Koga F, Kageyama Y, Kihara K. Spatial and isoform specific p63 expression in the male human urogenital tract. J Urol. 2006;176:2268–2273. doi: 10.1016/j.juro.2006.07.057. [DOI] [PubMed] [Google Scholar]
- Seiler P, Wenzel I, Wagenfeld A, Yeung CH, Nieschlag E, Cooper TG. The appearance of basal cells in the developing murine epididymis and their temporal expression of macrophage antigens. Int J Androl. 1998;21:217–226. doi: 10.1046/j.1365-2605.1998.00116.x. [DOI] [PubMed] [Google Scholar]
- Hermo L, Krzeczunowicz D, Ruz R. Cell specificity of aquaporins 0, 3, and 10 expressed in the testis, efferent ducts, and epididymis of adult rats. J Androl. 2004;25:494–505. doi: 10.1002/j.1939-4640.2004.tb02820.x. [DOI] [PubMed] [Google Scholar]
- Baranova A, Ivanov D, Petrash N, Pestova A, Skoblov M, Kelmanson I, Shagin D, Nazarenko S, Geraymovych E, Litvin O, Tiunova A, Born TL, et al. The mammalian pannexin family is homologous to the invertebrate innexin gap junction proteins. Genomics. 2004;83:706–716. doi: 10.1016/j.ygeno.2003.09.025. [DOI] [PubMed] [Google Scholar]
- Garraway IP, Sun W, Tran CP, Perner S, Zhang B, Goldstein AS, Hahm SA, Haider M, Head CS, Reiter RE, Rubin MA, Witte ON. Human prostate sphere-forming cells represent a subset of basal epithelial cells capable of glandular regeneration in vivo. Prostate. 2010;70:491–501. doi: 10.1002/pros.21083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein AS, Lawson DA, Cheng D, Sun W, Garraway IP, Witte ON. Trop2 identifies a subpopulation of murine and human prostate basal cells with stem cell characteristics. Proc Natl Acad Sci U S A. 2008;105:20882–20887. doi: 10.1073/pnas.0811411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinefelter GR, Amann RP. Metabolism of testosterone by principal cells and basal cells isolated from the rat epididymal epithelium. Biol Reprod. 1980;22:1149–1154. doi: 10.1093/biolreprod/22.5.1149. [DOI] [PubMed] [Google Scholar]
- Cheung KH, Leung GP, Leung MC, Shum WW, Zhou WL, Wong PY. Cell-cell interaction underlies formation of fluid in the male reproductive tract of the rat. J Gen Physiol. 2005;125:443–454. doi: 10.1085/jgp.200409205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva N, Cortez-Retamozo V, Reinecker HC, Wildgruber M, Hill E, Brown D, Swirski FK, Pittet MJ, Breton S. A dense network of dendritic cells populates the murine epididymis. Reproduction. 2011;141:653–663. doi: 10.1530/REP-10-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh M, Helm KM, Smith RW, Giordanengo MS, Li B, Shen H, Reynolds SD. A single cell functions as a tissue-specific stem cell and the in vitro niche-forming cell. Am J Respir Cell Mol Biol. 2011;45:459–469. doi: 10.1165/rcmb.2010-0314OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghebeh H, Sleiman GM, Manogaran PS, Al-Mazrou A, Barhoush E, Al-Mohanna FH, Tulbah A, Al-Faqeeh K, Adra CN. Profiling of normal and malignant breast tissue show CD44high/CD24low phenotype as a predominant stem/progenitor marker when used in combination with Ep-CAM/CD49f markers. BMC Cancer. 2013;13:289. doi: 10.1186/1471-2407-13-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson DA, Xin L, Lukacs RU, Cheng D, Witte ON. Isolation and functional characterization of murine prostate stem cells. Proc Natl Acad Sci U S A. 2007;104:181–186. doi: 10.1073/pnas.0609684104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, Li HI, Eaves CJ. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- Dubé E, Hermo L, Chan PT, Cyr DG. Alterations in gene expression in the caput epididymides of nonobstructive azoospermic men. Biol Reprod. 2008;78:342–351. doi: 10.1095/biolreprod.107.062760. [DOI] [PubMed] [Google Scholar]
- Johnston DS, Jelinsky SA, Bang HJ, DiCandeloro P, Wilson E, Kopf GS, Turner TT. The mouse epididymal transcriptome: transcriptional profiling of segmental gene expression in the epididymis. Biol Reprod. 2005;73:404–413. doi: 10.1095/biolreprod.105.039719. [DOI] [PubMed] [Google Scholar]
- Jelinsky SA, Turner TT, Bang HJ, Finger JN, Solarz MK, Wilson E, Brown EL, Kopf GS, Johnston DS. The rat epididymal transcriptome: comparison of segmental gene expression in the rat and mouse epididymides. Biol Reprod. 2007;76:561–570. doi: 10.1095/biolreprod.106.057323. [DOI] [PubMed] [Google Scholar]
- Turner TT, Johnston DS, Finger JN, Jelinsky SA. Differential gene expression among the proximal segments of the rat epididymis is lost after efferent duct ligation. Biol Reprod. 2007;77:165–171. doi: 10.1095/biolreprod.106.059493. [DOI] [PubMed] [Google Scholar]
- Guyonnet B, Dacheux F, Dacheux JL, Gatti JL. The epididymal transcriptome and proteome provide some insights into new epididymal regulations. J Androl. 2011;32:651–664. doi: 10.2164/jandrol.111.013086. [DOI] [PubMed] [Google Scholar]
- Dean MD, Good JM, Nachman MW. Adaptive evolution of proteins secreted during sperm maturation: an analysis of the mouse epididymal transcriptome. Mol Biol Evol. 2008;25:383–392. doi: 10.1093/molbev/msm265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JY, Wang HY, Liu J, Liu Q, Zhang JS, Wan FC, Liu FJ, Jin SH, Zhang YL. Transcriptome analysis of a cDNA library from adult human epididymis. DNA Res. 2008;15:115–122. doi: 10.1093/dnares/dsn005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan R, Legare C, Thabet M, Thimon V. Gene expression in the epididymis of normal and vasectomized men: what can we learn about human sperm maturation? J Androl. 2011;32:686–697. doi: 10.2164/jandrol.110.012575. [DOI] [PubMed] [Google Scholar]
- Da Silva N, Silberstein C, Beaulieu V, Pietrement C, Van Hoek AN, Brown D, Breton S. Postnatal expression of aquaporins in epithelial cells of the rat epididymis. Biol Reprod. 2006;74:427–438. doi: 10.1095/biolreprod.105.044735. [DOI] [PubMed] [Google Scholar]
- Song MG, Hwang SY, Park JI, Yoon S, Bae HR, Kwak JY. Role of aquaporin 3 in development, subtypes and activation of dendritic cells. Mol Immunol. 2011;49:28–37. doi: 10.1016/j.molimm.2011.07.015. [DOI] [PubMed] [Google Scholar]
- Nahmod KA, Vermeulen ME, Raiden S, Salamone G, Gamberale R, Fernandez-Calotti P, Alvarez A, Nahmod V, Giordano M, Geffner JR. Control of dendritic cell differentiation by angiotensin II. FASEB J. 2003;17:491–493. doi: 10.1096/fj.02-0755fje. [DOI] [PubMed] [Google Scholar]
- Nie W, Yan H, Li S, Zhang Y, Yu F, Zhu W, Fan F, Zhu J. Angiotensin-(1-7) enhances angiotensin II induced phosphorylation of ERK1/2 in mouse bone marrow-derived dendritic cells. Mol Immunol. 2009;46:355–361. doi: 10.1016/j.molimm.2008.10.022. [DOI] [PubMed] [Google Scholar]
- Mandon M, Cyr DG. Tricellulin and its role in the epididymal epithelium of the rat. Biol Reprod. 2015;92:66. doi: 10.1095/biolreprod.114.120824. [DOI] [PubMed] [Google Scholar]
- Dube E, Dufresne J, Chan PT, Cyr DG. Epidermal growth factor regulates connexin 43 in the human epididymis: role of gap junctions in azoospermia. Hum Reprod. 2012;27:2285–2296. doi: 10.1093/humrep/des164. [DOI] [PubMed] [Google Scholar]
- Hejmej A, Kotula-Balak M, Sadowska J, Bilinska B. Expression of connexin 43 protein in testes, epididymides and prostates of stallions. Equine Vet J. 2007;39:122–127. doi: 10.2746/042516407x169393. [DOI] [PubMed] [Google Scholar]
- Lydka M, Kopera-Sobota I, Kotula-Balak M, Chojnacka K, Zak D, Bilinska B. Morphological and functional alterations in adult boar epididymis: effects of prenatal and postnatal administration of flutamide. Acta Vet Scand. 2011;53:12. doi: 10.1186/1751-0147-53-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyr DG. Connexins and pannexins: coordinating cellular communication in the testis and epididymis. Spermatogenesis. 2011;1:325–338. doi: 10.4161/spmg.1.4.18948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon OJ, Zhang L, Ittmann MM, Xin L. Prostatic inflammation enhances basal-to-luminal differentiation and accelerates initiation of prostate cancer with a basal cell origin. Proc Natl Acad Sci U S A. 2014;111:E592–600. doi: 10.1073/pnas.1318157111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR. Basal cells are a multipotent progenitor capable of renewing the bronchial epithelium. Am J Pathol. 2004;164:577–588. doi: 10.1016/S0002-9440(10)63147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita T, Medina RT, Mills AA, Cunha GR. Role of p63 and basal cells in the prostate. Development. 2004;131:4955–4964. doi: 10.1242/dev.01384. [DOI] [PubMed] [Google Scholar]
- Yang A, Schweitzer R, Sun D, Kaghad M, Walker N, Bronson RT, Tabin C, Sharpe A, Caput D, Crum C, McKeon F. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398:714–718. doi: 10.1038/19539. [DOI] [PubMed] [Google Scholar]
- Mills AA, Zheng B, Wang XJ, Vogel H, Roop DR, Bradley A. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398:708–713. doi: 10.1038/19531. [DOI] [PubMed] [Google Scholar]
- Senoo M, Pinto F, Crum CP, McKeon F. p63 is essential for the proliferative potential of stem cells in stratified epithelia. Cell. 2007;129:523–536. doi: 10.1016/j.cell.2007.02.045. [DOI] [PubMed] [Google Scholar]
- Wang K, Li N, Yeung CH, Li JY, Wang HY, Cooper TG. Oncogenic Wnt/beta-catenin signalling pathways in the cancer-resistant epididymis have implications for cancer research. Mol Hum Reprod. 2013;19:57–71. doi: 10.1093/molehr/gas051. [DOI] [PubMed] [Google Scholar]
- DeBellefeuille S, Hermo L, Gregory M, Dufresne J, Cyr DG. Catenins in the rat epididymis: their expression and regulation in adulthood and during postnatal development. Endocrinology. 2003;144:5040–5049. doi: 10.1210/en.2002-0139. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Wang X, Kruithof-de Julio M, Economides KD, Walker D, Yu H, Halili MV, Hu YP, Price SM, Abate-Shen C, Shen MM. A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature. 2009;461:495–500. doi: 10.1038/nature08361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipitsin M, Campbell LL, Argani P, Weremowicz S, Bloushtain-Qimron N, Yao J, Nikolskaya T, Serebryiskaya T, Beroukhim R, Hu M, Halushka MK, Sukumar S, et al. Molecular definition of breast tumor heterogeneity. Cancer Cell. 2007;11:259–273. doi: 10.1016/j.ccr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Keymeulen A, Rocha AS, Ousset M, Beck B, Bouvencourt G, Rock J, Sharma N, Dekoninck S, Blanpain C. Distinct stem cells contribute to mammary gland development and maintenance. Nature. 2011;479:189–193. doi: 10.1038/nature10573. [DOI] [PubMed] [Google Scholar]
- Clermont Y, Flannery J. Mitotic activity in the epithelium of the epididymis in young and old adult rats. Biol Reprod. 1970;3:283–292. doi: 10.1093/biolreprod/3.3.283. [DOI] [PubMed] [Google Scholar]
- Kristensen DM, Nielsen JE, Kalisz M, Dalgaard MD, Audouze K, Larsen ME, Jacobsen GK, Horn T, Brunak S, Skakkebaek NE, Leffers H. OCT4 and downstream factors are expressed in human somatic urogenital epithelia and in culture of epididymal spheres. Mol Hum Reprod. 2010;16:835–845. doi: 10.1093/molehr/gaq008. [DOI] [PubMed] [Google Scholar]
- Pastrana E, Silva-Vargas V, Doetsch F. Eyes wide open: a critical review of sphere-formation as an assay for stem cells. Cell Stem Cell. 2011;8:486–498. doi: 10.1016/j.stem.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo S, Attard G, Hudson DL. Prostate epithelial stem cells. Cell Prolif. 2005;38:363–374. doi: 10.1111/j.1365-2184.2005.00356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadokoro T, Wang Y, Barak LS, Bai Y, Randell SH, Hogan BL. IL-6/STAT3 promotes regeneration of airway ciliated cells from basal stem cells. Proc Natl Acad Sci U S A. 2014;111:E3641–E3649. doi: 10.1073/pnas.1409781111. [DOI] [PMC free article] [PubMed] [Google Scholar]