Abstract
CALCB, ADM, and ADM2 are potent vasodilators that share a seven-transmembrane GPCR, calcitonin receptor-like receptor (CALCRL), whose ligand specificity is dictated by the presence of one of the three receptor activity-modifying proteins (RAMPs). We assessed the relative pharmacologic potency of these peptides in mesenteric artery smooth muscle cells (VSMCs) and the specific RAMP that mediates the effect of ADM in VSMCs. VSMCs, with or without RAMP knockdown, were treated with CALCB, ADM, or ADM2 in the presence or absence of their antagonists, CALCB8-37, ADM22-52, and ADM217-47, respectively, to assess the relative effect of peptides on cAMP production and their pharmacologic potency. Proximity ligation assay was used to assess the specific RAMP that associates with CALCRL to mediate the actions of ADM in VSMCs. All three peptides induced cAMP generation in VSMCs and the order of their potency is CALCB > ADM > ADM2. Effects of CALCB were blocked by CALCB8-37, ADM effects were blocked by CALCB8-37 and ADM217-47 but not ADM22-52, and ADM2 effects were blocked by all three antagonists. Knockdown of RAMP2 was ineffective, whereas knockdown of RAMP3 inhibited ADM-induced cAMP production in VSMCs, suggesting involvement of RAMP3 with CALCRL to mediate ADM effects. Absence of both RAMP2 and RAMP3 further increased CALCB-induced cAMP synthesis compared to control (P < 0.05). ADM increased CALCRL and RAMP3 association and RAMP3 knockdown inhibited the interaction of ADM with CALCRL.
Keywords: adrenomedullin, CALCB family peptides, RAMP3, VSMC
INTRODUCTION
Calcitonin gene-related peptides (CALCB1 and CALCB2), adrenomedullin (ADM), and intermedin/adrenomedullin 2 (ADM2) belong to the calcitonin family of peptides [1–5]. Although these peptides have little sequence homology, they share a similar secondary structure, consisting of an amino acid ring formed by a single disulfide bond and an amidated carboxyl terminus [3, 6–8]. In addition to their structural similarities, the receptors for these peptides consist of components that are commonly shared by these peptides, further adding to the functionality overlapping. Recent studies implicate involvement of CALCB family peptides in multiple essential roles in a variety of functions, including cardiovascular adaptations during pregnancy and utero-placental functions to facilitate a successful and healthy pregnancy [9–15].
CALCB, ADM, and ADM2 are potent vasodilators that share the signaling mechanisms in vascular endothelium and smooth muscle [15–18]. We reported previously that vascular relaxation effects of these peptides are greater during pregnancy compared to the nonpregnant state and that their signaling pathways involve the cAMP/Akt pathway in smooth muscle cells and cAMP/calcium calmodulin signaling in endothelial cells [15]. Among the three peptides, CALCB effects are primarily observed in smooth muscle cells that are antagonized by CALCB antagonist CALCB8-37, whereas ADM is shown to exert its effects on both endothelium and smooth muscle cells and the majority of ADM2 effects on the vasculature appear to be endothelium dependent [15–18]. Pharmacological evaluation shows that the effects of ADM in endothelium and smooth muscle are blocked by the antagonists CALCB8-37 and ADM22-52, respectively, whereas the endothelial effects of ADM2 are blocked by antagonist ADM217-47 in segments of the rat mesenteric artery [13, 17, 19].
A hetero-dimeric receptor complex consisting of a seven-transmembrane (7TM) G protein-coupled receptor (GPCR), calcitonin receptor-like receptor (CALCRL), and one of the receptor activity-modifying proteins (RAMPs) mediates the biological actions of these peptides. Activation of these receptors leads to the stimulation of intracellular cAMP synthesis. McLatchie et al. [20] showed that transport of CALCRL by RAMP1 to the plasma membrane forms a CALCB receptor, which is antagonized by CALCB8-37 [8, 11, 20]. Translocation of CALCRL by RAMP2 or RAMP3 results in ADM receptors, where the association of CALCRL with RAMP2 results in ADM1 receptor and CALCRL with RAMP3 forms the ADM2 receptor. Studies of the reconstituted CALCB and ADM receptors in yeast have suggested that the antagonists CALCB8-37 and ADM22-52 are selective for the CALCRL/RAMP1 and the CALCRL/RAMP2 and CALCRL/RAMP3 associations, respectively [21]. Interestingly, unlike CALCB and ADM, ADM2 actions are mediated via formation of a hetero-dimeric complex of CALCRL with any one of the three RAMPs. However, actions of ADM2 are more potent in the presence of CALCRL/RAMP1 or CALCRL/RAMP3 compared to CALCRL/RAMP2 receptor complex. A truncated form of ADM2, ADM217-47, is the suggested antagonist of ADM2, which is capable of blocking the effects of CALCRL/RAMP1, CALCRL/RAMP2, or CALCRL/RAMP3 [3]. Thus, ADM2 functions through CALCB and ADM receptors, and therefore some of the effects of ADM2 are antagonized by both CALCB and ADM antagonists. Although binding of these peptides to their 7TM-GPCR, CALCRL, is the prime requirement for their biological activity, presence of RAMPs is critical for their signaling [22, 23].
In recent years, ADM and CALCB and their respective receptors have gained considerable attention and have become targets for new drug development [24, 25], but their progress is hampered by the complex nature of the involved receptor system. As a result, despite clear evidence of an important role for ADM in vasorelaxation, the mechanism of ADM action and the specific RAMP that mediates ADM effects in smooth muscle cells are unknown. Both CALCB and ADM are reported to be more potent than ADM2 in many of the vascular beds [15–18]. Although the majority of the functional effects of ADM are mediated through CALCRL/RAMP2 or CALCRL/RAMP3, it is not clear which RAMP is specific for ADM-induced vascular effects in rat mesenteric artery smooth muscle [9, 13, 18, 19, 26–28]. Therefore, the goal of this study was to demonstrate the relative pharmacologic effects of CALCB, ADM, and ADM2 in vascular smooth muscle cells isolated from rat mesenteric artery and characterize the specific RAMP that mediates the effects of ADM in these cells.
MATERIALS AND METHODS
Animal Welfare and Ethical Statements
All procedures were approved by the Animal Care and Use Committee at the University of Texas Medical Branch in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Research Ethics Committee of Baylor College of Medicine. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals [29, 30]. Nonpregnant rats were obtained from Harlan Sprague Dawley. Rats were maintained in the colony room with a fixed photoperiod of 12L:12D and with access to water and rodent chow ad libitum and euthanized when needed.
Isolation of Mesenteric Artery Smooth Muscle Cells and Cell Culture
VSMCs were harvested from enzymatically dissociated rat mesenteric arteries as per the method described [31]. All procedures were carried out under aseptic conditions. The superior mesenteric artery with all its major branches was excised, en bloc, from its origin at the aorta to the mesenteric border of the intestine and placed in a Petri dish containing ice-cold Hanks balanced salt solution (HBSS, Ca2+ and Mg2+ free) with 0.2 mM added Ca2+. The cleaned mesenteric artery arcades (from at least two animals) were transferred into a 50-ml plastic tissue-culture flask containing 4.0 ml of enzymatic dissociation mixture: HBSS (Ca2+ and Mg2+ free) with 0.2 mM added Ca2+, 15 mM HEPES buffer (pH 7.2–7.3), 0.125 mg/ml elastase, 0.375 mg/ml soybean trypsin inhibitor, 1 mg/ml collagenase, and 2 mg/ml bovine albumin. After incubation at 37°C for 90 min in a gyratory shaker water bath, the tissues were triturated 10 times into a 10-ml plastic syringe with a 16½-gauge needle and passed through a 100-μm nylon mesh to separate the dispersed cells from undigested vessel wall fragments and debris. The filtered suspension was centrifuged in a siliconized conical plastic tube for 5 min at 200 × g, and the pellet was washed once with Dulbecco modified Eagle medium (DMEM) with all supplements. The cell pellet was resuspended in 5 ml of DMEM (high glucose) supplemented with 25 mM HEPES buffer, 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% (vol/vol) heat-inactivated calf serum, and the dispersed cell suspension was aliquoted into a 25-cm2 flask. After 18–24 h, the cultures were washed once with HBSS. Cells were cultured in DMEM containing 10% serum with media changes at 48–72-h intervals and typical confluent monolayers formed within 7 days. Each preparation typically yielded 5–6 × 105 cells with 80%–90% viability assessed by trypan blue exclusion. VSMCs were maintained at 37°C in a humidified incubator in an atmosphere of 95% air and 5% carbon dioxide and studied at subconfluence (2 days after they had been plated). Before experimentation, cells were rendered quiescent by deprivation of serum and maintained in a serum-free medium for 24 h. Cyclic AMP generation and proximity ligation assay (PLA) studies show consistent responses through the sixth passage and beyond.
RAMP Knockdowns in Mesenteric Artery Smooth Muscle Cell
VSMCs (passage 3–5) from mesenteric artery were cultured in six-well plates and transfected with a 19-nt short hairpin RNA (shRNA) sequence specific for rat RAMP1, RAMP2, or RAMP3 (Origene) by nucleofection using a nucleofection kit specific for primary smooth muscle cells as per manufacturer's instructions (Lonza). A universal scramble shRNA provided by the company was used as the control. Briefly, after nucleofection, cells were transferred to complete growth medium (DMEM [high glucose] supplemented with 25 mM HEPES buffer, 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% [vol/vol] heat-inactivated calf serum) for 24 h, followed by addition of puromycin (3 μg/ml; Sigma) for selection of cells transfected with shRNA. The effect of shRNA transfection on target gene knockdown was assessed by real-time PCR. All the clones used in this study had greater than 70% knockdown.
Isolation of Total RNA and Quantitative Real-Time PCR
Total RNA was isolated from the tissues using TRIzol (Life Technologies) and from the cells using an RNA extraction kit (Qiagen). Total RNA was digested with DNase I (cat. 79254; Qiagen), followed by cleanup procedures using a RNeasy minikit (cat. 74104; Qiagen) as per manufacturer's instructions, and cDNA was made as reported earlier [32]. Quantitative real-time PCR using SYBR Green (BioRad) was performed on a CFX96 Real-Time PCR Detection System (cat. 184-5096; Bio-Rad) for ADM2 and GAPDH using gene-specific primer assays (SA Biosciences). Amplification of GAPDH served as an endogenous control. Reactions were incubated at 95°C for 10 min and cycled according to the following parameters: 95°C for 30 sec (melt) and 60°C for 1 min (anneal/extend) for a total of 40 cycles. Negative control without cDNA was performed to test primer specificity. The relative gene expression was calculated by use of the threshold cycle (CT) for GAPDH/CT for target gene. For no-RT control, nuclease-free water was used in place of the reverse transcriptase.
cAMP Assay
The cAMP assays for the initial dose-response and antagonist studies were done using 12-well plates, whereas mesenteric artery VSMCs transfected with or without shRAMPs were cultured in 96-well plates in DMEM high glucose with 10% FBS. Cultured cells were starved overnight in serum-free DMEM followed by the addition of 100 μM phosphodiesterase inhibitor, isobutyl-1-methyl-xanthine (IBMX; Sigma) to all wells prior to the specified treatments at 37°C under 95% O2 and 5% CO2. After equilibration for 1 min in IBMX, the cells were incubated with specified concentrations of each of the three peptides for 5 min followed by the addition of lysis buffer as per the manufacturer's instructions (Amersham Biosciences, Inc.). Specificity of each of the antagonists, CALCB8-37, ADM22–52, and ADM217, was tested by incubating cells with 100 μM of these antagonists in the presence of these peptides. The dose of antagonists was selected based on our earlier reports [15, 16, 19]. The basal and peptide-stimulated intracellular cAMP levels in VSMCs were measured by radioimmunoassay using cAMP [125I] assay system (GE Healthcare Bio-sciences) as per manufacturer's instructions.
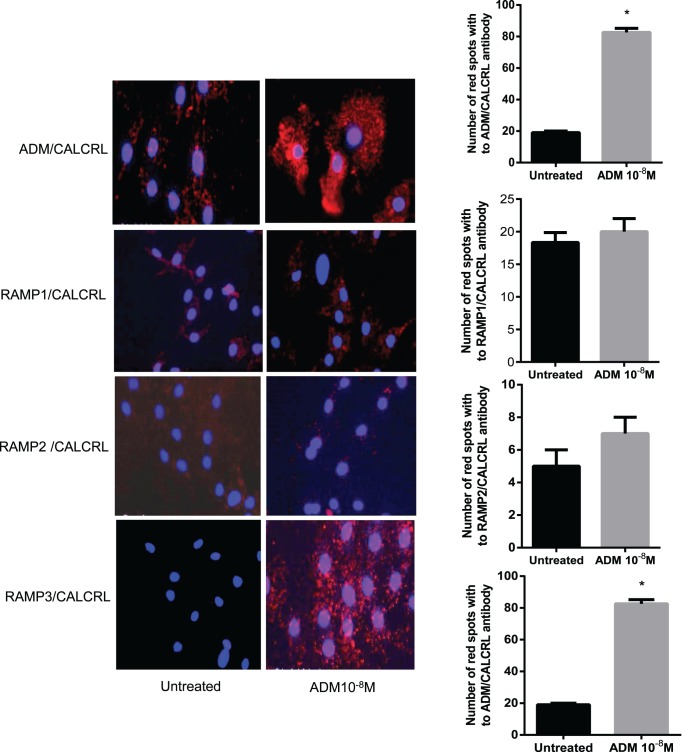
Proximity Ligation Assay
In situ PLA is a technology that extends the capabilities of traditional immunoassays to include direct detection of protein-protein interactions with high specificity and sensitivity. Utilizing only a few cells, even transient or weak interactions are revealed in situ and subpopulations of cells can be differentiated. Protein targets can be readily detected and localized with single-molecule resolution and objectively quantified in unmodified cells and tissues. PLA involves use of two antibodies (rather than a single antibody in immunoassays) raised in different species against the target antigen or antigens of interest. Species-specific secondary antibodies, called PLA probes, each with a unique short DNA strand attached to it, bind to the primary antibodies. When the PLA probes are in close proximity, the DNA strands can interact through a subsequent addition of two other circle-forming DNA oligonucleotides. Every single interaction between the two proteins results in amplification of circularized DNA molecules, which can then be detected as bright fluorescent dots. The amplification of the signal as a fluorescent spot provides a unique capability to study both stable and transient interactions at endogenous expression levels of the target protein(s). Use of two antibodies against different proteins allows for both localization and quantification of protein-protein interactions [33]. PLA was performed using a Duolink II Fluorescence kit (Olink Biosciences) according to manufacturer's instructions. Briefly, cells sparsely grown on 16-well Lab-Tek chamber slides were treated with or without ADM (10−8 M) for 2 min, fixed using 4% paraformaldehyde, and incubated sequentially with primary antibodies (one monoclonal and one polyclonal) followed by incubation with rabbit plus and mouse minus PLA probes, and finally with ligation and amplification mixtures. Slides were mounted with coverslips using Duolink II mounting medium and images were observed under fluorescence microscope (U-TV1 X; Olympus) under 20× objective. Images were processed, and red spots were counted using Image-Pro Plus software (Media Cybernetics) in five randomly selected images with a total of 200 cells per replicate. Appropriate negative controls included no primary antibody or −ve IgG isotype of primary antibody. Peptide treatments were done for 2 min based on our preliminary studies that showed that this time was optimal for visualization of receptor associations.
Statistical Analysis
Data sets were analyzed by SigmaPlot 9.0 and Prism GraphPad Software employing appropriate statistical tools. The means of the various groups were analyzed by unpaired t-test or one-way ANOVA and subjected to the Newman-Keuls multiple comparison test or Bonferroni post hoc test. P ≤ 0.05 was considered statistically significant.
RESULTS
Relative Effects of CALCB, ADM, and ADM2 in cAMP Generation in Mesenteric Artery VSMCs
VSMCs isolated from rat mesenteric artery were challenged with 1, 10, and 100 nM CALCB, ADM, or ADM2 for 5 min as described in Materials and Methods. As shown in Figure 1, all three peptides stimulated cAMP generation in a dose-dependent manner (P < 0.05). The effect of ADM2 on cAMP production was minimal compared to those of CALCB and ADM, and these effects were observed only at higher doses. CALCB-induced cAMP levels were 8-fold higher than those of ADM2 and 1.4-fold greater than those of ADM, whereas ADM-induced cAMP levels were >5-fold higher than those of ADM2. Thus, the comparative order of efficacy of these three peptides in VSMCs isolated from mesenteric artery is CALCB > ADM > ADM2.
FIG. 1.
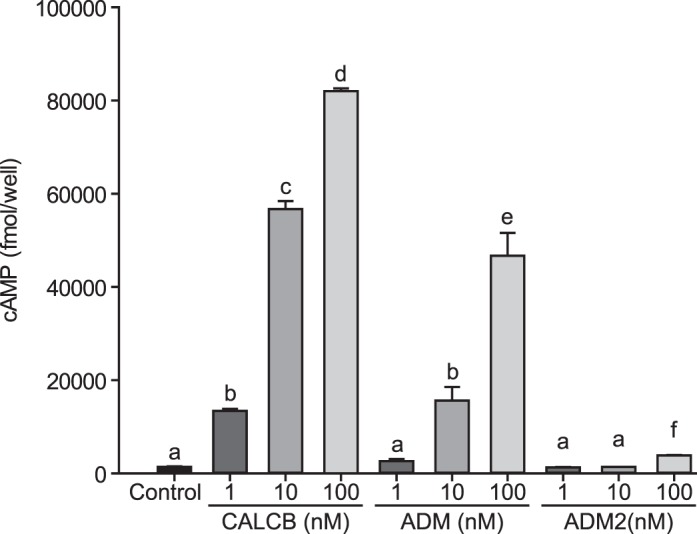
CALCB, ADM, and ADM2 dose-dependently increase cAMP generation in mesenteric artery VSMC cells. Intracellular cAMP was quantified using radioimmunoassay kit. Data are presented as mean ± SEM for three replicates and bars with different letters differ significantly among groups (P ≤ 0.05).
Pharmacological Assessment of RAMP Specificity Involved in cAMP Generation in Response to CALCB, ADM, and ADM2 in Rat Mesenteric Artery VSMCs
Smooth muscle cells isolated from mesenteric artery were challenged with CALCB (1 nM), ADM (100 nM), or ADM2 (100 nM) for 5 min in presence or absence of antagonist (1 μM CALCB8-37, ADM22-52, or ADM217-47). The dose of CALCB used in this experiment was 1 nM, as this dose resulted in a significant increase in cAMP production that was more than the highest dose of ADM2 (Fig. 1). As would be expected, effects of CALCB were blocked by CALCB8-37 but not by ADM22-52 or ADM217-47 (Fig. 2). Surprisingly, ADM effects were blocked by CALCB8-37 and ADM217-47, whereas ADM22-52 was ineffective in blocking ADM-induced increases in cAMP production in VSMCs. To confirm and validate the inability of ADM22-52 in blocking the effects of ADM, we tested different doses of ADM22-52 (10−6–10−4 M) on the relaxation response of ADM in endothelium-denuded rat mesenteric artery. Treatment with ADM22-52 at all doses was unable to block ADM-induced relaxation in rat mesenteric artery (data not shown). In light of the facts that RAMP1 is not a cofactor for ADM receptor and that CALCB8-37 can inhibit RAMP1 and RAMP3-related cAMP production, these data suggest that the majority of ADM effects in this system are mediated by RAMP3, whereas CALCB may function through RAMP1. In addition, the effects of ADM2, although minimal compared to those of CALCB and ADM, are inhibited in the presence of CALCB, ADM, or ADM2 antagonists, suggesting nonselective involvement of either of the RAMPs in ADM2-induced cAMP production in VSMCs.
FIG. 2.
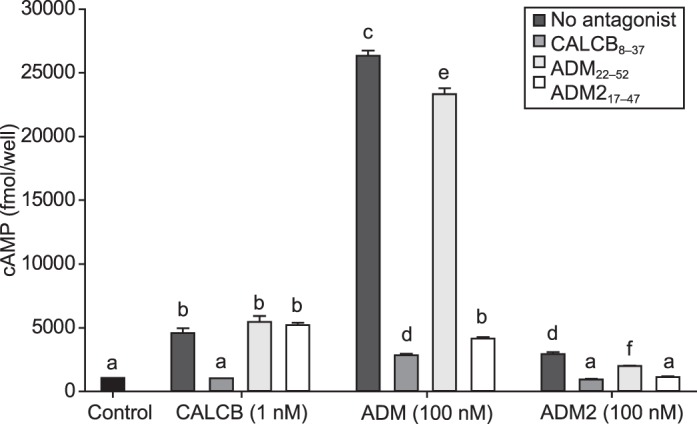
Effect of CALCB family peptide receptor antagonists on CALCB-, ADM-, and ADM2-induced cAMP generation in mesenteric artery VSMCs. As shown, CALCB-induced cAMP generation in mesenteric artery VSMCs is blocked by CALCB8-37, but not by ADM22-52 or ADM217-47. ADM-induced cAMP increases are blocked by CALCB8-37 and ADM217-47. ADM2-induced cAMP increases, although relatively small, are blocked by all three antagonists. All antagonists were used at a concentration of 100 μM. Data are presented as mean ± SEM for three replicates and bars with different letters differ significantly (P ≤ 0.05)
cAMP Responses to CALCB and ADM in Mesenteric Artery VSMCs Transfected with shRNAs Specific to RAMP2 and RAMP3
As shown in Figure 3A, VSMCs transfected with shRAMP2 and shRAMP3 showed ∼80% inhibition in mRNA expression of the target RAMP compared to their scramble controls. Figure 3B shows that both CALCB (10 nM) and ADM (100 nM) stimulated cAMP in the VSMCs transfected with the scramble shRNA used as control. In cells with RAMP2 knockdown, responses for ADM as well as CALCB treatments were similar and significantly higher than the responses observed in scramble (P < 0.05). In cells transfected with shRAMP3, responses to ADM were lower than the levels in shRAMP2 but were still higher than those for shscramble, whereas responses for CALCB still remained elevated (P < 0.05). These observations further suggest that vascular actions of ADM in VSMCs primarily involve RAMP3 rather than RAMP2. Moreover, elevated levels of CALCB-induced cAMP generation in shRAMP2- and shRAMP3-transfected cells suggest increased involvement of CALCRL with RAMP1 in the absence of RAMP2 and RAMP3 to generate CALCB receptor.
FIG. 3.
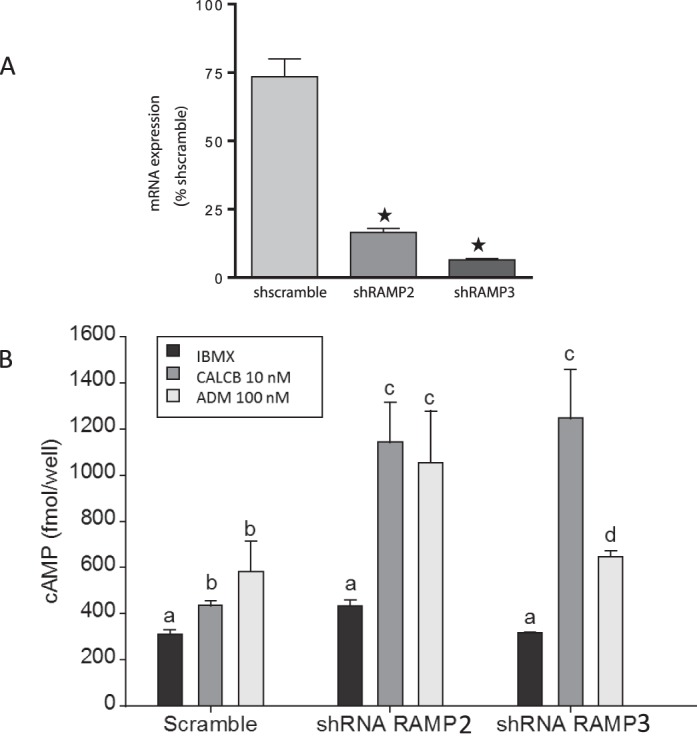
A) Short hairpin RNA-mediated knockdown of RAMPs in mesenteric artery VSMCs. Asterisks indicate significant knockdown of RAMP2 and RAMP3 mRNA in VSMC compared to shscramble. B) Effect of RAMP2 and RAMP3 knockdown on CALCB- and ADM-induced cAMP generation in VSMC. As shown, RAMP2 knockdown with shRNA in VSMCs enhances cAMP generation by both CALCB and ADM, whereas with RAMP3 knockdown, responses to ADM are reduced but responses to CALCB are still elevated. Data are presented as mean ± SEM for three replicates, and bars with different letters differ significantly (P ≤ 0.05)
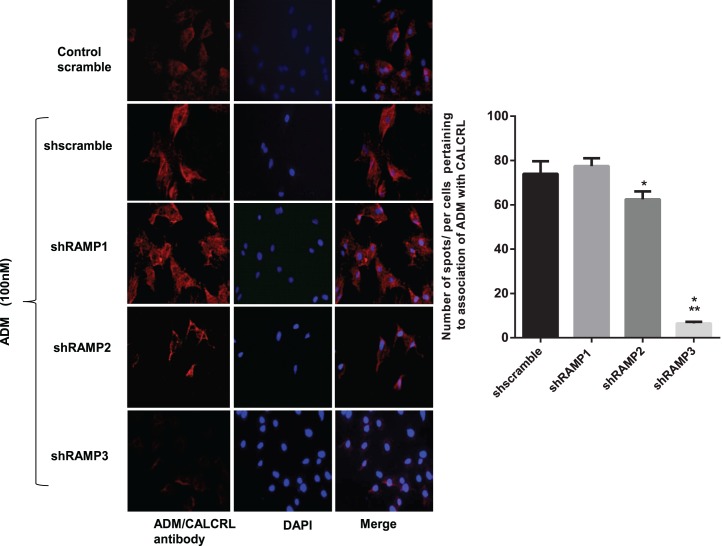
Cell Surface Association of CALCRL with RAMPs in Mesenteric Artery Smooth Muscle Cells
In situ PLA was utilized to assess if there is an endogenous cell surface association of CALCRL and different RAMP isotypes in a resting cell and characterize the RAMP isotype that is specific for ADM action in mesenteric artery VSMCs. As described in the methods, PLA is a technology that extends the capabilities of traditional immunoassays to include direct detection of protein-protein interactions with single-molecule resolution represented by fluorescent red spots. PLA demonstrated that RAMP1, RAMP2, and RAMP3 are each associated with CALCRL at very close proximity in the resting state of cells (Fig. 4). Addition of ADM 10−8 M to VSMC for 2 min increases the number of red fluorescent spots pertaining to combination of CALCRL and RAMP3 compared to CALCRL and RAMP1 or CALCRL and RAMP2 antibody. As shown, the number of red spots amplified in response to ADM treatment pertaining to combination of CALCRL and ADM antibody is similar to that for CALCRL/RAMP3 combinations, which are significantly higher than the number of red spots obtained with CALCRL/RAMP1 or CALCRL/RAMP2 antibody combinations. This indicates that ADM treatment enhances close associations of CALCRL and RAMP3 compared to CALCRL/RAMP1 or CALCRL/RAMP2 in mesenteric artery VSMCs. Further, association of ADM with CALCRL was significantly inhibited in VSMCs transfected with shRAMP3 and treated with ADM (10−8 M) (Fig. 5; P < 0.05) compared to the cells transfected with shRAMP2 or shRAMP3.
FIG. 4.
PLA showing effect of ADM on association of ADM with CALCRL and CALCRL with RAMPs. PLA was performed in mesenteric artery VSMCs treated with or without ADM. As shown, ADM treatment caused increases in the number of red spots, indicating ADM-induced increase in association of ADM with CALCRL and CALCRL with RAMP3 on the cell surface compared to CALCRL with RAMP1 or RAMP2; blue is the nuclear staining with 4′,6-diamidino-2-phenylindole. Negative controls included no primary antibody or −ve IgG isotype of primary antibody. Bar graph shows the number of spots per cell that correspond to number of associations or heterodimer complexes for ADM with CALCRL and each of the RAMPs with CALCRL. Asterisks indicate significant differences compared to control, P < 0.05. Original magnification ×200; n = 3.
FIG. 5.
Effect of RAMP knockdown on cell surface expression of ADM receptor in mesenteric artery VSMC. PLA showing red spots that corresponds to the combination of ADM + CALCRL antibody pertaining to association of ADM with CALCRL. Blue is the nuclear staining with 4′,6-diamidino-2-phenylindole. Negative controls included no primary antibody or −ve IgG isotype of primary antibody. The bar graph represents number of red spots per cell in ADM-treated VSMCs transfected with shscramble or shRAMPs. Asterisks indicate significant differences compared to shscramble, *P < 0.05, ***P < 0.001. Original magnification ×200; n = 3.
DISCUSSION
The current study shows that 1) CALCB, ADM, and ADM2 increase cAMP levels in VSMCs consistent with the relative rank order of their reported vasorelaxant effects (being CALCB > ADM > ADM2); 2) CALCB effects are blocked only by CALCB antagonist (CALCB8-37) and ADM effects are blocked by CALCB8-37 and ADM2 antagonist (ADM217-47) but not by ADM antagonist (ADM22-52), whereas ADM2 effects are blocked by all three antagonists; 3) inhibition of ADM-induced cAMP generation in VSMCs is more pronounced when RAMP3 mRNA expression in knocked down but not when RAMP2 is knocked down; 4) CALCB-induced cAMP generation is further increased when either RAMP2 or RAMP3 mRNA expression is knocked down, suggesting that absence of RAMP2 or RAMP3 results in an increased association of CALCRL with RAMP1 in response to CALCB in VSMCs; 5) cell surface associations of CALCRL and RAMPs are present in resting cells; and 6) ADM treatment increases the proximal association of ADM with CALCRL and CALCRL with RAMP3; however, knockdown of RAMP3 expression disrupts this association in VSMCs. Taken together with our previous reports, we suggest that CALCB, ADM, and ADM2 stimulate cAMP in VSMCs, with their order of potency being CALCB > ADM > ADM2, and that CALCRL/RAMP1 mediates CALCB effect, CALCRL/RAMP3 is specific for ADM, and ADM2 effects may involve RAMP1, RAMP2, or RAMP3.
Because of the structural similarities, CALCB, ADM, and ADM2 bind and activate the same G protein-coupled receptor, CALCRL, to elicit similar yet distinct physiological responses in a variety of cell types [13–19, 28, 32, 34–36]. The phenotype of ADM-null mice [37] is categorically different than that of CALCB-null mice [38–40], as ADM-null mice show extreme hydrops fetalis whereas CALCB-null mice are normal in cardiac development, suggesting that these peptides play distinct roles in vivo. Similarly, we have previously demonstrated that CALCRL and RAMPs are expressed in mesenteric artery and that although all three peptides cause relaxation of isolated rat mesenteric artery segments with an increased sensitivity during pregnancy, they exhibit different potencies in the presence or absence of endothelium in mesenteric artery segments, which are further enhanced in pregnancy [13, 15–19, 28]. We reported that in rat mesenteric arteries, the pD2 value (−log EC50 of the molar concentration of the agonist) for ADM-induced relaxation was 7.49 ± 0.08 in pregnant compared to pD2 = 7.13 ± 0.15 in nonpregnant at diestrus [13]; for CALCB the pD2 values were 8.51 ± 0.09 in pregnant compared to 7.61 ± 0.09 in nonpregnant mesenteric artery[41] whereas for ADM2-induced vasorelaxation the pD2 values were 7.07 ± 0.1 in pregnant compared to pD2 = 6.46 ± 0.2 in nonpregnant at diestrus [18], thus suggesting a rank order of potency for these peptides as CALCB > ADM > ADM2 in these vessels. Although several studies in animals as well as humans demonstrate a potential role for ADM in pregnancy and cardiovascular functions, the mechanisms of ADM action in these biological functions are not clearly understood because of the shared receptor system and existing overlap in the biological activities of CALCB, ADM, and ADM2. A recent study showed vascular endothelial cell deformities in ADM and RAMP2 knockdown mice that are embryonically lethal, suggesting that endothelial effects of ADM are primarily mediated through RAMP2 [42]. However, the direct contribution of smooth muscle cells in the vascular actions of ADM, the relative potency and pharmacological characteristics of these three peptides in VSMCs, and the specific receptor heterodimer that mediates the effects of ADM in VSMCs are not known. Therefore, in this study we assessed the comparative pharmacologic potencies of these peptides in stimulating cAMP generation as a measure of the contribution of smooth muscle cells in the vascular response elicited by these three peptides in mesenteric artery, and characterized the receptor phenotype that mediates ADM-specific effects in mesenteric artery VSMCs.
Similar to our earlier studies with segments of mesenteric arteries [13, 15–19, 28, 41], all three peptides stimulated an increase in cAMP production in VSMC isolated from mesenteric artery, with the magnitude of increases that follows the rank order of their potency as CALCB > ADM > ADM2 (Fig. 1; P < 0.05). This observation further strengthens our previous study showing that the majority of CALCB effects in the mesenteric artery are mediated through direct effects of CALCB on VSMCs, ADM effects are through both endothelium and VSMCs, and ADM2 effects are primarily endothelium dependent in the mesenteric artery [15–18]. Pharmacological evaluation of CALCB-, ADM-, and ADM2-induced cAMP generation performed in the presence of their receptor antagonists (Fig. 2) shows that CALCB-mediated effects on cAMP generation are blocked only by CALCB antagonist (CALCB8-37) and not by ADM22-52 or ADM217-47, suggesting a potential involvement of CALCRL/RAMP1 interaction in the formation of CALCB receptor in VSMCs. In our earlier studies using isolated segments of mesenteric artery, we showed that ADM-induced relaxation in endothelium-denuded mesenteric artery is blocked by the antagonist ADM22-52 [13], which has been suggested to act on both ADM1 and ADM2 receptors, where ADM1 receptor consists of CALCRL/RAMP2 heterodimer and ADM2 receptor consists of CALCRL/RAMP3 [43]. However, with ADM22-52 capable of blocking the effects of CALCRL/RAMP2 as well as CALCRL/RAMP3 receptor complexes in segments of mesenteric artery, the specific RAMP involved in ADM-induced effects in smooth muscle cells is not known. Figure 2 shows that in VSMCs, ADM-induced cAMP generation is blocked by CALCB8-37 and ADM217-47 but not by ADM22-52. Previous studies have suggested that CALCB8-37 can block the actions associated with both RAMP1 and RAMP3 and ADM217-47 can block actions associated with all three RAMPs, whereas ADM22-52 can block both RAMP2-and RAMP3-associated actions of ADM [15, 44]. Because ADM22-52 was unable to block ADM actions in VSMCs, and both CALCB8-37 and ADM217-47 were able to block ADM actions, we speculated that ADM acts through RAMP3-associated mechanisms in these cells. This notion is further confirmed in Figure 3B, where ADM-induced cAMP generation in VSMCs transfected with shRAMP3 was significantly lower compared to that in the cells transfected with shRAMP2. However, although ADM-induced cAMP levels in shRAMP3-transfected cells were lower than those in shRAMP2-transfected cells, these levels were higher than the control shscramble. In addition, knockdown of RAMP2 and RAMP3, instead of being inhibitory or ineffective, further enhanced the CALCB-induced cAMP generation in VSMCs, suggesting a potential increase in CALCRL/RAMP1 complex in absence of RAMP2 or RAMP3 resulting in an increased CALCB-induced cAMP levels. This is in accordance with the studies reported by Buhlmann et al. [45], where they show that although endogenous RAMP2 is sufficient to reveal an ADM receptor in osteoblast-like cells, CALCRL-RAMP2-mediated effects of ADM receptor are blocked in the presence of RAMP1 and CALCB receptor is generated, suggesting involvement of competitive interactions of different RAMPs with CALCRL. It would be worthwhile to assess in cells/tissues with pathophysiological conditions if CALCB effects are enhanced where ADM or ADM-specific RAMPs are inactive/absent, to facilitate an ADM fail-safe mechanism.
Studies using overexpression of CALCRL and RAMP proteins with different tags to facilitate protein visualization have shown that a significant amount of CALCRL or RAMP is present on the cell surface, thus arguing against the chaperone function of RAMPs [46–48]. However, there are no reports showing direct evidence of physical presence or expression of CALCRL/RAMP complex on the cell surface in response to the ligand activity. Using state-of-the-art PLA, the current study further provides evidence for the involvement of CALCRL/RAMP3 heterodimer in ADM-mediated effects in VSMC (Fig. 4). PLA assays show that addition of ADM peptide stimulates the formation and cell surface expression of CALCRL/RAMP3 receptor heterodimer in VSMCs and confirm previous reports suggesting that cell surface association of CALCRL with all three different RAMPs exists even in a resting state (untreated) of the cells. Further, in VSMCs transfected with shRAMP1, shRAMP2, or shRAMP3, we assessed if blocking the expression of RAMP1, RAMP2, or RAMP3 would inhibit the cell surface association of ADM with CALCRL resulting from lack of cell surface expression of ADM-specific CALCRL/RAMP complex; VSMCs were transfected with shRAMP1, shRAMP2, or shRAMP3 to inhibit the endogenous expression of RAMPs. As shown in Figure 5, the number of ADM receptors is significantly decreased in shRAMP3-transfected cells compared to cells transfected with shRAMP1 or shRAMP2. The inhibitory effect of RAMP3 and very minimal effect of RAMP2 knockdown on the number of red spots suggest primary involvement of RAMP3 in facilitating formation of a functional receptor for ADM in VSMCs. Therefore, we suggest that RAMP3 is the primary receptor activity-modifying protein in mediating vascular actions of ADM in mesenteric VSMCs. However, in Figure 2, where ADM-induced cAMP levels in VSMCs with RAMP3 knockdown are significantly lower than the cells with RAMP2 knockdown but remain higher compared to the shscramble (control), it cannot be ruled out that because of competitive binding, CALCRL may associate with RAMP2 in the absence of RAMP3 [45]. The current study is the first of its kind to demonstrate a primary role for RAMP3 in forming receptor heterodimer that is specific for ADM actions in vascular smooth muscle cells. Further, the fact that knocking down of RAMP2 or RAMP3 was not only ineffective in blocking CALCB-induced cAMP generation in VSMCs, but instead caused an increase in cAMP production, suggests that absence of one isotype of RAMP may allow for recruitment of the other RAMP isotypes to associate with CALCRL, resulting in a shift of ligand binding that is specific to the newly generated receptor complex. Thus, it is likely that the differential expression of RAMP isotypes on cell surface may results in a competitive association of RAMPs with CALCRL in VSMCs in response to CALCB family peptides in mesenteric artery.
In summary, all three peptides induce cAMP generation in VSMCs isolated from mesenteric artery, and the order of their potency is CALCB > ADM > ADM2. The pharmacological and biochemical characterization of ADM receptor phenotype in smooth muscle cells suggest that receptor heterodimer CALCRL and RAMP3 mediate the effects of ADM and CALCRL/RAMP1 mediates the CALCB effects in smooth muscle cells. Although ADM2-induced cAMP generation in VSMC was minimal, pharmacological evaluation suggests involvement of CALCRL/RAMP1 or CALCRL/RAMP3 receptor complex in ADM2-induced cAMP production. Furthermore, we suggest existence of competitive binding of CALCRL with RAMPs, and in the absence of RAMP2 or RAMP3, effects of CALCB are amplified because of increased CALCRL/RAMP1 association. This allows us to speculate that this phenomenon could form the basis for increased sensitivity of CALCB observed in pathological conditions such as migraine [49], where defective expression of RAMP2 and RAMP3 and/or their association with CALCRL could be the underlying cause of the pathology.
ACKNOWLEDGMENT
The authors would like to thank Ms. Sandra Garcia Dale for her incredible administrative assistance.
Footnotes
Supported by National Institutes of Health Grants (C.Y.) HL58144 and HL 102866.
REFERENCES
- Muff R, Born W, Fischer JA. Adrenomedullin selectivity of calcitonin-like receptor/receptor activity modifying proteins. Hypertens Res. 2003;26(suppl):S3–S8. doi: 10.1291/hypres.26.s3. [DOI] [PubMed] [Google Scholar]
- Wimalawansa SJ. Calcitonin gene-related peptide, calcitonin and amylin: a peptide super family. Crit Rev Neurobiol. 1997;11:167–239. doi: 10.1615/critrevneurobiol.v11.i2-3.40. [DOI] [PubMed] [Google Scholar]
- Roh J, Chang CL, Bhalla A, Klein C, Hsu SYT. Intermedin is a calcitonin/calcitonin gene-related peptide family peptide acting through the calcitonin receptor-like receptor/receptor activity-modifying protein receptor complexes. J Biol Chem. 2004;279:7264–7274. doi: 10.1074/jbc.M305332200. [DOI] [PubMed] [Google Scholar]
- Muff R, Born W, Fischer JA. Calcitonin, calcitonin gene-related peptide, adrenomedullin and amylin: homologous peptides, separate receptors and overlapping biological actions. Eur J Endocrinol. 1995;133:17–20. doi: 10.1530/eje.0.1330017. [DOI] [PubMed] [Google Scholar]
- Muff R, Leuthauser K, Buhlmann N, Foord SM, Fischer JA. Receptor activity modifying proteins regulate the activity of a calcitonin gene-related peptide receptor in rabbit aortic endothelial cells. FEBS Lett. 1998;441:366–368. doi: 10.1016/s0014-5793(98)01587-7. [DOI] [PubMed] [Google Scholar]
- Poyner DR. Calcitonin gene-related peptide: multiple actions, multiple receptors [review] Pharmacol Ther. 1992;56:23–51. doi: 10.1016/0163-7258(92)90036-y. [DOI] [PubMed] [Google Scholar]
- Poyner DR. Molecular pharmacology of receptors for calcitonin-gene-related peptide, amylin and adrenomedullin [review] Biochem Soc Trans. 1997;25:1032–1036. doi: 10.1042/bst0251032. [DOI] [PubMed] [Google Scholar]
- Poyner DR, Sexton PM, Marshall I, Smith DM, Quirion R, Born W, Muff R, Fischer JA, Foord SM. International union of pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol Rev. 2002;54:233–246. doi: 10.1124/pr.54.2.233. [DOI] [PubMed] [Google Scholar]
- Brain SD, Grant AD. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol Rev. 2004;84:903–934. doi: 10.1152/physrev.00037.2003. [DOI] [PubMed] [Google Scholar]
- Muff R, Born W, Lutz TA, Fischer JA. Biological importance of the peptides of the calcitonin family as revealed by disruption and transfer of corresponding genes. Peptides. 2004;25:2027–2038. doi: 10.1016/j.peptides.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Conner AC, Simms J, Hay DL, Mahmoud K, Howitt SG, Wheatley M, Poyner DR. Heterodimers and family-B GPCRs: RAMPs, CGRP and adrenomedullin. Biochem Soc Trans. 2004;32:843–846. doi: 10.1042/BST0320843. [DOI] [PubMed] [Google Scholar]
- Gangula PRR, Dong YL, Wimalawansa SJ, Yallampalli C. Infusion of pregnant rats with calcitonin gene-related peptide (CGRP)8-37, a CGRP receptor antagonist, increases blood pressure and fetal mortality and decreases fetal growth. Biol Reprod. 2002;67:624–629. doi: 10.1095/biolreprod67.2.624. [DOI] [PubMed] [Google Scholar]
- Ross GR, Yallampalli C. Vascular hyperresponsiveness to adrenomedullin during pregnancy is associated with increased generation of cyclic nucleotides in rat mesenteric artery. Biol Reprod. 2007;76:118–123. doi: 10.1095/biolreprod.106.053777. [DOI] [PubMed] [Google Scholar]
- Witlin A, Li ZY, Wimalawansa SJ, Grady JJ, Grafe MR, Yallampalli C. Placental and fetal growth and development in late rat gestation is dependent on adrenomedullin. Biol Reprod. 2002;67:1025–1031. doi: 10.1095/biolreprod.101.002196. [DOI] [PubMed] [Google Scholar]
- Yallampalli C, Chauhan M, Sathishkumar K. Calcitonin gene-related family peptides in vascular adaptations, uteroplacental circulation, and fetal growth. Curr Vasc Pharmacol. 2013;11:641–654. doi: 10.2174/1570161111311050007. [DOI] [PubMed] [Google Scholar]
- Ross GR, Chauhan M, Gangula PR, Reed L, Thota C, Yallampalli C. Female sex steroids increase adrenomedullin-induced vasodilation by increasing the expression of adrenomedullin2 receptor components in rat mesenteric artery. Endocrinology. 2005;147:389–396. doi: 10.1210/en.2005-0664. [DOI] [PubMed] [Google Scholar]
- Ross GR, Yallampalli C. Endothelium-independent relaxation by adrenomedullin in pregnant rat mesenteric artery: role of cAMP-dependent protein kinase A and calcium-activated potassium channels. J Pharmacol Exp Ther. 2006;317:1269–1275. doi: 10.1124/jpet.106.101790. [DOI] [PubMed] [Google Scholar]
- Chauhan M, Ross GR, Yallampalli U, Yallampalli C. Adrenomedullin-2, a novel calcitonin/calcitonin-gene-related peptide family peptide, relaxes rat mesenteric artery: influence of pregnancy. Endocrinology. 2007;148:1727–1735. doi: 10.1210/en.2006-1105. [DOI] [PubMed] [Google Scholar]
- Ross GR, Yallampalli U, Yallampalli C. Cyclic AMP-independent CGRP8-37-sensitive receptors mediate adrenomedullin-induced decrease of CaCl2-contraction in pregnant rat mesenteric artery. J Vasc Res. 2008;45:33–44. doi: 10.1159/000109075. [DOI] [PubMed] [Google Scholar]
- McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, Solari R, Lee MG, Foord SM. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- Miret JJ, Rakhilina L, Silverman L, Oehlen B. Functional expression of heteromeric calcitonin gene-related peptide and adrenomedullin receptors in yeast. J Biol Chem. 2002;277:6881–6887. doi: 10.1074/jbc.M107384200. [DOI] [PubMed] [Google Scholar]
- Christopoulos A, Christopoulos G, Morfis M, Udawela M, Laburthe M, Couvineau A, Kuwasako K, Tilakaratne N, Sexton PM. Novel receptor partners and function of receptor activity-modifying proteins. J Biol Chem. 2003;278:3293–3297. doi: 10.1074/jbc.C200629200. [DOI] [PubMed] [Google Scholar]
- Christopoulos G, Perry KJ, Morfis M, Tilakaratne N, Gao Y, Fraser NJ, Main MJ, Foord SM, Sexton PM. Multiple amylin receptors arise from receptor activity-modifying protein interaction with the calcitonin receptor gene product. Mol Pharm. 1999;56:235–242. doi: 10.1124/mol.56.1.235. [DOI] [PubMed] [Google Scholar]
- Doggrell SA. Pharmacology down under in 2001. Drug News Perspect. 2001;14:630–640. [PubMed] [Google Scholar]
- Ishimitsu T, Ono H, Minami J, Matsuoka H. Pathophysiologic and therapeutic implications of adrenomedullin in cardiovascular disorders. Pharmacol Ther. 2006;111:909–927. doi: 10.1016/j.pharmthera.2006.02.004. [DOI] [PubMed] [Google Scholar]
- DiPette DJ, Wimalawansa SJ. Cardiovascular actions of calcitonin gene-related peptide In Crass J, Avioli LV. (eds.), Calcium Regulating Hormones and Cardiovascular Function Boca Raton, FL: CRC Press; 1994. 239 252 [Google Scholar]
- Chauhan M, Gangula PR, Wimalawansa SJ, Yallampalli C. Studies on the effects of the N-terminal domain antibodies of calcitonin receptor-like receptor and receptor activity-modifying protein 1 on calcitonin gene-related peptide-induced vasorelaxation in rat uterine artery. Biol Reprod. 2004;70:1658–1663. doi: 10.1095/biolreprod.103.023895. [DOI] [PubMed] [Google Scholar]
- Ross GR, Yallampalli U, Gangula PR, Reed L, Sathishkumar K, Gao H, Chauhan M, Yallampalli C. Adrenomedullin relaxes rat uterine artery: mechanisms and influence of pregnancy and estradiol. Endocrinology. 2010;151:4485–4493. doi: 10.1210/en.2010-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JC, Drummond GB, McLachlan EM, Kilkenny C, Wainwright CL. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther S, Alexander RW, Atkinson WJ, Gimbrone MA., Jr. Functional angiotensin II receptors in cultured vascular smooth muscle cells. J Cell Biol. 1982;92:289–298. doi: 10.1083/jcb.92.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan M, Yallampalli U, Dong YL, Hankins GD, Yallampalli C. Expression of adrenomedullin 2 (ADM2)/intermedin (IMD) in human placenta: role in trophoblast invasion and migration. Biol Reprod. 2009;81:777–783. doi: 10.1095/biolreprod.108.074419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banadakoppa M, Goluszko P, Liebenthal D, Yallampalli C. Nitric oxide induces segregation of decay accelerating factor (DAF or CD55) from the membrane lipid-rafts and its internalization in human endometrial cells. Cell Biol Int. 2012;36:901–907. doi: 10.1042/CBI20110586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan M, Yallampalli U, Reed L, Yallampalli C. Adrenomedullin 2 antagonist infusion to rats during midgestation causes fetoplacental growth restriction through apoptosis. Biol Reprod. 2006;75:940–947. doi: 10.1095/biolreprod.106.053322. [DOI] [PubMed] [Google Scholar]
- Chauhan M, Elkins R, Balakrishnan M, Yallampalli C. Potential role of intermedin/adrenomedullin 2 in early embryonic development in rats. Regul Pept. 2011;170:65–71. doi: 10.1016/j.regpep.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witlin A, Gangula PRR, Wimalawansa SJ, Grady JJ, Grafe M, Yallampalli C. Adrenomedullin requires an intact nitric oxide system to function as an endogenous vasodilator in rat gestation. Hypertens Pregnancy. 2003;22(1):9–24. doi: 10.1081/PRG-120016789. [DOI] [PubMed] [Google Scholar]
- Caron KM, Smithies O. Extreme hydrops fetalis and cardiovascular abnormalities in mice lacking a functional Adrenomedullin gene. Proc Natl Acad Sci U S A. 2001;98:615–619. doi: 10.1073/pnas.021548898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh-hashi Y, Shindo T, Kurihara Y, Imai T, Wang Y, Morita H, Imai Y, Kayaba Y, Nishimatsu H, Suematsu Y, Hirata Y, Yazaki Y, et al. Elevated sympathetic nervous activity in mice deficient in alphaCGRP. Circ Res. 2001;89:983–990. doi: 10.1161/hh2301.100812. [DOI] [PubMed] [Google Scholar]
- Gibbons C, Dackor R, Dunworth W, Fritz-Six K, Caron KM. Receptor activity-modifying proteins: RAMPing up adrenomedullin signaling. Mol Endocrinol. 2007;21:783–796. doi: 10.1210/me.2006-0156. [DOI] [PubMed] [Google Scholar]
- Lu JT, Son YJ, Lee J, Jetton TL, Shiota M, Moscoso L, Niswender KD, Loewy AD, Magnuson MA, Sanes JR, Emeson RB. Mice lacking alpha-calcitonin gene-related peptide exhibit normal cardiovascular regulation and neuromuscular development. Mol Cell Neurosci. 1999;14:99–120. doi: 10.1006/mcne.1999.0767. [DOI] [PubMed] [Google Scholar]
- Gangula PR, Lanlua P, Bukoski RD, Wimalawansa SJ, Yallampalli C. Mesenteric arterial relaxation to calcitonin gene-related peptide is increased during pregnancy and by sex steroid hormones. Biol Reprod. 2004;71:1739–1745. doi: 10.1095/biolreprod.104.031369. [DOI] [PubMed] [Google Scholar]
- Koyama T, Sakurai T, Kamiyoshi A, Ichikawa-Shindo Y, Kawate H, Shindo T. Adrenomedullin-RAMP2 system in vascular endothelial cells. J Atheroscler Thromb. 2015;22:647–653. doi: 10.5551/jat.29967. [DOI] [PubMed] [Google Scholar]
- Hay DL, Howitt SG, Conner AC, Schindler M, Smith DM, Poyner DR. CL/RAMP2 and CL/RAMP3 produce pharmacologically distinct adrenomedullin receptors: a comparison of effects of adrenomedullin22-52, CGRP8-37 and BIBN4096BS. Br J Pharmacol. 2003;140:477–486. doi: 10.1038/sj.bjp.0705472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay DL, Conner AC, Howitt SG, Smith DM, Poyner DR. The pharmacology of adrenomedullin receptors and their relationship to CGRP receptors. J Mol Neurosci. 2004;22:105–113. doi: 10.1385/JMN:22:1-2:105. [DOI] [PubMed] [Google Scholar]
- Buhlmann N, Leuthauser K, Muff R, Fischer JA, Born W. A receptor activity modifying protein (RAMP)2-dependent adrenomedullin receptor is a calcitonin gene-related peptide receptor when coexpressed with human RAMP1. Endocrinology. 1999;140:2883–2890. doi: 10.1210/endo.140.6.6783. [DOI] [PubMed] [Google Scholar]
- Kuwasako K, Shimekake Y, Masuda M, Nakahara K, Yoshida T, Kitaura M, Kitamura K, Eto T. Visualization of the calcitonin receptor-like receptor and its receptor activity-modifying proteins during internalization and recycling. J Biol Chem. 2000;275:29602–29609. doi: 10.1074/jbc.M004534200. [DOI] [PubMed] [Google Scholar]
- Hilariet S, Foord SM, Marshall FH, Bouvier M. Protein-protein interaction and not glycosylation determines the binding selectivity of heterodimers between the calcitonin receptor-like receptor and the receptor activity-modifying proteins. J Biol Chem. 2001;276:29575–29581. doi: 10.1074/jbc.M102722200. [DOI] [PubMed] [Google Scholar]
- Buhlmann N, Aldecoa A, Leuthauser K, Gujer R, Muff R, Fischer JA, Born W. Glycosylation of the calcitonin receptor-like receptor at Asn(60) or Asn(112) is important for cell surface expression. FEBS Lett. 2000;486:320–324. doi: 10.1016/s0014-5793(00)02259-6. [DOI] [PubMed] [Google Scholar]
- Wrobel GS, Silberstein SD. Targeting CGRP: a new era for migraine treatment. CNS Drugs. 2015;29:443–452. doi: 10.1007/s40263-015-0253-z. [DOI] [PubMed] [Google Scholar]