Abstract
Expansion of the cumulus complex surrounding the oocyte is critical for ovulation of a fertilizable egg. The ovulation-inducing surge of luteinizing hormone leads to an increased expression of genes such as prostaglandin-endoperoxide synthase 2 (Ptgs2), pentraxin-related protein 3 (Ptx3), and tumor necrosis factor alpha-induced protein 6 (Tnfaip6) that support cumulus expansion. Factors released by mural granulosa and cumulus granulosa cells into the follicular fluid induce paracrine signaling within the follicular compartment. The follicular fluid that separates these distinct granulosa cell types is an enriched fluid containing numerous proteins, nucleic acids, and other macromolecules. Extracellular vesicles (EVs) are also present; however, no physiologically relevant functions of follicular EVs have yet been demonstrated. In our study, the effect of follicular EVs on cumulus-oocyte complex (COC) expansion and relevant gene expression was assayed. Follicular EVs were isolated using ultracentrifugation from follicular fluid of small (3–5 mm) and large (>9 mm) antral bovine follicles, then characterized by nanoparticle tracking analysis, electron microscopy, and Western blot analysis. To test for bioactivity, mouse and bovine COCs were cultured with follicular EVs. Cumulus expansion and Ptgs2, Ptx3, and Tnfaip6 gene expression were measured following COC maturation culture. The results demonstrated that follicular EVs can support both measurable cumulus expansion and increased gene expression.
Keywords: cumulus, exosome, extracellular vesicles, follicular fluid, granulosa, oocyte
INTRODUCTION
Antral follicles in the mammalian ovary enlarge with the secretion of follicular fluid that fills the growing cavity between the layers of granulosa cells [1]. This viscous fluid is a complex solution containing a mixture of water, electrolytes, proteins, RNA, and extracellular vesicles (EVs) providing a route for autocrine and paracrine communication between theca, mural granulosa, and cumulus cells and the maturing oocyte [2]. The content of follicular fluid correlates with the stage of the follicular growth and the developmental potential of the oocyte [3, 4].
EVs such as exosomes and microvesicles (ectosomes) have been identified in follicular fluid in several mammalian species including human, bovine, and equine [4–7]. Studies of cancer cell lines have demonstrated that cargos such as proteins and RNA within EVs can influence behavior and gene expression of cells both near and far [8, 9]. Formation of EVs occurs either by budding from the cell surface (microvesicles) or release from multivesicular bodies (exosomes) following fusion with the plasma membrane [8]. The size range of these two distinct types of EVs overlaps, with exosomes ranging from 30 to 200 nm in diameter, and microvesicles, typically considered to range from 50 to 1000 nm [10]. EVs are characterized by the presence of specific membrane-associated proteins, which are enriched in the EVs as compared to intact cells. These markers include several tetraspanins, CD81, CD63 and CD9, as well as other proteins, such as Alix, a protein involved in endosomal transport and tumor suppressor gene 101 (Tsg101) [11, 12]. Follicular fluid EVs have been associated with varying protein and RNA cargos depending on such factors as a woman's age [7] or the presence of ovarian pathologies such as polycystic ovarian syndrome [6]. Follicular fluid EVs carry miRNA and can be taken up by cultured granulosa cells [4, 5], demonstrating that they could potentially act as a method for cell-to-cell communication within the antral follicle. Isolated EVs from follicular fluid have been shown to affect the expression of a select few genes in cultured granulosa cells [4, 13]; however, evidence of physiological (cellular) effects on granulosa cell function following exposure to follicular fluid EV has yet to be shown.
Communication between mural granulosa and the cumulus-oocyte complex (COC) is known to occur bidirectionally across the antral follicular fluid [14, 15]. As an example, the luteinizing hormone (LH) surge stimulates release of mural granulosa epidermal growth factor (EGF) ligands that must then traverse the follicular fluid to affect the cumulus cells in order to induce the characteristic gene expression changes and expansion of the COC [14, 15]. The precise mechanism by which these and other signaling molecules move through the follicular fluid to rapidly affect cumulus cells remains unknown. Transmission of signals through an EV-mediated system is a possible mechanism of signal transduction within the follicle. In cancer cell models, EVs released into the microenvironment can increase cell motility and cause rapid modifications to the extracellular matrix [16, 17]. Similarly, cumulus expansion in response to the LH surge involves increased cumulus cell motility [18, 19] and rapid secretion of a hyaluronan-enriched extracellular matrix [20, 21]. Although the cargoes carried by follicular EVs vary greatly under different physiologic states, no direct effects of EVs on ovarian physiology have been demonstrated to date. The objective of the present study was to determine whether follicular EVs could affect the physiology and morphology of the COC and to elicit changes in expression of key genes known to be involved in the maturation (i.e., expansion) of the cumulus cells.
MATERIALS AND METHODS
COC Collection
COCs were collected from CF1 female mice (21–24 days old; obtained from Harlan Sprague-Dawley or Charles River Labs). Mice were housed in a temperature- and light-controlled room on a 14L:10D light cycle and experiments were conducted in accordance with National Institutes of Health guidelines for the care and use of laboratory animals [22, 23] and approved by the University of Kansas Medical Center Internal Animal Care and Use Committee. To stimulate synchronous follicular development, mice were administered 5 IU of equine chorionic gonadotropin (eCG; Calbiochem) i.p. Mice were euthanized by isoflurane inhalation anesthesia followed by cervical dislocation. Ovaries were collected at 44–46 h post-eCG and COCs were released from large antral follicles by rupturing the follicles with a sterile needle into HEPES-buffered KSOM medium [24] supplemented with 4 mg/ml polyvinyl alcohol (containing no bovine serum albumin [BSA] or fetal bovine serum [FBS]). Pools of COCs were created from 5–10 females, and only COCs that contained a germinal vesicle (GV)-stage oocyte (∼>75 μm) surrounded by 2 or more intact layers of cumulus cells were selected for culture. COCs containing dark, small, or non-GV oocytes or discontinuous cumulus layers were discarded. Unless otherwise stated, all chemicals and reagents were purchased from Sigma Chemical Corporation.
Murine COC Culture
Sets of 3–5 COCs were transferred to KSOMaa (Evolve; Zenith Biotech USA) supplemented with 4 mg/ml polyvinyl alcohol (molecular weight 10 000; no BSA) with or without FBS and/or follicular EVs (100 μg protein/ml) according to the individual experimental protocol (see results for details of each treatment). Protein levels were determined using Bradford Assay (Bio-Rad Protein Assay Dye Reagent Concentrate) as per manufacturer's protocol. Follicular fluid EV levels approximated 80–120 μg protein/ml and preliminary dose-response studies indicated that 100 μg/ml dose of follicular EVs yielded consistent responses in mouse COC assays. Mouse COCs were cultured for 16 h in 20-μl drops of media under oil in 35-mm Petri dishes (NUNC; Thermo Scientific) at 37.2°C in an incubator with 6% CO2 in humidified air. Each dish contained 3 to 5 culture drops of COC and each treatment was cultured in a separate dish to ensure no passage of treatment factors between drops of COC. The number of COCs in each drop was determined on the day of culture according to the total number of COCs collected on that day, and all drops cultured on that day contained the same number of COCs. COCs collected each day were randomly distributed across all treatments.
Bovine Follicular Fluid and COC Collection
Cow ovaries were obtained from a local abattoir in Omaha, Nebraska. Because the animals were not used for research purposes, our Institutional Animal Care and Use Committee ruled that the collection of ovaries from the abattoir did not constitute animal research and was exempt from further review. Ovaries were transported to the University of Kansas Medical Center in PBS at room temperature in preparation for ovarian follicular fluid aspiration. Ovarian follicular fluid was collected by needle aspiration from antral follicles with diameters of 3–5 mm (small) and >9 mm (large). Four independent collections of follicular fluid were conducted in a 2-mo period. Follicular fluids from small or large antral follicles were pooled separately on each collection day. All COCs were collected by pulled glass pipette and transferred to 400 μl of warm HEPES-buffered TCM199. High-quality COCs from the small antral follicles were used for COC cultures in TCM199 media using the same supplements and culture setup protocol as described for the mouse except that bovine COCs were matured for 24 h at 39°C and always in sets of 3 COCs/drop with or without follicular EVs (200 μg protein/ml). Because very few COCs (3–5/collection day) were found in follicular fluid from large follicles, none of the COCs from large follicles were used in the culture experiments.
Follicular EV Isolation by Differential Ultracentrifugation
Follicular EVs isolated from 15 ml of ovarian follicular fluid were obtained via a series of differential ultracentrifugation steps as described in [25]. Follicular fluid was diluted with an equal volume of PBS prior to centrifugation. To eliminate residual granulosa cells and oocytes, samples were spun at 800 × g for 10 min followed by 2000 × g for 20 min. Fluid was then centrifuged at 12 000 × g for 45 min to remove cell debris and large particles. The samples were then filtered through a 0.22-μm pore filter to remove particles larger than 200 nm. Ultracentrifugation was performed at 110 000 × g for 3 h in a Beckman X100 ultracentrifuge, using a swinging-bucket SW32Ti rotor, to pellet the follicular EVs. The pellets were then washed twice in PBS by centrifuging at 110 000 × g for 1.5 h. All centrifugations were performed at 4°C. The obtained pellets were resuspended in PBS for further analysis.
Nanoparticle Tracking Analysis
To determine particle size and concentration within follicular fluid from follicles of varying sizes, nanoparticle tracking analysis (NTA) was performed with a NanoSight LM10 instrument (Malvern Instruments). Follicular EV preparations were first diluted in PBS to meet the optimal concentration between 105 and 108 particles/ml. At least 300 μl of diluted sample was needed for each analysis and was mixed by vortexing before injection into the chamber. Three individual videos were collected and the resulting counts were averaged for each diluted sample. Triplicates of the same dilution were performed and the overall average of these dilutions was used as the experimental result for each sample. Each video of moving particles was 60 sec in duration, with a shutter speed of 30 ms and camera gain of 680. Software settings for analysis were as follows: detection threshold, 6; blur, auto; minimum expected particle size, 50 nm. A minimum of 200 particle tracks were completed for each video and the data were analyzed using the NTA 2.3 analytical software (Malvern). Data are presented as the average and standard deviation of the triplicate.
Western Blot Analysis
EV samples (10 μg protein) were lysed in SDS sample buffer with 50 mM dithiothreitol, heated for 5 min at 95°C, and subjected to electrophoresis using 12% SDS-PAGE in running buffer at constant 120 V for 1.5 h. Protein standards were included (#1610374; Bio-Rad). Proteins were electro-transferred onto polyvinylidene difluoride membranes, and the membranes were blocked with TTBS (50 mM Tris, 150 mM NaCl, 0.05% Tween-20) for 1 h at room temperature. Membranes were then probed with primary anti-CD81 (sc-166029; Santa Cruz Biotechnology Inc.) antibody for 1 h in TTBS followed by incubation with secondary goat anti-mouse IgG antibody for 1 h. Membranes were washed three times in TTBS for 10 min after each incubation step and detected by enhanced chemiluminescence (GE Healthcare Bio-science) per manufacturer's instructions. To ensure that similar levels of total proteins were loaded onto Western blots we stained the membranes with Swift Membrane Stain (G Biosciences) per manual instructions.
Nomarsky Live Microscopy
All COCs were imaged by Nomarski optics on a Nikon TE200 or by differential interference contrast (DIC) on an Olympus IX71 inverted microscope immediately after transfer to culture drops and again after 16 (murine) or 24 h (bovine) of maturation. Images were captured with 110× UplanFl 0.30 Ph1 and 20× LCplanFl 0.40 Ph1 objectives and an Olympus DP71 camera. The diameter of each murine COC was measured using ImageJ software [26]. For each COC, two diameters were measured and then averaged, resulting in an average diameter. The overall measure of expansion was the average change from 0 (start of culture) to 16 h for all COCs within each drop.
EV Uptake and Confocal Microscopy
EVs were labeled with PKH67 Green Fluorescent Cell Linker Kit (Sigma Chemical Corporation) per manual protocol and pelleted by ultracentrifugation following several washes in PBS before final resuspension in PBS. The labeled EVs were added to COC cultures the same as for standard cultures, and the last wash supernatant was used as the negative control. For imaging, COCs were fixed in 2% paraformaldehyde at room temperature overnight, then transferred into wash solution (PBS + 0.2% sodium azide + 1% BSA) supplemented with phalloidin conjugated with alexa568 (1:100 labels f-actin; Molecular Probes Life Technologies) and Hoechst 33258 (1:100 labels DNA) [27]. COCs were washed for 2 h, then whole mounted onto glass slides in wash solution. A small amount of paraffin-lanolin mixture was placed under the coverslips to prevent crushing of the COC; then, serial z-sections were imaged (1-μm thickness) on a Zeiss Pascal 510 confocal microscope with a 40× objective. Negative controls were COCs cultured with the last supernatant drawn off of the labeled EVs.
Transmission Electron Microscopy
EVs (10 mg/ml) were fixed overnight (2% glutaraldehyde and 0.1 M cacodylate buffer) and washed with 0.1 M sodium cacodylate buffer. The pellet was postfixed in 1% osmium tetroxide with 1% potassium ferric cyanide buffered in 0.1 M cacodylate buffer for 1 h. A glow-discharge-treated carbon-film 300-mesh grid was inverted and floated upside down on the drop containing fixed EVs (20 min). Each grid was rinsed seven times with filtered distilled water, then stained with 1% uranyl acetate. All samples were examined with a JEOL-JEM-1400 transmission electron microscope at 80–100 kV with 25 000× magnification.
RNA Isolation and Quantitative RT-PCR Analysis
RNA was isolated using the Arcturus PicoPure RNA Isolation kit (Life Technologies), with an on-column DNase I digestion. Concentration and purity of RNA were determined by using the Agilent RNA 6000 Pico kit (Agilent Technologies) and Bioanalyzer (Agilent Technologies). Total RNA was reverse transcribed using SuperScript II Reverse Transcriptase (Life Technologies) with random primer per manufacturer's instruction. Quantitative PCR was then performed with 1:5 dilution of cDNA on an Applied Biosystems HT7900 sequence detector. Primer sets for mouse Ptgs2 (forward: TGA CCC CCA AGG CTC AAA TA, reverse: CCC AGG TCC TCG CTT ATG ATC), Ptx3 (forward: CCC GCA GGT TGT GAA ACA G, reverse: TGC ACG CTT CCA AAA ATC TTC), Tnfaip6 (forward: ATA CAA GCT CAC CTA CGC CGA, reverse: ATC CAT CCA GCA GCA CAG ACA T), bovine Ptgs2 (forward: TGG GTG TGA AAG GGA GGA AA, reverse: GTG AAA GCT GGT CCT CGT TC), Ptx3 (forward: CCG GCA GGT TGT GAA ACA G, reverse: CAG CGA CCA GTC TGT TTT CC), Tnfaip6 (forward: AAC CCA CAT GCA AAG GAG TG, reverse: GCC GTG GAC ATC ATC GTA AC) and U6 (forward: CTC GCT TCG GCA GCA CA, reverse: AAC GCT TCA CGA ATT TGC GT) were designed using Primer Express 3.0 software (Applied Biosystems) and GAPDH primers and probe were purchased from Life Technologies. Samples were run in triplicate, and the ΔΔCt method was used to calculate the relative fold change between the samples after normalization with GAPDH or U6 for mouse or bovine COCs, respectively. The presence of a single dissociation curve confirmed the amplification of a single transcript and lack of primer dimers.
Statistics
Results of multiple repeats were presented as means ± SEM. A one-way ANOVA followed by a Newman-Keuls multiple-comparison test was used to determine statistical differences between groups. A Bartlett test was included to ensure equal variance in the group. If the data did not follow a normal distribution, the data sets were log transformed before performing the statistical analyses.
RESULTS
Characterization of Follicular EVs
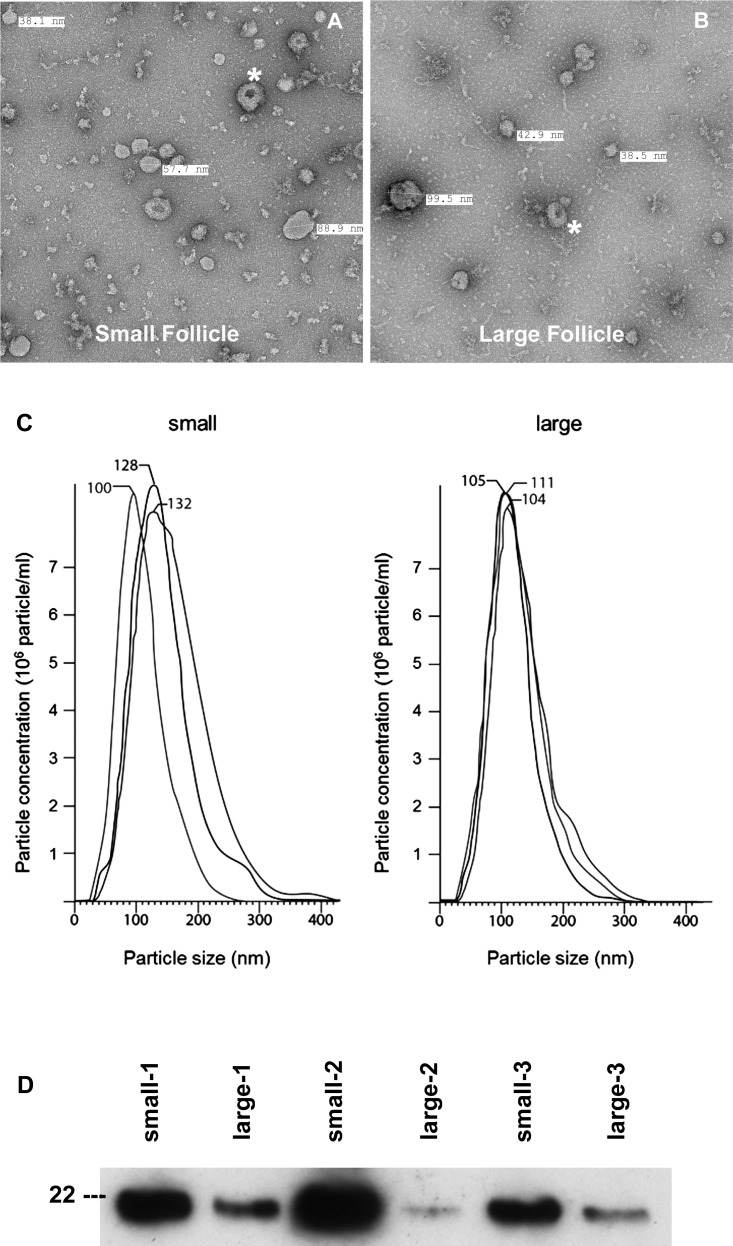
To confirm that follicular EVs were present in our isolates, we used a combination of techniques. Electron microscopy revealed a heterogeneously sized population of circular vesicles, 50–200 nm in diameter (Fig. 1A from small antral follicles; Fig. 1B from large antral follicles), many with the characteristic concave centers that result from the collapsing of the EV upon drying on the film (Fig. 1, A* and B*) [28]. The purity of the follicular EV preparation was confirmed, as few protein conglomerates were observed (dense/dark objects). We further characterized the follicular EVs from small and large follicles using NTA (Fig. 1C). Follicular EVs ranged in size between ∼30 and 325 nm with average sizes of 142 and 128 nm for EVs collected from small and large antral follicles, respectively. CD81, an established exosome marker, was used to confirm the presence and enrichment of exosomes in the EV preparations. We found that CD81 was highly enriched in EVs, with a higher level in EVs derived from small follicles (Fig. 1D). Total proteins were similarly (Supplemental Fig. S1; Supplemental Data are available online at www.biolreprod.org) present in both small- and large-follicle EV preparations, which suggests that levels of CD81 in EVs change with follicle size.
FIG. 1.
Characterization of follicular EVs from bovine follicular fluid. After centrifugation and washing, isolated follicular EVs were examined by negative staining on an electron microscope to determine the presence and characteristics of the follicular EVs. Both small (A) and large (B) antral follicles contained EVs of varying sizes, mostly spherical and some with the concave centers that are characteristic of exosomes (*). C) NTA indicated that EVs collected from small and large follicles are similar in size. NTA was repeated on three isolates of follicular EVs collected on different days, which are overlaid in this figure to graphically demonstrate the variation between these follicular EV isolates. The average diameters (nm) of particles are shown for each of the three independent preparations of small and large antral follicle EVs. D) Western blots of three collections of EVs from small and large antral follicles were probed with the CD81 antibody.
Uptake of Follicular EVs by Cumulus Cells
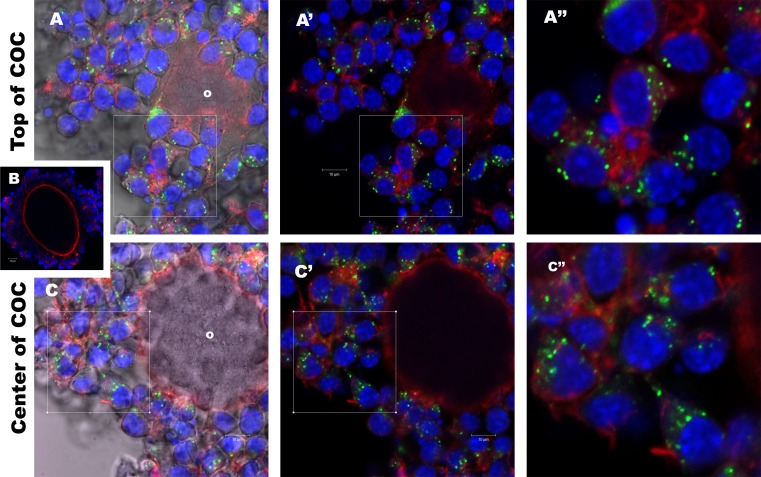
To determine if bovine follicular EVs are taken up by mouse cumulus cells, PKH67-labeled follicular EVs were cultured with COCs for 16 h. Green fluorescent punctate spots (internalized EVs) were observed in the cytoplasm of cumulus cells and localized near the nucleus (Fig. 2). Follicular EVs were detected in all layers of the expanded cumulus, including those cells farthest from the oocyte (Fig. 2A) as well as those cells next to the zona pellucida (i.e., corona radiata; Fig. 2C). To ensure that nonspecific staining of cells from leftover dye was not occurring, we also cultured COCs with the supernatant from the last wash, and no staining was detected (Fig. 2B). We also did not detect uptake of follicular EVs by the oocytes of intact COCs, although we did observe evidence of EVs associated with the transzonal processes, which span the zona pellucida to contact the oocyte (not shown).
FIG. 2.
Cumulus cells uptake follicular fluid EVs during COC culture. Mouse COCs were cultured 16 h with PKH67-labeled bovine follicular EVs. Individual COCs were imaged on a Zeiss Pascal confocal microscope (×40) in sections 1 μm thick and examined for internalization of follicular EVs. In the expanded COC, internalized follicular EVs are evident as green spots within cumulus cells in both the outer layers of cumulus cells (A) and inner layers adjacent (C) to the oocyte (o). As negative control to insure that the COC labeling was a result of EV uptake and not residual free-floating dye in the media, the final supernatant (wash) from the follicular EV PKH67 labeling process was added to COC cultures, and these cumulus cells were all negative for PKH67 (B). Distance between the sections in A and C is 30 μm within the same COC. A′ and C′ are the same as A and C but with DIC turned off. A″ and C″ are enlargements from the boxes identified in A′ and C′. Bar = 10 μm.
Follicular EVs Induce Cumulus Expansion
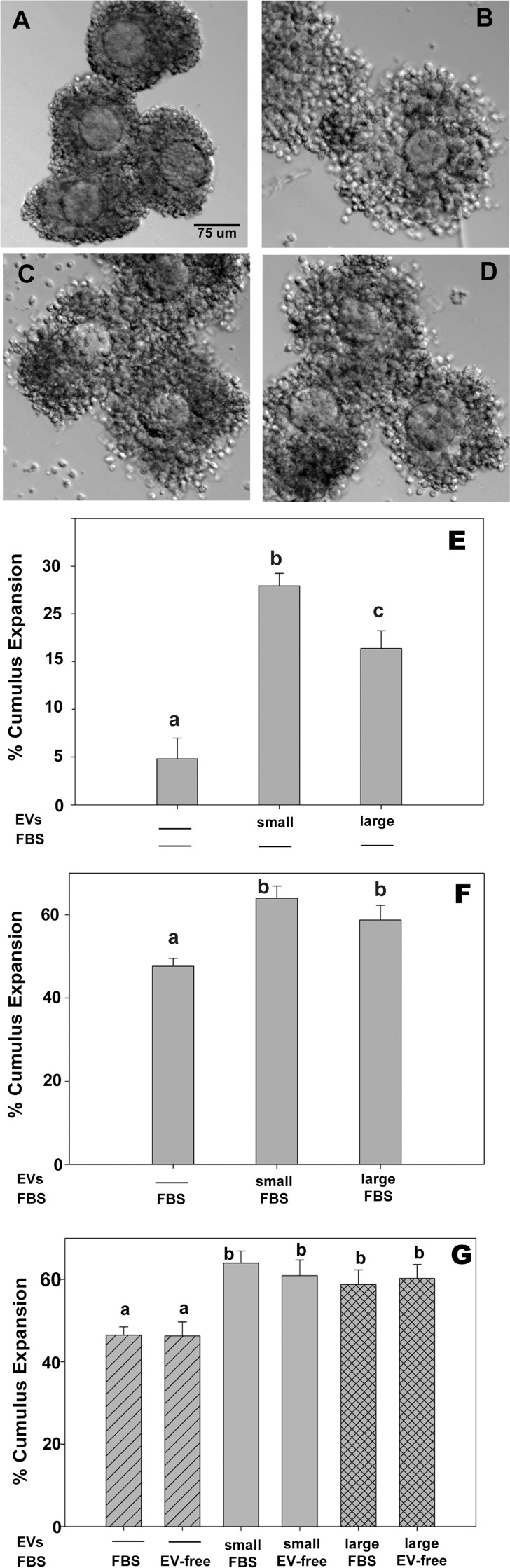
To determine if follicular EVs have a physical effect on the expansion of the COC, we used mouse COCs because we can synchronize the mice to produce large numbers of well-defined COCs that can then be in vitro matured with or without the addition of bovine follicular EVs (Fig. 3). Differential interference contrast (Nomarski) imaging was used to determine the diameter of COCs at the beginning (0 h) and at the completion of the 16-h mouse IVM protocol. The COCs cultured without serum (control) did not expand during culture (negative control; Fig. 3A). Addition of 10% FBS (positive control) caused an increase in the diameter of the COC (cumulus expansion; Fig. 3B). Similarly to the positive control, follicular EVs from both small (3–5 mm) and large (>9 mm) bovine antral follicles induced cumulus expansion (Fig. 3, C and D). Analysis of changes in COC diameter (percentage) confirmed that follicular EVs from both small and large antral follicles could induce cumulus expansion significantly greater than occurred in medium alone (i.e., negative control; Fig. 3E). Paradoxically, EVs isolated from small antral follicles exhibited a greater effect on cumulus expansion than those isolated from larger preovulatory follicles when cultured without FBS. (Average diameters of COCs are shown in Supplemental Table S1.) To determine if the observed effects of follicular EVs were due to contaminants of EV from blood serum, we next tested COC expansion in media that contained 10% FBS plus follicular EVs. The follicular EVs from both small and large follicles increased cumulus expansion over that observed for COCs cultured with FBS (EV containing) alone, indicating that follicular EVs had an additive effect over any possible effects of FBS EVs (Fig. 3F). Because whole serum such as FBS contains endogenous EVs, we also cultured COCs with either whole FBS or FBS that was processed by ultracentrifugation and filtration to remove endogenous EVs (EV-free FBS). In these experiments there was no difference between FBS with or without the native serum EVs (Fig. 3G). These results indicate that cumulus expansion in response to EV from antral follicles is specific to follicular EVs and not a general effect of EVs from serum.
FIG. 3.
Bovine follicular EVs induce mouse cumulus cell expansion. Mouse COCs were matured with follicular EVs from either small or large antral bovine follicles. COCs were imaged by DIC at the beginning (0 h) and end (16 h) of culture. Negative control COCs cultured with no FBS and with no follicular EVs did not expand (A). COCs cultured with either FBS (B; positive controls), or with follicular EVs from small (C) or large (D) antral follicles induced cumulus expansion. Cumulus expansion was quantified and the percentage change in diameter of the COCs after 16 h of in vitro maturation is shown. Bar = 75 μm. E) COCs cultured with no serum or EV exhibited very little expansion. In the absence of serum, cumulus expansion induced by follicular EVs from the large antral follicles was significantly less than that induced by small antral follicle EVs (A). F) COCs matured with follicular EVs added to media containing FBS. COCs cultured in FBS exhibited some expansion and follicular EVs increased cumulus expansion over that of whole FBS (i.e., FBS containing endogenous EVs). G) COCs matured in whole FBS, EV-free FBS (EV-FBS), with and without follicular EVs from small or large follicles. Removing serum endogenous EVs from the FBS had no effect on cumulus expansion, but addition of follicular EVs still significantly increased COC expansion (C). a,b,cMeans ± SEM with different superscripts (a, b, or c) are significantly different (P < 0.05).
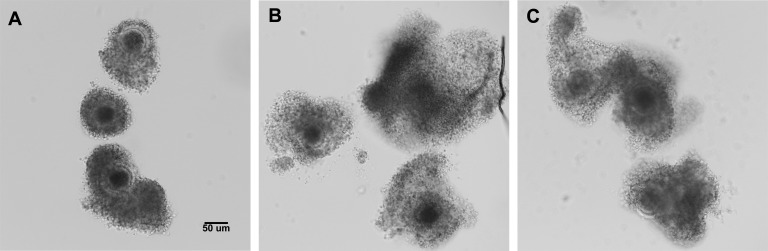
Because the above studies involved treatment of mouse COCs with bovine EVs, we wanted to evaluate whether cross-species effects could be either negatively or positively impacting our observations of cumulus expansion. Therefore, we similarly cultured bovine COCs with bovine EVs. Consistent with the mixed-species experiments using bovine EVs with mouse COCs, the bovine follicular EVs from both small (Fig. 4B) and large (Fig. 4C) antral follicles induced expansion of bovine COCs.
FIG. 4.
Bovine follicular EVs induce cumulus expansion of bovine COCs. Bovine COCs were matured with follicular EVs from either small or large antral bovine follicles. COCs were imaged by DIC at the beginning of culture (0 h) and following in vitro maturation (24 h). Negative control COCs cultured with no FBS and with no follicular EVs did not expand (A). COCs cultured with either small-follicle EVs (B) or large-follicle EVs (C) exhibited increased cumulus expansion. Bar = 50 μm.
Analysis of COC Expansion Marker Genes
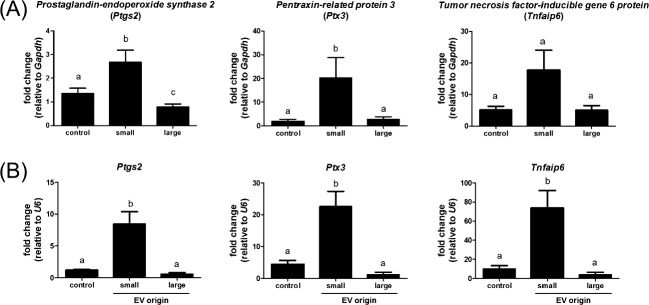
To determine if follicular EVs affect the expression of genes associated with cumulus expansion, we examined the expression of three genes with known functions in COC expansion: Ptgs2, Ptx3, and Tnfaisp6. Treatment of mouse COCs with bovine follicular EVs from small antral follicles significantly increased expression of Ptgs2 and Ptx3, with fold changes of 2.7 and 20.3, respectively (Fig. 5A). There was also a trend for increased expression of Tnfaisp6 (average fold change of 3.5) although this change did not reach significance (P < 0.08). Bovine COCs matured with bovine follicular EVs from small antral follicles also significantly up-regulated expression of Ptgs2 (7.1-fold), Ptx3 (5.1-fold) and Tnfaip6 (7.5-fold; Fig. 5B). Follicular EVs from large antral follicles did not induce the expression of Ptx3 or Tnfaip6; however, there was a decrease in the expression of Ptgs2 in mouse COCs. Thus, the bovine EVs had the same effect on gene expression patterns for both bovine and mouse COCs.
FIG. 5.
Follicular fluid EVs induce genes involved in cumulus expansion. COCs from mice (A) and cows (B) were matured for 16 and 24 h, respectively, followed by quantitative RT-PCR analysis of Ptgs2, Ptx3, and Tnfaip6 levels. Small-follicle EVs increased cumulus gene expression in both mouse (A) and bovine COCs (B), whereas large-follicle EVs did not. Mouse COC Tnfaip6 expression trended (P = 0.08) to increase. a,bMeans ± SEM with different superscripts are significantly different (P < 0.05).
DISCUSSION
Ovarian follicular fluid is enriched with proteins, RNA, and EVs. Our studies have demonstrated for the first time that follicular EVs can impact ovarian function. Follicular EVs were able to support cumulus expansion and modify the expression of genes with known associations to cumulus expansion in vivo. Interestingly, follicular EVs isolated from small antral follicles exhibited more activity than those from large follicles (Fig. 3), even though in vivo, COCs from small follicles do not undergo expansion. However, when bovine COCs from small antral follicles are released into culture they are functionally capable of undergoing expansion (Fig. 4). And, similar to the murine COCs, the bovine COCs exhibited significant increased gene expression when exposed to EV from small but not large antral follicles (Fig. 5, A and B). This is an interesting phenomenon, which is discussed below and poses many questions for examination in future studies.
Mammalian COCs secrete a hyaluronan-enriched extracellular matrix that separates the cumulus cells and the oocyte, leading to what is termed cumulus expansion in response to the LH surge. LH receptors are found primarily in the theca and mural granulosa of antral follicles but not in the early cumulus cells. It is theorized that cumulus expansion is triggered in vivo in response to signals that are generated first by the mural granulosa cells and transmitted through the follicular fluid to affect the cumulus cells. Indeed, many studies support a model by which EGF ligands, such as amphiregulin, epiregulin, and epigen, are released from mural granulosa cells to subsequently effect the cumulus cell gene expression and expansion [29, 30]. Whether these growth factors are simply released into the follicular fluid and work in a juxtacrine manner down a concentration gradient or whether they are also partially released as a constituent of EVs is currently unknown. Interestingly, EGF ligands and active receptors (ErbB1) have been detected in EVs released from cancer cells [31]. Although we did not detect any EGF ligands in the follicular fluid EVs, the negative results may be at least partially due to problems with antibody specificity to the bovine molecules (data not shown). RNAseq, proteomic, and lipidomic studies that can unbiasedly identify follicular EV RNAs, proteins, and phospholipids are under way in our laboratory to address this question. The fact that small-follicle EVs exhibited the greatest increase in cumulus expansion, however, argues against an EGF ligand as being the active component within or on the surface of the follicular EVs, as these ligands are not typically expressed by mural granulosa cells until after the LH surge [32, 33].
Our recent studies on the content of follicular EVs have identified changes in the EV miRNA content from bovine follicles of different sizes (Navakanitworakul, Hung, and Christenson, data not shown). Whether miRNA contents are responsible for the functional effects of the EVs is unknown. Two prior studies have implicated exosomal miRNA in gene expression of cultured granulosa cells [4, 13], although, upon careful inspection, these papers do not prove a direct cause and effect for the exosomal miRNA from EVs on the cultured granulosa cells. They do, however, suggest that exosome/EV exposure could be responsible for the gene expression changes. In the current study, we have shown changes in gene expression in cumulus cells, within a physiologically intact whole COC that clearly mimics the changes seen in normal COCs undergoing expansion in vivo following exposure to the ovulatory surge of LH. Identification of the factor(s) responsible for the changes in gene expression and cumulus expansion will not be a trivial task. Currently, the identified causative factor(s) in gene expression of granulosa cells and in our COC gene expression/expansion studies remain unknown. Whether it is proteins, lipids, miRNA, or other contents within the EVs, or whether it is a membrane-bound factor(s) or even factors that are loosely associated with the EVs, remains to be determined.
EVs isolated from follicles of different sizes differ in their ability to affect gene expression in the cumulus cells. We found increased expression of genes with known functions in cumulus expansion was activated by EVs from small but not large follicles (Fig. 5). This result is counterintuitive because it is the COC within the large follicles that normally reacts to the LH surge and undergoes cumulus expansion. However, previous studies in swine have similarly reported greater cumulus expansion induced by follicular fluid from small rather than large antral follicles [34]. One possible explanation is that small follicles contain EVs that support cumulus expansion and that these EVs are not bound/taken up by COCs during follicular development until after the LH surge, and the remaining (or newly produced) EVs in large follicles lack the ability to promote cumulus expansion. Another possibility is that follicular fluid from large follicles contains inhibitory factors that are not active in small antral follicles. This model is supported by the studies of Hosoda and Terada [35], who used filtration fractionation of follicle fluid from large bovine follicles and determined that a >100-kDa fraction was associated with an inhibitory factor that blocked the cumulus expanding activity of EGF. Because our isolation techniques used centrifugation primarily rather than filtration, the type of vesicles from our studies and the isolates from Hosoda and Terada [35] overlap in size. It is therefore possible that an inhibitory factor associated with our EVs isolated from large follicles might limit their biologic activity. Future studies will be aimed at determining the differences in follicular EV cargos as follicles mature.
Follicular EVs have been characterized by multiple groups [3–6], yet we still do not yet know which cells produce the EVs found in follicular fluid. EVs include exosomes that are released from multivesicular bodies [25], which are present in oocytes [36] and granulosa and cumulus cells [37]. Our Western blot shows that EVs are enriched for the tetraspanin CD81, a known exosome-associated protein. We theorize that follicular fluid EVs are likely released by the neighboring granulosa cells (mural and cumulus) [37] with lesser contributions from the oocyte and the theca cells, because it is the granulosa cells that contact the follicular fluid directly. However, until properly tested, the source of EVs remains unknown. It is generally considered that EVs would be too large to have entered from the theca vasculature, based on observations that large serum proteins >100 kDa are rate limited and cannot enter the follicular fluid freely (reviewed in [1]). Proteins of 100 kDa are in general less than 10 nm in diameter, whereas our NTA and electron microscopy (Fig. 2) found that follicular EVs range from 30 to 350 nm in diameter. Thus, EVs are much larger than the largest serum proteins and as a result may be limited in their ability to pass through the basement membrane separating the theca and granulosa. Of note, however, the lipid bilayer of EVs could also dramatically impact their transport. The passage of EVs through a cellular barrier by cell uptake, intracellular transport, and subsequent export cannot be ruled out, and ongoing studies are under way to determine the original source and transport mechanisms of follicular EVs [37].
In addition to our lack of knowledge regarding the cells that release follicular EVs, our current work, as well as previous published studies that describe follicular exosomes and microvesicles [3–6], does not directly address the mechanism of follicular EV biogenesis or release. Without this knowledge, it is impossible to differentiate between two types of EVs: large exosomes and small microvesicles. Therefore, we have chosen to call the bilipid vesicles we observe in follicular fluid, EVs, indicating that the material is probably a mixture of exosomes and/or microvesicles. In addition to an overlap in size, it is now known that many of the so-called exosome-specific markers can also be associated with some microvesicles; thus, there is also overlap in the protein markers. This may not be a minor concern, as major differences in the cargo and membrane components derived from these two different pathways and the specific effects on their target cells may exist.
Because our lab and others have shown differences in the miRNA and protein content in EVs derived from different cell types (discussed above), the question arose as to whether the EV-induced effects on cumulus cells was specific to EVs from follicular fluid or if this is a nonspecific effect of EVs in general. Therefore, in addition to the studies with EVs from follicular fluid, we also examined the potential effects of EVs from serum. FBS is a relatively undefined factor added to many culture systems. Different batches of FBS can have different effects on cell culture [38]. The methods used to isolate and preprocess the individual batches of FBS is especially critical in determining the types of factors (proteins, steroids, RNA, etc.) that are present in the serum. FBS also contains EVs from undefined sources of the body, and removal of EVs from FBS can reduce the proliferative rate of cultured cells [39]. Follicular fluid contains proteins that enter the antral follicle from serum [40]. The purpose of the experiment in Fig. 3 was to determine if EVs from serum (FBS, a nonfollicular source of EVs) would have the same effect as EVs from follicle fluid. In other words, is the effect of EVs from follicular fluid specific or would EVs from other cell types have the same effect on cumulus expansion and gene expression? Thus, we used a single batch of FBS (the same batch used in all of the experiments in this manuscript) and processed it through ultracentrifugation to remove the endogenous EVs from the serum (EV-free serum). We then used follicular fluid EVs from either small antral follicles or large follicles and added them either to whole FBS that contains endogenous serum EVs (FBS) or to the same batch of serum but with the endogenous EVs removed (EV-free serum). By removing the endogenous EVs from the serum and then replacing them with the EVs from follicular fluid, the results show that serum EVs are not the same as EVs from follicular fluid. Sera with or without EVs were identical. Therefore, the effects of follicular EVs on cumulus expansion and gene expression appear to be specific to EVs from the follicle.
In conclusion, our studies have found a physiological effect of follicular EVs. These EVs were able to stimulate cumulus expansion and to up-regulate cumulus gene expression in vitro. Our current and future studies are directed towards further defining the miRNA and protein cargos associated with follicular EVs and mechanisms of uptake, release, and transport within and between cells of the ovarian antral follicle.
ACKNOWLEDGMENT
The authors would like to thank Dr. John Davis of the University of Nebraska Medical Center and Pan Zhang for assistance in collection of bovine ovaries at the Omaha, NE, packing plant. We would also like to thank Barbara Fegley and the KUMC electron microscopy (EM) facility for TEM sample processing and imaging; Stan Fernald (S-Fernald Illustration and Design, S-fernald.com) for assistance in creating the NTA data figure (Fig. 1C).
Footnotes
Supported by NIH grants HD082484 to L.K.M. and L.K.C. and HD061580 to L.K.C. The KUMC EM core is supported in part by NIH COBRE grant 9P20GM104936.
These authors contributed equally to these studies.
REFERENCES
- Rodgers RJ, Irving-Rodgers HF. Formation of the ovarian follicular antrum and follicular fluid. Biol Reprod. 2010;82:1021–1029. doi: 10.1095/biolreprod.109.082941. [DOI] [PubMed] [Google Scholar]
- Gosden RG, Hunter RH, Telfer E, Torrance C, Brown N. Physiological factors underlying the formation of ovarian follicular fluid. J Reprod Fertil. 1988;82:813–825. doi: 10.1530/jrf.0.0820813. [DOI] [PubMed] [Google Scholar]
- Revelli A, Delle Piane L, Casano S, Molinari E, Massobrio M, Rinaudo P. Follicular fluid content and oocyte quality: from single biochemical markers to metabolomics. Reprod Biol Endocrinol. 2009;7:40. doi: 10.1186/1477-7827-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohel MM, Hoelker M, Noferesti SS, Salilew-Wondim D, Tholen E, Looft C, Rings F, Uddin MJ, Spencer TE, Schellander K, Tesfaye D. Exosomal and non-exosomal transport of extra-cellular microRNAs in follicular fluid: implications for bovine oocyte developmental competence. PLoS One. 2013;8:e78505. doi: 10.1371/journal.pone.0078505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silveira JC, Veeramachaneni DN, Winger QA, Carnevale EM, Bouma GJ. Cell-secreted vesicles in equine ovarian follicular fluid contain miRNAs and proteins: a possible new form of cell communication within the ovarian follicle. Biol Reprod. 2012;86:71. doi: 10.1095/biolreprod.111.093252. [DOI] [PubMed] [Google Scholar]
- Sang Q, Yao Z, Wang H, Feng R, Wang H, Zhao X, Xing Q, Jin L, He L, Wu L, Wang L. Identification of microRNAs in human follicular fluid: characterization of microRNAs that govern steroidogenesis in vitro and are associated with polycystic ovary syndrome in vivo. J Clin Endocrinol Metab. 2013;98:3068–3079. doi: 10.1210/jc.2013-1715. [DOI] [PubMed] [Google Scholar]
- Diez-Fraile A, Lammens T, Tilleman K, Witkowski W, Verhasselt B, De Sutter P, Benoit Y, Espeel M, D'Herde K. Age-associated differential microRNA levels in human follicular fluid reveal pathways potentially determining fertility and success of in vitro fertilization. Hum Fertil (Camb) 2014;17:90–98. doi: 10.3109/14647273.2014.897006. [DOI] [PubMed] [Google Scholar]
- Cocucci E, Meldolesi J. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015;25:364–372. doi: 10.1016/j.tcb.2015.01.004. [DOI] [PubMed] [Google Scholar]
- Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012;1820:940–948. doi: 10.1016/j.bbagen.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Gyorgy B, Szabo TG, Pasztoi M, Pal Z, Misjak P, Aradi B, Laszlo V, Pallinger E, Pap E, Kittel A, Nagy G, Falus A, et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68:2667–2688. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D, Gupta D, Shankar S, Srivastava RK. Biomolecular characterization of exosomes released from cancer stem cells: possible implications for biomarker and treatment of cancer. Oncotarget. 2014;6:3280–3291. doi: 10.18632/oncotarget.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissig C, Gruenberg J. ALIX. and the multivesicular endosome: ALIX in Wonderland. Trends Cell Biol. 2014;24:19–25. doi: 10.1016/j.tcb.2013.10.009. [DOI] [PubMed] [Google Scholar]
- da Silveira JC, Carnevale EM, Winger QA, Bouma GJ. Regulation of ACVR1 and ID2 by cell-secreted exosomes during follicle maturation in the mare. Reprod Biol Endocrinol. 2014;12:44. doi: 10.1186/1477-7827-12-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti M, Hsieh M, Zamah AM, Oh JS. Novel signaling mechanisms in the ovary during oocyte maturation and ovulation. Mol Cell Endocrinol. 2012;356:65–73. doi: 10.1016/j.mce.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su YQ, Sugiura K, Li Q, Wigglesworth K, Matzuk MM, Eppig JJ. Mouse oocytes enable LH-induced maturation of the cumulus-oocyte complex via promoting EGF receptor-dependent signaling. Mol Endocrinol. 2010;24:1230–1239. doi: 10.1210/me.2009-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber J, Yeung V, Clayton A. Extracellular vesicles as modulators of the cancer microenvironment. Semin Cell Dev Biol. 2015;40:27–34. doi: 10.1016/j.semcdb.2015.01.013. [DOI] [PubMed] [Google Scholar]
- Mu W, Rana S, Zoller M. Host matrix modulation by tumor exosomes promotes motility and invasiveness. Neoplasia. 2013;15:875–887. doi: 10.1593/neo.13786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akison LK, Alvino ER, Dunning KR, Robker RL, Russell DL. Transient invasive migration in mouse cumulus oocyte complexes induced at ovulation by luteinizing hormone. Biol Reprod. 2012;86:125. doi: 10.1095/biolreprod.111.097345. [DOI] [PubMed] [Google Scholar]
- Kawashima I, Liu Z, Mullany LK, Mihara T, Richards JS, Shimada M. EGF-like factors induce expansion of the cumulus cell-oocyte complexes by activating calpain-mediated cell movement. Endocrinology. 2012;153:3949–3959. doi: 10.1210/en.2012-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura N, Hoshino Y, Totsukawa K, Sato E. Cellular and molecular events during oocyte maturation in mammals: molecules of cumulus-oocyte complex matrix and signalling pathways regulating meiotic progression. Soc Reprod Fertil Suppl. 2007;63:327–342. [PubMed] [Google Scholar]
- Russell DL, Salustri A. Extracellular matrix of the cumulus-oocyte complex. Semin Reprod Med. 2006;24:217–227. doi: 10.1055/s-2006-948551. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (U.S.) Committee on Care and Use of Laboratory Animals Guide for the Care and Use of Laboratory Animals Bethesda, MD: U.S. Dept. of Health and Human Services, Public Health Service; 1996. 140. [Google Scholar]
- National Research Council (U.S.) Committee for the Update of the Guide for the Care and Use of Laboratory Animals, Institute for Laboratory Animal Research (U.S.), National Academies Press (U.S.) Guide for the Care and Use of Laboratory Animals Washington, D.C. National Academies Press; 2011. [Google Scholar]
- Biggers JD, McGinnis LK. Evidence that glucose is not always an inhibitor of mouse preimplantation development in vitro. Hum Reprod. 2001;16:153–163. doi: 10.1093/humrep/16.1.153. [DOI] [PubMed] [Google Scholar]
- Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. Chapter 3:Unit 3.22. 2006 doi: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH. Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis LK, Pelech S, Kinsey WH. Post-ovulatory aging of oocytes disrupts kinase signaling pathways and lysosome biogenesis. Mol Reprod Dev. 2014;81:928–945. doi: 10.1002/mrd.22413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan MC, Manganelli L, Woollard JR, Masyuk AI, Masyuk TV, Tammachote R, Huang BQ, Leontovich AA, Beito TG, Madden BJ, Charlesworth MC, Torres VE, et al. Characterization of PKD protein-positive exosome-like vesicles. J Am Soc Nephrol. 2009;20:278–288. doi: 10.1681/ASN.2008060564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carletti MZ, Christenson LK. Rapid effects of LH on gene expression in the mural granulosa cells of mouse periovulatory follicles. Reproduction. 2009;137:843–855. doi: 10.1530/REP-08-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti M. Signaling networks in somatic cells and oocytes activated during ovulation. Ann Endocrinol (Paris) 2010;71:189–190. doi: 10.1016/j.ando.2010.02.010. [DOI] [PubMed] [Google Scholar]
- Sanderson MP, Keller S, Alonso A, Riedle S, Dempsey PJ, Altevogt P. Generation of novel, secreted epidermal growth factor receptor (EGFR/ErbB1) isoforms via metalloprotease-dependent ectodomain shedding and exosome secretion. J Cell Biochem. 2008;103:1783–1797. doi: 10.1002/jcb.21569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303:682–684. doi: 10.1126/science.1092463. [DOI] [PubMed] [Google Scholar]
- Sekiguchi T, Mizutani T, Yamada K, Kajitani T, Yazawa T, Yoshino M, Miyamoto K. Expression of epiregulin and amphiregulin in the rat ovary. J Mol Endocrinol. 2004;33:281–291. doi: 10.1677/jme.0.0330281. [DOI] [PubMed] [Google Scholar]
- Ito M, Iwata H, Kitagawa M, Kon Y, Kuwayama T, Monji Y. Effect of follicular fluid collected from various diameter follicles on the progression of nuclear maturation and developmental competence of pig oocytes. Anim Reprod Sci. 2008;106:421–430. doi: 10.1016/j.anireprosci.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Hosoda K, Terada T. Bovine follicular fluid contains antagonist activity for cumulus expansion promoting effect by epidermal growth factor. Reprod Domest Anim. 2007;42:225–229. doi: 10.1111/j.1439-0531.2006.00755.x. [DOI] [PubMed] [Google Scholar]
- Adams EC, Hertig AT. Studies on guinea pig oocytes: I. Electron microscopic observations on the development of cytoplasmic organelles in oocytes of primordial and primary follicles. J Cell Biol. 1964;21:397–427. doi: 10.1083/jcb.21.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silveira JC, de Andrade GM, Nogueira MF, Meirelles FV, Perecin F. Involvement of miRNAs and cell-secreted vesicles in mammalian ovarian antral follicle development Reprod Sci (in press) Published online ahead of print 2 March 2015. DOI 10.1177/1933719115574344. [DOI] [PubMed] [Google Scholar]
- Zheng X, Baker H, Hancock WS, Fawaz F, McCaman M, Pungor E., Jr. Proteomic analysis for the assessment of different lots of fetal bovine serum as a raw material for cell culture. Part IV. Application of proteomics to the manufacture of biological drugs. Biotechnol Prog. 2006;22:1294–1300. doi: 10.1021/bp060121o. [DOI] [PubMed] [Google Scholar]
- Alvarez ML, Khosroheidari M. Kanchi Ravi R, DiStefano JK. Comparison of protein, microRNA, and mRNA yields using different methods of urinary exosome isolation for the discovery of kidney disease biomarkers. Kidney Int. 2012;82:1024–1032. doi: 10.1038/ki.2012.256. [DOI] [PubMed] [Google Scholar]
- Chen L, Mao SJ, Larsen WJ. Identification of a factor in fetal bovine serum that stabilizes the cumulus extracellular matrix. A role for a member of the inter-alpha-trypsin inhibitor family. J Biol Chem. 1992;267:12380–12386. [PubMed] [Google Scholar]