Abstract
Lpar3 encodes LPA3, the third G protein-coupled receptor for lysophosphatidic acid (LPA). Lpar3−/− female mice had delayed embryo implantation. Their serum progesterone and estrogen levels were comparable with control on Gestation Day 3.5 (D3.5) at 1100 h. There was reduced cell proliferation in D3.5 and D4.5 Lpar3−/− stroma. Progesterone receptor (PGR) disappeared from D4.5 Lpar3+/+ uterine luminal epithelium (LE) but remained highly expressed in D4.5 Lpar3−/− LE. Pgr and PGR- target genes but not estrogen receptor alpha (ERalpha [Esr1]) or ESR target genes, were upregulated in D4.5 Lpar3−/− LE. It was hypothesized that suppression of PGR activity in LE could restore on-time uterine receptivity in Lpar3−/− mice. A low dose of RU486 (5 μg/mouse) given on D3.5 at 900 h rescued delayed implantation in all pregnant Lpar3−/− females and significantly increased number of implantation sites compared to vehicle-treated pregnant Lpar3−/− females detected on D4.5. E2 (25 ng/mouse) had a similar effect as 5 μg RU486 on embryo implantation in Lpar3−/− females. However, when the ovaries were removed on late D2.5 to create an experimentally induced delayed implantation model, 25 ng E2 activated implantation in Lpar3+/+ but not Lpar3−/− females detected on D4.5. These results demonstrate that deletion of Lpar3 leads to an increased ratio of progesterone signaling/estrogen signaling that can be optimized by low doses of RU486 or E2 to restore on-time implantation in Lpar3−/− females.
Keywords: E2, embryo implantation, Lpar3−/− mice, progesterone receptor, RU486, uterine luminal epithelium
INTRODUCTION
Embryo implantation is a mandatory step for the success of mammalian reproduction. It requires a synchrony between a competent embryo and a receptive uterus [1]. A receptive uterus is a hormonally controlled transient state during which the uterus can accept an embryo to implant. Defective uterine receptivity, including delayed uterine receptivity and non-receptive endometrium, is the key maternal factor for implantation failure in both natural and assisted human reproduction and is a main cause of infertility and early pregnancy loss during or immediately after implantation [2–7].
Although a precise molecular signaling mechanism of how a uterus transforms into a receptive state is still largely undefined [8], progesterone (P4) and estrogen (17β-estradiol [E2]) play critical roles in the establishment of uterine receptivity in both humans and rodents [1, 9]. P4 signaling is essential for the establishment of uterine receptivity in all mammals studied [10]. In humans, a luteinizing hormone surge from the pituitary induces ovulation (Day 0 [D0]), which is followed by secretions of both P4 and E2, which prepare a receptive uterus during D7 to D10. In mice, mating (D0) takes place in the early estrous stage and, meanwhile, ovulation occurs. P4 and E2 levels are low at post coitus. P4 level steadily increases from D2.5 and maintains a high level. There is a brief E2 surge on the morning of D3.5, and a receptive uterus is established but lasts only ∼24 h in mice [1, 9]. Timely balanced levels of P4 and E2 are critical for the establishment of uterine receptivity.
The actions of P4 and E2 are mainly mediated through their respective nuclear receptors, progesterone receptor (PGR) and estrogen receptor α (ERα/ESR1) in the uterus, which have unique spatiotemporal expression patterns in the mouse uterus during early pregnancy. PGR expression increases significantly in the luminal epithelium (LE) from D0.5 to D1.5 of pregnancy (D0 is mating night). It is upregulated in both LE and stroma from D2.5 to D3.5 but disappears from LE and is highly expressed in the primary decidual zone at the implantation sites on D4.5 [11, 12]. Our detailed time course study demonstrates that PGR disappears from the LE after implantation has initiated and before the histologic decidualization manifests, which occurs a few hours after embryo attachment around D4.0 in mice [13], suggesting an active role of continued PGR expression in the LE for the initial implantation process. The essential role of uterine epithelial PGR in embryo implantation has been demonstrated in uterine epithelial PGR conditional knockout mice [14]. Although the role of PGR in the LE could not be differentiated from that in the glandular epithelium (GE) because PGR is deleted in both LE and GE in this mouse model [14], failed embryo attachment would imply a critical role of LE PGR in embryo implantation because LE is the first layer of cells that a competent embryo communicates with for implantation and PGR disappears from LE after embryo attachment [13, 14]. Uterine tissue recombinant studies show that the expression of PGR in LE can be downregulated by E2 [15] via stromal ESR1 [16], the main ER in the uterus [11]; and such downregulation of PGR in LE by E2 can be prevented by P4 via stromal PGR [17]. Loss of PGR in the uterine epithelium is associated with the establishment of uterine receptivity in all mammals examined [18, 19], whereas sustained PGR expression in uterine epithelium during expected “implantation window” has been associated with defective uterine receptivity in both human and mouse [20–22].
Previously we demonstrated that deletion of Lpar3 led to delayed embryo implantation due to delayed uterine receptivity in mice [23]. Lpar3 encodes the third G protein-coupled receptor for lysophosphatidic acid (LPA), LPA3. LPA3 couples to the Gi and Gq downstream signaling pathways that can regulate cell proliferation, survival, and differentiation [24–26]. Studies in Lpar3−/− mice have demonstrated roles of LPA3 in embryo implantation and spacing [23], spermatogenesis [27], and mediating immature dendritic cell chemotaxis to unsaturated LPA species [28]. In this study, we revealed sustained PGR expression in the Lpar3−/− LE during the expected implantation window. Therefore, we hypothesized that suppression of PGR activity in LE could restore on-time uterine receptivity in Lpar3−/− mice. RU486 (mifepristone), a well-characterized PGR antagonist and partial agonist [29–32], and E2, which downregulates PGR expression in the uterine epithelium [16], were used to test this hypothesis.
MATERIALS AND METHODS
Animals
Lpar3+/+ (wild type), Lpar3+/−, and Lpar3−/− mice (C57BL/6 and 129svj mixed background) were generated from a colony at the University of Georgia, which was originally derived from Dr. Jerold Chun's colony at the Scripps Research Institute [23]. They were genotyped as described [23]. Females at 2 to 4 mo old were used in the study. Because Lpar3+/− females were indistinguishable from Lpar3+/+ females [23], both Lpar3+/− and Lpar3+/+ females were included in the control. Mice were housed in polypropylene cages with free access to regular food and water from sip tubes in a reverse osmosis system. The animal facility operates on a 12:L/12:D cycle (600 h to 1800 h) at 23°C ± 1°C with 30% to 50% relative humidity. All methods used in this study were approved by the University of Georgia Institutional Animal Care and Use Committee (IACUC) and conform to National Institutes of Health guidelines and public law.
Reagents
The following reagents were obtained as indicated in the parenthesis: TRIzol and Superscript III (Invitrogen, Carlsbad, CA); deoxynucleoside triphosphates (Biomiga, San Diego, CA); Taq DNA polymerase (Lucigen, Middleton, WI); E2 and P4 (Sigma-Aldrich, St. Louis, MO); Superfrost plus slides (Fisher Scientific, Pittsburgh, PA); power SYBR green PCR master and 384-well plates (Applied Biosystems, Carlsbad, CA); progesterone receptor antibody (code A0098; Dako, Denmark); estrogen receptor α antibody (code ab37438; Abcam, Cambridge, MA; and code sc-543; Santa Cruz Biotechnology, Dallas, TX); 5-bromo-2′-deoxyuridine (BrdU) antibody (code G3G4; Developmental Studies Hybridoma Bank, Iowa City, IA).
P4 and E2 Measurement
Blood was collected from anesthetized pregnant Lpar3+/+ and Lpar3−/− mice (4 mice/group, mated with Lpar3+/+ stud males) by retro-orbital bleeding on D3.5 at ∼1100 h. After clotting at 25°C for 30 min, serum was collected and kept at −80°C. Serum P4 and E2 concentrations were quantified using progesterone and estradiol enzyme immunoassay kits (Cayman Chemical, Ann Arbor, MI), respectively, following the manufacturer's protocol. Each sample was appropriately diluted to fall within the range of the standard curve and measured in triplicates.
Isolation of Uterine Luminal Epithelium
On D4.5 at ∼1100 h, 3 pregnant control females (Lpar3+/−) and 3 pregnant Lpar3−/− females were euthanized by CO2 inhalation. Pregnancy was confirmed by the presence of implantation sites using blue dye injection for control mice and the presence of healthy blastocysts in Lpar3−/− uteri. Uterine horns were immediately dissected. One-third of a uterine horn from each mouse was flash-frozen on dry ice for gene expression study in the whole uterus. The remaining 1 and 2/3 uterine horns from each mouse were sliced open longitudinally along the mesometrial side. LE was isolated as previously reported [33, 34] and immediately processed for RNA isolation.
BrdU labeling
RNA Isolation and Real-Time PCR
The LE pellet was used directly for total RNA isolation by using TRIzol reagent. The 1/3 uterine horn from each mouse not used for LE isolation above was powdered in liquid nitrogen with a mortar and pestle. Powdered samples were quickly and carefully transferred to TRIzol reagent for total RNA isolation following manufacture's instruction, except that each sample was extracted twice with chloroform. Total RNA was treated with RNase-free DNase I to remove trace DNA contamination, and RNA sample quality was examined on RNA gel. The concentration of RNA was determined using a NanoDrop cuvette free spectrophotometer (ThermoScientific, Columbia, SC). For real-time PCR, cDNA was transcribed from approximately 0.5 to 1.0 μg of total RNA using Superscript III reverse transcriptase with random primers. Real-time PCR reactions were performed in 384-well plates using Sybr-Green I intercalating dye on ABI 7900 (Applied Biosystems). The primer pairs were designed crossing introns in genomic DNA and ordered from Integrated DNA Technologies (Table 1) for the following genes: Pgr and PGR target genes Indian hedgehog (Ihh) and alcohol dehydrogenase 5 (Adh5); Esr1 and ESR target genes leukemia inhibitory factor (Lif) and complement component 3 (C3); as well as the two housekeeping genes glyceraldehyde 3-phosphate dehydrogenase (Gapdh) and hypoxanthine phosphoribosyltransferase 1 (Hprt1); n = 3.
TABLE 1.
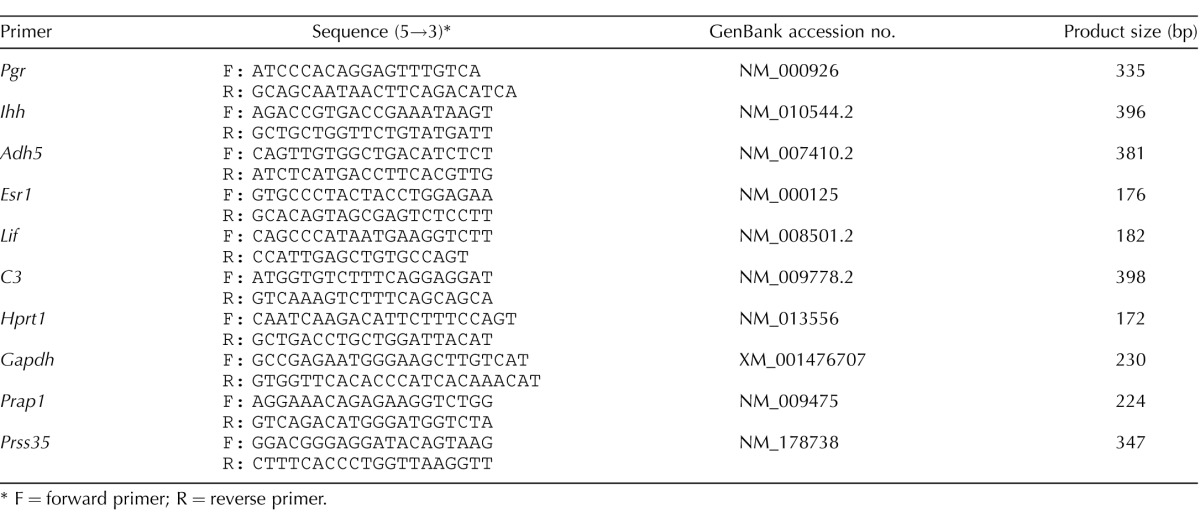
Primers used for real-time PCR and making probes for in situ hybridization.
F = forward primer; R = reverse primer.
Immunohistochemistry
Frozen uterine horns from D3.5 Lpar3+/+ and Lpar3−/− females were cross-sectioned, and those from D4.5 Lpar3+/+ and Lpar3−/− females or D4.5 RU486-treated Lpar3−/− females were longitudinally sectioned (10 μm). Only the sections with embryo(s) from D4.5 uteri were used in the study. Immunohistochemistry (IHC) of uterine PGR or ESR1 expression was performed as previously described [13, 36, 37]. Negative control were processed in exactly the same way, except that the primary antibodies were replaced with rabbit immunoglobulin G.
In Situ Hybridization
Serial sections from vehicle or RU486-treated D4.5 Lpar3−/− females were used for IHC of PGR (above) and in situ hybridization of Prss35, a decidual marker [38], and Prap1, an LE marker highly upregulated upon embryo implantation [39], as previously described [38, 39].
RU486 or E2 Treatment
To rescue delayed implantation in Lpar3−/− females, pregnant D3.5 Lpar3−/− females (mated with Lpar3+/+ stud males) were injected subcutaneously (s.c.) with vehicle (100 μl of oil), RU486 (5 μg/mouse in 100 μl of oil [∼0.2 mg/kg]), or E2 (25 ng/mouse in 100 μl of oil) at 900 h. Oil-injected mice and RU486-treated mice were randomly assigned to three groups each, dissected at D4.5, or D5.5, or left to give birth for determining gestation period and litter size. Mice in E2-treated group were dissected at D4.5. Implantation sites were visualized by intravenous (i.v.) injection of 200 μl of 1% Evans blue dye at D4.5 or D5.5, between 1100 and 1200 h, as previously described [23], and pregnancy was revealed by visible implantation sites and/or healthy looking blastocysts flushed from the uterus. The uterine horns were flash-frozen on dry ice. At least 4 pregnant females were included in each group.
Experimentally Induced Delayed Implantation Model
An experimentally induced delayed implantation mouse model can be generated by ovariectomizing pregnant mice on D2.5 and maintaining delayed implantation by daily injection of 1 or 2 mg of P4, and embryo implantation can be achieved with an injection of 3 to 25 ng of E2 [40]. The lowest recommended dose of P4 (1 mg) and the highest recommended dose of E2 (25 ng) were used in this study. Young Lpar3+/+ and Lpar3−/− female mice were mated with Lpar3+/+ stud males. On D2.5 at ∼1800 h, the ovaries from each mouse were removed to create an experimentally induced delayed implantation model. Delayed implantation was maintained by s.c. injection of P4 (1 mg/mouse in 100 μl oil) on D2.5 at 1800 h and D3.5 at 900 h. E2 (25 ng/mouse in 100 μl oil) was s.c. injected to P4 primed delayed implantation mice on D3.5 at 900 h. Implantation sites were detected in D4.5 mice using blue dye injection, and pregnancy status of mice without implantation sites was determined by the presence of blastocysts from uterine flush. Six to seven pregnant females were included in each group.
Histology
Fixed uterine horns sectioned (5 μm) longitudinally in order to increase the chance to obtain sections with embryo(s), especially from Lpar3−/− uteri that did not show implantation sites on D4.5. Sections were deparaffinized, rehydrated, and stained with hematoxylin and eosin.
Statistical Analyses
Statistical analyses were done using two-tail, unequal variance Student t tests. Significance level was set at a P value of <0.05.
RESULTS
Normal Serum Progesterone and Estrogen Levels in D3.5 Lpar3−/− Females
Deletion of Lpar3 leads to delayed uterine receptivity in mice [23]. Because uterine receptivity is controlled by the ovarian hormones P4 and E2 in mice [1], serum P4 and E2 levels in Lpar3+/+ and Lpar3−/− females were determined at 1100 h on D3.5, a time point right after an E2 surge [1]. No significant differences in serum P4 levels (Fig. 1A) or E2 levels (Fig. 1B) were detected between D3.5 Lpar3+/+ and Lpar3−/− females, indicating no obvious endocrine defect in the Lpar3−/− females that could be responsible for the implantation defects [23].
FIG. 1.
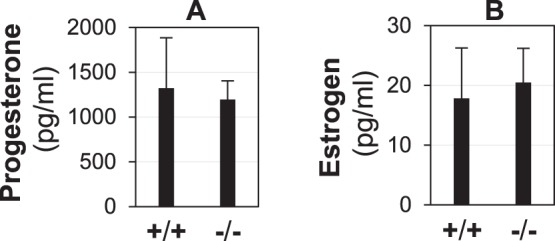
Levels of serum progesterone (A) and estrogen (B) on D3.5 Lpar3+/+ (+/+) and Lpar3−/− (−/−) females. n = 4; error bar = standard deviation.
Reduced Cell Proliferation in Lpar3−/− Stroma
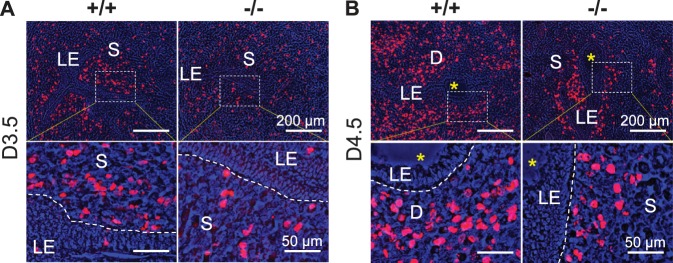
BrdU labeling revealed rare LE cell proliferation in D3.5 and D4.5 Lpar3+/+ and Lpar3−/− uteri. However, there was reduced stromal cell proliferation in the D3.5 and D4.5 Lpar3−/− uteri, especially in the subepithelial areas (Fig. 2).
FIG. 2.
BrdU labeling of cell proliferation on D3.5 and D4.5 Lpar3+/+ (+/+) and Lpar3−/− (−/−) endometrium. A) Representative images from D3.5 uteri. n = 4–6. B) Representative images from D4.5 uteri. n = 3. Red, BrdU labeling; blue, brightfield image merged in blue channel; rectangle, area enlarged in bottom panel; S, stroma; D, decidual zone; yellow star, embryo; dotted line on the bottom panel, border between LE and stroma. Bars = 200 μm (top panel) and 50 μm (bottom panel).
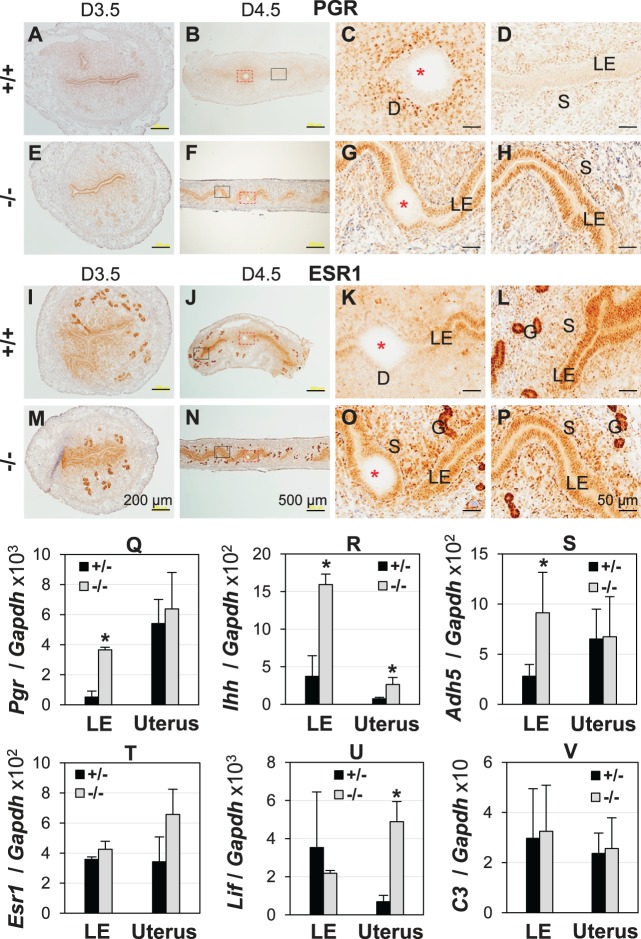
Upregulation of PGR and PGR Targeting Genes in D4.5 Lpar3−/− LE
P4 and E2 signals are tightly regulated through their respective receptors PGR and ESR1, in the uterus. IHC did not reveal any obvious difference in PGR expression patterns between D3.5 Lpar3+/+ and Lpar3−/− uteri (Fig. 3, A and E). PGR disappeared from D4.5 Lpar3+/+ LE and was strongly expressed in the primary decidual zone (Fig. 3, B–D). However, in D4.5 Lpar3−/− uteri, PGR remained strongly expressed in LE (Fig. 3, F–H). On D5.5, PGR was undetectable in the LE and highly expressed in the decidual zone in Lpar3−/− uteri, as implantation had already occurred then (data not shown) [23].
FIG. 3.
Uterine expression of PGR and estrogen receptor α (ESR1) and their target genes. D3.5/D4.5, Gestation Day 3.5/4.5; +/+, Lpar3+/+; +/-, Lpar3+/−; −/−, Lpar3−/− mice. A–H) Immunohistochemistry (IHC) of PGR. A) Cross-section of a uterus, +/+, D3.5. B) Longitudinal section of a uterus, +/+, D4.5. C) Enlarged view of the red rectangular area in B. D) Enlarged view of the black rectangular area in B. E) Cross-section of a uterus, −/−, D3.5. F) Longitudinal section of a uterus, −/−, D4.5. G) Enlarged view of the red rectangular area in F. H) Enlarged view of the black rectangular area in F. I–P) IHC of ESR1. I) Cross-section of a uterus, +/+, D3.5. J) Longitudinal section of a uterus, +/+, D4.5. K) Enlarged view of the red rectangular area in J. L) Enlarged view of the black rectangular area in J. M) Cross-section of a uterus, −/−, D3.5. N) Longitudinal section of a uterus, −/−, D4.5. O) Enlarged view of the red rectangular area in N. P) Enlarged view of the black rectangular area in N. Bars = 200 μm (A, E, I, M), 500 μm (B, F, J, N), 50 μm (C, D, G, H, K, L, O, P). Q–V) Quantitative PCR of mRNA expression in D4.5 LE and uterus. Q) Pgr. R) Indian hedgehog (Ihh). S) Alcohol dehydrogenase 5 (Adh5). T) Esr1. U) Leukemia inhibitory factor (Lif). V) Complement component 3 (C3). *P < 0.05; n = 3; error bars, standard deviation; glyceraldehyde 3-phosphate dehydrogenase (Gapdh), loading control.
ESR1 is the main ER expressed in the uterus [11]. No obvious differences in ESR1 expression levels were observed between D3.5 Lpar3+/+ and Lpar3−/− uteri (Fig. 3, I and M). On D4.5, the main difference was reduced ESR1 expression in the LE of Lpar3+/+ implantation site (Fig. 3, J, K, N, and O), but ESR1 expression was not lower in the rest of the Lpar3+/+ LE at interimplantation site compared to that in Lpar3−/− LE (Fig. 3, L and P). ESR1 was highly expressed in both Lpar3+/+ and Lpar3−/− GE on D3.5 and D4.5 (Fig. 3, I–P).
Quantification of D4.5 LE mRNA expression showed upregulation of Pgr (Fig. 3Q), and PGR target genes such as Indian hedgehog (Ihh) (Fig. 3R) [41] and alcohol dehydrogenase 5 (Adh5) (Fig. 3S) [42], but not Esr1 (Fig. 3T), or ESR1 target genes such as leukemia inhibitory factor (Lif) (Fig. 3U) [43] and complement factor 3 (C3) (Fig. 3V) [44] in the Lpar3−/− LE compared to control (Lpar3+/−) LE (n = 3). No significant difference in uterine expression levels was observed for Pgr and Adh5 due to their high expression levels in other uterine compartment(s) (Fig. 3, Q and S) [42]. Correlated expression patterns of Ihh between LE and uterus reflected main uterine epithelial localization of Ihh (Fig. 3R) [41]. Higher Lif expression in D4.5 Lpar3−/− uterus but not in LE (Fig. 3U) reflected delayed implantation because Lif expression peaks in GE prior to embryo implantation [45]. No significant differences were observed between control and Lpar3−/− LE or uterus for the housekeeping gene Hprt1 (data not shown). These results indicate that PGR signaling is upregulated in D4.5 Lpar3−/− LE at the transcriptional level.
5 μg of RU486 Restored On-Time Implantation in Lpar3−/− Females
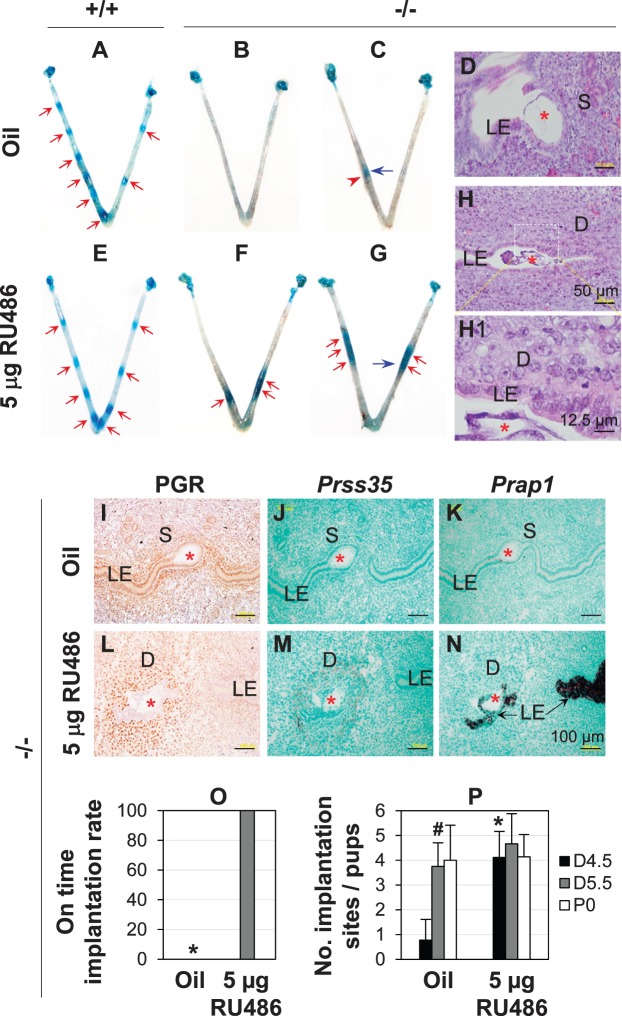
Because there was higher PGR signaling in D4.5 Lpar3−/− LE, we hypothesized that suppression of PGR signaling could restore on-time implantation in Lpar3−/− females and tested this hypothesis using RU486. A low dose of 5 μg RU486 given on D3.5 at 900 h did not affect embryo implantation in Lpar3+/+ females (Fig. 4, A and E) but restored on-time embryo implantation in all pregnant Lpar3−/− females detected on D4.5 (Fig. 4, B, C, F, and G). In vehicle and RU486-injected Lpar3−/− groups, the detectable implantation sites per uterus ranged from 0 to 2 (Fig. 4, B, C, and P) and 3 to 6 (Fig. 4, F, G, and P), respectively. All vehicle-injected Lpar3−/− pregnant females (n = 9) had delayed implantation whereas all RU486-injected Lpar3−/− pregnant females (n = 9) had on-time implantation detected on D4.5 (Fig. 4, B, C, F, G, and O), which was confirmed by histology and gene marker expression. Histology revealed that although an embryo had attached to LE in the implantation site in Figure 4C (vehicle-injected Lpar3−/− uterus), decidualization had not started yet (Fig. 4D), confirming delayed implantation [13]; and decidualization had occurred in the implantation sites in Figure 4G (RU486-treated Lpar3−/− uterus) (Fig. 4, H and H1). IHC indicated sustained PGR expression in the LE of vehicle-injected Lpar3−/− uterus (Fig. 4I) but absence of PGR expression in the LE of RU486-treated uterus (Fig. 4L). In situ hybridization of Prss35, a decidual marker [38], and Prap1, an LE marker highly upregulated upon embryo implantation [39], also confirmed restored on-time embryo implantation in the Lpar3−/− uterus upon RU486 treatment (Fig. 4, J, K, M, and N). However, this treatment failed to alleviate embryo crowding in the Lpar3−/− uterus (Fig. 4, F and G) [23].
FIG. 4.
RU486 (5 μg) rescuing delayed implantation in Lpar3−/− mice. +/+, Lpar3+/+; −/−, Lpar3−/−. A) A representative Gestation Day (D4.5) vehicle (oil)-injected Lpar3+/+ uterus. B and C) Two representative D4.5 pregnant Lpar3−/− uteri without an implantation site (B) or with a delayed implantation site (C) from oil injection. D) Histology of the implantation site in panel C indicated by a blue arrow. E) A representative D4.5 RU486-injected Lpar3+/+ uterus. F and G) Two representative D4.5 pregnant Lpar3−/− uteri from RU486 injection. H) Histology of the implantation site on G indicated by a blue arrow. H1) Enlarged view of the rectangular area in H. I–P) From Lpar3−/− mice. I–K) Serial sections of an implantation site from the vehicle control. L–N) Serial sections of an implantation site from the RU486-treated group. I and L) Immunohistochemistry of progesterone receptor (PGR). J and M) In situ hybridization (ISH) of serine protease 35 (Prss35). K and N) ISH of proline-rich acidic protein 1 (Prap1). Red arrow, on-time implantation site; red arrowhead, delayed implantation site; blue arrow, implantation site for histology; red star, embryo; S, stroma; D, decidual zone. Bars = 50 μm (D, H), 12.5 μm (H1), 100 μm (I–N). O) On-time implantation rate in oil and 5 μg RU486 treated Lpar3−/− mice. N = 9; * P < 0.05. P) Number of implantation sites at D4.5 or D5.5 and number of pups at Postnatal Day 0 (P0) in Lpar3−/− vehicle control group and Lpar3−/− RU486-treated group. n = 4–9; #P < 0.05 compared to D4.5 in vehicle control group; *P < 0.05 compared to vehicle control group; error bar, standard deviation.
The number of implantation sites in D5.5 vehicle-treated Lpar3−/− group (N = 4) was significantly higher than in D4.5 vehicle-treated group (n = 9) but comparable to those in D4.5 (n = 9) or D5.5 (n = 6) RU486-treated Lpar3−/− groups, supporting restoration of on-time implantation upon RU486 treatment. There was an average of 0.58 days reduction of gestation period in the RU486-treated group (20.21 ± 0.30 days, n = 7) compared to the vehicle-treated group (20.78 ± 0.64 days, n = 7, P = 0.049). However, no significant difference on the litter size at birth was observed between these two groups (Fig. 4P).
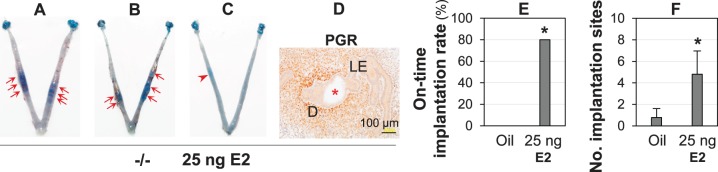
25 ng of E2 Restored On-Time Implantation in Lpar3−/− Females
A potential alternative approach to downregulate PGR expression in the uterine epithelium was E2 injection [16]. When D3.5 Lpar3−/− mice were given E2 (25 ng/mouse), 4 of 5 pregnant females had on-time implantation (Fig. 5, A and B) confirmed by PGR expression pattern in LE and decidual zone (Fig. 5D), and 1 female had delayed implantation (Fig. 5C). E2 treatment restored on-time implantation and the number of implantation sites (Fig. 5, E and F). However, like RU486 treatment (Fig. 4, F and G), E2 treatment had no obvious effect on alleviating embryo crowding in Lpar3−/− mice (Fig. 5, A and B). ESR1 antagonist ICI 182,780 (250 ng/kg) failed to rescue delayed implantation in Lpar3−/− mice (data not shown). These results revealed an increased progesterone-to-estrogen signaling ratio in the Lpar3−/− uterus during preparation for embryo implantation.
FIG. 5.
E2 (25 ng) restoring on-time implantation in Lpar3−/− mice detected on D4.5. −/−, Lpar3−/−. Representative images from vehicle (oil) injection were shown in Figure 4, B and C. A and B) Two representative uteri from Lpar3−/− females injected with 25 ng of E2 on D3.5. On-time implantation sites were indicated by red arrows. C) One E2-treated Lpar3−/− uterus showing a delayed implantation site, indicated by a red arrowhead. D) Immunohistochemistry of progesterone receptor (PGR) in a 25 ng E2-treated Lpar3−/− uterus with restored implantation timing. Bar = 100 μm. E) On-time implantation rate in oil and 25 ng E2-treated Lpar3−/− mice. n = 5–9; *P < 0.05. F) Number of implantation sites in oil and E2-treated Lpar3−/− mice. n = 5–9; *P < 0.05; error bar = standard deviation.
25 ng of E2 Failed to Activate Embryo Implantation in an Experimentally Induced Lpar3−/− Delayed Implantation Model
The unbalanced progesterone signaling and estrogen signaling in the Lpar3−/− uterus was further demonstrated in an experimentally induced delayed implantation mouse model. In the control Lpar3+/+ group, all seven pregnant females showed embryo implantation upon E2 treatment (Fig. 6, A–C). Six of them had on-time implantation with 5 to 9 implantation sites each (Fig. 6, A and B); and one had delayed implantation with 3 implantation sites (Fig. 6C). In the Lpar3−/− group, only 1 of 6 pregnant mice had one delayed implantation site (Fig. 6D), and the remaining 5 had no detectable implantation site but hatched blastocysts (Fig. 6, E and F). Both the implantation rate (Fig. 6G) and the number of implantation sites (Fig. 6H) were significantly lower in the Lpar3−/− group, further demonstrating an increased ratio of progesterone signaling/estrogen signaling in the Lpar3−/− uterus during embryo implantation.
FIG. 6.
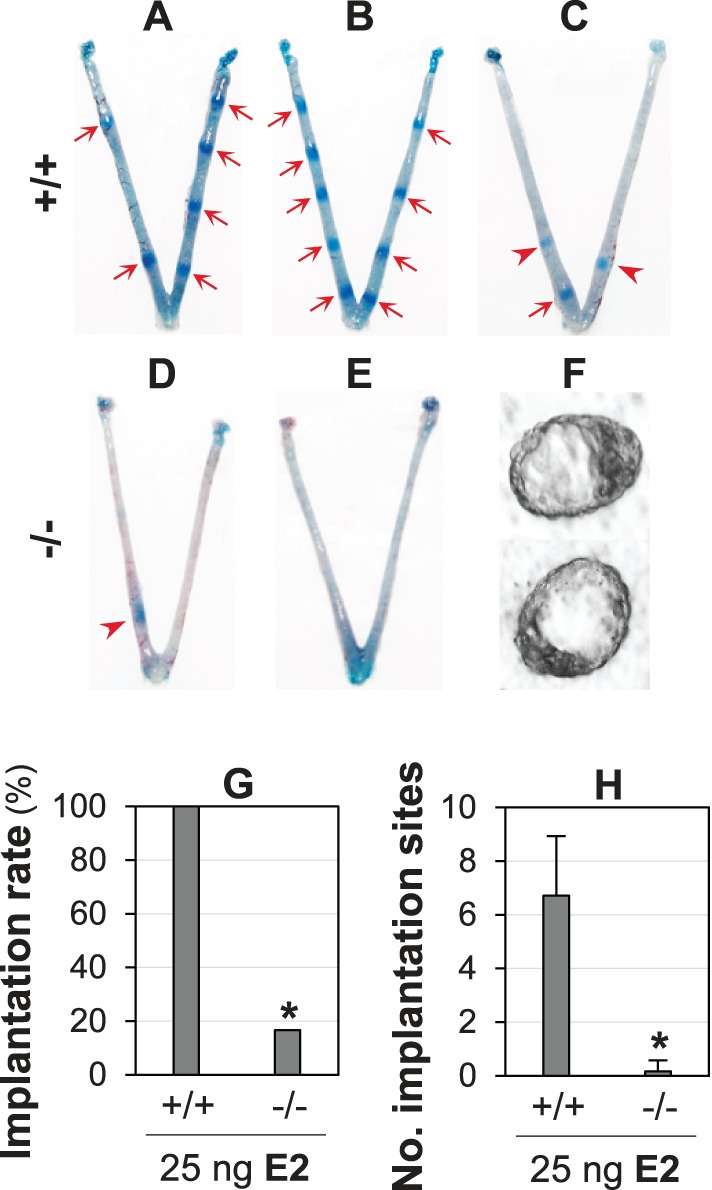
Effects of estrogen on embryo implantation in an experimentally induced delayed implantation mouse model. +/+, Lpar3+/+; −/−, Lpar3−/−. A–C) Lpar3+/+ uterine images 1 day after injection of 25 ng of E2. D and E) Lpar3−/− uterine 1 day after injection of 25 ng of E2. A–D) Red arrows indicate on-time implantation sites; red arrowheads indicate delayed implantation sites. F) Representative blastocysts flushed from Lpar3−/− uteri. G) Implantation rate. n = 6–7; *P < 0.05. E) Number of implantation sites. n = 6–7; *P < 0.05; error bar = standard deviation.
DISCUSSION
The critical roles of ovarian hormone signaling in the uterine preparation for embryo implantation have been demonstrated in animal models and clinical situations. Ovarian hormone signaling can be disrupted in animal models via altered ligand levels, such as exogenous application of agonists/antagonists for PGR or ESR [36, 46, 47] or changed local endogenous ligand levels [48], deletion of uterine PGR or ESR1 via global receptor deletion or uterine cell-specific deletion [14, 49–52], disruption of nuclear receptor coregulators [53–56], or PGR or ESR target genes [41, 57–59], to adversely affect the optimal uterine preparation for embryo implantation. Disrupted ovarian hormone signaling can also lead to impaired uterine receptivity in clinical situations, such as endometriosis and polycystic ovary syndrome. Women with endometriosis have progesterone resistance, which is most likely caused by reduced responsiveness to progesterone signaling instead of reduced progesterone levels [60, 61]. Polycystic ovary syndrome patients have a hormone imbalance, which may be accompanied by progesterone resistance [61–63]. In addition, imbalanced ovarian hormone signaling, such as the premature progesterone rise in stimulated in vitro fertilization cycles, can lead to impaired endometrial receptivity thus low pregnancy rate [64].
This study demonstrates that the fine balance of progesterone signaling and estrogen signaling is disrupted in the Lpar3−/− uterus and that this disrupted balance leads to delayed embryo implantation. Because the PGR antagonist RU486 and the ESR agonist E2, but not ESR antagonist ICI 182,780, can restore on-time implantation in the Lpar3−/− uterus, it indicates that there is an increased ratio of progesterone signaling/estrogen signaling in the Lpar3−/− uterus. It could not be distinguished if this is due to increased progesterone signaling or decreased estrogen signaling or both in Lpar3−/− uterus. However, the data from delayed implantation model, in which 25 ng E2 activated embryo implantation in the Lpar3+/+ mice but not Lpar3−/− mice, would suggest decreased estrogen signaling in the Lpar3−/− uterus, although it could not rule out increased progesterone signaling. Interestingly, another study demonstrated that a small dose of ESR antagonist, ICI 182,780, could counteract the elevated ESR1 activity in the LE and restore embryo implantation in mice deficient of chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII) [57]. Although it has not been explicitly demonstrated for the causes of the increased expression of PGR in the Lpar3−/− LE (Fig. 3G) or increased expression of ESR1 in the PRCre/+COUP-TFIIflox/flox LE [57], for example, at the level of ligands that regulate receptor expression [16] and/or at the level of receptor expression without the involvement of altered ligand levels, both studies using a pharmacological approach further demonstrate the essential role of balanced progesterone signaling and estrogen signaling in establishing uterine receptivity.
It remains unknown how LPA3-mediated signaling regulates the fine balance of progesterone signaling and estrogen signaling in the periimplantation uterus. Because serum P4 and E2 levels are comparable between D3.5 Lpar3+/+ and Lpar3−/− mice and the levels of PGR and ESR1 expression are comparable between D3.5 Lpar3+/+ endometrium and Lpar3−/− endometrium, it is possible that the activities of the endometrial local P4-to-E2 ratio is increased and/or there is altered activities of coregulators leading to increased progesterone signaling/decreased estrogen signaling in the D3.5 Lpar3−/− mice. LPA3 is a membrane protein [23, 65]. PGR and ESR1 function mainly as nuclear receptors to mediate the effects of progesterone and estrogen in the uterus during embryo implantation, although they may also have non-genomic functions and progesterone and estrogen may also act through membrane receptors (reviewed in references [66] and [67]). It is reasonable to speculate that there is no direct interactions between LPA3 and PGR or ESR1, although uterine Lpar3 expression is upregulated by P4 and downregulated by E2 [33, 68] and both LPA3-mediated signaling and PGR-mediated signaling can converge on MAPK [67, 69]. Considering the function of LPA3 in mediating cell proliferation [70], peak expression of Lpar3 in D3.5 LE [23] and reduced stromal cell proliferation in D3.5 Lpar3−/− uterus, it is most likely that LPA3 in LE has a paracrine effect on stromal cell proliferation prior to embryo implantation.
Studies in the endometria of patients with endometriosis suggest a potential role of LPA3 in progesterone resistance associated with endometriosis. Microarray analysis of human endometrium (identifier GSE6364) indicates significant upregulation of LPAR3 (EDG7) mRNA in normal early secretory phase endometrium (ESE) but such upregulation is abolished in ESE endometrium from patients with endometriosis [71]. LPA3 protein levels are marginally downregulated in the ESE and significantly downregulated in the middle and later secretory phase endometrium from patient with endometriosis [72]. We have previously demonstrated that Lpar3 in LE is upregulated by P4 in the ovariectomized mouse uterus [33]. If the regulation of LPA3 by P4 signaling in human endometrium is similar to that in the mouse endometrium, the decreased expression of LPAR3/LPA3 in the endometrium of patients with endometriosis would indicate reduced progesterone signaling, which is consistent with the common belief that endometriosis is associated with attenuated progesterone responsiveness.
RU486 and E2 can restore on-time implantation but not embryo spacing in Lpar3−/− mice. There have been multiple mechanisms proposed for embryo spacing [73, 74]. Our previous studies of cyclooxygenase-derived prostanoid signaling in Lpar3−/− females revealed that implantation timing and embryo spacing were two segregated events and that factors involved in myometrial contraction and relaxation contributed to embryo spacing [23, 74, 75]. Although progesterone signaling and estrogen signaling have opposing effects on myometrial activities during pregnancy [76] and embryo spacing in mice is a progressive event on D3.5 [75], the RU486 and E2 treatment regimens used in this study do not affect the net effect of ovarian signaling in embryo spacing. Because embryo spacing is not restored, it is expected that embryo crowding is still present in the RU486 or E2-treated Lpar3−/− females. Sustained embryo crowding would explain the comparable number of implantation sites in D4.5 RU486 or E2-treated Lpar3−/− females with D5.5 untreated Lpar3−/− females, which all have reduced numbers of implantation sites compared to that in D4.5 control females [23]. The molecular signaling mechanisms for LPA3 in implantation timing and embryo spacing remain to be elucidated.
Footnotes
Current address: Department of Obstetrics and Gynecology, Feinberg School of Medicine, Northwestern University, Chicago, IL 60611.
Current address: Reproductive Developmental Biology Group, National Institute of Environmental Health Sciences (NIEHS/NIH), 111 TW Alexander Drive, Research Triangle Park, NC 27709.
Supported by the National Institutes of Health grants R15HD066301 and R01HD065939 (co-funded by NIH/ Office of Research on Women's Health and National Institute of Child Health and Development) to X.Y.
REFERENCES
- Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet. 2006;7:185–199. doi: 10.1038/nrg1808. [DOI] [PubMed] [Google Scholar]
- Lee KY, Jeong JW, Tsai SY, Lydon JP, DeMayo FJ. Mouse models of implantation. Trends Endocrinol Metab. 2007;18:234–239. doi: 10.1016/j.tem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Wilcox AJ, Baird DD, Weinberg CR. Time of implantation of the conceptus and loss of pregnancy. N Engl J Med. 1999;340:1796–1799. doi: 10.1056/NEJM199906103402304. [DOI] [PubMed] [Google Scholar]
- Wilcox AJ, Weinberg CR, O'Connor JF, Baird DD, Schlatterer JP, Canfield RE, Armstrong EG, Nisula BC. Incidence of early loss of pregnancy. N Engl J Med. 1988;319:189–194. doi: 10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- Norwitz ER, Schust DJ, Fisher SJ. Implantation and the survival of early pregnancy. N Engl J Med. 2001;345:1400–1408. doi: 10.1056/NEJMra000763. [DOI] [PubMed] [Google Scholar]
- Simon C, Caballero-Campo P, Garcia-Velasco JA, Pellicer A. Potential implications of chemokines in reproductive function: an attractive idea. J Reprod Immunol. 1998;38:169–193. doi: 10.1016/s0165-0378(98)00031-x. [DOI] [PubMed] [Google Scholar]
- Sharkey AM, Smith SK. The endometrium as a cause of implantation failure. Best Pract Res Clin Obstet Gynaecol. 2003;17:289–307. doi: 10.1016/s1521-6934(02)00130-x. [DOI] [PubMed] [Google Scholar]
- Jones CJ, Aplin JD. Glycosylation at the fetomaternal interface: does the glycocode play a critical role in implantation? Glycoconj J. 2009;26:359–366. doi: 10.1007/s10719-008-9152-6. [DOI] [PubMed] [Google Scholar]
- Yoshinaga K. Research on blastocyst implantation essential factors (BIEFs) Am J Reprod Immunol. 2010;63:413–424. doi: 10.1111/j.1600-0897.2010.00853.x. [DOI] [PubMed] [Google Scholar]
- Bazer FW, Spencer TE, Johnson GA, Burghardt RC, Wu G. Comparative aspects of implantation. Reproduction. 2009;138:195–209. doi: 10.1530/REP-09-0158. [DOI] [PubMed] [Google Scholar]
- Tan J, Paria BC, Dey SK, Das SK. Differential uterine expression of estrogen and progesterone receptors correlates with uterine preparation for implantation and decidualization in the mouse. Endocrinology. 1999;140:5310–5321. doi: 10.1210/endo.140.11.7148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheon YP, Li Q, Xu X, DeMayo FJ, Bagchi IC, Bagchi MK. A genomic approach to identify novel progesterone receptor regulated pathways in the uterus during implantation. Mol Endocrinol. 2002;16:2853–2871. doi: 10.1210/me.2002-0270. [DOI] [PubMed] [Google Scholar]
- Diao H, Paria BC, Xiao S, Ye X. Temporal expression pattern of progesterone receptor in the uterine luminal epithelium suggests its requirement during early events of implantation. Fertil Steril. 2011;95:2087–2093. doi: 10.1016/j.fertnstert.2011.01.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco HL, Rubel CA, Large MJ, Wetendorf M, Fernandez-Valdivia R, Jeong JW, Spencer TE, Behringer RR, Lydon JP, Demayo FJ. Epithelial progesterone receptor exhibits pleiotropic roles in uterine development and function. FASEB J. 2012;26:1218–1227. doi: 10.1096/fj.11-193334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbetts TA, Mendoza-Meneses M, O'Malley BW, Conneely OM. Mutual and intercompartmental regulation of estrogen receptor and progesterone receptor expression in the mouse uterus. Biol Reprod. 1998;59:1143–1152. doi: 10.1095/biolreprod59.5.1143. [DOI] [PubMed] [Google Scholar]
- Kurita T, Lee KJ, Cooke PS, Taylor JA, Lubahn DB, Cunha GR. Paracrine regulation of epithelial progesterone receptor by estradiol in the mouse female reproductive tract. Biol Reprod. 2000;62:821–830. doi: 10.1093/biolreprod/62.4.821. [DOI] [PubMed] [Google Scholar]
- Kurita T, Lee KJ, Cooke PS, Lydon JP, Cunha GR. Paracrine regulation of epithelial progesterone receptor and lactoferrin by progesterone in the mouse uterus. Biol Reprod. 2000;62:831–838. doi: 10.1095/biolreprod62.4.831. [DOI] [PubMed] [Google Scholar]
- Bazer FW, Slayden OD. Progesterone-induced gene expression in uterine epithelia: a myth perpetuated by conventional wisdom. Biol Reprod. 2008;79:1008–1009. doi: 10.1095/biolreprod.108.072702. [DOI] [PubMed] [Google Scholar]
- Bazer FW, Wu G, Spencer TE, Johnson GA, Burghardt RC, Bayless K. Novel pathways for implantation and establishment and maintenance of pregnancy in mammals. Mol Hum Reprod. 2010;16:135–152. doi: 10.1093/molehr/gap095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomino WA, Fuentes A, Gonzalez RR, Gabler F, Boric MA, Vega M, Devoto L. Differential expression of endometrial integrins and progesterone receptor during the window of implantation in normo-ovulatory women treated with clomiphene citrate. Fertil Steril. 2005;83:587–593. doi: 10.1016/j.fertnstert.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Lessey BA, Killam AP, Metzger DA, Haney AF, Greene GL, McCarty KS., Jr Immunohistochemical analysis of human uterine estrogen and progesterone receptors throughout the menstrual cycle. J Clin Endocrinol Metab. 1988;67:334–340. doi: 10.1210/jcem-67-2-334. [DOI] [PubMed] [Google Scholar]
- Wakitani S, Hondo E, Phichitraslip T, Stewart CL, Kiso Y. Upregulation of Indian hedgehog gene in the uterine epithelium by leukemia inhibitory factor during mouse implantation. J Reprod Dev. 2008;54:113–116. doi: 10.1262/jrd.19120. [DOI] [PubMed] [Google Scholar]
- Ye X, Hama K, Contos JJ, Anliker B, Inoue A, Skinner MK, Suzuki H, Amano T, Kennedy G, Arai H, Aoki J, Chun J. LPA3-mediated lysophosphatidic acid signalling in embryo implantation and spacing. Nature. 2005;435:104–108. doi: 10.1038/nature03505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin ME, Herr DR, Chun J. Lysophosphatidic acid (LPA) receptors: signaling properties and disease relevance. Prostaglandins Other Lipid Mediat. 2010;91:130–138. doi: 10.1016/j.prostaglandins.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Chun J. Lysophosphatidic acid (LPA) signaling in vertebrate reproduction. Trends Endocrinol Metab. 2010;21:17–24. doi: 10.1016/j.tem.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JW, Herr DR, Noguchi K, Yung YC, Lee CW, Mutoh T, Lin ME, Teo ST, Park KE, Mosley AN, Chun J. LPA receptors: subtypes and biological actions. Annu Rev Pharmacol Toxicol. 2010;50:157–186. doi: 10.1146/annurev.pharmtox.010909.105753. [DOI] [PubMed] [Google Scholar]
- Ye X, Skinner MK, Kennedy G, Chun J. Age-dependent loss of sperm production in mice via impaired lysophosphatidic acid signaling. Biol Reprod. 2008;79:328–336. doi: 10.1095/biolreprod.108.068783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan LC, Peters W, Xu Y, Chun J, Farese RV, Jr, Cases S. LPA3 receptor mediates chemotaxis of immature murine dendritic cells to unsaturated lysophosphatidic acid (LPA) J Leukoc Biol. 2007;82:1193–1200. doi: 10.1189/jlb.0407221. [DOI] [PubMed] [Google Scholar]
- Meyer ME, Pornon A, Ji JW, Bocquel MT, Chambon P, Gronemeyer H. Agonistic and antagonistic activities of RU486 on the functions of the human progesterone receptor. EMBO J. 1990;9:3923–3932. doi: 10.1002/j.1460-2075.1990.tb07613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulieu EE. The antisteroid RU486: its cellular and molecular mode of action. Trends Endocrinol Metab. 1991;2:233–239. doi: 10.1016/1043-2760(91)90030-q. [DOI] [PubMed] [Google Scholar]
- Taylor RN, Savouret JF, Vaisse C, Vigne JL, Ryan I, Hornung D, Seppala M, Milgrom E. Promegestone (R5020) and mifepristone (RU486) both function as progestational agonists of human glycodelin gene expression in isolated human epithelial cells. J Clin Endocrinol Metab. 1998;83:4006–4012. doi: 10.1210/jcem.83.11.5214. [DOI] [PubMed] [Google Scholar]
- Das SK, Chakraborty I, Paria BC, Wang XN, Plowman G, Dey SK. Amphiregulin is an implantation-specific and progesterone-regulated gene in the mouse uterus. Mol Endocrinol. 1995;9:691–705. doi: 10.1210/mend.9.6.8592515. [DOI] [PubMed] [Google Scholar]
- Ye X, Herr DR, Diao H, Rivera R, Chun J. Unique uterine localization and regulation may differentiate LPA3 from other lysophospholipid receptors for its role in embryo implantation Fertil Steril 2011. 95 2107 2113 e2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S, Diao H, Zhao F, Li R, He N, Ye X. Differential gene expression profiling of mouse uterine luminal epithelium during periimplantation. Reprod Sci. 2014;21:351–362. doi: 10.1177/1933719113497287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Zowalaty AE, Baumann C, Li R, Chen W, De La Fuente R, Ye X. Seipin deficiency increases chromocenter fragmentation and disrupts acrosome formation leading to male infertility. Cell Death Dis. 2015;6:e1817. doi: 10.1038/cddis.2015.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S, Diao H, Smith MA, Song X, Ye X. Preimplantation exposure to bisphenol A (BPA) affects embryo transport, preimplantation embryo development, and uterine receptivity in mice. Reprod Toxicol. 2011;32:434–441. doi: 10.1016/j.reprotox.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Li R, Xiao S, Diao H, Viveiros MM, Song X, Ye X. Postweaning exposure to dietary zearalenone, a mycotoxin, promotes premature onset of puberty and disrupts early pregnancy events in female mice. Toxicol Sci. 2013;132:431–442. doi: 10.1093/toxsci/kfs343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao H, Xiao S, Li R, Zhao F, Ye X. Distinct spatiotemporal expression of serine proteases prss23 and prss35 in periimplantation mouse uterus and dispensable function of prss35 in fertility. PLoS One. 2013;8:e56757. doi: 10.1371/journal.pone.0056757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao H, Xiao S, Zhao F, Ye X. Uterine luminal epithelium-specific proline-rich acidic protein 1 (PRAP1) as a marker for successful embryo implantation Fertil Steril 2010. 94 2808 2811 e2801. [DOI] [PubMed] [Google Scholar]
- Deb K, Reese J, Paria BC. Methodologies to study implantation in mice. Methods Mol Med. 2006;121:9–34. doi: 10.1385/1-59259-983-4:007. [DOI] [PubMed] [Google Scholar]
- Lee K, Jeong J, Kwak I, Yu CT, Lanske B, Soegiarto DW, Toftgard R, Tsai MJ, Tsai S, Lydon JP, DeMayo FJ. Indian hedgehog is a major mediator of progesterone signaling in the mouse uterus. Nat Genet. 2006;38:1204–1209. doi: 10.1038/ng1874. [DOI] [PubMed] [Google Scholar]
- Jeong JW, Lee KY, Kwak I, White LD, Hilsenbeck SG, Lydon JP, DeMayo FJ. Identification of murine uterine genes regulated in a ligand-dependent manner by the progesterone receptor. Endocrinology. 2005;146:3490–3505. doi: 10.1210/en.2005-0016. [DOI] [PubMed] [Google Scholar]
- Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Kontgen F, Abbondanzo SJ. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359:76–79. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- Billon-Gales A, Krust A, Fontaine C, Abot A, Flouriot G, Toutain C, Berges H, Gadeau AP, Lenfant F, Gourdy P, Chambon P, Arnal JF. Activation function 2 (AF2) of estrogen receptor-alpha is required for the atheroprotective action of estradiol but not to accelerate endothelial healing. Proc Natl Acad Sci U S A. 2011;108:13311–13316. doi: 10.1073/pnas.1105632108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt H, Brunet LJ, Stewart CL. Uterine expression of leukemia inhibitory factor coincides with the onset of blastocyst implantation. Proc Natl Acad Sci U S A. 1991;88:11408–11412. doi: 10.1073/pnas.88.24.11408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemzell-Danielsson K, Svalander P, Swahn ML, Johannisson E, Bygdeman M. Effects of a single post-ovulatory dose of RU486 on endometrial maturation in the implantation phase. Hum Reprod. 1994;9:2398–2404. doi: 10.1093/oxfordjournals.humrep.a138458. [DOI] [PubMed] [Google Scholar]
- Thorpe JB, Burgess PS, Sadkowski M, deCatanzaro D. Estrogen-progesterone balance in the context of blastocyst implantation failure induced by predator stress. Psychoneuroendocrinology. 2013;38:3048–3056. doi: 10.1016/j.psyneuen.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Das A, Mantena SR, Kannan A, Evans DB, Bagchi MK, Bagchi IC. De novo synthesis of estrogen in pregnant uterus is critical for stromal decidualization and angiogenesis. Proc Natl Acad Sci U S A. 2009;106:12542–12547. doi: 10.1073/pnas.0901647106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton K, Arao Y, Korach K. Estrogen hormone physiology: reproductive findings from estrogen receptor mutant mice. Reprod Biol. 2014;14:3–8. doi: 10.1016/j.repbio.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel B, Elguero S, Thakore S, Dahoud W, Bedaiwy M, Mesiano S. Role of nuclear progesterone receptor isoforms in uterine pathophysiology. Hum Reprod Update. 2015;21:155–173. doi: 10.1093/humupd/dmu056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco HL, Jeong JW, Tsai SY, Lydon JP, DeMayo FJ. In vivo analysis of progesterone receptor action in the uterus during embryo implantation. Semin Cell Dev Biol. 2008;19:178–186. doi: 10.1016/j.semcdb.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Winuthayanon W, Hewitt SC, Orvis GD, Behringer RR, Korach KS. Uterine epithelial estrogen receptor alpha is dispensable for proliferation but essential for complete biological and biochemical responses. Proc Natl Acad Sci U S A. 2010;107:19272–19277. doi: 10.1073/pnas.1013226107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A, Soyal SM, Fernandez-Valdivia R, Gehin M, Chambon P, Demayo FJ, Lydon JP, O'Malley BW. Steroid receptor coactivator 2 is critical for progesterone-dependent uterine function and mammary morphogenesis in the mouse. Mol Cell Biol. 2006;26:6571–6583. doi: 10.1128/MCB.00654-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szwarc MM, Kommagani R, Lessey BA, Lydon JP. The p160/steroid receptor coactivator family: potent arbiters of uterine physiology and dysfunction. Biol Reprod. 2014;91:122. doi: 10.1095/biolreprod.114.125021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Yoon S, Zhao Y, Park SE, Liao L, Xu J, Lydon JP, DeMayo FJ, O'Malley BW, Bagchi MK, Katzenellenbogen BS. Uterine development and fertility are dependent on gene dosage of the nuclear receptor coregulator REA. Endocrinology. 2012;153:3982–3994. doi: 10.1210/en.2012-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Park S, Bagchi MK, Taylor RN, Katzenellenbogen BS. The coregulator, repressor of estrogen receptor activity (REA), is a crucial regulator of the timing and magnitude of uterine decidualization. Endocrinology. 2013;154:1349–1360. doi: 10.1210/en.2012-2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DK, Kurihara I, Jeong JW, Lydon JP, DeMayo FJ, Tsai MJ, Tsai SY. Suppression of ERalpha activity by COUP-TFII is essential for successful implantation and decidualization. Mol Endocrinol. 2010;24:930–940. doi: 10.1210/me.2009-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Kannan A, DeMayo FJ, Lydon JP, Cooke PS, Yamagishi H, Srivastava D, Bagchi MK, Bagchi IC. The antiproliferative action of progesterone in uterine epithelium is mediated by Hand2. Science. 2011;331:912–916. doi: 10.1126/science.1197454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara I, Lee DK, Petit FG, Jeong J, Lee K, Lydon JP, DeMayo FJ, Tsai MJ, Tsai SY. COUP-TFII mediates progesterone regulation of uterine implantation by controlling ER activity. PLoS Genet. 2007;3:e102. doi: 10.1371/journal.pgen.0030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sabbagh M, Lam EW, Brosens JJ. Mechanisms of endometrial progesterone resistance. Mol Cell Endocrinol. 2012;358:208–215. doi: 10.1016/j.mce.2011.10.035. [DOI] [PubMed] [Google Scholar]
- Young SL, Lessey BA. Progesterone function in human endometrium: clinical perspectives. Semin Reprod Med. 2010;28:5–16. doi: 10.1055/s-0029-1242988. [DOI] [PubMed] [Google Scholar]
- Li X, Feng Y, Lin JF, Billig H, Shao R. Endometrial progesterone resistance and PCOS. J Biomed Sci. 2014;21:2. doi: 10.1186/1423-0127-21-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudice LC. Endometrium in PCOS: Implantation and predisposition to endocrine CA. Best Pract Res Clin Endocrinol Metab. 2006;20:235–244. doi: 10.1016/j.beem.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Fatemi HM, Van Vaerenbergh I. Significance of premature progesterone rise in IVF. Curr Opin Obstet Gynecol. 2015 doi: 10.1097/GCO.0000000000000172. [DOI] [PubMed] [Google Scholar]
- Sheng X, Yung YC, Chen A, Chun J. Lysophosphatidic acid signalling in development. Development. 2015;142:1390–1395. doi: 10.1242/dev.121723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawar S, Hantak AM, Bagchi IC, Bagchi MK. Minireview: Steroid-regulated paracrine mechanisms controlling implantation. Mol Endocrinol. 2014;28:1408–1422. doi: 10.1210/me.2014-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetendorf M, DeMayo FJ. Progesterone receptor signaling in the initiation of pregnancy and preservation of a healthy uterus. Int J Dev Biol. 2014;58:95–106. doi: 10.1387/ijdb.140069mw. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama K, Aoki J, Bandoh K, Inoue A, Endo T, Amano T, Suzuki H, Arai H. Lysophosphatidic receptor, LPA3, is positively and negatively regulated by progesterone and estrogen in the mouse uterus. Life Sci. 2006;79:1736–1740. doi: 10.1016/j.lfs.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Ye X, Ishii I, Kingsbury MA, Chun J. Lysophosphatidic acid as a novel cell survival/apoptotic factor. Biochim Biophys Acta. 2002;1585:108–113. doi: 10.1016/s1388-1981(02)00330-x. [DOI] [PubMed] [Google Scholar]
- Qian L, Xu Y, Simper T, Jiang G, Aoki J, Umezu-Goto M, Arai H, Yu S, Mills GB, Tsukahara R, Makarova N, Fujiwara Y, et al. Phosphorothioate analogues of alkyl lysophosphatidic acid as LPA3 receptor-selective agonists. Chemmedchem. 2006;1:376–383. doi: 10.1002/cmdc.200500042. [DOI] [PubMed] [Google Scholar]
- Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, Lessey BA, Giudice LC. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;148:3814–3826. doi: 10.1210/en.2006-1692. [DOI] [PubMed] [Google Scholar]
- Wei Q, St Clair JB, Fu T, Stratton P, Nieman LK. Reduced expression of biomarkers associated with the implantation window in women with endometriosis. Fertil Steril. 2009;91:1686–1691. doi: 10.1016/j.fertnstert.2008.02.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Zhang Y, Elad D, Jaffa AJ, Cao Y, Ye X, Duan E. Navigating the site for embryo implantation: Biomechanical and molecular regulation of intrauterine embryo distribution. Mol Aspects Med. 2013;34:1024–1042. doi: 10.1016/j.mam.2012.07.017. [DOI] [PubMed] [Google Scholar]
- Ye X, Diao H, Chun J. 11-deoxy prostaglandin F(2alpha), a thromboxane A2 receptor agonist, partially alleviates embryo crowding in Lpar3((−/−)) females. Fertil Steril. 2012;97:757–763. doi: 10.1016/j.fertnstert.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama K, Aoki J, Inoue A, Endo T, Amano T, Motoki R, Kanai M, Ye X, Chun J, Matsuki N, Suzuki H, Shibasaki M, et al. Embryo spacing and implantation timing are differentially regulated by LPA3-mediated lysophosphatidic acid signaling in mice. Biol Reprod. 2007;77:954–959. doi: 10.1095/biolreprod.107.060293. [DOI] [PubMed] [Google Scholar]
- Williams KC, Renthal NE, Gerard RD, Mendelson CR. The microRNA (miR)-199a/214 cluster mediates opposing effects of progesterone and estrogen on uterine contractility during pregnancy and labor. Mol Endocrinol. 2012;26:1857–1867. doi: 10.1210/me.2012-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]