Abstract
For several decades, researchers have used erythrocytes for drug delivery of a wide variety of therapeutics in order to improve their pharmacokinetics, biodistribution, controlled release and pharmacodynamics. Approaches include encapsulation of drugs within erythrocytes, as well as coupling of drugs onto the red cell surface. This review focuses on the latter approach, and examines the delivery of red blood cell (RBC)-surface-bound anti-inflammatory, anti-thrombotic and anti-microbial agents, as well as RBC carriage of nanoparticles. Herein, we discuss the progress that has been made in surface loading approaches, and address in depth the issues relevant to surface loading of RBC, including intrinsic features of erythrocyte membranes, immune considerations, potential surface targets and techniques for the production of affinity ligands.
Introduction: red blood cells as carriers for vascular drug delivery
Drug delivery is frequently hindered by inadequate pharmacokinetics (PK) of the therapeutic agent. Rapid elimination impedes drug delivery to the intended targets, limits efficacy and may dictate the need for high doses and multiple administrations. These issues are especially pertinent to the use of expensive and labile biotherapeutics. Several approaches have been devised to enhance drug bioavailability within the bloodstream, including chemical modification, artificial carriers (such as polymers) and natural carriers (such as blood proteins and cells).
As a successful example of the first approach, chemical conjugation with polyethylene glycol (PEG), which prolongs circulation and reduces uptake by the reticuloendothelial system (RES), is widely used in research and clinically [1–7]. Loading drugs within PEG-coated carriers, especially durable polymeric carriers that adapt to flow, can further extend circulation times [8–14]. Still, no existing synthetic carrier can begin to match the intravascular longevity of erythrocytes [15,16].
Erythrocytes (red blood cells [RBCs]) are natural carriers for drugs that require sustained intravascular delivery [17–19], as they have evolved to deliver what might be considered the most vital biologic cargo, oxygen. The RBC is an enucleated biconcave disc that, in humans, has a diameter of approximately 7 µm, thickness of approximately 2 µm and plasma membrane surface area of approximately 160 µm2. One microliter of human blood contains about 4–5 million RBCs and the total number of RBCs, the most abundant cellular constituent of the blood (>99%), in the human body approaches 1013 cells. Human RBCs normally have a life span of 100–120 days (of note, mouse RBCs are smaller and have a life span of a month) and travel approximately 250 km through the cardiovascular system. These remarkable properties prompted researchers to investigate the use of RBCs as drug delivery vehicles beginning several decades ago.
Starting in the 1970s, several laboratories attempted to improve drug delivery by loading drugs within autologous or donor RBCs prior to transfusion [20]. Initial studies provided rather mixed results, likely due to damage to RBCs inflicted during drug loading [21,22]. During the 1980s, prospects of using carrier RBCs were impeded by the outbreak of HIV and other blood-transmitted infections. Meanwhile, use of erythrocytes was overshadowed by novel carriers, particularly liposomal systems. However, a few laboratories persisted in efforts to improve RBC delivery systems, devised less traumatic methods for loading and tested RBC carriers [18,23–25] in animal models [26,27], and, in pilot trials, in human patients [28–31]. At the present time, several RBC drug delivery strategies are in industrial development and clinical testing.
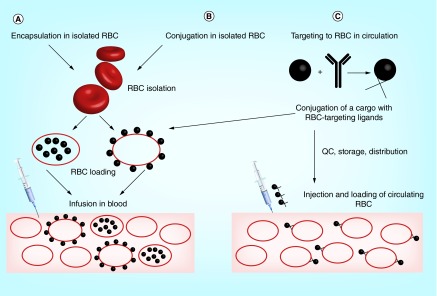
Three main strategies for RBC drug delivery are generally employed (Figure 1). Two approaches involve infusion of RBCs preloaded with a drug ex vivo, using either encapsulation within RBCs, or conjugation to the RBC surface. These strategies involve ex vivo manipulations with RBCs and create a situation wherein a relatively minor fraction of circulating RBCs carries the drug (unless exchange hemotransfusion is employed to load a greater fraction of the patient's RBCs). Hundreds of publications using this approach have been reported in the last four decades and have been the subject of comprehensive reviews, including important clinical studies showing the feasibility and medical promise of infusion of drug-loaded RBCs, such as RBCs loaded with encapsulated dexamethasone and therapeutic enzymes [32–42].
Figure 1. . Strategies for drug delivery by red blood cells.
Two approaches involve isolation of RBC (either from patient's autologous blood or from a donor blood), followed by drug loading using encapsulation or surface conjugation and injection of RBC-drug complex in the bloodstream (A & B). In the third approach (C), drug loading can be performed either in vivo by injecting the RBC-targeted drugs in bloodstream (thereby providing a homogenous loading on circulating RBC), or in vitro as a one-step modification of strategy B.
RBC: Red blood cell; QC: Quality control.
The third strategy involves targeting drugs directly to the RBC surface. Our article focuses on this novel approach. We will review: i) pertinent features of the RBC as a carrier for surface-bound drugs; ii) evolution, status and perspectives involving this strategy; and, iii) medical and translational aspects of the envisioned clinical use.
RBC features as drug carriers
RBC membrane endurance & plasticity
In order to examine RBCs as intravascular drug carriers, one should consider their innate properties that contribute to their utility as delivery vehicles. Chief among the structural features that RBCs possess to execute their function as oxygen carriers is their complex and unique membrane [43]. The RBC membrane is in a perpetual balance between tensile strength and deformability, which is required for RBC to repeatedly pass through capillaries with cross-sections as narrow as one-third of the cell diameter [44]. RBC must simultaneously maintain membrane integrity to avoid intravascular hemolysis with release of free hemoglobin, which may cause renal [45] and endothelial injury [46]. When extensive, hemolysis can be fatal, as evidenced by the significant mortality associated with acute hemolytic transfusion reactions [47].
The RBC membrane is supported from within by its cytoskeleton, in particular, the hexagonal actin–spectrin lattice that underlies the plasmalemma. This lattice interconnects with anchoring and membrane proteins, including glycophorin A and band 3 protein, two of the most prevalent integral glycoproteins among the more than 300 proteins found in the RBC plasma membrane [48]. Dynamic rearrangements of the cytoskeleton and plasma membrane plasticity enable the cells to undergo millions of reversible cycles of deformability through capillary networks [49]. Hydrodynamic forces drive RBCs to the center of the vascular lumen and the endothelial glycocalyx helps to minimize interactions with the vascular walls [50].
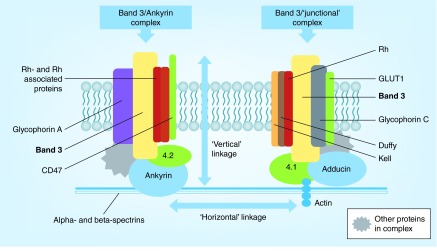
The organization of the RBC membrane that allows for deformation while maintaining integrity is based on a series of ‘vertical’ and ‘horizontal’ linkages between membrane protein complexes within the fluid phospholipid membrane bilayer (Figure 2). This structural organization allows the submembrane skeleton to undergo reversible changes in conformation from tight to expanded networks [51] that confer resistance to shear stress, while concurrently permitting deformation and maintenance of cell volume and surface area. As in other eukaryotic membranes, the phospholipid bilayer of RBCs also includes distinctly organized populations of cholesterol, lipid rafts and phospholipids including phosphatidyl serine (PS), a phospholipid that, although normally restricted to the inner leaflet of plasma membranes, can be exposed to the extracellular surface as cells senesce or incur damage [52,53].
Figure 2. . Organization of protein complexes associated with the red blood cell membrane.
Multiprotein complexes within the RBC membrane produce a network of vertical linkages with the phospholipid bilayer and the underlying spectrin network, and horizontal linkages between ankyrin and junction complexes.
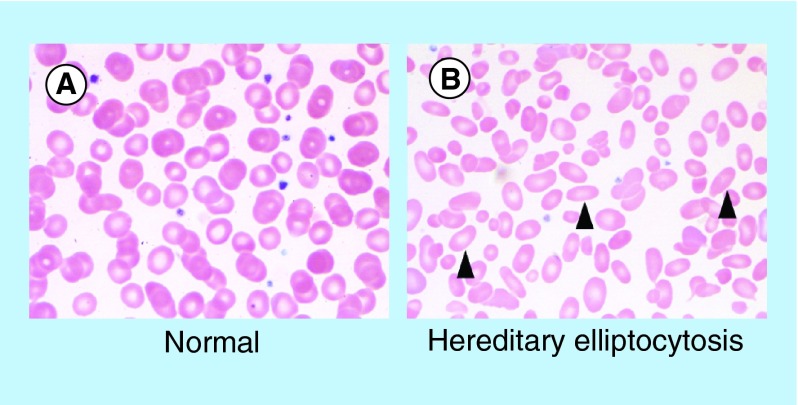
A spectrum of inherited genetic disorders involving structural components of the RBC membrane provides insight into the molecular basis of membrane deformability [54]. Altered membrane organization produces diverse clinical phenotypes that include hereditary spherocytosis (HS), hereditary elliptocytosis (HE) and hereditary ovalocytosis (HO) [55]. In HS, mutations in any of several genes produce a similar phenotype, ultimately leading to RBCs that assume a round shape rather than a biconcave disc. Deficiencies in ankyrin, spectrin, band 3, protein 4.2 and the Rh complex have all been demonstrated disrupt ‘vertical’ linkages between the skeletal network and membrane complexes, producing the HS phenotype. This phenotype results, in part, from loss of membrane surface area, which reduces the ability of the RBC to traverse the spleen, leading to sequestration and decreases lifespan [56]. In hereditary elliptocytosis, deficiencies in spectrin and protein 4.1 produce deficient ‘horizontal’ linkages, which lead the RBC to assume to an ovoid shape (see Figure 3), which also compromises deformability and lifespan.
Figure 3. . Morphologic features of normal red blood cells and congenital red blood cell defects.
Images demonstrate the peripheral blood smear from a (A) normal patient, and a (B) patient with hereditary elliptocytosis. Arrowheads highlight cells with characteristic morphologic defects.
Another feature central to efficient and safe transport by RBC is their ability to remain nonadherent to endothelium. In part, resistance to adhesion is mediated by the RBC glycocalyx, which contains abundant negatively charged sialic acids [57]. As might be predicted by these findings, it has proven to be extraordinarily difficult to replicate RBC flexibility and resistance to adherence using artificial blood substitutes, which likely has contributed to their limited clinical success notwithstanding decades of investigation [58,59].
Elimination of RBC: complement & RES
Having noted the important features of the RBC membrane, one must also consider the pathways by which aged and damaged RBCs are cleared, as they provide insight into potential adverse effects on cell lifespan and function when RBCs are employed as drug carriers. Erythrocytes do not leave the bloodstream except transiently during passage through hepatic sinuses and the interstitium in the splenic follicles. Tissue macrophages in the spleen and liver are the principal sites where ‘compromised’ RBCs are eliminated. Senescence, pathological changes (e.g., in malaria or sickle-cell disease) and, potentially, drug loading, all reduce RBC biocompatibility, enhancing their clearance and increasing the risk for harmful hemolysis.
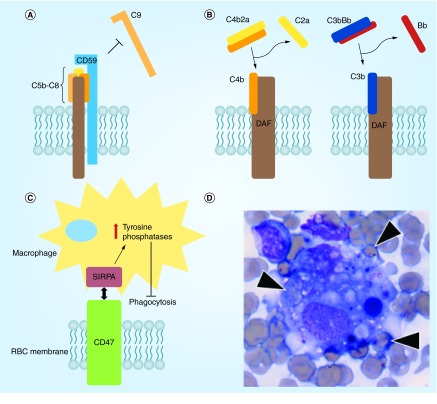
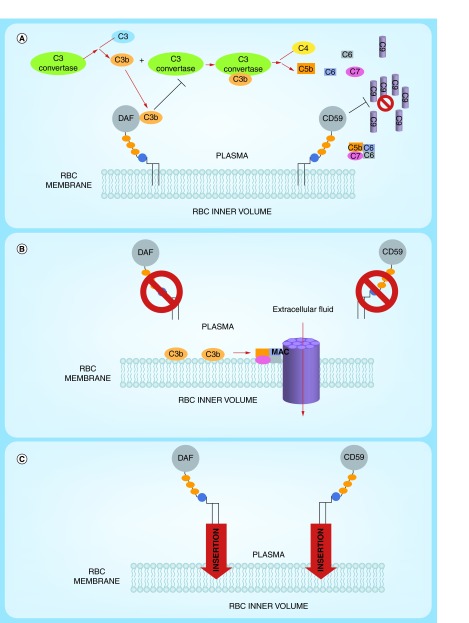
Some surface RBC glycoproteins that are expressed at low and variable levels protect RBCs from damage and elimination. These include complement inhibitors such as DAF and CD59 [60] and signaling molecules such as CD47 [61] and SHPS-1 [62]. CD47, a membrane protein, inhibits phagocytosis of RBCs by macrophages through binding to SIRPα, which transduces an inhibitory ‘do not eat me’ signal (Figure 4C) [63]. Loss or inhibition of CD47 may be a key step in the pathologic process known as hemophagocytic lymphohistiocytosis, a syndrome characterized by excessive immune activation that leads to engulfment of erythrocytes and other hematopoietic cells by macrophages in the bone marrow and elsewhere [64] (Figure 4D).
Figure 4. . Protective mechanisms in the red blood cell membrane.
(A) CD59 inhibits C9 multimerization and formation of the membrane attack complex by binding to nascent C5b-C8 complexes; (B) DAF inhibits early complement pathways by displacing C2a and Bb from C4b and C3b, respectively; (C) CD47 inhibits phagocytosis by macrophages through interaction with SIRPA and downstream upregulation of tyrosine phosphatase activity; (D) bone marrow aspirate from a patient with a hemophagocytic syndrome demonstrating engulfment of multiple erythrocytes (arrowheads) by a macrophage.
The complement system, consisting of more than 20 regulatory and executing proteins in blood plasma and cellular membranes, is part of innate immunity, mediating opsonization and destruction of pathogens, tumor and foreign cells, and activation of proinflammatory cascades at localized sites of invasion. However, both the involved and neighboring host cells can be damaged when activation of complement is disseminated.
RBCs are among the most frequently affected sites of bystander injury by complement, especially when there are deficiencies in membrane glycoproteins that inhibit this pathway: Decay Acceleration Factor (CD55 or DAF), CD59 and CR1 [65–70]. DAF and CR1 inhibit early stages of complement activation, thereby protecting cells from lysis and reducing generation of proinflammatory mediators (Figure 4B), whereas CD59 blocks formation of hemolytic pores in the plasmalemma (Figure 4A). Complement-mediated hemolysis of RBCs deficient in DAF and CD59 is the predominant pathophysiologic mechanism leading to intravascular hemolysis in paroxysmal nocturnal hemoglobinuria.
The complement system also functions physiologically to help eliminate senescent and damaged RBCs, such as those opsonized by immunoglobulins. Deposition of C1q and C3b on RBC facilitates binding and uptake of RBCs by phagocytes. Deposition of immunoglobulin molecules and IgG-containing immune complexes also accelerates uptake of RBC via Fc-receptors on phagocytes [71–74]. Phagocytosis of RBC is carried out mainly by Kuppfer cells, in other words, macrophages residing in the hepatic macrophage sinuses, and their counterparts in the spleen, two organs where RBCs pass from the systemic circulation to tissue compartments via vascular openings [75–77].
There have been intensive efforts made to understand how these and other processes contribute to loss of carrier RBC biocompatibility [60,78–82]. Conformational changes and abnormal clustering of membrane glycoproteins caused by cross-linking agents, and RBC membrane damage by osmotic stress during drug loading [82] have been shown to lead to cytoskeleton dysfunction, loss of RBC plasticity and mechanical stability [80], sensitization of RBCs to lysis [83] and provocation of IgG deposition and activation of complement [84]. Transposition of PS from the inner leaflet of the plasma membrane to the RBC surface [85] predisposes to phagocytosis and endothelial adhesion [86]. Hemolysis, aggregation, adhesion and phagocytosis of carrier RBCs compromise drug delivery and may cause hypoxia and acute vascular and renal damage by free hemoglobin and the toxic effects of RBC remnants [21,87–89].
Using RBC carriers for drug delivery to RES
Loading drugs within RBCs inevitably is associated with the risk of damage to the cell membrane and, in some cases, their internal contents (e.g., depletion of systems to store and utilize energy [90] and transport and metabolism of nitric oxide [91]). Making lemonade from lemons, modified RBC carriers destined for phagocytosis can be used to deliver drugs to macrophages [78,92]. Examples of these approaches include attempts to deliver enzymes therapy for lysosomal storage diseases, with variable success in animal studies [18,93], antimicrobial and antiviral agents [24,94–97], antigens to boost the immune response [98] and anti-inflammatory drugs [99]. Recently, studies testing drug delivery to host defense cells using modified RBCs have entered translational and clinical domains [15,100].
Coupling therapeutics to the RBC surface: prototype studies
Most drug delivery strategies, however, rely on avoiding RBC elimination. Coupling drugs to the RBC surface may allow for bypass of damage related to drug encapsulation, minimize RBC elimination and provide other potential advantages. Putting a drug on the RBC surface mitigates issues related to the need for release of drugs encapsulated within RBCs, which is difficult to control [101]. With this approach, the RBC membrane is no longer a barrier for the surface-bound enzymes and other agents. Experimental data confirmed that drugs, including enzymes, coupled to RBC surface are more accessible and active compared with encapsulated counterparts [102,103].
Chemical conjugation of drugs to RBC
Coupling antigens and antibodies to RBCs using chemical cross-linkers, including aldehyde, tannic acid and chromium chloride were employed in the 1950s to produce reagents suitable to study antibody-mediated agglutination. These methods were then used to conjugate drugs and other molecules to carrier RBCs [98,103–104]. However, these nonspecific cross-linking agents provided no control over attachment site and profoundly altered RBC membranes.
These methods were surpassed by conjugation to RBC amino acids [105–107], sulfhydryl groups [108], sugars [109] and lipids [110,111]. However, biocompatibility of modified RBCs remained a daunting issue [112–116]. Early RBC carriers activated complement [110,117], leading to hemolysis [66] and rapid elimination of RBCs [117–121].
Development of more precisely controlled conjugation methods [104,110,122–124], preserving DAF and CD59 and avoiding activation of complement [60,124–125] permitted coupling of drugs to RBCs with less damage. The circulation of RBC/drug complexes approximated those of control RBCs for at least several days after injection in rats and mice [122,126].
Activity & effect of RBC-conjugated drugs
Drugs whose delivery may be improved by coupling to RBCs include antigens and cytokines to stimulate the immune response [127], antibodies for vascular targeting of RBC-loaded cargoes [19,103,128], antibodies and other ligands to capture circulating pathological mediators such toxins and pathogens themselves [129–131], therapeutic enzymes and other biomolecules whose targets are localized within the bloodstream, and complement inhibitors to protect RBCs against pathological hemolysis, for example paroxysmal nocturnal hematuria [132].
Of note, DAF and CD59 are anchored onto the luminal surface of RBC membranes via a glycophosphatidyl inositol (GPI) anchor primarily within cholesterol-rich microdomains [133]. Methods to insert DAF, CD59 and other GPI-linked proteins into the RBC plasmalemma using lipid anchors have been developed (Figure 5) [134,135]. Insertion of GPI-anchored CD59 in the RBC plasmalemma protects against complement hemolysis [136]. The RBC harboring of artificial GPI-linked proteins is an attractive drug delivery paradigm [137], especially in the light of finding that these proteins transfer from RBCs to endothelium [138]. Recently, insertion of lipid-modified biomolecules into the RBC membrane is being explored to target various types of phagocytes [100].
Figure 5. . Approaches to ‘painting’ of red blood cells with protective complement regulatory proteins.
(A) Normal RBCs expresses complement-regulatory proteins including decay accelerating factor (DAF) and CD59 which are tethered to the RBC lipid bilayer of the cell membrane by a Glycosylphosphatidylinositol (GPI) anchor. The presence of both DAF and CD59 protects the cells from homologous complement-mediated lysis. (B) Paroxysmal nocturnal hemoglobinuria RBCs are more vulnerable to complement mediated lysis due to a reduction or complete absence of GPI-anchored DAF and CD59. Continuous complement activation leads to the formation of membrane attacking complex which forms pores in the RBC membrane, allowing extracellular fluid to the enter, resulting in the swelling and ultimately rupture of the RBC. (C) RBC ‘painting’ is a method of incorporating proteins such as DAF and CD59 via GPI anchors into the membrane, decreasing the RBC sensitivity towards complement-mediated lysis.
Agents that control blood fluidity and coagulation are attractive cargoes for RBC carriage. The first attempts to manage thrombosis included conjugating fibrinolytics [19] and heparin [139] to RBCs to prevent thrombosis and clotting agents to mitigate hemorrhagic disorders [17].
Plasminogen activators (PA, including tissue-type PA [tPA] and urokinase [uPA]), which generate plasmin which lysis thrombi, are used in emergent settings [140,141], but have the downside of rapid elimination [142], requiring high doses with the attendant risk of bleeding [143–146] and cerebral damage [147]. Biocompatible coupling of PAs to RBCs maintains fibrinolytic activity [17,19,139], prolongs their circulation and minimizes diffusion into the CNS and pre-existing hemostatic plugs. RBC/PA complexes injected in mice and rats circulate orders of magnitude longer than soluble PA counterparts, providing prophylactic delivery of PA to the interior of nascent thrombi, even when a tenfold higher dose of soluble PA is ineffective [148–150]. RBC/PA entrapped within growing clots rapidly form patent channels that permit perfusion of oxygen-carrying host RBCs prior to clot complete disintegration [148,149]. Delivery of PAs to the interior of nascent thrombi, while sparing preformed clots, make them suitable for thromboprophylaxis in settings where the risk of thrombosis and bleeding are both high [150,151].
RBC carriage favorably alters several important PA features. It switches PAs from activating proinflammatory receptors in the CNS parenchyma to protective signaling via intravascular receptors [152–155]. In addition, the RBC glycocalyx attenuates inactivation of coupled PAs by plasma inhibitors [57] and minimizes adverse interactions with vascular cells [156]. RBC/PA lyses cerebral thrombi in mice and rats, providing reperfusion, brain protection and improving survival. RBC/PA also alleviates brain injury in rats with intracranial hemorrhage and blunt trauma, and protects brain in pigs with cerebral thrombosis [154–155,157–159]. In stark contrast, free PA causes CNS bleeding, neurotoxicity and lethality in these settings. Figure 5 illustrates this strategy.
Targeting therapeutic agents to circulating RBC
As discussed above, chemical conjugation to RBCs requires cell isolation, modification and reinfusion, limiting its practical utility in clinical medicine. Targeting drugs to circulating RBCs offers an opportunity to avoid complications associated with ex vivo manipulations and transfusion of RBCs. This strategy involves conjugation of drugs to antibodies (or their engineered fragments) or polypeptides capable of binding to RBCs.
Coupling of RBC targeting ligands to therapeutics provides agents that can be loaded directly onto RBCs circulating in the bloodstream. Currently available publications focus on the effect of drugs coupled to RBC using this approach and examination of their efficacy with respect to free agents. Although characterization of the loading capacity and maximal efficacy possible with such an approach is important, these properties remain relatively poorly defined in RBC-coupling approaches, and early studies have instead focused on demonstration of the functional (i.e., therapeutic or prophylactic) superiority of RBC-coupled versus free agents. Indeed, several iterations of this approach have been devised and successfully tested in animal models and are described herein.
Targeting to RBCs via CR1-anchoring
Initial attempts to target therapeutics to circulating RBCs involved anchoring to complement receptor type 1 (CR1). Immune complexes containing activated component of complement C3b bind to CR1 on RBCs which are transferred to macrophages without damage [160]. In primates and humans, >90% of CR1 is expressed on RBCs at levels of approximately 500–1500 copies per cell, providing an anchorage site for C3b-containing complexes [161–164].
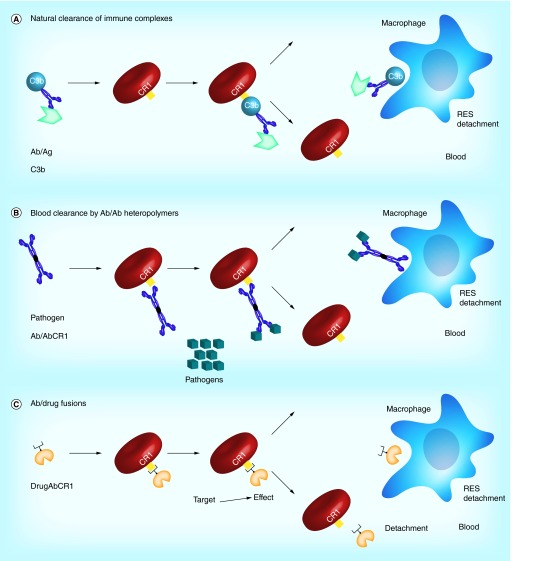
A quarter of a century ago, Ron Taylor exploited this natural mechanism using antibodies to CR1 conjugated with antibodies to a pathogen or toxin [131]. Injecting such conjugates enabled RBC-mediated clearance of pathogens in vitro [130–131,165] and in nonhuman primates [166] (rodents lack CR1 and in vivo studies of CR1-targeting involved monkeys prior to generation of a transgenic mouse expressing human CR1 [167]). Binding of anti-CR1 conjugates does not cause RBC phagocytosis [168], however, phagocytes recognize pathogens bound to CR1 [129], cleave off CR1-bound immune complexes [169] and thereby take C3b-pathogen complexes from RBC surfaces without damage to the cells [170–172]. Figure 6 illustrates this mechanism. Pathogens have been eliminated from the bloodstream using this approach in primates and transgenic mice without damage to RBCs [131,173–175]. This approach has been applied to both bacterial [174] and viral [176–178] pathogens, as well as natural toxins [179], and has been shown to attenuate infection [176,180].
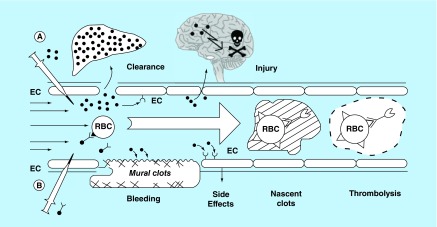
Figure 6. . A concept of prophylactic fibrinolysis by tissue-type PA targeting to red blood cells.
(A) Plasminogen activators (dots) are relatively ineffective clinically, in part due to rapid inactivation uptake by liver, requirement for high blood levels which predispose to bleeding through indiscriminate lysis of hemostatic mural clots, vascular side effects (e.g., activation of receptors on endothelial cells, EC) and injurious effects of tPA diffusing into the CNS. (B) Coupling to RBC (such as by engineered protein targeting ligands) dramatically prolongs the intravascular longevity of RBC/tPA variant. RBC/tPA will restrain tPA binding to cellular receptors, and restrict its access into mural hemostatic clots and the CNS. Propulsion of RBC towards the mainstream will further offset interactions of the prodrug with hemostatic clots and vessel walls. RBC/tPA will not restrict access to the interior of nascent pathological thrombi, which leads to lysis of nascent pathological intravascular clots while hemostatic clots remain impervious.
EC: Endothelial cell; RBC: Red blood cell.
Similarly, coupling anti-CR1/DNA conjugates to RBCs accelerated elimination of DNA antibodies in monkeys, providing a prototype treatment for lupus [173,181–182]. Coupling cytokines to RBCs via CR1 ligands has been proposed as an anti-inflammatory therapy [163,183]. tPA conjugated to a CR1 monoclonal antibody binds to circulating RBCs in mice without harming the cell and provides safe and effective prophylactic thrombolysis [184] comparable to infusion of RBC/tPA [151]. Figure 6B illustrates the strategy of CR1-targeted surface coupling of blood clearing agents and drugs to circulating RBCs.
Recombinant fusion proteins targeted to circulating RBCs
It will be challenging to translate antibody conjugates obtained through cross-linking chemistry to industrial scale production and clinical practice. In general, chemical conjugation yields larger, multimolecular and heterogeneous antibody-drug species, which are difficult to produce uniformly on a large scale, leading to regulatory and quality control challenges. These conjugates may also cause RBCs agglutination or impede the plasticity of RBC membranes. Antibody conjugates bearing Fc-fragments may activate complement and bind to a variety of Fc-receptor bearing cells, including phagocytes. Any of these adverse factors may impede the biocompatibility of carrier RBCs and cause toxicity. CR1-targeted antibody conjugates have not been reported to cause these problems, but CR1 expression levels in humans vary between 500–1500 copies/RBC, which may be suboptimal for effective dosing in some settings [184].
To circumvent these technical problems and achieve predictable loading of RBCs over a wide range of drug concentrations, drugs can be fused to the antigen-binding single chain variable fragment (scFv) of monoclonal antibodies (or similarly engineered derivatives) directed to RBC determinants such as glycophorin A (GPA) and associated transmembrane glycoproteins [185,186]. GPA expression exceeds 5 × 105 copies per RBC in all humans [187,188] (Table 1). Some GPA antibodies agglutinate RBC in part due to its high density its aforementioned role in supporting membrane integrity through interactions with the cytoskeleton [85,189], which might impede membrane plasticity and thereby accelerate cell clearance or promote vascular occlusion. Although nonagglutinating GPA antibodies have been described [190–192], the use of monovalent scFv fragments is a preferred means to load RBCs safely [193].
Table 1. . Representative erythrocyte antigens and key features.
| Protein family | Common antigen designations | Examples of high frequency epitopes | Function | Expression level | MW (kDa) | Other considerations |
|---|---|---|---|---|---|---|
|
Glycophorins (MNS system) | ||||||
| Glycophorin A |
M, N |
En(a) |
Structural membrane sialoglycoprotein |
High (8e5/cell) |
43 |
Most commonly used histologic marker of erythroid lineage; major sialic acid containing structure |
| Glycophorin B |
S, s, U |
U, En(a) |
Structural membrane sialoglycoprotein |
Moderate (2e5/cell) |
25 |
Significant sialic acid containing structure |
| Band 3 (Diego system) |
Di, Wr, Rb |
Wr(b) |
Anion transporter, structural |
Very high (>1e6/cell) |
95–105 |
Majority of ABO antigens are attached to band 3; Deficiency can result in HS, HO, HE phenotypes |
|
Rh family | ||||||
| RhCEce |
C, c, E, e |
Rh17 (Hr0), Rh29 |
Ammonia transporter |
Moderate |
30–32 |
Involved in regulation of ammonium transport, high polymorphism |
| RhD |
D |
None |
Ammonia transport; structural component |
Low to moderate (1e5-2e5/cell) |
30–32 |
Expressed by 85% of population, historically most important antigen in HDFN |
| RhAG |
Duclos |
Duclos, DSLK |
Drives expression of other Rh family members |
Low to moderate (1e5-2e5/cell) |
45–100 |
Ancestral precursor to other Rh family members |
| GLUT1 |
No significant antigen grouping |
No significant polymorphism |
Glucose transporter |
Moderate (2e5-5e5/cell) |
45–65 |
Mutations in GLUT1 associated with pathogenic phenotype including hemolytic anemia |
| Dombrock |
Do(a), Do(b), Jo(a) Gy(a) |
Jo(a), Gy(a), Hy |
Mono-ADP-ribosyltransferase |
Uncertain |
47–58 |
Apparent lack of enzymatic function in RBCs, clinical significance of Do-null phenotype uncertain |
| Kell |
K, k |
k |
Endothelin-3-converting zinc endopeptidase |
Low (1e4/cell) |
93 |
Deficiency known as McLeod phenotype; expressed on other tissues including brain, lymphoid organs, muscle |
| CD59, DAF (Cromer system) | Cr, Tc, Dr, Es | Cr(a), Tc(a), Dr(a) | Complement regulation | Low (2e4/cell) | 60–70 | Also expressed on leukocytes and platelets; low levels of soluble form in plasma |
Note that for simplicity several antigens systems are not included above. For more comprehensive discussion of red cell antigen families see Reid [194].
ABO: ABO blood group; HDFN: Hemolytic disease of the fetus and newborn; HE: Hereditary elliptocytosis; HO: Hereditary ovalocytosis; HS: Hereditary spherocytosis.
Advantages of scFv fusions include: lack of Fc-mediated side effects; lack of cross-linking of anchoring sites on RBC; relatively small scFv-fusions can be injected intramuscularly; established techniques for humanization and reduction of immunogenicity of scFv; and the modular format supports design of diverse scFv fusions [195]. Using modular gene engineering methods, these recombinant proteins can be fused via short connecting peptides with variety of executing proteins. Expression of scFv in diverse vectors enables large-scale, GMP-quality production of homogeneous monovalent scFv fusion proteins [140,196].
For example, Atkinson and co-workers produced a recombinant construct fusing human CR1 with scFv directed to the Rh(D) blood group antigen, which binds to CR1-deficient RBCs and restores their ability to bind immune complexes [197]. This approach has the potential to boost CR1-mediated functions (i.e., immune clearance and complement inhibition) and to boost function in CR1-deficient patients (˜10% of humans are CR1-negative). This team also devised a scFv composed of a monoclonal antibody TER-119 to a mouse analogue of human GPA [198], and fused this scFv with DAF [132] or CR1 [199]. These monovalent fusion constructs bound to RBCs after intravascular injection in mice without cell damage, and, furthermore, enhanced RBC resistance to lysis by complement [132,199]. More recently, the Atkinson's group performed neonatal gene transfer of TER-119 scFv/CR1 in mice using a retroviral gene transfer vector. They achieved prolonged synthesis of this fusion protein, sustained coupling to RBCs and restored protection against excessive complement lysis in genetically deficient mice [200].
In the field of antithrombotic interventions, a TER119-based scFv/tPA fusion provided biocompatible binding to circulating RBCs and antithrombotic effects in mouse models of thrombosis comparable to RBC/tPA and superior to tPA, with added advantage of a wide range of dosing [184,194,201]. To enhance therapeutic specificity, a mutant thrombin-activated prourokinase fragment has been fused with this scFv. In animal models, the resultant scFv/uPA-T fusion: binds safely to RBCs after IV injection; circulates as a RBC-bound prodrug; is activated by thrombin; and provides effective and safe thromboprophylaxis with efficacy higher than scFv/tPA [194]. Figure 7 illustrates this strategy.
Figure 7. . Strategies for targeting red blood cells via CR1.
(A) RBCs can be used to clear immune complexes by way of the interaction between complement protein C3b and CR1. (B) Bispecific antibodies to CR1 and pathogens can be used to capture pathogens via the RBC surface. (C) Antibody/drug fusions targeted to CR1 can be used to attach therapeutic moieties directly to the RBC surface.
RES: Reticuloendothelial system.
In addition to thrombolytic cargoes, multifaceted therapeutics for RBC carriage can also be envisioned. For example, soluble thrombomodulin (sTM) is a recombinant analogue of the natural anticoagulant and anti-inflammatory endothelial glycoprotein, which has shown encouraging clinical results [202]. The half-life of sTM in the circulation approaches several days after subcutaneous injection in mice, which exceeds the half-life of most of antithrombotic agents’ drug when given IV. Targeting of TM to carrier RBCs may enhance its longevity considerably. Studies in animal models showed that RBC-targeted sTM fused with scFv-anti-GPA: i) binds safely to RBC in the blood; ii) generates activated protein C (APC) from PC in the presence of thrombin; iii) has a half-life ˜100-fold longer than sTM after IV injection; and iv) prevents occlusive arterial and venous thrombi from forming at doses that are orders of magnitude lower than sTM [203]. TM and APC have multifaceted antithrombotic and anti-inflammatory activities and RBC-targeted scFv/TM may find therapeutic and prophylactic utility in disorders characterized by such intertwining pathologies.
Potential problems related to RBC coupling strategies
Targeting drugs to circulating RBCs may be hampered by undesirable side effects, such as to reduction of RBC biocompatibility. For example, excessive binding of scFv-fusions, despite their monovalency and inability to cross-link binding sites, may impose tension or conformational changes in the target molecule or otherwise affect its interaction with membrane elements including the RBC cytoskeleton. Studies of the effect of scFv/fusions on circulation of RBC have thus far not revealed harmful effects that would manifest in accelerated RBC clearance within the observed time frame [194,201].
On the other hand, drug molecules coupled to RBC may interact with other cells, such as endothelial cells, and other blood components, such as platelets and leukocytes, causing undesirable perturbations of the vascular system. Further, the PK and tissue distribution of RBC-coupled drugs may change so dramatically in comparison with free drugs that unanticipated off-target effects may occur.
In theory, molecules coupled to RBC surface may also modulate immune reactions and alter immunogenicity of administered fusion proteins, which suggests utmost caution in using this approach for multiple administrations. Humanization of targeting ligands (See section ‘New horizons: molecular engineering of affinity ligands for RBC targeting’, below) is likely to be important in minimizing such effects. Of note, just a single dose injection would be expected in many applications outlined above – stroke and other types of ischemia, sepsis, ALI/ARDS, acute thrombosis and bleeding. This lessens, although does not eliminate, these immunological concerns. Furthermore, recently, Jeff Hubbell's group showed that coupling to antigens fused to TER119 on RBCs confers immunological tolerance [204]. This result has dual importance. First, it opens a way to modulate immunological tolerance to selected antigens. Second, it implies that other scFv/cargoes targeted to RBCs may induce tolerance, thereby attenuating a potential concern over immune reactions that would preclude multiple administrations.
Choosing target binding sites on carrier RBCs
Currently, only a few of diverse RBC surface determinants have been assessed for their suitability as sites for drug targeting. The choice of target might alter dosing based on variable expression levels, intravascular kinetics and/or transfer to sites of action. Clinical disorders of RBC membranes highlight the importance of maintaining RBC membrane integrity, deformability, tensile strength and resistance to immune destruction when designing strategies to implement them as drug carriers. Indeed, if the long circulation time of RBCs is key to efficacy as a drug carrier, then perturbations of membrane integrity that shorten circulation time in vivo, as observed in nearly all RBC loading strategies to date (see section ‘Introduction: red blood cells as carriers for vascular drug delivery’, above), may be undesirable in some clinical settings. Care must also be taken to choose membrane determinants for which occupancy by targeting agent or cargo would not disrupt membrane integrity, fluidity, or deformability. In this section we provide a brief theoretical analysis of potential RBC determinants that may represent ‘good’ targets for RBC drug delivery.
The ideal RBC target would be an epitope present in nearly all populations and one that does not subsume an important physiologic function. Identification of potential RBC membrane targets is facilitated by over a century's worth of clinical experience in transfusion medicine, which provides a wealth of knowledge about the function, variability and prevalence of diverse red blood cell antigens in various ethnic groups [205,206]. The biologic impetus for the development of blood group polymorphisms is thought to involve population founder effects and natural selection based on susceptibility to infectious organisms, such as malaria [207].
Polymorphic antigens, such as the Rh antigen, have been identified based on alloantibodies arising in transfused patients. Alloantibodies put individuals at risk for hemolysis and hemolytic disease of the fetus and newborn, and highly polymorphic antigens are relatively poor choices as targets for drug delivery. In rare instances, however, alloantibodies recognize well-conserved sites on antigens that are widely expressed across the population. While these ‘high-frequency’ targets present a challenge with respect to transfusion, they also represent the proteins most likely to be of utility as broadly applicable drug targets [206].
Here, we examine some of the high-prevalence RBC targets in order to highlight the features that may make the ligand desirable or undesirable for a given drug delivery approach. Furthermore, because the targeting of some RBC determinants could theoretically perturb RBC physiology and affect their safety profile as drug carriers, the role of these proteins in normal RBC physiology is described herein. Table 1 briefly summarizes the most pertinent information. We provide below a digest of structural-functional and immunological features of RBC proteins that might be relevant to their suitability for drug carriage.
Rh & RhAG family
RhD was first recognized as a critical immunogen in hemolytic disease of the fetus and newborn over 70 years ago [208]. Originally described by Landsteiner and Wiener [209], the initial identification of a Rh (‘Rhesus’) factor was a result of the finding that antiserum from guinea pigs and rabbits immunized with blood from the rhesus macaque would also agglutinate human RBCs. This was, in fact, a misnomer as the antibody described was actually detecting a protein now known as LW (or ICAM4), an antigen that happened to be present in greater numbers on RhD+ RBCs.
Nevertheless, the family continues to be known by the collective term Rh, and includes the RhD, the closely related RhCE (also known as RhCcEe) and, in some classifications, the RhAG proteins, although the latter is now been designated as a separate blood group [210,211]. Rh proteins are approximately 30–40 kDa in size, and are part of multiprotein complexes on the RBC membrane (see Figure 2). In these complexes, particularly within band 3/ankyrin complexes, Rh proteins contribute to the mechanical integrity of the RBC membrane. The Rh associated glycoprotein, RhAG, is thought to represent the ancestral precursor to the RhD and RhCE proteins, and is essential for their expression on the RBC surface [212,213]. There are approximately 100,000–200,000 copies of RhD and RhCcEe per RBC, with minimal expression on other tissues [214], a desirable feature for specificity of RBC carriage. Non-erythroid homologues have, however, been identified [215].
Although the remarkable polymorphism of the RhD and RhCcEe proteins [216] limits their utility as target antigens for drug delivery, some Rh family member epitopes, such as Rh17 (also known as Hr0) and Rh29 [217], are present on nearly all human RBCs with the notable exception of the rare Rh-null phenotype that occurs in approximately 1 in 6 million individuals [218]. High-frequency epitopes have also been identified in the RhAG protein, for example, the Duclos antigen [219]. Recently, Rh family proteins have been implicated in ammonium transport across the cell membrane [220–222]. The Rh-null phenotype, which can arise from both mutations at the Rh locus itself (amorph type) and mutations at regulator sites such as RhAG (regulator type), is associated with a chronic hemolytic anemia and spherostomatocytosis, demonstrating that the activity of Rh family proteins is indispensable to normal RBC physiology and that significant inhibition should be avoided when designing a particular targeting approach.
Kell family
The Kell protein is a single-pass membrane glycoprotein present in low to moderate numbers on the RBC membrane [223]. It is thought to function as an endothelin-3-converting enzyme and has several high-frequency epitopes including the k antigen, also known as the Cellano antigen, present in >99% of individuals, as well as Kp(b) and Js(b). Deficiency of Kell proteins is known as the McLeod phenotype, manifested by acanthocytosis, elevated serum creatine kinase and neurologic disorders [224]. The Kell family may be a useful candidate for RBC targeting when low copy numbers are desired on the RBC surface. However, the expression of Kell protein on other tissues, including bone marrow, fetal liver, testes and parts of the brain, may limit its utility.
Band 3
Band 3, also known as anion exchanger 1 constitutes approximately 20% (by mass) of all RBC membrane proteins [225]. It is a transmembrane protein present both in isolation and anchored to the membrane cytoskeleton via ankyrin and protein 4.2 complexes. These complexes are central to maintaining ‘vertical’ linkages to the spectrin skeletal network [226] as well as mediating anion exchange. As previously noted, variants can result lead to spherocytosis and ovalocytosis [227].
The band 3 protein shows few polymorphisms, although some high-prevalence antigens (the Diego blood group [228]) have been described. This group includes Di(a), Di(b), Wr(a), Wr(b), among several others. The abundance of band 3 proteins on the RBC surface (>10⁁6 copies/cell) make it them an attractive target for RBC coupling. The critical importance of this protein in maintaining RBC membrane integrity and homeostasis suggests the need for caution, as does the expression of band 3 proteins on portions of the kidney. These potential downsides may be mitigated by the very high copy number on RBCs, such that in theory it would only be necessary for a therapeutic construct to occupy a small fraction of total available sites to be efficacious.
Glycophorin family
The glycophorin proteins are type I membrane bound sialoglycoproteins that function as receptors for complement, chaperones for band 3 transport to the RBC membrane and contribute negative charge to the RBC glycocalyx through sialyic acids. These proteins express the MNS blood group system [229]. The two main isoforms are glycophorin A (GPA), expressed at approximately 800,000 copies/RBC and glycophorin B, expressed at approximately 200,000 copies/RBC. GPA is part of the band 3/ankyrin complex, critical to membrane structure. GPA is among the most commonly utilized markers of erythrocytic lineage and a number of antibodies and antibody derivatives to this antigen have been described [198,230–231]. Glycophorin B contains the U epitope, designated as such due to its near ‘universal’ expression among individuals and across ethnic groups. Given their high expression levels and apparent lack of significant enzymatic activity, glycophorins are attractive candidates for RBC targeting. Success in anchoring recombinant therapeutic fusion proteins to GPA has been reported by our group [194,203,232]. The expression of glycophorin A on renal epithelium and renal endothelium will need to be considered in long-term safety analyses.
Glut1
The Glut sugar transporters are a family of 14 isoforms of transmembrane proteins that serve to transport sugars into cells. The Glut1 isoform (also known as band 4.5) is the main functional transporter of glucose in human RBCs and lymphocytes. RBCs express the highest level of the Glut1 transporter among cell lineages (greater than 200,000 copies per cell). Glut1 is found in both band 3/ankyrin complexes and band 3/junctional complexes [233], and shows little polymorphic variation across populations. However, Glut1-deficient individuals manifest a severe clinical phenotype characterized by infantile onset seizures, delayed neurological development, movement disorders and acquired microcephaly [7]. This raises concern that Glut1 will not tolerate physicochemical alteration if used as a target. Glut1 is also found on many tissues in addition to RBCs.
Dombrock blood group system
The Dombrock blood group system (Do) includes two antithetical antigens (Do[a] and Do[b]) and several other high-frequency antigens including Gy(a), Hy, Jo(a), DOYA and DOMR. These antigens are found on the Dombrock glycoprotein, a member of the mono-ADP-ribosyltransferase family that is attached to the RBC membrane via a GPI anchor, although it so far has no demonstrable enzyme activity in RBCs [234]. However, due to the rarity of the Dombrock-null phenotype, little is known about clinical manifestations due to deficiencies or disruption of function. The Do glycoprotein is thought to be expressed primarily on erythroid cells in adult bone marrow and in fetal liver.
Other high-prevalence antigens
Several other high-prevalence antigens have been identified on the RBC membrane based on clinical serologic testing, including Vel (SMIM1 protein [235,236]), Lan (an erythroid ATP binding cassette transporter ABCB6 [237]), At(a), Jr(a) and Emm antigens, among others. Characterization of the protein families to which these antigens belong is an area of ongoing research.
Modification of RBC as ‘universal donor’ cells
Immunological reactions to foreign RBC are of primary concern in transfusion medicine, as they can lead to cell agglutination or hemolysis, multiorgan failure, disseminated intravascular coagulation, shock and death. Antibodies to A and B blood group antigens are naturally occurring (predominantly IgM) antibodies in those who lack these carbohydrate-derived epitopes. Therefore, in order to avoid the severe intravascular hemolysis induced by these antibodies, the ABO system is routinely typed and matched when a transfusion is performed (with O units administered when ABO blood type cannot be determined). In addition, the RhD antigen is strongly immunogenic in RhD negative recipients (about 15% of the population), and antibodies to RhD are an important cause of hydrops fetalis and hemolytic disease of the newborn. Therefore, this antigen group is also routinely typed and matched in transfusion medicine. However, other non-ABO/RhD antigens (such as C, c, E and e, also within the Rh family but found on the RHCE gene product) are considered less immunogenic and are not routinely typed and matched.
Naive recipients generally tolerate (i.e., a symptomatic transfusion reaction does not occur) a single transfusion of blood mismatched for most blood group antigens other than ABO, as naturally occurring antibodies to these non-ABO antigens are rare. This contrasts with recipients who have been multiply transfused (e.g., people who suffer from sickle cell anemia, β-thalassemia or hematologic malignancies), or after pregnancy, who may develop alloantibodies to minor (non-ABO/RhD) antigens [238,239]. Allosensitization is especially common in the background of incongruence between the donor pool (predominantly Caucasian in the USA) and the recipient (e.g., African–American patients with sickle cell disease).
Extended matching protocols offers promise, but has not eliminated alloimmunization or its threat [216]. The chronic shortage of matched blood groups motivates attempts to modify RBCs in ways that would make them compatible across blood groups. Several approaches have been used to attempt to eliminate incompatibility. The first used was the addition of neuraminidases to hydrolyze the sialic acid residues from RBC glycoproteins. However, these RBCs were removed from circulation immediately after transfusion [240]. Specific exoglycosidases have been used to convert blood types B and A to type O. Studies by Lenny et al. [241–243] have shown that an exoglycosidase from green coffee bean is able convert blood type B to type O at pH 5.5 by removing the relevant terminal sugar. These RBCs appeared to survive normally in all recipients. Kruskall et al. utilized a recombinant form of this enzyme, which was successful in Phase II trials [244]. However, conversion of blood type A to type O has proved to be more challenging. Alpha-N-acetylgalactosamindase, a 72kD exoglycosidase degrades human blood A epitope, but the efficiency of conversion was low [245–253]. More recently, bacterial glycosidases that remove both A and B antigens efficiently have been identified [254], renewing interest in this approach, and clinical trials of enzyme-converted RBCs are in progress [255].
PEG-modification of RBC
An alternative approach to enhance compatibility is to camouflage antigenic RBC epitopes or targeting sites by covalent attachment of a long chain neutral hydrophilic nontoxic polymer such as PEG to its surface [256]. As described above, covalent attachment of PEG is commonly used to modify proteins, drugs, liposomes and artificial surfaces that come into contact with blood, prolonging their circulation times in addition to ‘repelling’ antibodies and phagocytes [1]. PEG, an extremely flexible and hydrophilic polymer composed of repeating units of ethylene oxide, creates a ‘sphere of hydration’ barrier in an aqueous environment that prevents binding of cells and antibodies to RBCs, essentially camouflaging surface charges. In addition to such ‘immune-camouflaging’ effects, conjugated PEG may improve rheological features of the donor RBCs, for example, by lowering their viscosity and propensity to aggregate at low shear rates [257].
In theory, both increasing the surface density of PEG molecules and their length should enhance the camouflaging effect, but excessive modification may inactivate cell-surface proteins. Alternative chemical approaches to conjugate PEG to RBC surfaces have been developed to avoid negative impact of cell modification. One of the first alternative polymers to be developed is methoxypoly(ethylene glycol) (mPEG). Unlike PEG, mPEG contains a terminal methyl group (-CH3) instead of a hydroxyl (-OH), which significantly reduces the potential for peroxide formation. Conjugation of mPEG (˜5 kD) to lysine residues on RBC surfaces reduces immunologic recognition of surface antigens. mPEG-derivatived mouse RBCs exhibited normal in vivo survival (˜50 days) with no sensitization after repeated transfusions [258–260].
Variations of PEG, such as linear, branched and development of bifunctional chains have been used [261,262]. Rossi et al., 2010 explored the conjugation of 3 kD to 101 kD hyperbranched and multifunctional polyglycerols (HPG) onto RBCs by reacting with primary amines on the cell surface [263]. Liu et al. modified HPG with C18 alkyl chains and mPEG (HPG-C18-PEG) [264]. Adsorption of HPG-C18-PEG onto human RBCs was mediated by hydrophobic interactions between C18 chains and the lipid membrane. In saline, and the maximum amount of HPG-C18-PEG adsorbed onto RBCs was greater than in plasma, due to the interactions between the polymer and plasma proteins (e.g., albumin). Janvier et al. reported that the use of amphiphilic star-shaped PEG polymers (four chains of PEG) with a cholic acid core with masses between 10–16 kDa efficiently reduced RBCs aggregation [265]. Unlike Pluronic F68, a PEG-PPG-PEG triblock copolymer that can also decrease RBC aggregation, the use of cholic acid as the core group did not induce any adverse reactions. Studies by Moghimi et al. and Szebeni et al. suggested that these adverse reactions from Pluronic F68 are associated with the PEG-PPG component of the polymer [266,267].
Other polymers such as polyethyloxazoline propionic acid (PEOZ) have been recently studied for their ability to ‘immunocamouflage’ cells. PEOZ polymers exhibit similar chemical, physical and immunological characteristics as PEG (e.g., low immunogenic potential, low toxicity) [268]. However, mPEG significantly improved the immunocamouflage efficacy of RBCs compared with PEOZ. mPEG and PEOZ can also camouflage unmodified antigens (indirect camouflage) found in RBC membrane protein complexes.
At this time, RBC modification by PEG and other polymers for immune camouflaging and rheological improvement of donor blood remains a promising avenue, but its clinical utility for transfusion medicine remains to be meticulously tested. This especially true in light of findings that PEG coating may inhibit the self-signaling function of CD47 on RBC surfaces and that humans may have natural antibodies to PEG [269].
RBC carriage of nanocarriers & engineered nanocarriers imitating RBCs
Fast clearance from the bloodstream is one of major factors limiting the utility of nanoparticles (NPs) for drug delivery. In theory, nondamaging coupling to RBCs may prolong the lifetimes of NPs in the circulation. Indeed, noncovalent adsorption onto RBC (‘piggybacking’) prolonged the circulation of NPs compared with free NP counterparts without detectable changes in RBC circulation; after several hours NPs detached from carrier RBCs, likely due to shear force and interactions with vascular cells, and eventually were taken up by the liver and the spleen [270]. More recently, it was reported that NPs noncovalently attached to RBC transfer from circulating carrier cells to vascular endothelium, which is manifested by the transient accumulation of NPs, but not RBCs, in the pulmonary vasculature [271].
Several laboratories are pursuing synthesis of polymeric drug carriers imitating RBC features, including elasticity [272–274]. Mitragotri and his team have created ‘artificial RBCs’, mimicking size, shape and elasticity of RBCs, which have oxygen carrying abilities (in vitro) and can squeeze through micron-size tubes. These artificial RBCs were also able to absorb heparin and release it continuously over a period of days in vitro. Discher and coworkers decorated the surfaces of polymeric nanocarriers with CD47 and peptides derived from this ‘self’-semaphoring glycoprotein, which is exposed on RBC surface and signals via specific receptors on host defense cells that the particle should not be taken up [275]. The ultimate test of artificial RBC and CD47-decorated polymeric carrier will come from experiments that show directly whether the behavior of these carriers is similar to RBCs in vivo.
New horizons: molecular engineering of affinity ligands for RBC targeting
As discussed in previous sections, one ‘ideal’ formulation for targeting drugs to RBC surface would comprise recombinant proteins consisting of drugs fused to antigen binding single-chain variable region antibody fragments (scFv). In most studies performed to date, the scFv was derived from a murine hybridoma. Oligonucleotide primers specific to the particular antibody's heavy and light chain variable regions are amplified by PCR to construct the scFv fragment of the hydridoma's original full-length IgG molecule [204].
For clinical use, the scFv should be human in origin to minimize immunogenicity. This is important even if multiple administrations are not anticipated because the prevalence of interfering heterophilic human antimouse antibodies (HAMAs) in the general population may typically be approximately 10% and as high as 80% in individuals exposed to therapeutic agents containing murine blood proteins [276,277]. The human scFv should be specific to a highly conserved nonpolymorphic target present on RBCs of all potential recipients.
These requirements present a conundrum. First, by definition, it will be difficult to identify a donor from whom one might attempt to clone an antibody to an antigen he lacks that nearly everyone else in the population expresses. Second, immunizing that individual with RBCs of common phenotype for the purpose of generating a source of antibody cDNA would be ethically unacceptable, even with donor consent. Alloimmunization of this individual to a commonly expressed RBC antigen could have serious health consequences in the future because cross-match-compatible blood would be nearly impossible to find in the event that individual required a transfusion. Third, even were such an individual available, cloning human peripheral blood B cells in vitro to provide the starting materials for scFv construction using conventional hybridoma approaches is problematic due to the absence of a suitable human myeloma fusion partner.
Alternatively, one could attempt to fuse the donor's cells with a mouse myeloma cell line to create a pool of heterohybridomas from which the desired anti-RBC alloantibody could be isolated. Unfortunately, human–mouse chimeras are unstable and often die or stop secreting antibodies before antigen-specific clones can be identified. Immortalizing human B cells by infection with Epstein–Barr virus is inefficient and selectively immortalizes lower affinity IgM antibodies [278]. Compounding the technical inefficiencies in transformation is the practical difficulty in obtaining sufficient numbers of human B cells from peripheral blood, as the spleen is the preferred source for production of monoclonal antibodies from mice.
To circumvent these obstacles, molecular methods have been developed to isolate human scFv fragments. Collectively referred to as ‘repertoire cloning’ or ‘antibody phage display’, such approaches immortalize the immunoglobulin genes, rather than the B cells from which the immunoglobulin genes were derived.
Phage display technology
Antibody phage display has been the subject of a number of recent reviews [279–281] and several excellent laboratory manuals are available that provide step-by-step protocols [282–285]. Typically, M13 filamentous bacteriophage, virions approximately 7 nm in diameter and up to 1 μm in length, are employed. The genome of M13 phage contains a set of genes that encode several viral coat proteins, notably pIII for which there are 3–5 copies at one end of the phage. Seminal experiments by George Smith almost 30 years ago [286] showed that foreign DNA inserted into the M13 genome just upstream of the gene for pIII produced phage particles that displayed the protein encoded by the foreign DNA at the aminoterminus of pIII. Though the significance of this experiment was not fully appreciated at the time, Smith had succeeded in demonstrating the physical linking of an exogenous protein (via fusion with pIII) with the DNA required for its replication (via the phage DNA within).
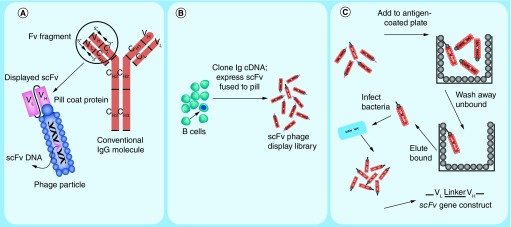
Methods have now been developed for expressing larger molecules, such as antibody scFv fragments, on pIII (Figure 8A). Starting with populations of peripheral blood B cells and using PCR to amplify their immunoglobulin heavy chain and light chain cDNA, it is possible to create large libraries of particles, each of which express an antibody fragment on its exterior and retained the DNA required for its replication in its interior (Figure 8B) [287,288]. Thus, antibody phenotype and genotype are linked, and such libraries can be panned on immobilized antigen to isolate and then amplify antigen-specific phage (Figure 9C). From the phage DNA, the antibody's scFv gene segment can be isolated directly.
Figure 8. . Creation and selection of antibody phage display libraries.
(A) Cartoon depicting a prototypical IgG immunoglobulin and corresponding antibody-displaying filamentous phage particle. IgG comprises a heavy chain variable region (VH) and 3 constant region domains (CH1, CH2 and CH3). The IgG light chain comprises a variable region (VL) and one constant region domain (CL). An Fv fragment is defined as the combination of heavy and light chain variable regions only (VH + VL). For simplicity in recombinant expression of Fv fragments, the VH and VL domains can be expressed as a single polypeptide separated by a short flexible glycine/serine-rich linker referred to as a single-chain Fv (scFv). Arrows on the IgG variable region represent the positions of forward and reverse polymerase chain reaction primers that are used to amplify the heavy and light chain gene segments. A second PCR step links the VH and VL into one genetic construct that can ligated into a phagemid expression vector that appends the gene for the phage pIII coat protein to the carboxy terminus of the scFv as depicted on the phage particle. (B) Cartoon depicting the construction of an antibody phage display library from a pool of B lymphocytes. Phage particles each express an scFv on their surface that is encoded by the DNA contained within the particle. Arrows indicate a potential correspondence between a particular B cell and a phage particle displaying that B cell's immunoglobulin. (C) Schematic representation of phage panning procedure in which an aliquot of phage library is incubated with immobilized antigen and adsorbed phage are eluted and amplified in bacterial culture (see text for details).
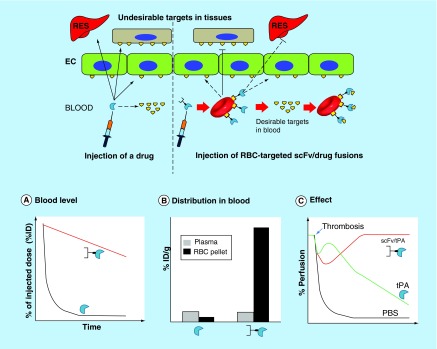
Figure 9. . Red blood cell targeting by fusion proteins.
Schematic of RBC drug delivery using engineered antibody/drug fusions and representative illustrations of the expected effect on: (A) blood level, (B) distribution within the blood and (C) therapeutic efficacy over time.
EC: Endothelial cell; PBS: Phosphate buffered saline; RBC: Red blood cell; RES: Reticuloendothelial system.
For the purposes of drug delivery, phage libraries can be selected or ‘panned’ on intact RBCs [289–292] (Figure 10A) to obtain cell-surface binding phage-displayed scFv (Figure 8B). A distinct advantage conferred by screening of antibody repertoires displayed as scFv on phage versus screening of full-length bivalent IgG's secreted by hybridomas is that one directly select for antibodies that bind well in a monovalent form and that fold correctly in the absence of immunoglobulin constant regions, properties that would be required by an scFv-containing RBC drug targeting agent. This is an important because the ability to convert any given hybridoma-derived bivalent IgG to a monovalent scFv that retains the properties of its parent IgG cannot be taken for granted.
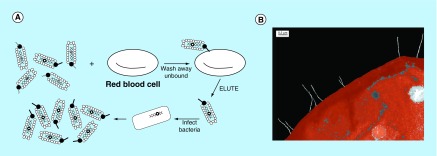
Figure 10. . Selecting anti-red blood cell antibodies from phage display libraries.
(A) Red cell-specific phage can be isolated by panning on intact cells. To obtain phage antibodies to common red cell determinants, sequential rounds of panning each with a different donor source of red cells can be performed. (B) Colorized transmission electron micrograph of anti-red cell Rh-expressing phage particles (white filaments) bound to the surface of a human Rh-positive red cell. Binding occurs at the tips of the phage particles where the scFv/pIII fusion protein is located. Original magnification × 44,000. Bar, 0.2 µm.
Adapted with permission from [292].
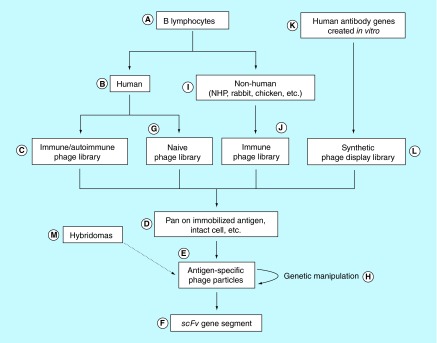
A general approach for the use of phage display to develop scFv for RBC targeting is presented in Figure 11. The most direct route for obtaining a phage display library from which a human red cell panreactive scFv could be isolated, would be to start with peripheral blood lymphocytes (A) from a human donor (B) alloimmunized to a common red cell membrane determinant and to construct an ‘immune’ phage library (C). Once in hand, the library could be panned (D) on purified, immobilized target protein (Figure 1C) to which the individual was immunized (either serendipitously or purposely) including those antigens described in the previous section. Alternatively, such a library could be selected using sequential rounds of panning on intact human RBCs (Figure 2A) each time alternating with antigen-negative RBCs of other species. This, by definition, would yield phage clones that react with common human RBC phenotypes. After completing several rounds of panning (typically 4), the resultant polyclonal phage preparations can be assayed for RBC reactivity using an anti-M13 antibody indirect agglutination reaction (indirect ‘Coombs test’) [291]. Titering of phage can then provide a set of monoclonal phages that can be individually assayed for binding by agglutination (E), and scFv DNA can be isolated from the phage clones that are positive (F) [290].
Figure 11. . Overview of antibody phage display technology for the isolation of scFv immunoglobulin fragments.
Though obtaining peripheral blood lymphocytes from such an immunized source is likely to be difficult for the reasons discussed above, phage display technology offers a number of other options. For example, phage display libraries can be constructed from patients with warm-type (IgG) autoimmune hemolytic anemia, which typically bind to determinants generally present on all human RBC [293]. Alternatively, naive human phage display libraries (G) can be panned on human RBC antigens directly (D). Naive phage libraries are libraries constructed from pools of peripheral blood B cells collected from nonimmunized healthy donors [294,295]. Naive libraries are designed to exploit the observation originally made with hybridomas that IgM antibodies that have not undergone somatic mutation can recognize a variety of antigens. The advantage of these libraries is that a single ‘universal’ library could be constructed and used to provide monoclonal antibodies with any desired specificity. Though the affinities of the antibodies obtained from such nonimmunized libraries may be suboptimal, a number of methods for ‘in vitro affinity maturation’ of the initial positive phage clones are available (H). Chemical mutagenesis, use of mutagenic strains of bacteria, incorporation of degenerate oligonucleotides, or error-prone PCR can be used to generate random or targeted nucleotide mutations in antibody gene segments [296,297].
Alternatively, panreactive antibodies to human RBCs can be obtained from immune phage libraries (J) constructed from nonhuman primates, rabbits, chickens, etc. (I), and ‘humanization’ protocols can be employed to reduce the immunogenicity of the scFv (H) [298,299]. In contradistinction to immune or naive libraries derived from B-cell immunoglobulin mRNA, in vitro generated human antibody gene segments (K) can be used to create so-called ‘synthetic’ phage display libraries (L). Such libraries are generally constructed by starting with one or more human scFv's of any specificity, and the polymerase chain reaction (or other strategy) is used to randomize the heavy and light chain hypervariable regions to create a collection of as many possible different antibodies as can be reasonably grown in bacteria [300,301]. Though antibodies isolated from such synthetic repertoires may also suffer from suboptimal affinity and require in vitro affinity maturation as described above, a significant advantage over immune and naive libraries is that the antibody composition of synthetic libraries is not constrained by the mechanisms at play in vivo that normally prevent the generation of antibodies to human self-antigens. Therefore, the synthetic phage library approach inherently possesses the ability to provide ‘human antibodies’ to common human antigens.
Lastly, phage display can be combined with conventional hybridoma technology (M) to convert a murine monoclonal antibody into a human-like antibody that retains the original binding specificity of the mouse antibody. These methods termed ‘guided selection’ or ‘epitope imprinting’ [302,303] start by cloning the hybridoma heavy and light chain variable regions into a scFv construct expressed on phage. This is followed by replacement of the murine light chain with a library of human (naive) light chains and selecting the new chimeric phage library on the desired antigen. In parallel, the light chain from the original murine antibody is paired with a repertoire of random human heavy chains, displayed on phage and similarly selected on the antigen. Finally, a set of ‘guided’ human heavy and light chains ‘selected’ from each human/mouse chimeric library are combined to form a third phage library (now completely human) that is panned on the target antigen. In vitro affinity maturation of the ‘best binder’ can then be used to generate a high affinity scFv that is a human representation of the original murine antibody.
Conclusion
RBCs are natural carriers with considerable potential for intravascular drug delivery. Surface loading (i.e., affinity targeting of drugs to the RBC surface) is an attractive alternative to encapsulation of drugs into carrier RBCs, as it avoids cell trauma and the need for ex vivo manipulations and reinfusion. Surface loading can also be used in tandem with transfusion of blood and blood products, already a widely used and generally safe therapeutic intervention. Chemical and recombinant approaches to drug conjugation with affinity polypeptides, such as scFvs and smaller peptides, enable RBC surface targeting to diverse epitopes. These epitopes include those present across diverse human subpopulations over a wide range of surface density, from thousands to millions of binding sites per RBC. Phage display technologies have enabled derivation of a broad array of engineered targeting ligands to desired targets.
RBC carriage alters pharmacologic features of drug cargoes in many key ways including pharmacokinetics (PK), biodistribution (BD), restriction of freedom to interact with cellular and molecular counterparts (even those localized in the vascular space, such as endothelial receptors), and masking of bound drugs by the RBC glycocalyx (thereby preventing inactivation by plasma inhibitors). In many cases, the changes in PK and BD prolong drug circulation and retention in the vascular compartment for sufficient time to cause a fundamental change in efficacy and, ultimately, clinical utility. However, several other factors, such as redistribution of drugs from plasma to the cellular fraction of blood, have myriad additional effects. For example, focal concentration of drugs on the surface of micron-size particles (i.e., the RBC plasma membrane) provides a new microenvironment for a drug and may serve as a natural assembly template or a catalyst for the activities of many biotherapeutic agents. In disorders such as sickle-cell disease, transfusion reactions, paroxysmal nocturnal hematuria, malaria and other maladies involving RBCs, the RBCs represent both the carrier and target for therapeutic interventions.
Future perspectives
Drug carriage by RBCs offers the potential to improve therapeutics and prophylactic interventions including modulation of host defense and immune responses, detoxification or elimination of toxins and pathogens in blood, enzyme replacement therapies, amelioration of inflammation and preventing and management of thrombosis. Although some of these potential uses have been characterized in vitro and in animal models, the next decade should see the clinical translation of these agents. We postulate that RBC-targeted pharmacotherapy, particularly strategies aimed at surface loading of therapeutics, will improve medical management of important pathological conditions, especially those manifested primarily within the vascular space. Advances in ex vivo cellular genetic engineering may also ultimately enable production of more sophisticated, multifunctional erythrocytes capable of drug delivery and multistep mechanisms of action [304]. Drug delivery by coupling to erythrocytes offers a new and exciting class of cellular and biomolecular therapy. While it is tempting to speculate that this approach will see successful clinical translation, at this stage it remains premature to anticipate timelines for milestones such as industrial production and clinical testing. These important steps will inevitably require joint translational efforts of academia and pharmaceutical partners.
Key terms.
Erythrocyte: The mature red blood cell (RBC). The human erythrocyte is an enucleated ˜6–8 um biconcave discoid cell central to systemic delivery and exchange of the blood gases oxygen and carbon dioxide.
Transfusion reaction: The clinical manifestation of incompatibility between a donor blood product and the recipient. Reactions include, but are not limited to, antibody mediated processes such as hemolytic transfusion reactions, and allergic reactions.
Complement: A component of the innate immune system involved in clearance of pathogens from the host. The complement systems consists of a number of proteins that ultimately produce a cascade of proteolytic cleavages and assembly of multi-protein effector complexes such as the membrane attack complex.
Thrombosis: A series of regulated enzymatic steps ultimately resulting in production of fibrin and generation of a thrombus, or blood clot.
Red blood cell antigen: A surface RBC protein epitope that has been demonstrated to generate an antibody response when administered to a host lacking the given protein epitope.
Antibody fragments: Engineered immunoglobulins, such as single chain variable fragments (scFv), that maintain their antigen specific binding while altering other parameters (for example, size, valency, or elimination of the constant region, Fc).
Phage display: A technique that utilizes bacteriophages to display a diverse library of proteins on their surface such that the proteins can be directly linked to the genetic information that encodes them.
Bacteriophage: A virus that infects bacteria and that is commonly used in phage display techniques (particularly phage M13) to present proteins on the viral particle surface by fusion to viral coat proteins (such as pIII).
Executive summary.
Introduction
Effect of therapeutics is limited by inadequate pharmacokinetics and biodistribution.
Conjugation of masking polymers prolongs circulation of macromolecular drugs and drug carriers.
Red blood cells (RBCs) may be used as long-circulating drug carriers (100-120 days in humans).
Early approaches focused mostly on loading of drugs within the RBCs.
An alternative approach is loading of drugs onto the RBC surface.
RBC features as drug carriers
RBCs membrane structure provides both high tensile strength and deformability due to a network of linkages between membrane protein complexes and the underlying actin-spectrin lattice.
RBC membrane defects cause pathological RBC phenotypes.
The reticuloendothelial system (RES) is responsible for clearance of senescent or damaged RBCs.
The innate defense system of complement can also target RBCs with altered surface proteins.
RBCs surface proteins control complement and prevent RBC destruction by the host.
The natural uptake of damaged RBCs to the RES system has been exploited to delivery diverse therapeutics to the RES organs such as the liver.
Coupling therapeutics to the RBC surface: prototype studies
Coupling drugs to the RBC surface may bypass issues related to drug encapsulation such as decreased membrane integrity.
Early strategies included non-specific chemical cross-linking to RBCs and insertion into RBC membrane.
Plasminogen activators were among the first therapeutics anchored to the RBC surface in a biocompatible fashion providing improved fibrinolytic interventions in animal models.
Targeting therapeutic agents to circulating RBC
Initial attempts to targeting circulating RBC involved anchoring to complement receptor 1 (CR1).
Anti-CR1 conjugates have been used to capture bacterial and viral pathogens, as well as pathogenic antibodies.
Anti-CR1/pathogen complexes can be eliminated by the immune system without damage to the carrier RBC.
Advances in recombinant protein engineering have enabled design of monovalent antibody fusions lacking Fc domains that avoid Fc mediated side effects and unintended cross-linking of RBC targets.
Engineered single chain variable fragment (scFv) fusions have been used to restore CR1 on CR1 deficient RBCs, deliver complement protective proteins, deliver anti-thrombotic plasminogen activators and deliver multi-faceted agents such as thrombomodulin.
Coupling to RBC may modulate immunogenicity and confer immunologic tolerance to certain antigenic cargoes.
Choosing target binding sites on carrier RBCs
The field of transfusion medicine has provided a wealth of information on RBC antigens, their prevalence and function.
Ideal RBC surface targets should be conserved across the population.
Potential coupling sites include Rh proteins, band 3, Kell, glycophorins, GLUT1, and other high-frequency epitopes.
Modification of RBC as ‘universal donor’ cells.
Immunologic reactions to allogenic red blood cells is a challenge in transfusion medicine.
Transfused RBC units are typically matched for ABO antigens because these antibodies are naturally occurring, as well as RhD due to its immunogenicity and association with hydrops fetalis and hemolytic disease of the newborn.
In chronically transfused patients, alloantibody responses often limit available compatible donor RBC units.
Extended matching protocols are difficult to implement in part due to the limited donor RBC supply.
Several strategies have been devised to minimize alloantibody responses and improve biocompatibility including enzymatic modification of allogenic epitopes on the RBC surface and grafting of hydrophilic polymers for immune ‘camouflaging’ and rheological modulations.
New horizons: molecular engineering of affinity ligands for RBC targeting
Clinically translatable RBC-targeted ligands should be human in origin, or humanized, to minimize immunological issues.
Bacteriophages (typically M13) can be used to ‘display’ foreign proteins on their surface by fusion to viral coat proteins (typically pIII).
Phage display has enabled physical linking of an exogenous protein with the DNA required for its replication.
It is possible to create ‘libraries’ of phage particles from populations of peripheral blood B cells by using PCR to amplify their immunoglobulin heavy chain and light chain cDNA.
Phage libraries can be selected or ‘panned’ on intact RBC to obtain cell-surface binding, antigen-specific, phage-displayed antibodies, including engineered derivatives (such as scFv) and peptides.
There are practical and ethical barriers to preparation of phage libraries from donors alloimmunized to high-frequency antigens.
Phage display can be used to prepare (and affinity mature) libraries from naive donors, as well as from immunized non-human primates.
Phage display can be used to convert mouse monoclonal antibodies to human-like antibodies.
Conclusion
Drug delivery by coupling to erythrocytes is an attractive alternative to internal drug loading.
Coupling drugs to affinity ligands by genetic fusion is likely to benefit from advantages of phage display and other evolving technologies.
RBC carriage alters pharmacokinetics, biodistribution and other features of drugs that are likely to fundamentally alter their mechanism of action.
Future perspectives
Progress in coupling several classes of drugs to erythrocytes is likely to see clinical translation in the next decade.
Genetic methods to engineer cellular therapies promises to further broaden the scope of erythrocyte mediated drug delivery.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: •• of considerable interest
- 1.Abuchowski A, McCoy JR, Palczuk NC, van Es T, Davis FF. Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. J. Biol. Chem. 1977;252(11):3582–3586. [PubMed] [Google Scholar]
- 2.Dumez H, Reinhart WH, Guetens G, de Bruijn EA. Human red blood cells: rheological aspects, uptake, and release of cytotoxic drugs. Crit. Rev. Clin. Lab. Sci. 2004;41(2):159–188. doi: 10.1080/10408360490452031. [DOI] [PubMed] [Google Scholar]
- 3.Farrell CL, Pardridge WM. Blood–brain barrier glucose transporter is asymmetrically distributed on brain capillary endothelial lumenal and ablumenal membranes: an electron microscopic immunogold study. Proc. Natl Acad. Sci. USA. 1991;88(13):5779–5783. doi: 10.1073/pnas.88.13.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haspel HC, Stephenson KN, Davies-Hill T, et al. Effects of barbiturates on facilitative glucose transporters are pharmacologically specific and isoform selective. J. Membr. Biol. 1999;169(1):45–53. doi: 10.1007/pl00005900. [DOI] [PubMed] [Google Scholar]
- 5.Waite CL, Roth CM. Nanoscale drug delivery systems for enhanced drug penetration into solid tumors: current progress and opportunities. Crit. Rev. Biomed. Eng. 2012;40(1):21–41. doi: 10.1615/critrevbiomedeng.v40.i1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D, Pascual JM, Yang H, et al. Glut-1 deficiency syndrome: clinical, genetic, and therapeutic aspects. Ann. Neurol. 2005;57(1):111–118. doi: 10.1002/ana.20331. [DOI] [PubMed] [Google Scholar]
- 7.Yang H, Wang D, Engelstad K, et al. Glut1 deficiency syndrome and erythrocyte glucose uptake assay. Ann. Neurol. 2011;70(6):996–1005. doi: 10.1002/ana.22640. [DOI] [PubMed] [Google Scholar]
- 8.Armstead WM, Ganguly K, Kiessling JW, et al. Signaling, delivery and age as emerging issues in the benefit/risk ratio outcome of tPA For treatment of CNS ischemic disorders. J. Neurochem. 2010;113(2):303–312. doi: 10.1111/j.1471-4159.2010.06613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collen D, Lijnen HR. Thrombolytic agents. Thromb. Haemost. 2005;93(4):627–630. doi: 10.1160/TH04-11-0724. [DOI] [PubMed] [Google Scholar]
- 10.Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano. 2009;3(1):16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- 11.Muro S, Garnacho C, Champion JA, et al. Control of endothelial targeting and intracellular delivery of therapeutic enzymes by modulating the size and shape of ICAM-1-targeted carriers. Mol. Ther. J. Am. Soc. Gene Ther. 2008;16(8):1450–1458. doi: 10.1038/mt.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nordt TK, Bode C. Thrombolysis: newer thrombolytic agents and their role in clinical medicine. Heart Br. Card. Soc. 2003;89(11):1358–1362. doi: 10.1136/heart.89.11.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010;9(8):615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 14.Shuvaev VV, Ilies MA, Simone E, et al. Endothelial targeting of antibody-decorated polymeric filomicelles. ACS Nano. 2011;5(9):6991–6999. doi: 10.1021/nn2015453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magnani M. Erythrocytes as carriers for drugs: the transition from the laboratory to the clinic is approaching. Expert Opin. Biol. Ther. 2012;12(2):137–138. doi: 10.1517/14712598.2012.650163. [DOI] [PubMed] [Google Scholar]
- 16.Muzykantov VR. Drug delivery by red blood cells: vascular carriers designed by mother nature. Expert Opin. Drug Deliv. 2010;7(4):403–427. doi: 10.1517/17425241003610633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coller BS, Springer KT, Beer JH, et al. Thromboerythrocytes. In vitro studies of a potential autologous, semi-artificial alternative to platelet transfusions. J. Clin. Invest. 1992;89(2):546–555. doi: 10.1172/JCI115619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dale GL, Kuhl W, Beutler E. Incorporation of glucocerebrosidase into Gaucher's disease monocytes in vitro . Proc. Natl Acad. Sci. USA. 1979;76(1):473–475. doi: 10.1073/pnas.76.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muzykantov VR, Sakharov DV, Smirnov MD, Samokhin GP, Smirnov VN. Immunotargeting of erythrocyte-bound streptokinase provides local lysis of a fibrin clot. Biochim. Biophys. Acta. 1986;884(2):355–362. doi: 10.1016/0304-4165(86)90184-4. [DOI] [PubMed] [Google Scholar]
- 20.Ihler GM, Glew RH, Schnure FW. Enzyme loading of erythrocytes. Proc. Natl Acad. Sci. USA. 1973;70(9):2663–2666. doi: 10.1073/pnas.70.9.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alvarez FJ, Herráez A, Murciano JC, Jordán JA, Díez JC, Tejedor MC. In vivo survival and organ uptake of loaded carrier rat erythrocytes. J. Biochem. (Tokyo) 1996;120(2):286–291. doi: 10.1093/oxfordjournals.jbchem.a021411. [DOI] [PubMed] [Google Scholar]
- 22.Pérez MT, Alvarez FJ, García-Pérez AI, Lucas L, Tejedor MC, Sancho P. Heterogeneity of hypotonically loaded rat erythrocyte populations as detected by counter-current distribution in aqueous polymer two-phase systems. J. Chromatogr. B Biomed. Appl. 1996;677(1):45–51. doi: 10.1016/0378-4347(95)00433-5. [DOI] [PubMed] [Google Scholar]
- 23.Ktavtzoff R, Desbois I, Doinel C, et al. Immunological response to L-asparaginase loaded into red blood cells. Adv. Exp. Med. Biol. 1992;326:175–182. doi: 10.1007/978-1-4615-3030-5_21. [DOI] [PubMed] [Google Scholar]
- 24.Rossi L, Serafini S, Cappellacci L, et al. Erythrocyte-mediated delivery of a new homodinucleotide active against human immunodeficiency virus and herpes simplex virus. J. Antimicrob. Chemother. 2001;47(6):819–827. doi: 10.1093/jac/47.6.819. [DOI] [PubMed] [Google Scholar]
- 25.Tonetti M, Astroff B, Satterfield W, De Flora A, Benatti U, DeLoach JR. Construction and characterization of adriamycin-loaded canine red blood cells as a potential slow delivery system. Biotechnol. Appl. Biochem. 1990;12(6):621–629. [PubMed] [Google Scholar]
- 26.Kim S-H, Kim E-J, Hou J-H, et al. Opsonized erythrocyte ghosts for liver-targeted delivery of antisense oligodeoxynucleotides. Biomaterials. 2009;30(5):959–967. doi: 10.1016/j.biomaterials.2008.10.031. [DOI] [PubMed] [Google Scholar]
- 27.Teisseire B, Ropars C, Villeréal MC, Nicolau C. Long-term physiological effects of enhanced O2 release by inositol hexaphosphate-loaded erythrocytes. Proc. Natl Acad. Sci. USA. 1987;84(19):6894–6898. doi: 10.1073/pnas.84.19.6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bax BE, Bain MD, Fairbanks LD, Webster AD, Chalmers RA. In vitro and in vivo studies with human carrier erythrocytes loaded with polyethylene glycol-conjugated and native adenosine deaminase. Br. J. Haematol. 2000;109(3):549–554. doi: 10.1046/j.1365-2141.2000.02059.x. [DOI] [PubMed] [Google Scholar]
- 29.Hambÿe AS, Verbeke KA, Vandermeiren RP, Joosens EJ, Verbruggen AM, De Roo MJ. Comparison of modified technetium-99m albumin and technetium-99m red blood cells for equilibrium ventriculography. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 1997;38(10):1521–1528. [PubMed] [Google Scholar]
- 30.Kravtzoff R, Colombat PH, Desbois I, et al. Tolerance evaluation of L-asparaginase loaded in red blood cells. Eur. J. Clin. Pharmacol. 1996;51(3–4):221–225. doi: 10.1007/s002280050187. [DOI] [PubMed] [Google Scholar]
- 31.Kravtzoff R, Desbois I, Lamagnere JP, et al. Improved pharmacodynamics of L-asparaginase-loaded in human red blood cells. Eur. J. Clin. Pharmacol. 1996;49(6):465–470. doi: 10.1007/BF00195932. [DOI] [PubMed] [Google Scholar]
- 32.Hamidi M, Zarrin A, Foroozesh M, Mohammadi-Samani S. Applications of carrier erythrocytes in delivery of biopharmaceuticals. J. Control. Release. 2007;118(2):145–160. doi: 10.1016/j.jconrel.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 33.Krantz A. Red cell-mediated therapy: opportunities and challenges. Blood Cells. Mol Dis. 1997;23(1):58–68. doi: 10.1006/bcmd.1997.0119. [DOI] [PubMed] [Google Scholar]
- 34.Patel PD, Dand N, Hirlekar RS, Kadam VJ. Drug loaded erythrocytes: as novel drug delivery system. Curr. Pharm. Des. 2008;14(1):63–70. doi: 10.2174/138161208783330772. [DOI] [PubMed] [Google Scholar]
- 35.Pierigè F, Serafini S, Rossi L, Magnani M. Cell-based drug delivery. Adv. Drug Deliv. Rev. 2008;60(2):286–295. doi: 10.1016/j.addr.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 36.Rossi L, Serafini S, Pierigé F, et al. Erythrocyte-based drug delivery. Expert Opin. Drug Deliv. 2005;2(2):311–322. doi: 10.1517/17425247.2.2.311. [DOI] [PubMed] [Google Scholar]
- 37.Bax BE, Bain MD, Fairbanks LD, et al. A 9-yr evaluation of carrier erythrocyte encapsulated adenosine deaminase (ADA) therapy in a patient with adult-type ADA deficiency. Eur. J. Haematol. 2007;79(4):338–348. doi: 10.1111/j.1600-0609.2007.00927.x. [DOI] [PubMed] [Google Scholar]
- 38.Bax BE, Bain MD, Scarpelli M, Filosto M, Tonin P, Moran N. Clinical and biochemical improvements in a patient with MNGIE following enzyme replacement. Neurology. 2013;81(14):1269–1271. doi: 10.1212/WNL.0b013e3182a6cb4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Godfrin Y, Horand F, Franco R, et al. International seminar on the red blood cells as vehicles for drugs. Expert Opin. Biol. Ther. 2012;12(1):127–133. doi: 10.1517/14712598.2012.631909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Domenech C, Thomas X, Chabaud S, et al. l-asparaginase loaded red blood cells in refractory or relapsing acute lymphoblastic leukaemia in children and adults: results of the GRASPALL 2005–01 randomized trial. Br. J. Haematol. 2011;153(1):58–65. doi: 10.1111/j.1365-2141.2011.08588.x. [DOI] [PubMed] [Google Scholar]
- 41.Bossa F, Latiano A, Rossi L, et al. Erythrocyte-mediated delivery of dexamethasone in patients with mild-to-moderate ulcerative colitis, refractory to mesalamine: a randomized, controlled study. Am. J. Gastroenterol. 2008;103(10):2509–2516. doi: 10.1111/j.1572-0241.2008.02103.x. [DOI] [PubMed] [Google Scholar]
- 42.Bossa F, Annese V, Valvano MR, et al. Erythrocytes-mediated delivery of dexamethasone 21-phosphate in steroid-dependent ulcerative colitis: a randomized, double-blind Sham-controlled study. Inflamm. Bowel Dis. 2013;19(9):1872–1879. doi: 10.1097/MIB.0b013e3182874065. [DOI] [PubMed] [Google Scholar]
- 43.Mohandas N, Gallagher PG. Red cell membrane: past, present, and future. Blood. 2008;112(10):3939–3948. doi: 10.1182/blood-2008-07-161166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sosa JM, Nielsen ND, Vignes SM, Chen TG, Shevkoplyas SS. The relationship between red blood cell deformability metrics and perfusion of an artificial microvascular network. Clin. Hemorheol. Microcirc. 2013;57(3):275–289. doi: 10.3233/CH-131719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tracz MJ, Alam J, Nath KA. Physiology and pathophysiology of heme: implications for kidney disease. J. Am. Soc. Nephrol. 2007;18(2):414–420. doi: 10.1681/ASN.2006080894. [DOI] [PubMed] [Google Scholar]
- 46.Schaer DJ, Buehler PW, Alayash AI, Belcher JD, Vercellotti GM. Hemolysis and free hemoglobin revisited: exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood. 2013;121(8):1276–1284. doi: 10.1182/blood-2012-11-451229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Janatpour KA, Kalmin ND, Jensen HM, Holland PV. Clinical outcomes of ABO-incompatible RBC transfusions. Am. J. Clin. Pathol. 2008;129(2):276–281. doi: 10.1309/VXY1ULAFUY6E6JT3. [DOI] [PubMed] [Google Scholar]
- 48.Pasini EM, Kirkegaard M, Mortensen P, Lutz HU, Thomas AW, Mann M. In-depth analysis of the membrane and cytosolic proteome of red blood cells. Blood. 2006;108(3):791–801. doi: 10.1182/blood-2005-11-007799. [DOI] [PubMed] [Google Scholar]
- 49.Kalfa TA, Pushkaran S, Mohandas N, et al. Rac GTPases regulate the morphology and deformability of the erythrocyte cytoskeleton. Blood. 2006;108(12):3637–3645. doi: 10.1182/blood-2006-03-005942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vink H, Duling BR. Identification of distinct luminal domains for macromolecules, erythrocytes, and leukocytes within mammalian capillaries. Circ. Res. 1996;79(3):581–589. doi: 10.1161/01.res.79.3.581. [DOI] [PubMed] [Google Scholar]
- 51.Nans A, Mohandas N, Stokes DL. Native ultrastructure of the red cell cytoskeleton by cryo-electron tomography. Biophys. J. 2011;101(10):2341–2350. doi: 10.1016/j.bpj.2011.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sathi A, Viswanad V, Aneesh TP, Kumar BA. Pros and cons of phospholipid asymmetry in erythrocytes. J. Pharm. Bioallied Sci. 2014;6(2):81–85. doi: 10.4103/0975-7406.129171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lubin B, Chiu D, Bastacky J, Roelofsen B, Van Deenen LL. Abnormalities in membrane phospholipid organization in sickled erythrocytes. J. Clin. Invest. 1981;67(6):1643–1649. doi: 10.1172/JCI110200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.An X, Mohandas N. Disorders of red cell membrane. Br. J. Haematol. 2008;141(3):367–375. doi: 10.1111/j.1365-2141.2008.07091.x. [DOI] [PubMed] [Google Scholar]
- 55.Barcellini W, Bianchi P, Fermo E, et al. Hereditary red cell membrane defects: diagnostic and clinical aspects. Blood Transfus. Trasfus. Sangue. 2011;9(3):274–277. doi: 10.2450/2011.0086-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reliene R, Mariani M, Zanella A, et al. Splenectomy prolongs in vivo survival of erythrocytes differently in spectrin/ankyrin- and band 3-deficient hereditary spherocytosis. Blood. 2002;100(6):2208–2215. [PubMed] [Google Scholar]
- 57.Ganguly K, Murciano J-C, Westrick R, Leferovich J, Cines DB, Muzykantov VR. The glycocalyx protects erythrocyte-bound tissue-type plasminogen activator from enzymatic inhibition. J. Pharmacol. Exp. Ther. 2007;321(1):158–164. doi: 10.1124/jpet.106.114405. [DOI] [PubMed] [Google Scholar]
- 58.Chang TMS. From artificial red blood cells, oxygen carriers, and oxygen therapeutics to artificial cells, nanomedicine, and beyond. Artif. Cells Blood Substit. Immobil. Biotechnol. 2012;40(3):197–199. doi: 10.3109/10731199.2012.662408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Habler O, Pape A, Meier J, Zwissler B. [Artificial oxygen carriers as an alternative to red blood cell transfusion] Anaesthesist. 2005;54(8):741–754. doi: 10.1007/s00101-005-0893-3. [DOI] [PubMed] [Google Scholar]
- 60.Zaltzman AB, Van den Berg CW, Muzykantov VR, Morgan BP. Enhanced complement susceptibility of avidin-biotin-treated human erythrocytes is a consequence of neutralization of the complement regulators CD59 and decay accelerating factor. Biochem. J. 1995;307(Pt 3):651–656. doi: 10.1042/bj3070651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science. 2000;288(5473):2051–2054. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- 62.Ishikawa-Sekigami T, Kaneko Y, Okazawa H, et al. SHPS-1 promotes the survival of circulating erythrocytes through inhibition of phagocytosis by splenic macrophages. Blood. 2006;107(1):341–348. doi: 10.1182/blood-2005-05-1896. [DOI] [PubMed] [Google Scholar]
- 63.Burger P, Hilarius-Stokman P, de Korte D, van den Berg TK, van Bruggen R. CD47 functions as a molecular switch for erythrocyte phagocytosis. Blood. 2012;119(23):5512–5521. doi: 10.1182/blood-2011-10-386805. [DOI] [PubMed] [Google Scholar]
- 64.Kuriyama T, Takenaka K, Kohno K, et al. Engulfment of hematopoietic stem cells caused by down-regulation of CD47 is critical in the pathogenesis of hemophagocytic lymphohistiocytosis. Blood. 2012;120(19):4058–4067. doi: 10.1182/blood-2012-02-408864. [DOI] [PubMed] [Google Scholar]
- 65.Kinoshita T, Medof ME, Silber R, Nussenzweig V. Distribution of decay-accelerating factor in the peripheral blood of normal individuals and patients with paroxysmal nocturnal hemoglobinuria. J. Exp. Med. 1985;162(1):75–92. doi: 10.1084/jem.162.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Medof ME, Kinoshita T, Nussenzweig V. Inhibition of complement activation on the surface of cells after incorporation of decay-accelerating factor (DAF) into their membranes. J. Exp. Med. 1984;160(5):1558–1578. doi: 10.1084/jem.160.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nicholson-Weller A, March JP, Rosenfeld SI, Austen KF. Affected erythrocytes of patients with paroxysmal nocturnal hemoglobinuria are deficient in the complement regulatory protein, decay accelerating factor. Proc. Natl Acad. Sci. USA. 1983;80(16):5066–5070. doi: 10.1073/pnas.80.16.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pangburn MK, Schreiber RD, Müller-Eberhard HJ. Deficiency of an erythrocyte membrane protein with complement regulatory activity in paroxysmal nocturnal hemoglobinuria. Proc. Natl Acad. Sci. USA. 1983;80(17):5430–5434. doi: 10.1073/pnas.80.17.5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schultz DR. Erythrocyte membrane protein deficiencies in paroxysmal nocturnal hemoglobinuria. Am. J. Med. 1989;87(3N):22N–29N. [PubMed] [Google Scholar]
- 70.Sugita Y, Ito K, Shiozuka K, et al. Recombinant soluble CD59 inhibits reactive haemolysis with complement. Immunology. 1994;82(1):34–41. [PMC free article] [PubMed] [Google Scholar]
- 71.Beppu M, Mizukami A, Nagoya M, Kikugawa K. Binding of anti-band 3 autoantibody to oxidatively damaged erythrocytes. Formation of senescent antigen on erythrocyte surface by an oxidative mechanism. J. Biol. Chem. 1990;265(6):3226–3233. [PubMed] [Google Scholar]
- 72.Galili U, Flechner I, Knyszynski A, Danon D, Rachmilewitz EA. The natural anti-alpha-galactosyl IgG on human normal senescent red blood cells. Br. J. Haematol. 1986;62(2):317–324. doi: 10.1111/j.1365-2141.1986.tb02935.x. [DOI] [PubMed] [Google Scholar]
- 73.Giger U, Sticher B, Naef R, Burger R, Lutz HU. Naturally occurring human anti-band 3 autoantibodies accelerate clearance of erythrocytes in guinea pigs. Blood. 1995;85(7):1920–1928. [PubMed] [Google Scholar]
- 74.Kay MM. Isolation of the phagocytosis-inducing IgG-binding antigen on senescent somatic cells. Nature. 1981;289(5797):491–494. doi: 10.1038/289491a0. [DOI] [PubMed] [Google Scholar]
- 75.Cambos M, Scorza T. Robust erythrophagocytosis leads to macrophage apoptosis via a hemin-mediated redox imbalance: role in hemolytic disorders. J. Leukoc. Biol. 2011;89(1):159–171. doi: 10.1189/jlb.0510249. [DOI] [PubMed] [Google Scholar]
- 76.Loegering DJ, Commins LM, Minnear FL, Gary LA, Hill LA. Effect of Kupffer cell phagocytosis of erythrocytes and erythrocyte ghosts on susceptibility to endotoxemia and bacteremia. Infect. Immun. 1987;55(9):2074–2080. doi: 10.1128/iai.55.9.2074-2080.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mebius RE, Kraal G. Structure and function of the spleen. Nat. Rev. Immunol. 2005;5(8):606–616. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- 78.Alvarez FJ, Jordán JA, Calleja P, et al. Cross-linking treatment of loaded erythrocytes increases delivery of encapsulated substance to macrophages. Biotechnol. Appl. Biochem. 1998;27(Pt 2):139–143. [PubMed] [Google Scholar]
- 79.Chiarantini L, Rossi L, Fraternale A, Magnani M. Modulated red blood cell survival by membrane protein clustering. Mol. Cell. Biochem. 1995;144(1):53–59. doi: 10.1007/BF00926740. [DOI] [PubMed] [Google Scholar]
- 80.Paulitschke M, Nash GB, Anstee DJ, Tanner MJ, Gratzer WB. Perturbation of red blood cell membrane rigidity by extracellular ligands. Blood. 1995;86(1):342–348. [PubMed] [Google Scholar]
- 81.Rancourt C, Robertson MW, Wang M, et al. Endothelial cell vehicles for delivery of cytotoxic genes as a gene therapy approach for carcinoma of the ovary. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 1998;4(2):265–270. [PubMed] [Google Scholar]
- 82.Turrini F, Arese P, Yuan J, Low PS. Clustering of integral membrane proteins of the human erythrocyte membrane stimulates autologous IgG binding, complement deposition, and phagocytosis. J. Biol. Chem. 1991;266(35):23611–23617. [PubMed] [Google Scholar]
- 83.Lisovskaya IL, Shcherbachenko IM, Volkova RI, Ataullakhanov FI. Clotrimazole enhances lysis of human erythrocytes induced by t-BHP. Chem. Biol. Interact. 2009;180(3):433–439. doi: 10.1016/j.cbi.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 84.Turrini F, Mannu F, Arese P, Yuan J, Low PS. Characterization of the autologous antibodies that opsonize erythrocytes with clustered integral membrane proteins. Blood. 1993;81(11):3146–3152. [PubMed] [Google Scholar]
- 85.Geldwerth D, Helley D, de Jong K, et al. Detection of phosphatidylserine surface exposure on human erythrocytes using annexin V-ferrofluid. Biochem. Biophys. Res. Commun. 1999;258(1):199–203. doi: 10.1006/bbrc.1999.0620. [DOI] [PubMed] [Google Scholar]
- 86.Closse C, Dachary-Prigent J, Boisseau MR. Phosphatidylserine-related adhesion of human erythrocytes to vascular endothelium. Br. J. Haematol. 1999;107(2):300–302. doi: 10.1046/j.1365-2141.1999.01718.x. [DOI] [PubMed] [Google Scholar]
- 87.Chiarantini L, Johnson J, Deloach JR. Optimized recirculation survival of mouse carrier erythrocytes. Blood Cells. 1991;17(3):607–617. discussion 618–622. [PubMed] [Google Scholar]
- 88.Eichler HG, Gasic S, Bauer K, Korn A, Bacher S. In vivo clearance of antibody-sensitized human drug carrier erythrocytes. Clin. Pharmacol. Ther. 1986;40(3):300–303. doi: 10.1038/clpt.1986.180. [DOI] [PubMed] [Google Scholar]
- 89.Grover GJ, Loegering DJ. Effect of red blood cell stroma on the reticuloendothelial system clearance and killing of Streptococcus pneumoniae. Circ. Shock. 1984;14(1):39–47. [PubMed] [Google Scholar]
- 90.Van Wijk R, van Solinge WW. The energy-less red blood cell is lost: erythrocyte enzyme abnormalities of glycolysis. Blood. 2005;106(13):4034–4042. doi: 10.1182/blood-2005-04-1622. [DOI] [PubMed] [Google Scholar]
- 91.Dejam A, Hunter CJ, Pelletier MM, et al. Erythrocytes are the major intravascular storage sites of nitrite in human blood. Blood. 2005;106(2):734–739. doi: 10.1182/blood-2005-02-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Alvarez FJ, Jordán JA, Herráez A, Díez JC, Tejedor MC. Hypotonically loaded rat erythrocytes deliver encapsulated substances into peritoneal macrophages. J. Biochem. (Tokyo) 1998;123(2):233–239. doi: 10.1093/oxfordjournals.jbchem.a021927. [DOI] [PubMed] [Google Scholar]
- 93.Humphreys JD, Ihler G. Enhanced stability of erythrocyte-entrapped glucocerebrosidase activity. J. Lab. Clin. Med. 1980;96(4):682–692. [PubMed] [Google Scholar]
- 94.Chiarantini L, Antonelli A, Rossi L, Fraternale A, Magnani M. Red blood cell phagocytosis following hexokinase inactivation. Cell Biochem. Funct. 1994;12(3):217–220. doi: 10.1002/cbf.290120310. [DOI] [PubMed] [Google Scholar]
- 95.Franchetti P, Cappellacci L, Petrelli R, et al. Inhibition of HIV-1 replication in macrophages by red blood cell-mediated delivery of a heterodinucleotide of lamivudine and tenofovir. Nucleosides Nucleotides Nucleic Acids. 2007;26(8–9):953–957. doi: 10.1080/15257770701508067. [DOI] [PubMed] [Google Scholar]
- 96.Magnani M, Casabianca A, Fraternale A, et al. Inhibition of murine AIDS by a new azidothymidine homodinucleotide. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1998;17(3):189–195. doi: 10.1097/00042560-199803010-00001. [DOI] [PubMed] [Google Scholar]
- 97.Rossi L, Brandi G, Schiavano GF, et al. Heterodimer-loaded erythrocytes as bioreactors for slow delivery of the antiviral drug azidothymidine and the antimycobacterial drug ethambutol. AIDS Res. Hum. Retroviruses. 1999;15(4):345–353. doi: 10.1089/088922299311312. [DOI] [PubMed] [Google Scholar]
- 98.Chiarantini L, Droleskey R, Magnani M, DeLoach JR. In vitro targeting of erythrocytes to cytotoxic T-cells by coupling of Thy-1.2 monoclonal antibody. Biotechnol. Appl. Biochem. 1992;15(2):171–184. [PubMed] [Google Scholar]
- 99.Magnani M, Rossi L, Fraternale A, et al. Erythrocyte-mediated delivery of drugs, peptides and modified oligonucleotides. Gene Ther. 2002;9(11):749–751. doi: 10.1038/sj.gt.3301758. [DOI] [PubMed] [Google Scholar]
- 100.Mukthavaram R, Shi G, Kesari S, Simberg D. Targeting and depletion of circulating leukocytes and cancer cells by lipophilic antibody-modified erythrocytes. J. Control. Release. 2014;183:146–153. doi: 10.1016/j.jconrel.2014.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Muzykantov VR, Zaltsman AB, Smirnov MD, Samokhin GP, Morgan BP. Target-sensitive immunoerythrocytes: interaction of biotinylated red blood cells with immobilized avidin induces their lysis by complement. Biochim. Biophys. Acta. 1996;1279(2):137–143. doi: 10.1016/0005-2736(95)00260-x. [DOI] [PubMed] [Google Scholar]
- 102.Magnani M, Mancini U, Bianchi M, Fazi A. Comparison of uricase-bound and uricase-loaded erythrocytes as bioreactors for uric acid degradation. Adv. Exp. Med. Biol. 1992;326:189–194. doi: 10.1007/978-1-4615-3030-5_23. [DOI] [PubMed] [Google Scholar]
- 103.Muzykantov VR, Sakharov DV, Domogatsky SP, Goncharov NV, Danilov SM. Directed targeting of immunoerythrocytes provides local protection of endothelial cells from damage by hydrogen peroxide. Am. J. Pathol. 1987;128(2):276–285. [PMC free article] [PubMed] [Google Scholar]
- 104.Muzykantov VR, Smirnov MD, Zaltzman AB, Samokhin GP. Tannin-mediated attachment of avidin provides complement-resistant immunoerythrocytes that can be lysed in the presence of activator of complement. Anal. Biochem. 1993;208(2):338–342. doi: 10.1006/abio.1993.1057. [DOI] [PubMed] [Google Scholar]
- 105.Godfrey W, Doe B, Wallace EF, Bredt B, Wofsy L. Affinity targeting of membrane vesicles to cell surfaces. Exp. Cell Res. 1981;135(1):137–145. doi: 10.1016/0014-4827(81)90306-2. [DOI] [PubMed] [Google Scholar]
- 106.Orr GA. The use of the 2-iminobiotin-avidin interaction for the selective retrieval of labeled plasma membrane components. J. Biol. Chem. 1981;256(2):761–766. [PubMed] [Google Scholar]
- 107.Roffman E, Meromsky L, Ben-Hur H, Bayer EA, Wilchek M. Selective labeling of functional groups on membrane proteins or glycoproteins using reactive biotin derivatives and 125I-streptavidin. Biochem. Biophys. Res. Commun. 1986;136(1):80–85. doi: 10.1016/0006-291x(86)90879-x. [DOI] [PubMed] [Google Scholar]
- 108.Bayer EA, Safars M, Wilchek M. Selective labeling of sulfhydryls and disulfides on blot transfers using avidin-biotin technology: studies on purified proteins and erythrocyte membranes. Anal. Biochem. 1987;161(2):262–271. doi: 10.1016/0003-2697(87)90450-7. [DOI] [PubMed] [Google Scholar]
- 109.Wilchek M, Ben-Hur H, Bayer EA. p-Diazobenzoyl biocytin – a new biotinylating reagent for the labeling of tyrosines and histidines in proteins. Biochem. Biophys. Res. Commun. 1986;138(2):872–879. doi: 10.1016/s0006-291x(86)80577-0. [DOI] [PubMed] [Google Scholar]
- 110.Muzykantov VR, Smirnov MD, Klibanov AL. Avidin attachment to red blood cells via a phospholipid derivative of biotin provides complement-resistant immunoerythrocytes. J. Immunol. Methods. 1993;158(2):183–190. doi: 10.1016/0022-1759(93)90212-p. [DOI] [PubMed] [Google Scholar]
- 111.Samokhin GP, Smirnov MD, Muzykantov VR, Domogatsky SP, Smirnov VN. Red blood cell targeting to collagen-coated surfaces. FEBS Lett. 1983;154(2):257–261. doi: 10.1016/0014-5793(83)80160-4. [DOI] [PubMed] [Google Scholar]
- 112.Cowley H, Wojda U, Cipolone KM, Procter JL, Stroncek DF, Miller JL. Biotinylation modifies red cell antigens. Transfusion. 1999;39(2):163–168. doi: 10.1046/j.1537-2995.1999.39299154730.x. [DOI] [PubMed] [Google Scholar]
- 113.Magnani M, Chiarantini L, Mancini U. Preparation and characterization of biotinylated red blood cells. Biotechnol. Appl. Biochem. 1994;20(Pt 3):335–345. doi: 10.1111/j.1470-8744.1994.tb00321.x. [DOI] [PubMed] [Google Scholar]
- 114.Magnani M, Rossi L, D'ascenzo M, Panzani I, Bigi L, Zanella A. Erythrocyte engineering for drug delivery and targeting. Biotechnol. Appl. Biochem. 1998;28(Pt 1):1–6. [PubMed] [Google Scholar]
- 115.Muzykantov VR, Sakharov DV, Smirnov MD, Domogatsky SP, Samokhin GP. Targeting of enzyme immobilized on erythrocyte membrane to collagen-coated surface. FEBS Lett. 1985;182(1):62–66. doi: 10.1016/0014-5793(85)81154-6. [DOI] [PubMed] [Google Scholar]
- 116.Smirnov MD, Samokhin GP, Muzykantov VR, Idelson GL, Domogatsky SP, Smirnov VN. Type I and III collagens as a possible target for drug delivery to the injured sites of vascular bed. Biochem. Biophys. Res. Commun. 1983;116(1):99–105. doi: 10.1016/0006-291x(83)90386-8. [DOI] [PubMed] [Google Scholar]
- 117.Muzykantov VR, Smirnov MD, Samokhin GP. Streptavidin-induced lysis of homologous biotinylated erythrocytes. Evidence against the key role of the avidin charge in complement activation via the alternative pathway. FEBS Lett. 1991;280(1):112–114. doi: 10.1016/0014-5793(91)80216-p. [DOI] [PubMed] [Google Scholar]
- 118.Hoffman JF. On red blood cells, hemolysis and resealed ghosts. Adv. Exp. Med. Biol. 1992;326:1–15. doi: 10.1007/978-1-4615-3030-5_1. [DOI] [PubMed] [Google Scholar]
- 119.Muzykantov VR, Smirnov MD, Samokhin GP. Avidin attachment to biotinylated erythrocytes induces homologous lysis via the alternative pathway of complement. Blood. 1991;78(10):2611–2618. [PubMed] [Google Scholar]
- 120.Muzykantov VR, Seregina N, Smirnov MD. Fast lysis by complement and uptake by liver of avidin-carrying biotinylated erythrocytes. Int. J. Artif. Organs. 1992;15(10):622–627. [PubMed] [Google Scholar]
- 121.Muzykantov VR, Smirnov MD, Klibanov AL. Avidin attachment to biotinylated amino groups of the erythrocyte membrane eliminates homologous restriction of both classical and alternative pathways of the complement. FEBS Lett. 1993;318(2):108–112. doi: 10.1016/0014-5793(93)80002-c. [DOI] [PubMed] [Google Scholar]
- 122.Muzykantov VR, Murciano JC. Attachment of antibody to biotinylated red blood cells: immuno-red blood cells display high affinity to immobilized antigen and normal biodistribution in rats. Biotechnol. Appl. Biochem. 1996;24(Pt 1):41–45. [PubMed] [Google Scholar]
- 123.Muzykantov VR, Taylor RP. Attachment of biotinylated antibody to red blood cells: antigen-binding capacity of immunoerythrocytes and their susceptibility to lysis by complement. Anal. Biochem. 1994;223(1):142–148. doi: 10.1006/abio.1994.1559. [DOI] [PubMed] [Google Scholar]
- 124.Muzykantov VR, Smirnov MD, Samokhin GP. Avidin acylation prevents the complement-dependent lysis of avidin-carrying erythrocytes. Biochem. J. 1991;273(Pt 2):393–397. doi: 10.1042/bj2730393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Muzykantov VR, Smirnov MD, Samokhin GP. Avidin-induced lysis of biotinylated erythrocytes by homologous complement via the alternative pathway depends on avidin's ability of multipoint binding with biotinylated membrane. Biochim. Biophys. Acta. 1992;1107(1):119–125. doi: 10.1016/0005-2736(92)90336-k. [DOI] [PubMed] [Google Scholar]
- 126.Muzykantov VR, Murciano JC, Taylor RP, Atochina EN, Herraez A. Regulation of the complement-mediated elimination of red blood cells modified with biotin and streptavidin. Anal. Biochem. 1996;241(1):109–119. doi: 10.1006/abio.1996.0384. [DOI] [PubMed] [Google Scholar]
- 127.Chiarantini L, Argnani R, Zucchini S, et al. Red blood cells as delivery system for recombinant HSV-1 glycoprotein B: immunogenicity and protection in mice. Vaccine. 1997;15(3):276–280. doi: 10.1016/s0264-410x(96)00181-8. [DOI] [PubMed] [Google Scholar]
- 128.Smirnov VN, Domogatsky SP, Dolgov VV, et al. Carrier-directed targeting of liposomes and erythrocytes to denuded areas of vessel wall. Proc. Natl Acad. Sci. USA. 1986;83(17):6603–6607. doi: 10.1073/pnas.83.17.6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gyimesi E, Bankovich AJ, Schuman TA, Goldberg JB, Lindorfer MA, Taylor RP. Staphylococcus aureus bound to complement receptor 1 on human erythrocytes by bispecific monoclonal antibodies is phagocytosed by acceptor macrophages. Immunol. Lett. 2004;95(2):185–192. doi: 10.1016/j.imlet.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 130.Kuhn SE, Nardin A, Klebba PE, Taylor RP. Escherichia coli bound to the primate erythrocyte complement receptor via bispecific monoclonal antibodies are transferred to and phagocytosed by human monocytes in an in vitro model. J. Immunol. 1998;160(10):5088–5097. [PubMed] [Google Scholar]
- 131.Taylor RP, Sutherland WM, Reist CJ, Webb DJ, Wright EL, Labuguen RH. Use of heteropolymeric monoclonal antibodies to attach antigens to the C3b receptor of human erythrocytes: a potential therapeutic treatment. Proc. Natl Acad. Sci. USA. 1991;88(8):3305–3309. doi: 10.1073/pnas.88.8.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Spitzer D, Unsinger J, Bessler M, Atkinson JP. ScFv-mediated in vivo targeting of DAF to erythrocytes inhibits lysis by complement. Mol. Immunol. 2004;40(13):911–919. doi: 10.1016/j.molimm.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 133.Suzuki K, Okumura Y. GPI-linked proteins do not transfer spontaneously from erythrocytes to liposomes. New aspects of reorganization of the cell membrane. Biochemistry (Mosc.) 2000;39(31):9477–9485. doi: 10.1021/bi000113v. [DOI] [PubMed] [Google Scholar]
- 134.Civenni G, Test ST, Brodbeck U, Bütikofer P. In vitro incorporation of GPI-anchored proteins into human erythrocytes and their fate in the membrane. Blood. 1998;91(5):1784–1792. [PubMed] [Google Scholar]
- 135.Medof ME, Nagarajan S, Tykocinski ML. Cell-surface engineering with GPI-anchored proteins. FASEB J. 1996;10(5):574–586. doi: 10.1096/fasebj.10.5.8621057. [DOI] [PubMed] [Google Scholar]
- 136.Hill A, Ridley SH, Esser D, et al. Protection of erythrocytes from human complement-mediated lysis by membrane-targeted recombinant soluble CD59: a new approach to PNH therapy. Blood. 2006;107(5):2131–2137. doi: 10.1182/blood-2005-02-0782. [DOI] [PubMed] [Google Scholar]
- 137.Chen R, Walter EI, Parker G, et al. Mammalian glycophosphatidylinositol anchor transfer to proteins and posttransfer deacylation. Proc. Natl Acad. Sci. USA. 1998;95(16):9512–9517. doi: 10.1073/pnas.95.16.9512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kooyman DL, Byrne GW, McClellan S, et al. In vivo transfer of GPI-linked complement restriction factors from erythrocytes to the endothelium. Science. 1995;269(5220):89–92. doi: 10.1126/science.7541557. [DOI] [PubMed] [Google Scholar]
- 139.Müller M, Büchi L, Woodtli K, Haeberli A, Beer JH. Preparation and characterization of “heparinocytes”: erythrocytes with covalently bound low molecular weight heparin. FEBS Lett. 2000;468(2–3):115–119. doi: 10.1016/s0014-5793(00)01204-7. [DOI] [PubMed] [Google Scholar]
- 140.Holvoet P, Laroche Y, Stassen JM, et al. Pharmacokinetic and thrombolytic properties of chimeric plasminogen activators consisting of a single-chain Fv fragment of a fibrin-specific antibody fused to single-chain urokinase. Blood. 1993;81(3):696–703. [PubMed] [Google Scholar]
- 141.Topol EJ, Morris DC, Smalling RW, et al. A multicenter, randomized, placebo-controlled trial of a new form of intravenous recombinant tissue-type plasminogen activator (activase) in acute myocardial infarction. J. Am. Coll. Cardiol. 1987;9(6):1205–1213. doi: 10.1016/s0735-1097(87)80457-6. [DOI] [PubMed] [Google Scholar]
- 142.Narita M, Bu G, Herz J, Schwartz AL. Two receptor systems are involved in the plasma clearance of tissue-type plasminogen activator (t-PA) in vivo . J. Clin. Invest. 1995;96(2):1164–1168. doi: 10.1172/JCI118105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Collen D, Van Hoef B, Schlott B, Hartmann M, Gührs KH, Lijnen HR. Mechanisms of activation of mammalian plasma fibrinolytic systems with streptokinase and with recombinant staphylokinase. Eur. J. Biochem. FEBS. 1993;216(1):307–314. [Google Scholar]
- 144.Holvoet P, Dewerchin M, Stassen JM, et al. Thrombolytic profiles of clot-targeted plasminogen activators. Parameters determining potency and initial and maximal rates. Circulation. 1993;87(3):1007–1016. doi: 10.1161/01.cir.87.3.1007. [DOI] [PubMed] [Google Scholar]
- 145.Muzykantov VR, Barnathan ES, Atochina EN, Kuo A, Danilov SM, Fisher AB. Targeting of antibody-conjugated plasminogen activators to the pulmonary vasculature. J. Pharmacol. Exp. Ther. 1996;279(2):1026–1034. [PubMed] [Google Scholar]
- 146.Runge MS, Harker LA, Bode C, et al. Enhanced thrombolytic and antithrombotic potency of a fibrin-targeted plasminogen activator in baboons. Circulation. 1996;94(6):1412–1422. doi: 10.1161/01.cir.94.6.1412. [DOI] [PubMed] [Google Scholar]
- 147.Wang YF, Tsirka SE, Strickland S, Stieg PE, Soriano SG, Lipton SA. Tissue plasminogen activator (tPA) increases neuronal damage after focal cerebral ischemia in wild-type and tPA-deficient mice. Nat. Med. 1998;4(2):228–231. doi: 10.1038/nm0298-228. [DOI] [PubMed] [Google Scholar]
- 148.Gersh KC, Zaitsev S, Muzykantov V, Cines DB, Weisel JW. The spatial dynamics of fibrin clot dissolution catalyzed by erythrocyte-bound vs. free fibrinolytics. J. Thromb. Haemost. JTH. 2010;8(5):1066–1074. doi: 10.1111/j.1538-7836.2010.03802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gersh KC, Zaitsev S, Cines DB, Muzykantov V, Weisel JW. Flow-dependent channel formation in clots by an erythrocyte-bound fibrinolytic agent. Blood. 2011;117(18):4964–4967. doi: 10.1182/blood-2010-10-310409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ganguly K, Krasik T, Medinilla S, et al. Blood clearance and activity of erythrocyte-coupled fibrinolytics. J. Pharmacol. Exp. Ther. 2005;312(3):1106–1113. doi: 10.1124/jpet.104.075770. [DOI] [PubMed] [Google Scholar]
- 151.Murciano J-C, Medinilla S, Eslin D, Atochina E, Cines DB, Muzykantov VR. Prophylactic fibrinolysis through selective dissolution of nascent clots by tPA-carrying erythrocytes. Nat. Biotechnol. 2003;21(8):891–896. doi: 10.1038/nbt846. [DOI] [PubMed] [Google Scholar]
- 152.Armstead WM, Cines DB, Higazi AA-R. Plasminogen activators contribute to impairment of hypercapnic and hypotensive cerebrovasodilation after cerebral hypoxia/ischemia in the newborn pig. Stroke J. Cereb. Circ. 2005;36(10):2265–2269. doi: 10.1161/01.STR.0000181078.74698.b0. [DOI] [PubMed] [Google Scholar]
- 153.Armstead WM, Christine AJ, Higazi AA-R, Cines DB. Urokinase plasminogen activator impairs SNP and PGE2 cerebrovasodilation after brain injury through activation of LRP and ERK MAPK. J. Neurotrauma. 2008;25(11):1375–1381. doi: 10.1089/neu.2008.0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Armstead WM, Ganguly K, Kiessling JW, et al. Red blood cells-coupled tPA prevents impairment of cerebral vasodilatory responses and tissue injury in pediatric cerebral hypoxia/ischemia through inhibition of ERK MAPK activation. J. Cereb. Blood Flow Metab. 2009;29(8):1463–1474. doi: 10.1038/jcbfm.2009.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Armstead WM, Ganguly K, Riley J, et al. Red blood cell-coupled tissue plasminogen activator prevents impairment of cerebral vasodilatory responses through inhibition of c-Jun-N-terminal kinase and potentiation of p38 mitogen-activated protein kinase after cerebral photothrombosis in the newborn pig. Pediatr. Crit. Care Med. 2011;12(6):e369–e375. doi: 10.1097/PCC.0b013e3181fe40a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Murciano J-C, Higazi AA-R, Cines DB, Muzykantov VR. Soluble urokinase receptor conjugated to carrier red blood cells binds latent pro-urokinase and alters its functional profile. J. Control. Release. 2009;139(3):190–196. doi: 10.1016/j.jconrel.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Danielyan K, Ganguly K, Ding B-S, et al. Cerebrovascular thromboprophylaxis in mice by erythrocyte-coupled tissue-type plasminogen activator. Circulation. 2008;118(14):1442–1449. doi: 10.1161/CIRCULATIONAHA.107.750257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Pisapia JM, Xu X, Kelly J, et al. Microthrombosis after experimental subarachnoid hemorrhage: time course and effect of red blood cell-bound thrombin-activated pro-urokinase and clazosentan. Exp. Neurol. 2012;233(1):357–363. doi: 10.1016/j.expneurol.2011.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Stein SC, Ganguly K, Belfield CM, et al. Erythrocyte-bound tissue plasminogen activator is neuroprotective in experimental traumatic brain injury. J. Neurotrauma. 2009;26(9):1585–1592. doi: 10.1089/neu.2008.0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hess C, Schifferli JA. Immune adherence revisited: novel players in an old game. News Physiol. Sci. 2003;18:104–108. doi: 10.1152/nips.01425.2002. [DOI] [PubMed] [Google Scholar]
- 161.Fearon DT. Identification of the membrane glycoprotein that is the C3b receptor of the human erythrocyte, polymorphonuclear leukocyte, B lymphocyte, and monocyte. J. Exp. Med. 1980;152(1):20–30. doi: 10.1084/jem.152.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Krych-Goldberg M, Atkinson JP. Structure-function relationships of complement receptor type 1. Immunol. Rev. 2001;180:112–122. doi: 10.1034/j.1600-065x.2001.1800110.x. [DOI] [PubMed] [Google Scholar]
- 163.Lindorfer MA, Hahn CS, Foley PL, Taylor RP. Heteropolymer-mediated clearance of immune complexes via erythrocyte CR1: mechanisms and applications. Immunol. Rev. 2001;183:10–24. doi: 10.1034/j.1600-065x.2001.1830102.x. [DOI] [PubMed] [Google Scholar]
- 164.Nickells M, Hauhart R, Krych M, et al. Mapping epitopes for 20 monoclonal antibodies to CR1. Clin. Exp. Immunol. 1998;112(1):27–33. doi: 10.1046/j.1365-2249.1998.00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Nardin A, Sutherland WM, Hevey M, Schmaljohn A, Taylor RP. Quantitative studies of heteropolymer-mediated binding of inactivated Marburg virus to the complement receptor on primate erythrocytes. J. Immunol. Methods. 1998;211(1–2):21–31. doi: 10.1016/s0022-1759(97)00168-3. [DOI] [PubMed] [Google Scholar]
- 166.Taylor RP, Reist CJ, Sutherland WM, Otto A, Labuguen RH, Wright EL. In vivo binding and clearance of circulating antigen by bispecific heteropolymer-mediated binding to primate erythrocyte complement receptor. J. Immunol. 1992;148(8):2462–2468. [PubMed] [Google Scholar]
- 167.Repik A, Pincus SE, Ghiran I, et al. A transgenic mouse model for studying the clearance of blood-borne pathogens via human complement receptor 1 (CR1) Clin. Exp. Immunol. 2005;140(2):230–240. doi: 10.1111/j.1365-2249.2005.02764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Reinagel ML, Gezen M, Ferguson PJ, Kuhn S, Martin EN, Taylor RP. The primate erythrocyte complement receptor (CR1) as a privileged site: binding of immunoglobulin G to erythrocyte CR1 does not target erythrocytes for phagocytosis. Blood. 1997;89(3):1068–1077. [PubMed] [Google Scholar]
- 169.Nardin A, Lindorfer MA, Taylor RP. How are immune complexes bound to the primate erythrocyte complement receptor transferred to acceptor phagocytic cells? Mol. Immunol. 1999;36(13–14):827–835. doi: 10.1016/s0161-5890(99)00103-0. [DOI] [PubMed] [Google Scholar]
- 170.Craig ML, Bankovich AJ, Taylor RP. Visualization of the transfer reaction: tracking immune complexes from erythrocyte complement receptor 1 to macrophages. Clin. Immunol. 2002;105(1):36–47. doi: 10.1006/clim.2002.5266. [DOI] [PubMed] [Google Scholar]
- 171.Craig ML, Waitumbi JN, Taylor RP. Processing of C3b-opsonized immune complexes bound to non-complement receptor 1 (CR1) sites on red cells: phagocytosis, transfer, and associations with CR1. J. Immunol. 2005;174(5):3059–3066. doi: 10.4049/jimmunol.174.5.3059. [DOI] [PubMed] [Google Scholar]
- 172.Taylor RP, Ferguson PJ, Martin EN, et al. Immune complexes bound to the primate erythrocyte complement receptor (CR1) via anti-CR1 mAbs are cleared simultaneously with loss of CR1 in a concerted reaction in a rhesus monkey model. Clin. Immunol. Immunopathol. 1997;82(1):49–59. doi: 10.1006/clin.1996.4286. [DOI] [PubMed] [Google Scholar]
- 173.Ferguson PJ, Martin EN, Greene KL, et al. Antigen-based heteropolymers facilitate, via primate erythrocyte complement receptor type 1, rapid erythrocyte binding of an autoantibody and its clearance from the circulation in rhesus monkeys. J. Immunol. 1995;155(1):339–347. [PubMed] [Google Scholar]
- 174.Lindorfer MA, Nardin A, Foley PL, et al. Targeting of Pseudomonas aeruginosa in the bloodstream with bispecific monoclonal antibodies. J. Immunol. 2001;167(4):2240–2249. doi: 10.4049/jimmunol.167.4.2240. [DOI] [PubMed] [Google Scholar]
- 175.Reist CJ, Liang HY, Denny D, Martin EN, Scheld WM, Taylor RP. Cross-linked bispecific monoclonal antibody heteropolymers facilitate the clearance of human IgM from the circulation of squirrel monkeys. Eur. J. Immunol. 1994;24(9):2018–2025. doi: 10.1002/eji.1830240913. [DOI] [PubMed] [Google Scholar]
- 176.Asher DR, Cerny AM, Finberg RW. The erythrocyte viral trap: transgenic expression of viral receptor on erythrocytes attenuates coxsackievirus B infection. Proc. Natl Acad. Sci. USA. 2005;102(36):12897–12902. doi: 10.1073/pnas.0506211102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hahn CS, French OG, Foley P, Martin EN, Taylor RP. Bispecific monoclonal antibodies mediate binding of dengue virus to erythrocytes in a monkey model of passive viremia. J. Immunol. 2001;166(2):1057–1065. doi: 10.4049/jimmunol.166.2.1057. [DOI] [PubMed] [Google Scholar]
- 178.Taylor RP, Sutherland WM, Martin EN, et al. Bispecific monoclonal antibody complexes bound to primate erythrocyte complement receptor 1 facilitate virus clearance in a monkey model. J. Immunol. 1997;158(2):842–850. [PubMed] [Google Scholar]
- 179.Sharma R, Zhao H, Al-Saleem FH, et al. Mechanisms of enhanced neutralization of botulinum neurotoxin by monoclonal antibodies conjugated to antibodies specific for the erythrocyte complement receptor. Mol. Immunol. 2014;57(2):247–254. doi: 10.1016/j.molimm.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Craig ML, Reinagel ML, Martin EN, Schlimgen R, Nardin A, Taylor RP. Infusion of bispecific monoclonal antibody complexes into monkeys provides immunologic protection against later challenge with a model pathogen. Clin. Immunol. 1999;92(2):170–180. doi: 10.1006/clim.1999.4739. [DOI] [PubMed] [Google Scholar]
- 181.Ferguson PJ, Reist CJ, Martin EN, et al. Antigen-based heteropolymers. A potential therapy for binding and clearing autoantibodies via erythrocyte CR1. Arthritis Rheum. 1995;38(2):190–200. doi: 10.1002/art.1780380207. [DOI] [PubMed] [Google Scholar]
- 182.Pincus SE, Lukacher N, Mohamed N, et al. Evaluation of antigen-based heteropolymer for treatment of systemic lupus erythematosus in a nonhuman primate model. Clin. Immunol. 2002;105(2):141–154. doi: 10.1006/clim.2002.5274. [DOI] [PubMed] [Google Scholar]
- 183.Buster BL, Mattes KA, Scheld WM. Monoclonal antibody-mediated, complement-independent binding of human tumor necrosis factor-alpha to primate erythrocytes via complement receptor 1. J. Infect. Dis. 1997;176(4):1041–1046. doi: 10.1086/516536. [DOI] [PubMed] [Google Scholar]
- 184.Zaitsev S, Danielyan K, Murciano J-C, et al. Human complement receptor type 1-directed loading of tissue plasminogen activator on circulating erythrocytes for prophylactic fibrinolysis. Blood. 2006;108(6):1895–1902. doi: 10.1182/blood-2005-11-012336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chasis JA, Mohandas N. Red blood cell glycophorins. Blood. 1992;80(8):1869–1879. [PubMed] [Google Scholar]
- 186.Reid ME. Some concepts relating to the molecular genetic basis of certain MNS blood group antigens. Transfus. Med. Oxf. Engl. 1994;4(2):99–111. doi: 10.1111/j.1365-3148.1994.tb00250.x. [DOI] [PubMed] [Google Scholar]
- 187.Poole J. Red cell antigens on band 3 and glycophorin A. Blood Rev. 2000;14(1):31–43. doi: 10.1054/blre.1999.0124. [DOI] [PubMed] [Google Scholar]
- 188.Ugorski M, Blackall DP, Påhlsson P, Shakin-Eshleman SH, Moore J, Spitalnik SL. Recombinant Miltenberger I and II human blood group antigens: the role of glycosylation in cell surface expression and antigenicity of glycophorin A. Blood. 1993;82(6):1913–1920. [PubMed] [Google Scholar]
- 189.Marchesi VT. The red cell membrane skeleton: recent progress. Blood. 1983;61(1):1–11. [PubMed] [Google Scholar]
- 190.Hillyard CJ, Rylatt DB, Kemp BE, Bundesen PG. Erythrocyte agglutination assay. www.google.com/patents/US5413913
- 191.Rozmyslowicz T, Donnell ME, Spitalnik SL, Blackall DP. Recombinant blood group antigens are useful for studying the fine specificity of antibodies against glycophorin A encoded antigens. Transfus. Clin. Biol. J. Société Fr. Transfus. Sang. 1997;4(1):77–80. doi: 10.1016/s1246-7820(97)80015-8. [DOI] [PubMed] [Google Scholar]
- 192.Song SC, Xie K, Czerwinski M, Spitalnik SL, Wedekind JE. Purification, crystallization and x-ray diffraction analysis of a recombinant Fab that recognizes a human blood-group antigen. Acta Crystallogr. D Biol. Crystallogr. 2004;60(Pt 4):788–791. doi: 10.1107/S0907444904002963. [DOI] [PubMed] [Google Scholar]
- 193.Czerwinski M, Krop-Watorek A, Siegel DL, Spitalnik SL. A molecular approach for isolating high-affinity Fab fragments that are useful in blood group serology. Transfusion. 1999;39(4):364–371. doi: 10.1046/j.1537-2995.1999.39499235667.x. [DOI] [PubMed] [Google Scholar]
- 194.Zaitsev S, Spitzer D, Murciano J-C, et al. Sustained thromboprophylaxis mediated by an RBC-targeted pro-urokinase zymogen activated at the site of clot formation. Blood. 2010;115(25):5241–5248. doi: 10.1182/blood-2010-01-261610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ding B-S, Gottstein C, Grunow A, et al. Endothelial targeting of a recombinant construct fusing a PECAM-1 single-chain variable antibody fragment (scFv) with prourokinase facilitates prophylactic thrombolysis in the pulmonary vasculature. Blood. 2005;106(13):4191–4198. doi: 10.1182/blood-2005-05-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Runge MS, Quertermous T, Zavodny PJ, et al. A recombinant chimeric plasminogen activator with high affinity for fibrin has increased thrombolytic potency in vitro and in vivo . Proc. Natl Acad. Sci. USA. 1991;88(22):10337–10341. doi: 10.1073/pnas.88.22.10337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Oudin S, Libyh MT, Goossens D, et al. A soluble recombinant multimeric anti-Rh(D) single-chain Fv/CR1 molecule restores the immune complex binding ability of CR1-deficient erythrocytes. J. Immunol. 2000;164(3):1505–1513. doi: 10.4049/jimmunol.164.3.1505. [DOI] [PubMed] [Google Scholar]
- 198.Kina T, Ikuta K, Takayama E, et al. The monoclonal antibody TER-119 recognizes a molecule associated with glycophorin A and specifically marks the late stages of murine erythroid lineage. Br. J. Haematol. 2000;109(2):280–287. doi: 10.1046/j.1365-2141.2000.02037.x. [DOI] [PubMed] [Google Scholar]
- 199.Spitzer D, Unsinger J, Mao D, Wu X, Molina H, Atkinson JP. In vivo correction of complement regulatory protein deficiency with an inhibitor targeting the red blood cell membrane. J. Immunol. 2005;175(11):7763–7770. doi: 10.4049/jimmunol.175.11.7763. [DOI] [PubMed] [Google Scholar]
- 200.Spitzer D, Wu X, Ma X, Xu L, Ponder KP, Atkinson JP. Cutting edge: treatment of complement regulatory protein deficiency by retroviral in vivo gene therapy. J. Immunol. 2006;177(8):4953–4956. doi: 10.4049/jimmunol.177.8.4953. [DOI] [PubMed] [Google Scholar]
- 201.Zaitsev S, Spitzer D, Murciano J-C, et al. Targeting of a Mutant Plasminogen Activator to Circulating Red Blood Cells for Prophylactic Fibrinolysis. J. Pharmacol. Exp. Ther. 2010;332(3):1022–1031. doi: 10.1124/jpet.109.159194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kumada T, Dittman WA, Majerus PW. A role for thrombomodulin in the pathogenesis of thrombin-induced thromboembolism in mice. Blood. 1988;71(3):728–733. [PubMed] [Google Scholar]
- 203.Zaitsev S, Kowalska MA, Neyman M, et al. Targeting recombinant thrombomodulin fusion protein to red blood cells provides multifaceted thromboprophylaxis. Blood. 2012;119(20):4779–4785. doi: 10.1182/blood-2011-12-398149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kontos S, Kourtis IC, Dane KY, Hubbell JA. Engineering antigens for in situ erythrocyte binding induces T-cell deletion. Proc. Natl Acad. Sci. USA. 2013;110(1):E60–E68. doi: 10.1073/pnas.1216353110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Reid ME, Mohandas N. Red blood cell blood group antigens: structure and function. Semin. Hematol. 2004;41(2):93–117. doi: 10.1053/j.seminhematol.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 206.Reid ME, Lomas-Francis C, Olsson ML. The Blood Group Antigen Factsbook. Elsevier/Academic Press; Amsterdam: 2012. [Google Scholar]
- 207.Anstee DJ. The relationship between blood groups and disease. Blood. 2010;115(23):4635–4643. doi: 10.1182/blood-2010-01-261859. [DOI] [PubMed] [Google Scholar]
- 208.Levine P, Stetson RE. AN unusual case of intra-group agglutination. J. Am. Med. Assoc. 1939;113(2):126–127. doi: 10.1001/jama.251.10.1316. [DOI] [PubMed] [Google Scholar]
- 209.Landsteiner K, Wiener AS. An agglutinable factor in human blood recognized by immune sera for rhesus blood. Exp. Biol. Med. 1940;43(1):223–223. [Google Scholar]
- 210.Avent ND, Reid ME. The Rh blood group system: a review. Blood. 2000;95(2):375–387. [PubMed] [Google Scholar]
- 211.Chou ST, Westhoff CM. The Rh and RhAG blood group systems. Immunohematol. Am. Red Cross. 2010;26(4):178–186. [PubMed] [Google Scholar]
- 212.Huang CH, Chen Y, Reid ME, Seidl C. Rhnull disease: the amorph type results from a novel double mutation in RhCe gene on D-negative background. Blood. 1998;92(2):664–671. [PubMed] [Google Scholar]
- 213.Mouro-Chanteloup I, D'Ambrosio AM, Gane P, et al. Cell-surface expression of RhD blood group polypeptide is posttranscriptionally regulated by the RhAG glycoprotein. Blood. 2002;100(3):1038–1047. [PubMed] [Google Scholar]
- 214.Chérif-Zahar B, Bloy C, Le Van Kim C, et al. Molecular cloning and protein structure of a human blood group Rh polypeptide. Proc. Natl Acad. Sci. USA. 1990;87(16):6243–6247. doi: 10.1073/pnas.87.16.6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Huang C-H, Liu PZ. New insights into the Rh superfamily of genes and proteins in erythroid cells and nonerythroid tissues. Blood Cells. Mol. Dis. 2001;27(1):90–101. doi: 10.1006/bcmd.2000.0355. [DOI] [PubMed] [Google Scholar]
- 216.Chou ST, Jackson T, Vege S, Smith-Whitley K, Friedman DF, Westhoff CM. High prevalence of red blood cell alloimmunization in sickle cell disease despite transfusion from Rh-matched minority donors. Blood. 2013;122(6):1062–1071. doi: 10.1182/blood-2013-03-490623. [DOI] [PubMed] [Google Scholar]
- 217.Rouger P, Edelman L. Murine monoclonal antibodies associated with Rh17, Rh29, and Rh46 antigens. Transfusion. 1988;28(1):52–55. doi: 10.1046/j.1537-2995.1988.28188127953.x. [DOI] [PubMed] [Google Scholar]
- 218.Nash R, Shojania AM. Hematological aspect of Rh deficiency syndrome: a case report and a review of the literature. Am. J. Hematol. 1987;24(3):267–275. doi: 10.1002/ajh.2830240306. [DOI] [PubMed] [Google Scholar]
- 219.Tilley L, Green C, Poole J, et al. A new blood group system, RHAG: three antigens resulting from amino acid substitutions in the Rh-associated glycoprotein. Vox Sang. 2010;98(2):151–159. doi: 10.1111/j.1423-0410.2009.01243.x. [DOI] [PubMed] [Google Scholar]
- 220.Gruswitz F, Chaudhary S, Ho JD, et al. Function of human Rh based on structure of RhCG at 2.1 A. Proc. Natl Acad. Sci. USA. 2010;107(21):9638–9643. doi: 10.1073/pnas.1003587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hemker MB, Cheroutre G, van Zwieten R, et al. The Rh complex exports ammonium from human red blood cells. Br. J. Haematol. 2003;122(2):333–340. doi: 10.1046/j.1365-2141.2003.04425.x. [DOI] [PubMed] [Google Scholar]
- 222.Westhoff CM. Deciphering the function of the Rh family of proteins. Transfusion. 2005;45(2 Suppl.):S117–S121. doi: 10.1111/j.1537-2995.2005.00535.x. [DOI] [PubMed] [Google Scholar]
- 223.Parsons SF, Gardner B, Anstee DJ. Monoclonal antibodies against Kell glycoprotein: serology, immunochemistry and quantification of antigen sites. Transfus. Med. Oxf. Engl. 1993;3(2):137–142. doi: 10.1111/j.1365-3148.1993.tb00051.x. [DOI] [PubMed] [Google Scholar]
- 224.Jung HH, Danek A, Frey BM. McLeod syndrome: a neurohaematological disorder: McLeod syndrome. Vox Sang. 2007;93(2):112–121. doi: 10.1111/j.1423-0410.2007.00949.x. [DOI] [PubMed] [Google Scholar]
- 225.Tanner MJ. The structure and function of band 3 (AE1): recent developments (review) Mol. Membr. Biol. 1997;14(4):155–165. doi: 10.3109/09687689709048178. [DOI] [PubMed] [Google Scholar]
- 226.Peters LL, Shivdasani RA, Liu SC, et al. Anion exchanger 1 (band 3) is required to prevent erythrocyte membrane surface loss but not to form the membrane skeleton. Cell. 1996;86(6):917–927. doi: 10.1016/s0092-8674(00)80167-1. [DOI] [PubMed] [Google Scholar]
- 227.Bruce LJ, Tanner MJ. Erythroid band 3 variants and disease. Baillières Best Pract. Res. Clin. Haematol. 1999;12(4):637–654. doi: 10.1053/beha.1999.0046. [DOI] [PubMed] [Google Scholar]
- 228.Figueroa D. The Diego blood group system: a review. Immunohematol. Am. Red Cross. 2013;29(2):73–81. [PubMed] [Google Scholar]
- 229.Reid ME. MNS blood group system: a review. Immunohematol. Am. Red Cross. 2009;25(3):95–101. [PubMed] [Google Scholar]
- 230.Chasis JA, Mohandas N, Shohet SB. Erythrocyte membrane rigidity induced by glycophorin A-ligand interaction. Evidence for a ligand-induced association between glycophorin A and skeletal proteins. J. Clin. Invest. 1985;75(6):1919–1926. doi: 10.1172/JCI111907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Knowles DW, Chasis JA, Evans EA, Mohandas N. Cooperative action between band 3 and glycophorin A in human erythrocytes: immobilization of band 3 induced by antibodies to glycophorin A. Biophys. J. 1994;66(5):1726–1732. doi: 10.1016/S0006-3495(94)80965-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Greineder CF, Howard MD, Carnemolla R, Cines DB, Muzykantov VR. Advanced drug delivery systems for antithrombotic agents. Blood. 2013;122(9):1565–1575. doi: 10.1182/blood-2013-03-453498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Rungaldier S, Oberwagner W, Salzer U, Csaszar E, Prohaska R. Stomatin interacts with GLUT1/SLC2A1, band 3/SLC4A1, and aquaporin-1 in human erythrocyte membrane domains. Biochim. Biophys. Acta. 2013;1828(3):956–966. doi: 10.1016/j.bbamem.2012.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Lomas-Francis C, Reid ME. The Dombrock blood group system: a review. Immunohematol. Am. Red Cross. 2010;26(2):71–78. [PubMed] [Google Scholar]
- 235.Ballif BA, Helias V, Peyrard T, et al. Disruption of SMIM1 causes the Vel−blood type. EMBO Mol. Med. 2013;5(5):751–761. doi: 10.1002/emmm.201302466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Storry JR, Jöud M, Christophersen MK, et al. Homozygosity for a null allele of SMIM1 defines the Vel-negative blood group phenotype. Nat. Genet. 2013;45(5):537–541. doi: 10.1038/ng.2600. [DOI] [PubMed] [Google Scholar]
- 237.Helias V, Saison C, Ballif BA, et al. ABCB6 is dispensable for erythropoiesis and specifies the new blood group system Langereis. Nat. Genet. 2012;44(2):170–173. doi: 10.1038/ng.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Sirchia G, Zanella A, Parravicini A, Morelati F, Rebulla P, Masera G. Red cell alloantibodies in thalassemia major. Results of an Italian cooperative study. Transfusion. 1985;25(2):110–112. doi: 10.1046/j.1537-2995.1985.25285169198.x. [DOI] [PubMed] [Google Scholar]
- 239.Vichinsky EP, Earles A, Johnson RA, Hoag MS, Williams A, Lubin B. Alloimmunization in sickle cell anemia and transfusion of racially unmatched blood. N. Engl. J. Med. 1990;322(23):1617–1621. doi: 10.1056/NEJM199006073222301. [DOI] [PubMed] [Google Scholar]
- 240.Aminoff D, Bruegge WF, Bell WC, Sarpolis K, Williams R. Role of sialic acid in survival of erythrocytes in the circulation: interaction of neuraminidase-treated and untreated erythrocytes with spleen and liver at the cellular level. Proc. Natl Acad. Sci. USA. 1977;74(4):1521–1524. doi: 10.1073/pnas.74.4.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Lenny LL, Hurst R, Goldstein J, Benjamin LJ, Jones RL. Single-unit transfusions of RBC enzymatically converted from group B to group O to A and O normal volunteers. Blood. 1991;77(6):1383–1388. [PubMed] [Google Scholar]
- 242.Lenny LL, Hurst R, Goldstein J, Galbraith RA. Transfusions to group O subjects of 2 units of red cells enzymatically converted from group B to group O. Transfusion. 1994;34(3):209–214. doi: 10.1046/j.1537-2995.1994.34394196617.x. [DOI] [PubMed] [Google Scholar]
- 243.Lenny LL, Hurst R, Zhu A, Goldstein J, Galbraith RA. Multiple-unit and second transfusions of red cells enzymatically converted from group B to group O: report on the end of phase 1 trials. Transfusion. 1995;35(11):899–902. doi: 10.1046/j.1537-2995.1995.351196110892.x. [DOI] [PubMed] [Google Scholar]
- 244.Kruskall MS, AuBuchon JP, Anthony KY, et al. Transfusion to blood group A and O patients of group B RBCs that have been enzymatically converted to group O. Transfusion. 2000;40(11):1290–1298. doi: 10.1046/j.1537-2995.2000.40111290.x. [DOI] [PubMed] [Google Scholar]
- 245.Falk P, Hoskins LC, Lindstedt R, Svanborg C, Larson G. Deantigenation of human erythrocytes by bacterial glycosidases – evidence for the noninvolvement of medium-sized glycosphingolipids in the Dolichos biflorus lectin hemagglutination. Arch. Biochem. Biophys. 1991;290(2):312–319. doi: 10.1016/0003-9861(91)90546-u. [DOI] [PubMed] [Google Scholar]
- 246.Goldstein J. Preparation of transfusable red cells by enzymatic conversion. Prog. Clin. Biol. Res. 1984;165:139–157. [PubMed] [Google Scholar]
- 247.Goldstein J. Conversion of ABO blood groups. Transfus. Med. Rev. 1989;3(3):206–212. doi: 10.1016/s0887-7963(89)70080-8. [DOI] [PubMed] [Google Scholar]
- 248.Hata J, Dhar M, Mitra M, et al. Purification and characterization of N-acetyl-alpha-D-galactosaminidase from Gallus domesticus. Biochem. Int. 1992;28(1):77–86. [PubMed] [Google Scholar]
- 249.Hoskins LC, Boulding ET. Changes in immunologic properties of group A RBCs during treatment with an A-degrading exo-α-N-acetylgalactosaminidase. Transfusion. 2001;41(7):908–916. doi: 10.1046/j.1537-2995.2001.41070908.x. [DOI] [PubMed] [Google Scholar]
- 250.Hoskins LC, Boulding ET, Larson G. Purification and characterization of blood group A-degrading isoforms of alpha-N-acetylgalactosaminidase from Ruminococcus torques strain IX-70. J. Biol. Chem. 1997;272(12):7932–7939. doi: 10.1074/jbc.272.12.7932. [DOI] [PubMed] [Google Scholar]
- 251.Hsieh H-Y, Smith D. Clostridium perfringens alpha-N-acetylgalactosaminidase blood group A2-degrading activity. Biotechnol. Appl. Biochem. 2003;37(2):157–163. doi: 10.1042/ba20020073. [DOI] [PubMed] [Google Scholar]
- 252.Izumi K, Yamamoto K, Tochikura T, Hirabayashi Y. Blood substitutes, present and future perspectives [Internet] α-N-acetylgalactosaminidase from Acremonium sp. Biochim. Biophys. Acta. 1992;1116(1):72–74. doi: 10.1016/0304-4165(92)90130-m. [DOI] [PubMed] [Google Scholar]
- 253.Zhu A, Monahan C, Wang ZK, Goldstein J. Expression, purification, and characterization of recombinant alpha-N-acetylgalactosaminidase produced in the yeast Pichia pastoris . Protein Expr. Purif. 1996;8(4):456–462. doi: 10.1006/prep.1996.0124. [DOI] [PubMed] [Google Scholar]
- 254.Liu QP, Sulzenbacher G, Yuan H, et al. Bacterial glycosidases for the production of universal red blood cells. Nat. Biotechnol. 2007;25(4):454–464. doi: 10.1038/nbt1298. [DOI] [PubMed] [Google Scholar]
- 255.Olsson ML, Clausen H. Modifying the red cell surface: towards an ABO-universal blood supply. Br. J. Haematol. 2008;140(1):3–12. doi: 10.1111/j.1365-2141.2007.06839.x. [DOI] [PubMed] [Google Scholar]
- 256.Tsuchida E. Blood Substitutes, Present and Future Perspectives [Internet] Elsevier Science; http://books.google.com/books?id=IvnREjqUiKIC [Google Scholar]
- 257.Armstrong JK, Meiselman HJ, Fisher TC. Covalent binding of poly(ethylene glycol) (PEG) to the surface of red blood cells inhibits aggregation and reduces low shear blood viscosity. Am. J. Hematol. 1997;56(1):26–28. doi: 10.1002/(sici)1096-8652(199709)56:1<26::aid-ajh5>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 258.Murad KL, Mahany KL, Brugnara C, Kuypers FA, Eaton JW, Scott MD. Structural and functional consequences of antigenic modulation of red blood cells with methoxypoly(ethylene glycol) Blood. 1999;93(6):2121–2127. [PubMed] [Google Scholar]
- 259.Scott MD, Murad KL, Koumpouras F, Talbot M, Eaton JW. Chemical camouflage of antigenic determinants: stealth erythrocytes. Proc. Natl Acad. Sci. USA. 1997;94(14):7566–7571. doi: 10.1073/pnas.94.14.7566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Wang D, Kyluik DL, Murad KL, Toyofuku WM, Scott MD. Polymer-mediated immunocamouflage of red blood cells: effects of polymer size on antigenic and immunogenic recognition of allogeneic donor blood cells. Sci. China Life Sci. 2011;54(7):589–598. doi: 10.1007/s11427-011-4190-x. [DOI] [PubMed] [Google Scholar]
- 261.Bradley AJ, Murad KL, Regan KL, Scott MD. Biophysical consequences of linker chemistry and polymer size on stealth erythrocytes: size does matter. Biochim. Biophys. Acta. 2002;1561(2):147–158. doi: 10.1016/s0005-2736(02)00339-5. [DOI] [PubMed] [Google Scholar]
- 262.Neu B, Armstrong JK, Fisher TC, Bäumler H, Meiselman HJ. Electrophoretic mobility of human red blood cells coated with poly(ethylene glycol) Biorheology. 2001;38(5–6):389–403. [PubMed] [Google Scholar]
- 263.Rossi NAA, Constantinescu I, Kainthan RK, Brooks DE, Scott MD, Kizhakkedathu JN. Red blood cell membrane grafting of multi-functional hyperbranched polyglycerols. Biomaterials. 2010;31(14):4167–4178. doi: 10.1016/j.biomaterials.2010.01.137. [DOI] [PubMed] [Google Scholar]
- 264.Liu Z, Janzen J, Brooks DE. Adsorption of amphiphilic hyperbranched polyglycerol derivatives onto human red blood cells. Biomaterials. 2010;31(12):3364–3373. doi: 10.1016/j.biomaterials.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 265.Janvier F, Zhu JXX, Armstrong J, Meiselman HJ, Cloutier G. Effects of amphiphilic star-shaped poly(ethylene glycol) polymers with a cholic acid core on human red blood cell aggregation. J. Mech. Behav. Biomed. Mater. 2013;18:100–107. doi: 10.1016/j.jmbbm.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Moghimi SM, Hunter AC, Dadswell CM, Savay S, Alving CR, Szebeni J. Causative factors behind poloxamer 188 (Pluronic F68, Flocor)-induced complement activation in human sera. A protective role against poloxamer-mediated complement activation by elevated serum lipoprotein levels. Biochim. Biophys. Acta. 2004;1689(2):103–113. doi: 10.1016/j.bbadis.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 267.Szebeni J. Complement activation-related pseudoallergy: a new class of drug-induced acute immune toxicity. Toxicology. 2005;216(2–3):106–121. doi: 10.1016/j.tox.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 268.Kyluik-Price DL, Li L, Scott MD. Comparative efficacy of blood cell immunocamouflage by membrane grafting of methoxypoly(ethylene glycol) and polyethyloxazoline. Biomaterials. 2014;35(1):412–422. doi: 10.1016/j.biomaterials.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 269.Armstrong JK, Hempel G, Koling S, et al. Antibody against poly(ethylene glycol) adversely affects PEG-asparaginase therapy in acute lymphoblastic leukemia patients. Cancer. 2007;110(1):103–111. doi: 10.1002/cncr.22739. [DOI] [PubMed] [Google Scholar]
- 270.Chambers E, Mitragotri S. Prolonged circulation of large polymeric nanoparticles by non-covalent adsorption on erythrocytes. J. Control. Release. 2004;100(1):111–119. doi: 10.1016/j.jconrel.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 271.Anselmo AC, Gupta V, Zern BJ, et al. Delivering nanoparticles to lungs while avoiding liver and spleen through adsorption on red blood cells. ACS Nano. 2013;7(12):11129–11137. doi: 10.1021/nn404853z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Chen K, Merkel TJ, Pandya A, et al. Low modulus biomimetic microgel particles with high loading of hemoglobin. Biomacromolecules. 2012;13(9):2748–2759. doi: 10.1021/bm3007242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Doshi N, Zahr AS, Bhaskar S, Lahann J, Mitragotri S. Red blood cell-mimicking synthetic biomaterial particles. Proc. Natl Acad. Sci. USA. 2009;106(51):21495–21499. doi: 10.1073/pnas.0907127106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Merkel TJ, Jones SW, Herlihy KP, et al. Using mechanobiological mimicry of red blood cells to extend circulation times of hydrogel microparticles. Proc. Natl. Acad. Sci. 2011;108(2):586–591. doi: 10.1073/pnas.1010013108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Rodriguez PL, Harada T, Christian DA, Pantano DA, Tsai RK, Discher DE. Minimal “self” peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science. 2013;339(6122):971–975. doi: 10.1126/science.1229568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Koshida S, Asanuma K, Kuribayashi K, et al. Prevalence of human anti-mouse antibodies (HAMAs) in routine examinations. Clin. Chim. Acta. 2010;411(5–6):391–394. doi: 10.1016/j.cca.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 277.Kricka LJ. Human anti-animal antibody interferences in immunological assays. Clin. Chem. 1999;45(7):942–956. [PubMed] [Google Scholar]
- 278.Winter G, Milstein C. Man-made antibodies. Nature. 1991;349(6307):293–299. doi: 10.1038/349293a0. [DOI] [PubMed] [Google Scholar]
- 279.Bratkovic T. Progress in phage display: evolution of the technique and its application. Cell. Mol. Life Sci. CMLS. 2010;67(5):749–767. doi: 10.1007/s00018-009-0192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Geyer CR, McCafferty J, Dübel S, Bradbury ARM, Sidhu SS. Recombinant antibodies and in vitro selection technologies. Methods Mol. Biol. 2012;901:11–32. doi: 10.1007/978-1-61779-931-0_2. [DOI] [PubMed] [Google Scholar]
- 281.Siegel DL. Phage display-based molecular methods in immunohematology. Transfusion. 2007;47(1 Suppl.):S89–S94. doi: 10.1111/j.1537-2995.2007.01318.x. [DOI] [PubMed] [Google Scholar]
- 282.Aitken R. Antibody Phage Display: Methods and Protocols. Humana; NY, USA: 2009. [Google Scholar]
- 283.Barbas CF. Phage Display: A Laboratory Manual. Cold Spring Harbor Laboratory Press; http://books.google.com/books?id=V7bwAAAAMAAJ [Google Scholar]
- 284.Clackson T, Lowman HB. Phage Display: A Practical Approach. Oxford University Press; http://books.google.com/books?id=CQ1rnQEACAAJ [Google Scholar]
- 285.Sidhu SS, Geyer CR. Phage Display In Biotechnology and Drug Discovery. Taylor & Francis; http://books.google.com/books?id=_xvXhluNfrkC [Google Scholar]
- 286.Smith GP. Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science. 1985;228(4705):1315–1317. doi: 10.1126/science.4001944. [DOI] [PubMed] [Google Scholar]
- 287.Burton DR, Barbas CF, Persson MA, Koenig S, Chanock RM, Lerner RA. A large array of human monoclonal antibodies to type 1 human immunodeficiency virus from combinatorial libraries of asymptomatic seropositive individuals. Proc. Natl Acad. Sci. USA. 1991;88(22):10134–10137. doi: 10.1073/pnas.88.22.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Persson MA, Caothien RH, Burton DR. Generation of diverse high-affinity human monoclonal antibodies by repertoire cloning. Proc. Natl Acad. Sci. USA. 1991;88(6):2432–2436. doi: 10.1073/pnas.88.6.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Chang TY, Siegel DL. Genetic and immunological properties of phage-displayed human anti-Rh(D) antibodies: implications for Rh(D) epitope topology. Blood. 1998;91(8):3066–3078. [PubMed] [Google Scholar]
- 290.Chang TY, Siegel DL. Isolation of an IgG anti-B from a human Fab-phage display library. Transfusion. 2001;41(1):6–12. doi: 10.1046/j.1537-2995.2001.41010006.x. [DOI] [PubMed] [Google Scholar]
- 291.Siegel DL, Chang TY, Russell SL, Bunya VY. Isolation of cell surface-specific human monoclonal antibodies using phage display and magnetically-activated cell sorting: applications in immunohematology. J. Immunol. Methods. 1997;206(1–2):73–85. doi: 10.1016/s0022-1759(97)00087-2. [DOI] [PubMed] [Google Scholar]
- 292.Siegel DL, Silberstein LE. Expression and characterization of recombinant anti-Rh(D) antibodies on filamentous phage: a model system for isolating human red blood cell antibodies by repertoire cloning. Blood. 1994;83(8):2334–2344. [PubMed] [Google Scholar]
- 293.Klein HG, Anstee DJ. Mollison's Blood Transfusion in Clinical Medicine. Wiley; NY, USA: 2013. [Google Scholar]
- 294.Lloyd C, Lowe D, Edwards B, et al. Modelling the human immune response: performance of a 1011 human antibody repertoire against a broad panel of therapeutically relevant antigens. Protein Eng. Des. Sel. PEDS. 2009;22(3):159–168. doi: 10.1093/protein/gzn058. [DOI] [PubMed] [Google Scholar]
- 295.Schofield DJ, Pope AR, Clementel V, et al. Application of phage display to high throughput antibody generation and characterization. Genome Biol. 2007;8(11):R254. doi: 10.1186/gb-2007-8-11-r254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Martineau P. Methods Mol. Biol. Vol. 178. Clifton NJ: 2002. Error-prone polymerase chain reaction for modification of scFvs; pp. 287–294. [DOI] [PubMed] [Google Scholar]
- 297.Thie H, Voedisch B, Dübel S, Hust M, Schirrmann T. Affinity maturation by phage display. In: Dimitrov AS, editor. Therapeutic Antibodies. Humana Press; NY, USA: 2009. pp. 309–322. [DOI] [PubMed] [Google Scholar]
- 298.Nishibori N, Horiuchi H, Furusawa S, Matsuda H. Humanization of chicken monoclonal antibody using phage-display system. Mol. Immunol. 2006;43(6):634–642. doi: 10.1016/j.molimm.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 299.Popkov M, Mage RG, Alexander CB, Thundivalappil S, Barbas CF, Rader C. Rabbit immune repertoires as sources for therapeutic monoclonal antibodies: the impact of kappa allotype-correlated variation in cysteine content on antibody libraries selected by phage display. J. Mol. Biol. 2003;325(2):325–335. doi: 10.1016/s0022-2836(02)01232-9. [DOI] [PubMed] [Google Scholar]
- 300.Knappik A, Ge L, Honegger A, et al. Fully synthetic human combinatorial antibody libraries (HuCAL) based on modular consensus frameworks and CDRs randomized with trinucleotides. J. Mol. Biol. 2000;296(1):57–86. doi: 10.1006/jmbi.1999.3444. [DOI] [PubMed] [Google Scholar]
- 301.Krebs B, Rauchenberger R, Reiffert S, et al. High-throughput generation and engineering of recombinant human antibodies. J. Immunol. Methods. 2001;254(1–2):67–84. doi: 10.1016/s0022-1759(01)00398-2. [DOI] [PubMed] [Google Scholar]
- 302.Jespers LS, Roberts A, Mahler SM, Winter G, Hoogenboom HR. Guiding the selection of human antibodies from phage display repertoires to a single epitope of an antigen. Biotechnol. Nat. Publ. Co. 1994;12(9):899–903. doi: 10.1038/nbt0994-899. [DOI] [PubMed] [Google Scholar]
- 303.Osbourn J, Groves M, Vaughan T. From rodent reagents to human therapeutics using antibody guided selection. Methods San Diego Calif. 2005;36(1):61–68. doi: 10.1016/j.ymeth.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 304.Shi J, Kundrat L, Pishesha N, et al. Engineered red blood cells as carriers for systemic delivery of a wide array of functional probes. Proc. Natl Acad. Sci. USA. 2014;111(28):10131–10136. doi: 10.1073/pnas.1409861111. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This study is among the first examples of ex-vivo cellular genetic engineering of erythrocytic cells for application in drug delivery.