Summary
Transcriptional deregulation plays a major role in acute myeloid leukemia, and therefore identification of epigenetic modifying enzymes essential for the maintenance of oncogenic transcription programs holds the key to better understanding of the biology and designing effective therapeutic strategies for the disease. Here we provide experimental evidence for the functional involvement and therapeutic potential of targeting PRMT1, an H4R3 methyltransferase, in various MLL and non-MLL leukemias. PRMT1 is necessary but not sufficient for leukemic transformation, which requires co-recruitment of KDM4C, an H3K9 demethylase, by chimeric transcription factors to mediate epigenetic reprogramming. Pharmacological inhibition of KDM4C/PRMT1 suppresses transcription and transformation ability of MLL fusions and MOZ-TIF2, revealing a tractable aberrant epigenetic circuitry mediated by KDM4C and PRMT1 in acute leukemia.
Keywords: histone methyltransferase, histone demethylase, leukemia epigenetics, KMT, PMT, KDM, PRMT1, KDM4C, MLL fusions, MOZ-TIF2, acute myeloid leukemia, AML
Graphical Abstract
Highlights
-
•
PRMT1 recruitment is necessary but not sufficient for transformation by AML fusions
-
•
MLL fusions and MOZ-TIF2 recruit KDM4C to remove H3K9me3 repressive marks
-
•
KDM4C and PRMT1 regulate aberrant epigenetic and transcriptional networks in AML
-
•
Molecular or pharmacology inhibition of KDM4C or PRMT1 suppresses AML
Cheung et al. show that PRMT1 is necessary but insufficient for acute myeloid leukemia induction by chimeric transcription factors, which also recruit KDM4C for epigenetic reprogramming. Pharmacological inhibition of KDM4C/PRMT1 suppresses transcription and transformation abilities of the MOZ-TIF2 and MLL fusions.
Significance
While the recent launch of phase I clinical trials with protein-methyltransferase (PMT) inhibitors has ignited the enthusiasm for targeting oncogenic transcription factors, our understanding of the functions of PMTs in cancer development is still in its infancy. This has limited the potential for exploiting this group of promising targets. Here, we reveal critical functions and preclinical in vivo evidence for targeting a second class of PMTs, PRMT, and histone demethylases (KDMs) in cancer. PRMT1 is necessary but not sufficient for leukemia induction by chimeric transcription factors, which also recruit KDM4C, for epigenetic reprogramming. Genetic or pharmacological inhibition of KDM4C/PRMT1 suppresses transcription and transformation abilities of the MOZ-TIF2 and MLL fusions, providing druggable therapeutic targets and molecular insights for the development of epigenetic therapy.
Introduction
Human leukemia is characterized by the prevalence of recurrent chromosomal translocations, resulting in the generation of chimeric fusion proteins with aberrant oncogenic activities (Look, 1997). Successful therapeutic exploitation of BCR-ABL fusion in chronic myeloid leukemia (CML) by the small molecular inhibitor imatinib has become the paradigm of targeted therapy (Kantarjian et al., 2002). In contrast, there has been very little progress in targeting classically intractable oncogenic transcription factors, which are the common drivers for many other malignancies including acute myeloid leukemia (AML) (Zeisig et al., 2012). With the exception of acute promyelocytic leukemia (APL) for which targeted therapy has been developed, transforming it from a highly fatal disease to a manageable condition (Arteaga et al., 2015, Wang and Chen, 2008), all AML patients still receive the same chemotherapy treatment developed more than half a century ago, which only induces long-term complete remission in less than 40% of young patients and is generally too toxic to use in patients aged older than 60 years (Zeisig et al., 2012). Therefore, there is an urgent need to understand the underlying transformation mechanisms and develop better therapeutic strategies for AML.
In contrast to kinases that already have functional enzymatic activities, transcription factors need to work in tandem with other co-factors to orchestrate an array of epigenetic modifications for regulating gene expression. Among these factors are protein methyltransferases (PMTs), consisting of lysine methyltransferases (KMTs) and arginine methyltransferases (PRMTs), which have recently taken the center stage as key players in transcription regulation during both normal and disease development (Abdel-Wahab and Levine, 2013, Cheung and So, 2011, Kouzarides, 2007). The involvements and therapeutic potential of targeting PMTs in human cancer were initially illustrated in MLL leukemia where the recruitments of DOT1L by MLL-AF10 (Okada et al., 2005) and PRMT1 by MLL-EEN (Cheung et al., 2007) were required for transcriptional deregulation and cellular transformation. Since then, additional members of the PMT family have been reported to be involved in different cancers (Campbell and Tummino, 2014, Cheung and So, 2011, Shih et al., 2012). The promise of targeting PMTs for cancer treatment has been highlighted by the successful development of chemical inhibitors against DOT1L for MLL leukemia (Daigle et al., 2011) and EZH2 for B-cell lymphoma carrying EZH2-activating mutations (Knutson et al., 2012, McCabe et al., 2012); these are now entering phase I clinical trials.
Despite the success in development of inhibitors, the field is still in its infancy and the involvements of PMTs, in particular PRMTs, in other leukemias are still largely unexplored. More importantly, we have very little knowledge about the mechanisms and molecular networks that underpin the oncogenic functions mediated by these individual PMTs. The discovery of JmjC domain-containing lysine demethylases (KDMs) has provided unique insights into dynamic regulation of histone methylation for gene regulation. KDMs can work together with specific KMTs to remove the opposing methylation marks to reinforce particular epigenetic programs for gene expression (Cloos et al., 2008). Consistent with this, a recent study reported an important role of KDM5B in suppressing the epigenetic program and function of leukemic stem cells, while its therapeutic value has yet to be demonstrated by pharmacological means (Wong et al., 2015). On the other hand, members of KDMs including JHDM1B (He et al., 2011) and JMJD1C (Sroczynska et al., 2014) have been shown to be required for leukemic transformation. In spite of these interesting observations indicating important and contrasting roles of KDMs in leukemogenesis, very little is known about their actual functions and underlying mechanisms. It is not clear whether and how the dynamic functional interplay between PMTs and KDMs takes part in regulating this critical process. More importantly, in contrast to the recent demonstration of specific in vivo efficacy of poly(ADP ribose) polymerase (PARP) inhibitors in certain subtypes of AML (Esposito et al., 2015), there have been no in vivo pharmacological inhibitor data showing the potential value of targeting KDMs for leukemia suppression. Together, these have significantly hindered the potential translation of these findings into the relevant clinical utility. Therefore, elucidation of the functional and mechanistic involvement of histone methylation machinery will shed light on the ongoing efforts to understand the mechanisms underlying the roles of these different epigenetic modifying enzymes in cancer biology, and the development of effective therapeutic strategies targeting the associated oncogenic transcription factors in human cancer.
Results
Identification of Prmt1-Dependent Leukemia Fusion Proteins
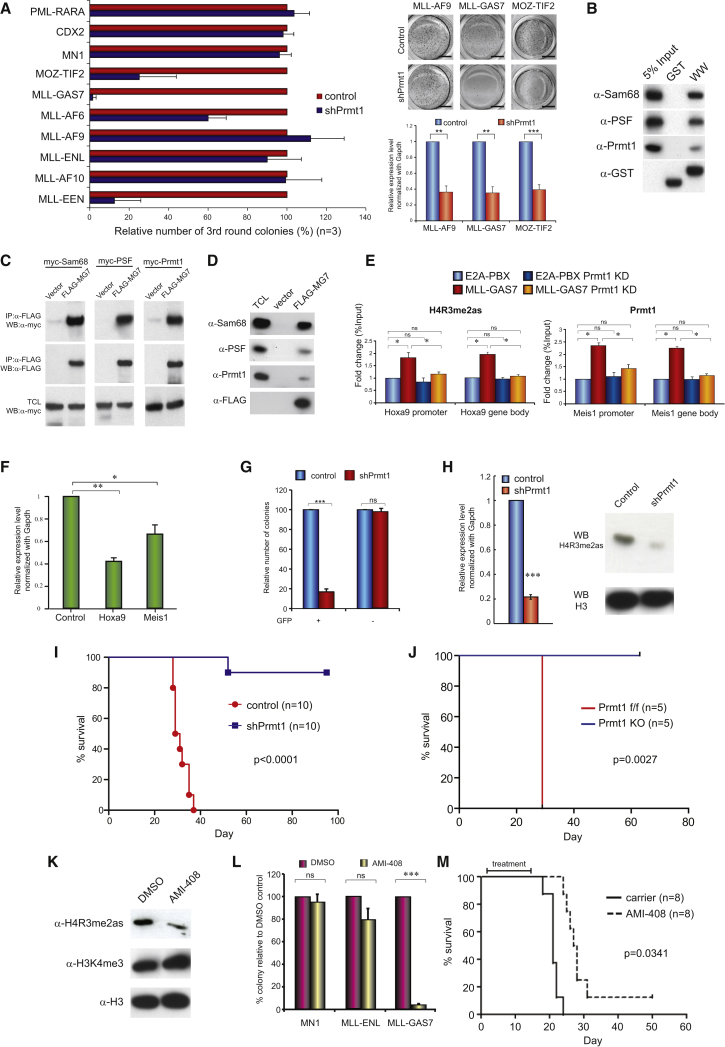
To define the functional involvement of Prmt1 in leukemias, we performed a systematic functional screen by retroviral transduction and transformation assay (RTTA) using validated Prmt1 small hairpin RNAs (shRNAs) on more than ten different MLL and non-MLL oncogenic transcription factors. As a control, downregulation of Prmt1 suppressed MLL-EEN-mediated transformation of primary c-kit enriched hematopoietic stem/progenitor cells (HSPCs) (Cheung et al., 2007). While transformation mediated by most of the other MLL fusions were not affected by Prmt1 knockdown, MLL-GAS7 (So et al., 2003a) and MOZ-TIF2 (Huntly et al., 2004) exhibited high degrees of Prmt1 dependence, resulting in a significant suppression of colony formation (Figure 1A). These data were reproduced using an independent Prmt1 shRNA (shPrmt1#2) (Figures S1A and S1B). In line with the RTTA data, suppression of Prmt1 resulted in an increased differentiation (Figure S1C), cell-cycle arrest particularly at G1 checkpoint (Figure S1D), and an enhanced apoptosis (Figure S1E) in both MLL-GAS7 and MOZ-TIF2 transformed cells. Discovery of additional Prmt1-dependent oncogenic fusions prompted us to speculate whether they utilized similar epigenetic machinery and, hence, recruitment of communal transcription complexes to transform HSPC. Consistent with this idea, GAS7 WW domain has been proposed to interact with Sam68 and PSF (Ingham et al., 2005), which are the key components of MLL-EEN/Prmt1 transcriptional complex (Cheung et al., 2007). To this end, both in vitro glutathione S-transferase (GST) pull-down using GAS7 WW domain (Figure 1B) and immunoprecipitation assay by co-expression of candidate proteins (Figure 1C) had successfully demonstrated the ability of GAS7 to recruit Sam68, PSF, and Prmt1. In addition, we also confirmed in vivo interactions between MLL-GAS7 and the endogenous Sam68, PSF, and Prmt1 by immunoprecipitation experiments using antibodies specific to the endogenous proteins (Figure 1D). To further evaluate the in vivo interaction in the context of chromatin and their epigenetic functions, we deployed chromatin immunoprecipitation (ChIP) to demonstrate the specific binding of Prmt1 and the associated asymmetric H4R3 dimethylation (H4R3me2as) activation mark to the downstream targets of MLL fusion, Hoxa9 (Figure 1E). As a result, we were able to detect significant enhancements of Prmt1 binding and the associated H4R3me2as marks in both the promoter and gene body regions of Hoxa9 in MLL-GAS7 transformed cells but not in the E2A-PBX control (Figure 1E). Conversely, loss of Prmt1 through shRNA-mediated knockdown resulted in a reduction of H4R3me2as mark (Figure 1E) and the suppressed expression of MLL downstream targets (Figure 1F), confirming a critical function of Prmt1 in MLL-GAS7-mediated transcription deregulation.
Figure 1.
Targeting of Prmt1 Suppresses MLL-GAS7 Leukemia
(A) Effect of Prmt1 knockdown on serial replating of transformed cells induced by various leukemic fusions. qRT-PCR analysis of Prmt1 knockdown in leukemic cells. Scale bars represent 0.5 cm.
(B) GST pull-down assays to show the interaction of GAS7 WW domain (WW) with Sam68, PSF, and Prmt1 in vitro.
(C and D) Co-immunoprecipitation of FLAG-MLL-GAS7 with myc-tagged Prmt1, Sam68, and PSF (C) and endogenous Sam68, Prmt1, and PSF (D).
(E) ChIP analysis on the effect of Prmt1 knockdown on H4R3me2as mark and Prmt1 binding in Hoxa9 promoter and gene body region of MLL-GAS7 and E2A-PBX.
(F) qRT-PCR analysis on Hoxa9 and Meis1 expression in MLL-GAS7 after Prmt1 knockdown.
(G) MLL-GAS7 leukemic cells transduced with control or shPrmt1 lentivirus expressing GFP markers. Transduced populations were sorted based on GFP expression and plated into methylcellulose to study colony-forming ability.
(H) Prmt1 knockdown was validated by qRT-PCR, and its effect on H4R3me2as marks was analyzed by western blot with histone H3 as the loading control.
(I) Kaplan-Meier survival analysis of the effect of Prmt1 knockdown on MLL-GAS7 leukemogenesis (log-rank test p < 0.0001). Median disease latency: control, 30 days; shPrmt1, undefined.
(J) Kaplan-Meier survival analysis of mice transplanted with wild type (WT) or Prmt1 knockout (KO) MLL-GAS7 leukemia cells (log-rank test p = 0.0027). Median disease latency: WT, 29 days; Prmt1 KO, undefined.
(K) Western blot analysis of H4R3me2as and H3K4me3 after AMI-408 treatment with histone H3 control for histone loading.
(L) Effect of AMI-408 on colony formation of murine leukemia cell lines.
(M) Kaplan-Meier survival analysis on the effect of AMI-408 treatment on MLL-GAS7 leukemogenesis (log-rank test p = 0.0341). Median disease latency: control, 21 days; AMI-408, 27.5 days.
All data shown are mean and SD (n = 3) unless otherwise specified. See also Figure S1. For all figures, asterisks indicate ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001; ns, not significant.
PRMT1 Is Required for Maintenance of MLL-GAS7 Leukemia
To investigate whether Prmt1 is required for not only initiation (Figure 1A) but also maintenance of the leukemic transformation, we transduced MLL-GAS7 full-blown leukemia cells from primary transplanted mice (So et al., 2003b) with lentivirus co-expressing a GFP marker and Prmt1 shRNA or a scramble control for in vitro and in vivo transformation assays. In contrast to GFP-negative cells, which did not show any significant difference in colony-forming ability regardless of shRNA constructs being used, GFP-positive cells carrying shPrmt1 had a severely compromised colony-forming ability compared with their scramble control (Figure 1G). The effectiveness of Prmt1 knockdown was confirmed by both qRT-PCR on Prmt1 mRNA and immunoblot on the associated H4R3me2as mark (Figure 1H). To assess the in vivo leukemogenic function of Prmt1, we transplanted MLL-GAS7 cells into syngeneic mice for disease development. Cohorts transplanted with Prmt1 knockdown leukemia cells exhibited increased disease latency and a reduced penetrance compared with the scramble control (log-rank test p < 0.0001) (Figures 1I, S1F, and S1G). Interestingly, the only mouse transplanted with Prmt1 knockdown cells that succumbed to leukemia re-expressed high levels of Prmt1 and Hoxa9 (Figure S1H), suggesting a high selective pressure against Prmt1 knockdown for leukemia development. To further address this point, we developed a Prmt1 Cre-ER conditional knockout mouse where exons 5–6 spanning the catalytic domain could be conditionally deleted upon tamoxifen treatment, resulting in a truncated protein. Using primary c-kit+ HSPCs from this Prmt1flox/flox Cre-ER mouse for RTTA, we observed an even more prominent suppression of MLL-GAS7 transformed cells both in vitro (Figures S1I and S1J) and in vivo (Figure 1J) whereby none of the mice developed leukemia upon Prmt1 deletion. Together, these independent approaches confirm a critical function of Prmt1 in both leukemia initiation and maintenance.
Pharmacological Inhibition of PRMT1 Suppresses AML In Vivo
To further demonstrate the therapeutic potential of targeting Prmt1, we examined the effect of an early-phase PRMT1 inhibitor, AMI-408 (Bonham et al., 2010) (Figure S1K) on the suppression of MLL-GAS7 mediated leukemogenesis. Consistently, treatment of MLL-GAS7 leukemia cells with AMI-408 resulted in the reduction of H4R3me2as mark (Figure 1K) and reduced colony-forming ability (Figure 1L). Importantly, in vivo administration of AMI-408 to mice transplanted with pretreated MLL-GAS7 leukemia cells significantly extended the survival and reduced disease penetrance compared with the carrier control (p = 0.0341) (Figure 1L), revealing the therapeutic potential of targeting Prmt1 by a small-molecule inhibitor.
Recruitment of PRMT1 Is Indispensable for MOZ-TIF2-Mediated Leukemogenesis
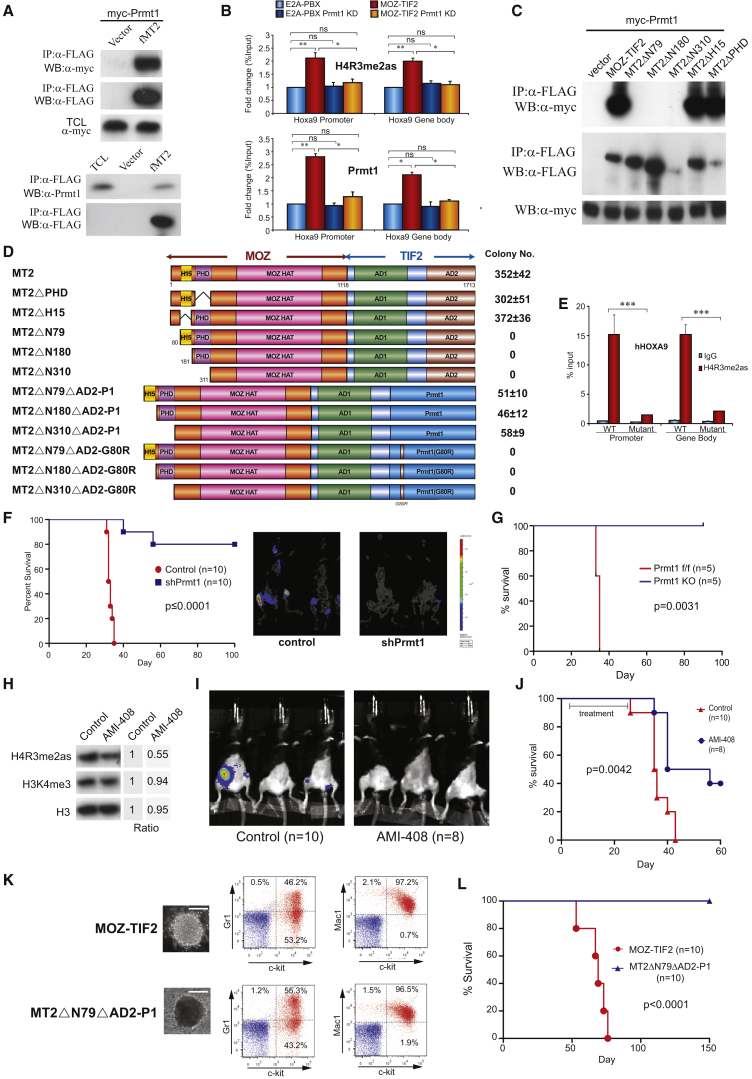
To further understand the functional role of Prmt1 in other leukemia subtypes, we sought to dissect the roles of Prmt1 in MOZ-TIF2-mediated transformation. Given that aberrant recruitment of Prmt1 appears to be a common feature shared by different MLL fusions, we intuitively examined the possible recruitment of Prmt1 by MOZ-TIF2. Using immunoprecipitation assays, we were able to show the specific interaction of MOZ-TIF2 with both ectopically expressed and endogenous Prmt1 (Figure 2A). To further demonstrate the in vivo functional interaction in MOZ-TIF2 leukemic cells, ChIP analysis revealed specific recruitment of Prmt1 and a high level of H4R3me2as mark on the downstream targets of MOZ-TIF2, Hoxa9 loci (Katsumoto et al., 2006, Kvinlaug et al., 2011), implicating a mechanistic similarity among those PRMT1-dependent leukemic fusions (Figure 2B). To gain insights into this Prmt1 interaction, we prepared various MOZ-TIF2 deletion mutants, which were used to map the Prmt1 interaction domain by co-immunoprecipitation assays. As a result, MOZ 5′ was sufficient to recruit Prmt1, and deletion of its N-terminal 310 amino acids (containing an N-terminal domain, H15 and PHD) completely abolished the interaction (Figures 2C and 2D). Further progressive deletion analysis refined the first 79 amino acids of the N-terminal domain but not H15 and PHD of the fusion as the minimal interaction domain required for Prmt1 recruitment, and conferring its epigenetic mark (Figures 2C–2E).
Figure 2.
Recruitment of Prmt1 by MOZ-TIF2 Is Necessary but Not Sufficient for HSPC Transformation
(A) Co-immunoprecipitation of FLAG-MOZ-TIF2 (fMT2) with myc-Prmt1 (upper) and endogenous Prmt1 (lower).
(B) ChIP analysis of H4R3me2as and Prmt1 localization on Hoxa9 promoter and gene body in MOZ-TIF2 after Prmt1 knockdown (KD).
(C) Co-immunoprecipitation of different FLAG-MOZ-TIF2 deletion mutants (indicated in D) and myc-Prmt1.
(D) RTTA to study the effect of N-terminal deletion mutants, active and inactive Prmt1 (P1) rescue fusions of MOZ-TIF2 on leukemic transformation.
(E) ChIP analysis of H4R3me2as mark on HOXA9 loci in HEK293 cells transfected with WT MOZ-TIF2 or ΔN79.
(F) Kaplan-Meier survival analysis of the effect of Prmt1 knockdown on MOZ-TIF2-mediated leukemogenesis (log-rank test p ≤ 0.0001). Bioluminescence imaging was performed at 21 days after transplantation. Median disease latency: control, 33 days; shPrmt1, undefined.
(G) Kaplan-Meier survival analysis of mice transplanted with WT or Prmt1 KO MOZ-TIF2 leukemia cells (log-rank test p = 0.0031). Median disease latency: WT, 35 days; Prmt1 KO, undefined.
(H) Western blot analysis on the effect of AMI-408 on H4R3me2as, H3K4me3, and the loading control histone H3. Band intensity ratio was determined by densitometry and normalized to vehicle control.
(I) Bioluminescence imaging of mice transplanted with MOZ-TIF2-luciferase leukemic cells 3 weeks after AMI-408 or carrier treatment.
(J) Kaplan-Meier survival analysis of AMI-408 and control treatment on MOZ-TIF2 leukemogenesis (log-rank test p = 0.0042). Median disease latency: control, 35.5 days; AMI-408, 48 days.
(K) Morphology of third-round colony of HSPC transformed by MOZ-TIF2 and its Prmt1 rescue fusion. FACS analysis of the transformed cells stained with c-kit, Gr1, and Mac1. Scale bars represent 50 μm.
(L) Kaplan-Meier survival analysis of mice transplanted with MOZ-TIF2 or MT2ΔN79ΔAD2-P1 transformed cells (log-rank test p < 0.0001). Median disease latency: MOZ-TIF2, 69 days; MT2ΔN79ΔAD2-P1, undefined.
All data shown are mean and SD (n = 3) unless otherwise specified. See also Figure S2.
To examine the significance of Prmt1 interaction in leukemic transformation, we performed structure-function analysis using the corresponding MOZ-TIF2 deletion mutants to evaluate their transformation ability (Figures 2D and S2A). An internal deletion of H15 or PHD did not compromise cellular transformation, whereas all the mutants with a deletion of the N-terminal Prmt1 interaction domain failed to transform HSPC (Figure 2D), consistent with a critical function of Prmt1 recruitment for MOZ-TIF2 transformation. To further assess the requirement of Prmt1 for leukemia maintenance, we transduced MOZ-TIF2 leukemic cells carrying a ubiquitin C promoter (UbC)-driven luciferase reporter (Becker et al., 2006) harvested from primary leukemia mice with either shPrmt1 or scramble control lentivirus prior to transplantation into syngeneic mice for leukemia development. As a result, in vivo imaging demonstrated reduced leukemia burdens for mice carrying Prmt1 knockdown leukemic cells (Figure 2F). Prmt1 knockdown also significantly extended the latency and reduced penetrance of the disease compared with the control cohort (log-rank test p < 0.0001) (Figures 2F, S2B, and S2C). Similar to the MLL-GAS7 studies, there was strong pressure on select leukemia clones to escape from Prmt1 knockdown as indicated by their re-expression of Prmt1, and Hoxa9 in the leukemic mice received MOZ-TIF2 Prmt1 knockdown cells (Figure S2B). Consistently, an irreversible inactivation of Prmt1 in MOZ-TIF2 transformed cells using conditional knockout approach in RTTA not only significantly suppressed their in vitro colony-forming ability (Figures S1I and S1J) but also abolished their in vivo leukemogenic potentials (Figure 2G). To further demonstrate the in vivo therapeutic potentials of targeting Prmt1 in the clinically relevant setting, we transplanted MOZ-TIF2 leukemia cells carrying UbC-luciferase reporter without any pretreatment into syngeneic mice and then subjected them to AMI-408 treatment. As expected, in vivo AMI-408 treatment suppressed H4R3me2as mark in MOZ-TIF2 leukemia cells (Figure 2H). More importantly, AMI-408 significantly reduced the tumor burdens (Figure 2I) and extended the leukemia latency (p = 0.0042) (Figure 2J). Although AMI-408 is an early-phase PRMT1 inhibitor that clearly requires further optimization to improve its potency, these results provide the proof-of-principle experimental data showing in vivo efficacy of pharmacological targeting of Prmt1 for leukemia suppression.
Aberrant Recruitment of PRMT1 Is Necessary but Not Sufficient for Induction of AML In Vivo
While structure-function analysis, shRNA-mediated knockdown, genetic knockout, and pharmacological inhibition experiments clearly indicate an essential role of Prmt1 in MOZ-TIF2 leukemia, it remains to be determined whether Prmt1 recruitment per se is sufficient and the sole function of the N-terminal minimal transformation domain required for MOZ-TIF2-mediated leukemogenesis. To this end, Prmt1 was covalently linked to transformation-defective MOZ-TIF2 N-terminal truncation mutants (ΔN79, ΔN180, and Δ310) to examine whether Prmt1 swapping is sufficient to resurrect their transformation activity. As a result, direct fusion of Prmt1 to those transformation-defective N-terminal deletion mutants was able to confer serial replating ability and established primary transformed cell lines, albeit the number of third-round colony was reduced (Figure 2D). In contrast, covalent fusion of Prmt1 catalytically inactive mutant carrying a single point mutation in the enzymatic domain failed to resurrect the transformation ability of any of these N-terminal deletion MOZ-TIF2 mutants (Figure 2D), despite their expression at a comparable level (Figure S2A). Immunophenotypic analysis of the MOZ-TIF2-Prmt1 transformed cells confirmed the phenotypes of early myeloid progenitors (c-kit, Gr1, and Mac1), which were similar to wild-type (WT) MOZ-TIF2 leukemic cells (Figure 2K). We then tested whether Prmt1 swapping could also rescue leukemogenesis in vivo by transplanting primary HSPC immortalized with WT MOZ-TIF2 or MOZ-TIF2-Prmt1 (MT2ΔN79ΔAD2-Prmt1) into syngeneic mice for leukemia development. Surprisingly, only mice transplanted with WT MOZ-TIF2 induced leukemia in vivo with a median disease latency of 61 days, whereas no leukemia was found in the cohort injected with MOZ-TIF2-Prmt1 fusion immortalized cells (Figure 2L), suggesting that additional hitherto unidentified molecules may also be recruited by the N-terminal transformation domain and are required for leukemogenesis.
MLL Fusions and MOZ-TIF2 Recruit KDM4C to Control H3K9me3 Status of Their Target Genes
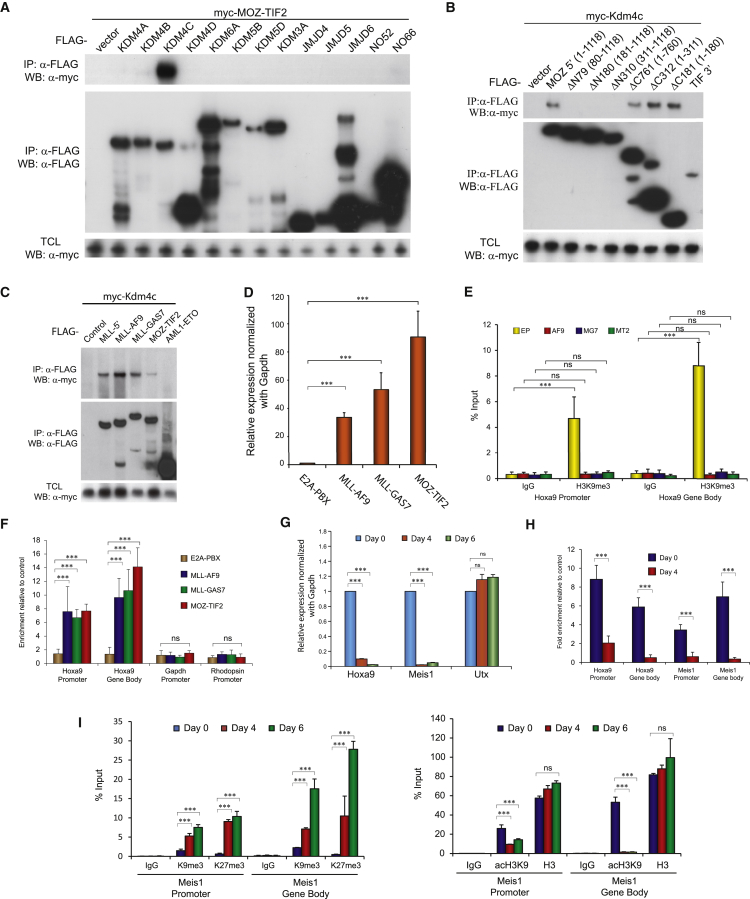
In addition to PMTs, KDMs frequently act in tandem with PMTs for regulating gene expression critical for normal and disease development (Cheung and So, 2011). This prompted us to explore whether MOZ-TIF2 could interact and collaborate with other KDMs to induce oncogenic transcriptional programs. To this end, we performed a systematic biochemical screen to identify potential KDMs that may interact with MOZ-TIF2. Co-immunoprecipitation using MOZ-TIF2 and different KDMs revealed a highly specific interaction with KDM4C but not any other tested demethylases (Figure 3A). Reciprocal co-immunoprecipitation experiments were then performed to map the KDM4C interaction domain within MOZ-TIF2 using different deletion constructs comprising the MOZ and TIF moiety of the fusion (Figure 3B). KDM4C interaction was maintained by MOZ 5′ but not TIF 3′ moiety. Further deletion analysis refined the minimal KDM4C interaction domain within the first N-terminal 79 amino acids of MOZ moiety, which overlaps with the PRMT1 interaction and the aforementioned minimal N-terminal transformation domain. This leads to the hypothesis that KDM4C may represent the additional epigenetic regulator that can cooperate with PRMT1 in mediating transcriptional deregulation and acute leukemogenesis. To this end, we further explored the role of KDM4C in other PRMT1-dependent MLL oncoproteins, and were able to demonstrate a strong interaction between KDM4C and MLL-GAS7 (Figure 3C). Biochemical mapping revealed the interaction domain located at MLL 5′, which is present in all MLL fusions. Consistently, KDM4C was recruited by other MLL fusions such as MLL-AF9 but not the non-MLL fusion control AML1-ETO (Figure 3C).
Figure 3.
MOZ-TIF2 and MLL Fusions Recruits Kdm4c to Regulate H3K9 Methylation Status of Their Target Genes
(A–C) Co-immunoprecipitation of FLAG-KDMs with myc-MOZ-TIF2 (A); FLAG-MOZ deletion constructs and myc-KDM4C (B); FLAG-MLL fusions, MOZ-TIF2, and AML1-ETO with myc-KDM4C (C).
(D) qRT-PCR analysis of Hoxa9 expression in murine leukemia cell lines.
(E) ChIP analysis of the H3K9me3 methylation on Hoxa9 loci of E2A-PBX (EP), MLL-AF9 (AF9), MLL-GAS7 (MG7), and MOZ-TIF2 (MT2).
(F) ChIP analysis of Kdm4c localization at the promoter and gene body region of Hoxa9 in murine leukemia cell lines.
(G) qRT-PCR analysis of Hoxa9, Meis1, and Utx expression 4 days after tamoxifen withdrawal in the inducible MLL-AF9-ER cells.
(H) ChIP analysis of Kdm4c at the promoter and gene body regions of Hoxa9 and Meis1 loci of MLL-AF9-ER 4 days after tamoxifen withdrawal.
(I) ChIP analysis of H3K9me3, H3K27me3 (left) and acH3K9 and H3 (right) on the promoter and gene body regions of Meis1 4 and 6 days after tamoxifen withdrawal.
All data shown are mean and SD (n = 3) unless otherwise specified. See also Figure S3.
To further demonstrate these interactions in vivo, we carried out transcriptional and epigenetic analyses using HSPC transformed by MLL fusions, MOZ-TIF2, or E2A-PBX control. KDM4C is a lysine demethylase that catalyzes the specific removal of the repressive H3K9 methylation marks and may be required to maintain its target genes in an open chromatin configuration for gene expression. In line with this hypothesis, the level of H3K9me3 mark was inversely correlated with the expression status of Hoxa9, a downstream transcriptional target of both MLL fusions and MOZ-TIF2 (Figures 3D and 3E). In contrast to the control E2A-PBX transformed cells, H3K9me3 mark on Hoxa9 loci was significantly lower in HSPC transformed by MLL fusions or MOZ-TIF2 that recruited endogenous Kdm4c to the promoter (Figures 3E and 3F). In addition, loss of H3K9me3 was concomitant with the increased H3K9 acetylation in MLL fusion or MOZ-TIF2 transformed cells (Figure S3A). To gain further insights into the dynamic interplay of H3K9 methylation and KDM4C in transcriptional regulation, we employed an inducible MLL-AF9-ER transformed primary cell line, in which MLL-AF9 was fused to ER, allowing its activity to be regulated by tamoxifen. Upon tamoxifen withdrawal, we detected significantly reduced expression of MLL downstream targets including Hoxa9 and Meis1 (Figure 3G) and a marked reduction of MLL fusion binding to the Hoxa9 and Meis1 promoters (Figure S3B). Lower MLL fusion binding also led to a concomitant reduced recruitment of endogenous Kdm4c on Hoxa9 and Meis1 loci (Figure 3H). Consistently, loss of Kdm4c binding was further accompanied by reduction of H3K9 acetylation and the accumulation of repressive H3K9me3 and H3K27me3, indicating repressive transcription complex domination in the absence of KDM4C (Figure 3I). Together, the results indicate that the dynamics of H3K9 methylation and acetylation is tightly regulated by the recruitment of KDM4C by the leukemic fusions.
Aberrant Transcriptional Networks Co-regulated by KDM4C and PRMT1 in MLL and MOZ-TIF2 Leukemia
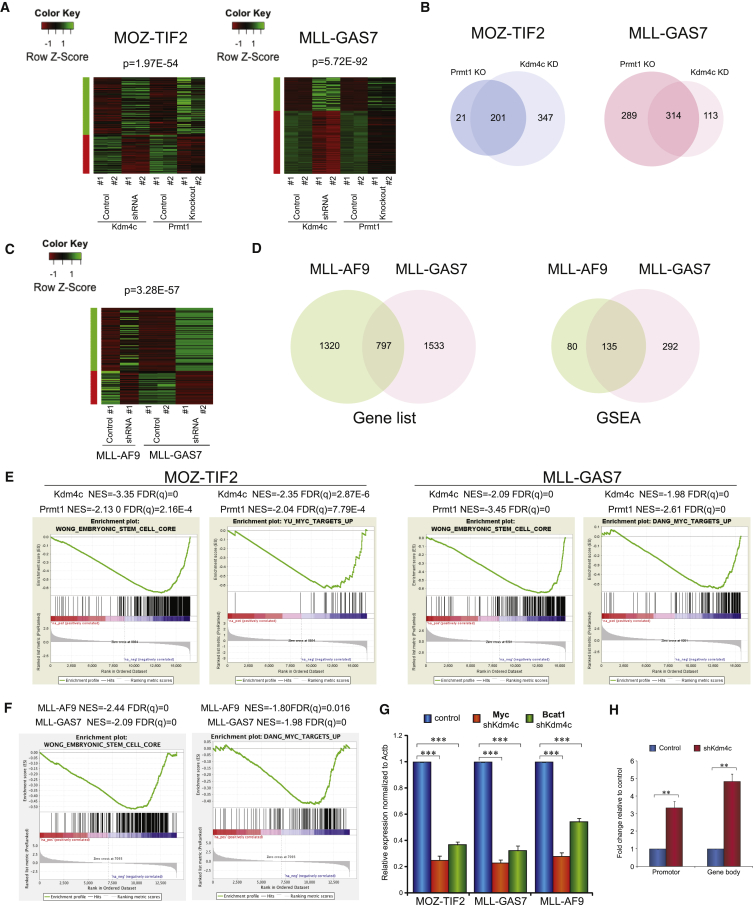
To investigate the transcriptional functions and potential crosstalk between KDM4C and PRMT1 in acute leukemogenesis, we performed global transcriptional analyses by RNA sequencing (RNA-seq) in both MOZ-TIF2 and MLL-GAS7 transformed cells in the presence or absence of Kdm4c or Prmt1 using shRNA knockdown and conditional knockout approach, respectively (Figures S4A and S4B). Differential expression gene lists from two biological replicates were used to generate heatmaps, which revealed highly similar and overlapping gene expression signatures associated with the loss of Kdm4c and Prmt1 (p = 1.97 × 10−54 for MOZ-TIF2 and 5.71 × 10−92 for MLL-GAS7) (Figure 4A), consistent with their critical functions in mediating transcriptional programs initiated by the fusions. To gain further insights into the molecular pathways co-regulated by these two epigenetic modifying enzymes, we deployed gene set enrichment analysis (GSEA) to identify potential pathways perturbed by the loss of function of Kdm4c and Prmt1. Consistently, a very large number of overlapping pathways were perturbed by the loss of function of Prmt1 and Kdm4c (Figure 4B). As transcriptional activators, about 37% (201 of 548) of the pathways activated by Kdm4c were also regulated by Prmt1 in MOZ-TIF2 transformed cells. Even more strikingly, 90% (201 of 222) of the pathways downregulated by Prmt1 inhibition were also suppressed upon Kdm4c knockdown, suggesting a strong transcriptional co-regulation mediated by these two different classes of epigenetic modifying enzymes. We also observed very similar transcriptional co-regulations between Prmt1 and Kdm4c in MLL-GAS7 transformed cells, in which 52% (314 of 603) of Prmt1 activated pathways and 74% (314 of 427) of Kdm4c activated pathways were co-regulated by each other (Figure 4B). These transcriptional analysis results are also consistent with biochemical study showing that interaction between Prmt1 and Kdm4c was largely dependent on MLL-GAS7 and MOZ-TIF2 (Figure S4C), supporting the hypothesis that these two epigenetic modifying enzymes are recruited by the leukemic fusions to execute aberrant transcription programs.
Figure 4.
RNA-Seq Reveals Overlapping Pathways Targeted by Both Prmt1 and Kdm4c
(A) Heatmap analysis of gene expression profile in MOZ-TIF2 and MLL-GAS7 leukemic cells after Prmt1knockout and Kdm4c knockdown.
(B) Venn diagram showing the common downregulated pathways after the loss of function of Prmt1 and Kdm4c (overlapped) identified by GSEA.
(C) Heatmap analysis of gene expression signature perturbed in both MLL-AF9 and MLL-GAS7 after Kdm4c knockdown.
(D) Venn diagram showing the commonly downregulated genes on MLL-AF9 and MLL-GAS7 after Kdm4c knockdown (left) as well as commonly downregulated pathways identified by GSEA (right).
(E) Embryonic stem cell signature and Myc pathway were downregulated after the loss of Prmt1 or Kdm4c in both MOZ-TIF2 (left) and MLL-GAS7 (right).
(F) Embryonic stem cell signature and Myc pathway were downregulated in both MLL fusions after Kdm4c knockdown.
(G) qRT-PCR analysis of Myc and Bcat1 expression after Kdm4c knockdown.
(H) ChIP analysis showing the effect of Kdm4c knockdown on H3K9me3 mark in Myc loci of MLL-AF9 leukemia cells.
All data shown are mean and SD (n = 3) unless otherwise specified. See also Figure S4.
KDM4C Is Required for Maintenance of Transcriptional Programs by MLL Fusions
Since MLL-AF9 recruits only Kdm4c but not Prmt1, we examined whether suppression of Kdm4c was sufficient to interrupt the oncogenic transcription programs maintained by the Prmt1-independent MLL fusion. Kdm4c knockdown in MLL-AF9 transformed cells resulted in transcriptional signatures similar to those in MLL-GAS7 transformed cells upon Prmt1/Kdm4c suppression (Figures 4C and 4D). More than 35% (797 of 2,217, p = 3.28 × 10−57) of the genes and 60% (135 of 215) of the pathways significantly downregulated in MLL-AF9 transformed cells upon Kdm4c knockdown overlapped with those in MLL-GAS7, indicating a common requirement of Kdm4c in the maintenance of transcription programs in different MLL leukemias. Interestingly, there was a number of pathways commonly regulated by both Prmt1 and Kdm4c in these fusions: these include the Myc pathway and embryonic stem cell program (Figures 4E and 4F), which are important for MLL leukemia (Dawson et al., 2011, Somervaille et al., 2009) and other AML (Zuber et al., 2011). To validate some of the findings from RNA-seq, qRT-PCR experiments confirmed that Kdm4c knockdown resulted in the suppression of expression of Myc as well as its target Bcat1 in MLL-GAS7, MLL-AF9, and MOZ-TIF2 leukemia cells (Figure 4G). Consistently, Kdm4c knockdown led to the upregulation of H3K9me3 marks on Myc loci in MLL transformed cells (Figure 4H), indicating a critical function of KDM4C in regulating oncogenic transcriptional networks.
KDM4C Is Essential for Initiation and Maintenance of MLL and MOZ-TIF2 Leukemia
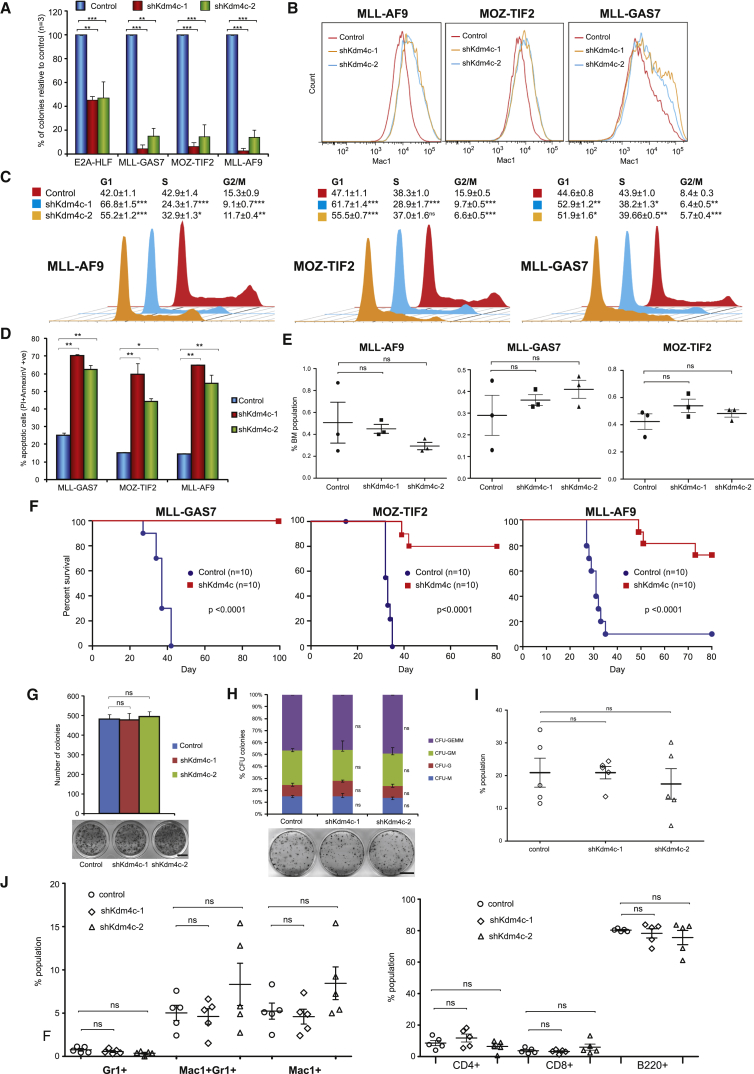
We next investigated the functional requirement of Kdm4c for leukemic transformation. Suppression of Kdm4c by two independent shRNAs (Figure S5A) resulted in a similar and significant reduction of the serial replating ability of MLL fusions and MOZ-TIF2 transformed cells compared with their relatively moderate effect on E2A-HLF transformed cells (Figure 5A). Inhibition of Kdm4c led to increased differentiation (Figure 5B), cell-cycle arrest (Figure 5C), and apoptosis (Figure 5D), which are reminiscent to the effects of Prmt1 knockdown in MLL-GAS7 and MOZ-TIF2 transformed cells (Figures S1C–S1E). Consistently, Kdm4c knockdown in MLL fusions and MOZ-TIF2 transformed cells resulted in the downregulation of Hoxa9 (Figure S5B) and the increased level of H3K9me3 repressive marks (Figure S5C). To further eliminate the possibility of any off-target effect of Kdm4c shRNA attributed to the observed transformation phenotype, we co-expressed shRNA-resistant human KDM4C with Kdm4c shRNA in MLL-AF9, MLL-GAS7, and MOZ-TIF2 leukemic cells. As a result, re-expression of KDM4C was able to rescue the transformation defects associated Kdm4c shRNAs (Figure S5D). To investigate whether Kdm4c is also required for maintenance of leukemia in vivo, we transduced MLL fusions and MOZ-TIF2 leukemia cells harvested from primary leukemic mice with a scramble control or the Kdm4c shRNA prior to their transplantation into syngeneic mice for disease development. 72 hours after transplantation, percentages of engraftment were assessed and showed no significant difference between the control and Kdm4c knockdown cells, indicating that Kdm4c knockdown has rather limited impact on homing of the leukemic cells (Figure 5E). In contrast to mice transplanted with control transduced MLL-GAS7 leukemic cells that all succumbed to leukemia within 6 weeks, Kdm4c knockdown in leukemic cells abolished their oncogenic activity, and all mice remained healthy even after 14 weeks of observation (Figures 5F and S5E–S5G). Similarly, inhibition of Kdm4c expression significantly delayed disease latency of MOZ-TIF2-induced leukemia (log-rank test p < 0.0001, Figures 5F and S5E–S5G). More importantly, we also observed drastic inhibition of the leukemogenic potentials of Prmt1-independent MLL-AF9 fusion, leading to a significant improvement of the disease-free survival and reduced penetrance (log-rank test p < 0.0001, Figures 5F and S5E–S5G). Similar to Prmt1 in vivo knockdown data, the few leukemias from the mice that received Kdm4c-knockdown cells actually re-expressed high levels of Kdm4c and Hoxa9 (Figure S5H), indicating a strong growth disadvantage of Kdm4c knockdown leukemia cells. Together, these results highlight an essential function of Kdm4c in leukemias driven by MLL fusions and MOZ-TIF2.
Figure 5.
Suppression of Kdm4c Inhibits Hematopoietic Transformation and Leukemogenesis
(A) RTTA showing the effect of two different Kdm4c shRNAs on leukemic transformation.
(B) FACS analysis showing the effect of Kdm4c knockdown on Mac1 expression.
(C) Cell-cycle analysis after Kdm4c knockdown.
(D) Apoptosis analysis after Kdm4c knockdown.
(E) Murine leukemia cells were transduced with the control or shKdm4c lentivirus and transplanted into syngeneic mice. Bone marrows were harvested after 72 hr and the percentage of donor cells was determined by FACS.
(F) Kaplan-Meier survival analysis on the effect of Kdm4c knockdown (shKdm4c#1) on leukemogenesis (log-rank test p < 0.0001). Median disease latency: control MLL-AF9, 31 days; MLL-GAS7, 37 days; MOZ-TIF2, 33 days; shKdm4c, undefined.
(G) Effect of Kdm4c knockdown on the colony-forming ability of HSPC. Scale bar represents 0.5 cm.
(H) CFU assay showing the effect of Kdm4c knockdown on the types and number of myeloid colonies derived from HSPC. Scale bar represents 0.5 cm.
(I) FACS analysis of HSPC engraftment using CD45.1 marker to detect the donor cell populations in peripheral blood 6 weeks after transplantation (n = 5).
(J) FACS analysis of the effect of Kdm4c knockdown on both myeloid (left) and lymphoid populations (right) in peripheral blood (n = 5).
All data shown are mean and SD (n = 3) unless otherwise specified. See also Figure S5.
To further investigate the potential therapeutic window of targeting Kdm4c in the hematopoietic system, we assessed the impact of Kdm4c suppression in normal hematopoiesis. Kdm4c knockdown in c-kit+ HSPCs did not lead to any significant reduction of colony-forming ability (Figure 5G) or any change in their ability to differentiate into different lineages (Figure 5H) in vitro. To assess the effect of Kdm4c suppression on in vivo hematopoietic development, we transplanted Kdm4c knockdown HSPCs into sublethally irradiated syngeneic mice for in vivo repopulation assay. Consistent with in vitro data, Kdm4c knockdown cells competently reconstituted the hematopoietic systems (Figure 5I) and gave rise to multiple hematopoietic lineages (including myeloid, B-lymphoid, and T-lymphoid) in a fashion indistinguishable to that in controls 6 weeks after the transplantation (Figure 5J). These results are in line with the dispensable embryonic function of Kdm4c for development into phenotypically normal Kdm4c knockout mouse (Pedersen et al., 2014), suggesting a potential therapeutic window for targeting Kdm4c for leukemia suppression.
Pharmacological Inhibition of Kdm4c Suppresses Leukemia Development in Both Syngeneic Mouse Model and Human AML Xenograft Model
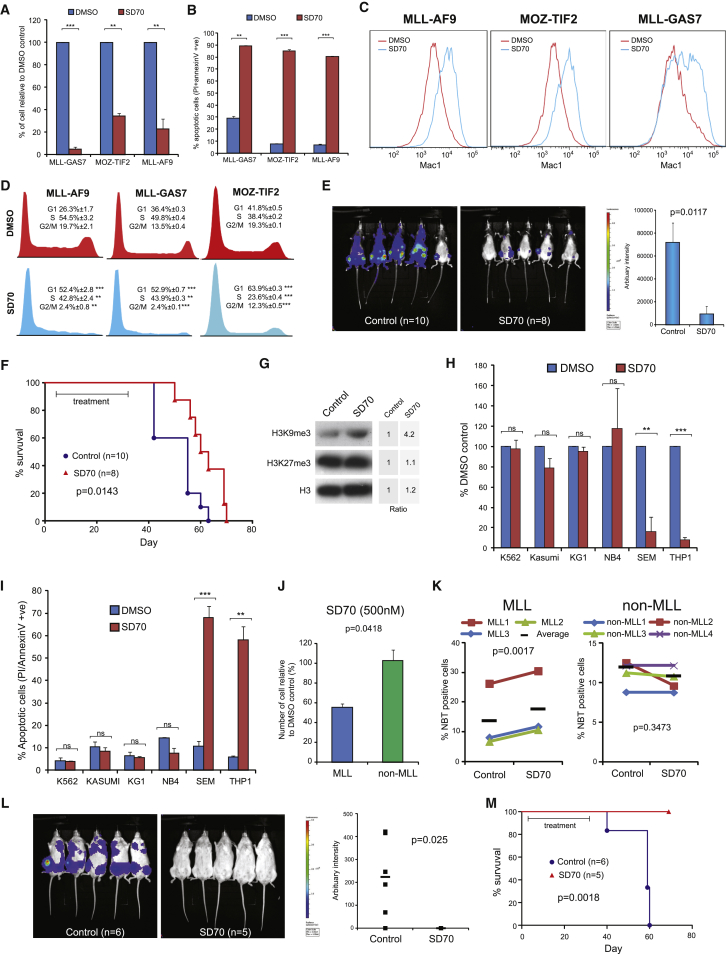
To further demonstrate the therapeutic potentials of targeting KDM4C in AML, we tested the leukemia inhibitory activity of a newly developed KDM4C inhibitor, SD70 (Jin et al., 2014). Using mouse primary cells transformed by MLL fusions and MOZ-TIF2 as the model systems, SD70 was able to significantly suppress their cell growth (Figure 6A), and induced apoptosis (Figure 6B), differentiation (Figure 6C), and cell-cycle arrest (Figure 6D), which are consistent with the effects of Kdm4c knockdown in these cells (Figures 5A–5D). To further access the in vivo efficacy of SD70 treatment, we transplanted MLL-AF9 leukemia cells carrying a luciferase reporter into irradiated syngeneic mice for in vivo treatment with either vehicle control or SD70. By in vivo imaging 6 weeks after the transplant, we detected significant leukemic burdens in the vehicle-treated cohort (Figure 6E). In contrast, SD70 drastically suppressed leukemic burdens (Figure 6E) and, more importantly, significantly extended the disease latency (Figure 6F). Consistent with Kdm4c knockdown data, SD70 was able to suppress H3K9me3 activity in vivo (Figure 6G) and inhibited the expression of MLL downstream target genes (Figure S6A).
Figure 6.
SD70 Inhibits Leukemogenesis In Vitro and In Vivo
(A) Murine leukemia cells were treated with SD70 or DMSO for 3 days with cell viability determined by trypan blue exclusion assay.
(B) Apoptosis analysis on SD70- or DMSO-treated murine leukemia cells.
(C) FACS analysis of myeloid marker Mac1 in murine leukemia cells after SD70 treatment.
(D) Cell-cycle analysis after SD70 treatment.
(E) MLL-AF9-luciferase leukemia cells were transplanted into syngeneic mice and subjected to either vehicle control or SD70 treatment. Bioluminescence imaging was performed at 39 days after transplantation.
(F) Kaplan-Meier survival analysis on the effect of SD70 treatment on MLL-AF9 mediated leukemogenesis (log-rank test p = 0.0143). Median disease latency: control, 55 days; SD70, 61.5 days.
(G) Western blotting analysis of H3K9me3 mark in murine MLL-AF9 leukemia cells after SD70 in vivo treatment. Intensity ratio was determined by densitometry.
(H) Cell viability of human leukemia cell lines were determined 3 days after SD70 or DMSO treatment.
(I) Apoptosis analysis of SD70-treated human leukemia cell lines.
(J) Cell viability of primary AML patient samples after SD70 treatment (MLL, n = 3; non-MLL, n = 4).
(K) NBT reduction assay to determine myeloid differentiation of MLL and non-MLL patient samples. The black bars show the mean.
(L) Primary human MLL leukemia (MLL3) was tagged with luciferase reporter and then transplanted into NSG mice. Bioluminescence imaging of control (n = 6) and SD70-treated (n = 5) cohorts were performed on day 44 after transplantation. Bars show the mean bioluminescence intensity.
(M) Kaplan-Meier survival analysis of vehicle or SD70-treated cohort transplanted with primary human MLL3 leukemia cells (log-rank test p = 0.0018). Median disease latency: control, 59 days; SD70, undefined.
All data shown are mean and SD (n = 3) unless otherwise specified. See also Figure S6.
To determine whether SD70 can also be effective in human MLL leukemia, we treated various human leukemia cell lines carrying different genetic mutations with SD70. As a result, we observed a specific and preferential suppression on human leukemia cell lines carrying MLL fusions (such as SEM and THP1) over the non-MLL leukemia cell lines (Figure 6H). Consistent with the data on mouse primary transformed cells, SD70 induced apoptosis (Figure 6I), differentiation (Figure S6B), and cell-cycle arrest (Figure S6C) accompanied with an increased level of H3K9me3 mark (Figure S6D) in MLL leukemia cell lines, suggesting a similar requirement of KDM4C activity in both human and mouse MLL leukemias.
To further demonstrate the utility of KDM4C inhibitor on the most relevant preclinical setting, we deployed primary AML cells from patients carrying MLL fusions for both in vitro and in vivo drug treatment studies. As a result, we observed that MLL primary leukemia cells (i.e., MLL1-3) were highly sensitive to SD70 (Figures 6J and 6K). A low dose of SD70 (500 nM) efficiently suppressed proliferation (Figure 6J) and induced differentiation (Figure 6K) of primary AML cells carrying MLL fusions (i.e., MLL1-3) but not the control primary AML primary cells without the translocations (non-MLL1-4). To further assess the effects of SD70 on leukemia cell growth and disease development in vivo, we labeled the primary AML cells carrying MLL fusion (MLL3) with a luciferase reporter prior to their transplantation into NSG mice for either the vehicle or SD70 in vivo treatment. Seven weeks after transplantation, in vivo bioluminescence imaging revealed a rapid leukemic growth and disease onset in the control cohort (Figure 6L). In contrast, the SD70-treated cohort had much lower tumor burdens (Figure 6L). More importantly, while the entire control cohort succumbed to leukemia within 59 days (Figures 6M, S6E, and S6F), the SD70-treated group did not show any sign of the disease and all mice survived throughout the observation period (Figures 6M, S6E, and S6F). These results could also be faithfully reproduced using an independent KDM4C shRNA approach on the human MLL3 primary leukemia cells (Figure S6G), where the control cohort with scramble shRNA succumbed to leukemia with a short latency whereas the entire KDM4C knockdown group survived throughout the 90-day observation period (Figure S6G). Together, these results provide the molecular and preclinical evidence for the potential clinical utility of SD70 in MLL leukemia.
Discussion
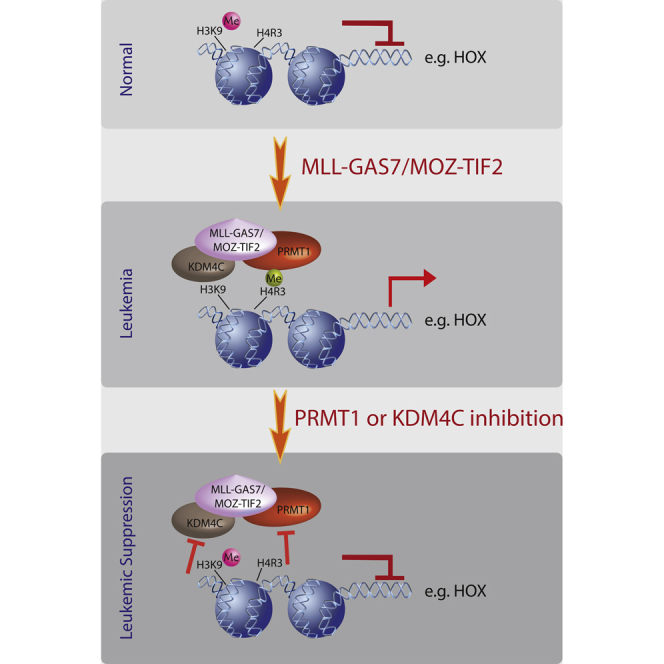
Elucidation of the mechanisms that orchestrate epigenetic reprogramming by oncogenic transcription factors is critical for understanding the molecular biology of the disease and designing effective therapeutic strategies (Cheung and So, 2011). In this study, we describe the co-recruitment of Prmt1 and Kdm4c by MLL-GAS7 and MOZ-TIF2, which exemplifies the dynamic interplay and cooperation between histone code writers and erasers for execution of specific transcriptional programs mediated by oncogenic transcription factors in acute leukemia (Figure 7). Histone methylation and demethylation as a key component of histone code is on constant flux, and perturbation of this dynamic event on chromatin can shift the equilibrium to alter transcription outcomes. While the collaboration between KMTs and KDMs (e.g., between MLL and JMJD3) (Agger et al., 2007) has been previously documented to facilitate the switch between different transcriptional states by reinforcing specific histone methylation codes on lysine residues (Cheung and So, 2011, Cloos et al., 2008), our study suggests that similar mechanisms also operate between histone arginine and lysine methylations. H4R3me2as encodes an activation mark that allows recruitment of histone acetyltransferases such as CBP and p300 to open up the chromatin structure for gene expression (An et al., 2004, Cheung et al., 2007). To facilitate such a modification, the lysine residues subjected to acetylation should be free from methyl groups. Consistently, recruitment of KDM4C mediates the removal of H3K9me3 repressive mark, allowing the replacement with the activating acetylation mark, suggesting that a coordinated functional recruitment of multiple distinctive epigenetic modifying enzymes is required for establishment of oncogenic transcriptional programs mediated by chimeric transcription factors in cancer development.
Figure 7.
Schematic Diagram Summarizes Aberrant Epigenetic Networks and Therapeutic Potentials of Targeting PRMT1 and KDM4C in AML
Left panel indicates the aberrant recruitment of PRMT1 and KDM4C by MLL fusions and MOZ-TIF2 to drive oncogenic transcriptional programs. Panels on the right depict the potential targeting of epigenetic modifying enzymes for leukemia suppression. MLL fusions in the diagram refer to MLL-GAS7 and MLL-EEN, although KDM4C inhibition can also be effective for other MLL fusions including MLL-AF9.
As proof of principle, we provide the long-sought-after in vivo preclinical data showing that inhibition of Prmt1 activity by shRNA or chemical inhibitor approaches can significantly suppress oncogenic transformation mediated by various AML fusions, and extend the latency of established disease in the transplanted animals. In recent years, PRMT1 has been implicated in AML1-ETO leukemia (Shia et al., 2012) and some solid tumors (Mathioudaki et al., 2011, Yoshimatsu et al., 2011); however, in vivo transformation data are still required to firmly establish a functional link in the actual disease pathogenesis. The current establishment of an in vivo preclinical model provides a strong rationale and platform to evaluate and develop more specific Prmt1 inhibitors in the future for targeted cancer therapy. Compared with BCR-ABL in CML and PML-RARα in APL, we have an extremely limited knowledge about the molecular functions of PMTs in normal and cancer development despite a preliminary indication of clinical efficacy of the KMT inhibitor against DOT1L in the phase I trial. The lessons from imatinib on CML (Balabanov et al., 2014) and ATRA on APL (Fung and So, 2013) indicate that the development of drug-resistant clones after achieving an initial clinical remission by highly effective and specific inhibitors against a particular molecule will be a major issue for most of the targeted therapies. Understanding the mechanisms of action is essential, and has been instrumental in designing more effective therapeutic strategies to minimize and overcome relapses (Cortes et al., 2012, Lo-Coco et al., 2013). The discovery of the functional crosstalk between PRMT1 and KDM4C in the establishment of an oncogenic transcriptional program in leukemia provides important insights into the molecular functions and underlying mechanisms of these critical PMTs and KMDs in oncogenesis.
The dynamics of H3K9 methylation is regulated by the intricate equilibrium of lysine methyltransferases and demethylases. Using in vitro cell line models, KDM4C has been implicated in different cancers including squamous cell carcinoma (Cloos et al., 2006), B-cell lymphoma (Rui et al., 2010), and prostate (Wissmann et al., 2007), and breast (Liu et al., 2009) cancers. Loss of Suv39h resulted in a reduction of H3K9me3 mark and accelerated tumor development (Braig et al., 2005, Peters et al., 2001), suggesting a tumor-suppressor function associated with H3K9me3 in critical but unknown loci. Consistent with this model, recruitment of KDM4C by chimeric transcription factors can counteract and remove the repressive H3K9 trimethylation marks on their target gene loci such as Hoxa9, which are critical for self-renewal and oncogenic transformation. This is supported by the concomitant increase in H3K9me3 mark and suppression of Hoxa9 expression upon Kdm4c knockdown. Recently, H3K9me2/1 demethylase PHF8 has been shown to govern ATRA treatment response in APL, and its activation helps to overcome treatment resistance (Arteaga et al., 2013). Our current studies provide the key in vivo experimental evidence demonstrating the requirement of KDM4C for cancer development and its functional crosstalk with PRMT1 in the establishment of histone codes for transcriptional deregulation in AML. Intriguingly, suppression of either of the epigenetic regulators compromises transcriptional programs and cellular transformation by MOZ-TIF2 or MLL-GAS7 fusions, indicating their critical and non-overlapping functions that are indispensable for the oncogenic transformation (Figure 7). This is also consistent with the finding that KDM4C is required for PRMT1-independent MLL leukemia. Importantly, transcriptional or pharmacological inhibition of KDM4C by molecular or small-molecule inhibitor approaches could significantly lower leukemic burdens and extend the disease latency, particularly in an MLL primary human AML cell xenograft in vivo model. Together, these findings provide strong experimental and preclinical in vivo evidence demonstrating an efficient MLL leukemia suppression by pharmacological inhibition of KDM4C, laying the foundation for future clinical application of KDM4C inhibitors in human cancer treatments.
Advancement in our knowledge of onco-epigenomics, together with the dissection of the dynamic interplay of chromatin modification and remodeling mediated by leukemic transcription factors, could pave the way to revolutionize our therapeutic options. Cracking the lethal histone code created by leukemic fusions and a strategy of rational therapeutic design targeting specific epigenetic modifying enzymes required for the oncogenic transcription factors hold the promise of eradicating this devastating disease.
Experimental Procedures
In Vitro and In Vivo Transformation Studies
RTTA was performed as previously described (Zeisig and So, 2009) and is detailed in Supplemental Experimental Procedures. For Kdm4c knockdown rescue experiments, cells were co-transduced with Kdm4c shRNA and shRNA-resistant human KDM4C lentiviruses, and co-selected with puromycin and blasticidin. To generate full-blown murine leukemia cells, we injected 106 immortalized cells via the tail vein into sublethally irradiated syngeneic C57BL/6 mice. Mice were injected with 105 murine leukemia cells to study the effect Prmt1 and Kdm4c knockdown on leukemogenesis. Leukemia cells were transduced with either control or shPrmt1 retrovirus, and GFP sorted before transplantation. Kdm4c knockdown cells were selected with antibiotic prior to transplantation. Prmt1 knockout induced by tamoxifen were confirmed by PCR genotyping prior to transplantation. Mice were monitored for development of leukemia by fluorescence-activated cell sorting (FACS) analysis with tissues processed for histological analysis. Primary human MLL3 leukemia cells were transduced with control or shKDM4C lentivirus, and antibiotic selected and transplanted (105) by intrafemoral injection into sublethally irradiated NOD/SCID/IL2Rg−/− (NSG) mice. For bioluminescence imaging and quantification of leukemia burden, murine leukemias were established using HSPC isolated from Ubc-luciferase reporter mice (Becker et al., 2006), whereas human leukemia was tagged with a lentiviral luciferase reporter. Transplanted mice were injected with 150 mg/kg of D-luciferin intraperitoneally and bioluminescence image acquired using IVIS Lumina II (Caliper; Perkin Elmer) with software Living Image Version 4.3.1 according to the manufacturer’s instructions. All the animal works were performed according to the guidelines and regulations of the Animal (Scientific Procedures) Act 1986, and approved by the KCL local ethics committee.
AMI-408 Drug Treatment
For AMI-408 drug treatment in vitro, 104 cells were plated in methylcellulose with 200 mM AMI-408 or DMSO control and cultured for 5–7 days. To study the effect of AMI-408 on leukemogenesis of MLL-GAS7 in vivo, we pretreated leukemia cells in vitro with the drug for 24 hr prior to injection. 105 MLL-GAS7 leukemia cells were then transplanted into C57BL/6 mice via tail vein and injected intraperitoneally with 20 mg/kg of AMI-408 or carrier control every other day for 2 weeks. To study the effect of AMI-408 on MOZ-TIF2 leukemogenesis in vivo, we transplanted 105 leukemia cells into syngeneic C57BL/6 mice via tail vein injection without pretreatment. Mice were subjected to a dosage of 10 mg/kg of AMI-408 in PEG300/D5W for 4 weeks with five consecutive injections per week. Bioluminescence imaging was performed every week.
SD70 Drug Treatment
Leukemia cells were seeded at 5 × 104/ml and incubated with SD70 (Xcess Biosciences) at a concentration of 0.8 μM for human cell lines, 2 μM for murine, and 0.5 μM for primary human samples for 48–72 hr. To study the effect of SD70 on leukemogenesis in vivo, we transplanted 105 murine MLL-AF9-luciferase leukemia cells 3 days prior to treatment. Human primary leukemia MLL3 was transplanted by intrafemoral injection into sublethally irradiated NSG mice 3 days before drug treatment. SD70 preparation and drug dosage used for in vivo animal experiments were performed as described by Jin et al. (2014). SD70 was administered intraperitoneally at 10 mg/kg in PEG300/D5W for 4 weeks with five consecutive injections in the first week and every alternative day for the next 3 weeks. Sick mice were euthanized and processed for FACS and histological analysis. SD70-treated mice were injected with 50 mg/kg of SD70 intraperitoneally, and bone marrow was harvested 5 hr later for western blot analysis.
Statistical Analysis
All the experiments were analyzed using two-way Student's t test. For the comparison of different specimens the unpaired t test was used. For the comparison of different treatments (e.g., drug, gene knockdown/knockout) within the same specimen, the paired t test was used. The log-rank test was used to compare survival curves. p Values of less than 0.05 were considered statistically significant. In the figures, asterisks indicate ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001. For the RNA-seq analysis, differentially expressed genes with p < 0.05 were used to generate heatmaps. The statistical significance of overlap between the gene expression patterns of two conditions was calculated using a hypergeometric test (Marioni et al., 2008).
Additional experimental procedures including description of plasmids, cell lines, conditions for qRT-PCR, GST pull-down assay, immunoprecipitation, chromatin immunoprecipitation, NTB reduction assay, flow cytometry, generation of Prmt1 conditional knockout mouse, RNA-seq, and RNA analysis are reported in Supplemental Experimental Procedures.
Acknowledgments
We would like to dedicate this paper to the memory of Prof. Li Chong Chan for his important contributions to the foundation of this work, which was started 20 years ago. Thanks are given to Jackie Chen and Amanda Wilson for assistance on various experimental procedures; Sam Tung, Magdalena Zarowiecki, and members of Lenhard's laboratory for advice on bioinformatics analysis; Jesus Gil, Hinrich Gronemeyer, Peter Parker, Joyce Taylor-Papdadimitriou, and Joy Burchell for critical advice on the manuscript; Erica Tse for graphic illustration; and KCL National Institute for Health Research Biomedical Research Center (NIHR BRC) for sequencing service. This research was funded by Bloodwise with additional support from Cancer Research UK (CRUK) to C.W.E.S.
Published: January 11, 2016
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, six figures, and three tables and can be found with this article online at http://dx.doi.org/10.1016/j.ccell.2015.12.007.
Accession Numbers
RNA-sequencing data can be accessed via ArrayExpress: E-MTAB-3322.
Supplemental Information
References
- Abdel-Wahab O., Levine R.L. Mutations in epigenetic modifiers in the pathogenesis and therapy of acute myeloid leukemia. Blood. 2013;121:3563–3572. doi: 10.1182/blood-2013-01-451781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agger K., Cloos P.A., Christensen J., Pasini D., Rose S., Rappsilber J., Issaeva I., Canaani E., Salcini A.E., Helin K. UTX and JMJD3 are histone H3K27 demethylases involved in HOX gene regulation and development. Nature. 2007;449:731–734. doi: 10.1038/nature06145. [DOI] [PubMed] [Google Scholar]
- An W., Kim J., Roeder R.G. Ordered cooperative functions of PRMT1, p300, and CARM1 in transcriptional activation by p53. Cell. 2004;117:735–748. doi: 10.1016/j.cell.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Arteaga M.F., Mikesch J.H., Qiu J., Christensen J., Helin K., Kogan S.C., Dong S., So C.W. The histone demethylase PHF8 governs retinoic acid response in acute promyelocytic leukemia. Cancer Cell. 2013;23:376–389. doi: 10.1016/j.ccr.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arteaga M.F., Mikesch J.H., Fung T.K., So C.W. Epigenetics in acute promyelocytic leukaemia pathogenesis and treatment response: a TRAnsition to targeted therapies. Br. J. Cancer. 2015;112:413–418. doi: 10.1038/bjc.2014.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balabanov S., Braig M., Brummendorf T.H. Current aspects in resistance against tyrosine kinase inhibitors in chronic myelogenous leukemia. Drug Discov. Today Technol. 2014;11:89–99. doi: 10.1016/j.ddtec.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Becker C.M., Wright R.D., Satchi-Fainaro R., Funakoshi T., Folkman J., Kung A.L., D'Amato R.J. A novel noninvasive model of endometriosis for monitoring the efficacy of antiangiogenic therapy. Am. J. Pathol. 2006;168:2074–2084. doi: 10.2353/ajpath.2006.051133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonham K., Hemmers S., Lim Y.H., Hill D.M., Finn M.G., Mowen K.A. Effects of a novel arginine methyltransferase inhibitor on T-helper cell cytokine production. FEBS J. 2010;277:2096–2108. doi: 10.1111/j.1742-4658.2010.07623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braig M., Lee S., Loddenkemper C., Rudolph C., Peters A.H., Schlegelberger B., Stein H., Dorken B., Jenuwein T., Schmitt C.A. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature. 2005;436:660–665. doi: 10.1038/nature03841. [DOI] [PubMed] [Google Scholar]
- Campbell R.M., Tummino P.J. Cancer epigenetics drug discovery and development: the challenge of hitting the mark. J. Clin. Invest. 2014;124:64–69. doi: 10.1172/JCI71605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung N., So C.W. Transcriptional and epigenetic networks in haematological malignancy. FEBS Lett. 2011;585:2100–2111. doi: 10.1016/j.febslet.2011.03.068. [DOI] [PubMed] [Google Scholar]
- Cheung N., Chan L.C., Thompson A., Cleary M.L., So C.W. Protein arginine-methyltransferase-dependent oncogenesis. Nat. Cell Biol. 2007;9:1208–1215. doi: 10.1038/ncb1642. [DOI] [PubMed] [Google Scholar]
- Cloos P.A., Christensen J., Agger K., Maiolica A., Rappsilber J., Antal T., Hansen K.H., Helin K. The putative oncogene GASC1 demethylates tri- and dimethylated lysine 9 on histone H3. Nature. 2006;442:307–311. doi: 10.1038/nature04837. [DOI] [PubMed] [Google Scholar]
- Cloos P.A., Christensen J., Agger K., Helin K. Erasing the methyl mark: histone demethylases at the center of cellular differentiation and disease. Genes Dev. 2008;22:1115–1140. doi: 10.1101/gad.1652908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes J.E., Kantarjian H., Shah N.P., Bixby D., Mauro M.J., Flinn I., O'Hare T., Hu S., Narasimhan N.I., Rivera V.M. Ponatinib in refractory Philadelphia chromosome-positive leukemias. N. Engl. J. Med. 2012;367:2075–2088. doi: 10.1056/NEJMoa1205127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daigle S.R., Olhava E.J., Therkelsen C.A., Majer C.R., Sneeringer C.J., Song J., Johnston L.D., Scott M.P., Smith J.J., Xiao Y. Selective killing of mixed lineage leukemia cells by a potent small-molecule DOT1L inhibitor. Cancer Cell. 2011;20:53–65. doi: 10.1016/j.ccr.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson M.A., Prinjha R.K., Dittmann A., Giotopoulos G., Bantscheff M., Chan W.I., Robson S.C., Chung C.W., Hopf C., Savitski M.M. Inhibition of BET recruitment to chromatin as an effective treatment for MLL-fusion leukaemia. Nature. 2011;478:529–533. doi: 10.1038/nature10509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito M.T., Zhao L., Fung T.K., Rane J.K., Wilson A., Martin N., Gil J., Leung A.Y., Ashworth A., So C.W. Synthetic lethal targeting of oncogenic transcription factors in acute leukemia by PARP inhibitors. Nat. Med. 2015;21:1481–1490. doi: 10.1038/nm.3993. [DOI] [PubMed] [Google Scholar]
- Fung T.K., So C.W. Overcoming treatment resistance in acute promyelocytic leukemia and beyond. Oncotarget. 2013;4:1128–1129. doi: 10.18632/oncotarget.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., Nguyen A.T., Zhang Y. KDM2b/JHDM1b, an H3K36me2-specific demethylase, is required for initiation and maintenance of acute myeloid leukemia. Blood. 2011;117:3869–3880. doi: 10.1182/blood-2010-10-312736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntly B.J., Shigematsu H., Deguchi K., Lee B.H., Mizuno S., Duclos N., Rowan R., Amaral S., Curley D., Williams I.R. MOZ-TIF2, but not BCR-ABL, confers properties of leukemic stem cells to committed murine hematopoietic progenitors. Cancer Cell. 2004;6:587–596. doi: 10.1016/j.ccr.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Ingham R.J., Colwill K., Howard C., Dettwiler S., Lim C.S.H., Yu J., Hersi K., Raaijmakers J., Gish G., Mbamalu G. WW domains provide a platform for the assembly of multiprotein networks. Mol. Cell Biol. 2005;25:7092–7106. doi: 10.1128/MCB.25.16.7092-7106.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C., Yang L., Xie M., Lin C., Merkurjev D., Yang J.C., Tanasa B., Oh S., Zhang J., Ohgi K.A. Chem-seq permits identification of genomic targets of drugs against androgen receptor regulation selected by functional phenotypic screens. Proc. Natl. Acad. Sci. USA. 2014;111:9235–9240. doi: 10.1073/pnas.1404303111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarjian H., Sawyers C., Hochhaus A., Guilhot F., Schiffer C., Gambacorti-Passerini C., Niederwieser D., Resta D., Capdeville R., Zoellner U. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N. Engl. J. Med. 2002;346:645–652. doi: 10.1056/NEJMoa011573. [DOI] [PubMed] [Google Scholar]
- Katsumoto T., Aikawa Y., Iwama A., Ueda S., Ichikawa H., Ochiya T., Kitabayashi I. MOZ is essential for maintenance of hematopoietic stem cells. Genes Dev. 2006;20:1321–1330. doi: 10.1101/gad.1393106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson S.K., Wigle T.J., Warholic N.M., Sneeringer C.J., Allain C.J., Klaus C.R., Sacks J.D., Raimondi A., Majer C.R., Song J. A selective inhibitor of EZH2 blocks H3K27 methylation and kills mutant lymphoma cells. Nat. Chem. Biol. 2012;8:890–896. doi: 10.1038/nchembio.1084. [DOI] [PubMed] [Google Scholar]
- Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Kvinlaug B.T., Chan W.I., Bullinger L., Ramaswami M., Sears C., Foster D., Lazic S.E., Okabe R., Benner A., Lee B.H. Common and overlapping oncogenic pathways contribute to the evolution of acute myeloid leukemias. Cancer Res. 2011;71:4117–4129. doi: 10.1158/0008-5472.CAN-11-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G., Bollig-Fischer A., Kreike B., van de Vijver M.J., Abrams J., Ethier S.P., Yang Z.Q. Genomic amplification and oncogenic properties of the GASC1 histone demethylase gene in breast cancer. Oncogene. 2009;28:4491–4500. doi: 10.1038/onc.2009.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo-Coco F., Avvisati G., Vignetti M., Thiede C., Orlando S.M., Iacobelli S., Ferrara F., Fazi P., Cicconi L., Di Bona E. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N. Engl. J. Med. 2013;369:111–121. doi: 10.1056/NEJMoa1300874. [DOI] [PubMed] [Google Scholar]
- Look A.T. Oncogenic transcription factors in the human acute leukemias. Science. 1997;278:1059–1064. doi: 10.1126/science.278.5340.1059. [DOI] [PubMed] [Google Scholar]
- Marioni J.C., Mason C.E., Mane S.M., Stephens M., Gilad Y. RNA-seq: an assessment of technical reproducibility and comparison with gene expression arrays. Genome Res. 2008;18:1509–1517. doi: 10.1101/gr.079558.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathioudaki K., Scorilas A., Ardavanis A., Lymberi P., Tsiambas E., Devetzi M., Apostolaki A., Talieri M. Clinical evaluation of PRMT1 gene expression in breast cancer. Tumour Biol. 2011;32:575–582. doi: 10.1007/s13277-010-0153-2. [DOI] [PubMed] [Google Scholar]
- McCabe M.T., Ott H.M., Ganji G., Korenchuk S., Thompson C., Van Aller G.S., Liu Y., Graves A.P., Della Pietra A., 3rd, Diaz E. EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature. 2012;492:108–112. doi: 10.1038/nature11606. [DOI] [PubMed] [Google Scholar]
- Okada Y., Feng Q., Lin Y., Jiang Q., Li Y., Coffield V.M., Su L., Xu G., Zhang Y. hDOT1L links histone methylation to leukemogenesis. Cell. 2005;121:167–178. doi: 10.1016/j.cell.2005.02.020. [DOI] [PubMed] [Google Scholar]
- Pedersen M.T., Agger K., Laugesen A., Johansen J.V., Cloos P.A., Christensen J., Helin K. The demethylase JMJD2C localizes to H3K4me3-positive transcription start sites and is dispensable for embryonic development. Mol. Cell Biol. 2014;34:1031–1045. doi: 10.1128/MCB.00864-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A.H., O'Carroll D., Scherthan H., Mechtler K., Sauer S., Schofer C., Weipoltshammer K., Pagani M., Lachner M., Kohlmaier A. Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell. 2001;107:323–337. doi: 10.1016/s0092-8674(01)00542-6. [DOI] [PubMed] [Google Scholar]
- Rui L., Emre N.C., Kruhlak M.J., Chung H.J., Steidl C., Slack G., Wright G.W., Lenz G., Ngo V.N., Shaffer A.L. Cooperative epigenetic modulation by cancer amplicon genes. Cancer Cell. 2010;18:590–605. doi: 10.1016/j.ccr.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shia W.J., Okumura A.J., Yan M., Sarkeshik A., Lo M.C., Matsuura S., Komeno Y., Zhao X., Nimer S.D., Yates J.R., 3rd, Zhang D.E. PRMT1 interacts with AML1-ETO to promote its transcriptional activation and progenitor cell proliferative potential. Blood. 2012;119:4953–4962. doi: 10.1182/blood-2011-04-347476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih A.H., Abdel-Wahab O., Patel J.P., Levine R.L. The role of mutations in epigenetic regulators in myeloid malignancies. Nat. Rev. Cancer. 2012;12:599–612. doi: 10.1038/nrc3343. [DOI] [PubMed] [Google Scholar]
- So C.W., Karsunky H., Passegue E., Cozzio A., Weissman I.L., Cleary M.L. MLL-GAS7 transforms multipotent hematopoietic progenitors and induces mixed lineage leukemias in mice. Cancer Cell. 2003;3:161–171. doi: 10.1016/s1535-6108(03)00019-9. [DOI] [PubMed] [Google Scholar]
- So C.W., Lin M., Ayton P.M., Chen E.H., Cleary M.L. Dimerization contributes to oncogenic activation of MLL chimeras in acute leukemias. Cancer Cell. 2003;4:99–110. doi: 10.1016/s1535-6108(03)00188-0. [DOI] [PubMed] [Google Scholar]
- Somervaille T.C., Matheny C.J., Spencer G.J., Iwasaki M., Rinn J.L., Witten D.M., Chang H.Y., Shurtleff S.A., Downing J.R., Cleary M.L. Hierarchical maintenance of MLL myeloid leukemia stem cells employs a transcriptional program shared with embryonic rather than adult stem cells. Cell Stem Cell. 2009;4:129–140. doi: 10.1016/j.stem.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sroczynska P., Cruickshank V.A., Bukowski J.P., Miyagi S., Bagger F.O., Walfridsson J., Schuster M.B., Porse B., Helin K. shRNA screening identifies JMJD1C as being required for leukemia maintenance. Blood. 2014;123:1870–1882. doi: 10.1182/blood-2013-08-522094. [DOI] [PubMed] [Google Scholar]
- Wang Z.Y., Chen Z. Acute promyelocytic leukemia: from highly fatal to highly curable. Blood. 2008;111:2505–2515. doi: 10.1182/blood-2007-07-102798. [DOI] [PubMed] [Google Scholar]
- Wissmann M., Yin N., Muller J.M., Greschik H., Fodor B.D., Jenuwein T., Vogler C., Schneider R., Gunther T., Buettner R. Cooperative demethylation by JMJD2C and LSD1 promotes androgen receptor-dependent gene expression. Nat. Cell Biol. 2007;9:347–353. doi: 10.1038/ncb1546. [DOI] [PubMed] [Google Scholar]
- Wong S.H., Goode D.L., Iwasaki M., Wei M.C., Kuo H.P., Zhu L., Schneidawind D., Duque-Afonso J., Weng Z., Cleary M.L. The H3K4-methyl epigenome regulates leukemia stem cell oncogenic potential. Cancer Cell. 2015;28:198–209. doi: 10.1016/j.ccell.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimatsu M., Toyokawa G., Hayami S., Unoki M., Tsunoda T., Field H.I., Kelly J.D., Neal D.E., Maehara Y., Ponder B.A. Dysregulation of PRMT1 and PRMT6, Type I arginine methyltransferases, is involved in various types of human cancers. Int. J. Cancer. 2011;128:562–573. doi: 10.1002/ijc.25366. [DOI] [PubMed] [Google Scholar]
- Zeisig B.B., So C.W. Retroviral/lentiviral transduction and transformation assay. Methods Mol. Biol. 2009;538:207–229. doi: 10.1007/978-1-59745-418-6_10. [DOI] [PubMed] [Google Scholar]
- Zeisig B.B., Kulasekararaj A.G., Mufti G.J., So C.W. Acute myeloid leukemia: snapshot. Cancer Cell. 2012;22:698. doi: 10.1016/j.ccr.2012.10.017. [DOI] [PubMed] [Google Scholar]
- Zuber J., Shi J., Wang E., Rappaport A.R., Herrmann H., Sison E.A., Magoon D., Qi J., Blatt K., Wunderlich M. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature. 2011;478:524–528. doi: 10.1038/nature10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.