Abstract
Even after incomplete myocardial recovery during mechanical circulatory support, long-term survival rates after ventricular assist device (VAD) explantation can be better than those expected after heart transplantation even for patients with chronic non-ischemic cardiomyopathy as the underlying cause for VAD implantation. The elective therapeutic use of ventricular assist devices for heart failure reversal in its early stage is a future goal. It may be possible to achieve it by developing tools to predict heart failure reversibility even before ventricular assist device implantation and increasing the number of weaning candidates by improvement of adjunctive therapies to optimize unloading-promoted recovery.
Special attention is focused on the long-term stability of cardiac remission after VAD removal, the clinical relevance unloading-promoted myocardial recovery and on the current knowledge about a potential prediction of myocardial recovery during long-term VAD support already before VAD implantation.
Keywords: heart failure, ventricular assist devices, ventricular function, myocardial recovery, survival, risk factors
Long-term patient outcome after VAD removal
The post-weaning survival probability of patients who had end-stage non-ischemicchronic heart failure (HF) before the implantation of ventricular assist device (VAD) is comparable with that of patients who recovered from acute myocarditis, non-coronary post-cardiotomy HF and peripartum cardiomyopathy, where reversible causes of HF can play major roles [1]. Our recent evaluation of 53 weaned patients with end-stage non-ischemic chronic cardiomyopathy (CCM) as the underlying cause for VAD implantation revealed 5 and 10 year post-explant survival probabilities (including post-heart-transplantation survival for those with HF recurrence) of 72.8±6.6% and 67.0±7.2%, respectively [1]. Assessment of post-weaning survival only from HF recurrence or weaning-related complications revealed even higher probabilities for 5 and 10-year survival, reaching 87.8±5.3%and 82.6±7.3%, respectively [1]. Of the first three patients who were electively weaned in 1995 in our department, one is still asymptomatic after 20 years and another survived 17 years without the need for heart transplantation (HTx), whereas the third, still alive, remained stable for 14 years before needing another VAD due to recurrence of HF. Of 33 patients with non-ischemic CCM as the underlying cause for VAD implantation who were weaned from VADs in our center before 2004, 24 (72.7%) were alive at the end of the 5th post-weaning year (79.2% of them with their native hearts) [2]. Comparing these data with the ISHLT (International Society for Heart and Lung Transplantation) post-HTx outcome data, with the option of HTx for patients with post-explantation HF recurrence, the long-term survival rates after weaning from VADs appear to be better than those expected after HTx [2, 3]. In a recentl ypublished study, which compared the long-term outcome of patients bridged to recovery and patients bridged to HTx, the actuarial survival rate at 5 years after left VAD (LVAD) explantation was 73.9%, whereas in the group bridged to HTx, where all patients finally received a transplant, the actuarial post-HTx survival rate at 5 years was 78.3% [4]. Thus, patients weaned from VADs appeared not to be at a higher risk for death in comparison to those who underwent HTx, even if the underlying cause for VAD implantation was chronic cardiomyopathy and not one of the more often reversible cardiac diseases such as acute myocarditis, post-cardiotomy HF or peripartum cardiomyopathy. However, for various reasons (availability of donor organs, contraindications for HTx etc.) not all patients can be bridged to HTxand to date the survival probability on VADs is lower than that after HTx. Thus, the recently published 5th INTERMACS Annual Report revealed for continuous flow LVADs an actuarial survival of 70% at 2 years, and of less than 50% before the end of the fourth year after implantation [5]. The survival probability with pulsatile LVADs was lower and reached only about 40% at the end of the third post-implantation year [5]. Fortunately, many of those who cannot be weaned from their VAD may be successfully bridged to HTx and thus the survival probability for patients who must remain on VAD support might be better. Indeed, for our patients with non-ischemic CCM as the underlying cause for VAD implantation, a comparison of long-term survival data of patients with and without explantation revealed a 5-year survival probability of 72.8% and 52.4%, respectively (p < 0.01)[6]. Since VAD explantation in the recovered patient group was performed after a mechanical support time of ≥ 4weeks, we included in the non-explanted group only those patients who also survived the first 4 post-implantation weeks. The prevalence of patients who underwent HTx during the evaluation period was nearly identical in the 2 groups (28.3% in the group with explantation and 28.7% in the group without) [6]. Thus, the survival probability of our weaned patients with non-ischemic CCM as the underlying cause for VAD implantation was better than that of patients with the same underlying cardiac disease who could not be weaned from their VAD. Post-explant HF recurrence appeared related to the duration of HF before VAD implantation and a pre-implant history of HF >5 years can be a relevant risk factor for post-weaning HF recurrence [7, 8]. The influence of the etiology of the underlying cardiac disease, responsible for HF development before VAD implantation on post-weaning patient outcome, is barely known. However, with the option of HTx for patients with post-explant HF recurrence, the 5-year survival probability of our weaned patients with idiopathic cardiomyopathy as the underlying cause for LVAD implantation, a disease that for a long time was considered to be almost irreversible, reached nearly 80%, suggesting that VAD explantation should be considered in all patients with relevant cardiac recovery, not only in those with potentially more reversible cardiac diaseases [7].
Optimizing unloading-promoted cardiac recovery
Whereas renin, angiotensin II (Ang-II) and aldosterone plasma levels usually decrease after LVAD implantation, in the unloaded myocardium both norepinephrine and Ang-II tissue levels are elevated and can promote cardiac interstitial fibrosis with an increase in myocardial stiffness [9, 10]. Angiotensin-converting enzyme (ACE) inhibitors, being able to reduce myocardial Ang-II and also the Ang-II-induced myocardial sympathetic activation, can prevent the progression of extra-cellular matrix remodeling and, in combination with VAD unloading, ACE-inhibitors may also be able to reverse, at least partially, that remodeling [9]. To promote recovery during VAD support, in addition to ACE-inhibitors, Ang-II-receptor antagonists, aldosterone-antagonists and β-blockers are recommended [8, 11, 12]. Medication doses should be individually adapted with the goal of reducing HR towards 55-60 bpm, and blood pressure to the lowest optimally tolerated value, as well as to maintain optimal renal function [1]. The target doses used by the Harefield group are:40mg daily for lisinopril, 25-50mg three times daily for carvedilol, 100mg daily for losartan, 25mg daily for spironolactone and 125μg daily for digoxin [11].
Clenbuterol (selective β2-adrenergic receptor-agonist) was supposed to be a possible promoter of myocardial recovery during VAD support [11, 13]. With clenbuterol as additional therapy the Harefield study reported a weaning rate from LVADs of >60% [11, 13]. However, the possibility that these high recovery rates might have been also facilitated by pre-implant patient selection could not be definitely excluded [14]. Thus, to confirm these excellentresults, a multi-center trial using the Harefield protocol was initiated in the U.S. (Harp trial) [11]. Unfortunately, only one of the 17 patients enrolled in the HARP study finally underwent explantation. The discrepancy between the results of the U.S. and Harefield studies have not been completely clarified and further studies are necessary to establish the therapeutic value ofclenbuterol.
A potential tool to facilitate unloading-promoted myocardial recovery might be the future development of automatic control strategies for the left ventricle (LV) afterload impedance, allowing optimization of unloading and controlled “myocardial training” [15].
Pre-explant prediction of cardiac stability without VAD support
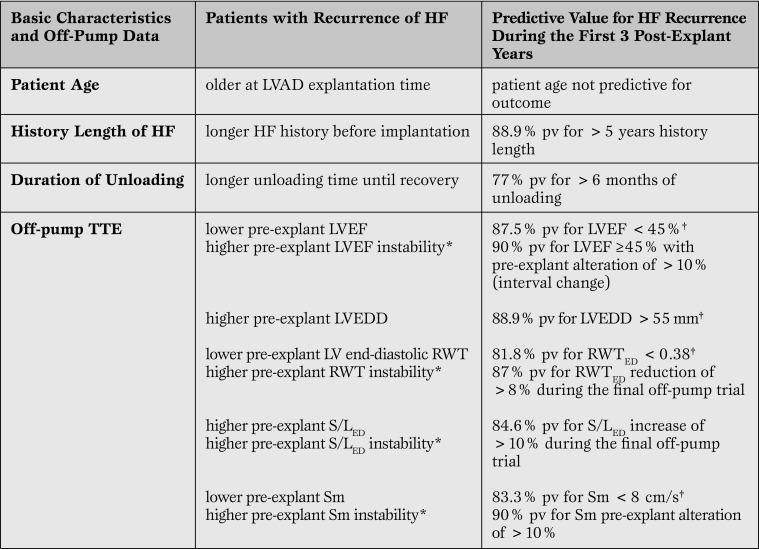
After VAD implantation, transthoracic echocardiography (TTE) parameters of “off-pump” cardiac function,LV size and geometry and their stability between and during off-pump trials after maximum improvement as well as HF duration before VAD implantation allow detection of patients with the potential to remain stable for >5 post-weaning years [1, 2, 8]. Final off-pump left ventricular ejection fraction (LVEF) of ≥45% at rest showed a predictive value of only 74% for post-explant cardiac stability of ≥5 years, but together with either HF history length of ≤5 years, or final off-pump LV end-diastolic diameter (LVEDD) ≤55mm, or LV end-diastolic relative wall-thickness (RWTED)≥0.38, or LV systolic peak wall-motion velocity (Sm) ≥8cm/sec the predictive value for post-explant cardiac stability of ≥5 years increased to 86%-87% [2, 8]. Taking into consideration also the pre-explant stability of LVEF, LV size and LV geometry during the time between maximum LV improvement and VAD explantation, as well as during the final off-pump trial before VAD removal, the predictive value of the main off-pump TTE parameters for post-explant cardiac stability of ≥5 years increases beyond 93% [1]. In patients with stable off-pump LVEF ≥45%at rest plus normal and stable LV size (LVEDD ≤ 55mm) and/or geometry (RWTED≥0.38) the predictive value for post-explant cardiac stability of ≥10 years can reach 90% [1].
Exercise testing also appeared predictive for recovery. Stable or increased mean arterial pressure (MAP) and pulse pressure, as well as LVEF ≥53% after the 6MW test appeared to be strong predictors of recovery, reaching sensitivity and specificity values of up to 93% and 80%, respectively [11].
Risk factors for post-weaning heart failure recurrence
Off-pump LVEF < 45% showed 88% predictive value for HF recurrence during the first 3 years after VAD removal and values < 40% even appeared 100% predictive for early recurrence of HF [1]. The same 100% predictive value for early recurrence of HF was also found for off-pump LVEF < 45% in patients with a history of HF >5years [2]. In patients with LVEF < 50%, an LVEDD >55mm and/or unstable LV geometry (RWTED decrease of >10% during the final off-pump trial before VAD removal) and/or history length of >5 years are relevant risk factors for HF recurrence (predictive values 83%-100%) [2].
Unstable LVEF alone (decrease of >10% during the time between maximum improvement and VAD explantation) is a relevant risk factor for early recurrence of HF even if pre-explant LVEF is >45%, the LV size and geometry are normal and the history length of HF is < 5 years [1, 2]. Also altered and/orunstable LV geometry, as well as low and/or unstable peak wall motion velocity (Sm), arerelevant risk factors for HF recurrence even in patients with LVEF ≥45% [1].
In patients with pre-explant LVEF ≥45%, off-pump diastolic arterial pressure < 50mmHg appeared to be a risk factor for post-weaning HF recurrence [16]. This can be explained by possible over estimations of LV systolic function due to the reduced afterload (LVEF is a load-dependent parameter).
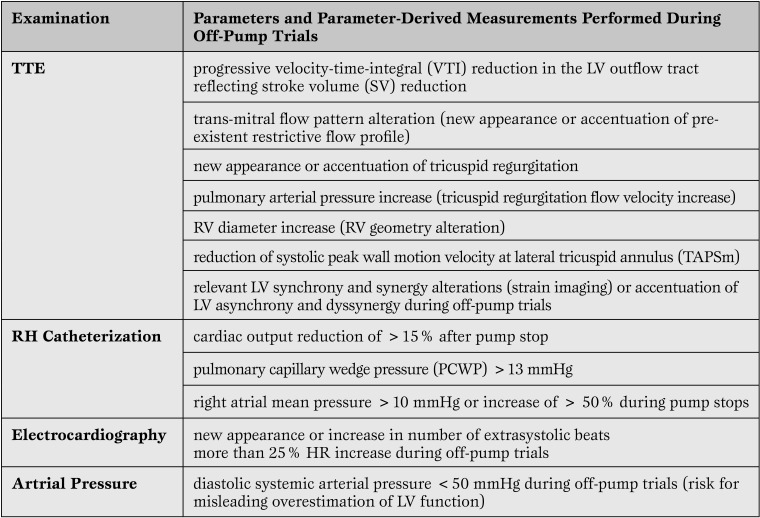
In addition to the definitely proved risk factors for post-weaning HF recurrence mentioned above and also shown in Table 1, we identified other risk factors detectable by TTE or RHC during the off-pump trials with potential relevance for weaning decisions (Table 2).
Table 1.
Major Risk Factors for Recurrence of Heart Failure after LVAD Explantation1,6,7
*parameter changes during the time between maximum improvement and LVAD explantation (“interval change”) and/or during the final off-pump trial (“pre-explant change”).
† parameters measured during the final off-pump trial.
TTE = trans-thoracic echocardiography; pv = predictive value; LVEF = left ventricular ejection fraction; LVEDD = left ventricularend-diastolic diameter; VAD = Ventricular Assist Device; RWT = relative wall-thickness; HTx = heart transplantation; RV = right ventricular.
Table 2.
Additional Risk Factors Detectable During “Off-Pump” Trials at Rest with Relevance for Weaning Decisions7,8,2,1
TTE = trans-thoracic echocardiography;LV = left ventricular; RH = right heart; HR = heart rate; RV = right ventricular.
Elective VAD insertion to promote recovery - a treatment goal
Long-term VADs are to date used as life-saving devices for patients with end-stage HF after all medical therapy options have been exhausted. After insertion, VADs will later become either a bridge-to-HTxor a definitive therapy for those who, for different reasons, cannot receive HTx. Nevertheless, the most attractive potential indication for VADs in the future might be their elective implantationas a therapeutic strategy for cardiac recovery. This will be possible only if the potential for recovery during VAD support becomes predictable before VAD implantation. Unfortunately, the low rates of relevant and stable cardiac recovery during VAD support and the lack of reliable methods to predict recovery before VAD implantation, on the one hand, and the highly invasive procedure of VAD implantation with possibly serious complications after surgery, on the other hand, do not allow VAD implantationsprimarily designed as a therapeutic option for cardiac recovery today.
Cardiac recovery occurred more often in patients with less LV dilation and it was also suggested that pre-implant LV size can predict the potential for VAD-promoted myocardial recovery [17]. However, in a large series of patients with dilated cardiomyopathy (DCM) as the underlying cause for HF before LVAD insertion who underwent LVAD explantation after recovery, we could not identify any TTE parameter measured before implantation which was able to predict recovery during LVAD support [2, 8]. We found that even preoperative LVEDD of >70mm does not exclude reverse remodeling and LVEF increase beyond 45% during LVAD support allowing long-term cardiac stability after LVAD removal [2, 8]. Thus, to date, standard echocardiographycannot predict cardiac recovery during mechanical unloading. The possible superiority of strain imagingin this matterneeds to be assessed in the future [18, 19].
In DCM patients, histological examination of myocardial tissue obtained at VAD implantation showed that less fibrosis and myocyte hypertrophy are associated with better cardiac recovery during VAD support, and fibrosis appeared to be an independent predictor of sustained myocardial recovery [20]. Our weaned patients with ≥5 year post-explant cardiac stability showed less fibrosis before VAD implantation than those with early post-weaning HF recurrence [2]. Nevertheless, more information is necessary before any elective LVAD implantation with the aim of cardiac recovery can be considered only on the basis of reduced pre-implant fibrosis. History length of HF≥5 years does not exclude reverse remodeling with EF increase beyond 45%, but such patients' risk for HF recurrence during the first 3 post-weaning years is several times higher than in those with history of HF < 5 years (probability of HF recurrence 89%) [2]. However, short history ofHFalone is not predictive for cardiac recovery during mechanical unloading [2, 8].
VAD implantation is a traumatic event for the myocardium due to cardiac surgery, creation of a hole in the LV apex, increasing fibrosis and secondarily increasing the incidence of ventricular arrhythmias. There is a realistic hope that smaller VADs will reduce the operative and postoperative complications associated with VAD implantations and open up prospects in the near future of VAD implantations in earlier stages of the disease when the reversibility of morphological and functional changes during mechanical unloading might be substantially higher than in the end stages of HF.
Summary and future directions
During VAD support, end-stage failing hearts can often recover at molecular and cellular level but translation of these changes into functionally stable cardiac recovery allowing long-term HTx/VAD-free outcome after VAD removal is relatively rare and appears to be related to the etiology, severity and duration of myocardial damage.
Weaning from VADs is a feasible clinical option with potential successful results for >15 years even if CCM was the underlying cause for VAD implantation and even if cardiac recovery remains incomplete. TTE and RHC are the cornerstone methods to assess clinically relevant cardiac recovery. There are many parameters that have proved to be useful for assessment of recovery and for prediction of long-termweaning success, but to date there is still no gold standard for recovery assessment. However, off-pump LVEF ≥45% and LVEDD ≤ 55mm, at rest, are generally accepted as basic criteria for LVAD explantation and their stability for 2-4 weeks after maximum improvement is also accepted as an important requirement. Other off-pump echocardiographic parameters of cardiac function (including tissue Doppler and strain imaging data) and LV geometry, as well as their pre-explant stability (between and during off-pump trials after maximum improvement) are helpful for weaning decisions. Normal and stable hemodynamics during off-pump RHC trials is necessary for weaning decisions, but not sufficiently predictive for long-term cardiac stability after VAD explantation. Off-pump CI >2.5L/min/m²and PCWP <14 mmHg are accepted as major requirements for VAD explantation. HF history length ≥5 years is one of the major risk factors for HF recurrence after VAD explantation.
There are two major limitations for a potential future use of VADs as a therapeutic strategy aimed to reverse HF: first, the low probability of relevant cardiac recovery, even after combination of unloading with drugs known to enhance reverse remodeling (ACE-inhibitors, β-blockers, Ang-II-receptor antagonists and aldosterone inhibitors) and second, the fact that recovery is not predictable before VAD implantation.
There are still several open questions on myocardial recovery after VAD implantation, which need to be answered in the future:
What causes the great discrepancy between the high recovery rates on cellular and molecular levels and the low rate of functionally stable cardiac recovery allowing VAD explantation?
Can future research on the molecular and cellular levels provide a platform for additional therapies (pharmacologic and/or cell-based therapy, gene transfer, etc.), aimed to optimize recovery and increase the number of weaning candidates?
Is it possible to facilitate weaning from VADs also in patients with chronic ischemic cardiomyopathy by promoting angiogenesis and myocyte regeneration?
Can future research on the molecular and cellular level provide data that might help detect patients with the potential for cardiac recovery under mechanical unloading already before VAD implantation?
Acknowledgments
We thank Anne Gale, Editor in the Life Sciences, of the Deutsches Herzzentrum Berlin for editorial assistance.
Footnotes
Source of Support Nil.
Disclosures None declared.
This three-part article is based on a lecture given at the 7th Expert Forum of the Roland Hetzer International Cardiothoracic and Vascular Surgery Society, Berlin, Germany, 5-6 April 2014.
Cite as: Dandel M, Hetzer R. Myocardial recovery during mechanical circulatory support: long-term outcome and elective ventricular assist device implantation to promote recovery as a treatment goal. Heart, Lung and Vessels. 2015;7(4):289-296
References
- Dandel M, Weng Y, Siniawski H, Potapov E, Krabatsch T, Lehmkuhl H B. et al. Pre-explant stability of unloading promoted cardiac improvement predicts outcome after weaning from ventricular assist devices. Circulation. 2012;126:3–19. doi: 10.1161/CIRCULATIONAHA.111.084640. [DOI] [PubMed] [Google Scholar]
- Dandel M, Weng Y, Siniawski H, Stepanenko A, Krabatsch T, Potapov E. et al. Heart failure reversal by ventricular unloading in patients with chronic cardiomyopathy: criteria for weaning from ventricular assist devices. Eur Heart J. 2011;32:1148–1160. doi: 10.1093/eurheartj/ehq353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D O, Edwards L B, Boucek M M, Trulock E P, Aurora P, Christie J. et al. The registry of the International Society for Heart and Lung Transplantation: twenty fourth official adult transplant report – 2007. J Heart Lung Transplant. 2007;26:769–781. doi: 10.1016/j.healun.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Birks E J, George R S, Firouzi A, Wright G, Bahrami T, Yacoub M H. et al. Long-term outcomes of patients bridged to recovery versus patients bridged to transplantation. J Thorac Cardiovasc Surg. 2012;144:190–196. doi: 10.1016/j.jtcvs.2012.03.021. [DOI] [PubMed] [Google Scholar]
- Kirklin J K, Naftel D C, Kormos R L, Stevensen L W, Pagani F D, Miller M M. et al. Fifth INTERMACS Annual Report: Risk factor analysis for more than 6000 mechanical circulatory support patients. J Heart Lung Transplant. 2013;32:141–156. doi: 10.1016/j.healun.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Dandel M, Knosalla C, Hetzer R. Contribution of ventricular assist devices to the recovery of failing hearts: a review and Berlin Heart Center experience. Eur J Heart Fail. 2014;16:248–263. doi: 10.1002/ejhf.18. [DOI] [PubMed] [Google Scholar]
- Dandel M, Weng Y, Sinawski H, Potapov E, Lehmkuhl H B, Hetzer R. Long-term results in patients with idiopathic dilated cardiomyopathy after weaning from left ventricular assist devices. Circulation. 2005;112:37–45. doi: 10.1161/CIRCULATIONAHA.104.525352. [DOI] [PubMed] [Google Scholar]
- Dandel M, Weng Y, Siniawski H, Potapov E, Drews T, Lehmkuhl H B. et al. Prediction of cardiac stability after weaning from ventricular assist devices in patients with idiopathic dilated cardiomyopathy. Circulation. 2008;118:94–105. doi: 10.1161/CIRCULATIONAHA.107.755983. [DOI] [PubMed] [Google Scholar]
- Klotz S, Burkhoff D, Garrelds I M, Boomsma F, Danser A H. The impact of left ventricular assist device induces left ventricular unloading on the myocardial renin-angiotensin-aldosterone system: therapeutic consequences? Eur Heart J. 2009;30:805–812. doi: 10.1093/eurheartj/ehp012. [DOI] [PubMed] [Google Scholar]
- Klotz S, Danser A H, Foroniy R F, Oz M C, Wang J, Mancini D. et al. The impact of angiotensin-converting enzyme inhibitor therapy on the extracellular collagen matrix during left ventricular assist device support in patients with end-stage heart failure. J Am Coll Cardiol. 2007;49:1166–1174. doi: 10.1016/j.jacc.2006.10.071. [DOI] [PubMed] [Google Scholar]
- Birks E J, Miller L W. Myocardial recovery with use of ventricular assist devices. In: Kormos RL and Miller LW (eds.) Mechanical Circulatory Support. A Companion to Braunwald’s Heart Disease. Elsevier, Philadelphia. 2012;258:71–71. [Google Scholar]
- Khan T, Okerberg K, Hernandez A, Miller K, Myers T J, Radovancevic B. et al. Assessment of myocardial recovery using Dobutamin stress echocardiography in LVAD patients. J Heart Lung Transplant. 2001;20:202–203. doi: 10.1016/s1053-2498(00)00432-0. [DOI] [PubMed] [Google Scholar]
- Birks E J, Tansley P D, Hardy J, George R S, Bowles C T, Burke M. et al. Left ventricular assist device and drug therapy for the reversal of heart failure. N Engl J Med. 2006;355:1873–1884. doi: 10.1056/NEJMoa053063. [DOI] [PubMed] [Google Scholar]
- Hetzer R, Dandel M, Knosalla C. Left ventricular assist devices and drug therapy in heart failure. N Engl J Med. 2007;356:869–870. doi: 10.1056/NEJMc063394. [DOI] [PubMed] [Google Scholar]
- Moscato F, Arabia M, Colacino F M, Naiyanetr P, Danieli G A, Schima H. Left ventricular afterload impedance control by an axial flow ventricular assist device: a potential tool for ventricular recovery. Artifical Organs. 2010;34:736–744. doi: 10.1111/j.1525-1594.2010.01066.x. [DOI] [PubMed] [Google Scholar]
- Dandel M, Potapov E, Vierecke J, Stepanenko A, Krabatsch T, Knosalla C. et al. Low diastolic arterial pressure during pre-explant off-pump trials can induce misleading overestimation of left ventricular function after left ventricular assist device removal. Circulation. 2012;126:9754–9754. [Google Scholar]
- Simon M A, Primack B A, Teutenberg J, Kormos R L, Bermudez C, Toyoda Y. et al. Left ventricular remodelling and myocardial recovery on mechanical circulatory support. J Card Fail. 2010;16:99–105. doi: 10.1016/j.cardfail.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandel M, Hetzer R. Echocardiographic strain and strain rate imaging - Clinical applications. Int J Cardiol. 2009;132:11–24. doi: 10.1016/j.ijcard.2008.06.091. [DOI] [PubMed] [Google Scholar]
- Dandel M, Lehmkuhl H, Stepanenko A, Krabatsch T, Potapov E, Weng Y. et al. Evaluation of left ventricular function by tissue Doppler and 2D strain imaging can facilitate the decision to wean patients with unloading induced cardiac recovery from mechanical assist devices. Eur Heart J. 2010;31:67–67. [(Abstract-Suppl)] [Google Scholar]
- Saito S, Matsumiya G, Sakaguchi T, Miyagawa S, Yamauchi T, Kuratani T. et al. Cardiac fibrosis and cellular hypertrophy decrease the degree of reverse remodeling and improvement in cardiac function during left ventricular assist. J Heart Lung Transplant. 2010;29:672–679. doi: 10.1016/j.healun.2010.01.007. [DOI] [PubMed] [Google Scholar]