Abstract
Survival of elderly patients with glioblastoma (GBM) is poor, but improves with tumor resection and radiotherapy (RT). Concurrent temozolomide (TMZ) chemotherapy during RT improves the survival of younger patients with GBM, but the benefit in elderly patients is unclear. Medical records of patients ≥65 years old with primary GBM, histologically confirmed at Memorial Sloan-Kettering Cancer Center and treated with RT, were reviewed. Survival was associated with patient (age, performance status), tumor (single or multiple), and treatment (extent of surgery, RT field, technique, fractionation and use of concurrent TMZ) characteristics in a multivariable Cox regression model. Grade ≥3 hematologic toxicity rates were compared to reported rates in younger patients. Median age of the 291 patients studied was 71 years. Longer survival was associated with younger age, tumor resection, and concomitant TMZ and RT (p < 0.01). Concurrent TMZ and RT improved median survival of patients with favorable prognostic factors from 12 to 21 months and from 10 to 13 months in patients 65–70 and ≥71 years old, respectively. Concomitant TMZ and RT increased the 2 year OS rate from 14 to 41 % and from 5 to 24 % in patients 65–70 and ≥71 years old, respectively. Grade 3–4 thrombocytopenia was significantly more frequent in the present cohort. Survival of elderly patients with GBM may be prolonged with the use of concomitant TMZ during RT. An ongoing randomized study will determine the benefit of this approach in a prospective fashion.
Keywords: Concurrent, Elderly, Glioblastoma, Radiotherapy, Temozolomide
Introduction
According to the central brain tumor registry of the US half of patients diagnosed with glioblastoma (GBM) are elderly (≥65 years old) [1]. Older patients with GBM fare worse than their younger counterparts [2]. The perception that older patients may not benefit from treatment and the reluctance to offer aggressive therapy is a possible reason for this observation. However, a small randomized controlled trial (RCT) of elderly patients (≥65 years old) confirmed the benefit of maximally safe tumor resection as first line therapy. For patients with a Karnofsky performance score (KPS) ≥60 [3] and radiographic evidence of a malignant supratentorial glioma, this study showed that survival was significantly longer in patients who underwent surgery versus biopsy (median 6 vs. 3 months) [4].
Radiation therapy (RT) has also been shown to improve survival in elderly patients as opposed to best supportive care (BSC). A RCT demonstrated that patients ≥70 years old with a histologically confirmed GBM and KPS ≥70 randomized to receive RT (50 Gy in 28 fractions) survived longer than those who received BSC (median 7 vs. 4 months) [5]. Two RCTs have investigated abbreviated courses of RT for elderly patients to improve convenience and minimize toxicity of protracted RT [6], [7]. The RCT by Roa et al. indicated that 40 Gy in 15 fractions was equivalent to 60 Gy in 30 fractions for RT alone.
The European organisation for research and treatment of cancer (EORTC) and the national cancer institute of Canada (NCIC) conducted a practice-changing study (26981-22981/CE3) of patients 18–70 years old with GBM with world health organization performance status ≤2 who underwent surgery (when possible) followed by RT (60 Gy in 30 fractions) and were randomly assigned to receive temozolomide (TMZ) given concurrently with and after RT. This study found that median survival was extended from 12 to 15 months with the addition of TMZ. Moreover, the 2 year survival rate was increased from 10 to 27 % with TMZ [8]. Long-term follow-up has confirmed the durability of these results [9] and similarly designed studies have replicated the findings independently [10]. Thus, in patients less than 70 years old who are in good health, the standard of care is surgery followed by concurrent TMZ and RT and an adjuvant course of TMZ.
But are the findings of the EORTC–NCIC study applicable to the large proportion of patients with GBM who are elderly? The original and updated results of the EORTC–NCIC trial suggest that the benefit of concomitant TMZ and RT is greatest in younger patients [8], [9]. An unpublished trend benefit analysis showed no benefit of adding TMZ to RT in the small subgroup of patients ≥65 years old in the EORTC–NCIC trial (HR 0.8, p = 0.340) [11]. However, subsequent analyses demonstrated a survival benefit in the group of patients 60–70 years old [9]. Several single-arm prospective and retrospective studies have determined that concurrent TMZ and conventional RT in selected elderly patients is a tolerable regimen, but little data exists regarding the relative benefit of RT given with or without TMZ in the elderly [12–18]. Therefore, we conducted this retrospective clinical study to determine the added value of TMZ concurrent with RT in elderly patients with GBM.
Methods and materials
Patients
With permission from the institutional review board at Memorial Sloan-Kettering Cancer Center (MSKCC) we conducted this retrospective clinical study. Patients ≥65 years old at the time of histologic diagnosis of GBM (which was confirmed at MSKCC) between 1987 and 2008, who were treated with definitive RT were included. We considered those patients that received a biologically equivalent dose of 60 Gy (equivalent to at least 40 Gy in 15 fractions), using the linear-quadratic modeling and alpha/beta of 5.6, to have received definitive (rather than palliative) RT [19], [20]. Patients were identified in various electronic institutional databases and were eligible for study. Patients with secondary GBMs, inadequate medical records for review, or who did not receive external beam RT were excluded from study. Year of diagnosis, age at diagnosis, KPS, and mental status prior to initiating RT was recorded. The number of supratentorial tumors noted on imaging prior to surgery was also recorded.
Patient and treatment characteristics were used to assign patients to a radiation therapy oncology group recursive partitioning analysis (RTOG RPA) class. The RTOG RPA is a validated prognostic classification system for patients with high-grade glioma undergoing RT [21], [22]. RTOG RPA class 6 consisted of patients with KPS <70 and abnormal mental status, or patients with KPS ≥70 who underwent biopsy only and received <90 % of the prescribed dose of RT. Class 5 consisted of all other patients because a criterion for inclusion in class 4 of the RTOG RPA is that a patient is “able to work.” In this cohort of elderly people, we were unable to determine which patients were “unable to work” from those that were simply retired. Therefore no patients were assigned to class 4. Patients were further categorized into age groups based on potential eligibility for the EORTC–NCIC trial; patients 65–70 would have been eligible, while those ≥71 would not have been eligible.
Treatment and toxicity
Treatment characteristics recorded included extent and date of surgery (biopsy only, or neurosurgeon-determined subtotal or gross total resection), RT field design (whole-brain RT or partial-brain RT), RT technique (conventional, three-dimensional conformal RT, or intensity-modulated RT), RT dose (total in Gy), and fractionation (hypofractionated, ≥2.5 Gy/fraction, or conventional, 1.8–2 Gy/fraction), and concomitant TMZ use.
Acute grade ≥3 hematologic toxicity (leukopenia, neutropenia, thrombocytopenia, and anemia) during and ≤28 days after the completion of RT (prior to starting any chemotherapy not delivered concomitantly with RT) was recorded for patients receiving concurrent TMZ using the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 3.0. Constitutional, dermatologic, infectious, neurologic, late, or other complications were not routinely assessed, and therefore were not recorded.
Statistical analysis
Overall survival (OS) was defined as duration of time from start of RT to death or last follow-up (collected until March 31, 2010). Cox regression models were built to evaluate the association of clinical factors with OS. Hazard ratios along with 95 % confidence intervals (CIs) were reported. OS was estimated using Kaplan–Meier methods, and log-rank tests were performed for patients with favorable prognostic features to compare the survival functions of concomitant TMZ during RT versus RT alone. Among patients that received a full course of conventionally fractionated RT, log-rank tests were also performed to compare survival between those that did or did not receive concurrent TMZ. Acute hematologic toxicity was compared with the toxicity observed in the EORTC-NCIC trial using Fisher’s exact test. All statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC, USA) and two-sided statistical inferences were employed throughout the analyses.
Results
There were 291 patients eligible for study. Patient, tumor, and treatment-related characteristics are listed in Table 1. The median age of patients was 71 (range 65–100) years. Patients were divided into two age groups (65–70 years and ≥71 years) to distinguish the patients who would have been eligible for the EORTC–NCIC trial, and those that were not. The distribution of patients by decade of age is presented in Supplement Fig. 1. Median KPS was 80 with a range of 40–100. Most patients exhibited favorable clinical features meeting criteria for RTOG RPA class 5. Few patients had multiple lesions present on imaging at diagnosis. Most patients underwent either gross total or subtotal resection. Subsequently, most were treated with conventionally fractionated conformal RT to part of the brain. The median interval from histologic diagnosis to initiation of RT was 4 weeks. Approximately 40 % of patients received concomitant TMZ during RT; among these patients, the average total duration of receiving TMZ was 6.5 months (median 4.3 months, range 0.3–25 months). Among patients that received adjuvant TMZ after receiving RT alone, the average total duration of receiving TMZ was 5.7 months (median 2.8 months, range 0.03–26 months) Characteristics of the patients receiving and not receiving TMZ are listed in Supplement Table 1.
Table 1.
Patient, tumor and treatment characteristics (n = 291)
| Patient and tumor characteristics | # Of patients (%) |
|---|---|
| Age group (years) | |
| 65–70 | 146 (50) |
| ≥71 | 145 (50) |
| Sex | |
| Male | 176 (60) |
| Female | 115 (40) |
| RTOG RPA class | |
| 5 | 263 (90) |
| 6 | 28 (10) |
| Number of lesions | |
| 1 | 264 (91) |
| ≥2 | 27 (9) |
| Treatment characteristics | |
| Extent of surgery | |
| Biopsy only | 51 (18) |
| Subtotal resection | 134 (46) |
| Gross total resection | 106 (36) |
| RT fields | |
| Unknown | 8 (3) |
| WBRT | 10 (3) |
| PBRT | 273 (94) |
| RT fractionation | |
| Conventional | 259 (89) |
| Hypofractionated | 32 (11) |
| RT technique | |
| Unknown | 16 (5) |
| 3DCRT | 132 (45) |
| IMRT | 111 (38) |
| Non-conformal | 32 (11) |
| Concomitant TMZ and RT | |
| Yes | 115 (40) |
| No | 176 (60) |
RTOG RPA radiation therapy oncology group recursive partitioning analysis, RT radiotherapy, WBRT whole-brain radiotherapy, PBRT partial-brain radiotherapy, 3DCRT 3-dimensional conformal radiotherapy, IMRT intensity-modulated radiotherapy, TMZ temozolomide
Prior to RT, mean KPS of the group of patients undergoing concurrent RT and TMZ was 77.5 (median 80, range 40–100); after RT the average KPS in this group was 74.1 (median 80, range 40–100) representing a statistically significant decline on paired t test (p = 0.0055). Among patients not receiving concurrent TMZ during RT, mean KPS prior to RT was 76.2 (median 80, range 40–100). After RT, mean KPS of this group was 74.0 (median 80, range 40–100), representing a marginally significant decline in KPS (p = 0.07).
Median follow-up and OS for the cohort was 10 and 12 months, respectively. Median follow-up for survivors was 22 months. Two-year OS for the entire cohort was 15 % (95 % CI, 11–20 %). As noted in Table 2, among 291 patients with clinical data available for inclusion in the Cox regression model, all patient, tumor, and treatment variables studied were associated with survival on univariable analysis with the exception of RT field and technique. However, on multivariable analysis longer survival was associated with younger age group, tumor resection, and concomitant TMZ and RT. RTOG RPA class 5 was marginally associated with improved survival. Multiple tumors, RT fractionation, and time period of diagnosis were not associated with longer survival on multivariable analysis.
Table 2.
Patient, tumor, and treatment variables associated with survival in a univariable and multivariable Cox regression model (n = 291)
| Univariable
|
Multivariable
|
|||||
|---|---|---|---|---|---|---|
| Patient, tumor and treatment variables | HR | 95 % CI | p value | HR | 95 % CI | p value |
| Age group (years) | ||||||
| 65–70 | 0.67 | (0.52–0.86) | <0.01 | 0.65 | (0.49–0.84) | 0.004 |
| ≥71 | 1 | 1 | ||||
| Number of lesions | ||||||
| 1 | 0.57 | (0.38–0.87) | <0.01 | 0.59 | NS | |
| ≥2 | 1 | 1 | ||||
| RTOG RPA | ||||||
| 5 | 0.35 | (0.23–0.53) | <0.01 | 0.59 | (0.35–1.00) | 0.05 |
| 6 | 1 | 1 | ||||
| Extent of surgery | ||||||
| Subtotal resection | 0.54 | (0.38–0.75) | <0.01 | 0.55 | (0.39–0.77) | <0.01 |
| Gross total resection | 0.42 | (0.29–0.6) | 0.48 | (0.32–0.69) | ||
| Biopsy only | 1 | |||||
| RT fields | ||||||
| PBRT | 0.54 | (0.25–1.14) | 0.11 | NS | ||
| WBRT | 1 | |||||
| RT fractionation | ||||||
| Conventional | 0.50 | (0.34–0.73) | <0.01 | NS | ||
| Hypofractionated | 1 | |||||
| RT technique | ||||||
| Non-conformal | 1.48 | (0.98–2.24) | 0.11 | NS | ||
| IMRT | 0.95 | (0.72–1.24) | ||||
| 3DCRT | 1 | |||||
| Concomitant TMZ and RT | ||||||
| Yes | 0.58 | (0.45–0.75) | <0.01 | 0.62 | (0.45–0.84) | 0.003 |
| No | 1 | 1 | ||||
| Time period of diagnosis | ||||||
| 1987–1991 | 1.95 | (1.14–3.34) | 0.04 | NS | ||
| 1992–1996 | 1.47 | (0.94–2.28) | ||||
| 1997–2001 | 1.15 | (0.84–1.57) | ||||
| 2002–2008 | 1 | |||||
RT radiotherapy, WBRT whole-brain radiotherapy, PBRT partial-brain radiotherapy, 3DCRT 3-dimensional conformal radiotherapy, IMRT intensity-modulated radiotherapy, TMZ temozolomide
Among the 291 patients studied, 208 were found to have favorable prognostic characteristics including RTOG RPA class 5, and a tumor that was resected. Figure 1 demonstrates the duration of survival and effect of TMZ in the favorable group of patients, aged 65–70 and ≥71 years, respectively. Log-rank tests revealed a statistically significant increase in the duration of survival in patients who received RT alone versus concomitant TMZ and RT, from 12 months (95 % CI, 10–15) to 21 months (95 % CI, 13–24) and from 10 months (95 % CI, 8–11) to 13 months (95 % CI, 8–17) months, in patients 65–70 and ≥71 years old, respectively. Compared to RT alone, concomitant TMZ and RT increased the 2 year OS rate from 14 % (95 % CI, 7–24 %) to 41 % (95 % CI, 26–56 %) and from 5 % (95 % CI, 1–13 %) to 24 % (95 % CI 11–40 %) in patients 65–70 and ≥71 years old, respectively.
Fig. 1.
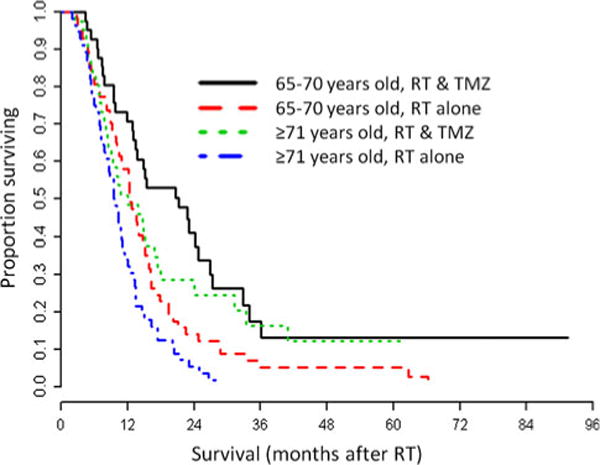
Overall survival of GBM in patients with favorable prognosis (single resected tumor and RTOG RPA class 5) that underwent RT (n = 208). Patients 65–70 years old treated with RT alone are represented by a red, long-dash line. Patients 65–70 years old treated with RT and concomitant TMZ are represented by a black, solid line. Patients ≥71 years old treated with RT alone are represented by a blue, dash-dot line. Patients ≥71 years old treated with RT and concomitant TMZ are represented by a green, dot line. RT, radiation therapy; TMZ, temozolomide. p < 0.01 for log rank test between the treatment groups within the age groups
Of all patients treated with a full course of conventionally fractionated RT (n = 259), a log-rank test revealed a statistically significant increase in survival among those that received concurrent TMZ during RT (p = 0.00001). Figure 2 demonstrates the effect of TMZ in patients who received a full course of conventionally fractionated RT.
Fig. 2.
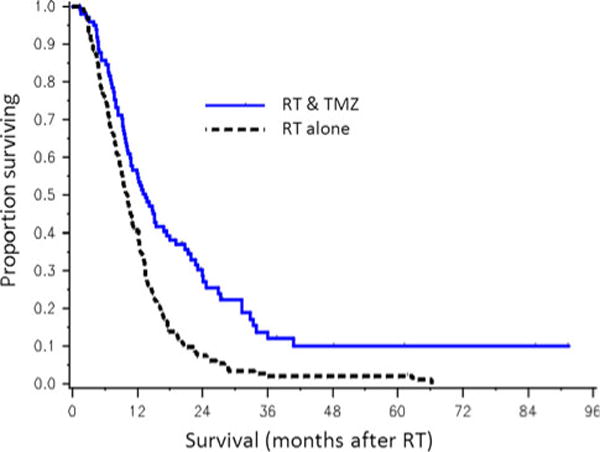
Overall survival of GBM in patients treated with a full course of conventionally fractionated RT (n = 245) that did (n = 98, blue, solid line) or did not (n = 147, black, dashed line) receive concurrent TMZ during RT. RT, radiation therapy; TMZ, temozolomide. p < 0.01 for log rank test between the treatment groups
Toxicity
Of the 115 patients treated with concomitant TMZ and RT, 84 had hematologic data available for toxicity assessment. Grade 3–4 leukopenia, neutropenia, thrombocytopenia, anemia, or any hematologic toxicity was noted in up to 12 % of patients, as noted in Table 3. Thrombocytopenia was significantly more common in the present cohort when compared to the rate of toxic effect observed in the EO-RTC-NCIC study (p < 0.05). Of the 115 patients treated with concurrent TMZ, one died of neutropenic sepsis. Of the 31 patients for whom hematologic data was not available, only one died ≤28 days after the completion of RT (of a non-hematologic cause). No other acute fatal hematologic toxicity related to TMZ use was observed.
Table 3.
Acute grade 3–4 hematologic toxicity among patients receiving concomitant temozolomide and radiotherapy in the present study and the European organisation for research and treatment of cancer (EORTC) 26981-22981/national cancer institute of Canada clinical trials group (NCIC) CE3 study
| Grade 3–4 hematologic toxicity | Number of patients (%) Present study (n = 84) |
Number of patients (%) EORTC–NCIC study (n = 284) |
|---|---|---|
| Leukopenia | 4 (4.8) | 7 (2.5) |
| Neutropenia | 2 (2.4) | 12 (4.2) |
| Thrombocytopenia* | 9 (10.7) | 9 (3.2) |
| Anemia | 2 (2.4) | 1 (0.4) |
| Any | 10 (11.9) | 19 (6.7) |
EORTC–NCIC European organisation for research and treatment of cancer—national cancer institute of Canada
p<0.05 for Fisher’s exact test between studies
Discussion
In this retrospective analysis we found that when TMZ is given concomitant with RT survival of elderly patients with GBM may be improved.
Previous RCTs supported a survival benefit of TMZ given concomitant with RT in younger patients with GBM but studies in patients ≥65 years old [12–18] have lacked a comparison group to assess the relative benefit of TMZ and have been too small to perform multivariable analyses to adjust for patient, tumor, and treatment variables prognostic of survival when determining the benefit of concomitant TMZ.
A previous study of elderly patients with GBM from our institution found several factors associated with longer survival after diagnosis [23]. On multivariable analysis these included younger age, KPS ≥70, single tumor, and tumor resection. The prior analysis included patients who did not undergo RT and therefore the cohort in the present analysis likely represents a population with a more favorable prognosis. We combined KPS, mental status, and treatment characteristics to assign patients to a RTOG RPA classification, a prognostic schema developed and validated in clinical trial participants with high-grade glioma who were less than 70 years old [21], [22]. We found that the RTOG RPA classification was a marginally effective prognostic factor in patients ≥65 years old. Analysis of the Cox regression model using KPS instead of RTOG RPA yielded the similar results (data not shown). While the previous study from our institution assessed the benefit of adjuvant chemotherapy after RT, the present study investigated the benefit of concomitant TMZ during RT and supports a benefit of this approach.
Our results also support several results of previously conducted RCTs in elderly patients with GBM. We found that tumor resection was associated with longer survival on multivariable analysis [4]. Similarly, after adjusting for other factors in multivariable models we found no difference in survival after conventional versus abbreviated hypofractionated RT consistent with a RCT from Canada [6]. After adjusting for other factors in multivariable models we also found no difference in survival of patients who received whole-brain versus partial-brain RT consistent with the results of a small RCT from Australia [7]. The latter finding must be interpreted with caution as full-dose whole-brain RT may be associated with increased toxicity in the elderly patient population and therefore should probably not be employed routinely.
Previous studies have suggested that concurrent RT and TMZ might yield excessive toxicity in the elderly [17]. We found that low-dose TMZ yielded severe and lethal hematologic toxicity in 12 and 1 % of patients, respectively. Consistent with previous studies [24] we found that treatment-related hematologic toxicity was almost twice as common in the elderly population (12 % vs. 7 %), compared to the younger population studied by the EORTC–NCIC. Of note, we had only had hematologic toxicity data available for 84 of 115 (73 %) patients that received TMZ, thus our estimate may not be accurate.
Despite our findings determining which elderly patients will benefit from concomitant chemoradiation remains a formidable challenge; clinical acumen and tumor biomarkers may aid in this decision-making process. We found a benefit of TMZ and RT in patients with favorable clinical characteristics (single resected tumor and RTOG RPA class 5). Methylation of the O-6-methylguanine methyltransferase gene promoter proved to be a valuable aid when predicting response to concomitant TMZ and RT in the EORTC–NCIC study [25] and also had prognostic power in a recent study of elderly patients with GBM treated with concomitant TMZ and RT [12]. Future studies of concomitant TMZ and RT in the elderly should incorporate these types of evaluations.
Selection of patients for treatment with concurrent TMZ during and after RT in the present study may have lead to biased results. As noted in Supplemental Table 1, most patients not receiving TMZ during RT were treated prior to 2001. However, a considerable number (n = 74, 42 % of those receiving concurrent TMZ during RT) received this treatment in the more recent era. The selection of these patients for less intensive therapy may reflect a difference in the patient population not adequately described in the present analysis. Future studies designed to limit bias will be necessary to further clarify this point.
A limitation of our report is that we did not ascertain exact details of the doses of TMZ prescribed or taken, nor did we describe the safety or efficacy of TMZ given after RT as was delivered in the EORTC-NCIC study. At MSKCC patients who receive concomitant TMZ during RT usually receive 50–75 mg/m2 as suggested by the RCTs. At MSKCC it is common practice to continue TMZ after RT, for 6–12 months or until treatment intolerance, progression, or death. Importantly, our report suggests a benefit of concomitant TMZ with RT but does not address the issue of whether additional TMZ after RT is beneficial. Although there is no high-level evidence to suggest a benefit for a short course of TMZ concomitant with RT exclusively this approach might be suitable for elderly or infirm patients with cognitive impairment who take multiple medicines. Preclinical studies have demonstrated that TMZ modifies the biologic effects of RT [26], [27] suggesting a benefit to concomitant delivery. This hypothesis has been supported by a clinical study which suggests added value of TMZ when given concomitantly with RT as opposed to after RT [28]. For these reasons the optimal approach to incorporating TMZ into the adjuvant regimens for elderly patients with GBM deserves further study.
The retrospective nature of this study is an obvious limitation to interpretation. The changes in data availability and patterns of clinical practice over the 20 years of the study period likely led to heterogeneity in the studied population. Nevertheless, we carefully constructed Cox regression models using the most important previously described patient, tumor, and treatment characteristics that affect survival in this disease. Using a large cohort of patients we were able to confirm previously described prognostic factors and in an exploratory fashion demonstrates the benefit of TMZ concomitant with RT. Results from an ongoing prospective RCT by the EORTC–NCIC (CE6, NCT00482677) will further elucidate the benefit of this approach. In the absence of the results of these studies we agree with other investigators that age alone should not preclude aggressive combined-modality adjuvant therapy with concomitant TMZ and RT [16].
Supplementary Material
Acknowledgments
Presented in part at the 52nd annual meeting of the American Society for Radiation Oncology meeting in San Diego, CA, on November 2, 2010. This study was supported in part by the Memorial Sloan-Kettering Cancer Center Brain Tumor Center Medical Student Summer Fellowship (M.C.).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s11060-012-0906-4) contains supplementary material, which is available to authorized users.
Conflict of interest The authors have no actual or potential conflicts of interest.
Contributor Information
Christopher A. Barker, Email: barkerc@mskcc.org, Department of Radiation Oncology, Memorial Sloan-Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA.
Maria Chang, Department of Radiation Oncology, Memorial Sloan-Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA.
Joanne F. Chou, Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY, USA
Zhigang Zhang, Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY, USA.
Kathryn Beal, Department of Radiation Oncology, Memorial Sloan-Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA.
Philip H. Gutin, Department of Neurosurgery, Memorial Sloan-Kettering Cancer Center, New York, NY, USA
Fabio M. Iwamoto, Neuro-Oncology Branch, National Cancer Institute, Bethesda, MD, USA
References
- 1.CBTRUS. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2004–2006. CBTRUS; Hinsdale: 2010. [Google Scholar]
- 2.Iwamoto FM, Reiner AS, Panageas KS, Elkin EB, Abrey LE. Patterns of care in elderly glioblastoma patients. Ann Neurol. 2008;64:628–634. doi: 10.1002/ana.21521. [DOI] [PubMed] [Google Scholar]
- 3.Karnofsky D, Burchenal J. The clinical evaluation of chemotherapeutic agents in cancer. In: Macleod C, editor. Evaluation of chemotherapeutic agents. Columbia University Press; New York: 1949. pp. 199–205. [Google Scholar]
- 4.Vuorinen V, Hinkka S, Farkkila M, Jaaskelainen J. Debulking or biopsy of malignant glioma in elderly people—a randomised study. Acta Neurochir (Wien) 2003;145:5–10. doi: 10.1007/s00701-002-1030-6. [DOI] [PubMed] [Google Scholar]
- 5.Keime-Guibert F, Chinot O, Taillandier L, Cartalat-Carel S, Frenay M, Kantor G, Guillamo JS, Jadaud E, Colin P, Bondiau PY, Menei P, Loiseau H, Bernier V, Honnorat J, Barrie M, Mokhtari K, Mazeron JJ, Bissery A, Delattre JY. Radiotherapy for glioblastoma in the elderly. N Engl J Med. 2007;356:1527–1535. doi: 10.1056/NEJMoa065901. [DOI] [PubMed] [Google Scholar]
- 6.Roa W, Brasher PM, Bauman G, Anthes M, Bruera E, Chan A, Fisher B, Fulton D, Gulavita S, Hao C, Husain S, Murtha A, Petruk K, Stewart D, Tai P, Urtasun R, Cairncross JG, Forsyth P. Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J Clin Oncol. 2004;22:1583–1588. doi: 10.1200/JCO.2004.06.082. [DOI] [PubMed] [Google Scholar]
- 7.Phillips C, Guiney M, Smith J, Hughes P, Narayan K, Quong G. A randomized trial comparing 35 Gy in ten fractions with 60 Gy in 30 fractions of cerebral irradiation for glioblastoma multiforme and older patients with anaplastic astrocytoma. Radiother Oncol. 2003;68:23–26. doi: 10.1016/s0167-8140(03)00206-8. [DOI] [PubMed] [Google Scholar]
- 8.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 9.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5 year analysis of the EORTC–NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 10.Athanassiou H, Synodinou M, Maragoudakis E, Paraskevaidis M, Verigos C, Misailidou D, Antonadou D, Saris G, Beroukas K, Karageorgis P. Randomized phase II study of temozolomide and radiotherapy compared with radiotherapy alone in newly diagnosed glioblastoma multiforme. J Clin Oncol. 2005;23:2372–2377. doi: 10.1200/JCO.2005.00.331. [DOI] [PubMed] [Google Scholar]
- 11.Laperriere N, O’Callaghan C, Ding K. Rationale and design for a phase III randomized controlled trial in elderly patients with glioblastoma multiforme: NCIC CTG CE.6. 13th biannual Canadian neuro-oncology meeting 2008 [Google Scholar]
- 12.Brandes AA, Franceschi E, Tosoni A, Benevento F, Scopece L, Mazzocchi V, Bacci A, Agati R, Calbucci F, Ermani M. Temozolomide concomitant and adjuvant to radiotherapy in elderly patients with glioblastoma: correlation with MGMT promoter methylation status. Cancer. 2009;115:3512–3518. doi: 10.1002/cncr.24406. [DOI] [PubMed] [Google Scholar]
- 13.Minniti G, De Sanctis V, Muni R, Filippone F, Bozzao A, Valeriani M, Osti MF, De Paula U, Lanzetta G, Tombolini V, Maurizi Enrici R. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma in elderly patients. J Neurooncol. 2008;88:97–103. doi: 10.1007/s11060-008-9538-0. [DOI] [PubMed] [Google Scholar]
- 14.Combs SE, Wagner J, Bischof M, Welzel T, Wagner F, Debus J, Schulz-Ertner D. Postoperative treatment of primary glioblastoma multiforme with radiation and concomitant temozolomide in elderly patients. Int J Radiat Oncol Biol Phys. 2008;70:987–992. doi: 10.1016/j.ijrobp.2007.07.2368. [DOI] [PubMed] [Google Scholar]
- 15.Fiorica F, Berretta M, Colosimo C, Stefanelli A, Ursino S, Zanet E, Palmucci T, Maugeri D, Malaguarnera M, Palmucci S, Grasso M, Tirelli U, Cartei F. Glioblastoma in elderly patients: safety and efficacy of adjuvant radiotherapy with concomitant temozolomide. Arch Gerontol Geriatr. 2010;51(1):31–35. doi: 10.1016/j.archger.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 16.Mangiola A, Maira G, De Bonis P, Porso M, Pettorini B, Sabatino G, Anile C. Glioblastoma multiforme in the elderly: a therapeutic challenge. J Neurooncol. 2006;76:159–163. doi: 10.1007/s11060-005-4711-1. [DOI] [PubMed] [Google Scholar]
- 17.Sijben AE, McIntyre JB, Roldan GB, Easaw JC, Yan E, Forsyth PA, Parney IF, Magliocco AM, Bernsen H, Cairncross JG. Toxicity from chemoradiotherapy in older patients with glioblastoma multiforme. J Neurooncol. 2008;89:97–103. doi: 10.1007/s11060-008-9593-6. [DOI] [PubMed] [Google Scholar]
- 18.Kimple RJ, Grabowski S, Papez M, Collichio F, Ewend MG, Morris DE. Concurrent temozolomide and radiation, a reasonable option for elderly patients with glioblastoma multiforme? Am J Clin Oncol. 2010;33(3):265–270. doi: 10.1097/COC.0b013e3181a76a24. [DOI] [PubMed] [Google Scholar]
- 19.Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol. 1989;62:679–694. doi: 10.1259/0007-1285-62-740-679. [DOI] [PubMed] [Google Scholar]
- 20.Qi XS, Schultz CJ, Li XA. An estimation of radiobiologic parameters from clinical outcomes for radiation treatment planning of brain tumor. Int J Radiat Oncol Biol Phys. 2006;64:1570–1580. doi: 10.1016/j.ijrobp.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 21.Curran WJ, Jr, Scott CB, Horton J, Nelson JS, Weinstein AS, Fischbach AJ, Chang CH, Rotman M, Asbell SO, Krisch RE, et al. Recursive partitioning analysis of prognostic factors in three radiation therapy oncology group malignant glioma trials. J Natl Cancer Inst. 1993;85:704–710. doi: 10.1093/jnci/85.9.704. [DOI] [PubMed] [Google Scholar]
- 22.Scott CB, Scarantino C, Urtasun R, Movsas B, Jones CU, Simpson JR, Fischbach AJ, Curran WJ., Jr Validation and predictive power of radiation therapy oncology group (RTOG) recursive partitioning analysis classes for malignant glioma patients: a report using RTOG 90–06. Int J Radiat Oncol Biol Phys. 1998;40:51–55. doi: 10.1016/s0360-3016(97)00485-9. [DOI] [PubMed] [Google Scholar]
- 23.Iwamoto FM, Cooper AR, Reiner AS, Nayak L, Abrey LE. Glioblastoma in the elderly: the Memorial Sloan-Kettering Cancer Center experience (1997–2007) Cancer. 2009;115:3758–3766. doi: 10.1002/cncr.24413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grant R, Liang BC, Page MA, Crane DL, Greenberg HS, Junck L. Age influences chemotherapy response in astrocytomas. Neurology. 1995;45:929–933. doi: 10.1212/wnl.45.5.929. [DOI] [PubMed] [Google Scholar]
- 25.Hegi ME, Diserens A, Gorlia T, Hamou M, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JEC, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R. MGMT gene silencing and benefit from temozolomide in glioblastoma. New Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 26.Wedge SR, Porteous JK, Glaser MG, Marcus K, Newlands ES. In vitro evaluation of temozolomide combined with X-irradiation. Anticancer Drugs. 1997;8:92–97. doi: 10.1097/00001813-199701000-00013. [DOI] [PubMed] [Google Scholar]
- 27.Kil WJ, Cerna D, Burgan WE, Beam K, Carter D, Steeg PS, Tofilon PJ, Camphausen K. In vitro and in vivo radiosensitization induced by the DNA methylating agent temozolomide. Clin Cancer Res. 2008;14:931–938. doi: 10.1158/1078-0432.CCR-07-1856. [DOI] [PubMed] [Google Scholar]
- 28.Sher DJ, Henson JW, Avutu B, Hochberg FH, Batchelor TT, Martuza RL, Barker FG, 2nd, Loeffler JS, Chakravarti A. The added value of concurrently administered temozolomide versus adjuvant temozolomide alone in newly diagnosed glioblastoma. J Neurooncol. 2008;88:43–50. doi: 10.1007/s11060-008-9530-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


