Abstract
BACKGROUND
Nitrates are commonly prescribed to enhance activity tolerance in patients with heart failure and a preserved ejection fraction. We compared the effect of isosorbide mononitrate or placebo on daily activity in such patients.
METHODS
In this multicenter, double-blind, crossover study, 110 patients with heart failure and a preserved ejection fraction were randomly assigned to a 6-week dose-escalation regimen of isosorbide mononitrate (from 30 mg to 60 mg to 120 mg once daily) or placebo, with subsequent crossover to the other group for 6 weeks. The primary end point was the daily activity level, quantified as the average daily accelerometer units during the 120-mg phase, as assessed by patient-worn accelerometers. Secondary end points included hours of activity per day during the 120-mg phase, daily accelerometer units during all three dose regimens, quality-of-life scores, 6-minute walk distance, and levels of N-terminal pro–brain natriuretic peptide (NT-proBNP).
RESULTS
In the group receiving the 120-mg dose of isosorbide mononitrate, as compared with the placebo group, there was a nonsignificant trend toward lower daily activity (−381 accelerometer units; 95% confidence interval [CI], −780 to 17; P = 0.06) and a significant decrease in hours of activity per day (−0.30 hours; 95% CI, −0.55 to −0.05; P = 0.02). During all dose regimens, activity in the isosorbide mononitrate group was lower than that in the placebo group (−439 accelerometer units; 95% CI, −792 to −86; P = 0.02). Activity levels decreased progressively and significantly with increased doses of isosorbide mononitrate (but not placebo). There were no significant between-group differences in the 6-minute walk distance, quality-of-life scores, or NT-proBNP levels.
CONCLUSIONS
Patients with heart failure and a preserved ejection fraction who received isosorbide mononitrate were less active and did not have better quality of life or submaximal exercise capacity than did patients who received placebo.
Approximately half of patients with heart failure have a preserved ejection fraction.1 Exercise intolerance is a cardinal feature of this syndrome and perpetuates sedentary behavior, deconditioning, and frailty.2–4 In early studies in patients with heart failure with a reduced ejection fraction, long-acting nitrates improved activity tolerance, as assessed by submaximal5,6 or maximal7 exercise tests. Although nitrates are commonly prescribed for symptom relief in heart failure,8–12 the effects of nitrates in patients with heart failure and a preserved ejection fraction have not been extensively studied. The hemodynamic effects of nitrates might attenuate pulmonary congestion with exertion and improve exercise capacity in heart failure with a preserved ejection fraction.13 However, the unique pathophysiology, associated coexisting illnesses, and polypharmacy that are characteristic of heart failure with a preserved ejection fraction may limit hemodynamic improvements and predispose patients to excessive hypotension or other side effects with nitrates.14–17 Thus, the overall effect of nitrates on activity tolerance in such patients is uncertain.
In assessing activity tolerance, intermittent supervised exercise tests may not reflect the full effect of a therapy on a patient’s daily functional status. Patient-worn accelerometers provide continuous assessment of physical activity during daily life and may more accurately reflect the effect of a therapy on functional status.18,19 Accordingly, we performed the Nitrate’s Effect on Activity Tolerance in Heart Failure with Preserved Ejection Fraction (NEAT-HFpEF) trial to test the hypothesis that extended-release isosor-bide mononitrate would enhance the daily activity level in patients with heart failure with a preserved ejection fraction, as assessed by patient-worn accelerometers.13
METHODS
STUDY OVERSIGHT
The NEAT-HFpEF trial was sponsored by the National Heart, Lung, and Blood Institute. The protocol was approved by the protocol review committee of the institute’s Heart Failure Clinical Research Network and monitored by the network’s data and safety monitoring board. The ethics committee at each participating site approved the trial design. Data collection, management, and analysis were performed at the network’s data coordinating center at Duke Clinical Research Institute. All the authors reviewed and approved the manuscript and assume full responsibility for the accuracy and completeness of the data and for the fidelity of this report to the study protocol, which is available with the full text of this article at NEJM.org.
STUDY PATIENTS
Ambulatory patients with a diagnosis of heart failure were eligible if they were 50 years of age or older and had heart failure while they were receiving stable medical therapy. Patients were required to have an ejection fraction of 50% or more and objective evidence of heart failure, as shown by one or more of the following criteria within 12 months before enrollment: previous hospitalization for heart failure with radiographic evidence of pulmonary congestion, elevated left ventricular end diastolic pressure at rest (≥15 mm Hg) or elevated pulmonary capillary wedge pressure at rest (≥20 mm Hg) or with exercise (≥25 mm Hg), an elevated level of N-terminal pro–brain natriuretic peptide (NT-proBNP) (>400 pg per milliliter) or brain natriuretic peptide (BNP) (>200 pg per milliliter), or Doppler echocardiographic evidence of diastolic dysfunction. In addition, patients were required to report on a screening questionnaire that the primary reason for their inability to be active was a history of dyspnea, fatigue, or chest pain (rather than orthopedic, neurologic, or lifestyle factors).
Exclusion criteria included a systolic blood pressure of less than 110 mm Hg or greater than 180 mm Hg or a previous adverse reaction to or current use of long-term nitrate or phosphodiesterase type 5 inhibitor therapy. The full entry criteria are provided in Tables S1 and S2 in the Supplementary Appendix, available at NEJM.org. All the patients provided written informed consent.
STUDY DESIGN
The design of the trial, which was a multicenter, randomized, double-blind, placebo-controlled, crossover study, has been described previously.13 After enrollment, patients underwent baseline studies, including echocardiography (with results read centrally at Duke Echocardiography Laboratory), and assessments of secondary end points, including quality-of-life scores, distance on the 6-minute walk test, and core laboratory assessment of NT-proBNP.13
Patients were supplied with a belt outfitted with two kinetic activity monitors (Kersh Health) containing high-sensitivity, triaxis accelerometers (KXUD9-2050, Kionix) (Fig. S1 in the Supplementary Appendix). Patients were instructed to wear the accelerometers 24 hours per day except while bathing or swimming. Each accelerometer was matched to a patient by means of a serial number and activated at the time it was dispensed, providing time- and date-stamped data synchronized to the study protocol.
The accelerometers measure movement, as described in the Supplementary Appendix. The accelerometer measurements are expressed as arbitrary accelerometer units. These are stored as 15-minute cumulative accelerometer units (96 data points per day). The 15-minute cumulative accelerometer units were totaled over a 24-hour period to provide daily accelerometer units.
Permuted-block randomization that was stratified according to study site was used to assign patients in a 1:1 ratio to one of two treatment groups (6 weeks of placebo first with crossover to 6 weeks of isosorbide mononitrate or 6 weeks of isosorbide mononitrate first with crossover to 6 weeks of placebo). According to the crossover design, the same patients were compared during the two treatment periods. The study drugs were prepared as 30-mg tablets of isosorbide mononitrate and matching placebo (University of Iowa Pharmaceuticals).
During each 6-week period, patients were instructed to take no study drug for the first 2 weeks (baseline phase during the first period and wash-out phase during the second period), followed by one tablet (30 mg daily) for 1 week, two tablets (60 mg once daily) for 1 week, and four tablets (120 mg once daily) until the next study visit, for a treatment duration of at least 2 weeks and up to 4 weeks. Patients were called weekly to assess any side effects and reinforce compliance with study procedures. Patients who had unacceptable side effects were allowed to return to the previous dose. After the first period, patients returned to the study center to repeat end-point assessments, receive the crossover study drug, and exchange accelerometers. After the second period, patients returned to repeat end-point assessments and to return the accelerometers and any unused tablets of isosorbide mononitrate or placebo provided during the second period.
OUTCOME MEASURES
The prespecified primary end point was a comparison of average daily accelerometer units during the period in which patients were receiving the 120-mg dose of isosorbide mononitrate as compared with placebo. In addition, two other accelerometer-derived activity end points were prespecified. The average hours of activity per day during the 120-mg dose were calculated from the daily number of 15-minute cumulative accelerometer units greater than 50 (activity threshold). (Details are provided in the Methods section in the Supplementary Appendix.) To assess activity during the entire duration of study-drug administration, the area under the curve for time and daily accelerometer units during the receipt of all three doses (30 mg, 60 mg, and 120 mg) of study drug was divided by the total days of the regimen. Finally, a dose–response analysis compared the change in average daily accelerometer units from baseline at each dose. The accelerometer core laboratory at the Mayo Clinic processed the data.
Other secondary end points included the 6-minute walk distance and the post-walk Borg dyspnea score, scores on the Kansas City Cardiomyopathy Questionnaire and the Minnesota Living with Heart Failure Questionnaire, and NT-proBNP levels. At the end of the study, patients completed a questionnaire indicating in which period they felt better (first period, second period, or no preference).
STATISTICAL ANALYSIS
Since no previous heart-failure studies have used the primary outcome measure that we used in our study, we based our power calculations on previous data for the secondary end points in heart-failure trials. In a crossover design, we estimated that the enrollment of 94 patients would have a power of 80% to detect a minimal clinically significant difference of 5 points on the clinical summary score of the Kansas City Cardiomyopathy Questionnaire and that the enrollment of 60 patients would provide a power of more than 90% to detect a minimal clinically significant difference of 43 m in the 6-minute walk dis-tance.13 Estimates derived from reproducibility data with a different accelerometer in 49 elderly, sedentary volunteers indicated that the enrollment of 100 patients would provide a power of 90% to detect a relative treatment effect of approximately 2.5% of the baseline measurement.13,20 The target enrollment was approximately 100 patients.
For all crossover end points, the analysis was performed on an intention-to-treat basis and used a linear mixed model with fixed-effect terms for the sequence, study period, and treatment.21 A random-effect term was included to account for the correlated measurements for each patient.22 For the primary end point, sensitivity analyses included the mixed model with 100 multiple imputations for missing data and an analysis that used a paired t-test in a crossover design structure (treatment and period effect terms) in patients with data for the two study periods. A general linear model was used to determine whether the average daily accelerometer units during each dose period differed from baseline in the two study groups. This analysis was not adjusted for any between-group differences at baseline. A two-sided P value of 0.05 was considered to indicate statistical significance. For the primary end point, interaction between treatment effect and a number of prespecified baseline characteristics was assessed.
RESULTS
STUDY PATIENTS
From April 7, 2014, to October 30, 2014, a total of 110 patients with chronic heart failure were enrolled at 20 sites in the United States, with 51 patients assigned to receive isosorbide mono-nitrate first and placebo second and 59 patients assigned to receive placebo first and isosorbide mononitrate second (Fig. S2 in the Supplementary Appendix). The median duration of heart failure was 1.8 years. The mean age of the patients was 69 years, and 57% were women. The majority of the patients were white and obese, with controlled blood pressure and multiple coexisting illnesses; most of the patients were taking multiple cardiovascular medications (Table 1). Nearly all the patients had New York Heart Association functional class II or III symptoms; 2 patients in the first placebo group had class I or IV symptoms. The mean ejection fraction was 63%, and 47% of the patients had evidence of concentric remodeling or hypertrophy, with a relative wall thickness (defined as the sum of the intraventricularseptum and posterior-wall diastolic thicknesses divided by the diastolic cavity dimension) of more than 0.41.
Table 1.
Characteristics of the Patients at Baseline.*
| Characteristic | Placebo First (N = 59) | Isosorbide Mononitrate First (N = 51) |
|---|---|---|
| Age — yr | 69±10 | 69±9 |
| Female sex — no. (%) | 38 (64) | 25 (49) |
| White race — no. (%)† | 54 (92) | 44 (86) |
| Body-mass index‡ | 35.1±8.7 | 36.2±8.0 |
| Functional measures | ||
| New York Heart Association classification — no. (%)§ | ||
| II | 33 (56) | 25 (49) |
| III | 24 (41) | 26 (51) |
| Overall score on Kansas City Cardiomyopathy Questionnaire¶ | 58.0±24.6 | 53.6±23.8 |
| Total score on Minnesota Living with Heart Failure Questionnaire|| | 43.3±22.0 | 46.0±25.6 |
| Six-minute walk distance — m | 321±112 | 300±127 |
| Daily no. of arbitrary accelerometer units** | 9607±5439 | 9889±4730 |
| No. of hours of activity per day** | 9.2±2.3 | 9.6±2.1 |
| Physical examination | ||
| Systolic blood pressure — mm Hg | 132±18 | 129±14 |
| Heart rate — beats/min | 70±12 | 73±13 |
| Elevated jugular venous pressure — no./total no. (%) | 17/59 (29) | 20/50 (40) |
| Edema — no./total no. (%) | 34/58 (59) | 32/51 (63) |
| Medical history — no./total no. (%) | ||
| Hospitalization for heart failure | 16/59 (27) | 12/51 (24) |
| Hypertension | 54/59 (92) | 45/51 (88) |
| Ischemic heart disease | 36/59 (61) | 32/51 (63) |
| History of atrial fibrillation | 20/59 (34) | 19/51 (37) |
| Diabetes mellitus | 21/59 (36) | 22/51 (43) |
| Chronic obstructive pulmonary disease | 10/59 (17) | 6/51 (12) |
| Sleep apnea | 28/56 (50) | 29/51 (57) |
| Anemia†† | 18/59 (31) | 18/50 (36) |
| Chronic kidney disease, stage ≥3‡‡ | 32/59 (54) | 21/50 (42) |
| Depression treated with prescription drug | 22/59 (37) | 14/51 (27) |
| Cardiovascular medication at enrollment — no. (%) | ||
| Loop diuretic | 36 (61) | 36 (71) |
| Hydrochlorothiazide | 6 (10) | 6 (12) |
| ACE inhibitor or ARB | 36 (61) | 34 (67) |
| Beta-blocker | 41 (69) | 36 (71) |
| Aldosterone antagonist | 13 (22) | 14 (27) |
| Calcium-channel blocker | 24 (41) | 10 (20) |
| Lipid-lowering agent | 42 (71) | 32 (63) |
| Antiplatelet or anticoagulant agent | 47 (80) | 43 (84) |
| Laboratory or echocardiographic measure | ||
| Local laboratory creatinine — mg/dl | 1.2±0.4 | 1.2±0.4 |
| Median core laboratory NT-proBNP (IQR) — pg/ml | 248 (120–644) | 210 (102–511) |
| Ejection fraction — % | 65±9 | 62±8 |
| Relative wall thickness >0.41 — no./total no. (%) | 26/58 (45) | 24/48 (50) |
| Ratio of early mitral inflow velocity to early diastolic medial mitral annular velocity | 15±7 | 15±10 |
| Left atrial volume — ml/m2 of body-surface area | 38.9±11.6 | 41.2±19.1 |
Plus–minus values are means ±SD. In this crossover study, patients were assigned to 6 weeks of placebo first with crossover to 6 weeks of isosorbide mononitrate or to 6 weeks of isosorbide mononitrate first with crossover to 6 weeks of placebo. The same patients were compared during the two treatment periods. There were no significant differences between the two groups except for the use of a calcium-channel blocker (P = 0.02) and the ejection fraction (P = 0.03). ACE denotes angiotensin-converting enzyme, ARB angiotensin-receptor blocker, and NT-proBNP N-terminal pro–brain natriuretic peptide. To convert the values for creatinine to micromoles per liter, multiply by 88.4.
Race was self-reported.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
In the placebo-first group, one patient was classified as having class I symptoms and another as having class IV symptoms.
Overall scores on the Kansas City Cardiomyopathy Questionnaire range from 1 to 100, with higher scores indicating better function.
Total scores on the Minnesota Living with Heart Failure Questionnaire range from 0 to 105, with higher scores indicating worse function.
This category was assessed during the baseline phase of period 1 before the initiation of isosorbide mononitrate or placebo.
Anemia was defined as a hemoglobin level of less than 13 g per deciliter for men and less than 12 g per deciliter for women.
Chronic kidney disease of stage 3 or greater was defined as a glomerular filtration rate of 60 ml per minute per 1.73 m2 of body-surface area or less, as calculated by the Modification of Diet in Renal Disease equation.
PRIMARY AND SECONDARY ACTIVITY END POINTS
During the 120-mg phase, 101 patients during the first period and 91 during the second period had usable accelerometer data, with a median of 16 complete days of accelerometer data (inter-quartile range, 12 to 20) during the first period and 14 complete days (interquartile range, 10 to 18) during the second period (P<0.001) (Fig. S2 in the Supplementary Appendix). The average daily accelerometer units, as assessed by the two accelerometers worn by each patient, were highly correlated (r=0.99) (Fig. S3 in the Supplementary Appendix).
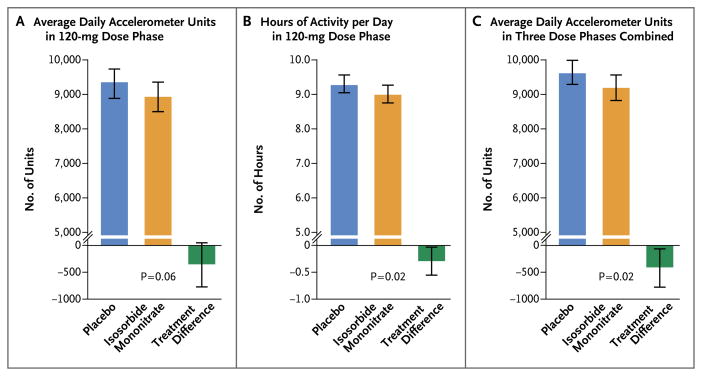
For the primary end point of average daily accelerometer units during the 120-mg phase, there was a nonsignificant trend (P = 0.06) toward lower activity during receipt of isosorbide mononitrate than during receipt of placebo, with a treatment effect of −381 accelerometer units per day (95% confidence interval [CI], −780 to 17) (Table 2 and Fig. 1A). Similar treatment effects were revealed on the basis of sensitivity analyses using multiple imputation (−365 accelerometer units per day; 95% CI, −754 to 23; P = 0.07), paired t-test crossover analysis (−402 accelerometer units per day; 95% CI, −794 to −9; P = 0.04), and analysis restricted to patients who were receiving a study drug during the end-point assessment (−306 accelerometer units per day; 95% CI, −745 to 141; P = 0.18).
Table 2.
Efficacy and Safety End Points.
| End Point | Placebo (N = 110) | Isosorbide Mononitrate (N = 110) | Treatment Difference* | P Value |
|---|---|---|---|---|
| mean (95% CI) | ||||
| Efficacy | ||||
| Activity as assessed on accelerometry | ||||
| Daily arbitrary accelerometer units during 120-mg phase: primary end point | 9303 (8884–9723) | 8922 (8500–9345) | −381 (−780 to 17) | 0.06 |
| No. of hours of activity per day | 9.31 (9.05–9.56) | 9.01 (8.75–9.27) | −0.30 (−0.55 to −0.05) | 0.02 |
| Daily arbitrary accelerometer units for all treatment doses | 9623 (9271–9976) | 9185 (8822–9547) | −439 (−792 to −86) | 0.02 |
| Six-minute walk test | ||||
| Distance — m | 321 (307–336) | 322 (307–336) | 0.57 (−9.63 to 10.78) | 0.91 |
| Borg dyspnea score† | 3.97 (3.59–4.34) | 3.89 (3.52–4.26) | −0.07 (−0.50 to 0.36) | 0.74 |
| Quality of life | ||||
| Overall score on Kansas City Cardiomyopathy Questionnaire | 61.6 (58.9– 64.4) | 59.7 (57.0–62.5) | −1.91 (−4.55 to 0.74) | 0.16 |
| Total score on Minnesota Living with Heart Failure Questionnaire | 35.4 (31.6– 39.2) | 37.0 (33.3–40.6) | 1.62 (−1.98 to 5.23) | 0.37 |
| NT-proBNP — pg/ml | 497 (422– 572) | 550 (475–625) | 53 (−33 to 138) | 0.22 |
| Blood pressure — mm Hg | ||||
| Systolic | 129 (125–132) | 125 (122–128) | −3.7 (−7.2 to −0.3) | 0.04 |
| Diastolic | 70 (69–72) | 69 (67–71) | −1.6 (−3.5 to 0.3) | 0.10 |
| Mean arterial blood pressure — mm Hg | 90 (88–92) | 88 (86–90) | −2.3 (−4.4 to −0.2) | 0.03 |
| Safety | ||||
| no. of patients with event | ||||
| Any event of interest | 6 | 14 | ||
| Arrhythmia | 2 | 2 | ||
| Worsening heart failure | 1 | 5 | ||
| Stroke or transient ischemic attack | 0 | 1 | ||
| Presyncope or syncope | 3 | 6 | ||
| Worsening renal function | 0 | 0 | ||
| Serious adverse event | ||||
| Death | 0 | 0 | ||
| Other serious adverse event‡ | 1 | 2 | ||
The treatment difference is the value in the isosorbide mononitrate group minus the value in the placebo group.
The Borg dyspnea score ranges from 1 to 10, with higher scores indicating greater severity.
One patient had fecaloma and urinary retention during the baseline period before starting any study drug and cellulitis while taking isosor-bide mononitrate, one patient had herpes zoster while taking isosorbide mononitrate, and one patient had an exacerbation of chronic obstructive pulmonary disease while taking placebo.
Figure 1. Primary and Secondary End Points for Activity Levels.
Shown are the absolute values and differences between the two treatments (isosorbide mononitrate minus placebo) for the average daily arbitrary accelerometer units (Panel A) and hours of activity per day (Panel B) during the 120-mg phase and for daily arbitrary accelerometer units for all three doses (30 mg, 60 mg, and 120 mg) combined (Panel C). The arbitrary accelerometer units were stored as 15-minute cumulative accelerometer units (96 data points per day) and were totaled over a 24-hour period to provide daily accelerometer units. The I bars indicate 95% confidence intervals.
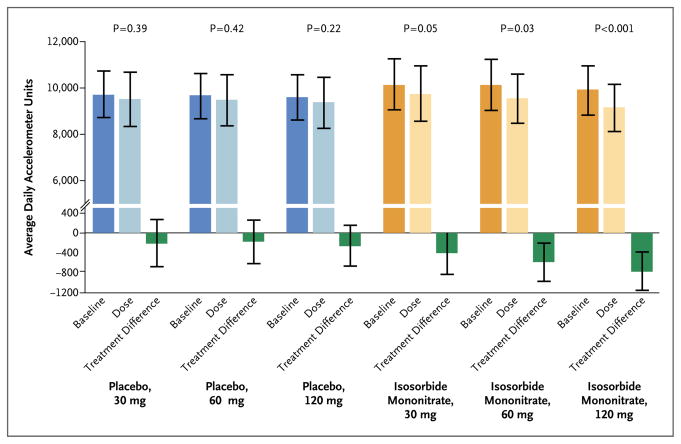
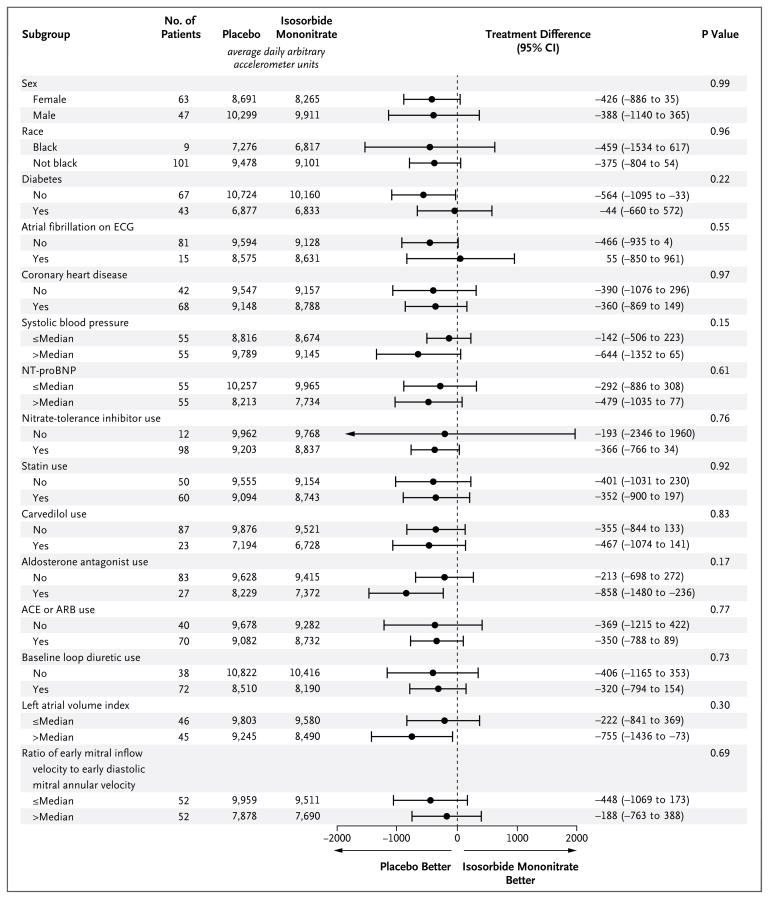
Patients were active for fewer hours of the day (−0.30 hours; 95% CI, −0.55 to −0.05; P = 0.02) during the 120-mg phase of receipt of isosorbide mononitrate as compared with placebo (Table 2 and Fig. 1B). During all study-drug regimens combined (30 mg to 120 mg), patients were less active (−439 accelerometer units per day; 95% CI, −792 to −86; P = 0.02) during receipt of isosorbide mononitrate as compared with placebo (Table 2 and Fig. 1C). As compared with baseline, average daily accelerometer units decreased with increasing doses of isosorbide mononitrate but not placebo (Fig. 2). For the primary end point, there were no significant interactions between treatment effect and baseline characteristics (Fig. 3).
Figure 2. Change in Activity Levels with Increasing Doses of Isosorbide Mononitrate or Placebo.
Shown are the changes from baseline in the average daily arbitrary accelerometer units according to the dose of iso-sorbide mononitrate and placebo in the two study groups and the corresponding treatment differences (dose value minus baseline value). Among the patients who received placebo, 89 received the 30-mg dose, 91 received the 60-mg dose, and 93 received the 120-mg dose. Among the patients who received isosorbide mononitrate, 85 received the 30-mg dose, 87 received the 60-mg dose, and 89 received the 120-mg dose. The I bars indicate 95% confidence intervals.
Figure 3. Average Daily Arbitrary Accelerometer Units, According to Subgroup.
Shown are the average daily arbitrary accelerometer units in each of the prespecified subgroups and the between-group treatment differences and 95% confidence intervals. The term “nitrate tolerance” refers to the decreasing effect of nitrates in patients after long-term exposure to this drug class. Inhibitors of nitrate tolerance include renin–angiotensin–aldosterone antagonists, carvedilol, statins, and hydralazine. Since only four patients were treated with hydralazine, the subgroup analysis for this variable alone is not shown. ACE denotes angiotensin-converting enzyme, ARB angiotensin-receptor blocker, ECG electrocardiography, and NT-proBNP N-terminal pro–brain natriuretic peptide.
OTHER SECONDARY END POINTS
As compared with placebo, there was no significant effect of isosorbide mononitrate on the 6-minute walk distance or post-walk Borg dyspnea score, the clinical summary score on the Kansas City Cardiomyopathy Questionnaire, the total score on the Minnesota Living with Heart Failure Questionnaire, or the NT-proBNP level (Table 2). Although the treatment differences were not significant, the direction of the numerical effects on quality-of-life scores and NT-proBNP levels were unfavorable for isosorbide mononitrate. As compared with placebo, isosorbide mono-nitrate decreased blood pressure (Table 2). Similar proportions of patients indicated that they felt better during receipt of isosorbide mononitrate (36%) or receipt of placebo (30%), and 33% had no preference.
Numerically, more patients discontinued isosorbide mononitrate than placebo (Fig. S2 in the Supplementary Appendix). Numerically, more patients had adverse events of interest while receiving isosorbide mononitrate than while receiving placebo (Table 2). There were five serious adverse events in three patients during the study (in two patients during the isosorbide mononitrate phase and one patient during the placebo phase) (Table 2).
DISCUSSION
Data from previous studies indicate that 15 to 50% of patients with heart failure and a preserved ejection fraction are treated with nitrates.8–12 However, in our study, isosorbide mononitrate did not improve the daily activity level, sub-maximal exercise capacity (6-minute walk distance), or perceptive exercise tolerance (post-walk dyspnea score), quality-of-life scores, or NT-proBNP levels in such patients. Indeed, dose-dependent decreases in daily activity levels were seen among patients receiving isosorbide mono-nitrate.
Although the most effective entry criteria for clinical trials involving patients with heart failure with a preserved ejection fraction remain controversial,23 the entry criteria and characteristics of the patients in our study were consistent with those in several recent studies (Tables S2, S3, and S4 in the Supplementary Appendix). The NT-proBNP levels were lower in our study than in trials requiring an elevated NT-proBNP level for enrollment,24,25 since we did not require elevated NT-proBNP levels at study entry if there was other objective evidence of heart failure. Because the primary outcome assessment was based on the activity levels of patients, we required that patients report heart-failure–related symptoms as the primary reason they were less active than they desired to be.
We speculated that measures of daily activity using accelerometer-derived data might be more sensitive to the overall effect of a therapy than intermittent repetition of coached exercise tests or memory-dependent quality-of-life questionnaires. Accelerometer data are high-density, quantitative, and collected continuously under conditions of daily living.18,19 Furthermore, the ultimate goal of therapies that are prescribed to improve exercise tolerance is indeed to facilitate activity. Inactivity promotes further deconditioning and frailty among patients with heart failure3 and is independently associated with both the incidence and deleterious outcomes of heart failure.26–28
If behavioral or environmental factors prominently influence a patient’s willingness or ability to be active, improved exercise tolerance may not lead to increased activity. However, our patients indicated that their activity was primarily limited by their heart-failure symptoms. Furthermore, we observed a decrease in activity levels with isosorbide mononitrate, rather than no change. The decrease in activity occurred in the absence of adverse effects on submaximal exercise capacity or perceptive exercise tolerance, as assessed on the 6-minute walk test and in association with directionally adverse, albeit not significant, effects on quality-of-life scores. These findings suggest that activity levels were sensitive to adverse effects of isosorbide mononitrate beyond the numerically higher rate of overt symptoms requiring study-drug discontinuation.
Absolute values for accelerometer units and the activity time per day that is based on such units are highly sensitive to the device design, body location, data-acquisition mode, analytics, activity threshold values, and patient population.18,19 Thus, a comparison with other studies is difficult. During the 120-mg phase, patients who received isosorbide mononitrate were active for 0.3 hours (18 minutes) less per day than were those who received placebo. Observational studies using uniaxial, chest-worn accelerometer data from implanted pacing or defibrillator devices in patients with heart failure and a reduced ejection have shown that even a 10-minute reduction in activity per day was associated with adverse outcomes.28 These data would suggest that the reductions in activity with isosorbide mononitrate that we observed were clinically relevant. Each accelerometer was calibrated during production, but there are no validation studies with this accelerometer in patients with heart failure. Similar accelerometers have been shown to capture low levels of daily physical activity with a high degree of sensitivity.29–32
The lack of improvement in exercise tolerance and the adverse effect on daily activity levels may relate to the pathophysiology of heart failure with a preserved ejection fraction. Increased ventricular systolic and vascular stiffness, autonomic dysfunction, chronotropic incompetence, and altered baroreflex sensitivity are common and may limit the hemodynamic benefits of nitrates.14,15,33 Our post hoc analysis indicated decreases in blood pressure with isosorbide mono-nitrate. In addition, the potential for drug interactions and adverse drug reactions increases with an older age, obesity, coexisting illnesses, and polypharmacy, all of which are characteristic of our study population.16,17
One limitation of our trial is that we used a rapid dose escalation of the study drug. Isosor-bide mononitrate or placebo was initiated at a dose of 30 mg daily for 1 week and 60 mg daily for 1 week before reaching the target dose of 120 mg daily. Given the sensitivity of patients with heart failure with a preserved ejection fraction to changes in hemodynamics, it is possible that a more gradual dose escalation might have yielded different results. We also speculate that inorganic nitrite or nitrate34–36 may enhance nitric oxide bioavailability preferentially during exercise and be more effective and have fewer side effects than do organic nitrates.
In conclusion, in patients with heart failure with a preserved ejection fraction, the receipt of isosorbide mononitrate, as compared with placebo, decreased daily activity levels. In addition, receipt of isosorbide mononitrate did not improve submaximal exercise capacity, quality-of-life scores, or NT-proBNP levels in these patients.
Supplementary Material
Acknowledgments
Funded by the National Heart, Lung, and Blood Institute; ClinicalTrials.gov number, NCT02053493.
Supported by grants from the National Heart, Lung, and Blood Institute (NHLBI) (coordinating center: U10 HL084904; regional clinical centers: U01 HL084861, U10 HL110312, U109 HL110337, U01 HL084889, U01 HL084890, U01 HL084891, U10 HL110342, U10 HL110262, U01 HL084931, U10 HL110297, U10 HL110302, U10 HL110309, U10 HL110336, and U10 HL110338).
Footnotes
The views expressed by the authors do not represent the official position of the National Heart, Lung, and Blood Institute.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–9. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 2.Smith GL, Masoudi FA, Vaccarino V, Radford MJ, Krumholz HM. Outcomes in heart failure patients with preserved ejection fraction: mortality, readmission, and functional decline. J Am Coll Cardiol. 2003;41:1510–8. doi: 10.1016/s0735-1097(03)00185-2. [DOI] [PubMed] [Google Scholar]
- 3.Upadhya B, Haykowsky MJ, Eggebeen J, Kitzman DW. Exercise intolerance in heart failure with preserved ejection fraction: more than a heart problem. J Geriatr Cardiol. 2015;12:294–304. doi: 10.11909/j.issn.1671-5411.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maurer MS, Schulze PC. Exercise intolerance in heart failure with preserved ejection fraction: shifting focus from the heart to peripheral skeletal muscle. J Am Coll Cardiol. 2012;60:129–31. doi: 10.1016/j.jacc.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leier CV, Huss P, Magorien RD, Unver-ferth DV. Improved exercise capacity and differing arterial and venous tolerance during chronic isosorbide dinitrate therapy for congestive heart failure. Circulation. 1983;67:817–22. doi: 10.1161/01.cir.67.4.817. [DOI] [PubMed] [Google Scholar]
- 6.Elkayam U, Johnson JV, Shotan A, et al. Double-blind, placebo-controlled study to evaluate the effect of organic nitrates in patients with chronic heart failure treated with angiotensin-converting enzyme inhibition. Circulation. 1999;99:2652–7. doi: 10.1161/01.cir.99.20.2652. [DOI] [PubMed] [Google Scholar]
- 7.Franciosa JA, Goldsmith SR, Cohn JN. Contrasting immediate and long-term effects of isosorbide dinitrate on exercise capacity in congestive heart failure. Am J Med. 1980;69:559–66. doi: 10.1016/0002-9343(80)90468-4. [DOI] [PubMed] [Google Scholar]
- 8.Adamson PB, Abraham WT, Bourge RC, et al. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail. 2014;7:935–44. doi: 10.1161/CIRCHEARTFAILURE.113.001229. [DOI] [PubMed] [Google Scholar]
- 9.Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777–81. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 10.Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–67. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 11.Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J. The Perindopril in Elderly People with Chronic Heart Failure (PEP-CHF) study. Eur Heart J. 2006;27:2338–45. doi: 10.1093/eurheartj/ehl250. [DOI] [PubMed] [Google Scholar]
- 12.Shah SJ, Heitner JF, Sweitzer NK, et al. Baseline characteristics of patients in the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial. Circ Heart Fail. 2013;6:184–92. doi: 10.1161/CIRCHEARTFAILURE.112.972794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zakeri R, Levine JA, Koepp GA, et al. Nitrate’s effect on activity tolerance in heart failure with preserved ejection fraction trial: rationale and design. Circ Heart Fail. 2015;8:221–8. doi: 10.1161/CIRCHEARTFAILURE.114.001598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwartzenberg S, Redfield MM, From AM, Sorajja P, Nishimura RA, Borlaug BA. Effects of vasodilation in heart failure with preserved or reduced ejection fraction implications of distinct pathophysiologies on response to therapy. J Am Coll Cardiol. 2012;59:442–51. doi: 10.1016/j.jacc.2011.09.062. [DOI] [PubMed] [Google Scholar]
- 15.Kawaguchi M, Hay I, Fetics B, Kass DA. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation. 2003;107:714–20. doi: 10.1161/01.cir.0000048123.22359.a0. [DOI] [PubMed] [Google Scholar]
- 16.Fleg JL, Aronow WS, Frishman WH. Cardiovascular drug therapy in the elderly: benefits and challenges. Nat Rev Cardiol. 2011;8:13–28. doi: 10.1038/nrcardio.2010.162. [DOI] [PubMed] [Google Scholar]
- 17.von Lueder TG, Atar D. Comorbidities and polypharmacy. Heart Fail Clin. 2014;10:367–72. doi: 10.1016/j.hfc.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Gorman E, Hanson HM, Yang PH, Khan KM, Liu-Ambrose T, Ashe MC. Accelerometry analysis of physical activity and sedentary behavior in older adults: a systematic review and data analysis. Eur Rev Aging Phys Act. 2014;11:35–49. doi: 10.1007/s11556-013-0132-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheung VH, Gray L, Karunanithi M. Review of accelerometry for determining daily activity among elderly patients. Arch Phys Med Rehabil. 2011;92:998–1014. doi: 10.1016/j.apmr.2010.12.040. [DOI] [PubMed] [Google Scholar]
- 20.Thompson WG, Kuhle CL, Koepp GA, McCrady-Spitzer SK, Levine JA. “Go4Life” exercise counseling, accelerometer feedback, and activity levels in older people. Arch Gerontol Geriatr. 2014;58:314–9. doi: 10.1016/j.archger.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Mills EJ, Chan AW, Wu P, Vail A, Guyatt GH, Altman DG. Design, analysis, and presentation of crossover trials. Trials. 2009;10:27. doi: 10.1186/1745-6215-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Senn S, editor. Cross-over trials in clinical research. 2. Chichester, United Kingdom: Wiley; 2002. [Google Scholar]
- 23.Kelly JP, Mentz RJ, Mebazaa A, et al. Patient selection in heart failure with preserved ejection fraction clinical trials. J Am Coll Cardiol. 2015;65:1668–82. doi: 10.1016/j.jacc.2015.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solomon SD, Zile M, Pieske B, et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet. 2012;380:1387–95. doi: 10.1016/S0140-6736(12)61227-6. [DOI] [PubMed] [Google Scholar]
- 25.Redfield MM, Chen HH, Borlaug BA, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309:1268–77. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biswas A, Oh PI, Faulkner GE, et al. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: a systematic review and meta-analysis. Ann Intern Med. 2015;162:123–32. doi: 10.7326/M14-1651. [DOI] [PubMed] [Google Scholar]
- 27.Young DR, Reynolds K, Sidell M, et al. Effects of physical activity and sedentary time on the risk of heart failure. Circ Heart Fail. 2014;7:21–7. doi: 10.1161/CIRCHEARTFAILURE.113.000529. [DOI] [PubMed] [Google Scholar]
- 28.Conraads VM, Spruit MA, Braunschweig F, et al. Physical activity measured with implanted devices predicts patient outcome in chronic heart failure. Circ Heart Fail. 2014;7:279–87. doi: 10.1161/CIRCHEARTFAILURE.113.000883. [DOI] [PubMed] [Google Scholar]
- 29.Levine JA, Lanningham-Foster LM, McCrady SK, et al. Interindividual variation in posture allocation: possible role in human obesity. Science. 2005;307:584–6. doi: 10.1126/science.1106561. [DOI] [PubMed] [Google Scholar]
- 30.Levine JA. Measurement of energy expenditure. Public Health Nutr. 2005;8:1123–32. doi: 10.1079/phn2005800. [DOI] [PubMed] [Google Scholar]
- 31.Levine J, Melanson EL, Westerterp KR, Hill JO. Tracmor system for measuring walking energy expenditure. Eur J Clin Nutr. 2003;57:1176–80. doi: 10.1038/sj.ejcn.1601673. [DOI] [PubMed] [Google Scholar]
- 32.Levine J, Melanson EL, Westerterp KR, Hill JO. Measurement of the components of nonexercise activity thermogenesis. Am J Physiol Endocrinol Metab. 2001;281(4):E670–E675. doi: 10.1152/ajpendo.2001.281.4.E670. [DOI] [PubMed] [Google Scholar]
- 33.Borlaug BA, Melenovsky V, Russell SD, et al. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation. 2006;114:2138–47. doi: 10.1161/CIRCULATIONAHA.106.632745. [DOI] [PubMed] [Google Scholar]
- 34.Zamani P, Rawat D, Shiva-Kumar P, et al. Effect of inorganic nitrate on exercise capacity in heart failure with preserved ejection fraction. Circulation. 2015;131:371–80. doi: 10.1161/CIRCULATIONAHA.114.012957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–67. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 36.Borlaug BA, Koepp KE, Melenovsky V. Sodium nitrite improves exercise hemo-dynamics and ventricular performance in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2015;66:1672–82. doi: 10.1016/j.jacc.2015.07.067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.