Abstract
Objectives
Depot medroxyprogesterone acetate (DMPA) was associated with increased HIV transmission and accelerated disease progression in untreated women. The potential underlying mechanisms include immune-modulation. We evaluated the effect of a single DMPA injection on cell-mediated immunity (CMI), T cell activation (Tact), regulation (Treg) and inflammation in HIV-infected women on cART.
Methods
Women with HIV plasma RNA ≤400 c/mL on stable cART received DMPA and had immunologic and MPA measurements at baseline, 4 weeks [peak MPA concentration (Cmax)] and 12 weeks [highest MPA area under the concentration curve (AUC)].
Results
At baseline, among 24 women with median 32 years of age and 622 CD4+ cells/μL, ≥68% had HIV, VZV, PHA and CD3/CD28 CMI measured by lymphocyte proliferation and/or IFNγ/IL2 dual-color fluorospot. CMI did not significantly change after DMPA administration except for a 1.4-fold increase in IL2/IFNγ VZV fluorospot at week 12. Tact decreased after DMPA administration, reaching statistical significance at week 12 for CD4+CD25+%. Treg behaved heterogeneously with an increase in CD8+FOXP3+% at week 4 and a decrease in CD4+IL35+% at week 12. There was a decrease in TGFβ at week 12 and no other changes in plasma biomarkers. Correlation analyses showed that high MPA Cmax and/or AUC were significantly associated with increases of IFNγ HIV ELISPOT, CD4+IL35+% and CD4+TGFβ+% Treg and decreases of plasma IL10 from baseline to weeks 4 and/or 12.
Conclusions
A single dose of DMPA did not have immune-suppressive or pro-inflammatory effects in HIV-infected women on cART. Additional studies need to assess the effect of multiple doses.
Introduction
Ensuring access to preferred contraceptive methods for women and couples is essential to securing the well-being and autonomy of women [1, 2]. This aspect is particularly relevant to HIV infection, where family planning reduces the risk of unintended pregnancies among women living with HIV. Although the strategies to prevent mother-to-child HIV transmission have been extremely successful, there is still a limited risk of intra uterine and breast milk HIV transmission and also a risk of children being orphaned due to premature maternal death that could be prevented by adequate contraception. Among the different methods of contraception, condoms are highly recommended, because they have the advantage of providing a physical barrier against sexually transmitted infections, including HIV. However, the effectiveness of condoms as contraceptives is <80%, whereas hormonal contraceptives (HC) have an effectiveness of 94% and 91% for injectable and oral preparations, respectively [1]. In addition, compared to condoms, the use of HC is more readily controlled by women conferring them a degree of choice and autonomy.
In the context of HIV infection, the use of HC has been hotly debated. There is evidence that the use of HC may increase the risk of HIV transmission, acquisition and may accelerate progression of HIV infection in women who are not on antiretroviral (ARV) therapy [3–12]. In addition, compared with HIV-infected women using non-hormonal birth control methods, HIV-infected women on HC had higher HIV loads in genital secretions [13]. Pregnancy, a state of heightened female hormone secretion, has also been associated with modest increases both in the risk of HIV acquisition and in HIV loads in the genital tract [14, 15]. The results of these studies might have been influenced by socioeconomic and behavioral characteristics of the study population that are difficult to separate from those directly generated by the use of HC on the risk of HIV acquisition, transmission or progression. However, studies of HC in animal models of simian immunodeficiency virus (SIV) or simian-HIV (SHIV) acquisition also found an increased risk of infection in animals treated with supraphysiologic doses of female hormones [16, 17]. It should be noted that the effect of hormonal contraceptives on transmission of HIV or other primate lentiviruses and on disease progression remains controversial, with multiple studies failing to demonstrate any associations [4, 5, 18–23].
Human and animal studies sought to elucidate the mechanism(s) that mediate the potential increased risk of HIV infection and disease progression associated with the use of HC. These ultimately found two important, non-mutually exclusive potential mechanisms: 1) changes in the female reproductive tract [24–28] and 2) attenuation of cell-mediated immunity (CMI) [16, 17, 29–33].
Among the HC, depot medoxyprogesterone acetate (DMPA) is one of the most widely used worldwide including areas of high prevalence of HIV infection, such as sub-Saharan Africa and Southeast Asia [34]. In the US, women enrolled in the Women’s Interagency HIV Study reported roughly equal use of DMPA and oral HC [35]. DMPA is administered intra-muscularly every 3 months, ensuring better compliance compared with oral HC, which typically require daily administration. Furthermore, DMPA maintains effectiveness when co-administered with efavirenz, which typically decreases the effectiveness of progestin implants [36]. However, DMPA is also commonly used to promote female genital tract infection with SHIV and SIV in non-human primates taking advantage of its local effects on the female reproductive tract [16]. It is also purported to depress CMI through its dual action on progestin and glucocorticoid receptors, which are widely expressed by lymphocytes and other mononuclear cells [37–41].
In this study, we investigated the CMI in participants of the AIDS Clinical Trials Group (ACTG) study A5283 [42]. The goal of the immunologic component of the study was to compare functional CMI against HIV and varicella-zoster virus (VZV), T cell activation and regulation, and inflammatory biomarkers before and after DMPA administration and to examine potential associations of immunologic and inflammatory characteristics with medroxyprogesterone acetate (MPA) plasma concentrations.
Subjects and methods
Study Design
The study was a 12-week, multicenter, open-label, non-randomized trial in which a single dose of 150 mg DMPA was administered intramuscularly to non-pregnant, pre-menopausal HIV-1-infected women ≥15-year old, who did not use DMPA for at least 6 months prior to enrollment and were on a stable combination antiretroviral regimen (cART) containing lopinavir with ritonavir boost (LPV/r) administered twice daily for at least 12 weeks prior to enrollment. All subjects had plasma HIV-1 RNA ≤ 400 copies/mL within 30 days of study entry and were required to continue on cART for the 12 weeks of the study. Blood samples for MPA and progesterone concentrations, plasma HIV RNA levels, CD4+ cell counts and immunologic and inflammatory measurements were collected at study entry (week 0, pre-DMPA administration), and 4 (putative peak of MPA) and 12 (putative trough of MPA) weeks after DMPA administration.
Processing of samples for Immunologic Assays
Peripheral blood mononuclear cells (PBMC) were cryopreserved at the clinical site laboratories following a standardized protocol (http://www.hanc.info/labs/Pages/SOPs.aspx). All laboratories were in good standing with the Immunology Cryopreservation Quality Assurance program [43]. Cryopreserved PBMC were shipped within 7 days of collection to a central repository where cells were stored in liquid N2 until shipment in liquid N2 dewars to the testing laboratory at the University of Colorado Anschutz Medical Center. This procedure ensured optimal viability and functionality of the PBMC [44]. Plasma was separated and cryopreserved in 1 mL aliquots by the processing laboratories and batch-shipped on dry ice. All specimens from each subject were tested in the same run to avoid confounders potentially introduced by inter-assay variability.
Fluorospot
Dual-color fluorospot kits for IFNγ and IL2 (Mabtech) were used as per manufacturer’s instructions. PBMC were thawed and rested over night. Cells with viability ≥70% before and after resting were added at 250,000 PBMC/well in 100 μL of RPMI 1640 with glutamine (Gibco) containing 10% human AB serum (Nabi) and 1% antibiotics (Gibco) and stimulated in duplicate wells with HIV inactivated virions and control (gift of Dr. Jeff Lifson [45]; 6 μg/mL), VZV inactivated cell lysate and mock-infected control prepared as previously described [46] at a pre-optimized concentration, phytohemagglutinin A (PHA; Sigma; 0.01 μg/mL) or anti-CD3 and anti-CD28 mAb (CD3/CD28; Mabtech; 0.1 μg/mL). After 36 h at 37°C in a 5% CO2 humidified atmosphere, plates were washed; bound IFNγ was detected with 7-B6-1-FS FITC and bound IL-2 with 11-Biotin. Spots were revealed using a mixture of anti-FITC-Green fluorochrome (IFNγ) and SA-Red fluorochrome (IL-2) and analyzed with an Immunospot II plate reader (CTL). Results were reported as mean spot-forming cells (SFC)/105 PBMC in antigen- or mitogen-stimulated wells after subtraction of the mean SFC in control wells.
Lymphocyte proliferation assay (LPA)
The LPA was performed on freshly thawed PBMC with a viability ≥70% as previously described [47]. Stimulants consisted of HIV antigen and control (1.5 μg/mL), VZV antigen and control (0.1 μg/mL), CD3/CD28 (0.1 μg/mL) and PHA (2.5 μg/mL). Results are presented as stimulation indices (SI) calculated by dividing the median counts per minute (cpm) in the antigen-stimulated wells, by the median cpm in the control wells.
Flow cytometry assays
T-cell subsets were enumerated in freshly thawed cryopreserved PBMC. After washing and counting viable cells, PBMCs were surface-stained with the following conjugated mAbs: anti-CD3-AF488 (Biolegend; clone HIT3a), anti CD4-APC/Cy7 (Biolegend; RPA-T4), anti-CD25-PE/Cy7 (Biolegend; BC96), anti-HLA-DR-PerCP/Cy5.5 (Biolegend; L243), anti-CD39-APC (Biolegend; A1), and anti-CD38-PECy7 (Biolegend; HIT2). Cells were fixed and permeabilized with Cytofix/Cytoperm (BD Biosciences), and stained with anti-IL10-APC (R & D Systems; 127107), anti-FOXP3-PE (Biolegend; 206D), anti-TGFβ-PerCP/Cy5.5 (Biolegend; TW4-2F8) and anti-IL35-PE (eBioscience; ebic6) and analyzed with Guava easyCyte 8HT and FlowJo (Treestar).
Subsets were expressed as percentages of the parent CD4+ and CD8+ T-cell populations. The gating strategy is presented in Fig S1.
Soluble cytokines
IL6, IL8, IL10, IFNγ and TNFα plasma levels were measured by multiplex bead array and TGFβ by ELISA as previously described [48]. The bead array assays used the MILLIPLEX MAP High Sensitivity Human Cytokine Magnetic Bead Panel kit (Millipore; HSCYTMAG-60SK) on the Bio-Rad Bio-Plex 200 instrument following the manufacturers’ instructions. The lower level of detection (LLOD) was 0.08–1.01 pg/mL and the dynamic range 13–2,000 pg/mL. Data were analyzed using Bio-Plex manager 5.0 software (Bio Rad) and concentrations were interpolated on the manufacturer’s standard curve using PRISM software (Graphpad). The LLOD for the TGFβ ELISA was 31 pg/mL and the dynamic range 31–4,000 pg/mL. The optical density (OD) was measured with a Multiscan FC ELISA reader (Thermo Fisher) using a 450 nm filter. Test TGFβ concentrations were calculated by interpolating the test ODs on the standard curve built with quantitative controls provided by the manufacturer.
Statistical analysis
All analyses were conducted on subjects with assay results available for immunologic and inflammatory endpoints using non-parametric statistical approaches, unless otherwise noted. The effects of hormonal contraceptive MPA on those immunologic markers were evaluated based on intra-subject changes using nonparametric Wilcoxon signed-rank test. Spearman’s correlation test was used to assess associations between immunologic biomarkers and associations of immunologic markers against MPA pharmacokinetic parameters. All analyses were conducted using SAS (SAS Institute Inc.).
Results
Characteristics of the study population
The study used blood samples from 24 HIV-infected women with a median of 32 years of age (Table 1). As per inclusion criteria, all participants had HIV plasma RNA ≤400 copies/mL on LPV/r-containing cART. CD4+ cell numbers varied from 326 to 1367 cells/μL (median=622 cells/μL) at study entry.
Table 1.
Baseline Characteristics of the Study Group
| Characteristics | N = 24 |
|---|---|
| Years of age: median [range] | 32 [15–47] |
| Race/ethnicity, n (%) | |
| White | 3 (13%) |
| Black | 14 (58%) |
| Hispanic | 6 (25%) |
| Native American | 1 (4%) |
| Median CD4+ cells/μL [range] | 622 [326, 1367] |
| HIV-1 plasma RNA≤ 400 copies/mL, n (%) | 24 (100%) |
Functional cell-mediated immunity of HIV-infected women before and after a single dose of DMPA
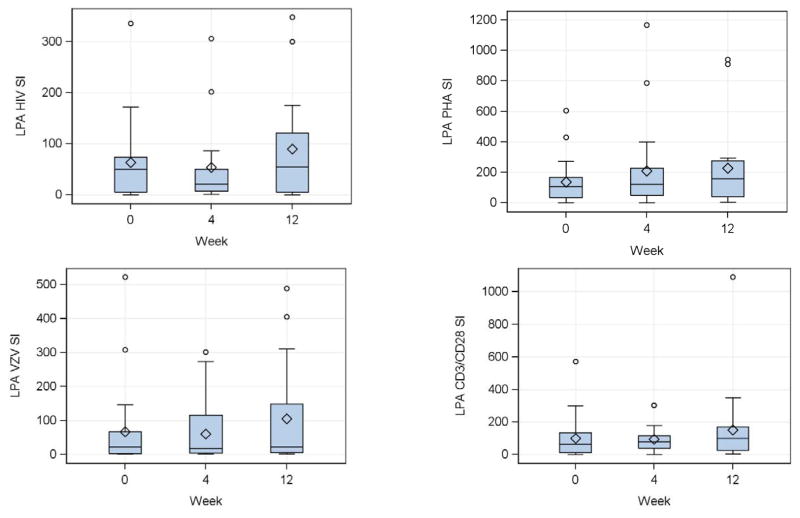
At entry, women had robust HIV-, VZV-, PHA- and CD3/CD28-stimulated LPA responses (Fig 1), including median (interquartile ranges; IQR) LPA SI for HIV of 51 (5, 74), for VZV 22 (3, 67), for CD3/CD28 65 (11, 135) and for PHA 105 (33, 166). Using a threshold ≥3 for HIV and VZV and ≥5 for CD3/CD28 and PHA to define positive results, 78% had positive qualitative results for HIV, 77% for VZV, 86% for CD3/CD28 and 90% for PHA. There were no significant quantitative or qualitative changes in the LPA responses after DMPA administration (Fig 1).
Figure 1. Effect of a single dose of DMPA on lymphocyte proliferative responses in HIV-infected women.
Data were derived from 24 HIV-infected women. Proliferation was measured by 3H-Thymidine incorporation after 6 days of in vitro stimulation of PBMC collected at the indicated time points. Stimulation indices (SI) calculated by the median incorporation in stimulated PBMC divided by the median incorporation in unstimulated or mock-stimulated controls, for HIV antigen (upper left), VZV antigen (lower left), PHA mitogen (upper right) and CD3/CD28 ligands (lower right) are summarized in box plots showing the medians as horizontal lines inside the boxes; means as diamonds inside the boxes; upper and lower quartiles as the box boundaries; minimum and maximum values, excluding potential outliers, as whiskers and outliers as open circles. There were no significant changes from week 0 (DMPA administration) to week 4 (peak MPA concentration) or week 12 (maximum exposure to MPA).
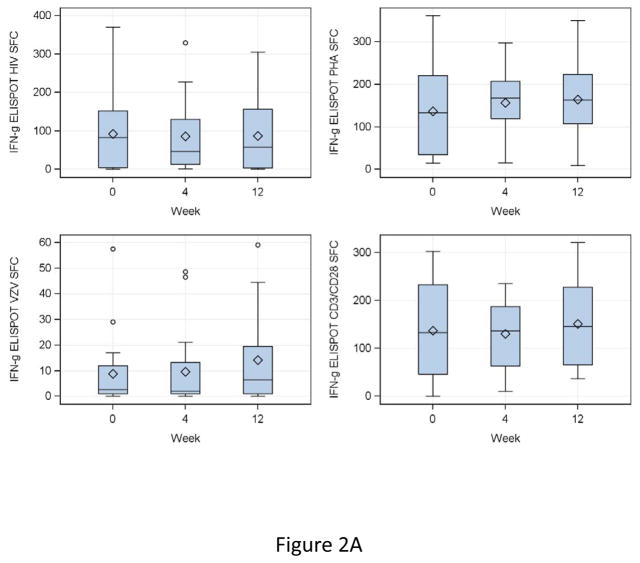
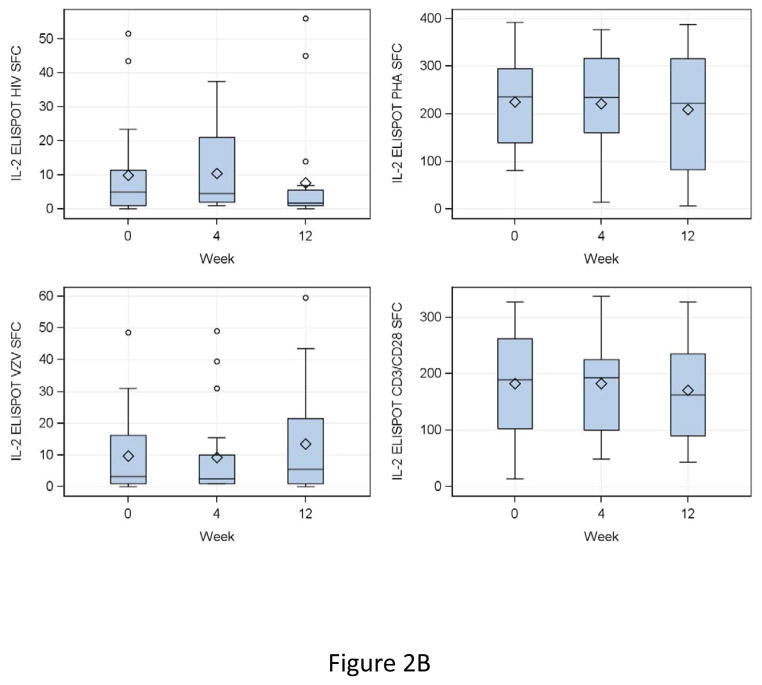
IFNγ FLUOROSPOT results at entry showed median (IQR) SFC/105 PBMC of 83 (4, 152) for HIV, 3 (1, 12) for VZV, 133 (47, 233) for CD3/CD28 and 133 (34, 221) for PHA (Fig 2A). At 4 and 12 weeks after DMPA administration, HIV, CD3/CD28 and PHA IFNγ SFC did not appreciably change compared to baseline (Fig 2A). VZV IFNγ SFC did not change from baseline to week 4, but increased at week 12 by a median of 2 SFC/105 PBMC (fold-rise = 1.4, p = 0.007). IL2 SFC were highly correlated with those of IFNγ and showed similar changes or lack thereof over time (Fig 2B).
Figure 2. Effect of a single dose of DMPA on ELISPOT responses in HIV-infected women.
Data were derived from 24 HIV-infected women. Interferon γ (IFNg; Panel A) and IL2 secretion (Panel B) were measured by dual-color fluorospot. Adjusted spot forming cells (SFC)/105 PBMC calculated by subtraction of the mean SFC in control unstimulated or mock-stimulated wells from the mean SFC in stimulated PBMC, for HIV antigen (upper left), VZV antigen (lower left), PHA mitogen (upper right) and CD3/CD28 ligands (lower right) are summarized in box plots showing the medians as horizontal lines inside the boxes; means as losanges inside the boxes; upper and lower quartiles as the box boundaries; minimum and maximum values, excluding potential outliers, as whiskers and outliers as open circles. There were significant increases from week 0 (DMPA administration) to week 12 (maximum exposure to MPA) in VZV IFNγ and IL2 SFC (p=0.007 for both). SFC in other conditions remained unchanged.
CD4+ and CD8+ T cell subsets of HIV-infected women before and after a single dose of DMPA
Activated T cells (Tact) were identified by the expression of CD25 and by dual expression of CD38 and HLADR (Table 2). There were small changes in the distribution of activated T cell subsets in the course of the study including a median decrease of 0.5% in CD4+CD25+% at week 12 that reached statistical significance (from a median of 3.42% to 2.84%, p=0.03); and lesser decreases at week 12 of 0.05% in CD8+CD25+% (from 0.39% to 0.34%; p=0.08) and 0.7% in CD8+CD38+HLADR+% (from 4.47% to 3.79%, p = 0.06).
Table 2.
Kinetics of T-cell subsets after a single dose of DMPA in HIV-infected women on effective cART.
| Anchor | T-cell subset | Week | N | Median (%) | IQR (Q1, Q3) | p-value1 |
|---|---|---|---|---|---|---|
| CD4 | CD4+CD25+ | 0 | 21 | 3.42 | ( 2.02, 5.05 ) | |
| 4 | 21 | 2.85 | ( 1.73, 6.25 ) | 0.533 | ||
| 12 | 21 | 2.84 | ( 1.63, 4.33 ) | 0.029 | ||
| CD4+FOXP3+ | 0 | 21 | 1.26 | ( 0.80, 2.73 ) | ||
| 4 | 21 | 1.30 | ( 0.90, 1.46 ) | 0.828 | ||
| 12 | 21 | 1.29 | ( 1.01, 1.80 ) | 0.511 | ||
| CD4+CD25+FOXP3+ | 0 | 21 | 0.41 | ( 0.17, 0.80 ) | ||
| 4 | 21 | 0.24 | ( 0.11, 0.62 ) | 0.176 | ||
| 12 | 21 | 0.33 | ( 0.13, 0.45 ) | 0.100 | ||
| CD4+CD39+ | 0 | 21 | 10.1 | ( 5.95, 15.9 ) | ||
| 4 | 21 | 10.1 | ( 6.31, 13.2 ) | 0.763 | ||
| 12 | 21 | 11.6 | ( 7.65, 16.5 ) | 0.078 | ||
| CD4+CD38+HLADR+ | 0 | 21 | 7.52 | ( 4.19, 9.55 ) | ||
| 4 | 21 | 5.98 | ( 4.05, 10.1 ) | 0.206 | ||
| 12 | 21 | 5.65 | ( 3.35, 9.39 ) | 0.269 | ||
| CD4+TGFB+ | 0 | 21 | 4.32 | ( 2.66, 6.02 ) | ||
| 4 | 21 | 3.62 | ( 1.93, 7.96 ) | 0.334 | ||
| 12 | 21 | 3.08 | ( 2.30, 5.62 ) | 0.308 | ||
| CD4+IL10+ | 0 | 21 | 4.66 | ( 3.52, 6.16 ) | ||
| 4 | 21 | 6.01 | ( 3.41, 7.68 ) | 0.511 | ||
| 12 | 21 | 5.64 | ( 3.56, 8.43 ) | 0.427 | ||
| CD4+IL35+ | 0 | 21 | 1.86 | ( 1.22, 3.36 ) | ||
| 4 | 21 | 1.59 | ( 0.93, 3.19 ) | 0.352 | ||
| 12 | 21 | 1.35 | ( 0.78, 2.08 ) | 0.024 | ||
| CD8 | CD8+CD25+ | 0 | 21 | 0.39 | ( 0.23, 0.48 ) | |
| 4 | 21 | 0.38 | ( 0.22, 0.57 ) | 0.427 | ||
| 12 | 21 | 0.34 | ( 0.14, 0.41 ) | 0.078 | ||
| CD8+FOXP3+ | 0 | 21 | 0.85 | ( 0.54, 1.04 ) | ||
| 4 | 21 | 0.91 | ( 0.68, 1.49 ) | 0.023 | ||
| 12 | 21 | 0.74 | ( 0.67, 1.43 ) | 0.176 | ||
| CD8+CD25+FOXP3+ | 0 | 21 | 0.07 | ( 0.03, 0.19 ) | ||
| 4 | 21 | 0.06 | ( 0.03, 0.27 ) | 0.388 | ||
| 12 | 21 | 0.08 | ( 0.01, 0.26 ) | 0.556 | ||
| CD8+CD39+ | 0 | 21 | 3.38 | ( 2.53, 4.52 ) | ||
| 4 | 21 | 4.20 | ( 2.37, 4.89 ) | 0.848 | ||
| 12 | 21 | 3.12 | ( 2.31, 5.20 ) | 0.675 | ||
| CD8+CD38+HLADR+ | 0 | 21 | 4.47 | ( 2.35, 5.72 ) | ||
| 4 | 21 | 4.28 | ( 2.44, 7.26 ) | 0.300 | ||
| 12 | 21 | 3.79 | ( 2.47, 5.55 ) | 0.056 | ||
| CD8+TGFB+ | 0 | 21 | 1.26 | ( 0.74, 1.90 ) | ||
| 4 | 21 | 1.22 | ( 0.76, 2.02 ) | 0.159 | ||
| 12 | 21 | 1.18 | ( 0.60, 1.66 ) | 0.404 | ||
| CD8+IL10+ | 0 | 21 | 1.46 | ( 1.10, 1.87 ) | ||
| 4 | 21 | 1.53 | ( 1.13, 2.60 ) | 0.290 | ||
| 12 | 21 | 1.45 | ( 1.10, 2.21 ) | 0.701 | ||
| CD8+IL35+ | 0 | 21 | 1.22 | ( 0.87, 1.61 ) | ||
| 4 | 21 | 1.52 | ( 0.53, 1.99 ) | 0.490 | ||
| 12 | 21 | 0.89 | ( 0.65, 1.83 ) | 0.500 |
Wilcoxon signed-rank test of changes from baseline.
Bold font highlights significant differences and strong trends.
Regulatory T cell subsets were characterized by expression of FOXP3, dual expression of CD25 and FOXP3, CD39, IL10, IL35 and TGFβ (Table 2). At week 4 after DMPA administration, there was a small, but statistically significant increase of 0.06% in CD8+FOXP3+% (from 0.85% to 0.91%, p=0.02). At week 12, there was a statistically significant decrease of 0.5% in CD4+IL35+% (from 1.86% to 1.35%, p=0.02) and a trend increase of 1.5% in CD4+CD39+% (from 10.1% to 11.6%, p=0.08).
Soluble biomarkers of HIV-infected women before and after a single dose of DMPA
Pregnancy, which is a state of heightened female hormone secretion, is also characterized by increased production of inflammatory and regulatory cytokines [49]. Pro-inflammatory (IL6, IL8, IFNγ and TNFα) and regulatory cytokines (IL10 and TGFβ) were measured before and after DMPA administration. There were no significant changes over time in plasma concentrations of IL6, IL8, IL10, IFNγ or TNFα. TGFβ had a small, but significant decrease at week 12 (p=0.04).
Relationship between MPA plasma concentrations and immunologic parameters in HIV-infected women
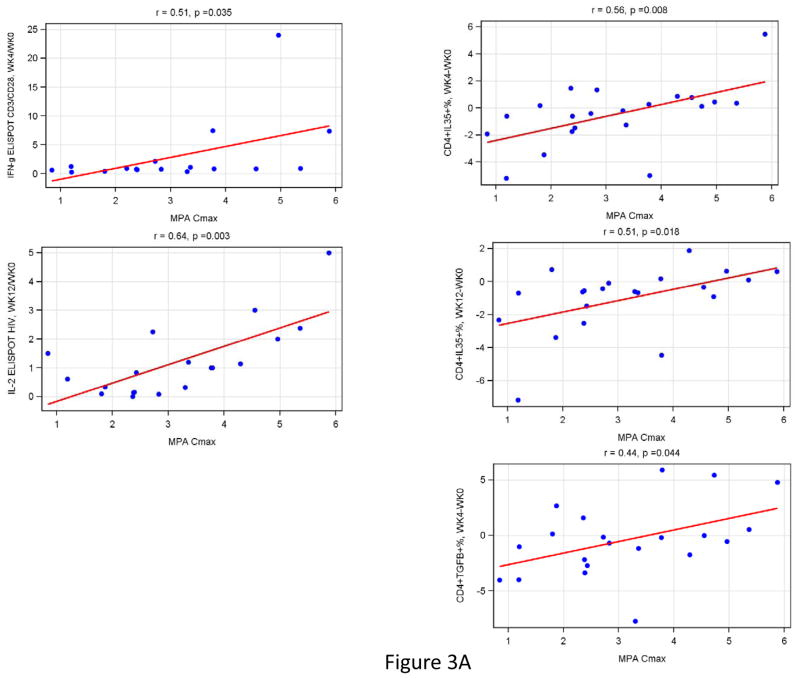
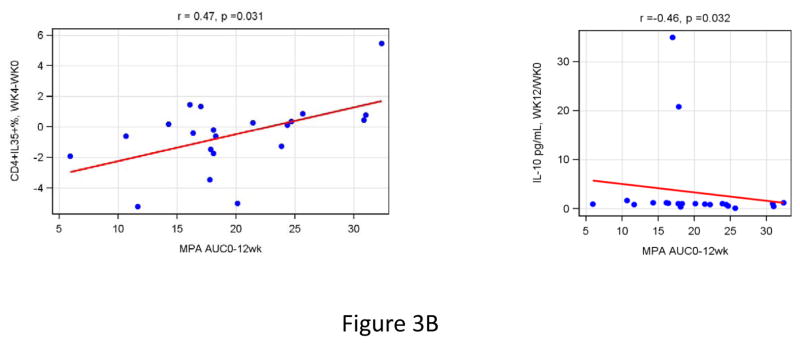
Correlation analyses were performed to determine the relationship of MPA Cmax and AUC with changes in functional immune responses (ratios of post-DMPA over baseline results), T cell subsets (differences in percentages from baseline to post-DMPA) and/or plasma biomarkers (ratios of post-DMPA over baseline results). High MPA Cmax were associated with increased HIV IFNγ FLUOROSPOT fold-rises at week 12 over baseline (r=0.64, p=0.003; Fig 3), CD3/CD28 IFNγ at week 4 (r= 0.51, p= 0.04; Fig 3), CD4+IL35+% at week 4 and 12 (r≥0.51, p≤0.02; Fig 3) and CD4+TGFβ+% at week 4 (r=0.44, p=0.04; Fig 3). In addition, high MPA AUC was associated with increases in CD4+IL35+% at week 4 and 12 compared to baseline (r=0.47, p=0.03 for both; Fig 3) and with a decrease in the plasma concentration of IL10 (r=−0.46, p=0.03; Fig 3).
Figure 3. Effect of the magnitude of MPA Cmax (Panel A) and T-cell subsets (Panel B) on CMI, T-cell subsets and soluble cytokines in HIV-Infected women.
Data were derived from 24 HIV-infected women who received a single dose of DMPA. MPA Cmax was measured at week 4 and AUC at week 12 after DMPA administration. Correlation analyses were performed between changes from baseline to week 4 or 12 of all the measures of CMI, T-cell subsets and soluble cytokines evaluated in this study. The graphs show only the significant correlations. Estimated linear regression lines, correlation coefficients (r) and p values calculated using Spearman’s correlation test are shown on each graph.
Discussion
This exploratory analysis of the effect of DMPA on CMI of HIV-infected women on effective cART failed to show any attenuation of their CMI after a single dose of DMPA. This result was unexpected based on the accumulated evidence that suggested a downregulatory and anti-inflammatory effect of progestins in general and of MPA in particular on CMI [16, 17, 29, 33, 37–40, 50–54]. In contrast to these studies, we observed an increase in VZV-specific IL2 and IFNγ FLUOROSPOT responses after DMPA administration compared to baseline. In addition, we found positive associations of the MPA Cmax with the fold-increase of HIV-specific IFNγ responses from baseline to week 12 and of CD3/CD28-stimulated IFNγ responses from baseline to week 4. A mechanism to explain the association of DMPA administration with the CMI increases that we observed is unclear. Based on previous in vitro data, it is unlikely that it involved a direct effect of MPA on the cells of the immune system. However, an indirect effect mediated by the decrease of estrogen secretion in DMPA recipients [55] is a potential explanation due to the strong immune regulatory effect of estrogen [56–66].
It is important to note that our study evaluated women on effective cART, a characteristic shared by other studies that failed to detect any detrimental effects of DMPA on HIV disease progression or transmission studies [18, 21, 67]. In contrast, studies that documented detrimental effects of DMPA were generally conducted in the absence of cART [7, 8]. A stimulatory direct effect of MPA and of other steroidal hormones on HIV replication has been described [68]. The use of cART might attenuate the direct effect of MPA on HIV replication thus contributing to the difference in findings between DMPA studies conducted in the presence or absence of cART. If the deleterious effect of MPA on the CMI of HIV-infected women is indeed mediated by the enhancement of HIV replication, the current trend towards increased cART utilization may improve the safety of DMPA administration to HIV-infected women.
T-cell activation markers decreased after DMPA administration, an effect that was predicted by in vitro studies showing that MPA decreased dendritic cell and monocyte-activation and production of pro-inflammatory cytokines [30, 31, 33, 39, 40]. However, we were not able to demonstrate a decrease in pro-inflammatory cytokine plasma levels. A recent study showed increased T-cell activation in HIV-uninfected women receiving DMPA compared with women using oral or no hormonal contraceptives [69]. The divergence may be related to differences in study populations, HIV-infected vs. uninfected, and designs, longitudinal vs. cross-sectional. The longitudinal study design allowed us to use the subjects as their own controls, thus avoiding potential biases introduced by differences in exposures to other factors that may promote T-cell activation.
Based on previous studies [53, 54], we expected an increase in circulating Treg subsets after DMPA administration. In fact, we observed a correlation between high MPA Cmax and AUC with an increase in CD4+IL35+% and in CD4+TGFβ+% after DMPA administration compared to baseline, which was consistent with a stimulatory effect of MPA on Tregs. However, the changes in the proportion of Tregs were heterogeneous among study participants and at the group level, there was no consistent increase in the proportion of Tregs over time on DMPA. This suggests that the stimulatory effect of MPA on Treg subsets may be offset by the DMPA-mediated decrease in estrogen, which is also a Treg inducer, perhaps even more potent than MPA [64, 65].
Our study had limitations and virtues. Because this was an exploratory analysis we did not adjust for multiple comparisons. This increased our ability to detect changes and generate hypotheses, but might have also allowed the introduction of spurious associations. Although the number of participants in this study was limited, their ages, which varied from 15 to 47 years, overlap with the age range of 15 to 44 years considered by the CDC as representative for the use of contraceptive methods in the U.S. and with previously published demographics of DMPA in HIV-infected users [70, 71]. The limited number of participants in this analysis was counterbalanced by the longitudinal design using intra-subject comparison approaches, which minimized the introduction of DMPA-unrelated variables. Furthermore, longitudinal samples of each participant were assayed jointly in order to minimize the effect of inter-assay variability.
Although our findings need to be confirmed, these preliminary results are encouraging, because they support the notion that the effectiveness of DMPA as a contraceptive agent in the context of cART overpowers its potential attenuation of immune defenses in HIV-infected women. Our results also show that the effect of in vivo administration of hormones have consequences that may not be anticipated from their effect in vitro, probably due to feed back mechanisms that are intact in vivo and change the overall hormonal homeostasis and cannot be readily reproduced in vitro. More studies are needed to evaluate the cumulative effect of multiple doses of DMPA on the immune system of HIV-infected women and its effect on HIV transmission.
Acknowledgments
The authors are grateful for the patients’ commitment and participation in this study. We thank Dr. Karin Klingman for her overall contributions to the A5283 protocol and for critical review of this manuscript. The laboratory work reported in this publication was supported by N01HD33162 (97-07) to AW; the clinical work by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Numbers UM1 AI068634, UM1 AI068636 and UM1 AI106701; Grants 1U01AI069511 and CRC grant UL-1RR02460 to the University of Rochester; 1U01AI069471 and CRC Grant UL 1TR000150 to Northwestern University; 1U01AI069513 to Cincinnati CRS; 1U01AI069481 to the University of Washington; UM1- AI069423-08, CTSA Grant 1UL1TR001111, CFAR Grant P30 AI50410 to UNC Global CTU: Chapel Hill CRS and by UCSL PSL is under NIH grant 1 U01 AI068636. Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC) and UM1AI106716 (IMPAACT LC), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH); and the statistical work by the National Institute of Allergy and Infectious Diseases cooperative agreement UM1 AI068634 to the Statistical and Data Analysis Center at Harvard School of Public Health. The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the views of the Office of the Global AIDS Coordinator, the National Institutes of Health, or the U.S. Department of State. The following individuals assisted in conducting A5283: Becky Straub, RN, MPH and Miriam Chicurel-Bayard, RN, BSN-UNC Global CTU: Chapel Hill CRS (Site3201); Rachel K. Scott, MD, MPH and Patricia Tanjutco, MD-MedStar Washington Hospital Center (Site 5023); Jenny Baer, RN and Jennifer Forrester, MD, Cincinnati CRS (Site 2401); Mariam Aziz, MD and Maureen McNichols, RN-Rush University Medical Center/Ruth M. Rothstein CORE Center (Site5083); Mary Adams, RN and Christine Hurley, RN- University of Rochester CRS (Site1101); Sheila Dunaway, MD and Eric Helgeson, RN- University of Washington ACTG CRS (Site1401); Donna McGregor, NP- Northwestern University CRS( Site 2701); Steven Zeichner, MD and Connie Trexler, RN-Children’s National (Site5015); Rodrigo Diaz-Velasco, MD and Elvia Perez-Hernandez, MPH-San Juan Hospital (Site 5031); Sharon Nachman, MD, Denise Ferraro, FNP and Erin Infanzon-SUNY Stony Brook NICHD CRS (Site 5040) and Patricia Riley and Sheila Bradford-Tulane University New Orleans NICHD CRS (Site 5095).
Footnotes
Conflicts of Interest: None of the authors had any conflicts of interest related to the work described in this manuscript.
References
- 1.World Health Organization. Hormonal Contraception and HIV: Technical Statement. Geneva: 2012. [PubMed] [Google Scholar]
- 2.Darroch JE. Trends in contraceptive use. Contraception. 2013;87:259–263. doi: 10.1016/j.contraception.2012.08.029. [DOI] [PubMed] [Google Scholar]
- 3.Heffron R, Donnell D, Rees H, Celum C, Mugo N, Were E, et al. Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. The Lancet Infectious diseases. 2012;12:19–26. doi: 10.1016/S1473-3099(11)70247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polis CB, Curtis KM. Use of hormonal contraceptives and HIV acquisition in women: a systematic review of the epidemiological evidence. The Lancet Infectious diseases. 2013;13:797–808. doi: 10.1016/S1473-3099(13)70155-5. [DOI] [PubMed] [Google Scholar]
- 5.Polis CB, Phillips SJ, Curtis KM. Hormonal contraceptive use and female-to-male HIV transmission: a systematic review of the epidemiologic evidence. AIDS. 2013;27:493–505. doi: 10.1097/QAD.0b013e32835ad539. [DOI] [PubMed] [Google Scholar]
- 6.Stringer E, Antonsen E. Hormonal contraception and HIV disease progression. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2008;47:945–951. doi: 10.1086/591697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stringer EM, Kaseba C, Levy J, Sinkala M, Goldenberg RL, Chi BH, et al. A randomized trial of the intrauterine contraceptive device vs hormonal contraception in women who are infected with the human immunodeficiency virus. American journal of obstetrics and gynecology. 2007;197:144. e141–148. doi: 10.1016/j.ajog.2007.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stringer EM, Levy J, Sinkala M, Chi BH, Matongo I, Chintu N, et al. HIV disease progression by hormonal contraceptive method: secondary analysis of a randomized trial. AIDS. 2009;23:1377–1382. doi: 10.1097/QAD.0b013e32832cbca8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumwenda JJ, Makanani B, Taulo F, Nkhoma C, Kafulafula G, Li Q, et al. Natural history and risk factors associated with early and established HIV type 1 infection among reproductive-age women in Malawi. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2008;46:1913–1920. doi: 10.1086/588478. [DOI] [PubMed] [Google Scholar]
- 10.Lavreys L, Baeten JM, Martin HL, Jr, Overbaugh J, Mandaliya K, Ndinya-Achola J, et al. Hormonal contraception and risk of HIV-1 acquisition: results of a 10-year prospective study. AIDS. 2004;18:695–697. doi: 10.1097/00002030-200403050-00017. [DOI] [PubMed] [Google Scholar]
- 11.Morrison CS, Chen PL, Kwok C, Richardson BA, Chipato T, Mugerwa R, et al. Hormonal contraception and HIV acquisition: reanalysis using marginal structural modeling. AIDS. 2010;24:1778–1781. doi: 10.1097/QAD.0b013e32833a2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watson-Jones D, Baisley K, Weiss HA, Tanton C, Changalucha J, Everett D, et al. Risk factors for HIV incidence in women participating in an HSV suppressive treatment trial in Tanzania. AIDS. 2009;23:415–422. doi: 10.1097/QAD.0b013e32831ef523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mostad SB, Overbaugh J, DeVange DM, Welch MJ, Chohan B, Mandaliya K, et al. Hormonal contraception, vitamin A deficiency, and other risk factors for shedding of HIV-1 infected cells from the cervix and vagina. Lancet. 1997;350:922–927. doi: 10.1016/S0140-6736(97)04240-2. [DOI] [PubMed] [Google Scholar]
- 14.Mugo NR, Heffron R, Donnell D, Wald A, Were EO, Rees H, et al. Increased risk of HIV-1 transmission in pregnancy: a prospective study among African HIV-1-serodiscordant couples. AIDS. 2011;25:1887–1895. doi: 10.1097/QAD.0b013e32834a9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gardella B, Roccio M, Maccabruni A, Mariani B, Panzeri L, Zara F, et al. HIV shedding in cervico-vaginal secretions in pregnant women. Current HIV research. 2011;9:313–320. doi: 10.2174/157016211797636017. [DOI] [PubMed] [Google Scholar]
- 16.Abel K, Rourke T, Lu D, Bost K, McChesney MB, Miller CJ. Abrogation of attenuated lentivirus-induced protection in rhesus macaques by administration of depo-provera before intravaginal challenge with simian immunodeficiency virus mac239. The Journal of infectious diseases. 2004;190:1697–1705. doi: 10.1086/424600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trunova N, Tsai L, Tung S, Schneider E, Harouse J, Gettie A, et al. Progestin-based contraceptive suppresses cellular immune responses in SHIV-infected rhesus macaques. Virology. 2006;352:169–177. doi: 10.1016/j.virol.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Chu JH, Gange SJ, Anastos K, Minkoff H, Cejtin H, Bacon M, et al. Hormonal contraceptive use and the effectiveness of highly active antiretroviral therapy. American journal of epidemiology. 2005;161:881–890. doi: 10.1093/aje/kwi116. [DOI] [PubMed] [Google Scholar]
- 19.Heikinheimo O, Lahteenmaki P. Contraception and HIV infection in women. Human reproduction update. 2009;15:165–176. doi: 10.1093/humupd/dmn049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myer L, Denny L, Wright TC, Kuhn L. Prospective study of hormonal contraception and women’s risk of HIV infection in South Africa. International journal of epidemiology. 2007;36:166–174. doi: 10.1093/ije/dyl251. [DOI] [PubMed] [Google Scholar]
- 21.Radzio J, Hanley K, Mitchell J, Ellis S, Deyounks F, Jenkins L, et al. Depot-medroxyprogesterone acetate does not reduce the prophylactic efficacy of emtricitabine and tenofovir disoproxil fumarate in macaques. Journal of acquired immune deficiency syndromes. 2014;67:365–369. doi: 10.1097/QAI.0000000000000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radzio J, Hanley K, Mitchell J, Ellis S, Deyounks F, Jenkins LT, et al. Physiologic doses of depot-medroxyprogesterone acetate do not increase acute plasma simian HIV viremia or mucosal virus shedding in pigtail macaques. AIDS. 2014;28:1431–1439. doi: 10.1097/QAD.0000000000000294. [DOI] [PubMed] [Google Scholar]
- 23.Sanders-Beer B, Babas T, Mansfield K, Golightly D, Kramer J, Bowlsbey A, et al. Depo-Provera does not alter disease progression in SIVmac-infected female Chinese rhesus macaques. AIDS research and human retroviruses. 2010;26:433–443. doi: 10.1089/aid.2009.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bradshaw CS, Vodstrcil LA, Hocking JS, Law M, Pirotta M, Garland SM, et al. Recurrence of bacterial vaginosis is significantly associated with posttreatment sexual activities and hormonal contraceptive use. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2013;56:777–786. doi: 10.1093/cid/cis1030. [DOI] [PubMed] [Google Scholar]
- 25.Ghanem KG, Shah N, Klein RS, Mayer KH, Sobel JD, Warren DL, et al. Influence of sex hormones, HIV status, and concomitant sexually transmitted infection on cervicovaginal inflammation. The Journal of infectious diseases. 2005;191:358–366. doi: 10.1086/427190. [DOI] [PubMed] [Google Scholar]
- 26.Goode D, Aravantinou M, Jarl S, Truong R, Derby N, Guerra-Perez N, et al. Sex hormones selectively impact the endocervical mucosal microenvironment: implications for HIV transmission. PloS one. 2014;9:e97767. doi: 10.1371/journal.pone.0097767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitchell CM, McLemore L, Westerberg K, Astronomo R, Smythe K, Gardella C, et al. Long-term effect of depot medroxyprogesterone acetate on vaginal microbiota, epithelial thickness and HIV target cells. The Journal of infectious diseases. 2014;210:651–655. doi: 10.1093/infdis/jiu176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao D, Lebovic DI, Taylor RN. Long-term progestin treatment inhibits RANTES (regulated on activation, normal T cell expressed and secreted) gene expression in human endometrial stromal cells. The Journal of clinical endocrinology and metabolism. 2002;87:2514–2519. doi: 10.1210/jcem.87.6.8526. [DOI] [PubMed] [Google Scholar]
- 29.Cherpes TL, Busch JL, Sheridan BS, Harvey SA, Hendricks RL. Medroxyprogesterone acetate inhibits CD8+ T cell viral-specific effector function and induces herpes simplex virus type 1 reactivation. Journal of immunology. 2008;181:969–975. doi: 10.4049/jimmunol.181.2.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hughes GC, Thomas S, Li C, Kaja MK, Clark EA. Cutting edge: progesterone regulates IFN-alpha production by plasmacytoid dendritic cells. Journal of immunology. 2008;180:2029–2033. doi: 10.4049/jimmunol.180.4.2029. [DOI] [PubMed] [Google Scholar]
- 31.Huijbregts RP, Helton ES, Michel KG, Sabbaj S, Richter HE, Goepfert PA, et al. Hormonal contraception and HIV-1 infection: medroxyprogesterone acetate suppresses innate and adaptive immune mechanisms. Endocrinology. 2013;154:1282–1295. doi: 10.1210/en.2012-1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones LA, Kreem S, Shweash M, Paul A, Alexander J, Roberts CW. Differential modulation of TLR3- and TLR4-mediated dendritic cell maturation and function by progesterone. Journal of immunology. 2010;185:4525–4534. doi: 10.4049/jimmunol.0901155. [DOI] [PubMed] [Google Scholar]
- 33.Enomoto LM, Kloberdanz KJ, Mack DG, Elizabeth D, Weinberg A. Ex vivo effect of estrogen and progesterone compared with dexamethasone on cell-mediated immunity of HIV-infected and uninfected subjects. J Acquir Immune Defic Syndr. 2007;45:137–143. doi: 10.1097/QAI.0b013e3180471bae. [DOI] [PubMed] [Google Scholar]
- 34.Darroch JE, Singh S. Trends in contraceptive need and use in developing countries in 2003; 2008; and 2012: an analysis of national surveys. The Lancet. 2013;381:1756–1762. doi: 10.1016/S0140-6736(13)60597-8. [DOI] [PubMed] [Google Scholar]
- 35.Weinberg A, Allshouse AA, Mawhinney S, Canniff J, Benning L, Wentz EL, et al. Responses to hepatitis A virus vaccine in HIV-infected women: effect of hormonal contraceptives and HIV disease characteristics. J Acquir Immune Defic Syndr. 2012;60:e15–18. doi: 10.1097/QAI.0b013e31824d30bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scarsi KNS, Byakika-Kibwika P, Lamorde M, Darin K, Cohn S, Back D, Else L, Dilly-Penchala S, Merry C. Intracellular and Clinical Pharmacology, Drug Interactions, and Adherence. SEATTLE, WASHINGTON: Copyright c 2015 IAS–USA/CROI Foundation; 2015. Levonorgestrel Implant + EFV-Based ART: Unintended Pregnancies and Associated PK Data. [Google Scholar]
- 37.Africander D, Louw R, Verhoog N, Noeth D, Hapgood JP. Differential regulation of endogenous pro-inflammatory cytokine genes by medroxyprogesterone acetate and norethisterone acetate in cell lines of the female genital tract. Contraception. 2011;84:423–435. doi: 10.1016/j.contraception.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 38.Bamberger CM, Else T, Bamberger AM, Beil FU, Schulte HM. Dissociative glucocorticoid activity of medroxyprogesterone acetate in normal human lymphocytes. The Journal of clinical endocrinology and metabolism. 1999;84:4055–4061. doi: 10.1210/jcem.84.11.6091. [DOI] [PubMed] [Google Scholar]
- 39.Hapgood JP, Ray RM, Govender Y, Avenant C, Tomasicchio M. Differential glucocorticoid receptor-mediated effects on immunomodulatory gene expression by progestin contraceptives: implications for HIV-1 pathogenesis. American journal of reproductive immunology. 2014;71:505–512. doi: 10.1111/aji.12214. [DOI] [PubMed] [Google Scholar]
- 40.Huijbregts RP, Michel KG, Hel Z. Effect of progestins on immunity: medroxyprogesterone but not norethisterone or levonorgestrel suppresses the function of T cells and pDCs. Contraception. 2014;90:123–129. doi: 10.1016/j.contraception.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tomasicchio M, Avenant C, Du Toit A, Ray RM, Hapgood JP. The progestin-only contraceptive medroxyprogesterone acetate, but not norethisterone acetate, enhances HIV-1 Vpr-mediated apoptosis in human CD4+ T cells through the glucocorticoid receptor. PloS one. 2013;8:e62895. doi: 10.1371/journal.pone.0062895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luque AE, Cohn SE, Park JG, Cramer Y, Weinberg A, Livingston E, et al. Depot Medroxyprogesterone Acetate in Combination with a Twice-Daily Lopinavir-Ritonavir-Based Regimen in HIV-Infected Women Showed Effective Contraception and a Lack of Clinically Significant Interactions, with Good Safety and Tolerability: Results of the ACTG 5283 Study. Antimicrob Agents Chemother. 2015;59:2094–2101. doi: 10.1128/AAC.04701-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sarzotti-Kelsoe M, Needham LK, Rountree W, Bainbridge J, Gray CM, Fiscus SA, et al. The Center for HIV/AIDS Vaccine Immunology (CHAVI) multi-site quality assurance program for cryopreserved human peripheral blood mononuclear cells. Journal of Immunological Methods. 2014;409:21–30. doi: 10.1016/j.jim.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weinberg A, Song LY, Wilkening CL, Fenton T, Hural J, Louzao R, et al. Optimization of storage and shipment of cryopreserved peripheral blood mononuclear cells from HIV-infected and uninfected individuals for ELISPOT assays. J Immunol Methods. 2010;363:42–50. doi: 10.1016/j.jim.2010.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rossio JL, Esser MT, Suryanarayana K, Schneider DK, Bess JW, Jr, Vasquez GM, et al. Inactivation of human immunodeficiency virus type 1 infectivity with preservation of conformational and functional integrity of virion surface proteins. J Virol. 1998;72:7992–8001. doi: 10.1128/jvi.72.10.7992-8001.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zaia JA, Leary PL, Levin MJ. Specificity of the blastogenic response of human mononuclear cells to herpesvirus antigens. Infect Immun. 1978;20:646–651. doi: 10.1128/iai.20.3.646-651.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weinberg A, Song LY, Wilkening C, Sevin A, Blais B, Louzao R, et al. Optimization and limitations of use of cryopreserved peripheral blood mononuclear cells for functional and phenotypic T-cell characterization. Clin Vaccine Immunol. 2009;16:1176–1186. doi: 10.1128/CVI.00342-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richardson K, Weinberg A. Dynamics of regulatory T-cells during pregnancy: effect of HIV infection and correlations with other immune parameters. PLoS One. 2011;6:e28172. doi: 10.1371/journal.pone.0028172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Protonotariou E, Chrelias C, Kassanos D, Kapsambeli H, Trakakis E, Sarandakou A. Immune response parameters during labor and early neonatal life. In Vivo. 24:117–123. [PubMed] [Google Scholar]
- 50.Ehring GR, Kerschbaum HH, Eder C, Neben AL, Fanger CM, Khoury RM, et al. A nongenomic mechanism for progesterone-mediated immunosuppression: inhibition of K+ channels, Ca2+ signaling, and gene expression in T lymphocytes. The Journal of experimental medicine. 1998;188:1593–1602. doi: 10.1084/jem.188.9.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kleynhans L, Du Plessis N, Black GF, Loxton AG, Kidd M, van Helden PD, et al. Medroxyprogesterone acetate alters Mycobacterium bovis BCG-induced cytokine production in peripheral blood mononuclear cells of contraceptive users. PloS one. 2011;6:e24639. doi: 10.1371/journal.pone.0024639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koubovec D, Vanden Berghe W, Vermeulen L, Haegeman G, Hapgood JP. Medroxyprogesterone acetate downregulates cytokine gene expression in mouse fibroblast cells. Molecular and cellular endocrinology. 2004;221:75–85. doi: 10.1016/j.mce.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 53.Weinberg A, Enomoto L, Marcus R, Canniff J. Effect of menstrual cycle variation in female sex hormones on cellular immunity and regulation. Journal of reproductive immunology. 2011;89:70–77. doi: 10.1016/j.jri.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 54.Lee JH, Ulrich B, Cho J, Park J, Kim CH. Progesterone promotes differentiation of human cord blood fetal T cells into T regulatory cells but suppresses their differentiation into Th17 cells. J Immunol. 2011;187:1778–1787. doi: 10.4049/jimmunol.1003919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ildgruben A, Sjoberg I, Hammarstrom ML, Backstrom T. Steroid receptor expression in vaginal epithelium of healthy fertile women and influences of hormonal contraceptive usage. Contraception. 2005;72:383–392. doi: 10.1016/j.contraception.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 56.Arruvito L, Sanz M, Banham AH, Fainboim L. Expansion of CD4+CD25+and FOXP3+ regulatory T cells during the follicular phase of the menstrual cycle: implications for human reproduction. Journal of immunology. 2007;178:2572–2578. doi: 10.4049/jimmunol.178.4.2572. [DOI] [PubMed] [Google Scholar]
- 57.Dai R, Phillips RA, Zhang Y, Khan D, Crasta O, Ahmed SA. Suppression of LPS-induced Interferon-gamma and nitric oxide in splenic lymphocytes by select estrogen-regulated microRNAs: a novel mechanism of immune modulation. Blood. 2008;112:4591–4597. doi: 10.1182/blood-2008-04-152488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duncan GS, Brenner D, Tusche MW, Brustle A, Knobbe CB, Elia AJ, et al. 2-Methoxyestradiol inhibits experimental autoimmune encephalomyelitis through suppression of immune cell activation. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:21034–21039. doi: 10.1073/pnas.1215558110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Escribese MM, Kraus T, Rhee E, Fernandez-Sesma A, Lopez CB, Moran TM. Estrogen inhibits dendritic cell maturation to RNA viruses. Blood. 2008;112:4574–4584. doi: 10.1182/blood-2008-04-148692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lambert KC, Curran EM, Judy BM, Milligan GN, Lubahn DB, Estes DM. Estrogen receptor alpha (ERalpha) deficiency in macrophages results in increased stimulation of CD4+ T cells while 17beta-estradiol acts through ERalpha to increase IL-4 and GATA-3 expression in CD4+ T cells independent of antigen presentation. Journal of immunology. 2005;175:5716–5723. doi: 10.4049/jimmunol.175.9.5716. [DOI] [PubMed] [Google Scholar]
- 61.Liu HB, Loo KK, Palaszynski K, Ashouri J, Lubahn DB, Voskuhl RR. Estrogen receptor alpha mediates estrogen’s immune protection in autoimmune disease. Journal of immunology. 2003;171:6936–6940. doi: 10.4049/jimmunol.171.12.6936. [DOI] [PubMed] [Google Scholar]
- 62.Michalek RD, Gerriets VA, Nichols AG, Inoue M, Kazmin D, Chang CY, et al. Estrogen-related receptor-alpha is a metabolic regulator of effector T-cell activation and differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:18348–18353. doi: 10.1073/pnas.1108856108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pazos MA, Kraus TA, Munoz-Fontela C, Moran TM. Estrogen mediates innate and adaptive immune alterations to influenza infection in pregnant mice. PloS one. 2012;7:e40502. doi: 10.1371/journal.pone.0040502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Polanczyk MJ, Carson BD, Subramanian S, Afentoulis M, Vandenbark AA, Ziegler SF, et al. Cutting edge: estrogen drives expansion of the CD4+CD25+ regulatory T cell compartment. Journal of immunology. 2004;173:2227–2230. doi: 10.4049/jimmunol.173.4.2227. [DOI] [PubMed] [Google Scholar]
- 65.Polanczyk MJ, Hopke C, Vandenbark AA, Offner H. Estrogen-mediated immunomodulation involves reduced activation of effector T cells, potentiation of Treg cells, and enhanced expression of the PD-1 costimulatory pathway. Journal of neuroscience research. 2006;84:370–378. doi: 10.1002/jnr.20881. [DOI] [PubMed] [Google Scholar]
- 66.Wang C, Dehghani B, Li Y, Kaler LJ, Proctor T, Vandenbark AA, et al. Membrane estrogen receptor regulates experimental autoimmune encephalomyelitis through up-regulation of programmed death 1. Journal of immunology. 2009;182:3294–3303. doi: 10.4049/jimmunol.0803205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Watts DH, Park JG, Cohn SE, Yu S, Hitti J, Stek A, et al. Safety and tolerability of depot medroxyprogesterone acetate among HIV-infected women on antiretroviral therapy: ACTG A5093. Contraception. 2008;77:84–90. doi: 10.1016/j.contraception.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Asin SN, Heimberg AM, Eszterhas SK, Rollenhagen C, Howell AL. Estradiol and progesterone regulate HIV type 1 replication in peripheral blood cells. AIDS research and human retroviruses. 2008;24:701–716. doi: 10.1089/aid.2007.0108. [DOI] [PubMed] [Google Scholar]
- 69.Tsibris ASG, Wang C, Young M, Murphy K, Mehri Z, Greenblatt R, Cohen M, Golub E, Watts H. CCR5 Expression in HIV-Uninfected Women Receiving Hormo. Seattle, Washington: Copyright c 2015 IAS–USA/CROI Foundation; 2015. CCR5 Expression in HIV-Uninfected Women Receiving Hormonal Contraception. [Google Scholar]
- 70.Overton ET, Shacham E, Singhatiraj E, Nurutdinova D. Incidence of sexually transmitted infections among HIV-infected women using depot medroxyprogesterone acetate contraception. Contraception. 2008;78:125–130. doi: 10.1016/j.contraception.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 71.Daniels K, Mosher WD. Contraceptive methods women have ever used: United States, 1982–2010. Natl Health Stat Report. 2013:1–15. [PubMed] [Google Scholar]