SUMMARY
Morphogenesis protein C (MorC) of Aggregatibacter actinomycetemcomitans is important for maintaining the membrane morphology and integrity of the cell envelope of this oral pathogen. The MorC sequence and operon organization was found to be conserved in Gammaproteobacteria, based on a bioinformatic analysis of 435 sequences from representative organisms. Functional conservation of MorC was investigated utilizing an A. actinomycetemcomitans morC mutant as a model system to express MorC homologs from four phylogenetically diverse representatives of the Gammaproteobacteria: Haemophilus influenzae; Escherichia coli; Pseudomonas aeruginosa; and Moraxella catarrhalis. The A. actinomycetemcomitans strains expressing the homologous proteins were assessed for sensitivity to bile salts, leukotoxin secretion, autoaggregation and membrane morphology. MorC from the most closely related organism (H. influenzae) was functionally identical to MorC from A. actinomycetemcomitans. However, the genes from more distantly related organisms restored some but not all A. actinomycetemcomitans mutant phenotypes. In addition, deletion mutagenesis indicated that the most conserved portion of the protein, the carboxyl terminus DUF490 domain, was necessary to maintain the integrity of the membrane. Deletion of the last ten amino acids of this domain of the A. actinomycetemcomitans MorC protein was sufficient to disrupt membrane stability and leukotoxin secretion. The data suggest that the MorC sequence is functionally conserved across Gammaproteobacteria and the carboxyl terminus of the protein is essential for maintaining membrane physiology.
Keywords: Membrane Morphology, Toxin secretion, TamB, YtfN, DUF490
INTRODUCTION
Aggregatibacter actinomycetemcomitans, a member of the Pasteurellaceae family, is a capnophilic, facultatively anaerobic bacterium implicated as the causative agent of human adult and localized aggressive periodontitis in adolescents (Fine et al. 2007; Socransky et al. 1998). In addition, A. actinomycetemcomitans, together with Haemophilus spp., Cardiobacterium hominis, Eikenella corrodens, and Kingella spp. are classified as HACEK organisms, which represent a group of oropharyngeal bacilli causing infective endocarditis (Paturel et al. 2004). A. actinomycetemcomitans is the most commonly isolated member of this group. This bacterium is also implicated in other systemic infections such as pneumonia and even brain infections (Rahamat-Langendoen et al. 2011; Scannapieco 1999)
The ability of this bacterium to survive within and colonize multiple tissues is highly dependent on the protein composition of the cell envelope. The protein/lipid composition of the envelope allows for the passage of specific molecules for growth and maintenance of homeostasis, while excluding environmental insults (Silhavy et al. 2010). A. actinomycetemcomitans expresses a novel membrane protein, morphogenesis protein C (MorC), that is essential for maintaining the distinct outer membrane morphology and membrane function of this organism. The deletion of this 141 kDa inner membrane protein in A. actinomycetemcomitans changes the membrane morphology from rugose to flat, reduces the secretion of leukotoxin posttranscriptionally, decreases cell size and increases autoaggregation (Gallant et al. 2008). Transformation with a replicating plasmid containing the endogenous morC gene restores all phenotypes and complemented strains are identical to wild-type (Gallant et al. 2008). Although the absence of MorC results in the pleiotropic phenotypes, analysis of the A. actinomycetemcomitans cell envelope composition indicates that the protein is found in low quantities and absence of this protein only affects a specific subset of membrane proteins (Smith et al. 2015). Interestingly, the proteins of the leukotoxin secretion apparatus and characterized autotransporter proteins are unchanged in the mutant (Smith et al. 2015).
morC in A. actinomycetemcomitans is a member of a three gene operon including an outer membrane protein (omp67) and an exopolysaccharide phosphatase (ppx) (Gallant et al. 2008). Bioinformatic analysis indicates conservation of the MorC sequence and operon organization in multiple phylogenetically and physiologically diverse bacterial families (Gallant et al. 2008; Selkrig et al. 2012). Work in representative organisms of the Enterobacteriaceae family suggests an additional role for a MorC homolog (TamB/YftN) in protein translocation of the Flu autotransporter to the outer membrane (Selkrig et al. 2012). The membrane-related phenotypes of the A. actinomycetemcomitans morC mutant and the presence of homologous sequences in other organisms suggest that MorC function is conserved across diverse Gammaproteobacteria. Although MorC appears to be integral to the maintenance of cellular homeostasis, little is known about the protein domains and the functional conservation of this protein.
In the present study, a complementation strategy was used to determine the functional conservation of MorC using A. actinomycetemcomitans as a model organism. Homologous morC sequences were amplified, transformed into an A. actinomycetemcomitans morC mutant strain and assayed for complementation of morC phenotypes. MorC from the most closely related organism was functionally identical to that from A. actinomycetemcomitans. Interestingly, MorC from more distantly related organisms restored some but not all mutant phenotypes. In addition, the carboxyl terminal DUF490 domain was necessary to maintain the integrity of the membrane. Deletion of the last 10 amino acids of this domain as well as conserved cysteine residues outside of the DUF490 domain were found to be required for proper protein function in A. actinomycetemcomitans. Taken together, these results suggest that MorC function is conserved across Gammaproteobacteria and the functional domain is associated with the carboxyl terminus of the protein.
MATERIALS AND METHODS
Bacterial Strains and Growth Conditions
Bacterial strains and plasmids used in this study are listed in Table 1. A. actinomycetemcomitans strain VT1169 (wild-type) was grown statically at 37°C in a humidified 10% CO2 atmosphere using TSBYE medium (3% trypticase soy broth, 0.6% yeast extract; Becton Dickinson, Franklin Lakes, NJ). Escherichia coli, Moraxella catarrhalis, and Pseudomonas aeruginosa were grown using LB medium (1% tryptone, 0.5% yeast extract, 0.5% NaCl; Becton Dickinson) with agitation at 37°C. H. influenzae was grown statically at 37°C in a humidified 5% CO2 atmosphere in BHI medium (3.7% brain heart infusion; Becton Dickinson) supplemented with 10 µg nicotinamide adenine dinucleotide ml−1 and hemin ml−1 (Sigma Aldrich, St. Louis, MO). Plasmids were maintained by addition to the medium of: 1 µg chloramphenicol ml−1 and 50 µg kanamycin ml−1 for A. actinomycetemcomitans; 50 µg kanamycin ml−1 and 20 µg chloramphenicol ml−1 for E. coli. Where necessary, 0.3 mM diaminopimelic acid (DAP) was added to the medium to maintain E. coli strain β-2163.
Table 1.
Bacterial strains and plasmids.
| Bacterial Strain or Plasmid |
Description | Reference or Source |
|---|---|---|
| E. coli | ||
| TOP10F-, DH10B | General cloning, lac− | Invitrogen |
| DH5α(λpir) | General cloning, λpir | |
| β-2163 | DAP auxotroph, λpir | (Demarre et al. 2005) |
| A. actinomycetemcomitans | ||
| VT1169 | Spontaneous rifampin and nalidixic acid resistant mutant of SUNY465 | (Mintz and Fives-Taylor 1994) |
| VT1650 | morC insertional inactivation strain. Specr | (Gallant et al. 2008) |
| KM555 | morC deletion strain. Specr | This study |
| morC complement | morC mutant containing pKM303-A.a. Cmr | (Gallant et al. 2008) |
| Plasmids | ||
| pCR2.1-TOPO | TA cloning vector that replicates only in E. coli. Ampr, Kanr | Invitrogen |
| pKM1 | E. coli and A. actinomycetemcomitans shuttle vector. Kanr | (Ruiz et al. 2006) |
| pKM2 | E. coli and A. actinomycetemcomitans shuttle vector. Cmr | (Gallant et al. 2008) |
| pVT1460 | Conjugative plasmid, replicates in λpir strains of E. coli, Kmr | (Mintz et al. 2002) |
| pKM550 | pVT1460 containing morC deletion construct | This study |
| pKM303 | pKM2 containing the 165bp morC promoter sequence. Cmr | (Gallant et al. 2008) |
| pKM475 | pKM1 containing the 165bp morC promoter sequence. Kanr | This study |
| pKM557 | pKM475 containing A. actinomycetemcomitans morC. Kanr | This study |
| pKM408 | pKM303 containing A. actinomycetemcomitans morC. Cmr | (Gallant et al. 2008) |
| pKM303 – H.i. | pKM303 containing the morC homolog from Haemophilus influenzae 86-028NP. Cmr | This study |
| pKM303 –E.c. | pKM303 containing the morC homolog from Escherichia coli K-12. Cmr | This study |
| pKM303 – P.a. | pKM303 containing the morC homolog from Pseudomonas aeruginosa PAO1. Cmr | This study |
| pKM303 – M.c. | pKM303 containing the morC homolog from Moraxella catarrhalis ATCC 43628. Cmr | This study |
| C26S | Substitution of cysteine 26 to serine in pKM475 | This Study |
| C103S, C110S | Substitution of cysteine 103 and 110 to serine in pKM475. | This Study |
| C732S, C742S | Substitution of cysteine 732 and 741 to serine in pKM475. | This Study |
| C732S | Substitution of cysteine 732 to serine in pKM475. | This Study |
| C741S | Substitution of cysteine 741 to serine in pKM475. | This Study |
| Δ1050–1292 | In-frame deletion of bp 3148 to 3876 of morC in pKM475 | This Study |
| Δ1235–1292 | In-frame deletion of bp 3703 to 3876 of morC in pKM475 | This Study |
| Δ1283–1292 | In-frame deletion of bp 3847 to 3876 of morC in pKM475 | This Study |
| Δ1292 | In-frame deletion of bp 3874 to 3876 of morC in pKM475 | This Study |
Amp = Ampicillin, Cm = Chloramphenicol, Kan = Kanamycin, Spec = Spectinomycin.
Construction of morC deletion strain
Preliminary data indicated an increase in the propensity for homologous recombination in the insertion mutant (VT1650), when transformed with replicating plasmids carrying truncations of the A. actinomycetemcomitans morC. Therefore, it was necessary to develop a morC deletion strain of A. actinomycetemcomitans to eliminate homologous recombination of the truncated gene. An isogenic mutant of VT1169 with the morC gene deleted was generated by conjugation using a non-replicating broad host range plasmid (Mintz et al. 2002). The plasmid constructed for conjugation in A. actinomycetemcomitans is based on the mobilizable plasmid pGP704 (Miller and Mekalanos 1988). The kanamycin resistance gene from pUC-4k (Pharmacia, Kalamazoo, MI) was used as a selective marker in place of β-lactamase. The kanamycin cassette was restricted by BamHI followed by treatment with Klenow (New England Biolabs, Ipswich, MA). The cassette was ligated with pGP704 that had been digested with EcoRV and treated with shrimp alkaline phosphatase (Amersham Life Sciences, Buckinghamshire, UK). The ligation mixture was transformed into electrocompetent DH5α(λpir) E. coli cells. The resulting plasmid, pVT1460, was used as the template for inverse PCR to delete the β-lactamase gene as described previously (Mintz et al. 2002).
A. actinomycetemcomitans genomic DNA from VT1169 was purified using the Puregene genomic DNA isolation kit (Qiagen, Valencia, CA) and used as a template for PCR. A 1.5 kb fragment of the downstream ppx gene was amplified with a 5’XbaI restriction site (Table 2) and ligated with the spectinomycin adenyltransferase aad9 gene, derived from pSL60 (Lukomski et al. 2000), amplified with primers containing 5’XhoI and 3’XbaI restriction sites (Table 2). A PCR product generated using the 5’ aad9 primer and the 3’ppx primer was ligated with the PCR2.1 TOPO vector (Invitrogen, Carlsbad, CA). The 5’XhoI/3’EcoRI PCR product was released and ligated with an amplified 1 kb fragment of the upstream omp67 containing a 3’XhoI restriction site. The construct was introduced into the PCR2.1 TOPO vector (Invitrogen, Carlsbad, CA) and transformed into electrocompentent TOP10F- cells. Plasmids were purified from selected colonies (Qiagen, Valencia, CA) and characterized by restriction mapping and agarose gel electrophoresis. Digestion with EcoRI released the corresponding ~3.3 kb band, which was purified using the Qiagen gel purification kit (Qiagen, Valencia, CA, USA). The DNA was ligated with pVT1460 previously restricted with EcoRI and treated with shrimp alkaline phosphatase (USB, Santa Clara, CA), and the mixture was transformed into electrocompetent DH5α(λpir). Plasmids were isolated from this strain and transformed into β-2163 E. coli cells, auxotrophic for diaminopimelic acid (DAP) (Demarre et al. 2005).
Table 2.
Oligonucleotide primers.
| Oligonucleotide | Sequence (5’ -3’) |
|---|---|
| HimorCF | GCAAAGCTTATGACTACAACCATCAG |
| HimorCR | GCTCTAGACTAAATATAAAGTAAATC |
| EcmorCF | CCGCTCGAGATGAATGGAAAAAAATC |
| EcmorCR | GCTCTAGACTAAACTCGAACTGATAG |
| PamorCF | CCGCTCGAGGTGAAGGCGCTGAAG |
| PamorCR | CCGGAATTCTCAGTCGCGCTTCAAGA |
| McmorCF | CCGCTCGAGATGACTCATCAGGATAATTC |
| McmorCR | GCTCTAGACTAAAACTTCCAACGATAA |
| Omp67F | TCTGGACGTATTGCTTTATCCGC |
| Omp67R | CTTCCTCGAGCTTATTATCCGTTCTTGTTGA |
| SpecF | TAAGCTCGAGTGACTAAATAGTGAGG |
| SpecR | CTTCTCTAGACATGTGATTTTCCTCC |
| PpxF | CTTCTCTAGATTATGAATAACGAAAATTTA |
| PpxR | TCAACGTGCCGACAGGCTTA |
| BamHI.P165.For | CGCGGATCCGCCCGTTTATTGAATCTACCTTCC |
| A.morC.C103S.For | GCAAATGCAATTAAGCAGCTTATGGAAATTAAAGGTTTGCG |
| A.morC.C110S.For | GCAAATGCAATTAAGCAGCTTATGGAAATTAAAGGTTTGCG |
| A.morC.C732S.For | GCCACTATTTCCGCGCACAGCTGGATACACAG |
| A.morC.C741S.For | CGGATTTAAGTTTCCCGCAAAGCTTTAAC |
| A.morC.C103S.Rev | CGCAAACCTTTAATTTCCATAAGCTGCTTAATTGCATTTGC |
| A.morC.C103S.Rev | CGCAAACCTTTAATTTCCATAAGCTGCTTAATTGCATTTGC |
| A.morC.C110S.Rev | CGCAAACCTTTAATTTCCATAAGCTGCTTAATTGCATTTGC |
| A.morC.C732S.Rev | CTGTGTATCCAGCTGTGCGCGGAAATAGTGGC |
| A.morC.C741S.Rev | GTTAAAGCTTTGCGGGAAACTTAAATCCG |
| MorC.SpHI.1049Rev | ACATGCATGCTTATTCGCTCGGCAATGTTTTC |
| MorC.SpHI.1234Rev | ACATGCATGCTTATCTGTCGCCCACCCCG |
| MorC.SpHI.1282Rev | ACATGCATGCTTATTGATTAACACCCGAAACGGATTG |
| MorC.SpHI.1291Rev | ACATGCATGCTTATTCAAATTGATAGAGTAG |
| MorC.SpHI.TTA.Rev | ACATGCATGCTTAAAATTCAAATTGATAGAGTAG |
Underlined sequences indicate restriction endonuclease sites.
Chromosomal deletion of morC
E. coli β-2163 containing pKM550 was used as a donor strain for conjugation with A. actinomycetemcomitans strain VT1169 as described by Gallant (2008) with modifications. Donor and recipient cells were grown to mid-logarithmic phase, centrifuged, washed and suspended at a 1:1 ratio in 100 µl TSBYE. The bacterial suspension was spotted onto a TSBYE plate containing DAP and air dried prior to incubation for 6 hours at 37°C in a humidified 10% CO2 environment. After incubation, cells were plated onto TSBYE agar lacking DAP and containing 50 µg spectinomycin ml−1 for counter selection of the donor and recipient cells, respectively. Double homologous recombination events were identified by antibiotic resistance profiles and confirmed by PCR.
Modification of the morC sequence and amplification of morC homologs
Designated in-frame deletion constructs were generated by PCR using relevant primers (Table 2). Single nucleotide substitution mutants were generated using a commercially available mutagenesis kit following the manufacturer’s instructions (Stratagene, La Jolla, CA). All plasmids were transformed into the morC deletion strain by electroporation (Gallant et al. 2008).
Genomic DNA from all organisms was prepared using the Puregene genomic DNA isolation kit (Qiagen, Valencia, CA) and used as a template for PCR with associated primers (Table 2) at an annealing temperature of 58°C. Products were introduced into the PCR2.1 TOPO vector (Invitrogen, Carlsbad, CA), transformed, and colonies were selected on LB agar containing kanamycin. Plasmids from selected transformants were isolated (Qiagen, Valencia, CA) and the amplicon was confirmed by DNA sequencing. morC was isolated and ligated into the appropriate restriction sites of a shuttle vector containing the morC promoter (Gallant et al. 2008). Transformants were screened by PCR and plasmids were transformed into the morC− strain of A. actinomycetemcomitans by electroporation (Gallant et al. 2008). All constructs were verified by nucleotide sequencing before and after transformation into A. actinomycetemcomitans at the Vermont Cancer Center DNA Analysis Facility, University of Vermont.
Detection of A. actinomycetemcomitans MorC
Affinity purified polyclonal antiserum raised against the amino terminal half of the protein (Smith et al. 2015) was used to determine synthesis of MorC in strains expressing the modified forms of the protein. For whole cell lysate preparations, cells were grown to mid-logarithmic phase (OD495 = 0.2–0.3), collected by centrifugation and lysed by the addition of SDS. Protein concentrations were determined by the BCA method (Pierce, Rockford, IL). Membrane isolation was performed based on the method previously described (Smith et al. 2014). Cells were grown to mid-logarithmic phase cells (OD495 = 0.3) in liquid culture (250 ml) and lysed using a French pressure cell (Thermo Scientific, Waltham, MA) at 18,000 psi. Cell debris was removed by centrifugation at 12 000 g for 20 min. Membranes were recovered by repeated centrifugation at 100 000 g for 30min with the intermediary pellets suspended in PBS (10mM sodium phosphate, 150 mM NaCl, pH7.4). Equivalent amounts of protein were resolved by SDS-PAGE and transferred to PVDF membranes (Millipore, Billerica, MA)(Smith et al. 2015). Immunoblots were imaged using an Odyssey CLx infrared imaging system (LiCor Biosciences, Lincoln NE).
Autoaggregation assay
Autoaggregation was monitored using an assay modified from Ulett et. al (2007). A single colony of A. actinomycetemcomitans was inoculated into 5 ml TSBYE broth and grown overnight. The next day cells were suspended by gentle swirling for 10 s and the OD495 was recorded immediately using a Thermo Scientific Spectronic 20+ spectrophotometer with a culture tube adaptor (ThermoFisher Scientific, Waltham, MA) as time point 0. Cells were placed at 4 °C to prevent additional growth and OD495 readings were taken after 15 and 30 min. The optical density readings were standardized by setting the initial time point to 100%. Data for each strain was taken from a minimum of three trials and representative data was graphed.
Bile salt assay
Sensitivity to bile salts was measured using a broth microdilution assay based on the method of Floyd et al. with modifications (Floyd et al. 2010). Concentrations ranging from 20 mg bile salts ml−1 to 0.2 mg bile salts ml−1 (50% glycoholic acid, 45% taurocholic acid; ThermoFisher Scientific, Waltham, MA) were generated by two-fold serial dilution of a stock solution (20 mg ml−1) in TSBYE broth in a sterile 96-well tissue culture plate (Nunc, Roskilde, Denmark). Overnight A. actinomycetemcomitans cultures were diluted to OD495 = 0.1 and 20 µl (approximately 106 cells) were added to each well to a final volume of 200 µl. Plates were incubated for 24 hours and the minimal inhibitory concentration (MIC) was determined.
morC deletions and substitution mutants were tested for sensitivity to bile salt by relative growth in 2.5 mg bile salts ml−1. Growth in bile salt was performed as above and measured at OD495 using an ELx 800 plate reader (Biotek, Winooski, VT). The effect of bile salt on bacterial growth was expressed as a percentage (OD495(bile salt)/ OD495(no bile salt) × 100%). Strains were compared by ANOVA with Dunnett’s post-test with significance defined as a p-value < 0.05 (GraphPad Prism 6.0, GraphPad Software, La Jolla, CA).
Leukotoxin detection
Leukotoxin was detected using the method of Tang and Mintz (2012). Cell associated toxin was determined using mid-logarithmic phase cultures of A. actinomycetemcomitans (OD495 = 0.3). Whole cell lysates were prepared, and aliquots removed for protein determination (BCA; Pierce, Rockford, IL). Secreted leukotoxin was prepared by filtration (0.22 µM filter; Corning Life Sciences, Tewksbury, MA) of 4 ml of growth medium concentrated 40-fold by ultrafiltration (50 kDa molecular weight cutoff; Millipore, Billerica, MA). Proteins, representing 20 µg of whole cell lysate or secreted proteins from 1×109 cells, were separated by electrophoresis on 4–15% gradient denaturing gels (Bio-Rad, Hercules, CA). For morC truncation and substitution mutants, secreted leukotoxin was normalized to whole cell lysate protein concentration (BCA assay, Pierce, Rockford, IL) as a measure of cell number. Proteins were separated by SDS-PAGE, transferred to PVDF or Immobilon-FL membranes (Millipore, Billerica, MA) and probed with an anti-leukotoxin antibody (provided by E. T. Lally, University of Pennsylvania). Immune complexes were bound by horseradish peroxidase or IRDye 800CW conjugated goat anti-rabbit antibodies (Jackson ImmunoResearch, West Grove, PA and LiCor Biosciences, Lincoln NE, respectively). Detection was completed by addition of SuperSignal West Pico substrate (Pierce, Rockford, IL) followed by exposure to film (Eastman Kodak, Rochester, NY) or by secondary antibody fluorescent emissions measured using the Odyssey CLx system (LiCor Biosciences, Lincoln NE).
Ultrastructure analysis
Membrane ultrastructure was visualized by transmission electron microscopy (TEM) of negatively stained whole mount bacterial preparations as described previously (Azari et al. 2013). Bacteria were grown overnight in the appropriate broth medium. Cultures were diluted 1:10 with sterile media and allowed to grow to log phase (OD495 = 0.2). A 2 ml aliquot of cells was removed and centrifuged at 800 × g for 5 minutes at 4°C. The supernatant was removed and the cell pellet was gently suspended in cold phosphate buffered saline (PBS, 10mM sodium phosphate, 150 mM NaCl, pH7.4). A 5 µl aliquot of the cell suspension was added to carbon coated copper grids (400 mesh), rinsed with PBS, and stained with Nano-W (Nanoprobes, Yaphank, NY). The representative Gammaproteobacteria used for the morC homolog studies were visualized from colonies grown overnight on agar plates. Cells were transferred to carbon coated grids by first placing 5 µl of PBS on a group of colonies and then lowering a grid, carbon-side down, on top of the humidified colonies. 3 µl of buffer was added to the grid containing the transferred colonies to prevent drying and were further processed as described above prior to visualization. Grids were observed on a Tecnai 12 electron microscope (FEI, Portland, OR) operating at an acceleration voltage of 100 kV. Images were collected at nominal magnifications of 52 kx on a 2,048-by-2048-pixel charge-coupled camera with a pixel size of 14 µm (TVIPS, Gaunting, Germany).
Bioinformatic analysis
The BLASTP algorithm (e-value cutoff = 10−50) (Altschul et al. 1990) was used to select homologous MorC sequences from the NCBI database using as query the A. actinomycetemcomitans sequence (Genbank Accession: AAY86707.1). Multiple phylogenetic trees were constructed using either neighbor joining, maximum likelihood or minimal evolution methods in the MEGA5 software package (Tamura et al. 2011). Transmembrane and signal anchor domains were predicted using Transmembrane Helix prediction based on hidden Markov Models (TMHMM) (Krogh et al. 2001). Conserved domains were identified using the NCBI Conserved Domain Search algorithm (Marchler-Bauer et al. 2011). Multiple sequence alignments were conducted using CLUSTALW or COBALT (Papadopoulos and Agarwala 2007; Sievers et al. 2011). Amino acid sequence of MorC was compared to similar sequences in solved 3-D strucutres using HHPred (Soding et al. 2005). Predicted organization of the morC operon was retrieved from the Prokaryotic Operon Database (Taboada et al. 2012; Taboada et al. 2010).
RESULTS
Bioinformatic analysis of MorC
MorC plays an important role in the architecture of the outer membrane of A. actinomycetemcomitans (Gallant et al. 2008). A BLAST search revealed a total of 435 MorC homologs from a variety of Gram-negative bacteria. In this study, four MorC homologs from clinically relevant pathogens were used for further analysis. In order of phylogenetic relatedness to A. actinomycetemcomitans, the sequences chosen were: H. influenzae of the Pasteurellaceae (Genbank Accession: YP_248384.1); E. coli of the Enterobacteriaceae (NP_418642.1); P. aeruginosa of the Pseudomonadaceae (NP_251232.1); and M. catarrhalis of the Moraxellaceae (EKF83187.1). All the translated protein sequences have relatively the same size (1,200–1,300 amino acids) except for the M. catarrhalis protein, which contains 1696 amino acids. In relation to the A. actinomycetemcomitans MorC sequence, the sequence homology (identical and conserved residues) varied among the proteins: 69% (H. influenzae), 57% (E. coli), 46% (P. aeruginosa) and 42% (M. catarrhalis).
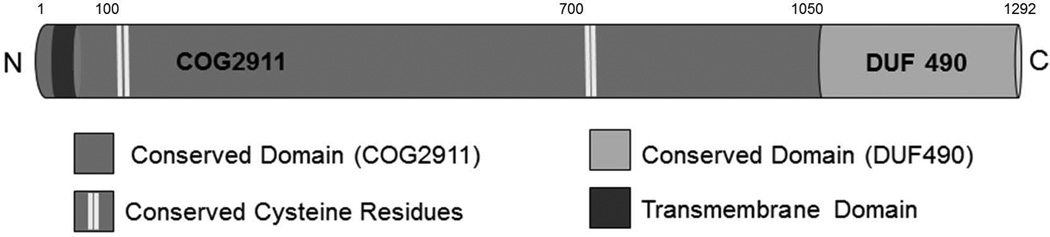
Analysis of the protein sequences revealed two conserved domain architectures despite primary sequence variability (Figure 1). An N-terminal domain belonging to the cluster of orthologous group, COG2911, is present in all sequences. A single transmembrane segment is predicted (TMHMM) in this domain, which supports the inner membrane localization previously reported (Gallant et al. 2008; Selkrig et al. 2012). In the A. actinomycetemcomitans MorC sequence five cysteines are present: C26, C103, C110, C732 and C741. The C26 is present only in the most closely related homolog of H. influenzae. While the Moraxella sequence contains only the second pair of conserved cysteines, the rest of the sequences contain two pairs of conserved cysteines: C103, C110 and C732, C741. The second conserved domain is located at the C-terminus and is categorized as DUF490, a domain of unknown function. This region of the protein contains the greatest homology: 85% (H. influenzae), 53% (E. coli), 58% (P. aeruginosa) and 50% (M. catarrhalis).
Figure 1. Diagram of a prototypic MorC.
Domains common to all MorC proteins are shown to scale. Domain structure was predicted using the NCBI conserved domain search tool. Conserved cysteines were identified using CLUSTALW and transmembrane helices were predicted using the TMHMM algorithm.
Conservation of the primary amino acid sequence is also predicted to be maintained at the structural level. Protein structure prediction based on solved 3D structures (HHPred) indicated shared structural conformations between the five sequences. The A. actinomycetemcomitans and H. influenzae sequence predictions are similar in the transmembrane domain and amino acids 880–1012 (Table 4). The remaining proteins share these conformations and contain unique structural elements. The E. coli protein contains unique elements between amino acids 1059–1120. The P. aeruginosa protein contains elements between amino acids 1015–1062 and is the only sequence with a predicted structure at the carboxyl terminal end of the protein (1160–1221). The M. catarrhalis structure prediction is more closely related to A. actinomycetemcomitans and H. influenzae but contains additional elements between amino acids 180–265 (also present in the P. aeruginosa protein) and 468–716 (present in P. aeruginosa and E. coli).
Table 4.
MorC three-dimensional structure predictions based on HHPred.
| Aa | Hi | Ec | Pa | Mc | Reference Structure |
|---|---|---|---|---|---|
| *1–18 | 1–19 | 1–29 | 1–22 | - | §2JPW (1–23), 2JPW (1–23), 1V54 (13–41), 1M56 (29–50) |
| 21–43 | 24–46 | - | - | - | 1SPF (10–32), 1XRD (2–41) |
| - | - | - | - | 34–63 | 3HD7 (79–108) |
| - | - | - | 146–201 | - | 2NQN (66–123) |
| - | - | 515–634 | 453–554 | 485–695 | 3PET (53–178), 3LYC (96–209), 3LYC (50–220) |
| 880–1012 | 879–1011 | 857–988 | 830–961 | 1258–1389 | 3LYC (85–239) |
| - | - | - | 1015–1062 | - | 4FZL (168–240) |
| - | - | 1059–1120 | - | - | 3BB6 (34–97) |
| - | - | - | 1160–1221 | - | 4FQE (56–115) |
| - | - | - | - | 1323–1502 | 3SZE (537–514) |
Numbers represent the amino acid sequence from the MorC proteins.
PDB IDs for the reference structures are listed in order from left to right. Numbers in parentheses indicate the corresponding sequence of the reference structure.
“-“absence of predicted structural homologs
Characterization of the heterologous expression of morC from different genera of bacteria in the A. actinomycetemcomitans morC mutant strain
Afimbriated A. actinomycetemcomitans grows relatively homogenously in broth. In contrast, the morC mutant strain exhibits autoaggregation and grows in macroscopic clumps (Gallant et al. 2008). This phenotype explains the difference in the maximum optical density of the isogenic mutant (OD495 = 0.45) when compared with the parent strain (OD495 = 0.65)(Figure 2A). The growth phenotype, particularly the lag phase, was differentially impacted by the introduction of the various morC homologs. The strain containing the H. influenzae gene consistently displayed a higher maximum optical density and a more rapid growth rate than the A. actinomycetemcomitans complemented strain. Strains complemented with P. aeruginosa or M. catarrhalis morC displayed maximal growth similar to the strain complemented with A. actinomycetemcomitans morC. However, during the lag phase of growth (time points between 0 and 12 hours), these strains consistently displayed optical densities less than that of the A. actinomycetemcomitans complemented strain. The E. coli morC strain displayed growth characteristics similar to the A. actinomycetemcomitans mutant. Based on these growth curves, all subsequent experiments were performed using cells in early logarithmic phase (OD495nm = 0.2–0.3).
Figure 2. Representative growth curves and autoaggregation of transformed A. actinomycetemcomitans strains.
A. Overnight cultures of A. actinomycetemcomitans morC mutant strains containing homologous morC genes on a replicating plasmid were diluted to OD495 = 0.1 and incubated at 37 °C in a 10% CO2 atmosphere for 24 hours. Optical density was recorded at the time points indicated and data is representative of at least three independent experiments. B. Cells were grown overnight in broth and gently suspended. OD495 readings were taken at 0, 15, and 30 minutes and standardized to time point 0 as 100%. Percentages indicate the relative amount of cells in solution at the indicated time point. Data is representative of three independent experiments. Legend: A. actinomycetemcomitans morC mutant containing, empty vector (square), endogenous morC (cross), H. influenzae morC (plus), E. coli morC (circle), P. aeruginosa morC (diamond), M. catarrhalis morC (triangle).
Autoaggregation of each strain was assayed by monitoring the percentage of cells remaining in the supernatant of a well-mixed culture over time using optical density measurements (Figure 2B). Fewer cells in suspension indicates an increase in aggregation. In the morC mutant, 30% of cells remain in suspension after half an hour compared to 80% of cells complemented with endogenous morC. Strains transformed with morC from H. influenzae, E. coli, or M. catarrhalis, exhibited autoaggregation similar to a strain complemented with the endogenous morC with 70% to 80% of cells remaining in the supernatant. Interestingly, mutant cells transformed with Pseudomonas homologs displayed no discernable autoaggregation and all cells remained in suspension during the time period tested.
Bile salt sensitivity was determined to examine the relative membrane stability of the different morC expressing strains. The minimal inhibitory concentration (MIC) of the wild-type A. actinomycetemcomitans was shown to be 10 mg bile salts ml−1. The bile salt sensitivity of the other bacteria used in this study was determined to be > 20 mg bile salts ml−1. Inactivation of the A. actinomycetemcomitans morC results in a four-fold reduction in the MIC to 2.5 mg bile salts ml−1. Mutants transformed with the homologous morC sequences were tested for the restoration of the bile salt phenotype. The H. influenzae homolog rescued the bile salt sensitivity phenotype and restored the MIC to wild-type levels. This is in comparison to the remaining homologs, which did not restore bile salt sensitivity and the MIC remained at mutant levels (data not shown).
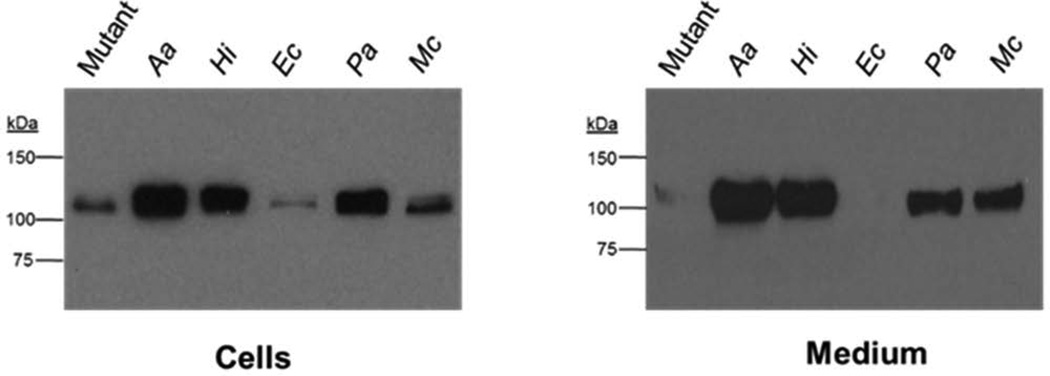
The loss of MorC in A. actinomycetemcomitans decreases the amount of both intracellular and secreted leukotoxin (Gallant et al. 2008). Intracellular and secreted toxin was detected by immunoblot using a leukotoxin-specific antibody (Figure 3). Leukotoxin was found to be reduced the morC mutant cells relative to a control producing A. actinomycetemcomitans MorC. Complementation with the heterologous gene from H. influenzae restored the secretion of the toxin in both compartments. Conversely, transformation of the mutant with the E. coli homolog showed no detectable increase in leukotoxin abundance and the amount appeared to be reduced when compared with the A. actinomycetemcomitans mutant. The strains expressing the P. aeruginosa homolog were observed to contain increased amounts of toxin in both compartments compared with the mutant strain. In the M. catarrhalis expressing strain, leukotoxin was only slightly elevated above mutant levels. Interestingly, the magnitude of the increase was substantially greater in the medium.
Figure 3. Immunoblot analysis of leukotoxin synthesis and secretion in morC homologs.
Strains were grown to mid-log phase and 20 µg of protein (cell associated toxin) or secreted protein from 109 cells (secreted toxin) was probed with anti-leukotoxin antibody. Immunoblots are representative of three separate experiments. A. actinomycetemcomitans morC mutant (Mutant), morC mutant complemented (Aa), H. influenzae morC (Hi), E. coli morC (Ec), P. aeruginosa morC (Pa), and M. catarrhalis morC (Mc).
The outer membrane of A. actinomycetemcomitans displays a rugose or convoluted phenotype when whole bacteria are observed by TEM. This membrane phenotype is shared by some but not all of the parent bacteria used in this investigation. H. influenzae, M. catarrhalis, and E. coli were all observed to have a rugose morphology. In contrast to these organisms, the outer membrane of P. aeruginosa displays a flat morphology (data not shown). Inactivation of the A. actinomycetemcomitans morC results in a change in the outer membrane from a rugose to a flat morphology (Figure 4). The wild type phenotype can be restored by transformation with the A. actinomycetemcomitans morC on a replicating plasmid containing the endogenous promoter. Transformation of the A. actinomycetemcomitans mutant with a plasmid encoding the homologs of MorC from H. influenzae and E. coli resulted in complementation of the membrane phenotype and displayed wild-type features. Homologous MorC from M. catarrhalis resulted in a subpopulation of cells with a wild-type outer membrane morphology, while the majority retained a flat outer membrane. In contrast, transformation with a similar plasmid encoding the P. aeruginosa homolog resulted in a flat outer membrane. In this strain, a number of elongated and t-shaped cells were observed, suggestive of a defect in cell division. A summary of phenotypes for each strain is presented in Table 3.
Figure 4. Electron micrographs of negatively stained whole mount preparations of morC complementation strains.
Transmission electron micrographs of representative A. actinomycetemcomitans cells transformed with the morC homologs. A. actinomycetemcomitans morC mutant (Mutant), morC mutant transformed with morC from: A. actinomycetemcomitans (Aa), H. influenzae (Hi), E. coli (Ec), P. aeruginosa (Pa), and M. catarrhalis (Mc). Scale bar = 100 nm.
Table 3.
Summary of phenotypes.
| Source of morC | Bile Salt Resistance |
Leukotoxin | Membrane Morphology |
Maximal Growth |
Aggregation |
|---|---|---|---|---|---|
| morC mutant | − | − | − | − | − |
| A. actinomycetemcomitans | + | + | + | + | + |
| H. influenzae | + | + | + | + | + |
| E. coli | − | − | + | − | + |
| P. aeruginosa | − | + | − | + | + |
| M. catarrhalis | − | + | + | + | + |
Characterization of the DUF490 domain and conserved cysteine residues of A. actinomycetemcomitans MorC
The heterologous expression of morC from different genera of bacteria can substitute for the A. actinomycetemcomitans gene in the restoration of most of the mutant phenotypes. The DUF490 domain is the most highly conserved region of the protein. To determine if this domain is required for the physiological functions associated with this protein, amino acid substitution and deletion analysis were performed.
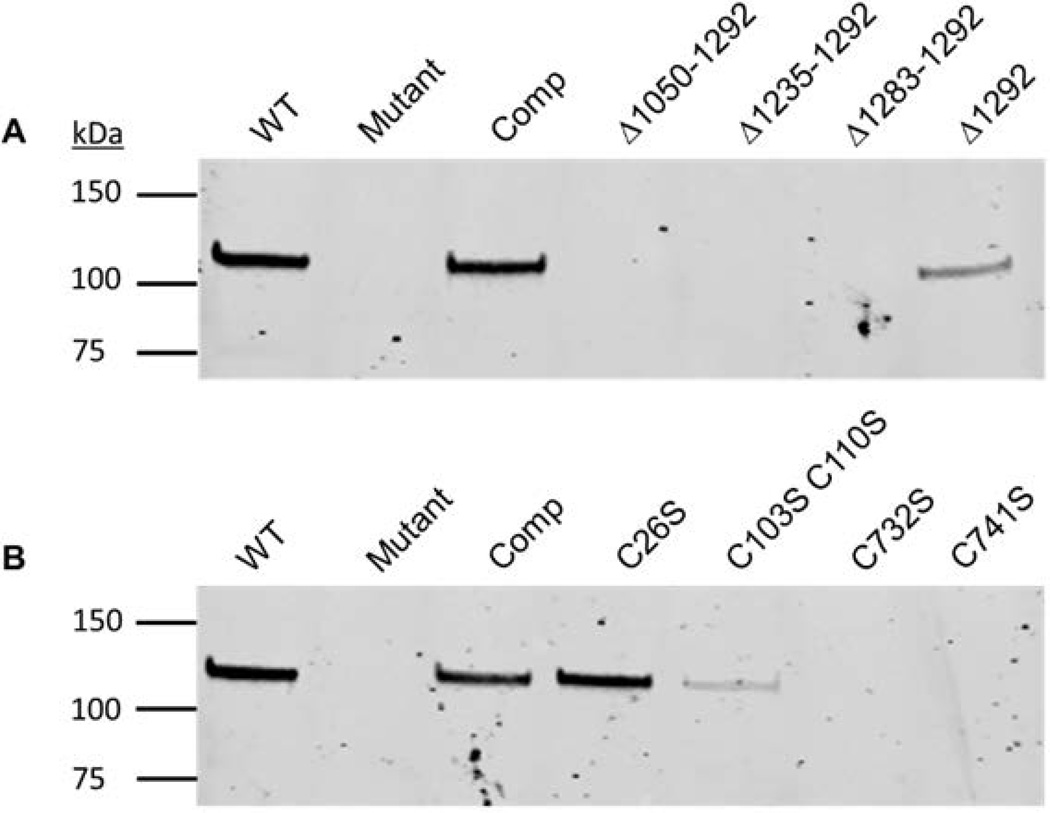
Truncated proteins corresponding to different regions of the DUF490 domain, based on secondary structure predictions, were generated by in-frame deletions of the corresponding nucleotide sequences and expressed in the morC mutant strain. The strain expressing MorC with the DUF490 domain (residues 1050–1292) deleted displayed phenotypes identical to the mutant strain for both leukotoxin secretion and bile salt sensitivity (Figure 5 A & 6, respectively). Similar phenotypes were also observed in a strain expressing the Δ1235–1292 construct. However, deletion of the last 10 amino acids (Δ1283–1292: AFDLLYQFEF) resulted in a strain displaying intermediate bile salt sensitivity, which is significantly different from both the strain expressing A. actinomycetemcomitans morC and mutant strains (Figure 5, ANOVA with Dunnett’s post-test, p<0.05). Interestingly, this strain did not restore leukotoxin secretion (Figure 6) and showed a mixed population of cells with rugose and flat, mutant-like, outer membranes (data not shown). The strain expressing a protein containing a deletion of the terminal phenylalanine (Δ1292) secretes more leukotoxin than the mutant and partially complements the leukotoxin secretion phenotype.
Figure 5. Immunoblot analysis of leukotoxin secretion in A. actinomycetemcomitans MorC truncation and substitution mutants.
Secreted leukotoxin was prepared by filtration of 4 ml of growth medium from mid-log phase cells and concentrated 40-fold by ultrafiltration. Samples were normalized to whole cell lysate protein concentration as a measure of cell number and immunoblots were probed with anti-leukotoxin antibody. Immunoblots are representative of three separate experiments. A. A. actinomycetemcomitans morC mutant strains containing truncations in the DUF490 domain. B. A. actinomycetemcomitans morC mutant strains containing cysteine substitutes.
Figure 6. Bile salt sensitivity of A. actinomycetemcomitans MorC truncation and substitution mutants.
Sensitivity to bile salts was measured in a 96 well plate growth assay. Mid-logarithmic phase cells were diluted into 2.5 mg bile salts ml−1 and growth for 24 hours. Optical density was measured at 495 nm. Growth of each strain in bile salt was compared to a control in TSBYE alone and expressed as a percentage. The (*) symbol indicates a significant difference from the strain expressing endogenous morC (ANOVA, Dunnett’s post-test, p < 0.05).
The role of the cysteine residues in determining the functionality of the A. actinomycetemcomitans protein was determined in complementation assays using substitution mutagenesis (conversion of cysteine to a serine residue) of the protein. Strains expressing proteins with substitutions at C26S (Figure 5B & 6), C103S, C110S (data not shown), or the C103S/C110S double mutant (Figure 5B &6) were indistinguishable from the wild type strain. However, expression of proteins with a substitution of either cysteine at 732 or 741 with serine (data not shown), or the double substitution construct (Figure 5 B & 6), failed to complement the mutant phenotypes.
MorC production was verified in the truncation strains by probing an immunoblot with affinity purified polyclonal anti-MorC serum. MorC of the expected size was found in the whole cell lysate preparations of all strains expressing a truncated protein (Figure 7A). However, we were unable to detect MorC production in the strains expressing MorC with cysteine substitutions C732S and C741S (Figure 7B). We tested for membrane localization of MorC in strains that produced the protein in whole cell lysate samples and displayed mutant phenotypes. Membrane-associated MorC of the expected molecular mass was detected in all of these samples (Figure 7C).
Figure 7. MorC expression in A. actinomycetemcomitans truncation and substitution mutants.
Strains were grown to mid-logarithmic phase and 20 µg of whole cell lysate or 50 µg of whole membrane proteins were probed with affinity purified polyclonal antiserum. Bacterial cell envelopes were prepared for strains that exhibited MorC expression in whole cell lysate preparations but were otherwise mutant for MorC phenotypes. The * indicates the predicted position of the 141 kDa MorC protein A. Whole cell lysates of carboxyl-terminal truncations of the DUF490 domain: Δ1050–1292 (115 kDa), Δ1235–1292 (134 kDa) Δ1283–1292 (139 kDa) and Δ1292 (141 kDa). B. Whole cell lysates of cysteine substitutes. C. Cell envelope preparations of carboxyl-terminal truncations.
DISCUSSION
The bacterial membrane protein composition is affected by physiological and environmental demands. Particular subsets of proteins are present or conserved to fulfill general membrane functions. Cellular homeostasis is maintained by the targeting and insertion of these proteins into the membrane. A variety of macromolecular complexes ensures proper protein integration and results in the generation of a functional membrane. We have previously identified a novel protein, MorC, in A. actinomycetemcomitans that impacts membrane morphology, barrier function and protein secretion (Gallant et al. 2008). Recently, MorC has been implicated in the modulation of fimbriae secretion and the architecture of the biofilm associated with fimbrial production (unpublished data). A homologous protein in the Enterobacteriaceae, TamB, has also been shown to be involved in protein secretion to the outer membrane (Selkrig et al. 2012). The MorC protein sequence is highly conserved across species and a bioinformatic analysis was used to select sequences present in Gammaproteobacteria to study the functional conservation of this protein.
MorC sequences from all families of Gammaproteobactera investigated display the same arrangement of two conserved domains. A single transmembrane helix consisting of ~20 amino acids (25–42 in A. actinomycetemcomitans and found in most of the species) is predicted at the N-terminal region of the protein within the COG2911 domain. This domain is found in hundreds of Gram-negative bacterial proteins, yet has no known function. The COG2911 is distinct from any other COGs and is not assigned to a protein family (Marchler-Bauer et al. 2011; Punta et al. 2012). A second conserved domain, DUF490, is predicted to reside at the C-terminus of all investigated MorC homologs and exhibits the greatest sequence homology between MorC proteins. In contrast to COG2911, DUF490 is assigned to the AsmA (assembly suppressor mutations) protein family. AsmA is the prototypic protein of this family and is localized to the inner membrane of E. coli (Deng and Misra 1996). E. coli AsmA is involved in the insertion of proteins into the outer membrane (Deng and Misra 1996). Inactivation of the morC homolog in Citrobacter rodentium affects the display of a specific autotransporter on the cell surface (Selkrig et al. 2012). However, MorC does not appear to influence the integration of autotransporters in A. actinomycetemcomitans as the trimeric autotransporter EmaA was still observed attached to the outer membrane of the morC mutant (Azari et al. 2013; Gallant et al. 2008; Yu et al. 2009). A second autotransporter, Aae, was found to be equal in abundance between the wild-type and morC mutant (Smith et al. 2015). Interestingly, no AsmA homologs are predicted in the Pasteurellaceae as determined by BLAST analysis. Collectively, the available data implies that MorC is the only AsmA-like protein in A. actinomycetemcomitans and therefore could play a central role in membrane organization in this organism.
Resistance to chaotropic agents such as detergents or bile salts determines membrane stability (Nikaido 2003). Bile salt sensitivity is mediated by outer membrane proteins, which serve to stabilize the membrane by precluding intercalation of solubilizing agents or playing a role in active efflux (Begley et al. 2005). The ability of the H. influenzae morC gene product to restore the MIC in the A. actinomycetemcomitans mutant suggests a similar mechanism for bile salt resistance in the Pasteurellacae, which may be dependent on a membrane protein composition that is not shared by distantly related organisms. The MorC derived from non-Pasteurellacae bacteria may not properly interact with and assemble the outer membrane proteins required for bile salt resistance found in A. actinomycetemcomitans.
Some secretion systems are maintained among diverse bacterial species. Hemolytic strains of E. coli secrete a repeats in toxin (RTX) or hemolysin that is dependent on a type I secretion system. Secretion requires the assembly of an ATPase (HlyB), a periplasmic channel (HlyD) and an outer membrane trimeric protein controlling secretion (TolC) (Delepelaire 2004). To the best of our knowledge, no accessory proteins associated with type I secretion systems have been identified in E. coli. The secretion of LtxA via an analogous type I secretion system in A. actinomycetemcomitans is dependent on the presence of MorC in the membrane, as indicated by the reduced levels of toxin secreted by the morC mutant. However, a similar role for MorC in a hemolytic E. coli strain is not evident as inactivation of morC in E. coli did not affect hemolysin secretion (data not shown). Therefore, the A. actinomycetemcomitans MorC appears to play a distinct role in the secretion of a type I effector molecule when compared with a homologous E. coli effector molecule secreted by an analogous system.
MorC homologs expressed in the A. actinomycetemcomitans mutant strain demonstrated the functional diversity of this protein. The expression of MorC derived from P. aeruginosa, an organism that secretes type I effector molecules (Duong et al. 2001; Guzzo et al. 1991; Wandersman and Delepelaire 2004) restored LtxA secretion. In addition, homologs derived from organisms expressing closely (H. influenzae) or more distantly (M. catarrhalis) related MorC proteins, which do not have characterized type I secretion systems, also restored leukotoxin secretion in the A. actinomycetemcomitans mutant strain. In contrast, the bacteria expressing the E. coli MorC homolog failed to restore LtxA secretion. Disparity in the function of E.coli MorC is further illustrated by the inability of this homolog to restore LtxA secretion when expressed in the A. actinomycetemcomitans mutant strain. The inability of the E. coli MorC homolog to restore LtxA secretion may be attributed to the presence of a structural element that is absent in the other proteins. Based on structure predictions by HHPred, a distinct structural element composed of beta sheets and random coils is predicted between amino acids 1059–1120 of the E. coli sequence, which is not present in the other sequences (Table 4). This additional element may change the conformation of the E. coli MorC structure in such a manner that the protein does not interact either directly with a protein(s) of the secretion apparatus or indirectly with other proteins required for maintaining a membrane environment competent for LtxA secretion.
Electron microscopy images of A. actinomycetemcomitans reveal a rugose appearance of the bacterial membrane (Azari et al. 2013; Gallant et al. 2008). The rugose or convoluted surface of the bacteria is a trait shared with other members of the Pasteurellaceae and the Moraxellaceae families (data not shown). The molecules involved in the formation of membrane convolutions have not yet been identified. However, our data supports a role for MorC in the biogenesis of these structures. Inactivation of the A. actinomycetemcomitans morC renders the surface of the bacterium smooth, without obvious convolutions in 2D electron micrographs (Azari et al. 2013; Gallant et al. 2008). The smooth outer membrane morphology of the mutant strain can be restored to the rugose or wild-type membrane phenotype by plasmids expressing morC derived from A. actinomycetemcomitans, H. influenzae, E. coli and M. catarrhalis. Expression of the P. aeruginosa morC in the mutant strain did not restore the rugose phenotype associated with the A. actinomycetemcomitans parent strain. The inability of the MorC from P. aeruginosa to restore the morphology of the outer membrane may be attributed to the formation of a structural element at the carboxyl terminus of the sequence (amino acids 1160–1221), which is absent in all other MorC sequences investigated. The change in the conformation of the protein may interfere with the binding of the P. aeruginosa MorC directly or indirectly to proteins that are involved in the formation of the convolutions associated with A. actinomycetemcomitans strains. Alternatively, the inability of the P. aeruginosa MorC to restore convolutions maybe related to the inherent lack of convolutions observed in P. aeruginosa.
Cells over-expressing P. aeruginosa or M. catarrhalis MorC exhibited aberrant cell division and altered cell shape. These observations may explain the difference in the growth curves of these strains when compared with the parent or mutant strains. These changes suggest the transcription and translation of the homologous genes in A. actinomycetemcomitans. Semi-quantitative RT-PCR indicated that all morC genes are equally transcribed (data not shown). However, we have not been successful in visualizing the synthesis of MorC homologs with N-terminal epitope tags (6xHis, FLAG, or HA) whereas the synthesis of A. actinomycetemcomitans MorC with the identical tags are easily detected (data not shown). Nevertheless, the ability of these constructs to complement some or all of the mutant phenotypes implies the synthesis and membrane localization of MorC.
A signature sequence, present in the majority of outer membrane proteins (Struyve et al. 1991) necessary for the interaction with BamA/Omp85, is present in the A. actinomycetemcomitans MorC sequence. The sequence contains a terminal phenylalanine (phe) and a hydrophobic residue at positions -3,-5,-7 and -9 relative to the C-terminus of the protein. The terminal phe is important for the function of the prototypic protein (PhoE)(de Cock et al. 1997). The MorC sequence, AFDLLYQFEF, contains the requisite Phe (F) at the C-terminal and hydrophobic amino acids at positions -3 (F), -5 (Y),-7(L) and -9(F) relative to the C-terminus of the protein. Expression of a protein with this sequence deleted did not restore the mutant phenotypes to wild type, indicating the importance of this sequence for the function of MorC. Unlike the prototypic protein, deletion of the C-terminal Phe of the MorC sequence is not essential for restoration of bile salt resistance (Figure 6) but may play a role in leukotoxin secretion (Figure 5). Together, the data suggest that the DUF490 domain, in particular the terminal 10 amino acids, is essential for membrane function in A. actinomycetemcomitans.
In addition to the high degree of conservation found in the DUF490 domain, MorC also contains a pair of cysteines in the COG2911 domain (C103, C110) that are conserved among some species of Gammaproteobacteria. Expression of proteins containing mutation of these residues does not appear to be important in protein structure or function. Similarly, a cysteine (C26) found in A. actinomycetemcomitans and H. influenzae in the predicted signal anchor domain, was also shown not to be important for the MorC phenotypes tested. However, in strains expressing the mutation of either C732 or C741, which are conserved in all of the homologs, do not complement the mutant phenotypes and we predict that these cysteine residues are important in maintaining the structure/function of the MorC protein. Therefore, we hypothesize that disulfide bonding between C732 and C741 is important for MorC function. Although these residues are highly conserved, they are not part of the DUF490 domain. This implies that the DUF490 domain is necessary but not sufficient for restoration of the morC phenotypes and that the COG2911 domain may play an indirect role in MorC function.
In this study, we have used A. actinomycetemcomitans as a model organism to investigate the function of MorC proteins derived from evolutionarily related and unrelated bacterial species. The proteins share varying levels of sequence similarity with the greatest homology located at the carboxyl region of the molecule. Though related by sequence, we have demonstrated a variation in the functional activity of the proteins, which may be attributed to the differences in secondary structures formed by specific sequences of amino acids in the DUF490 domain. In addition, we have determined that the DUF490 domain of the A. actinomycetemcomitans protein is required for function. Based on our data, we propose that MorC interacts with other membrane proteins to coordinate the biological functions associated with the A. actinomycetemcomitans membrane. Studies are ongoing to determine the interactions associated with MorC in the A. actinomycetemcomitans membrane.
ACKNOWLEDGEMENTS
We thank Matthew Wargo (University of Vermont) for his help in generating and providing the P. aeruginosa morC constructs associated with this project, and Xiaoli Fu and Thomas Freeman for technical support. The anti-LtxA antibody was a generous gift of Edward Lally (University of Pennsylvania). This study was supported by NIH grant RO1-DE018889 (K.P.M.) and has benefited from developments supported by NIH Grant RO1-DE017474 (T.R.)
REFERENCES CITED
- Altschul SF, Gish W, Miller W, et al. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Azari F, Nyland L, Yu C, et al. Ultrastructural analysis of the rugose cell envelope of a member of the Pasteurellaceae family. J Bacteriol. 2013;195:1680–1688. doi: 10.1128/JB.02149-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley M, Gahan CG, Hill C. The interaction between bacteria and bile. FEMS Microbiol Rev. 2005;29:625–651. doi: 10.1016/j.femsre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- de Cock H, Struyve M, Kleerebezem M, et al. Role of the carboxy-terminal phenylalanine in the biogenesis of outer membrane protein PhoE of Escherichia coli K-12. J Mol Biol. 1997;269:473–478. doi: 10.1006/jmbi.1997.1069. [DOI] [PubMed] [Google Scholar]
- Delepelaire P. Type I secretion in gram-negative bacteria. Biochim Biophys Acta. 2004;1694:149–161. doi: 10.1016/j.bbamcr.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Demarre G, Guerout AM, Matsumoto-Mashimo C, et al. A new family of mobilizable suicide plasmids based on broad host range R388 plasmid (IncW) and RP4 plasmid (IncPalpha) conjugative machineries and their cognate Escherichia coli host strains. Res Microbiol. 2005;156:245–255. doi: 10.1016/j.resmic.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Deng M, Misra R. Examination of AsmA and its effect on the assembly of Escherichia coli outer membrane proteins. Mol Microbiol. 1996;21:605–612. doi: 10.1111/j.1365-2958.1996.tb02568.x. [DOI] [PubMed] [Google Scholar]
- Duong F, Bonnet E, Geli V, et al. The AprX protein of Pseudomonas aeruginosa: a new substrate for the Apr type I secretion system. Gene. 2001;262:147–153. doi: 10.1016/s0378-1119(00)00541-2. [DOI] [PubMed] [Google Scholar]
- Fine DH, Markowitz K, Furgang D, et al. Aggregatibacter actinomycetemcomitans and its relationship to initiation of localized aggressive periodontitis: longitudinal cohort study of initially healthy adolescents. J Clin Microbiol. 2007;45:3859–3869. doi: 10.1128/JCM.00653-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd JL, Smith KP, Kumar SH, et al. LmrS is a multidrug efflux pump of the major facilitator superfamily from Staphylococcus aureus. Antimicrob Agents Chemother. 2010;54:5406–5412. doi: 10.1128/AAC.00580-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant CV, Sedic M, Chicoine EA, et al. Membrane morphology and leukotoxin secretion are associated with a novel membrane protein of Aggregatibacter actinomycetemcomitans. J Bacteriol. 2008;190:5972–5980. doi: 10.1128/JB.00548-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzzo J, Pages JM, Duong F, et al. Pseudomonas aeruginosa alkaline protease: evidence for secretion genes and study of secretion mechanism. J Bacteriol. 1991;173:5290–5297. doi: 10.1128/jb.173.17.5290-5297.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogh A, Larsson B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol. 2001;305:567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- Lukomski S, Hoe NP, Abdi I, et al. Nonpolar inactivation of the hypervariable streptococcal inhibitor of complement gene (sic) in serotype M1 Streptococcus pyogenes significantly decreases mouse mucosal colonization. Infect Immun. 2000;68:535–542. doi: 10.1128/iai.68.2.535-542.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchler-Bauer A, Lu S, Anderson JB, et al. CDD: a Conserved Domain Database for the functional annotation of proteins. Nucleic Acids Res. 2011;39:D225–D229. doi: 10.1093/nar/gkq1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller VL, Mekalanos JJ. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz KP, Brissette C, Fives-Taylor PM. A recombinase A-deficient strain of Actinobacillus actinomycetemcomitans constructed by insertional mutagenesis using a mobilizable plasmid. FEMS Microbiol Lett. 2002;206:87–92. doi: 10.1111/j.1574-6968.2002.tb10991.x. [DOI] [PubMed] [Google Scholar]
- Mintz KP, Fives-Taylor PM. Adhesion of Actinobacillus actinomycetemcomitans to a human oral cell line. Infect Immun. 1994;62:3672–3678. doi: 10.1128/iai.62.9.3672-3678.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol Mol Biol Rev. 2003;67:593–656. doi: 10.1128/MMBR.67.4.593-656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos JS, Agarwala R. COBALT: constraint-based alignment tool for multiple protein sequences. Bioinformatics. 2007;23:1073–1079. doi: 10.1093/bioinformatics/btm076. [DOI] [PubMed] [Google Scholar]
- Paturel L, Casalta JP, Habib G, et al. Actinobacillus actinomycetemcomitans endocarditis. Clin Microbiol Infect. 2004;10:98–118. doi: 10.1111/j.1469-0691.2004.00794.x. [DOI] [PubMed] [Google Scholar]
- Punta M, Coggill PC, Eberhardt RY, et al. The Pfam protein families database. Nucleic Acids Res. 2012;40:D290–D301. doi: 10.1093/nar/gkr1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahamat-Langendoen JC, van Vonderen MG, Engstrom LJ, et al. Brain abscess associated with Aggregatibacter actinomycetemcomitans: case report and review of literature. J Clin Periodontol. 2011;38:702–706. doi: 10.1111/j.1600-051X.2011.01737.x. [DOI] [PubMed] [Google Scholar]
- Ruiz T, Lenox C, Radermacher M, Mintz KP. Novel surface structures are associated with the adhesion of Actinobacillus actinomycetemcomitans to collagen. Infect Immun. 2006;74:6163–6170. doi: 10.1128/IAI.00857-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scannapieco FA. Role of oral bacteria in respiratory infection. J Periodontol. 1999;70:793–802. doi: 10.1902/jop.1999.70.7.793. [DOI] [PubMed] [Google Scholar]
- Selkrig J, Mosbahi K, Webb CT, et al. Discovery of an archetypal protein transport system in bacterial outer membranes. Nat Struct Mol Biol. 2012;19:506–510. S1. doi: 10.1038/nsmb.2261. [DOI] [PubMed] [Google Scholar]
- Sievers F, Wilm A, Dineen D, et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol. 2011;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhavy TJ, Kahne D, Walker S. The bacterial cell envelope. Cold Spring Harb Perspect Biol. 2010;2:a000414. doi: 10.1101/cshperspect.a000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KP, Fields JG, Voogt RD, et al. The cell envelope proteome of Aggregatibacter actinomycetemcomitans. Mol Oral Microbiol. 2014 doi: 10.1111/omi.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KP, Fields JG, Voogt RD, et al. Alteration in abundance of specific membrane proteins of Aggregatibacter actinomycetemcomitans is attributed to deletion of the inner membrane protein MorC. Proteomics. 2015 doi: 10.1002/pmic.201400505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD, Cugini MA, et al. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- Soding J, Biegert A, Lupas AN. The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res. 2005;33:W244–W248. doi: 10.1093/nar/gki408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struyve M, Moons M, Tommassen J. Carboxy-terminal phenylalanine is essential for the correct assembly of a bacterial outer membrane protein. J Mol Biol. 1991;218:141–148. doi: 10.1016/0022-2836(91)90880-f. [DOI] [PubMed] [Google Scholar]
- Taboada B, Ciria R, Martinez-Guerrero CE, Merino E. ProOpDB: Prokaryotic Operon DataBase. Nucleic Acids Res. 2012;40:D627–D631. doi: 10.1093/nar/gkr1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taboada B, Verde C, Merino E. High accuracy operon prediction method based on STRING database scores. Nucleic Acids Res. 2010;38:e130. doi: 10.1093/nar/gkq254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G, Kawai T, Komatsuzawa H, Mintz KP. Lipopolysaccharides mediate leukotoxin secretion in Aggregatibacter actinomycetemcomitans. Mol Oral Microbiol. 2012;27:70–82. doi: 10.1111/j.2041-1014.2011.00632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulett GC, Valle J, Beloin C, et al. Functional analysis of antigen 43 in uropathogenic Escherichia coli reveals a role in long-term persistence in the urinary tract. Infect Immun. 2007;75:3233–3244. doi: 10.1128/IAI.01952-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandersman C, Delepelaire P. Bacterial iron sources: from siderophores to hemophores. Annu Rev Microbiol. 2004;58:611–647. doi: 10.1146/annurev.micro.58.030603.123811. [DOI] [PubMed] [Google Scholar]
- Yu C, Mintz KP, Ruiz T. Investigation of the three-dimensional architecture of the collagen adhesin EmaA of Aggregatibacter actinomycetemcomitans by electron tomography. J Bacteriol. 2009;191:6253–6261. doi: 10.1128/JB.00563-09. [DOI] [PMC free article] [PubMed] [Google Scholar]