Abstract
Background
Vascularized composite allotransplantation (VCA) represents an important advancement in the field of reconstructive microsurgery and has continued to increase in popularity. The significant clinical morbidity associated with flap failure represents an important barrier to even more widespread use of these techniques. Early identification of vascular compromise has been associated with a higher salvage rate, yet most surgeons rely only on clinical assessment intraoperatively. Spatial frequency domain imaging (SFDI) presents a non-contact, objective measurement of tissue oxygenation over a large field of view. This study aims to evaluate the use of SFDI technology in hemifacial composite flap compromise as could occur during facial transplant.
Methods
Six composite hemifacial flaps were created in three 35 kg Yorkshire pigs and continuously imaged using SFDI before, during, and after 15-minute selective vascular pedicle occlusion. Arterial and venous clamping trials were performed for each flap. Changes in oxyhemoglobin concentration (ctO2Hb), deoxyhemoglobin concentration (ctHHb), and total hemoglobin (ctHbT) were quantified over time.
Results
SFDI successfully measured changes in oxygenation parameters in all six composite tissue flaps. Significant changes in ctO2Hb, ctHHb, and ctHbT were seen relative to controls. Early and distinct patterns of alteration were noted in arterial and in venous compromise relative to one another.
Conclusions
The need for non-invasive, reliable assessment of composite tissue graft viability is apparent, given the morbidity associated with flap failure. The results of this study suggest that SFDI technology shows promise in providing intraoperative guidance with regard to pedicle vessel integrity during reconstructive microsurgery.
Keywords: Facial transplant, spatial frequency domain imaging, near infrared imaging
INTRODUCTION
Vascularized composite allotransplantation (VCA) has continued to increase in popularity over the last two decades as a means of providing functional restoration of complex defects.1 VCA methods are uniquely advantageous compared with conventional techniques in that they offer replacement of defective or absent structures with anatomically identical tissues.2 Advancing microsurgical and immunosuppressive techniques have led to composite hand and face transplantation with encouraging results.3,4 Since the first reported partial face transplantation in 2005, over 30 human facial VCAs have been performed worldwide.5–7
Despite distinct clinical utility, more complex VCAs present inherent risks, both perioperatively and over the long term. The risk of flap failure in head and neck free autologous tissue transfer has been reported at 3.8% and represents significant clinical morbidity.8 Emergent surgical re-exploration is indicated for post-operative flap compromise, and is most often performed for venous or arterial insufficiency.8,9 A majority of salvage procedures performed for vascular compromise in a recent series of autologous head and neck flaps were reported to be successful in preventing complete flap loss.8 Earlier identification of flap compromise and prompt re-exploration have led to improvement in rates of successful flap salvage.10 The importance of early detection and salvage of facial allografts cannot be understated, as loss of the graft mandates removal, and may leave patients in a worse functional and aesthetic state than prior to transplantation.2
Although the field of reconstructive microsurgery has seen remarkable advances in vascularized composite allotransplantation, standard intraoperative monitoring techniques have not evolved. In most institutions, flap viability is determined clinically by the surgical team, typically in terms of color, temperature, edema, and capillary refill. Unfortunately, these measures are subjective and rely on relatively late signs of tissue compromise. Often, clinical assessment is supplemented with the use of a handheld Doppler probe when pedicle vessels are easily accessible. Although other techniques, such as implantable Doppler systems, 11,12 laser Doppler flowmetry, 12 fluorescence angiography, 13–15 and near-infrared tissue oximetry 11,16 have been described, each has important limitations and none has achieved widespread adoption into routine clinical practice.
Spatial frequency domain imaging (SFDI) is a non-contact optical imaging method which provides accurate assessment of tissue optical properties using rapid acquisition over a large field of view (>100 cm2).17,18 Measurement of specific near-infrared (NIR) wavelengths allows quantification of tissue constituents - oxyhemoglobin, deoxyhemoglobin, lipids, and water - at depths up to 5 mm.19 Our group has previously validated the SFDI system in porcine abdominal skin flap, bowel, and liver ischemia models, and performed a first-in-human study during perforator flap breast reconstruction.20 Here, we present a novel evaluation of this technology in a porcine hemifacial composite transplantation model.
Methods
Animals
The use of animals in this study was performed under the supervision of the Institutional Animal Care and Use Committee, and in accordance with approved institutional protocol number 046-2010. Three female, 35 kg Yorkshire pigs (E.M. Parsons and Sons, Hadley, MA) were included in this study. All animals were healthy, with no prior history of allosensitization. Each pig was housed in its own cage and provided with a standard diet and water ad libitum. An initial physical examination was performed on each animal prior to beginning the procedure.
Surgical procedure
Anesthesia was induced using 4.4 mg/kg intramuscular Telazol (Fort Dodge Animal Health, Fort Dodge, IA), and maintained with 2% isoflurane (Baxter Healthcare Corp., Deerfield, IL) in oxygen following intubation. Mechanical ventilation via Quantiflex ventilator (Matrix Medical, Inc, Orchard Park, NY) was maintained throughout the procedure. Femoral central venous access was established for intravenous hydration and a urinary catheter was placed for urine output monitoring prior to the start of surgery. Physiologic parameters - heart rate, blood pressure, temperature, and urine output - were monitored during all experiments.
A total of six hemifacial composite flaps were elevated, and included skin, muscle, nerve, ear cartilage, parotid gland, and surrounding soft tissue as previously described.21 In the lateral position, following hair removal, skin was incised to the depth of the platysma anteriorly, cleido-occipitalis and cleido-mastoideus posteriorly, and to the periosteum cranially. The upper and lower eyelids were excluded from the flap. Anteriorly, the platysma was divided and external jugular vein carefully preserved as the pedicle vein. The tendon of the sternomastoideus muscle was transected, and the bony paracondylar process removed to reveal the common carotid artery and its branches. The internal carotid artery and lingual artery were ligated and the external carotid was preserved as the pedicle artery. The superficial temporal artery, one of two terminal branches of the external carotid, was preserved to supply the flap; the other branch, the maxillary artery was identified and ligated. Facial dissection was carried out superficial to the masseter muscle toward the ear, with preservation and inclusion of the parotid gland and ligation of the facial nerve trunk. Posteriorly, the flap was elevated in a plane superficial to the trapezius, cleido-occipitalis, and cleido-mastoideus muscles. External ear cartilage was detached at the osteocartilaginous junction and included in the flap. Following complete elevation about the external jugular vein and external carotid artery pedicle, the flap was secured using a running 4-0 nylon cuticular suture to prevent inadvertent pedicle tension or torsion (Figure 1).
Figure 1. Hemifacial Flap Elevation.

SFDI field of view is shown following elevation of a right hemifacial composite flap in a 35 kg Yorkshire pig. The anterior most aspect of the face is included for control measurement and comparison.
In order to evaluate the ability to detect tissue oxygenation defects associated with acute compromise of the vascular pedicle, after an initial 3–5 minutes of data was acquired, a vascular clamp was applied to either the pedicle artery or vein for a period of 10 to 15 minutes and subsequently released.
Imaging
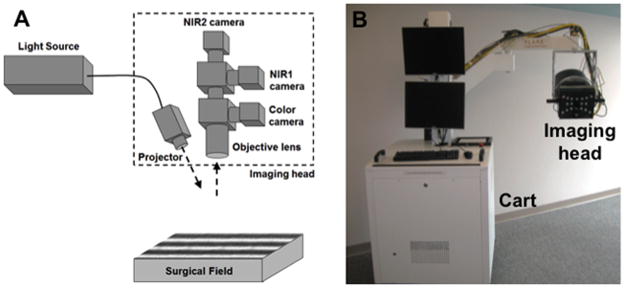
As shown in Figure 2, the SFDI system consists of an imaging cart containing a multispectral NIR light source, control electronics, computer, and adjustable imaging head which enables data acquisition over a 16 × 12 cm field at a 45 cm working distance. The imaging head is further composed of a projector which shines patterns of light onto the surgical field and cameras that record color images co-registered with NIR images for further processing (2 channels, NIR1 from 650 to 780 nm and NIR2 from 780 to 1000 nm). SFDI data is acquired by projecting 6 wavelengths onto the field: 670, 730 and 760 collected on the NIR1 channel, and 808, 860, and 980 nm collected on the NIR2 channel. Three distinct patterns are projected: two of which are used to extract optical properties, and the other which is used for profilometry measurement and correction for variation in surface profile.22 Acquisition is performed in real-time intraoperatively, then processed to extract spatial maps of tissue oxyhemoglobin (ctO2Hb) and deoxyhemoglobin (ctHHb) concentration. Complete details of the SFDI clinical system have been previously reported.19 SFDI oxygen saturation measurements have also been validated directly against a clinical, FDA-approved oxygenation probe in an earlier study using a skin flap model, and values were found to correlate within 10%.19
Figure 2. SFDI imaging system.
The SFDI imaging system is composed of a cart containing all electronics and a light source, and of an imaging head containing a projector and 3 co-registered cameras (color, NIR1 and NIR2).
A. Schematics of the imaging head
B. Picture of the actual imaging system
In this study, SFDI data was acquired continuously during vascular pedicle clamping, and for 10 minutes following clamp release. Regions of interest were established in two areas of the facial composite flap as well as in a control area of the face not included in the flap. Data was processed using a custom code in MATLAB (Mathworks, Natick, MA), and component analysis of oxyhemoglobin concentration, deoxyhemoglobin concentration, and total hemoglobin was performed.
Statistical Analysis
Change in flap oxygenation measurements were compared with controls using unpaired t-tests. A p value of < 0.05 was considered statistically significant. Flaps that experienced significant change in control tissue measurements during the imaging period were not included.
RESULTS
A total of six composite hemifacial flaps were successfully elevated and evaluated using SFDI. Intraoperative SFDI demonstrated clear change in all three parameters during both arterial and venous pedicle clamping. Unique and consistent profiles using these elements were identified for arterial occlusion and venous occlusion relative to controls and to one another.
Arterial occlusion
As expected clinically, the concentration of oxyhemoglobin following arterial pedicle occlusion decreased within seconds by a mean of 20.0 μM SD 5.5. The concentration subsequently appeared to reach a steady trough, which lasted until clamp release. Interestingly, the post-occlusion concentration of oxyhemoglobin exceeded baseline flap measurements by an average of 3.1 μM SD 2.0 (Figure 3A).
Figure 3. Arterial Occlusion.

Representative SFDI measurements of oxygenation parameters are shown over the study period for flap (green) and control (blue) regions. The pedicle artery was clamped at 3 min and released at 16 min (red arrows). Numbers 1–4 indicate key time points for which spatial maps are shown in Figure 4.
A. Tracing of oxyhemoglobin concentration (ctO2Hb) over the study period.
B. Tracing of deoxyhemoglobin concentration (ctHHb) over the study period.
C. Tracing of hemoglobin (ctHbT) during the study period.
Deoxyhemoglobin concentration within the flap also decreased within seconds following arterial pedicle occlusion, and steadily declined by a mean of 4.6 μM SD 2.9 during the clamping period. Following clamp release, ctHHb increased rapidly, peaking an average of 2.8 μM SD 2.1 above the baseline flap measurement before declining again (Figure 3B).
Total hemoglobin concentration decreased by an average of 23.0 μM SD 4.0 during arterial pedicle clamping, quickly reaching a relatively steady value. Release of the vascular clamp produced an immediate increase in total hemoglobin, which exceeded initial flap measurements by 5.0 μM SD 4.6 (Figure 3C).
Spatial mapping of alterations in ctO2Hb, ctHHb, and ctHbT during arterial occlusion are shown in Figure 4. Although the flap color may appear slightly pale to the surgeon in late occlusion (color images), examination of the SFDI component maps demonstrate visible change at the earlier time point. All three components return to or exceed baseline measurements following release of the pedicle clamp.
Figure 4. Spatial Map of Perfusion Defects during Arterial Occlusion.
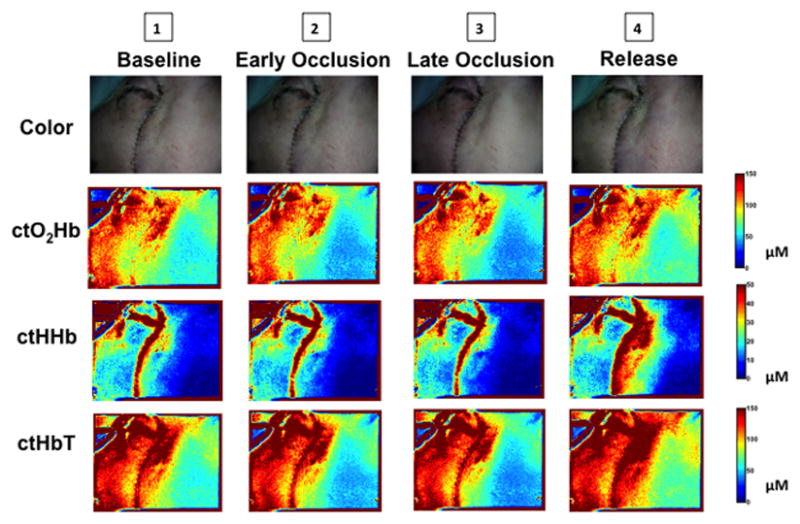
Changes in flap color, ctO2Hb, ctHHb, and ctHbT over the SFDI field of view are shown at key time points (1–4) during arterial pedicle occlusion. The anterior aspect of the face is included as a control region for comparison.
Venous occlusion
Unlike the arterial occlusion pattern, venous pedicle clamping produced an immediate increase in oxyhemoglobin concentration by a mean of 13.0 μM SD 3.3. The concentration was noted to fall slowly during the clamping period until reaching approximately the initial flap value. Only a very small increase in concentration was noted when the venous clamp was released (Figure 5A).
Figure 5. Venous Occlusion.

Representative SFDI measurements of oxygenation parameters are shown over the study period for flap (green) and control (blue) regions. The pedicle vein was clamped at 3 min and released at 13 min (red arrows). Numbers 1–4 indicate key time points for which spatial maps are shown in Figure 6.
A. Tracing of oxyhemoglobin concentration (ctO2Hb) over the study period.
B. Tracing of deoxyhemoglobin concentration (ctHHb) over the study period.
C. Tracing of hemoglobin (ctHbT) during the study period.
Deoxyhemoglobin measurements followed a dramatic and consistent path during venous occlusion, with a progressive increase by an average of 39.0 μM SD 14.0 over the period leading up to clamp release. Release of the venous clamp resulted in a sharp decrease in concentration followed by a gradual return to initial values. Unlike the changes in ctHHb with arterial occlusion, flap ctHHb during venous occlusion actually increased to values well above that of the control tissue (Figure 5B).
Initial ctHbT measurements in the flap were 105.0 μM SD 14.3. Total hemoglobin concentration during pedicle vein occlusion was noted to increase quickly at first then more slowly, reaching an average of 134.0 μM SD 18.4. Clamp release produced a steep decrease in ctHbT, which subsequently approached initial flap values at 106.0 μM SD 16.3 (Figure 5C). This pattern was noted to correlate inversely with changes during arterial occlusion (Figure 3C).
Spatial mapping of alterations in ctO2Hb, ctHHb, and ctHbT during venous occlusion are shown in Figure 6. Although changes in flap color would be apparent to the surgeon in late occlusion (color images), examination of the SFDI component maps demonstrate visible change at the earlier time point. Return to baseline appearance is seen in all four components following release of the pedicle clamp.
Figure 6. Spatial Map of Perfusion Defects during Venous Occlusion.
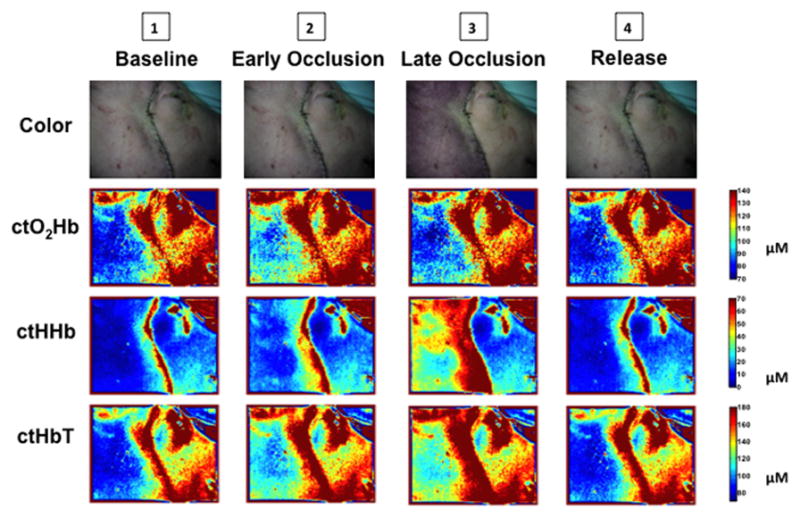
Changes in flap color, ctO2Hb, ctHHb, and ctHbT over the SFDI field of view are shown at key time points (1–4) during venous pedicle occlusion. The anterior aspect of the face is included as a control region for comparison.
DISCUSSION
SFDI technology provides a potential method for reliably imaging changes in ctO2Hb, ctHHb, and ctHbT during vascularized composite allotransplantation in this model of hemifacial flap vascular compromise. Characteristic patterns identified using these measurements were seen quickly after vascular clamping and may be reliably differentiated based on which pedicle vessel was clamped. This technique could provide a means of not only rapidly detecting pedicle occlusion, but also directing revascularization of the affected vessel.
The opportunity to view spatial representations of changes in tissue oxygenation associated with pedicle compromise provides additional valuable information to the surgeon. As regions of the flap further from the pedicle vessels are typically affected more rapidly by vascular insufficiency, evaluation of the larger surgical field is important for early detection. Figure 6 demonstrates the pattern of alteration in each aspect of tissue oxygenation during key phases of experimental venous pedicle occlusion. Appreciable change in ctO2Hb, ctHHb, and ctHbT occur quickly during early occlusion - perhaps sooner than might be detected using more localized techniques or with tissue oximetry measurements alone.
As demonstrated in the ctO2Hb, ctHHb, and ctHbT tracings during pedicle vessel clamping (Figures 3 and 5), the ability to extract these values individually provides valuable insight into the presence and type of vascular compromise beyond simply monitoring tissue oxygenation. Access to this information intraoperatively could aid surgeons during initial microsurgical anastomosis and flap inset as well as during revision, if necessary. As shown in Table 1, significant change in ctO2Hb, ctHHB, and ctHbT was seen in both arterial and venous flap pedicle occlusion relative to control tissue. By analyzing ctO2Hb, ctHHb, and ctHbT individually, the SFDI system has the ability to differentiate between arterial and vascular compromise within 2 minutes; far earlier than any potential irreversible damage from thrombosis.
Table 1. Component Change with Vascular Occlusion.
A. Absolute change in ctO2Hb, ctHHb, and ctHbT in control tissue and the hemifacial flap during pedicle artery occlusion are shown (mean, standard deviation). P values < 0.05 are considered statistically significant.
B. Change in ctO2Hb, ctHHb, and ctHbT in control tissue and the hemifacial flap during pedicle vein occlusion are shown (mean, standard deviation). P values < 0.05 are considered statistically significant.
| A. Arterial Occlusion | |||
|---|---|---|---|
| ΔctO2Hb (μM) | Δ ctHHb (μM) | Δ ctHbT (μM) | |
| Flap | − 20.0 SD 5.5 | − 4.6 SD 2.9 | − 23.0 SD 4.0 |
| Control | − 0.3 SD 0.5 | − 0.7 SD 0.8 | 0.0 SD 0.0 |
| P value | < 0.01* | < 0.01* | < 0.01* |
| B. Venous Occlusion | |||
|---|---|---|---|
| ΔctO2Hb (μM) | Δ ctHHb (μM) | Δ ctHbT (μM) | |
| Flap | 13.0 SD 3.3 | 27.0 SD 13.0 | 29.0 SD 10.0 |
| Control | 12.0 SD 1.9 | 0.2 SD 0.4 | 1.0 SD 1.1 |
| P value | < 0.01* | < 0.01* | < 0.01* |
There are limitations to this study. In order to compare SFDI flap monitoring against the gold standard of clinical assessment as well as methods of tissue oximetry currently in use at some institutions, we will need to perform simultaneous measurements of flap oxygenation parameters using all three techniques. Future study is planned in this regard in order to assess the time to detection of vessel occlusion using each method. Only complete vascular compromise was evaluated; additional investigation of partial pedicle compromise is planned in order to assess the use of SFDI in varying degrees of flap failure. Effects of microvascular anastomosis were not evaluated, as native pedicle vessels were clamped following flap elevation. The experimental clamp time of 15 minutes, though important for early detection of pedicle compromise, would not detect patterns of change in oxygenation parameters that require longer ischemia time. In these preliminary experiments, we did not attempt to predict long-term flap outcome in the setting of specific vascular insults. Our group is planning additional study of more complex auto- and allografted composite tissue using SFDI, though, based on the results of this initial evaluation. We are also pursuing development of software capable of providing real-time processing of the SFDI data in order to further increase the opportunity for intraoperative application.
The need for non-invasive, reliable, immediate assessment of composite tissue graft viability is clear, given the morbidity associated with flap failure. The ability to rapidly detect and simultaneously characterize vascular pedicle defects represents an important advancement in the evaluation of composite tissue transfer. SFDI technology shows promise in providing reconstructive surgeons with critical intraoperative guidance with regard to pedicle vessel integrity.
Acknowledgments
Sources of Funding:
NIH/NIDCR Award Number R01-DE-022820 (JVF and BTL)
NIH/NIDDK Award Number K01-DK-093603 (SG)
NSF Award Number DGE-1247312 (JA)
Footnotes
Presented at the 2013 American Society for Reconstructive Microsurgery meeting, Naples FL and the 2014 World Society for Reconstructive Microsurgery meeting, Chicago IL
Financial Disclosure:
Dr. Frangioni is currently CEO of Curadel, LLC, which has licensed FLARE imaging systems and contrast agents from the Beth Israel Deaconess Medical Center.
Author Contributions:
JVF, SG and BTL designed the study. CV, JTN, YA, JA, VV, FK and FN performed the experiments and processed the data. CV, JVF, SG and BTL reviewed, analyzed, and interpreted the data. CV, JVF, SG and BTL wrote and revised the paper. All authors discussed the results and commented on the manuscript. JVF, SG and BTL approved the submitted version.
References
- 1.Hettiaratchy S, Randolph MA, Petit F, et al. Composite tissue allotransplantation--a new era in plastic surgery? Br J Plast Surg. 2004 Jul;57(5):381–391. doi: 10.1016/j.bjps.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 2.Pomahac B, Bueno EM, Sisk GC, Pribaz JJ. Current principles of facial allotransplantation: the Brigham and Women’s Hospital Experience. Plast Reconstr Surg. 2013 May;131(5):1069–1076. doi: 10.1097/PRS.0b013e3182865cd3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siemionow MZ, Kulahci Y, Bozkurt M. Composite tissue allotransplantation. Plast Reconstr Surg. 2009 Dec;124(6 Suppl):e327–339. doi: 10.1097/PRS.0b013e3181bf8413. [DOI] [PubMed] [Google Scholar]
- 4.Shanmugarajah K, Hettiaratchy S, Clarke A, Butler PE. Clinical outcomes of facial transplantation: a review. International journal of surgery. 2011;9(8):600–607. doi: 10.1016/j.ijsu.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Devauchelle B, Badet L, Lengele B, et al. First human face allograft: early report. Lancet. 2006 Jul 15;368(9531):203–209. doi: 10.1016/S0140-6736(06)68935-6. [DOI] [PubMed] [Google Scholar]
- 6.Siemionow MZ, Papay F, Djohan R, et al. First U.S. near-total human face transplantation: a paradigm shift for massive complex injuries. Plast Reconstr Surg. 2010 Jan;125(1):111–122. doi: 10.1097/PRS.0b013e3181c15c4c. [DOI] [PubMed] [Google Scholar]
- 7.Khalifian S, Brazio PS, Mohan R, et al. Facial transplantation: the first 9 years. Lancet. 2014 Apr 25; doi: 10.1016/S0140-6736(13)62632-X. [DOI] [PubMed] [Google Scholar]
- 8.Wu CC, Lin PY, Chew KY, Kuo YR. Free tissue transfers in head and neck reconstruction: Complications, outcomes and strategies for management of flap failure: Analysis of 2019 flaps in single institute. Microsurgery. 2013 Dec 8; doi: 10.1002/micr.22212. [DOI] [PubMed] [Google Scholar]
- 9.Wei FC, Demirkan F, Chen HC, et al. The outcome of failed free flaps in head and neck and extremity reconstruction: what is next in the reconstructive ladder? Plast Reconstr Surg. 2001 Oct;108(5):1154–1160. doi: 10.1097/00006534-200110000-00007. discussion 1161–1152. [DOI] [PubMed] [Google Scholar]
- 10.Disa JJ, Cordeiro PG, Hidalgo DA. Efficacy of conventional monitoring techniques in free tissue transfer: an 11-year experience in 750 consecutive cases. Plast Reconstr Surg. 1999 Jul;104(1):97–101. [PubMed] [Google Scholar]
- 11.Lohman RF, Langevin CJ, Bozkurt M, et al. A prospective analysis of free flap monitoring techniques: physical examination, external Doppler, implantable Doppler, and tissue oximetry. J Reconstr Microsurg. 2013 Jan;29(1):51–6. doi: 10.1055/s-0032-1326741. [DOI] [PubMed] [Google Scholar]
- 12.Hallock GG. Acoustic Doppler sonography, color duplex ultrasound, and laser Doppler flowmetry as tools for successful autologous breast reconstruction. Clin Plast Surg. 2011 Apr;38(2):203–11. doi: 10.1016/j.cps.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Lee BT, Matsui A, Hutteman M, et al. Intraoperative near-infrared fluorescence imaging in perforator flap reconstruction: current research and early clinical experience. J Reconstr Microsurg. 2010 Jan;26(1):59–65. doi: 10.1055/s-0029-1244805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee BT, Hutteman M, Gioux S, et al. The FLARE intraoperative near-infrared fluorescence imaging system: a first-in-human clinical trial in perforator flap breast reconstruction. Plast Reconstr Surg. 2010 Nov;126(5):1472–81. doi: 10.1097/PRS.0b013e3181f059c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsui A, Lee BT, Winer JH, et al. Image-guided perforator flap design using invisible near-infrared light and validation with x-ray angiography. Ann Plast Surg. 2009 Sep;63(3):327–30. doi: 10.1097/SAP.0b013e318193493d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin SJ, Nguyen MD, Chen C, et al. Tissue oximetry monitoring in microsurgical breast reconstruction decreases flap loss and improves rate of flap salvage. Plast Reconstr Surg. 2011 Mar;127(3):1080–5. doi: 10.1097/PRS.0b013e31820436cb. [DOI] [PubMed] [Google Scholar]
- 17.Cuccia DJ, Bevilacqua F, Durkin AJ, et al. Quantitation and mapping of tissue optical properties using modulated imaging. J Biomed Opt. 2009 Mar-Apr;14(2):024012. doi: 10.1117/1.3088140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vervandier J, Gioux S. Single snapshot imaging of optical properties. Biomedical optics express. 2013;4(12):2938–2944. doi: 10.1364/BOE.4.002938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gioux S, Mazhar A, Lee BT, et al. First-in-human pilot study of a spatial frequency domain oxygenation imaging system. J Biomed Opt. 2011 Aug;16(8):086015. doi: 10.1117/1.3614566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen JT, Lin SJ, Tobias AM, et al. A novel pilot study using spatial frequency domain imaging to assess oxygenation of perforator flaps during reconstructive breast surgery. Ann Plast Surg. 2013 Sep;71(3):308–315. doi: 10.1097/SAP.0b013e31828b02fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen JT, Ashitate Y, Buchanan IA, et al. Face transplant perfusion assessment using near-infrared fluorescence imaging. J Surg Res. 2012 Oct;177(2):e83–8. doi: 10.1016/j.jss.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gioux S, Mazhar A, Cuccia DJ, et al. Three-dimensional surface profile intensity correction for spatially modulated imaging. J Biomed Opt. 2009 May-Jun;14(3):034045. doi: 10.1117/1.3156840. [DOI] [PMC free article] [PubMed] [Google Scholar]