SUMMARY
There is an increasing appreciation that complement dysregulation lies at the heart of numerous immune-mediated and inflammatory disorders. Complement inhibitors are therefore being evaluated as new therapeutic options in various clinical translation programs and the first clinically approved complement-targeted drugs have profoundly impacted the management of certain complement-mediated diseases. Among the many members of the intricate protein network of complement, the central component C3 represents a ‘hot-spot’ for complement-targeted therapeutic intervention. C3 modulates both innate and adaptive immune responses and is linked to diverse immunomodulatory systems and biological processes that affect human pathophysiology. Compelling evidence from preclinical disease models has shown that C3 interception may offer multiple benefits over existing therapies or even reveal novel therapeutic avenues in disorders that are not commonly regarded complement-driven, such as periodontal disease. Using the clinically-developed compstatin family of C3 inhibitors and periodontitis as illustrative examples, this review highlights emerging therapeutic concepts and developments in the design of C3-targeted drug candidates as novel immunotherapeutics for oral and systemic inflammatory diseases.
Keywords: complement, C3, therapeutics, compstatin Cp40, primate models, inflammation, periodontitis
INTRODUCTION
Complement exemplifies an evolutionarily ancient innate immune sentinel whose role is undergoing a profound paradigm shift in recent years. Its traditional perception as a serum-borne auxiliary system that merely potentiates humoral and cellular antimicrobial responses has been superseded in the light of robust evidence that complement plays a key role in tissue homeostasis and immunosurveillance (Ricklin et al., 2010). However, when excessively activated or dysregulated, complement drives pathogenesis in a wide spectrum of acute or chronic inflammatory disorders and immune-mediated diseases (Ricklin & Lambris, 2013a;Ricklin & Lambris, 2013b). The growing appreciation of complement’s key involvement in disease pathogenesis has spearheaded systematic efforts to develop potent, highly selective, and tailored complement therapeutics with potential for clinical translation (Ricklin & Lambris, 2013c;Mastellos et al., 2015;Ricklin & Lambris, 2015). Indeed, never before has complement research witnessed so diverse and mechanistically subtle approaches for modulating complement’s activity in a therapeutic context, let alone a surge of highly cross-disciplinary programs for implementing novel complement-based anti-inflammatory therapies with translational potential. The launch of the first complement-targeted drugs, anti-C5 (Eculizumab; Soliris, Alexion) and C1-inhibitor (C1-INH; available from various manufacturers) has changed the clinical landscape of chronic debilitating diseases whose pathology is driven by complement dysregulation such as paroxysmal nocturnal hemoglobinuria (PNH), and atypical hemolytic uremic syndrome (aHUS) (Mastellos et al., 2014;Risitano, 2012). This profound therapeutic leverage has also reignited a vibrant interest in the biopharmaceutical development and clinical evaluation of a new generation of complement-based inhibitors (Ricklin & Lambris, 2013b;Ricklin & Lambris, 2007;Qu et al., 2009b).
In addition to the classic serum proteins (C1-C9), the integrated complement system includes pattern-recognition molecules, convertases and other proteases, cofactors, regulators, and receptors for interactions with diverse immune mediators (Ricklin et al., 2010). The complement cascade can be activated by distinct mechanisms (classical, lectin, or alternative), all of which converge at the third complement component (C3) leading to the generation of effectors with diverse functions. These include the recruitment and activation of inflammatory cells (via the C3a and C5a anaphylatoxins that activate the G-protein-coupled receptors, C3aR and C5aR1 [CD88], respectively), microbial opsonization and phagocytosis (e.g., through the C3b or C4b opsonins), and direct lysis of susceptible targeted microbes (via assembly of the C5b-9 membrane attack complex [MAC]) (Ricklin et al., 2010). Comprising more than 50 proteins, the complement cascade offers multiple targets for therapeutic modulation. However, the design of anti-complement therapeutics is faced with formidable challenges given the context-specific involvement of complement in disease progression and the fine spatiotemporal regulation of its activity. Undeniably, nevertheless, the field is witnessing an impressive lineup of approaches that aim to modulate complement activity at various steps of the cascade using a diversified toolbox of therapeutics that range from large biologicals (e.g., antibodies and fusion proteins) to small interfering RNAs, aptamers and low-molecular-weight peptidic and non-peptidic inhibitors (Ricklin & Lambris, 2015;Ricklin & Lambris, 2013b;Risitano, 2015). In this Review, we discuss recent developments in complement therapeutics, with an emphasis on C3-targeted approaches, and their potential for the treatment of oral and systemic inflammatory diseases.
TRENDS AND CHALLENGES IN COMPLEMENT DRUG DESIGN
Interception at the early stages of complement initiation, such as inhibition of C1s, affords effective blockade of a single pathway (i.e., the classical pathway in the case of C1s), while allowing other complement pathways operate and preserve their homeostatic and immune surveillance functions (Ricklin & Lambris, 2013c). However, this approach might bear minimal therapeutic merit, if the classical pathway is not exclusively responsible for complement activation in the clinical disorder under question. On the other hand, strategies that target C3 as the point of intervention effectively block all activation, amplification and downstream receptor-triggered effector mechanisms. Such an approach may have greater therapeutic value in clinical conditions associated with systemic complement activation, excessive C3 fragment deposition and perpetually sustained autologous tissue injury, but may also blunt beneficial activities, such as microbial opsonophagocytosis and tissue immune surveillance (Ricklin & Lambris, 2015;Ricklin & Lambris, 2013c). Conversely, inhibition at the level of C5 appears more modular, affording protection from the detrimental effects of C5a generation and MAC formation, while preserving activation/amplification at the level of C3. Alternatively, specific blockade of C5aR1 signaling (using small-molecule inhibitors such as PMX53) targets the detrimental consequences of C5aR-mediated inflammatory activation but allows the terminal pathway proceed to the formation of MAC with its ensuing cytolytic effects. Interception of complement at later stages of the cascade (such as blocking C5 and C5aR1 signaling) balances on a ‘tight rope’, with elimination of downstream effector functions that may drive pathology on one end, but also perpetuation of C3 activation/alternative pathway amplification and C3 fragment deposition on the other end.
Emerging evidence from various disease models and human pathologies suggests that inborn or acquired complement dysregulation rather than a complete functional shutdown of the system is mainly responsible for the detrimental proinflammatory ramifications that exacerbate pathology and may lead to chronic medical complications and a deteriorated quality of life (Ricklin & Lambris, 2013c). Therefore, any therapeutic intervention that targets complement activity should aim to a prudent regulation/monitoring of complement’s activity to maintain homeostatic control and avoid disruption of long-term immunosurveillance. Given the pivotal role of various complement components (e.g. C3 and C5) in the opsonophagocytic clearance of microbial intruders, a treatment scheme including antimicrobial prophylaxis should also be considered as an essential ‘side arm’ in any complement-targeted therapeutic protocol.
Complement-based therapeutic design is dominated by two prevalent ‘schools of thought’: Namely, i) the ‘systemic interception’ approach that exerts its therapeutic effects using biologics or small-sized inhibitors that target complement proteins in the fluid phase and ii) the ‘surface-targeting’ approach that directs inhibitors closer to the complement-opsonized surface. In general terms, the adverse consequences of complement activation are manifested as proinflammatory reactions in the vicinity or directly on host cell surfaces that sustain the activation/amplification process. In several cases, systemically delivered complement inhibitors have proven quite effective in containing these surface-induced inflammatory or procoagulant complications (Ricklin & Lambris, 2013b;Kourtzelis et al., 2010;Ekdahl et al., 2011;Klapper et al., 2014). However, recent studies have illustrated a wide array of highly innovative and mechanistically subtle strategies for targeting complement inhibitors to the diseased surface (Ricklin & Lambris, 2013c). Such a targeting approach is believed to offer a number of advantages by selectively blocking complement at the tissue or site that triggers local activation, instead of saturating the circulation with high doses of inhibitor in order to achieve lasting therapeutic efficacy (Ricklin et al., 2013). Indeed, a successful paradigm illustrating the therapeutic potential of this approach is the design of fusion proteins (e.g., TT30, Alexion) consisting of functional modules of regulator of complement activation proteins such as factor H (FH; CCP domains 1–5), combined with the iC3b/C3dg-binding domains of complement receptor 2 (CR2; CCP1–4) (Fridkis-Hareli et al., 2011). In principle, such chimeric regulators help localize the inhibitory effect to iC3b/C3dg-opsonized surfaces by tethering the inhibitory module to the surface through the CR2 moiety. These modular inhibitors have shown great promise as therapeutic agents in alternative pathway-mediated disease models (Holers et al., 2013;Banda et al., 2009). Another example underscoring the emerging therapeutic potential of surface-targeted inhibitors is mini-FH, a miniaturized version of human FH (directly linking the modules CCP1–4 and CCP19–20) with unique triple-targeting properties for recognition of complement-opsonized surfaces, host cell markers and products of oxidative damage (Schmidt et al., 2013). This new inhibitor has recently shown promising therapeutic results in a disease model of PNH, protecting patient erythrocytes from C3 opsonization and intravascular hemolysis (Schmidt et al., 2013). Other therapeutic approaches have exploited endogenous C3 regulators to increase the biocompatibility of non-self or artificial surfaces. For instance, recruitment of factor H to surfaces coated with a factor H-binding peptide can prevent AP-mediated complement activation/amplification and attenuate blood-mediated thromboinflammatory reactions and may thus have important implications for transplantation medicine and biomaterial-triggered pathologies (Wu et al., 2011;Nilsson et al., 2013).
Notably, the genetic profiling of large patient cohorts has revealed another level of complexity that should be taken into consideration in complement-based drug design efforts. Genome-wide association studies and whole exome sequencing approaches have recently unveiled previously elusive regulatory circuits that appear to be fine-tuned by subtle genetic variations in distinct complement-related gene loci (Haines et al., 2005;Schramm et al., 2014;Harris et al., 2012). In this respect, complement polymorphic variants in conjunction with other immune-related genetic traits appear to forge intricate associations that have unmasked novel pathogenetic mechanisms underlying disease predisposition and prognosis (Schramm et al., 2014). In some cases, such polymorphic gene variants have even been held responsible for the manifestation of subsided responses to therapy by patients treated with first-in-class anti-complement agents (Nishimura et al., 2014). These developments have clearly underscored the importance of integrating population-wide genetic profiling, clinical monitoring and preclinical drug validation in appropriate disease models into the design of effective therapeutic interventions.
TARGETS AND SAFETY IN THERAPEUTIC MODULATION OF COMPLEMENT
Given that complement proteins cross-talk with other innate immune and proinflammatory systems (e.g. TLRs, cytokines, the contact/coagulation system, angiogenic and growth factors), complement dysregulation is likely to affect the course of disease in a context-specific manner (Hajishengallis & Lambris, 2010;Ricklin & Lambris, 2013a). Therefore targeting the same complement component may not be optimal for all cases and therapeutic interventions that intercept upstream, central (C3-targeted) or terminal (lytic pathway) components may each have their own advantages and limitations, depending on specific context (Mastellos et al., 2014).
Among different therapeutic targets, C3 is strategically positioned in the complement cascade. It serves as a central ‘hub’ that transduces initiating signals into downstream activation of effectors and rapid amplification of complement responses on diseased cell surfaces (Ricklin & Lambris, 2015). C3 interception may thus afford potent and broad complement inhibition in any inflammatory disorder that engages complement either as an early innate ‘sensor’ of danger signals (e.g. products of cell oxidative damage) or as a mediator that perpetuates the vicious cycle of inflammation. C3 mediates a plethora of protein-protein interactions that can be modulated by small-sized inhibitors through allosteric changes and/or steric hindrance, such as in the case of compstatin and its latest derivatives (see below) (Fig. 1). Dysbiotic microbial communities leading to sustained inflammation as seen in periodontitis have recently emerged as a novel paradigm of how C3 inhibition may intercept crucial pathways driving disease pathogenesis (key pathophysiological aspects are discussed later in this review) (Hajishengallis, 2014a).
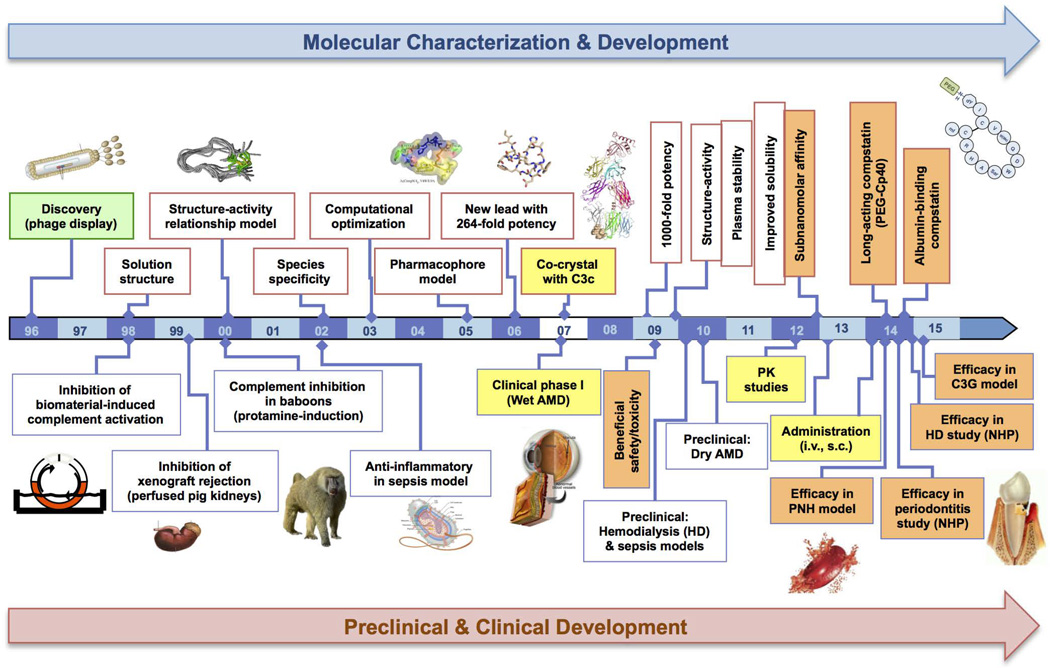
Figure 1. Roadmap of compstatin’s structure-function optimization and important milestones achieved towards its clinical translation.
The crossdisciplinary effort placed on compstatin’s molecular characterization and structure-guided refinement culminated in a series of improved, more potent derivatives displaying favourable pharmacokinetic profiles, increased plasma residence and sustained therapeutic efficacy in various NHP disease models. This comprehensive timeline reflects the tight reciprocity and consolidation of key molecular developments (i.e. the resolution of the C3c-compstatin crystal structure by Janssen et al., 2007) with the preclinical and clinical evaluation program of compstatin.
(Figure 1 has been adapted from the article “Compstatin: a C3-targeted complement inhibitor reaching its prime for bedside intervention’, Eur J Clin Invest. 2015, 45(4):423–40, with permission from Wiley)
By targeting the central component of complement, C3-based therapeutic intervention may raise concerns in terms of maintaining antimicrobial defense during long-term clinical intervention. Despite increased susceptibility to pyogenic infections observed in patients with primary C3 deficiencies, there is still limited clinical experience concerning the potential ramifications of long-term and systemic anti-C3 therapy (Pickering et al., 2000;Mastellos et al., 2015). It should be noted however that individuals with primary C3 deficiencies display increased risk for infections primarily in the early stages of life (Reis et al., 2006). When these patients reach adulthood, their susceptibility typically subsides, suggesting the operation of compensatory mechanisms once immunity is fully developed (Reis et al., 2006). Importantly, C3 interception using small-sized inhibitors can be readily phased out in a clinical protocol allowing for rapid recovery of C3’s opsonic activity during an infection. Experience from currently approved anti-complement therapies (e.g. eculizumab) has taught us that a tailored vaccination program against encapsulated bacteria and perhaps long-term prophylactic use of antibiotics would certainly suffice for applying prolonged C3 interception in a chronic setting (e.g., in PNH treatment). On the other hand, acute protocols involving transient C3 interception (e.g., during hemodialysis (Reis et al., 2014) are not expected to increase the risk of infection, nor require prior meningococcal vaccination. Whereas other considerations include the potential impact of C3 intervention on immune complex clearance and autoimmune reactions, the lack of robust clinical data on any of these aspects renders such discussions over safety rather hypothetical (Mastellos et al, 2015). In fact, immune complex diseases are comparatively rare even in C3-deficient patients (Pickering et al., 2000), and recent evidence from mouse models suggests that the absence of functional C3 may even show preventive effects in autoimmune conditions (Scott & Botto, 2015). As C3 inhibitors make their way into clinical trials, definitive clinical experience will be obtained regarding the safety of C3-targeted interventions.
NEXT-GENERATION C3-TARGETED THERAPEUTICS: THE PARADIGM OF COMPSTATIN
Protecting C3 from cleavage by convertase complexes provides an attractive opportunity for comprehensive control of complement activation and amplification. However, the high plasma concentration of this protein and the intricate involvement of protein-protein interactions in its activation cycle impose substantial challenges for the development of C3 inhibitors. Thus far, members of the compstatin family are the only clinical drug candidates acting directly on C3 (Mastellos et al., 2015). Compstatin was discovered through a phage library screen as a 13-residue cyclic peptide that selectively binds to native C3 and to its bioactive fragments C3b, iC3b and C3c (Sahu et al., 1996). It prevents the convertase-dependent cleavage of C3, thereby blunting complement activation at the very core of this cascade (Ricklin & Lambris, 2013c). A major milestone in the roadmap towards the optimization of compstatin was the resolution of its crystal structure in complex with its binding partner C3c (Janssen et al., 2007) (Fig. 1). The co-crystal structure not only illuminated our understanding of the conformational dynamics of C3, but it also unraveled the structural basis of compstatin’s binding and inhibitory mode on C3 (Janssen et al., 2007). Cumulative structural and biochemical evidence strongly suggests that compstatin acts as an inhibitor of protein-protein interactions by binding to the β-chain of C3 and sterically hindering the binding of native C3 to the C3 convertases (Janssen et al., 2007;Ricklin & Lambris, 2008). This unique mode of action essentially explained how this small-sized inhibitor can single-handedly block all pathways of complement activation affording a broad and potent inhibitory effect in therapeutic protocols.
Capitalizing on the structural insight gained from target-bound compstatin, intensive rounds of structure-function refinement and optimization culminated in improved compstatin derivatives with enhanced inhibitory potency and target binding affinity almost 6000-fold greater than the original peptide (i.e., sub-nanomolar KD values have been reported for the latest derivative Cp40 and the clinically developed therapeutic AMY-101); for a comprehensive review see (Ricklin & Lambris, 2008;Mastellos et al., 2015;Qu et al., 2009b). Backbone N-methylation and C-terminal substitution approaches afforded a more constrained, ‘bound-like’ solution conformation to these derivatives (e.g., Cp20 analog) affecting their thermodynamic stability, and increasing their inhibitory potency and binding affinity for their target C3 protein. Moreover, structure-guided extension of compstatin’s N-terminus with non-proteinogenic amino-acids led to a significant enhancement of the peptide’s inhibitory activity and propelled a remarkable increase (in the subnanomolar scale) of its binding affinity for C3 (e.g., Cp40 analog) (Qu et al., 2013).
Throughout the development of compstatin, it was evident that both the parental peptide and its derivatives exhibit a narrow species specificity profile by binding exclusively to human and non-human primate (NHP) C3 (Sahu et al., 2003). This finding was originally considered surprising, given the high degree of phylogenetic conservation among C3 from various mammalian species. However, the resolution of the C3c-compstatin structure accounted for the high selectivity of compstatin for human and NHP C3 by localizing the binding site of compstatin to the MG4 and MG5 domains of the β-chain of C3 (Janssen et al., 2007). Critical residues located within these domains that were shown to be involved in the interaction with compstatin, i.e., Gly-345, His-392, Pro-393, Leu-454, and Arg-459, are highly conserved in human and primate C3 but deviate in other mammalian species (Janssen et al., 2007). The presence of different residues in these positions in the C3 of non-primate mammals would most likely lead to subtle structural changes obstructing compstatin’s access to the binding site.
In 2006, first-generation analogs of compstatin were licensed to Potentia Pharmaceuticals for clinical development in age-related macular degeneration (AMD) and have been evaluated in phase I and phase II trials. Meanwhile, the technology has been acquired by Apellis, which is developing these compstatin analogs for AMD and other indications. In contrast to local injection in AMD, systemic administration in inflammatory diseases puts higher demands concerning pharmacokinetics and stability. To enable swift therapeutic translation, the pharmacokinetic profile of compstatin analogs was evaluated in NHP and strategies for increasing plasma residence were explored [reviewed in (Mastellos et al., 2015)]. These studies showed that compstatin analogs display a two-phase elimination profile in circulation (Qu et al., 2013) with an initial rapid phase of clearance of the excess of free (unbound) peptide followed by a second, slower phase of plasma elimination that is largely mediated by the tight binding of compstatin to its abundant target protein, C3. Due to this ‘target-driven elimination profile’, next-generation compstatin analogs with binding affinities in the low or sub-nanomolar range such as Cp40 show exceptional pharmacokinetic behavior (Qu et al., 2013;Mastellos et al., 2015). Proof-of-concept studies showed that the plasma retention of compstatin analogs may be even further improved through conjugation to plasma carrier proteins by using albumin-binding moieties (Huang et al., 2014;Qu et al., 2009a) and PEGylation strategies that have led to an impressive increase of the peptide’s half-life (Huang et al., 2014;Risitano et al., 2014;Qu et al., 2009a). Yet even without those modifications, the beneficial pharmacokinetic properties and suitability for subcutaneous administration render Cp40 and other next-generation analogs, which have been licensed by Amyndas Pharmaceuticals and build the base for the clinical candidate AMY-101, suitable for prolonged therapeutic intervention in chronic inflammatory diseases such as PNH.
Overall, this structure-guided optimization has led to an impressive lineup of C3-targeted therapeutics that display favourable pharmacokinetic profiles and sustained biological efficacy both in acute and chronic protocols. Indeed, compstatin analogs are now advancing through clinical trials for diseases such as AMD and also show promise for clinical intervention in PNH, hemodialysis-associated thromboinflammation, periodontal disease, acute graft rejection, sepsis and various renal diseases [reviewed in (Mastellos et al., 2015)].
C3 INTERCEPTION AS A VALID THERAPEUTIC MODALITY IN INFLAMMATORY DISEASES
The development of compstatin also enabled the evaluation of C3-targeted complement inhibition in various inflammatory disorders. Meanwhile, compstatin analogs have been evaluated in a plethora of preclinical disease models characterized by dysregulated or excessive complement activation in a proinflammatory milieu (for an updated review see (Mastellos et al., 2015;Ricklin & Lambris, 2013c) (Fig. 1). Below are a few examples illustrating the therapeutic potential of C3-targeted intervention in acute or chronic inflammatory disorders and orphan indications with a prominent involvement of complement in their pathogenic landscape.
Periodontal disease
Periodontitis is a chronic inflammatory disease that leads to the destruction of the tooth-supporting structures (e.g., gingiva, periodontal ligament, and the alveolar bone), collectively known as the periodontium (Hajishengallis, 2014a). The disease is driven by inflammation induced by bacterial communities forming on subgingival tooth sites (Lamont & Hajishengallis, 2015). Recent human microbiome analyses (both metagenomic and metatranscriptomic) and animal model-based mechanistic studies collectively suggest that periodontitis is not a bacterial infection in the classical sense, i.e., caused by a single or a limited number of pathogens, but rather is the result of a polymicrobial community-induced disruption of the host’s homeostasis leading to destructive inflammation in susceptible individuals (Abusleme et al., 2013;Dewhirst et al., 2010;Duran-Pinedo et al., 2014;Griffen et al., 2012;Hajishengallis & Lamont, 2012;Hajishengallis et al., 2011;Jiao et al., 2013;Jorth et al., 2014;Kumar et al., 2006;Lamont & Hajishengallis, 2015;Maekawa et al., 2014b;Perez-Chaparro et al., 2014;Settem et al., 2012). According to the polymicrobial synergy and dysbiosis (PSD) model, the host immune response is initially subverted by keystone pathogens with the help of accessory pathogens and is subsequently over-activated by pathobionts, leading to destructive inflammation in susceptible hosts (Lamont & Hajishengallis, 2015).
Chronic periodontitis affects >47% of U.S. adults (Eke et al., 2012). In its severe form which afflicts 8.5% of adults, periodontitis is not only a common cause of tooth loss, but is also associated with increased risk for certain systemic disorders (e.g., atherosclerosis, diabetes, rheumatoid arthritis, and adverse pregnancy outcomes) (Hajishengallis, 2015;Han et al., 2014;Kebschull et al., 2010;Lalla & Papapanou, 2011). Due to its high prevalence and significant economic burden (Beikler & Flemmig, 2011;Eke et al., 2012) and the fact that not all cases are responsive to combined mechanical and antimicrobial therapy (scaling and root planning, surgery, and systemically administered antibiotics) (Colombo et al., 2012), it is important to develop innovative and cost-effective therapeutic interventions as adjuncts to standard treatment.
Early clinical studies have shown a strong association between periodontitis and complement activation. Gingival crevicular fluid (GCF) collected from periodontitis patients was shown to contain activated complement fragments at significantly higher concentrations than in GCF from healthy individuals (Patters et al., 1989;Schenkein & Genco, 1977). Consistently, chronically inflamed gingivae are characterized by the abundant presence of complement components and cleavage products, whereas complement is undetectable or present at lower abundance in healthy gingival biopsy samples (Lally et al., 1982;Nikolopoulou-Papaconstantinou et al., 1987;Toto et al., 1978). Importantly, induction of experimental gingivitis in human volunteers caused progressive complement activation (as determined by C3 conversion) correlating with increased clinical inflammatory parameters (Patters et al., 1989). Conversely, the resolution of inflammation in periodontitis patients undergoing therapy resulted in decreased complement activation, as revealed by reduced C3-to-C3c conversion in the GCF (Niekrash & Patters, 1985). Using an integrative gene prioritization method and databases from genome-wide association studies and microarray experiments, another recent study has identified C3 among the top 21 most promising candidate genes involved in periodontitis (Zhan et al., 2014).
Though very important, the above-discussed clinical observations are correlative and do not necessarily imply a cause-and-effect relationship between complement activation and periodontal tissue destruction. In this regard, it could be argued that complement activation in periodontitis represents a host attempt to control the periodontal microbiota through complement-dependent opsonophagocytic mechanisms. Conclusive evidence for a destructive role of complement in periodontitis was provided by recent intervention studies in animal models of periodontitis, including NHP. Specifically, it has been shown that complement is involved in both the dysbiotic transformation of the periodontal microbiota and the inflammatory response that leads to destruction of periodontal bone (Abe et al., 2012;Hajishengallis & Lambris, 2011;Hajishengallis et al., 2011;Maekawa et al., 2014a;Maekawa et al., 2014b;Wang et al., 2010) (Fig. 2).
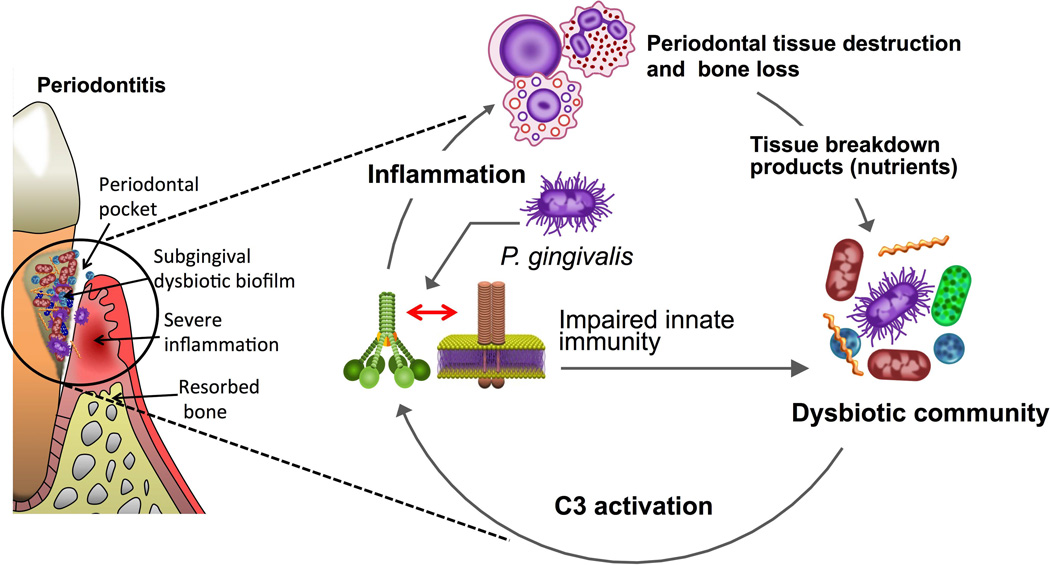
Figure 2. Complement involvement in periodontal dysbiosis and inflammation.
Periodontitis is induced by a polymicrobial bacterial community, wherein different members have distinct roles that synergize to cause destructive inflammation in periodontal pockets. Keystone pathogens manipulate the host response (for instance, P. gingivalis induces a subversive complement-Toll-like receptor cross-talk; see text for details) leading to the dysbiotic transformation of the microbiota. In dysbiosis, pathobionts over-activate the inflammatory response in a complement C3-dependent manner resulting in destructive periodontal inflammation and bone loss. Inflammation and dysbiosis reinforce each other since inflammatory tissue breakdown products are used as nutrients by the dysbiotic microbiota, which further exacerbates inflammation and perpetuates a disease-provoking vicious cycle. Therapeutic intervention at the C3 level with specific inhibitors (Cp40) has blocked periodontitis in non-human primates.
In a mouse model of periodontitis, Porphyromonas gingivalis– while at low colonization levels– was shown to act as a ‘keystone pathogen’, that is, to alter the numbers and composition of the entire microbial community leading to dysbiosis and periodontitis (Hajishengallis et al., 2011). P. gingivalis fails to cause periodontitis in the absence of commensal bacteria, i.e., in germ-free mice, despite its ability to colonize this host (Hajishengallis et al., 2011), suggesting that the P. gingivalis-altered microbiota is required for induction of disease in this model. The capacity of P. gingivalis to instigate dysbiosis and bone loss is dependent on complement manipulation and specifically required the presence of C5aR1 (CD88) (Hajishengallis et al., 2011). Genetic or pharmacological ablation of C5aR1 prevented P. gingivalis-induced dysbiosis and periodontal bone loss (Hajishengallis et al., 2011;Maekawa et al., 2014b). Mechanistically, P. gingivalis was shown to instigate a subversive cross-talk between C5aR1 and Toll-like receptor 2 (TLR2) in neutrophils that selectively inhibits their killing activity without suppressing their inflammatory responses (Maekawa et al., 2014b). Specifically, both in vitro (in human or mouse neutrophils) and in vivo (in mice), P. gingivalis-induced C5aR1-TLR2 crosstalk disarms and disassociates a host-protective TLR2–MyD88 pathway from a TLR2–MyD88-adaptor-like (Mal)–PI(3)K pathway, which enhances the fitness of P. gingivalis and bystander bacteria by blocking phagocytosis and promoting inflammation (Maekawa et al., 2014b), a requirement for nutrient acquisition by the bacteria in the form of tissue-breakdown products (Hajishengallis, 2014b). It should be noted that, despite enhanced inflammation in periodontitis (hence increased complement activation), P. gingivalis and other periodontitis-associated bacteria (such as Tannerella forsythia, Treponema denticola, and Prevotella intermedia) have several mechanisms to protect themselves from complement-mediated opsonophagocytosis or lysis, including prevention of opsonization and capturing and co-opting physiological inhibitors of complement (e.g., FH or C4b-binding protein) (Jusko et al., 2012;Miller et al., 2012;Popadiak et al., 2007;Potempa et al., 2009;Schenkein et al., 1995).
Interestingly, P. gingivalis can activate C5aR1 independently of the immunologically activated complement cascade, as this bacterium can release biologically active C5a from C5 through the action of its arginine-specific gingipains (Liang et al., 2011;Wang et al., 2010;Wingrove et al., 1992). Accordingly, P. gingivalis was shown to retain its capacity to colonize the periodontium of C3-deficient (C3–/–) mice, which express normal levels of C5 and C5aR1 that are required for P. gingivalis colonization and induction of dysbiosis (Maekawa et al., 2014a). Remarkably, although P. gingivalis was able to colonize C3–/– mice, its dysbiotic effect was only transient and the periodontal microbiota was not sustained at high levels throughout the experimental period, in contrast to wild-type mice where dysbiosis was stable. Moreover, P. gingivalis-colonized C3–/– mice exhibited diminished periodontal inflammation and bone loss as compared to P. gingivalis-colonized wild-type mice (Maekawa et al., 2014a). These findings suggest that C3 is crucial for the long-term sustenance of the dysbiotic microbiota and for maximal inflammatory bone loss. The reason why P. gingivalis-induced dysbiosis cannot be sustained in C3–/– mice is likely related to the severely attenuated periodontal inflammation, which− as alluded to above− is required for nutrient acquisition (e.g., degraded collagen peptides and heme-containing compounds). Consistent with the concept that periodontitis-associated bacteria are inflammophilic and thrive under inflammatory conditions, the bacterial biomass of human periodontitis-associated biofilms increases with escalating periodontal inflammation (Abusleme et al., 2013). Moreover, anti-inflammatory treatments in animal models reduce the periodontal bacterial load (Abe et al., 2014;Eskan et al., 2012;Hasturk et al., 2007;Moutsopoulos et al., 2014).
The suitability of C3 as a therapeutic target in periodontitis was assessed in a NHP model (cynomolgus monkeys) using compstatin analog Cp40 (Maekawa et al., 2014a). In this intervention study, silk ligatures were placed around posterior teeth on both halves of the lower jaw (mandible); this treatment results in massive local accumulation of bacteria and development of inflammation and bone loss in a few weeks (Assuma et al., 1998). The Cp40 study had a 6-week duration and involved a split-mouth experimental design. Specifically, one side was treated with active drug (Cp40) and the other with inactive analog (control peptide), therefore, each animal served as its own control. Local intragingival injections of Cp40 resulted in decreased clinical indices that measure periodontal inflammation and tissue destruction, such as clinical attachment loss and gingival index. The decreased clinical inflammation correlated with lower GCF levels of proinflammatory or osteoclastogenic cytokines (e.g., TNF, IL-1β, IL-17, and RANKL) and decreased numbers of osteoclasts in bone biopsy specimens (Maekawa et al., 2014a). Accordingly, radiographic analysis showed that Cp40 caused a significant inhibition of periodontal bone loss. In contrast to RANKL, the GCF levels of osteoprotegerin (OPG), a natural inhibitor of RANKL-induced osteoclastogenesis, were sustained at higher levels in Cp40-treated sites than control sites during the course of the study. Therefore, Cp40 caused a favorable reversal of the RANKL/OPG ratio, which is considered a useful biomarker of human periodontitis (Belibasakis & Bostanci, 2012). More recently, locally administered Cp40 was shown to arrest preexisting, naturally occurring chronic periodontitis in cynomolgus monkeys (Maekawa et al, unpublished observations). It should be noted that local C3 inhibition is not likely to lead to uncontrolled bacterial growth. In this regard, 6 weeks after P. gingivalis-induced periodontitis, C3−/− mice had decreased total counts in their periodontal microbiota as compared to C3+/+ controls (Maekawa et al., 2014a), suggesting that defective complement activation does not predispose to defective immune surveillance in the periodontium. In fact, the decreased bacterial burden in C3−/− mice may be attributed to the reduced inflammation, which would be expected to deprive the bacteria of nutrients they can potentially derive from inflammatory tissue breakdown.
Importantly, the immune system and periodontal anatomy of the cynomolgus monkey are very similar to that of humans, and periodontitis in the cynomolgus monkey exhibits bacteriological, immunohistological, and clinical features that are highly similar to those of human periodontitis (Page & Schroeder, 1982). Hence, this model is considerably more predictive of drug efficacy in human periodontitis compared to widely used models such as those involving rodents, rabbits, or dogs. The study by Maekawa et al therefore provides strong proof-of-concept for the therapeutic potential of Cp40 in human periodontitis. At present, there is no satisfactory adjunctive therapy to scaling and root planing for the treatment of chronic periodontitis. The use of antimicrobials and generic antibiotics as adjunctive therapies has met with limited success (Krayer et al., 2010;Rams et al., 2014). As a host modulation-based approach, complement inhibition is advantageous to antimicrobial strategies since it is the host response that ultimately inflicts damage upon the periodontal tissues. Moreover, as documented above, the inhibition of periodontal inflammation also exerts indirect antimicrobial effects, since the periodontitis-associated microbiota requires an inflammatory environment to obtain essential nutrients for its growth and sustenance (Eskan et al., 2012;Hasturk et al., 2007;Maekawa et al., 2014a;Moutsopoulos et al., 2014). In summary, periodontitis may be a promising clinical application for Cp40 and the clinically developed drug candidate AMY-101. This possibility merits investigation in future clinical trials.
Paroxysmal nocturnal hemoglobinuria (PNH)
PNH is a rare but chronic debilitating hematological disorder whose cardinal clinical symptom is intravascular hemolysis perpetuated by complement dysregulation on PNH erythrocytes due to a lack of surface-bound complement regulators (Risitano, 2013). The anti-C5 monoclonal antibody eculizumab (Soliris; Alexion) is currently used for the treatment of PNH. Eculizumab blocks complement-dependent intravascular hemolysis by preventing C5 cleavage and MAC formation on susceptible erythrocytes (Luzzatto et al., 2010). However, a significant fraction of PNH patients fail to sufficiently respond to anti-C5 therapy, thus remaining dependent on blood transfusions (Risitano, 2013). This clinical observation has suggested that extravascular hemolysis of PNH erythrocytes due to persistent C3 opsonization and recognition by phagocytic cells might exacerbate pathology in this chronic disease (Lin et al., 2015). C3 interception therefore has emerged as a promising strategy for controlling the whole spectrum of detrimental consequences of complement dysregulation in PNH (Mastellos et al., 2014). In this respect, the compstatin analog Cp40 has shown efficacy in preventing opsonization with C3 fragments and in abrogating MAC-mediated hemolysis of PNH erythrocytes (Risitano et al., 2014). These studies indicate that C3 interception might afford greater therapeutic benefit to PNH patients than standard anti-C5 therapy by also preventing extravascular phagocytosis of C3-opsonized PNH cells (Lin et al., 2015;Mastellos et al., 2014). The favorable pharmacokinetic and safety profiles and the sustained efficacy of Cp40/AMY-101 in NHP models point to a viable option for chronic administration of C3 inhibitors in PNH patients.
Sepsis
Polymicrobial sepsis may lead to multi-organ dysfunction through a generalized impairment of the innate antimicrobial response, marked by pronounced thromboinflammation and massive complement activation (Bosmann & Ward, 2013). Compstatin derivatives have shown consistent efficacy in ex vivo and NHP models of bacterial sepsis (Mastellos et al., 2015). In a baboon model of sepsis closely resembling human pathology, compstatin conferred significant organ protection intercepting key proinflammatory and procoagulant markers, thereby suggesting that it might serve as a new treatment option even in late ‘rescue’ regimens (Silasi-Mansat et al., 2010). Moreover, combined C3 interception and CD14 blockade has shown promising results as a novel anti-inflammatory intervention in murine and human whole-blood models of sepsis (Egge et al., 2014).
Hemodialysis-induced inflammation and other conditions
Biomaterial-induced complement activation has been linked to thromboinflammatory complications that may evoke concerns for end-stage renal disease patients undergoing hemodialysis (Nilsson et al., 2010;Kourtzelis et al., 2010;DeAngelis et al., 2012). Recent studies in an NHP model of hemodialysis-induced inflammation have shown that a single injection of Cp40 can effectively block complement activation throughout the hemodialysis session; the treatment also upregulated the anti-inflammatory cytokine IL-10 (Reis et al., 2014). Besides these examples of acute or chronic inflammatory conditions that can be controlled by small-sized C3 inhibitors, promising results have also been generated in models of allogeneic solid organ transplantation, cell-based xenotransplantation and in renal disease models (e.g. C3 glomerulopathy) (Kourtzelis et al., 2014;Zhang et al., 2015;Mastellos et al., 2015). Compstatin derivatives have shown promise as effective therapeutics that can attenuate antibody-mediated graft rejection by downregulating proinflammatory markers and attenuating the detrimental effects of thromboinflammation and sustained cellular immune activation (i.e. leukocyte infiltration) (Mastellos et al., 2015).
Notably, therapeutic C3 interception by compstatin has received endorsement by international regulatory authorities such as the FDA and EMA (Mastellos et al., 2015). The recently disclosed C3-targeted therapeutic AMY-101 (Amyndas Pharmaceuticals) has been granted orphan drug designation for the treatment of PNH and is currently under clinical development as a treatment option for PNH and ABO-incompatible kidney transplantation (Mastellos et al., 2015).
CONCLUDING REMARKS AND OUTLOOK
Compelling clinical evidence has indicated that inborn or acquired complement dysregulation drives pathology in many inflammatory and immune-mediated disorders. Appreciating complement’s contribution in human pathophysiology has spawned new opportunities for developing anti-inflammatory therapeutics that could be amenable to clinical use. The launch of the first complement-targeting drugs has reshaped the clinical landscape of orphan diseases with a prominent inflammatory signature. C3 interception has emerged as a promising therapeutic option for diseases involving complement dysregulation and persistent C3-fragment opsonization of host surfaces such as PNH, biomaterial-induced pathologies, renal diseases and organ transplantation. A new class of C3-targeted inhibitors of the compstatin family (including the recently disclosed Cp40 derivative and the Cp40-based therapeutic AMY-101) displays improved pharmacokinetic profiles and sustained biological potency that make them suitable for further clinical development and bedside intervention. These drug candidates show promise as potent therapeutics for systemic or local application in complement-related diseases, and also point to a more affordable treatment option for a wide spectrum of clinical indications, nowadays still relying on costly antibody-based biologics (Shaughnessy, 2012). From an oral perspective, the local application of compstatin (i.e. Cp40) in sites of ongoing periodontal inflammation triggered by dysbiotic microbial communities has shown promising results in terms of biological efficacy in preclinical NHP models.
In view of the emerging impact of polymorphic genetic variations on patient drug responses, these new therapeutic options should be cautiously evaluated in a personalized medicine context through scrutinous monitoring of multiple patient biomarkers. Overall, small-sized C3 inhibitors underscore the therapeutic potential of C3 intervention and open up new avenues for developing tailored immunotherapeutics for acute or chronic indications.
Acknowledgements
The authors are supported by grants from the U.S. National Institutes of Health: DE015254, DE017138, DE021685, and DE024716 (GH); AI003040, AI068730, EY020633, and GM097747 (JDL), the European Community’s Seventh Framework Programme under grant agreement number 602699 (DIREKT) (JDL) and the Greek General Secretariat for Research and Technology and the European Regional Development Fund under the Action “Development Grants For Research Institutions–KRIPIS” of OPCE II (DCM).
REFERENCES
- Abe T, Hosur KB, Hajishengallis E, et al. Local complement-targeted intervention in periodontitis: proof-of-concept using a C5a receptor (CD88) antagonist. J Immunol. 2012;189:5442–5448. doi: 10.4049/jimmunol.1202339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe T, Shin J, Hosur K, Udey MC, Chavakis T, Hajishengallis G. Regulation of osteoclast homeostasis and inflammatory bone loss by MFG-E8. J Immunol. 2014;193:1383–1391. doi: 10.4049/jimmunol.1400970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abusleme L, Dupuy AK, Dutzan N, et al. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME. J. 2013;7:1016–1025. doi: 10.1038/ismej.2012.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assuma R, Oates T, Cochran D, Amar S, Graves DT. IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. J Immunol. 1998;160:403–409. [PubMed] [Google Scholar]
- Banda NK, Levitt B, Glogowska MJ, et al. Targeted inhibition of the complement alternative pathway with complement receptor 2 and factor H attenuates collagen antibody-induced arthritis in mice. J Immunol. 2009;183:5928–5937. doi: 10.4049/jimmunol.0901826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beikler T, Flemmig TF. Oral biofilm-associated diseases: trends and implications for quality of life, systemic health and expenditures. Periodontol. 2000. 2011;55:87–103. doi: 10.1111/j.1600-0757.2010.00360.x. [DOI] [PubMed] [Google Scholar]
- Belibasakis GN, Bostanci N. The RANKL-OPG system in clinical periodontology. J. Clin. Periodontol. 2012;39:239–248. doi: 10.1111/j.1600-051X.2011.01810.x. [DOI] [PubMed] [Google Scholar]
- Bosmann M, Ward PA. The inflammatory response in sepsis. Trends Immunol. 2013;34:129–136. doi: 10.1016/j.it.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo AP, Bennet S, Cotton SL, et al. Impact of periodontal therapy on the subgingival microbiota of severe periodontitis: comparison between good responders and individuals with refractory periodontitis using the human oral microbe identification microarray. J. Periodontol. 2012;83:1279–1287. doi: 10.1902/jop.2012.110566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deangelis RA, Reis ES, Ricklin D, Lambris JD. Targeted complement inhibition as a promising strategy for preventing inflammatory complications in hemodialysis. Immunobiology. 2012;217:1097–1105. doi: 10.1016/j.imbio.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewhirst FE, Chen T, Izard J, et al. The human oral microbiome. J. Bacteriol. 2010;192:5002–5017. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran-Pinedo AE, Chen T, Teles R, et al. Community-wide transcriptome of the oral microbiome in subjects with and without periodontitis. ISME. J. 2014;8:1659–1672. doi: 10.1038/ismej.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egge KH, Thorgersen EB, Lindstad JK, et al. Post challenge inhibition of C3 and CD14 attenuates Escherichia coli-induced inflammation in human whole blood. Innate. Immun. 2014;20:68–77. doi: 10.1177/1753425913482993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekdahl KN, Lambris JD, Elwing H, et al. Innate immunity activation on biomaterial surfaces: a mechanistic model and coping strategies. Adv. Drug Deliv. Rev. 2011;63:1042–1050. doi: 10.1016/j.addr.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J. Dent. Res. 2012;91:914–920. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- Eskan MA, Jotwani R, Abe T, et al. The leukocyte integrin antagonist Del-1 inhibits IL-17-mediated inflammatory bone loss. Nat. Immunol. 2012;13:465–473. doi: 10.1038/ni.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridkis-Hareli M, Storek M, Mazsaroff I, et al. Design and development of TT30, a novel C3d–targeted C3/C5 convertase inhibitor for treatment of human complement alternative pathway-mediated diseases. Blood. 2011;118:4705–4713. doi: 10.1182/blood-2011-06-359646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffen AL, Beall CJ, Campbell JH, et al. Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. ISME. J. 2012;6:1176–1185. doi: 10.1038/ismej.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines JL, Hauser MA, Schmidt S, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- Hajishengallis G. Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends Immunol. 2014a;35:3–11. doi: 10.1016/j.it.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G. The inflammophilic character of the periodontitis-associated microbiota. Mol. Oral Microbiol. 2014b;29:248–257. doi: 10.1111/omi.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015;15:30–44. doi: 10.1038/nri3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Lambris JD. Crosstalk pathways between Toll-like receptors and the complement system. Trends Immunol. 2010;31:154–163. doi: 10.1016/j.it.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Lambris JD. Microbial manipulation of receptor crosstalk in innate immunity. Nat. Rev. Immunol. 2011;11:187–200. doi: 10.1038/nri2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Lamont RJ. Beyond the red complex and into more complexity: the polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol. Oral Microbiol. 2012;27:409–419. doi: 10.1111/j.2041-1014.2012.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Liang S, Payne MA, et al. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host. Microbe. 2011;10:497–506. doi: 10.1016/j.chom.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YW, Houcken W, Loos BG, Schenkein HA, Tezal M. Periodontal disease, atherosclerosis, adverse pregnancy outcomes, and head-and-neck cancer. Adv. Dent. Res. 2014;26:47–55. doi: 10.1177/0022034514528334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris CL, Heurich M, Rodriguez de CS, Morgan BP. The complotype: dictating risk for inflammation and infection. Trends Immunol. 2012;33:513–521. doi: 10.1016/j.it.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasturk H, Kantarci A, Goguet-Surmenian E, et al. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J. Immunol. 2007;179:7021–7029. doi: 10.4049/jimmunol.179.10.7021. [DOI] [PubMed] [Google Scholar]
- Holers VM, Rohrer B, Tomlinson S. CR2-mediated targeting of complement inhibitors: bench-to-bedside using a novel strategy for site-specific complement modulation. Adv. Exp. Med. Biol. 2013;735:137–154. doi: 10.1007/978-1-4614-4118-2_9. [DOI] [PubMed] [Google Scholar]
- Huang Y, Reis ES, Knerr PJ, van der Donk WA, Ricklin D, Lambris JD. Conjugation to albumin-binding molecule tags as a strategy to improve both efficacy and pharmacokinetic properties of the complement inhibitor compstatin. ChemMedChem. 2014;9:2223–2226. doi: 10.1002/cmdc.201402212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen BJ, Halff EF, Lambris JD, Gros P. Structure of compstatin in complex with complement component C3c reveals a new mechanism of complement inhibition. J. Biol. Chem. 2007;282:29241–29247. doi: 10.1074/jbc.M704587200. [DOI] [PubMed] [Google Scholar]
- Jiao Y, Darzi Y, Tawaratsumida K, et al. Induction of bone loss by pathobiont-mediated Nod1 signaling in the oral cavity. Cell Host. Microbe. 2013;13:595–601. doi: 10.1016/j.chom.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorth P, Turner KH, Gumus P, Nizam N, Buduneli N, Whiteley M. Metatranscriptomics of the human oral microbiome during health and disease. MBio. 2014;5:e01012–e01014. doi: 10.1128/mBio.01012-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jusko M, Potempa J, Karim AY, et al. A metalloproteinase karilysin present in the majority of Tannerella forsythia isolates inhibits all pathways of the complement system. J. Immunol. 2012;188:2338–2349. doi: 10.4049/jimmunol.1101240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebschull M, Demmer RT, Papapanou PN. "Gum bug, leave my heart alone!"--epidemiologic and mechanistic evidence linking periodontal infections and atherosclerosis. J. Dent. Res. 2010;89:879–902. doi: 10.1177/0022034510375281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapper Y, Hamad OA, Teramura Y, et al. Mediation of a non-proteolytic activation of complement component C3 by phospholipid vesicles. Biomaterials. 2014 doi: 10.1016/j.biomaterials.2013.12.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourtzelis I, Ferreira A, Mitroulis I, et al. Complement Inhibition in a Xenogeneic Model of Interactions Between Human Whole Blood and Porcine Endothelium. Horm. Metab Res. 2014 doi: 10.1055/s-0034-1390452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourtzelis I, Markiewski MM, Doumas M, et al. Complement anaphylatoxin C5a contributes to hemodialysis-associated thrombosis. Blood. 2010;116:631–639. doi: 10.1182/blood-2010-01-264051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krayer JW, Leite RS, Kirkwood KL. Non-surgical chemotherapeutic treatment strategies for the management of periodontal diseases. Dent. Clin. North Am. 2010;54:13–33. doi: 10.1016/j.cden.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar PS, Leys EJ, Bryk JM, Martinez FJ, Moeschberger ML, Griffen AL. Changes in periodontal health status are associated with bacterial community shifts as assessed by quantitative 16S cloning and sequencing. J. Clin. Microbiol. 2006;44:3665–3673. doi: 10.1128/JCM.00317-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalla E, Papapanou PN. Diabetes mellitus and periodontitis: a tale of two common interrelated diseases. Nat. Rev. Endocrinol. 2011;7:738–748. doi: 10.1038/nrendo.2011.106. [DOI] [PubMed] [Google Scholar]
- Lally ET, McArthur WP, Baehni PC. Biosynthesis of complement components in chronically inflamed gingiva. J. Periodontal Res. 1982;17:257–262. doi: 10.1111/j.1600-0765.1982.tb01152.x. [DOI] [PubMed] [Google Scholar]
- Lamont RJ, Hajishengallis G. Polymicrobial synergy and dysbiosis in inflammatory disease. Trends Mol. Med. 2015;21:172–183. doi: 10.1016/j.molmed.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S, Krauss JL, Domon H, et al. The C5a receptor impairs IL-12-dependent clearance of Porphyromonas gingivalis and is required for induction of periodontal bone loss. J. Immunol. 2011;186:869–877. doi: 10.4049/jimmunol.1003252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, Schmidt CQ, Koutsogiannaki S, et al. Complement C3dg-mediated erythrophagocytosis: implications for paroxysmal nocturnal hemoglobinuria. Blood. 2015 doi: 10.1182/blood-2015-02-625871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzatto L, Risitano AM, Notaro R. Paroxysmal nocturnal hemoglobinuria and eculizumab. Haematologica. 2010;95:523–526. doi: 10.3324/haematol.2009.017848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa T, Abe T, Hajishengallis E, et al. Genetic and intervention studies implicating complement c3 as a major target for the treatment of periodontitis. J. Immunol. 2014a;192:6020–6027. doi: 10.4049/jimmunol.1400569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa T, Krauss JL, Abe T, et al. Porphyromonas gingivalis manipulates complement and TLR signaling to uncouple bacterial clearance from inflammation and promote dysbiosis. Cell Host. Microbe. 2014b;15:768–778. doi: 10.1016/j.chom.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastellos DC, Ricklin D, Yancopoulou D, Risitano A, Lambris JD. Complement in paroxysmal nocturnal hemoglobinuria: exploiting our current knowledge to improve the treatment landscape. Expert. Rev. Hematol. 2014;7:583–598. doi: 10.1586/17474086.2014.953926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastellos DC, Yancopoulou D, Kokkinos P, et al. Compstatin: a C3-targeted complement inhibitor reaching its prime for bedside intervention. Eur. J. Clin. Invest. 2015;45:423–440. doi: 10.1111/eci.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DP, Bell JK, McDowell JV, et al. Structure of factor H-binding protein B (FhbB) of the periopathogen, Treponema denticola: insights into progression of periodontal disease. J. Biol. Chem. 2012;287:12715–12722. doi: 10.1074/jbc.M112.339721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutsopoulos NM, Konkel J, Sarmadi M, et al. Defective neutrophil recruitment in leukocyte adhesion deficiency type I disease causes local IL-17-driven inflammatory bone loss. Sci. Transl. Med. 2014;6:229ra40. doi: 10.1126/scitranslmed.3007696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niekrash CE, Patters MR. Simultaneous assessment of complement components C3, C4, and B and their cleavage products in human gingival fluid. II. Longitudinal changes during periodontal therapy. J. Periodontal Res. 1985;20:268–275. doi: 10.1111/j.1600-0765.1985.tb00434.x. [DOI] [PubMed] [Google Scholar]
- Nikolopoulou-Papaconstantinou AA, Johannessen AC, Kristoffersen T. Deposits of immunoglobulins, complement, and immune complexes in inflamed human gingiva. Acta Odontol. Scand. 1987;45:187–193. doi: 10.3109/00016358709098858. [DOI] [PubMed] [Google Scholar]
- Nilsson B, Korsgren O, Lambris JD, Ekdahl KN. Can cells and biomaterials in therapeutic medicine be shielded from innate immune recognition? Trends Immunol. 2010;31:32–38. doi: 10.1016/j.it.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson PH, Ekdahl KN, Magnusson PU, et al. Autoregulation of thromboinflammation on biomaterial surfaces by a multicomponent therapeutic coating. Biomaterials. 2013;34:985–994. doi: 10.1016/j.biomaterials.2012.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura J, Yamamoto M, Hayashi S, et al. Genetic variants in C5 and poor response to eculizumab. N. Engl. J. Med. 2014;370:632–639. doi: 10.1056/NEJMoa1311084. [DOI] [PubMed] [Google Scholar]
- Page RC, Schroeder HE. Periodontitis in man and other animals - A comparative review. Basel, Switzerland: Karger; 1982. [Google Scholar]
- Patters MR, Niekrash CE, Lang NP. Assessment of complement cleavage in gingival fluid during experimental gingivitis in man. J. Clin. Periodontol. 1989;16:33–37. doi: 10.1111/j.1600-051x.1989.tb01609.x. [DOI] [PubMed] [Google Scholar]
- Perez-Chaparro PJ, Goncalves C, Figueiredo LC, et al. Newly identified pathogens associated with periodontitis: a systematic review. J. Dent. Res. 2014;93:846–858. doi: 10.1177/0022034514542468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering MC, Botto M, Taylor PR, Lachmann PJ, Walport MJ. Systemic lupus erythematosus, complement deficiency, and apoptosis. Adv. Immunol. 2000;76:227–324. doi: 10.1016/s0065-2776(01)76021-x. [DOI] [PubMed] [Google Scholar]
- Popadiak K, Potempa J, Riesbeck K, Blom AM. Biphasic effect of gingipains from Porphyromonas gingivalis on the human complement system. J. Immunol. 2007;178:7242–7250. doi: 10.4049/jimmunol.178.11.7242. [DOI] [PubMed] [Google Scholar]
- Potempa M, Potempa J, Kantyka T, et al. Interpain A, a cysteine proteinase from Prevotella intermedia, inhibits complement by degrading complement factor C3. PLoS. Pathog. 2009;5:e1000316. doi: 10.1371/journal.ppat.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu H, Magotti P, Ricklin D, Lambris JD. Development of Compstatin Derivative-Albumin Binding Peptide Chimeras for Prolonged Plasma Half-Life. 21st American Peptide Symposium; Proceedings of the 21st American Peptide Symposium; 2009a. pp. 219–220. [Google Scholar]
- Qu H, Ricklin D, Bai H, et al. New analogs of the clinical complement inhibitor compstatin with subnanomolar affinity and enhanced pharmacokinetic properties. Immunobiology. 2013;218:496–505. doi: 10.1016/j.imbio.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu H, Ricklin D, Lambris JD. Recent developments in low molecular weight complement inhibitors. Mol. Immunol. 2009b;47:185–195. doi: 10.1016/j.molimm.2009.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rams TE, Feik D, Mortensen JE, Degener JE, van Winkelhoff AJ. Antibiotic susceptibility of periodontal Streptococcus constellatus and Streptococcus intermedius clinical isolates. J. Periodontol. 2014;85:1792–1798. doi: 10.1902/jop.2014.130291. [DOI] [PubMed] [Google Scholar]
- Reis ES, Deangelis RA, Chen H, Resuello RR, Ricklin D, Lambris JD. Therapeutic C3 inhibitor Cp40 abrogates complement activation induced by modern hemodialysis filters. Immunobiology. 2014;220:476–482. doi: 10.1016/j.imbio.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis S, Falcao DA, Isaac L. Clinical aspects and molecular basis of primary deficiencies of complement component C3 and its regulatory proteins factor I and factor H. Scand. J. Immunol. 2006;63:155–168. doi: 10.1111/j.1365-3083.2006.01729.x. [DOI] [PubMed] [Google Scholar]
- Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat. Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklin D, Lambris JD. Complement-targeted therapeutics. Nat. Biotechnol. 2007;25:1265–1275. doi: 10.1038/nbt1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklin D, Lambris JD. Compstatin: a complement inhibitor on its way to clinical application. Adv. Exp. Med. Biol. 2008;632:273–292. doi: 10.1007/978-0-387-78952-1_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklin D, Lambris JD. Complement in immune and inflammatory disorders: pathophysiological mechanisms. J. Immunol. 2013a;190:3831–3838. doi: 10.4049/jimmunol.1203487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklin D, Lambris JD. Complement in immune and inflammatory disorders: therapeutic interventions. J. Immunol. 2013b;190:3839–3847. doi: 10.4049/jimmunol.1203200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklin D, Lambris JD. Progress and trends in complement therapeutics. Adv. Exp. Med. Biol. 2013c;735:1–22. doi: 10.1007/978-1-4614-4118-2_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklin D, Lambris JD. Therapeutic control of complement activation at the level of the central component C3. Immunobiology. 2015 doi: 10.1016/j.imbio.2015.06.012. S0171-2985(15)30012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risitano AM. Paroxysmal nocturnal hemoglobinuria and other complement-mediated hematological disorders. Immunobiology. 2012;217:1080–1087. doi: 10.1016/j.imbio.2012.07.014. [DOI] [PubMed] [Google Scholar]
- Risitano AM. Paroxysmal nocturnal hemoglobinuria and the complement system: recent insights and novel anticomplement strategies. Adv. Exp. Med. Biol. 2013;735:155–172. doi: 10.1007/978-1-4614-4118-2_10. [DOI] [PubMed] [Google Scholar]
- Risitano AM. Current and Future Pharmacologic Complement Inhibitors. Hematol. Oncol. Clin. North Am. 2015;29:561–582. doi: 10.1016/j.hoc.2015.01.009. [DOI] [PubMed] [Google Scholar]
- Risitano AM, Ricklin D, Huang Y, et al. Peptide inhibitors of C3 activation as a novel strategy of complement inhibition for the treatment of paroxysmal nocturnal hemoglobinuria. Blood. 2014;123:2094–2101. doi: 10.1182/blood-2013-11-536573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu A, Kay BK, Lambris JD. Inhibition of human complement by a C3-binding peptide isolated from a phage-displayed random peptide library. J. Immunol. 1996;157:884–891. [PubMed] [Google Scholar]
- Sahu A, Morikis D, Lambris JD. Compstatin, a peptide inhibitor of complement, exhibits species-specific binding to complement component C3. Mol. Immunol. 2003;39:557–566. doi: 10.1016/s0161-5890(02)00212-2. [DOI] [PubMed] [Google Scholar]
- Schenkein HA, Fletcher HM, Bodnar M, Macrina FL. Increased opsonization of a prtH-defective mutant of Porphyromonas gingivalis W83 is caused by reduced degradation of complement-derived opsonins. J. Immunol. 1995;154:5331–5337. [PubMed] [Google Scholar]
- Schenkein HA, Genco RJ. Gingival fluid and serum in periodontal diseases. II. Evidence for cleavage of complement components C3, C3 proactivator (factor B) and C4 in gingival fluid. J. Periodontol. 1977;48:778–784. doi: 10.1902/jop.1977.48.12.778. [DOI] [PubMed] [Google Scholar]
- Schmidt CQ, Bai H, Lin Z, et al. Rational engineering of a minimized immune inhibitor with unique triple-targeting properties. J. Immunol. 2013;190:5712–5721. doi: 10.4049/jimmunol.1203548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm EC, Clark SJ, Triebwasser MP, Raychaudhuri S, Seddon JM, Atkinson JP. Genetic variants in the complement system predisposing to age-related macular degeneration: a review. Mol. Immunol. 2014;61:118–125. doi: 10.1016/j.molimm.2014.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott D, Botto M. The paradoxical roles of C1q and C3 in autoimmunity. Immunobiology. 2015 doi: 10.1016/j.imbio.2015.05.001. S0171-2985(15)00069-8. [DOI] [PubMed] [Google Scholar]
- Settem RP, El-Hassan AT, Honma K, Stafford GP, Sharma A. Fusobacterium nucleatum and Tannerella forsythia induce synergistic alveolar bone loss in a mouse periodontitis model. Infect. Immun. 2012;80:2436–2443. doi: 10.1128/IAI.06276-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaughnessy AF. Monoclonal antibodies: magic bullets with a hefty price tag. BMJ. 2012;345:e8346. doi: 10.1136/bmj.e8346. [DOI] [PubMed] [Google Scholar]
- Silasi-Mansat R, Zhu H, Popescu NI, et al. Complement inhibition decreases the procoagulant response and confers organ protection in a baboon model of Escherichia coli sepsis. Blood. 2010;116:1002–1010. doi: 10.1182/blood-2010-02-269746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toto PD, Lin L, Gargiulo A. Identification of C3a, IgG, IgM in inflamed human gingiva. J. Dent. Res. 1978;57:696. doi: 10.1177/00220345780570050501. [DOI] [PubMed] [Google Scholar]
- Wang M, Krauss JL, Domon H, et al. Microbial hijacking of complement-toll-like receptor crosstalk. Sci. Signal. 2010;3:ra11. doi: 10.1126/scisignal.2000697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingrove JA, DiScipio RG, Chen Z, Potempa J, Travis J, Hugli TE. Activation of complement components C3 and C5 by a cysteine proteinase (gingipain-1) from Porphyromonas (Bacteroides) gingivalis. J. Biol. Chem. 1992;267:18902–18907. [PubMed] [Google Scholar]
- Wu YQ, Qu H, Sfyroera G, et al. Protection of nonself surfaces from complement attack by factor H-binding peptides: implications for therapeutic medicine. J. Immunol. 2011;186:4269–4277. doi: 10.4049/jimmunol.1003802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y, Zhang R, Lv H, et al. Prioritization of candidate genes for periodontitis using multiple computational tools. J. Periodontol. 2014;85:1059–1069. doi: 10.1902/jop.2014.130523. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Shao D, Ricklin D, et al. Compstatin analog Cp40 inhibits complement dysregulation in vitro in C3 glomerulopathy. Immunobiology. 2015;220:993–998. doi: 10.1016/j.imbio.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]