Abstract
BACKGROUND AND PURPOSE
We report a patient with abnormal diffusion tensor imaging (DTI) and tractography of the corticospinal tract caused by mass effect from adjacent enlarged Virchow–Robin spaces.
METHODS
DTI was performed using 25 noncollinear directions. Fractional anisotropy (FA) and mean diffusivity (MD) maps were generated. Region-of-interest measurements of the corticospinal tracts were organized in histograms, and comparisons were made between sides. Statistical analysis consisted of a Wilcoxon rank-sum nonparametric test and a two-sample test of proportions to compare the relative percentage of voxels >.8.
RESULTS
The patient had no signs or symptoms of motor weakness. The corticospinal tract adjacent to the enlarged Virchow–Robin spaces showed significant changes in the proportion of FA > .8, distribution of FA and distribution of MD (P < .001).
CONCLUSIONS
Diffusion tensor changes may be caused by enlarged Virchow–Robin spaces in the absence of clinical signs or symptoms. We hypothesize that the DTI changes are due to alterations in the extravascular extracellular space. Tensor changes should be interpreted with caution in patients with space occupying mass lesions such as brain tumors.
Keywords: DTI, corticospinal tract, enlarged Virchow-Robin spaces
Introduction
Enlarged Virchow–Robin spaces are extensions of the subpial cerebrospinal space surrounding small penetrating arteries and veins. The vast majority are incidental and clinically unimportant. Diffusion tensor imaging (DTI) examines water motion in vivo. DTI changes in the corticospinal tract changes have been correlated with sensorimotor deficits and postoperative improvement in patients with brain tumors,1–3 and motor function in patients with ischemic strokes.4,5 We describe a patient with unilateral enlarged Virchow–Robin spaces in the thalamus and ventral midbrain. The patient did not have any motor symptoms despite compression of the adjacent corticospinal tract, abnormal diffusion tensor metrics, and abnormal tractography.
Case Report
A 54-year-old female with peritoneal and breast carcinomas presented with headaches, memory loss, and confusion. She had no signs or symptoms of motor weakness after complete neurological evaluation. MRI at 1.5 Tesla (Signa HDx, GE Medical Systems, Milwaukee, WI, USA) showed multiple brain metastases and edema distant from the motor cortices and corticospinal tracts. The patient had already received systemic chemotherapy and whole brain radiation therapy. Despite additional chemotherapy and stereotactic radiosurgery to a right frontal lobe metastasis, the patient succumbed to her disease 18 months after diagnosis of the brain metastases.
MRI also revealed cystic dilatations in the left thalamus and ventral midbrain adjacent to the corticospinal tract. (Fig 1A). These lesions followed cerebro-spinal fluid (CSF) intensity on all sequences including diffusion-weighted imaging (excluding dermoid or epidermoid), were in the brain (excluding arachnoid cyst), and did not enhance (excluding cystic neoplasm or infection). Consistent with enlarged Virchow–Robin spaces along collicular and accessory collicular arteries,6 comparison with prior studies showed them to be stable for >1 year and present before the brain metastases.
Fig 1.
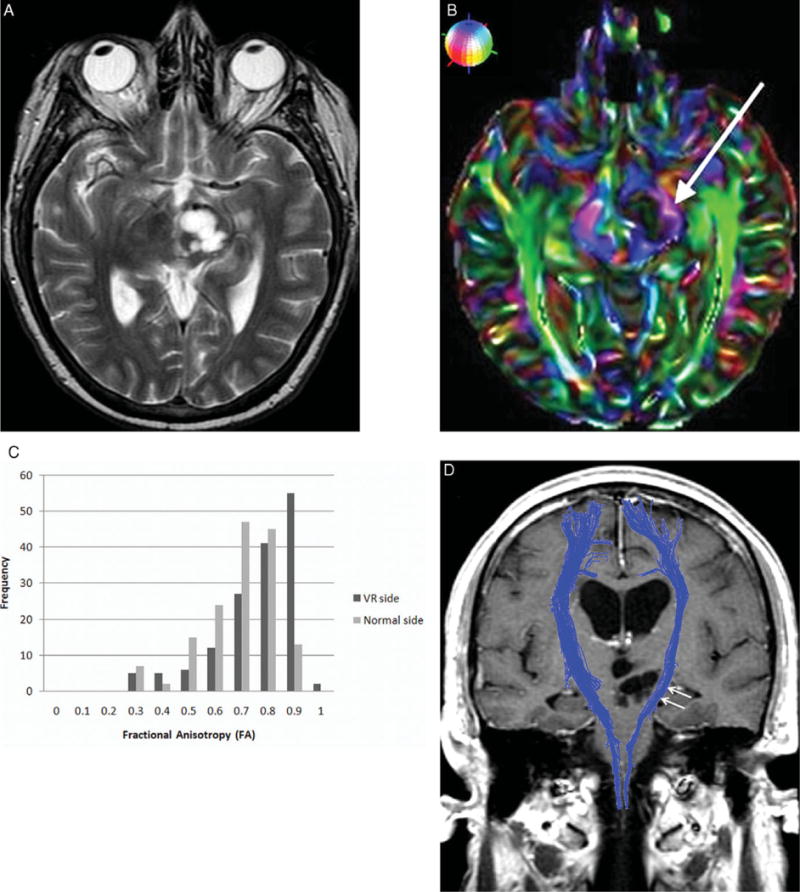
(A) Axial T2-weighted image shows an expansile CSF isointense lesion in the left thalamus and cerebral peduncle. No metastasis or edema is found in the expected location of the left corticospinal tract. (B) Axial directionally encoded color FA map illustrates thinning and deformity of the left corticospinal tract (arrow) caused by the enlarged Virchow–Robin spaces. As shown in the small globe in the upper left corner, blue represents craniocaudal, green anteroposterior, and red transverse orientations. (C) FA histogram shows the peak frequency for the enlarged Virchow–Robin space side is .9 (dark gray), and for the normal side is .7 (light gray). (D) Tractography overlaid on a coronal contrast T1-weighted image shows subtle mass effect upon the left corticospinal tract (arrows).
DTI was acquired using a single-shot spin-echo echo-planar sequence with: TR/TE 13,500/100 ms, matrix 128 × 128, field of view (FOV) 240 mm, slice thickness 3 mm, b-value 1,000 s/mm2, and number of excitations (NEX) 1 in 25 noncollinear directions. Using DTI Studio v2.5,7 fractional anisotropy (FA), mean diffusivity (MD), and directionally encoded color FA maps were generated (Fig 1B). Regions-of-interest (ROIs) were drawn around each corticospinal tract on axial slices in the posterior limb of the internal capsule and in the ventral midbrain. Values generated for each voxel within the ROIs were exported in Analyze format into Analysis of Functional Neuroimages,8 and organized in histograms according to frequency of values per side.
Comparisons were made between sides for proportion of FA > .8, distribution of FA, distribution of MD, longitudinal diffusivity (λ0), and radial diffusivity [(λ1+λ2)/2]. Statistical analysis was performed using two-sided Wilcoxon rank-sum nonparametric tests and two-sample tests of proportions (to compare the relative percentage of voxels >.8). Median FA and MD were .82 and 2.2 × 10−3 mm2/second as compared to .74 and 2.5 × 10−3 mm2/second on the normal side. Increases in the proportion of FA > .8 (P < .0001), distribution of FA (P < .0001), and distributions of longitudinal (P < .0001) and radial diffusivity (P < .0001) were observed. Histogram analysis showed increased peak frequency of FA (Fig 1C). Decreases in the distribution of MD (P < .0001) also occurred.
Fiber tracking using fiber assignment by continuous tracking9 was performed with termination criteria set to FA < .15 and angle >60°. Tractography showed displacement and compression of the corticospinal tract by the enlarged Virchow–Robin spaces (Fig 1D). Fiber density index was calculated as the number of fiber paths passing through the ROI divided by the area of the ROI in pixels. Fiber density index was 14.6 (1157/8973) as compared to 8.8 (fibers/voxels = 746/6029) for the normal contralateral tract.
Discussion
We report a case with enlarged Virchow–Robin spaces adjacent to the corticospinal tract, which showed increased FA, decreased MD, and thinning and compression at tractography. Increased FA suggests augmented diffusion anisotropy and structural coherence, whereas decreased MD suggests diminished diffusion magnitude. These tensor abnormalities, however, did not correlate with any clinical signs or symptoms.
Zhu and colleagues10 found Virchow–Robin spaces were ubiquitous in 1,818 elderly patients, of whom 32% had enlarged Virchow–Robin spaces. In a patient with enlarged Virchow–Robin spaces, Ugawa and colleagues11 reported normal clinical findings and sensorimotor conduction. In 2 patients with enlarged Virchow–Robin spaces just below the Rolandic cortex, Mathias et al12 described decreased white matter tract vectors on the side of the enlarged spaces. Given normal neuropsychological evaluations, they postulated that these represented technical limitations of tractography.
We instead hypothesize that the DTI changes are due to alterations in the extravascular extracellular space (EES). Increased FA may occur from increased myelination, increased axonal diameter, increased axonal density, and/or increased directionality.13 The case patient had increased FA that was confirmed by increased peak frequency and increased skewness at histogram analysis, and by increased longitudinal and radial diffusivity. Mass effect by enlarged Virchow–Robin spaces decreases unrestricted water in the EES that has low anisotropy. This suggests compression or increased density of normal axons, as corroborated by the increased fiber density index. DTI in acute ischemia has correlated transient increases in FA with changes in the EES.14 In vitro nerve research has also shown that decreased EES causes decreased MD.15 Calculation of the EES fraction VE is possible using T1 perfusion imaging but beyond the scope of this study.
The main limitation is the complicated oncological history. The case patient had two primary cancers and multiple brain metastases, but none near the corticospinal tracts or motor gyri. Previous chemotherapy and whole brain radiation therapy may potentially affect global DTI measurements. She never received a targeted treatment, however, expected to differentially affect sides.
In conclusion, enlarged Virchow–Robin spaces may induce asymptomatic diffusion tensor and tractography changes in the corticospinal tract through mass effect and compression. This suggests that imaging changes even when significantly different do not necessarily explicate clinical signs and symptoms in patients with space occupying mass lesions–imaging abnormalities always require careful clinical correlation. This has implications on the growing applications of DTI and tractography to predict the location and function of white matter tracts for presurgical planning and image-modulated radiation therapy planning in patients with brain tumors.
Footnotes
Conflict of Interest: The authors have nothing to disclose.
References
- 1.Jellison BJ, Field AS, Medow J, et al. Diffusion tensor imaging of cerebral white matter: a pictorial review of physics, fiber tract anatomy, and tumor imaging patterns. AJNR Am J Neuroradiol. 2004;25:356–369. [PMC free article] [PubMed] [Google Scholar]
- 2.Stadlbauer A, Nimsky C, Gruber S, et al. Changes in fiber integrity, diffusivity, and metabolism of the pyramidal tract adjacent to gliomas: a quantitative diffusion tensor fiber tracking and MR spectroscopic imaging study. AJNR Am J Neuroradiol. 2007;28:462–469. [PMC free article] [PubMed] [Google Scholar]
- 3.Laundre BJ, Jellison BJ, Badie B, et al. Diffusion tensor imaging of the corticospinal tract before and after mass resection as correlated with clinical motor findings: preliminary data. AJNR Am J Neuroradiol. 2005;26:791–796. [PMC free article] [PubMed] [Google Scholar]
- 4.Tang PF, Ko YH, Luo ZA, et al. Tract-specific and region of interest analysis of corticospinal tract integrity in subcortical ischemic stroke: reliability and correlation with motor function of affected lower extremity. AJNR Am J Neuroradiol. 2010;31:1023–1030. doi: 10.3174/ajnr.A1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu LL, Lindenberg R, Alexander MP, et al. Lesion load of the corticospinal tract predicts motor impairment in chronic stroke. Stroke. 2010;41:910–915. doi: 10.1161/STROKEAHA.109.577023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwee RM, Kwee TC. Virchow-Robin spaces at MR imaging. Radiographics. 2007;27:1071–1086. doi: 10.1148/rg.274065722. [DOI] [PubMed] [Google Scholar]
- 7.Jiang H, van Zijl PC, Kim J, et al. DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed. 2006;81:106–116. doi: 10.1016/j.cmpb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 9.Mori S, Crain BJ, Chacko VP, et al. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 10.Zhu Y-C, Dufouil C, Mazoyer B, et al. Frequency and location of dilated Virchow-Robin Spaces in elderly people: a population-based 3D MR imaging study. AJNR Am J Neuroradiol. 2011;32:709–713. doi: 10.3174/ajnr.A2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ugawa Y, Shirouzu I, Terao Y, et al. Physiological analyses of a patient with extreme widening of Virchow-Robin spaces. J Neurol Sci. 1998;159:25–27. doi: 10.1016/s0022-510x(98)00140-3. [DOI] [PubMed] [Google Scholar]
- 12.Mathias J, Koessler L, Brissart H, et al. Giant cystic widening of Virchow-Robin spaces: an anatomofunctional study. AJNR Am J Neuroradiol. 2007;28:1523–1525. doi: 10.3174/ajnr.A0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimony JS, McKinstry RC, Akbudak E, et al. Quantitative diffusion-tensor anisotropy brain MR imaging: normative human data and anatomic analysis. Radiology. 1999;212:770–784. doi: 10.1148/radiology.212.3.r99au51770. [DOI] [PubMed] [Google Scholar]
- 14.Ozsunar Y, Grant PE, Huisman TA, et al. Evolution of water diffusion and anisotropy in hyperacute stroke: significant correlation between fractional anisotropy and T2. AJNR Am J Neuroradiol. 2004;25:699–705. [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson AW, Zhong J, Petroff OA, et al. Effects of osmotically driven cell volume changes on diffusion-weighted imaging of the rat optic nerve. Magn Reson Med. 1996;35:162–167. doi: 10.1002/mrm.1910350206. [DOI] [PubMed] [Google Scholar]


