Abstract
Background
Ethanol causes neurotoxicity via several mechanisms including neuroinflammation (during ethanol exposure), and excitotoxicity (during ethanol withdrawal – EWD). Alpha7 nicotinic acetylcholine receptor (nAChR) selective agonists have the potential to reduce both. The aim of this study was to evaluate the anti-inflammatory and neuroprotective potential of rhamnetin, a dietary flavonoid with alpha7 nAChR selective activity, in an in vitro model of ethanol-induced neurotoxicity.
Methods
The anti-inflammatory and neuroprotective properties of rhamnetin were assessed in neonatal organotypic hippocampal slice cultures (OHSC) undergoing EWD (or not) and challenged with N-methyl-D-aspartate (NMDA) and/or lipopolysaccharide (LPS). Neurotoxicity was determined using propidium iodide uptake and the inflammatory response was evaluated by measuring the release of TNF-alpha (quantified by ELISA) and nitric oxide (quantified by the Griess reaction) into culture media.
Results
As predicted, rhamnetin reduced LPS-induced release of TNF-alpha and nitric oxide both under control conditions and during EWD. Additionally, rhamnetin had no effect on NMDA induced neurotoxicity under control conditions but significantly reduced NMDA toxicity during EWD. In contrast, rhamnetin had no effect on neurotoxicity induced by NMDA and LPS combined despite reducing TNF-alpha and nitric oxide levels under these conditions.
Conclusions
Rhamnetin is anti-inflammatory and neuroprotective during EWD and therefore has potential value in treating neurotoxicity caused by ethanol.
Keywords: rhamnetin, alpha7 nicotinic acetylcholine receptors, excitotoxicity, neuroinflammation, ethanol induced neurotoxicity
1. Introduction
Ethanol-induced neurodegeneration has a complex etiology involving several neurotoxic mechanisms (Crews et al., 2004), including neuroinflammation (Crews and Nixon, 2009) and excitotoxicity (Lovinger, 1993). These two mechanisms have been studied extensively and are both thought to significantly contribute to ethanol-induced neurotoxicity. This suggests the need to develop therapeutic strategies that can reduce both these pathological mechanisms. One such strategy is by pharmacologically targeting the alpha7 nicotinic acetylcholine receptor (nAChR).
The alpha7 nAChR has recently emerged as a pharmacological target for the treatment of neurodegenerative disorders as it is expressed on both neurons and neuroimmune cells (De Simone et al., 2005, Xiu et al., 2005) and can be activated to attenuate excitotoxicity and neuroinflammation. For example, nicotine reduces excitotoxic injury induced by NMDA on hippocampal neurons and this effect is blocked by methyllycaconitine (MLA), an alpha7 nAChR selective antagonist (Dajas-Bailador et al., 2000). In parallel, nicotine attenuates proinflammatory signaling induced by lipopolysaccharide (LPS) on primary microglia cultures, an effect that is significantly blocked by alpha-bungarotoxin, another alpha7 nAChR selective antagonist (Shytle et al., 2004). In support, 3-(2,4-dimethoxy-benzylidene)anabaseine (DMXB), a relatively selective alpha7 nAChR agonist, protects neocortical neurons from glutamate toxicity (Shimohama et al., 1998) and inhibits LPS-induced TNF-alpha release from cultured microglia (Thomsen and Mikkelsen, 2012). Taken together, an alpha7 nAChR selective agonist should act on neurons and microglia simultaneously to reduce excitotoxicity and neuroinflammation.
In order to discover novel alpha7 nAChR selective natural products, we developed a differential pharmacological high throughput screen and applied it to a large Kentucky plant extract library of about 1000 different species (Littleton et al., 2005). Interestingly, we discovered that Solidago nemoralis extracts exhibited selectivity for alpha7 nAChRs relative to alpha4beta2 nAChRs, the other major nAChR subtype in the brain, and that the compounds responsible for this activity were methyl-quercetin derivatives (Lutz et al., 2014). Moreover, by screening a pure flavonoid library we discovered that specific flavonoids, such as rhamnetin, were able to selectively displace alpha7 nAChR selective [3H]MLA whereas related structures, such as sakuranetin, do not (Lutz et al., 2014). Rhamnetin and sakuranetin were compared for their anti-inflammatory properties against LPS induced inflammatory release from immortalized BV2 microglia and rhamnetin was found to inhibit the response in part via alpha7 nAChRs (Lutz et al., 2014). In sum, rhamnetin is capable of reducing neuroinflammation but also has the potential to reduce excitotoxicity by activating alpha7 nAChRs and therefore constitutes a good therapeutic candidate to reduce ethanol-induced neurotoxicity.
In the current study, rhamnetin is evaluated for its anti-inflammatory and potential neuroprotective properties in a recently developed model of in vitro ethanol-induced neurotoxicity which includes neuroinflammatory and excitotoxic component (Lutz et al., 2015). This model takes advantage of organotypic hippocampal slice cultures (OHSC) that contain both neurons and neuroimmune cells (Benediktsson et al., 2005, Haber et al., 2009, Dailey and Waite, 1999), have been previously used to study the effects of ethanol on excitotoxicity (Thomas et al., 1998) and neuroinflammation (Moon et al., 2014), and on which drugs can be directly applied to brain tissue without pharmacokinetic confounds. This model is well suited to evaluate the potential anti-inflammatory and neuroprotective properties of natural products such as rhamnetin. The anti-inflammatory effects of rhamnetin can be assessed on LPS-induced inflammatory mediator release and the neuroprotective effects of rhamnetin can be assessed on NMDA-induced toxicity, both under control conditions and during EWD. In addition, the overall effects of rhamnetin can be evaluated on both outcome measures simultaneously when OHSC are exposed to both LPS and NMDA.
In the original study (Lutz et al., 2015), we found that while LPS enhanced NMDA toxicity under control conditions, the reverse was observed during EWD. Additionally, prior ethanol exposure reduced subsequent inflammatory response to LPS. These data suggest that changes to neuroimmune processes induced by ethanol exposure may be protective against excitotoxicity during EWD. Additional experiments on BV2 microglia exposed to the same ethanol regimen suggested that ethanol exposure induces a non-classical microglial phenotype that may be neuroprotective. In support, Marshall and colleagues (Marshall et al., 2013) report that in rats exposed to a 4-day binge ethanol paradigm, microglia are partially activated and take on an anti-inflammatory phenotype. Exposure to flavonoids can induce similar changes in microglia. For example, luteolin, another plant derived flavonoid with a very similar structure to rhamnetin, induces global transcriptome changes in microglia indicative of an anti-inflammatory and neuroprotective phenotype (Dirscherl et al., 2010). Therefore, it is unclear how rhamnetin exposure will affect neuroimmune changes induced by ethanol and how those changes in turn affect excitotoxicity during EWD.
Based on the anti-inflammatory effects of rhamnetin on BV2 microglia (Lutz et al., 2014), this compound is predicted to reduce LPS-induced inflammatory mediator release under control conditions. However, whether rhamnetin (1) retains anti-inflammatory properties during EWD, (2) inhibits NMDA toxicity under control conditions, or (3) prevents enhanced NMDA toxicity during EWD remains to be established. The hypothesis is that rhamnetin will reduce LPS-induced inflammatory mediator release and NMDA-induced toxicity under control conditions and during EWD in hippocampal slice cultures. These studies are designed to test this hypothesis in order to establish whether rhamnetin and similar dietary flavonoids have potential value in the prevention and/or treatment of ethanol-induced neurodegeneration.
2. Materials & methods
2.1. Organotypic hippocampal slice culture (OHSC) preparation
OHSC were prepared essentially as described by Stoppini et al. (Stoppini et al., 1991). Briefly, hippocampi were aseptically removed from 8-day-old Sprague-Dawley male and female rat pups and sliced at a transverse thickness of 200 μm using a McIlwain tissue chopper (Campden Instruments Ltd., Lafayette, ID). Slices were transferred to sterile culture inserts (4 slices per insert) and placed in 6-well-plates containing culture medium (Minimum Essential Medium (MEM; Life Technologies Corporation, Grand Island, NY), 200mM glutamine (Invitrogen, Carlsbad, CA), 25mM HEPES (ATCC, Manassas, VA), 50uM penicillin/streptomycin (ATCC, Manassas, VA), 36mM glucose, 25% (v/v) Hank's buffered salt solution (HBSS; Gibco BRL, Gaithersburg, MD), 25% heat-inactivated horse serum (HIHS; Sigma, St. Louis, MO)). Cultures were maintained at 37°C in an atmosphere of 5% CO2/95% air in 95% humidity for 5 days to allow slices to adhere to the insert membrane. The care of animals was carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23, revised 1996), as well as the University of Kentucky's Institutional Animal Care and Use Committee.
2.2. Ethanol exposure, rhamnetin exposure and ethanol withdrawal (EWD)
At 6 days in vitro, slices were placed in culture media with or without the addition of 100mM ethanol, incubated for 10 days and then subjected to EWD for 24h. During exposure, the plates were placed in topless polypropylene containers containing 50mL of 100mM ethanol or dH2O accordingly, and placed inside sealable plastic bags filled with 5% CO2, 21% oxygen, and a balance of nitrogen. The ethanol solution is used as an evaporating source of ethanol to counter the evaporation of ethanol from the culture wells which has been shown to result in an average ethanol concentration of 65mM during the 5 days between media changes (Prendergast et al., 2004). At 11 days in vitro, slices were sub-cultured into their respective culture media (control and ethanol), further subdivided and exposed to control media or media supplemented with rhamnetin (25uM/100uM) for the last 5 days of ethanol exposure. At the onset of EWD, slices were separated into treatment groups with or without rhamnetin and challenged accordingly: control media, 5uM NMDA (Sigma Aldrich Co. LCC., St. Louis, MO), 10ug/mL lipopolysaccharide from Escherichia coli 026:B6 (LPS, Lot #: 021M4072V; Sigma Aldrich Co. LCC., St. Louis, MO) or both NMDA and LPS combined (Fig. 1).
Figure 1.
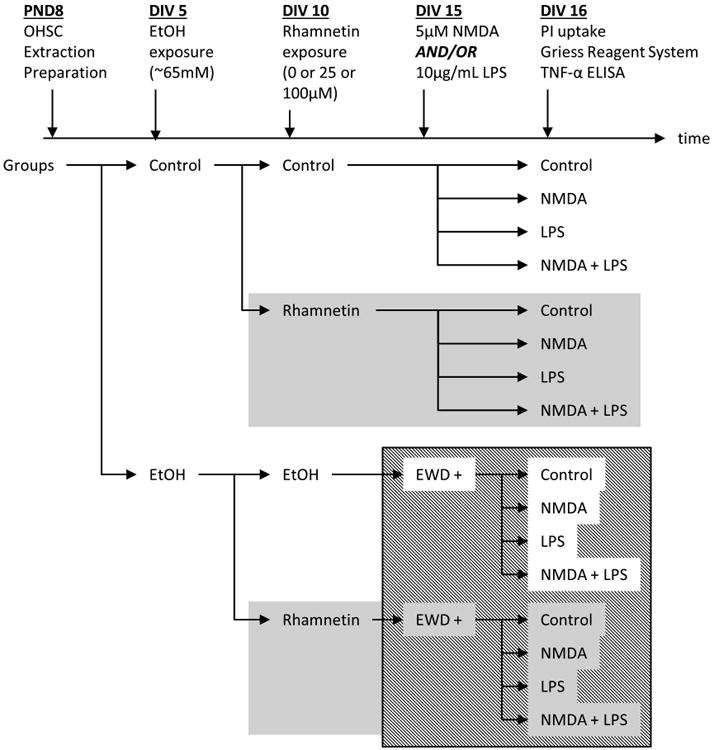
Flow diagram showing a timeline of the procedures and experimental groups.
2.3. Assessment of toxicity by propidium iodide uptake
Propidium iodide (PI - Sigma Aldrich Co. LCC., St. Louis, MO) is a membrane impermeable, DNA intercalating fluorescent molecule that is commonly used in OSHC as a semi-quantitative stain for cellular toxicity and has been significantly correlated to other reliable markers of cell death (Zimmer et al., 2000). It has been extensively used to screen neuroprotective compounds in OHSC (Noraberg et al., 2005) and we previously used it to evaluate the combined effects of NMDA and LPS during EWD (Lutz et al., 2015). Therefore, PI was chosen to evaluate the neuroprotective properties of rhamnetin in this study. During EWD, slices were treated in culture media containing 3.74uM PI. Slice images were captured using SPOT Advanced software (Version 4.0.9; W. Nuhsbaum Inc., McHenry, IL) connected to an inverted Leica DMIRB microscope (W. Nuhsbaum Inc.) fitted for fluorescence detection (mercury-arc lamp) and connected to a computer via a SPOT 7.2 color mosaic camera (W. Nuhsbaum Inc). PI uptake in the CA1, CA3, and DG cell layers was measured using ImageJ software (Version 1.46; National Institute of Health, Bethesda, MD). Background signal was subtracted from intensities obtained for each cell layer resulting in specific intensities which were used for statistical analysis. These values were then converted to % control (no EWD, no NMDA, and no LPS) within each preparation for graphical representation.
2.4. Assessment of inflammatory mediator release
Once slices were imaged, inserts were discarded and the resulting media was collected for assessment of inflammatory mediator release. Nitric oxide (NO) release was assessed by the Griess Reagent System (Promega Corporation, Madison, WI) according to the manufacturer's instructions. Briefly, samples were mixed sequentially with sulfanilamide and N-1-napthylethylenediamine dihydrochloride and incubated for 5min. Absorbance was measured at 550nm using a Wallac 1420 VICTOR plate reader (PerkinElmer, MA, USA). All samples were assayed in duplicate and nitrite content was estimated using a reference NaNO2 standard curve performed with each assay. TNF-alpha content was assessed by enzyme linked immunosorbent assay kit (ELISA; Ready-Set-Go!® ELISA, eBioscience Inc., San Diego, CA) according to the manufacturer's instructions. Briefly, samples were pipetted on 96-well plates coated with rat anti-TNF-alpha antibodies and detected using the sandwich method (anti-TNF-alpha primary antibody, avidin-HRP linked secondary antibody and tetramethylbenzidine substrate). All samples were assayed in duplicate and TNF-alpha content was estimated from a reference TNF-alpha standard curve performed with each assay.
2.5. Statistical analysis
Data were analyzed using IBM Statistical Package for the Social Sciences (SPSS) Version 21 (IBM Corporation, Armonk, NY) and graphed using Prism (Graphpad Software Inc., La Jolla, CA). All outcome measures were analyzed by multi-factorial analysis of variance (ANOVA) with EWD, NMDA, LPS and rhamnetin as fixed factors. Data were obtained from different preparations so preparation was used as a covariate to control for differences across litters/culture preparations. PI uptake was measured in three different regions (DG, CA3 and CA1). Thus, for analysis of PI uptake, region was included as a repeated, within-subjects variable. Post hoc analyses were conducted using Fisher's least significant difference (LSD) test with a level of significance set at p<0.05.
3. Results
Overall multi-factorial ANOVAs on NO release and TNF-alpha release revealed that the highest order interactions included all factors except NMDA (for NO release: EWD × LPS [F(1,502) = 103.6, p < 0.001]; EWD × rhamnetin [F(2,502) = 7.6, p < 0.01]; LPS × rhamnetin [F(2,502) = 28.1, p < 0.001]; for TNF-alpha release: EWD × rhamnetin [F(2,237) = 5.6, p < 0.01]). Therefore, differences in inflammatory mediator release between treatment groups, excluding NMDA groups, were compared post hoc where indicated. The repeated-measures multi-factorial ANOVA on PI uptake revealed a main effect of region ([F(1.2,2545.9) = 102.01, p < 0.001] corrected using Greenhouse-Geisser). This effect was mainly driven by differences in the CA1 region of the hippocampus where toxicity was most significant compared to DG and CA3. Therefore, assessment of cellular damage was focused on the CA1. A multi-factorial ANOVA on PI uptake in the CA1 revealed that the highest order interaction included all factors (EWD × NMDA × LPS × rhamnetin [F(2,2101) = 6.7, p < 0.01]). Therefore, differences in PI uptake between the different treatment groups were compared post hoc where indicated.
3.1. Rhamnetin inhibits LPS-induced inflammatory mediator release but has no effect on NMDA-induced toxicity under control conditions
The anti-inflammatory and neuroprotective properties of rhamnetin were first evaluated under control conditions. Both 25uM and 100uM rhamnetin equally inhibited inflammatory mediator release induced by LPS (Fig. 2A & 2B) but it did not afford neuroprotection against NMDA toxicity under these conditions (Fig. 2C). TNF-alpha and NO levels measured in media from cultures treated with LPS and rhamnetin were significantly lower than those measured in media from cultures treated with LPS alone (post hoc p < 0.0001). As in the original study (Lutz et al., 2015), under control conditions, LPS treatment potentiated NMDA-induced PI uptake (post hoc p < 0.05). 25uM or 100uM rhamnetin had no effect on NMDA-induced toxicity. Moreover, despite its anti-inflammatory effects, rhamnetin had no effect on toxicity induced by NMDA and LPS in combination.
Figure 2.
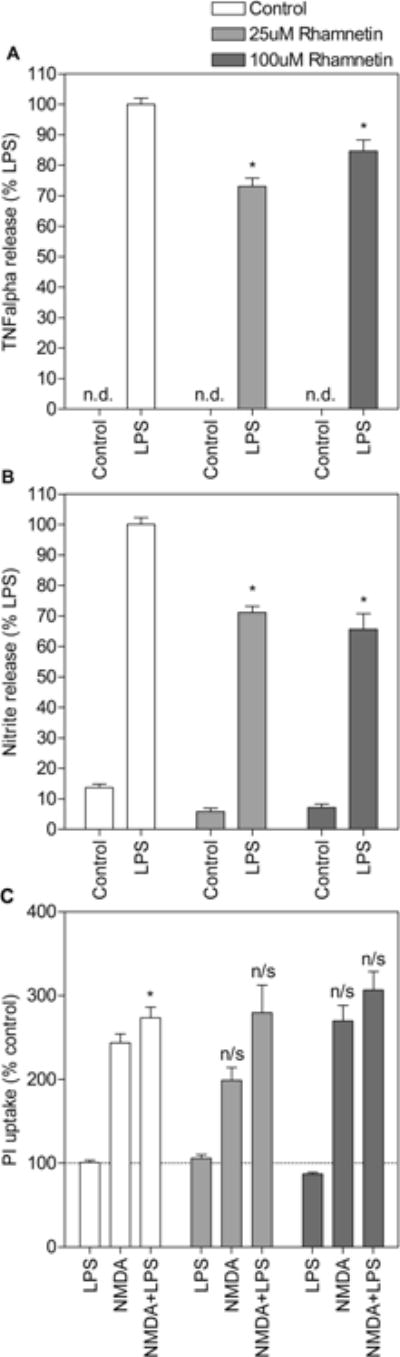
Rhamnetin is anti-inflammatory but not neuroprotective under control conditions. Slices were treated with lipopolysaccharide (LPS) and/or N-methyl-D-aspartate (NMDA) in the absence (white bars) or presence (grey bars) of rhamnetin (25uM and 100uM). Culture media was collected after 24h and assayed for TNF-alpha (A) and NO (B) content following PI uptake measurement (C). For (A) and (B) * p<0.001 compared to LPS alone, n/d not detected. Data are expressed as percent release induced by LPS alone (means ± SEM). n > 12 media samples from 2 independent experiments for each treatment group. For (C) * p<0.05 compared to NMDA alone, n/s no significant difference with equivalent treatment group (LPS, NMDA, NMDA+LPS) in the absence of rhamnetin. Data are expressed as percent of untreated control (means ± SEM). Dotted line represents untreated control. n > 27 hippocampal slices from 2 independent experiments for each treatment group.
3.2. Effects of EWD on LPS-induced inflammatory mediator release and NMDA-induced toxicity
As in the original study (Lutz et al., 2015), EWD reduced the release of inflammatory mediators induced by LPS in comparison to control (Fig. 3A & 3B). TNF-alpha and NO levels measured in media from EWD cultures treated with LPS were significantly lower than LPS control levels (post hoc p < 0.001). In addition, EWD enhanced NMDA-induced toxicity and the effects of LPS on NMDA-induced toxicity were reversed under these conditions (Fig. 3C). EWD cultures treated with NMDA exhibited significantly more PI uptake than NMDA controls (post hoc p < 0.001) and EWD cultures treated with a combination of NMDA and LPS exhibited significantly less PI uptake than EWD cultures treated with NMDA alone (post hoc p < 0.01).
Figure 3.
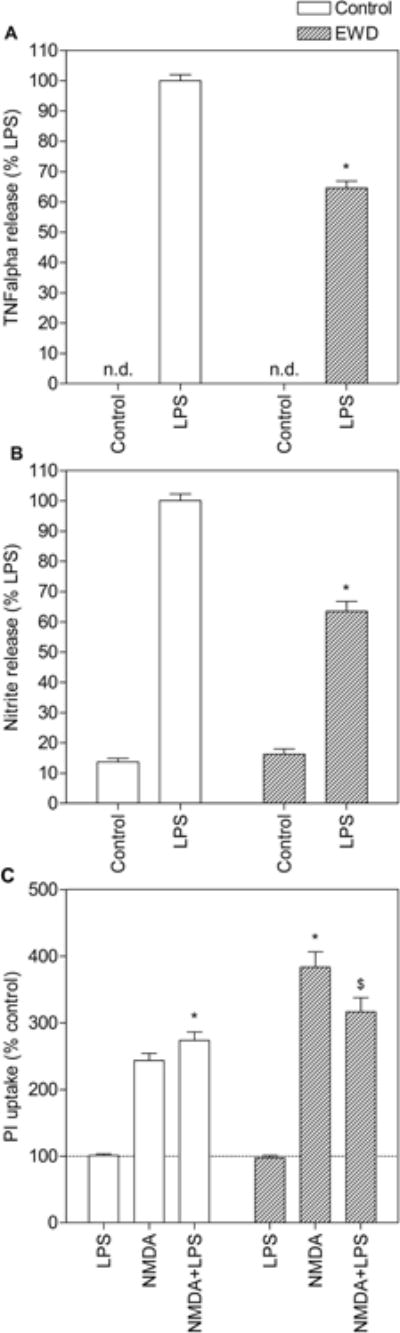
Effects of ethanol withdrawal (EWD) on proinflammatory release and toxicity induced by lipopolysaccharide (LPS) and/or N-methyl-D-aspartate (NMDA) in organotypic hippocampal cultures. Slices were treated with LPS and/or NMDA under control conditions (empty bars) and during EWD (dashed bars). Culture media was collected after 24h and assayed for TNF-alpha (A) and NO (B) content following PI uptake measurement (C). For (A) and (B), *p<0.001 compared to LPS alone. Data are expressed as percent release induced by LPS alone (means ± SEM). n > 27 hippocampal slices from 2 independent experiments for each treatment group. For (C), *p<0.05 compared to NMDA alone; $p<0.01 compared to NMDA+EWD. Data are expressed as percent of untreated control (means ± SEM). Dotted line represents untreated control. n > 117 hippocampal slices from 5 independent experiments for each treatment group.
3.3. Rhamnetin inhibits LPS-induced inflammatory mediator release and NMDA-induced toxicity during EWD
The anti-inflammatory and neuroprotective properties of rhamnetin were evaluated under EWD conditions. Despite the fact that the response to LPS is reduced during EWD, both 25uM and 100uM rhamnetin further inhibited inflammatory mediator release during EWD (Fig. 4A & 4B). TNF-alpha and NO levels measured in media from EWD cultures treated with LPS and rhamnetin were significantly lower than those measured in media from cultures treated with LPS alone (post hoc p < 0.001). Moreover, both 25uM and 100uM reduced NMDA-induced toxicity during EWD (Fig. 4C). PI uptake in slices treated with NMDA and rhamnetin was significantly lower than in slices treated with NMDA alone under these conditions (post hoc p < 0.001). However, rhamnetin had no effect on toxicity induced by NMDA and LPS in combination during EWD.
Figure 4.
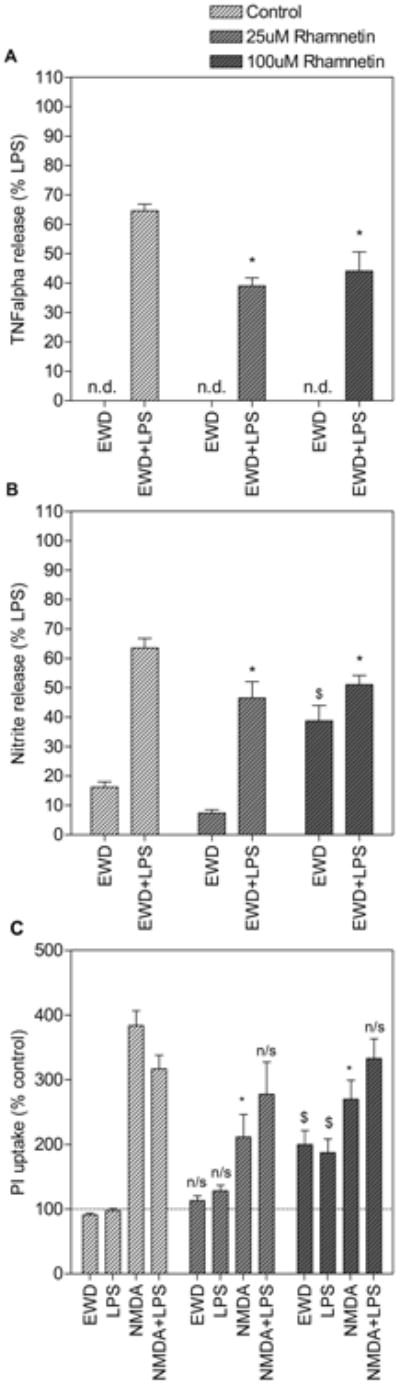
Rhamnetin is anti-inflammatory and neuroprotective against N-methyl-D-aspartate (NMDA) toxicity during ethanol withdrawal (EWD). Slices were treated with lipopolysaccharide (LPS) and/or NMDA in the absence (white dashed bars) or presence (grey dashed bars) of rhamnetin (25uM and 100uM). Culture media was collected after 24h and assayed for TNF-alpha (A) and NO (B) content following PI uptake measurement (C). For (A) and (B), *p<0.01 compared to LPS+EWD; $p<0.001 compared to EWD alone. Data are expressed as percent release induced by LPS alone under control conditions (means ± SEM). n > 11 media samples from 2 independent experiments for each treatment group. For (C), *p<0.001 compared to NMDA+EWD; $p<0.001 compared to EWD alone, n/s no significant difference with equivalent treatment group in the absence of rhamnetin. Data are expressed as percent of untreated control (means ± SEM). Dotted line represents untreated control. n > 45 hippocampal slices from 2 independent experiments for each treatment group.
3.4. 100uM rhamnetin spontaneously induces NO release and toxicity during EWD
Interestingly, 100uM rhamnetin interacted with ethanol exposure and/or EWD to spontaneously induce NO release and toxicity (Fig. 5). NO levels measured in media from EWD cultures treated with 100uM rhamnetin was significantly higher than NO measured in media from EWD control cultures (post hoc p < 0.001). Similarly, PI uptake was significantly higher in slices treated with 100uM rhamnetin compared to EWD control slices (post hoc p < 0.001). On the other hand, 25uM rhamnetin did not interact with EWD and levels of NO release in media or PI uptake in EWD cultures treated with this concentration of rhamnetin did not differ from EWD controls.
Figure 5.
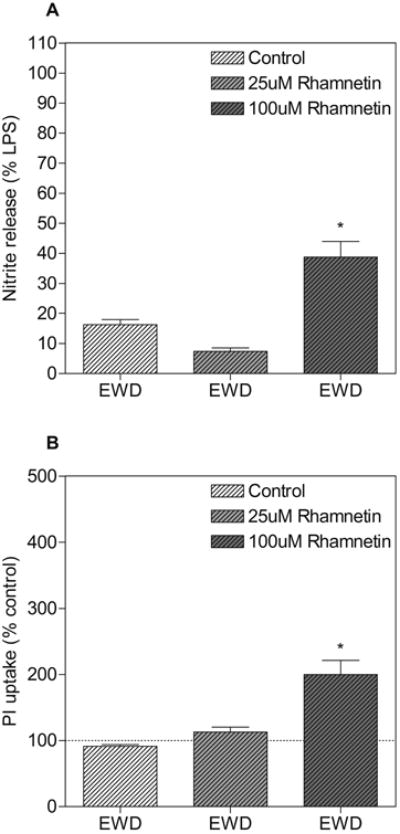
High concentration of rhamnetin induces spontaneous NO release and toxicity during ethanol withdrawal (EWD) Slices were subjected to EWD in the absence (white dashed bars) or presence (grey dashed bars) of rhamnetin (25uM and 100uM). (A) Culture media was collected after 24h and assayed for NO content. *p<0.001 compared to EWD alone. Data are expressed as percent release induced by LPS alone (means ± SEM). n > 12 media samples from 2 independent experiments for each treatment group. (B) PI uptake was measured after 24h. *p<0.001 compared to EWD alone. Data are expressed as percent of untreated control (means ± SEM). Dotted line represents untreated control. n > 47 hippocampal slices from 2 independent experiments for each treatment group.
4. Discussion
The present studies were undertaken to evaluate the anti-inflammatory and potentially neuroprotective properties of rhamnetin, a dietary flavonoid found in spices such as cloves, in an in vitro model of ethanol-induced neurotoxicity that includes both neuroinflammatory and excitotoxic components. We have previously shown that rhamnetin is anti-inflammatory in LPS-stimulated immortalized BV2 microglia (Lutz et al., 2014). Thus, we originally predicted that rhamnetin would inhibit LPS-induced proinflammatory mediator release under control conditions and during EWD. The results support both predictions. Rhamnetin reduced LPS elicited TNF-alpha and NO release under control conditions (fig. 2a & 2B) as well as during EWD (fig. 4A & 4B). Flavonoids have been extensively studied for their anti-inflammatory properties (Tunon et al., 2009) and have been proposed as treatments for neuroinflammatory disorders (Spencer et al., 2012). The current study supports the potential use of dietary flavonoids such as rhamnetin to reduce neuroinflammation and suggests that they may also be effective in reducing neuroinflammation during EWD.
In addition to evaluating the anti-inflammatory effects of rhamnetin, the current study aimed to assess its potential for reducing excitotoxicity during EWD. We have previously shown that rhamnetin has agonist activity at alpha7 nAChRs (Lutz et al., 2014) and this predicts that the flavonoid should reduce NMDA toxicity both under control conditions and during EWD. In support, nicotine exposure reduces excitotoxic injury in OHSC under control conditions (Prendergast et al., 2001a) and during EWD (Prendergast et al., 2000) most probably via alpha7 nAChRs (Prendergast et al., 2001b). In the current study, rhamnetin reduced NMDA-induced toxicity during EWD (fig. 4C), but was inactive under control conditions (fig. 2C). The lack of effect under control conditions is surprising because flavonoids are well known for their antioxidant properties (Bubols et al., 2013) and they have been shown to be protective against a variety of excitotoxic insults by reducing oxidative stress (Cho and Lee, 2004, Silva et al., 2008, Campos-Esparza et al., 2009). However, the neuroprotective effects of rhamnetin during EWD suggest that the mechanism of enhanced excitotoxicity following ethanol exposure is a target for rhamnetin. The specific mechanism targeted remains unknown but these data suggest that dietary flavonoids such as rhamnetin have the potential to reduce excitotoxicity during EWD.
The current study also aimed to evaluate the impact of rhamnetin on the combined effects of NMDA and LPS. Rhamnetin afforded no protection against the combined insults under control conditions (fig. 2C) or during EWD (fig. 4C). The mechanisms by which rhamnetin reduce inflammatory responses are not certain. Our previous studies on BV2 cells suggest that direct effects via alpha7 nAChR is one component. However, changes in microglial phenotype may play a prominent role here. Microglia exposed chronically to flavonoids and alpha7 nAChR agonists have been shown to shift towards an anti-inflammatory and neuroprotective phenotype (Dirscherl et al., 2010, Hua et al., 2014). From our previous studies (Lutz et al., 2015) it was apparent that chronic exposure to ethanol also produced changes in microglia towards a neuroprotective phenotype and similar conclusions have been reached by others using an in vivo model (Marshall et al., 2013). It is therefore likely that the interactions between ethanol, flavonoids, and alpha7 nAChR agonists on microglial phenotype are complex and it will be necessary to explore these in order to explain the results obtained here.
Throughout the current study, 25uM and 100uM rhamnetin were tested. Both concentrations were equipotent at inhibiting LPS-induced inflammatory mediator release under control conditions and during EWD as well as against NMDA toxicity during EWD. However, exposure to 100uM rhamnetin spontaneously induced NO release and toxicity during EWD (fig. 5). The mechanism is unknown but this effect confounds interpretation of results on NO and PI uptake with 100uM rhamnetin during EWD on slices treated with NMDA and/or LPS. This interaction is a potential concern for the proposed use of this class of flavonoids in preventing ethanol-induced neurotoxicity and suggests a potential shift in their therapeutic index following ethanol exposure given the fact that 100uM rhamnetin had no toxic effect under control conditions. However, since flavonoids are absorbed in the low uM range in animals (Morand et al., 1998), concentrations as high as 100uM are unlikely to be achieved in vivo and the lower concentration of 25uM rhamnetin, which was just as anti-inflammatory and neuroprotective as 100uM, had no effect on spontaneous NO release or toxicity. Similar toxicity issues have been reported for quercetin, another flavonoid structurally related to rhamnetin, at high concentrations (40uM) despite being protective at lower concentrations (5uM) against amyloid beta induced toxicity in cultured neurons (Ansari et al., 2009).
It is important to note that OHSC used in these studies are obtained from neonatal animals and as such the effects of ethanol and/or rhamnetin on excitotoxic and neuroinflammation observed herein have important implications for brain development. The current study therefore supports the involvement of glutamate and neuroimmune systems in ethanol-induced neurotoxicity in the developing brain (Barron et al., 2008, Lutz et al., 2015, Moon et al., 2014, Drew and Kane, 2014) and suggests that dietary flavonoids, such as rhamnetin, may have therapeutic potential for ethanol related pathologies in the developing brain. In support, anti-inflammatory drugs (Drew and Kane, 2014), including dietary flavonoids (Moreland et al., 2002, Tiwari et al., 2010, Ke et al., 2011) have been shown to be neuroprotective in models of ethanol-induced neurotoxicity in developing animals. It is also important to note that preparation of OHSC causes neuronal damage and induction of neuroinflammatory processes (Huuskonen et al., 2005). However, OHSC are incubated in vitro for several days before the experiments are initiated at which point neuronal damage and neuroinflammatory signaling associated with explantation have been shown to have subsided (Huuskonen et al., 2005).
In summary, rhamnetin reduced LPS induced inflammation both under control conditions and during EWD, as well as preventing EWD-enhanced excitotoxicity. However, rhamnetin did not reduce excitotoxicity under control conditions and had no effect on the combined effects of NMDA and LPS. Thus, the results support only some of our original predictions. However, the current study shows that rhamnetin (1) retains its anti-inflammatory effects during EWD and (2) specifically targets a mechanism by which ethanol exposure enhances excitotoxicity during EWD. As such, rhamnetin is anti-inflammatory and neuroprotective during EWD. Therefore, rhamnetin and dietary flavonoids with similar pharmacology have potential value in the treatment of ethanol-induced neurotoxicity.
Acknowledgments
Funding sources: This work was supported in part by NIAAA (National Institute on Alcohol Abuse and Alcoholism) grants (R21-AA020188, R42-AA014555, and R42-AA015475) awarded to Dr. Littleton as Principal Investigator. The authors would like to thank Dr. Mark Prendergast for allowing use of his microscope.
Contributor Information
Joseph A. Lutz, Department of Pharmaceutical Sciences, College of Pharmacy, University of Kentucky, Lexington, Kentucky.
Megan Carter, Department of Psychology, College of Arts and Sciences, University of Kentucky, Lexington, Kentucky.
Logan Fields, Department of Psychology, College of Arts and Sciences, University of Kentucky, Lexington, Kentucky.
Susan Barron, Department of Psychology, College of Arts and Sciences, University of Kentucky, Lexington, Kentucky.
John M. Littleton, Department of Psychology, College of Arts and Sciences, University of Kentucky, Lexington, Kentucky.
References
- Ansari MA, Abdul HM, Joshi G, Opii WO, Butterfield DA. Protective effect of quercetin in primary neurons against Abeta(1-42): relevance to Alzheimer's disease. J Nutr Biochem. 2009;20:269–75. doi: 10.1016/j.jnutbio.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron S, Mulholland PJ, Littleton JM, Prendergast MA. Age and Gender Differences in Response to Neonatal Ethanol Withdrawal and Polyamine Challenge in Organotypic Hippocampal Cultures. Alcoholism: Clinical and Experimental Research. 2008;32:929–936. doi: 10.1111/j.1530-0277.2008.00649.x. [DOI] [PubMed] [Google Scholar]
- Benediktsson AM, Schachtele SJ, Green SH, Dailey ME. Ballistic labeling and dynamic imaging of astrocytes in organotypic hippocampal slice cultures. J Neurosci Methods. 2005;141:41–53. doi: 10.1016/j.jneumeth.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Bubols GB, Vianna Dda R, Medina-Remon A, von Poser G, Lamuela-Raventos RM, Eifler-Lima VL, Garcia SC. The antioxidant activity of coumarins and flavonoids. Mini Rev Med Chem. 2013;13:318–34. doi: 10.2174/138955713804999775. [DOI] [PubMed] [Google Scholar]
- Campos-Esparza MR, Sanchez-Gomez MV, Matute C. Molecular mechanisms of neuroprotection by two natural antioxidant polyphenols. Cell Calcium. 2009;45:358–68. doi: 10.1016/j.ceca.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Cho J, Lee HK. Wogonin inhibits excitotoxic and oxidative neuronal damage in primary cultured rat cortical cells. Eur J Pharmacol. 2004;485:105–10. doi: 10.1016/j.ejphar.2003.11.064. [DOI] [PubMed] [Google Scholar]
- Crews FT, Collins MA, Dlugos C, Littleton J, Wilkins L, Neafsey EJ, Pentney R, Snell LD, Tabakoff B, Zou J, Noronha A. Alcohol-induced neurodegeneration: when, where and why? Alcohol Clin Exp Res. 2004;28:350–64. doi: 10.1097/01.alc.0000113416.65546.01. [DOI] [PubMed] [Google Scholar]
- Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol. 2009;44:115–27. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey ME, Waite M. Confocal imaging of microglial cell dynamics in hippocampal slice cultures. Methods. 1999;18:222–30. 177. doi: 10.1006/meth.1999.0775. [DOI] [PubMed] [Google Scholar]
- Dajas-Bailador FA, Lima PA, Wonnacott S. The [alpha]7 nicotinic acetylcholine receptor subtype mediates nicotine protection against NMDA excitotoxicity in primary hippocampal cultures through a Ca2+ dependent mechanism. Neuropharmacology. 2000;39:2799–2807. doi: 10.1016/s0028-3908(00)00127-1. [DOI] [PubMed] [Google Scholar]
- De Simone R, Ajmone-Cat MA, Carnevale D, Minghetti L. Activation of alpha7 nicotinic acetylcholine receptor by nicotine selectively up-regulates cyclooxygenase-2 and prostaglandin E2 in rat microglial cultures. J Neuroinflammation. 2005;2:4. doi: 10.1186/1742-2094-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirscherl K, Karlstetter M, Ebert S, Kraus D, Hlawatsch J, Walczak Y, Moehle C, Fuchshofer R, Langmann T. Luteolin triggers global changes in the microglial transcriptome leading to a unique anti-inflammatory and neuroprotective phenotype. J Neuroinflammation. 2010;7:3. doi: 10.1186/1742-2094-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew PD, Kane CJM. Chapter Three - Fetal Alcohol Spectrum Disorders and Neuroimmune Changes. In: Changhai Cui DS, Harris RA, editors. International Review of Neurobiology. Academic Press; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber M, Vautrin S, Fry EJ, Murai KK. Subtype-specific oligodendrocyte dynamics in organotypic culture. Glia. 2009;57:1000–13. doi: 10.1002/glia.20824. [DOI] [PubMed] [Google Scholar]
- Hua S, Ek CJ, Mallard C, Johansson ME. Perinatal hypoxia-ischemia reduces alpha 7 nicotinic receptor expression and selective alpha 7 nicotinic receptor stimulation suppresses inflammation and promotes microglial Mox phenotype. Biomed Res Int. 2014;2014:718769. doi: 10.1155/2014/718769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huuskonen J, Suuronen T, Miettinen R, van Groen T, Salminen A. A refined in vitro model to study inflammatory responses in organotypic membrane culture of postnatal rat hippocampal slices. J Neuroinflammation. 2005;2:25. doi: 10.1186/1742-2094-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke Z, Liu Y, Wang X, Fan Z, Chen G, Xu M, Bower KA, Frank JA, Ou X, Shi X, Luo J. Cyanidin-3-glucoside ameliorates ethanol neurotoxicity in the developing brain. J Neurosci Res. 2011;89:1676–84. doi: 10.1002/jnr.22689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littleton J, Rogers T, Falcone D. Novel approaches to plant drug discovery based on high throughput pharmacological screening and genetic manipulation. Life Sci. 2005;78:467–75. doi: 10.1016/j.lfs.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Lovinger DM. Excitotoxicity and alcohol-related brain damage. Alcohol Clin Exp Res. 1993;17:19–27. doi: 10.1111/j.1530-0277.1993.tb00720.x. [DOI] [PubMed] [Google Scholar]
- Lutz JA, Carter M, Fields L, Barron S, Littleton JM. Altered Relation Between Lipopolysaccharide-Induced Inflammatory Response and Excitotoxicity in Rat Organotypic Hippocampal Slice Cultures During Ethanol Withdrawal. Alcoholism: Clinical and Experimental Research. 2015;39:827–835. doi: 10.1111/acer.12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz JA, Kulshrestha M, Rogers DT, Littleton JM. A nicotinic receptor-mediated anti-inflammatory effect of the flavonoid rhamnetin in BV2 microglia. Fitoterapia. 2014;98c:11–21. doi: 10.1016/j.fitote.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall SA, McClain JA, Kelso ML, Hopkins DM, Pauly JR, Nixon K. Microglial activation is not equivalent to neuroinflammation in alcohol-induced neurodegeneration: The importance of microglia phenotype. Neurobiol Dis. 2013;54:239–51. doi: 10.1016/j.nbd.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon KH, Tajuddin N, Brown J, 3rd, Neafsey EJ, Kim HY, Collins MA. Phospholipase A2, oxidative stress, and neurodegeneration in binge ethanol-treated organotypic slice cultures of developing rat brain. Alcohol Clin Exp Res. 2014;38:161–9. doi: 10.1111/acer.12221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morand C, Crespy V, Manach C, Besson C, Demigné C, Rémésy C. Plasma metabolites of quercetin and their antioxidant properties. 1998 doi: 10.1152/ajpregu.1998.275.1.R212. [DOI] [PubMed] [Google Scholar]
- Moreland N, La Grange L, Montoya R. Impact of in utero exposure to EtOH on corpus callosum development and paw preference in rats: protective effects of silymarin. BMC Complement Altern Med. 2002;2:10. doi: 10.1186/1472-6882-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noraberg J, Poulsen FR, Blaabjerg M, Kristensen BW, Bonde C, Montero M, Meyer M, Gramsbergen JB, Zimmer J. Organotypic hippocampal slice cultures for studies of brain damage, neuroprotection and neurorepair. Curr Drug Targets CNS Neurol Disord. 2005;4:435–52. doi: 10.2174/1568007054546108. [DOI] [PubMed] [Google Scholar]
- Prendergast MA, Harris BR, Mayer S, Holley RC, Hauser KF, Littleton JM. Chronic nicotine exposure reduces N-methyl-D-aspartate receptor-mediated damage in the hippocampus without altering calcium accumulation or extrusion: evidence of calbindin-D28K overexpression. Neuroscience. 2001a;102:75–85. doi: 10.1016/s0306-4522(00)00450-4. [DOI] [PubMed] [Google Scholar]
- Prendergast MA, Harris BR, Mayer S, Holley RC, Pauly JR, Littleton JM. Nicotine exposure reduces N-methyl-D-aspartate toxicity in the hippocampus: relation to distribution of the alpha7 nicotinic acetylcholine receptor subunit. Med Sci Monit. 2001b;7:1153–60. [PubMed] [Google Scholar]
- Prendergast MA, Harris BR, Mayer S, Littleton JM. Chronic, but not acute, nicotine exposure attenuates ethanol withdrawal-induced hippocampal damage in vitro. Alcohol Clin Exp Res. 2000;24:1583–92. [PubMed] [Google Scholar]
- Prendergast MA, Harris BR, Mullholland PJ, Blanchard JA, Ii, Gibson DA, Holley RC, Littleton JM. Hippocampal CA1 region neurodegeneration produced by ethanol withdrawal requires activation of intrinsic polysynaptic hippocampal pathways and function of N-methyl-d-aspartate receptors. Neuroscience. 2004;124:869–877. doi: 10.1016/j.neuroscience.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Shimohama S, Greenwald DL, Shafron DH, Akaika A, Maeda T, Kaneko S, Kimura J, Simpkins CE, Day AL, Meyer EM. Nicotinic alpha 7 receptors protect against glutamate neurotoxicity and neuronal ischemic damage. Brain Res. 1998;779:359–63. doi: 10.1016/s0006-8993(97)00194-7. [DOI] [PubMed] [Google Scholar]
- Shytle RD, Mori T, Townsend K, Vendrame M, Sun N, Zeng J, Ehrhart J, Silver AA, Sanberg PR, Tan J. Cholinergic modulation of microglial activation by alpha 7 nicotinic receptors. J Neurochem. 2004;89:337–43. doi: 10.1046/j.1471-4159.2004.02347.x. [DOI] [PubMed] [Google Scholar]
- Silva B, Oliveira PJ, Dias A, Malva JO. Quercetin, kaempferol and biapigenin from Hypericum perforatum are neuroprotective against excitotoxic insults. Neurotox Res. 2008;13:265–79. doi: 10.1007/BF03033510. [DOI] [PubMed] [Google Scholar]
- Spencer JP, Vafeiadou K, Williams RJ, Vauzour D. Neuroinflammation: modulation by flavonoids and mechanisms of action. Mol Aspects Med. 2012;33:83–97. doi: 10.1016/j.mam.2011.10.016. [DOI] [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–82. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Thomas MP, Davis MI, Monaghan DT, Morrisett RA. Organotypic brain slice cultures for functional analysis of alcohol-related disorders: novel versus conventional preparations. Alcohol Clin Exp Res. 1998;22:51–9. [PubMed] [Google Scholar]
- Thomsen MS, Mikkelsen JD. The alpha7 nicotinic acetylcholine receptor ligands methyllycaconitine, NS6740 and GTS-21 reduce lipopolysaccharide-induced TNF-alpha release from microglia. J Neuroimmunol. 2012;251:65–72. doi: 10.1016/j.jneuroim.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Tiwari V, Kuhad A, Chopra K. Epigallocatechin-3-gallate ameliorates alcohol-induced cognitive dysfunctions and apoptotic neurodegeneration in the developing rat brain. Int J Neuropsychopharmacol. 2010;13:1053–66. doi: 10.1017/S146114571000060X. [DOI] [PubMed] [Google Scholar]
- Tunon MJ, Garcia-Mediavilla MV, Sanchez-Campos S, Gonzalez-Gallego J. Potential of flavonoids as anti-inflammatory agents: modulation of pro-inflammatory gene expression and signal transduction pathways. Curr Drug Metab. 2009;10:256–71. doi: 10.2174/138920009787846369. [DOI] [PubMed] [Google Scholar]
- Xiu J, Nordberg A, Zhang JT, Guan ZZ. Expression of nicotinic receptors on primary cultures of rat astrocytes and up-regulation of the alpha7, alpha4 and beta2 subunits in response to nanomolar concentrations of the beta-amyloid peptide(1-42) Neurochem Int. 2005;47:281–90. doi: 10.1016/j.neuint.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Zimmer J, Kristensen BW, Jakobsen B, Noraberg J. Excitatory amino acid neurotoxicity and modulation of glutamate receptor expression in organotypic brain slice cultures. Amino Acids. 2000;19:7–21. doi: 10.1007/s007260070029. [DOI] [PubMed] [Google Scholar]


