Abstract
Background
Mycobacterium abscessus causes lung infection in patients with cystic fibrosis. M. abscessus stimulates the host innate immune response via TLR2 on respiratory epithelial cells. Signaling through TLR2 requires the formation of TLR2/TLR1 heterodimers on the cell surface.
Methods
The ability of M. abscessus to stimulate the innate immune response of cystic fibrosis CFBE41o- respiratory epithelial cells was measured as expression of HβD2 by RT PCR, and release of IL-8 by ELISA. Genotyping of CFBE41o- TLR polymorphisms was carried out.
Results
CFBE41o- cells are hyporesponsive to M. abscessus. They are homozygous for the TLR1 SNP I602S which has been demonstrated to cause diminished cellular responses to TLR2 agonists.
Conclusions
Homozygosity for I602S is prevalent in Western Europeans and North American Caucasians, the same demographic in which the ΔF508 mutation is present. This SNP may play a role in the pathogenesis of M. abscessus lung infection in patients with cystic fibrosis.
1. Introduction
The CFBE41o- respiratory epithelial cell line was derived from the bronchus of a patient with cystic fibrosis and immortalized with SV40 [1,2]. We have found that this cell line fails to generate an innate immune response to M. abscessus, a pathogen causing progressive lung disease in patients with cystic fibrosis.
Respiratory epithelium has been increasingly recognized as playing an important role in the innate immune response to pulmonary pathogens. These cells are among the various cell types which express toll-like receptors (TLRs); receptors which mediate the innate immune response to invading pathogens [3]. Both interleukin-8 (IL-8) and human β-defensin 2 (HβD2) are expressed by respiratory epithelial cells in response to TLR stimulation [4–6].
M. abscessus exists as either a glycopeptidolipid (GPL) expressing variant (smooth phenotype) in which GPL masks underlying bioactive cell wall lipids, or as a variant lacking GPL (rough phenotype) which is immunostimulatory and invasive in macrophage infection models [7–9]. We have demonstrated that the rough phenotype stimulates host cell innate immune responses via TLR2 [6,8,9]. Importantly, it has been demonstrated that optimal mycobacterial TLR2 signaling involves formation of heterodimers with TLR1 at the cell surface [10,11]. Recent studies indicate that a single nucleotide polymorphism (SNP) in the TLR1 gene at 1805T/G (rs5743618) i.e., Ile602Ser (isoleucine to serine, non-synonymous polymorphism) (I602S), confers impaired function on TLR1 [12,13]. This SNP is common in the Caucasian population of Western Europe and in Caucasians of European descent in the United States and Canada [14]. This is the same demographic in which the cystic fibrosis CFTR ΔF508 mutation occurs. In our study, we demonstrate hyporesponsiveness of CFBE41o- cells to M. abscessus and Pam3Cys, another TLR2 ligand. Importantly, we demonstrate that CFBE41o- cells are homozygous for TLR1 SNP I602S.
2. Materials and methods
2.1. Bacteria
The isogenic M. abscessus 390S (smooth colony morphotype expressing GPL), 390R and 390V (rough colony morphotype lacking GPL expression) variants have been previously characterized [15]. Bacteria were cultured and stored at −70 °C as described [7].
2.2. Cell lines
The CFBE41o-, CFBE41o-/CEP-CFTR, CFTE29o- and 16HBE14o- respiratory epithelial cell lines were obtained from Dr. Dieter Gruenert at the University of California San Francisco with origins as described [1,2,16–18]. Upon receipt, CFBE41o-cells were passage 4.80, CFBE41o-/CEP-CFTR cells were passage 4.77.96, CFTE29o- cells were passage 2.49, and 16HBE14o- cells were passage 2.51. The CFBE41o- cell line has been extensively characterized and used in numerous studies examining how the CFTR ΔF508 mutation affects the normal functions of respiratory epithelium. Importantly, this cell line remains homozygous for CFTR ΔF508 over multiple passages in culture [2]. Upon receipt, cells were passaged 3 times, cryopreserved and stored in multiple vials. For each set of experiments, a new vial was thawed and cells passaged not more than 5 times. Cells were cultured in a T-75 tissue culture flask in 6 ml of media — MEM with 10% FBS (Invitrogen 16000–044) and 1% Antibiotic/Antimycotic (Gibco 15240), CFBE41o-/CEP-CFTR cells were grown in the same medium plus hygromycin (cell culture medium). Specific additives to tissue culture flasks were not required for growth of confluent cell monolayers. Cells were split every 3–4 days. Confluent cell monolayers were trypsinized with 0.5 ml 0.25% trypsin (Invitrogen 25200056) at 37 °C. New flasks were seeded at 1:10 ratio from trypsinized confluent flasks.
2.3. Treatment of respiratory epithelial cells for measurement of HβD2 gene expression
Respiratory cells were plated at a concentration of 1.5–2×105 cells per well in cell culture medium in 6 well tissue culture plates and incubated at 37 °C, 5% CO2 for 72 h. After washing, individual wells containing cell monolayers then received 1×107 cfu of the M. abcessus variant 390R. Additional wells received MALP-2 (Imgenex IMG2206) as a control for functioning TLR2, via stimulation of TLR2/TLR6 heterodimers, at a final concentration of 100 ng/ml and Pam3Cys (Imgenex IMG2201), which signals through TLR2/TLR1 heterodimers, at concentrations ranging from 100 ng/ml to 10,000 ng/ml. IL-1β (PeproTech 20001B) bypasses TLRs but signals through distal common pathways leading to HβD2 gene expression. It was added to some wells at a final concentration of 20 ng/ml as an additional control. The negative control wells received media alone. All wells received their respective treatments in a final volume of 2 ml Iscove’s Modified Dulbecco’s Medium +5% NHS (Sera Care Life Sciences Human AB serum male cc-520-100), in triplicate. Following incubation at 37 °C, 5% CO2 for 8 h, cells were rinsed 3 times with Iscove’s Modified Dulbecco’s Medium, and harvested for RNA isolation using the RNeasy Mini Kit (Qiagen 74104).
2.4. Real-time PCR assessment of HβD2 gene expression
The HβD2 primer and probe sequences, and final reaction concentrations, were based on previous reports and were as follows: forward: 5′-GAGGAGGCCAAGAAGCTGC-3′ (300 nM); reverse: 5′-CGCACGTCTCTGATGAGGG-3′ (300 nM); probe: 5′-FAM-TGGCTGATGCGGATTCAGAAAGGG-TAMRA-3′ (250 nM) [5]. The ABI Human 18S rRNA primer/probe mix (ABI 4319413e) was used per the manufacturer’s instruction for detection of 18S. qRTPCR of the RNA was performed using the ABI Taqman One Step RT-PCR Master Mix Reagents kit (4309169), per manufacturer’s instruction. Relative quantity was determined using the ddCT method, using the untreated control as the calibrator sample.
2.5. Treatment of respiratory epithelial cells for measurement of IL-8 levels in cell supernates
CFBE41o-, CFBE41o-/CEP-CFTR, and CFTE29o- respiratory epithelial cells were plated at a concentration of 2× 105 cells per well in cell culture medium in 24 well tissue culture plates and incubated at 37 °C, 5% CO2 for 24 h. After washing, wells were treated with either 10,000 ng/ml Pam3Cys, 100 ng/ml MALP-2, or M. abcessus variant 390R at a density of 2.5×106 cfu/well. The negative control wells received no treatment. All wells received their respective treatments in a final volume of 0.5 ml Iscove’s Modified Dulbecco’s Medium +5% NHS, in triplicate. Following incubation at 37 °C, 5% CO2 for 8 h, cell supernates were collected, filtered with 0.2 μm centrifugal filters (VWR 82031-358) and frozen at −80 °C. Supernatants were analyzed with the BD Human IL8 ELISA set (BD555244). The remaining cells were lysed with 150 μl 1% cetyltrimethyl ammonium bromide in 0.1 M citric acid with 0.5% napthol blue black (cetyltrimethyl ammonium bromide: ISC 0833; citric acid: Sigma C1909; napthol blue black: Sigma N3005). Stained nuclei from lysed cells were transferred to a tube containing 50 μl formaldehyde. Nuclei were counted to determine the number of cells in each monolayer and these data were used to standardize reporting of results as IL-8 released per 105 cells.
2.6. Genotyping TLR1 polymorphisms
The genomic region on chromsome 4 from 38,798,485–38,798,812 (327 bp) was PCR amplified using the forward primer-AAGTGTTAGAGGGCTGGCCTGATT and reverse primer-TTCACCCAGAAAGAATCGTGCCCA. The PCR conditions were: the initial denaturation step was one cycle for 8 min at 94 °C, followed by 34 cycles at 94 °C for 30 s, annealing for 30 s at 60 °C, and extension for 50 s at 72 °C, followed by a final extension step for 7 min at 72 °C. The resulting PCR products were purified over Microcon® centrifugal filter devices (10,000 mwco, Millipore). The aforementioned primer set was used to determine the presence or absence of T1805G polymorphism using the BigDye® Terminator Cycle Sequencing Kit in a Model 377 Sequencing System (Applied Biosystems, Foster City, CA) at the DNA sequencing and analysis facility (http://hsc.unm.edu/som/programs/cidi/dna.shtml). In addition, the presence of the variant allele was confirmed by restriction fragment length polymorphism (RFLP) analysis. Briefly, NEBcutter V2.0 was used to determine the restriction enzyme site for the TLR1 SNP. As such, Alu1 and Pst1 were determined to cut only samples with the TLR1 SNP, and were used to digest PCR samples, at 37 °C for 16–24 h. Digests were then run on a 3% agarose gel to visualize digested DNA. Samples digested by Alu1 produced bands that were 150 bp and 177 bp long, and samples digested by Pst1 produced bands that were 151 bp and 176 bp long. Samples that did not carry the TLR1 SNP were not digested, and produced an uncut 327 bp band.
3. Results
3.1. CFBE41o- cells are hyporesponsive to M. abscessus 390R and the TLR2/TLR1 agonist Pam3Cys as measured by HβD2 gene expression
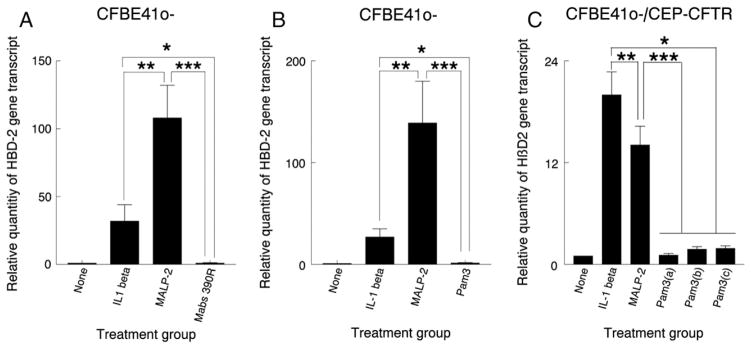
We have previously verified the specificity of HβD2 as a readout for M. abscessus TLR2 stimulation by siRNA knockdown of TLR2 which abrogated HβD2 release in response to M. abscessus [6]. In addition, we have reported that phosphatidyl inositol mannosides (PIMs) are one of the M. abscessus ligands for TLR2. Importantly, we have demonstrated that M. abscessus smooth expressing variants fail to elicit a TLR2 response as a result of expression of GPL, which masks underlying PIMs preventing their interaction with TLR2 [8]. Based on this evidence, epidemiologic evidence [19], and clinical evidence [20], we have proposed that M. abscessus smooth variants are initial colonizers of the lung [6–8,21]. Spontaneous loss of GPL leads to the rough variant which is invasive and stimulates TLR2 [6,8,9]. Signaling by mycobacterial lipopeptides (triacylated lipopeptides) occurs predominantly through TLR2/TLR1 heterodimers [10,11]. Our results indicate that CFBE41o- cells generate a diminished innate immune response to the M. abscessus rough variant 390R (Fig. 1A) and the TLR2/TLR1 agonist Pam3Cys (Fig. 1B). Furthermore, Pam3Cys at concentrations of 1000 μg/ml and 10,000 μg/ml failed to significantly increase HβD2 expression above untreated control (data not shown). This hyporesposiveness to M. abscessus and Pam3Cys occurred in spite of a robust response to MALP-2, a TLR2/TLR6 agonist, and IL-1β which signals downstream via a common final pathway independent of the TLRs.
Fig. 1.
CFBE41o- and CFBE41o-/CEP-CFTR cells are hyporesponsive to M. abscessus and the TLR2/TLR1 ligand Pam3Cys as measured by HβD2 gene expression. CFBE41o- cell monolayers received no treatment or were treated with IL-1β, MALP-2 and the rough M. abscessus variant 390R (A), or the TLR2/TLR1 ligand Pam3Cys (100 ng/ml) (B). After 8 h, HβD2 gene expression was quantified by real-time PCR. The results of real-time PCR are expressed as the relative fold increase in HβD2 gene expression over that of the untreated group and presented as mean+/−SEM of measurements from duplicate experiments performed in triplicate. Asterisks note differences, p<0.05, t-test. In addition, CFBE41o-/CEP-CFTR cells received the same treatment, substituting increasing concentrations of Pam3Cys (100, 1000 and 10,000 ng concentrations) for Pam3Cys 100 ng/ml and M. abscessus 390R (C). Asterisks denote differences, p<0.05, t-test.
3.2. CFBE41o- cells complemented with a plasmid containing wild type CFTR (CFBE41o-/CEP-CFTR cells) remain hyporesponsive to the TLR2/TLR1 agonist Pam3Cys as measured by HβD2 gene expression
Our findings raised the question of whether this lack of response by CFBE41o- cells could be due to the fact that these cells are homozygous for the CFTR ΔF508 mutation, the most common genetic variant which causes cystic fibrosis [22]. We thus examined the response to CFBE41o- cells transformed with a plasmid containing wild type CFTR (CFBE41o-/CEP-CFTR cells). In spite of expressing wild type CFTR these cells failed to respond to a range of Pam3Cys concentrations, suggesting that hyporesponsiveness to M. abscessus is not the result of the CFTR Δ508 mutation (Fig. 1C).
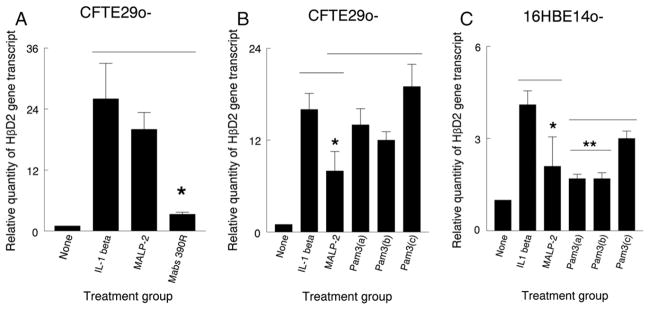
3.3. CFTE29o- and 16HBE14o- cell monolayers respond to both Mabs 390R and the TLR2/TLR1 agonist Pam3Cys as measured by HβD2 gene expression
Although the defect in signaling to M. abscessus and Pam3Cys exhibited by CFBE41o- cells was not reversed by wild type CFTR, we sought additional evidence that this defect was not specific to cells homozygous for the ΔF508 mutation using the CFTE29o-cell line which is also homozygous for the ΔF508 mutation. Challenge with M. abscessus generated a response as measured by HβD2 gene expression which was 3.3-fold above baseline (Fig. 2A), in contrast to the complete lack of a response exhibited by CFBE41o- cells (Fig. 1A). Furthermore, these cells responded to Pam3Cys with a 19-fold increase over baseline at a Pam3Cys concentration of 10,000 ng/ml (Fig. 2B) compared with a 1.4- and 1.9-fold increase over baseline at this concentration in the CFBE41o- and CFBE41o-/CEP-CFTR cells, respectively. The non-cystic fibrosis bronchial epithelial cell line 16HBE14o-manifested low level responses to all treatments (Fig. 2C). The decreased magnitude of these responses could relate to early reports which indicated that this cell line overexpresses wild type CFTR [23], and recent data indicating that CFTR has a general dampening effect on the immune response [24,25]. However, the responses of this cell line to Pam3Cys were above those of untreated cells, with Pam3Cys at a concentration of 10,000 ng/ml inducing a 3-fold increase in HβD2 gene expression over baseline (Fig. 2C).
Fig. 2.
CFTE29o- cells respond to M. abscessus and the TLR2/TLR1 ligand Pam3Cys as measured by HβD2 gene expression. CFTE29o- cell monolayers received no treatment or were treated with IL-1β, MALP-2 and the rough M. abscessus variant 390R (A), or the TLR2/TLR1 ligand Pam3Cys at increasing concentrations (100, 1000 and 10,000 ng/ml)(B). After 8 h, HβD2 gene expression was quantified by real-time PCR. The results of real-time PCR are expressed as the relative fold increase in HβD2 gene expression over that of the untreated group and presented as mean+/−SEM of measurements from duplicate experiments performed in triplicate. Asterisks note differences, p<0.05, t-test. In addition, 16HBE14o- cell monolayers (non-cystic fibrosis cell line) (C) received the same treatment as in (B). Asterisks denote differences, p<0.05, t-test.
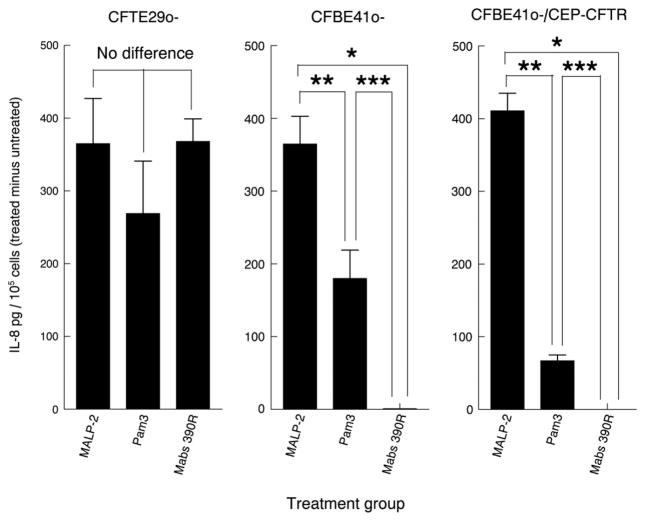
3.4. CFBE41o- cells release decreased amount of IL-8 in response to M. abscessus and Pam3Cys compared to CFTE29o-cells
As further confirmation of a defect in the innate immune response of CFBE41o- cells to TLR2/TLR1 ligands, we assessed release of IL-8 as a readout for TLR stimulation. Consistent with our HβD2 gene expression measurements, CFBE41o- cells and CFBE41o-/CEP-CFTR cells released significantly reduced amounts of IL-8 in response to both M. abscessus and Pam3Cys (Fig. 3). In contrast, CFTE29o- cells released similar amounts of IL-8 in response to MALP-2, Pam3Cys and M. abscessus 390R (Fig. 3).
Fig. 3.
CFBE41o- and CFBE41o-/CEP-CFTR cells are hyporesponsive to M. abscessus and the TLR2/TLR1 ligand Pam3Cys as measured by IL-8 release when compared to CFTE29o- cells. CFTE29o-, CFBE41o- and CFBE41o-/CEP-CFTR cells monolayers received no treatment, or were incubated with MALP-2 (100 ng/ml), Pam3Cys (10,000 ng/ml) or M. abscessus 390R (5.0×106/ml). After 8 h, supernates were harvested and assayed for IL-8 by ELISA. Results are IL-8 levels corrected to 1×105 cells minus the IL-8 levels of untreated control wells and are presented as the mean+/−SEM of measurements from duplicate experiments performed in triplicate. Asterisks note differences, p<0.05 t-test.
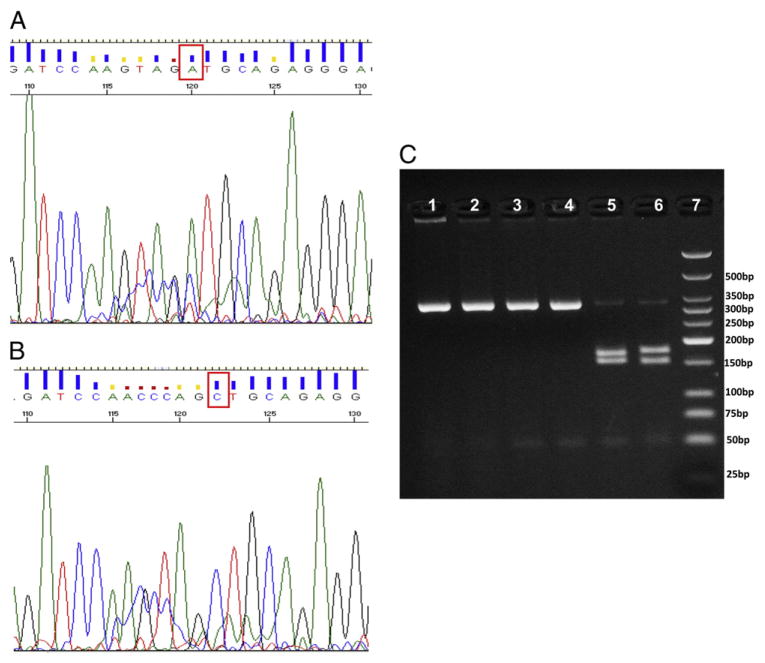
3.5. CFBE41o- cells are homozygous for the TLR1 SNP I602S, whereas CFTE29o- cells and 16HBE14o- cells possess wild type TLR1
Since CFBE41o- cells respond to the TLR2/TLR6 ligand MALP-2 this suggests that the TLR2 receptor is functional in these cells. Conversely, the lack of response of CFBE41o- cells to M. abscessus and the TLR2/TLR1 ligand, Pam3Cys, suggests a problem with the TLR1 receptor in the TLR signaling pathway of these cells. Recent studies have documented an extremely high prevalence of the SNP TLR1 I602S which alters a specific region of the TLR1 molecule such that it is unable to traffic to the cell membrane and participate in TLR2/TLR1 heterodimer formation [14]. It has been conclusively demonstrated that this SNP results in suboptimal TLR2 signaling in response to mycobacterial lipopeptides [12,13]. We found that CFTE29o- cells and 16HBE14o- cells have wild type TLR1 at the 1805T/G locus (Fig. 4A, C), whereas CFBE41o- has the I602S SNP at this locus (Fig. 4B, C). In addition, we demonstrated that CFBE41o- cells are homozygous for this allele (Fig. 4C).
Fig. 4.
CFBE41o- cells are homozygous for SNP I602S whereas CFTE29o- cells possess the wild type allele. The genomic DNA from both cell lines were subjected to PCR amplification followed by sequencing and RFLP analyses to determine the allele and genotypes. (A), CFTE29o- cell line possesses the wild type ′A′ allele (shown in box using the reverse primer sequence chromatogram), whereas the CFBE41o- cell line has a mutant ′C′ allele (shown similarly in box using the reverse primer sequence chromatogram) (B). The PCR products were also subjected to RFLP analyses to confirm the genotype of this 1805T/G SNP based on the allele specific digestion and distinct pattern on the agarose gel electrophoresis. (C), 16HBE14o- and CFTE29o- cell lines are homozygous wild type (AA or TT, lane 1, 3 digested with Pst1 and lane 2, 4 digested with Alu1 restriction enzymes) and CFBE41o- cell line is homozygous mutant (CC or GG, lane 5 digested with Pst1 and lane 6 digested with Alu1 restriction enzymes), the lane 7 loaded with low molecular weight ladder (NEB N3233S) to facilitate the band identification.
4. Discussion
Patients with cystic fibrosis are susceptible to lung infection with NTM; the most common species being M. avium and M. abscessus [26,27]. Susceptibility is felt to be due in part to inspissated pulmonary secretions and resultant decreased mucociliary clearance. This enables bacterial pathogens to colonize the airways and invade the lung parenchyma. The results of our study demonstrate another mechanism which may contribute to the susceptibility of cystic fibrosis patients to lung infection with M. abscessus and perhaps other NTM; homozy-gosity for the TLR1 1602S SNP prevents an important component of the host cell innate immune sensing mechanism from recognizing this pathogen.
Our study demonstrates that the CFBE41o- cell line generates a diminished innate immune response to M. abscessus. The innate immune system recognizes bacteria through TLRs. TLRs are present in alveolar macrophages, dendritic cells and mucosal epithelial cells in the lung. TLRs recognize pathogen-associated molecular patterns, which are conserved motifs expressed by microorganisms, but not by higher eukaryotes [3]. TLRs cooperate in binding ligands which initiate innate immune signaling; the TLR2/TLR1 heterodimer is required for signaling in response to triacylated lipopeptides found in virulent mycobacteria and gram negative bacteria, while the TLR2/TLR6 heterodimer is required for signaling in response to diacylated lipopeptides found in gram positive bacteria and mycoplasma species [10,11]. Engagement of TLRs on respiratory epithelial cells leads to expression of the chemokines IL-8 and MCP-1, which are involved in recruitment of circulating neutrophils and monocytes to sites of infection/inflammation in the lung, and expression of the human β-defensin family of antimicrobial peptides [4–6].
Respiratory epithelial cells have been increasingly recognized as playing an important sentinel role in innate immune recognition of respiratory pathogens. We have recently demonstrated that respiratory epithelial cells respond to M. abscessus rough variants with HβD2 gene expression and release of IL-8. Furthermore, we demonstrated that TLR2 is necessary for this interaction to occur. Conversely, M. abscessus expressing GPL does not stimulate expression of IL-8 or HβD2 by respiratory epithelial cells which is consistent with “masking” of underlying bioactive cell wall lipids by GPL [6]. Because GPL-expressing smooth variants are the predominant phenotype existing in the environment [19], this provides an explanation whereby initial M. abscessus colonization of abnormal lung airways escapes detection by the innate immune system. Whereas the interaction of M. abscessus with TLR2/TLR1 heterodimers is influenced by GPL expression, there is also evidence that host variation, as manifested by SNPs in TLR genes, plays a critical role in the outcome of mycobacterial infections. The SNP, I602S, is a nonsynonymous polymorphism which results in defective TLR1 trafficking to the cell membrane. This results in an inability of TLR1 to participate in heterodimer formation with TLR2, and suboptimal signaling in response to mycobacterial lipopeptides [13]. The clinical relevance of this SNP is underscored by the finding that it is strongly associated with protection against the development of leprosy, and there is evidence that selection pressure as a result of Mycobacterium leprae infection resulted in the emergence of this SNP in the ancestral European population. Remarkably, 40% of European Caucasians have been found to be homozygous for this SNP, with American and Canadian Caucasians in the range of 60–70% for homozygosity [14]. The mechanism by which defective TLR1 signaling in response to M. leprae leads to protection against leprosy has not yet been elucidated [12,14]. Although hypotheses for this effect have been proposed, the protective effect against leprosy is counterintuitive. One might predict that loss of function of TLR1 would lead to a diminished protective effect rather than the reverse. This is an unresolved paradox and may relate to the immunologic complexity of the host-pathogen interplay that occurs in patients with leprosy. We speculate that this loss of function may have a significant impact on susceptibility to infection with other mycobacteria as well. Importantly, CF is an autosomal recessive inherited disorder characterized by mutations in the gene encoding the CFTR protein. Of relevance to our findings is the fact that it is one of the most common lethal hereditary disorders among Caucasians of European descent. The most frequent mutation in CF is a deletion of phenylalanine at position 508 of the CFTR protein, the ΔF508 mutation [22], which is present in CFBE41o- cells [1]. Given the fact that both CFTR ΔF508 and TLR1 SNP I602S occur in the same demographic, this association may be an additional factor predisposing cystic fibrosis patients to lung airway infection, in particular, airway infection with NTM such as M. abscessus and M. avium.
Acknowledgments
The authors thank Dr. Dieter Gruenert for providing the cell lines used in this study.
Funding
Dr. Thomas F. Byrd receives support from the U.S.A. Department of Veterans Affairs, with this project funded by the University of New Mexico Research Allocation Committee (RAC) Grant, and a grant from New Mexico Sonographics administered by the Biomedical Research Institute of New Mexico. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Meng QH, Springhall DR, Bishop AE, Morgan K, Evans TJ, Habib S, et al. Lack of inducible nitric oxide synthase in bronchial epithelium: a possible mechanism of susceptibility to infection in cystic fibrosis. J Pathol. 1998;184:323–31. doi: 10.1002/(SICI)1096-9896(199803)184:3<323::AID-PATH2>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 2.Ehrhardt C, Collnot EM, Baldes C, Becker U, Laue M, Kim KJ, et al. Towards an in vitro model of cystic fibrosis small airway epithelium: characterisation of the human bronchial epithelial cell line CFBE41o−. Cell Tissue Res. 2006;323(3):405–15. doi: 10.1007/s00441-005-0062-7. [DOI] [PubMed] [Google Scholar]
- 3.Bals R, Hiemstra PS. Innate immunity in the lung: how epithelial cells fight against respiratory pathogens. Eur Respir J. 2004;23:327–33. doi: 10.1183/09031936.03.00098803. [DOI] [PubMed] [Google Scholar]
- 4.Sha Q, Truong-Tran AQ, Plitt JR, Beck LA, Schleimer RP. Activation of airway epithelial cells by toll-like receptor agonists. Am J Respir Cell Mol Biol. 2004;31:358–64. doi: 10.1165/rcmb.2003-0388OC. [DOI] [PubMed] [Google Scholar]
- 5.Birchler T, Seibl R, Buchner K, Loeliger S, Seger R, Hossle JP, et al. Human Toll-like receptor 2 mediates induction of the antimicrobial peptide human beta defensin 2 in response to bacterial lipoprotein. Eur J Immunol. 2001;31:3131–7. doi: 10.1002/1521-4141(200111)31:11<3131::aid-immu3131>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 6.Davidson LB, Nessar R, Kempaiah P, Perkins DJ, Byrd TF. Mycobacterium abscessus glycopeptidolipid prevents respiratory epithelial TLR2 signaling as measured by HβD2 gene expression and IL-8 release. PLoS One. 2011;6(12):e29148. doi: 10.1371/journal.pone.0029148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howard ST, Rhoades E, Recht J, Pang X, Alsup A, Kolter R, et al. Spontaneous reversion of Mycobacterium abscessus from a smooth to a rough morphotype is associated with reduced expression of glycopeptidolipid and reacquisition of an invasive phenotype. Microbiology. 2006;152(Pt 6):1581–90. doi: 10.1099/mic.0.28625-0. [DOI] [PubMed] [Google Scholar]
- 8.Rhoades ER, Archambault AS, Greendyke R, Hsu FF, Streeter C, Byrd TF. Mycobacterium abscessus glycopeptidolipids mask underlying cell wall phosphatidyl-myo-inositol mannosides blocking induction of human macrophage TNF-alpha by preventing interaction with TLR2. J Immunol. 2009;183:1997–2007. doi: 10.4049/jimmunol.0802181. [DOI] [PubMed] [Google Scholar]
- 9.Nessar R, Reyrat JM, Davidson LB, Byrd TF. Deletion of the mmpL4b gene in the Mycobacterium abscessus glycopeptidolipid biosynthetic pathway results in loss of surface colonization capability, but enhanced ability to replicate in human macrophages and stimulate their innate immune response. Microbiology. 2011;157:1187–95. doi: 10.1099/mic.0.046557-0. [DOI] [PubMed] [Google Scholar]
- 10.Takeuchi O, Sato S, Horiuchi T, Hoshino K, Takeda K, Dong Z, et al. Cutting edge: role of toll-like receptor 1 in mediating immune response to microbial lipoproteins. J Immunol. 2002;169:10–4. doi: 10.4049/jimmunol.169.1.10. [DOI] [PubMed] [Google Scholar]
- 11.Jin MS, Kim SE, Heo JY, Lee ME, Kim HM, Paik S, et al. Crystal structure of the TLR1–TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell. 2007;130:1071–82. doi: 10.1016/j.cell.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Johnson CM, Lyle EA, Omueti KO, Stepensky VA, Yegin O, Alpsoy E, et al. Cutting edge: a common polymorphism impairs cell surface trafficking and functional responses of TLR1 but protects against leprosy. J Immunol. 2007;178:7520–4. doi: 10.4049/jimmunol.178.12.7520. [DOI] [PubMed] [Google Scholar]
- 13.Hawn TR, Misch EA, Dunstan SJ, Thwaites GE, Lan NT, Quy HT, et al. A common human TLR1 polymorphism regulates the innate immune response to lipopeptides. Eur J Immunol. 2007;37:2280–9. doi: 10.1002/eji.200737034. [DOI] [PubMed] [Google Scholar]
- 14.Wong SH, Gochhait S, Malhotra D, Pettersson FH, Teo YY, Khor CC, et al. Leprosy and the adaptation of human toll-like receptor 1. PLoS Pathog. 2010;6:e1000979. doi: 10.1371/journal.ppat.1000979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Byrd TF, Lyons CR. Preliminary characterization of a Mycobacterium abscessus mutant in human and murine models of infection. Infect Immun. 1999;67:4700–7. doi: 10.1128/iai.67.9.4700-4707.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Illek B, Maurisse R, Wahler L, Kunzelmann K, Fischer H, Gruenert DC. Cl transport in complemented CF bronchial epithelial cells correlates with CFTR mRNA expression levels. Cell Physiol Biochem. 2008;22:57–68. doi: 10.1159/000149783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunzelmann K, Schwiebert EM, Zeitlin PL, Kuo WL, Stanton BA, Gruenert DC. An immortalized cystic fibrosis tracheal epithelial cell line homozygous for the delta F508 CFTR mutation. Am J Respir Cell Mol Biol. 1993;8:522–9. doi: 10.1165/ajrcmb/8.5.522. [DOI] [PubMed] [Google Scholar]
- 18.Haws C, Krouse ME, Xia Y, Gruenert DC, Wine JJ. CFTR channels in immortalized human airway cells. Am J Physiol. 1992;263:L692–707. doi: 10.1152/ajplung.1992.263.6.L692. [DOI] [PubMed] [Google Scholar]
- 19.Jonsson BE, Gilljam M, Lindblad A, Ridell M, Wold AE, Welinder-Olsson C. Molecular epidemiology of Mycobacterium abscessus, with focus on cystic fibrosis. J Clin Microbiol. 2007;45:1497–504. doi: 10.1128/JCM.02592-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Catherinot E, Roux AL, Macheras E, Hubert D, Matmar M, Dannhoffer L, et al. Acute respiratory failure involving an R variant of Mycobacterium abscessus. J Clin Microbiol. 2009;47:271–4. doi: 10.1128/JCM.01478-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greendyke R, Byrd TF. Differential antibiotic susceptibility of Mycobacterium abscessus variants in biofilms and macrophages compared to that of planktonic bacteria. Antimicrob Agents Chemother. 2008;52:2019–26. doi: 10.1128/AAC.00986-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Population variation of common cystic fibrosis mutations. The cystic fibrosis genetic analysis consortium. Hum Mutat. 1994;4:167–77. doi: 10.1002/humu.1380040302. [DOI] [PubMed] [Google Scholar]
- 23.Cozens AL, Yezzi MJ, Kunzelmann K, Ohrui T, Chin L, Eng K, et al. CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells. Am J Respir Cell Mol Biol. 1994;10:38–47. doi: 10.1165/ajrcmb.10.1.7507342. [DOI] [PubMed] [Google Scholar]
- 24.Vij N, Mazur S, Zeitlin PL. CFTR is a negative regulator of NFkappaB mediated innate immune response. PLoS One. 2009;4:e4664. doi: 10.1371/journal.pone.0004664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hunter MJ, Treharne KJ, Winter AK, Cassidy DM, Land S, Mehta A. Expression of wild-type CFTR suppresses NF-kappaB-driven inflammatory signaling. PLoS One. 2010;5:e11598. doi: 10.1371/journal.pone.0011598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olivier KN, Weber DJ, Wallace RJ, Jr, Faiz AR, Lee JH, Zhang Y, et al. Nontuberculous mycobacteria. I: multicenter prevalence study in cystic fibrosis. Am J Respir Crit Care Med. 2003;167(6):828–34. doi: 10.1164/rccm.200207-678OC. [DOI] [PubMed] [Google Scholar]
- 27.Sermet-Gaudelus I, Le Bourgeois M, Pierre-Audigier C, Offredo C, Guillemot D, Halley S, et al. Mycobacterium abscessus and children with cystic fibrosis. Emerg Infect Dis. 2003;9:1587–91. doi: 10.3201/eid0912.020774. [DOI] [PMC free article] [PubMed] [Google Scholar]