Abstract
Prostate cancer is a significant public health burden and a major cause of morbidity and mortality among men worldwide. Analyzing geographic patterns and temporal trends may help identify high-risk populations, suggest the degree of PSA testing, and provide clues to etiology. We used incidence data available from the International Agency for Research on Cancer (IARC) and certain cancer registries for 43 populations across five continents during a median period of 24 years. Trends in overall prostate cancer rates showed five distinct patterns ranging from generally monotonic increases to peaking of rates followed by declines, which coincide somewhat with changes in the prevalence of PSA testing. Trends in age-specific rates generally mirrored those in the overall rates, with several notable exceptions. For populations where overall rates increased rapidly and then peaked, exemplified in North America and Oceania, the highest incidence tended to be most pronounced and occurred during earlier calendar years among older men compared with younger ones. For populations with almost continual increases in overall rates, exemplified in Eastern Europe and Asia, peaks were evident among men aged ≥75 years in many instances. Rates for ages 45–54 years did not clearly stabilize or decline in the majority of studied populations. Global geographic variation remained substantial for both overall and age-specific incidence rates regardless of levels of PSA testing, with the lowest rates consistently in Asia. Explanations for the persistent geographic differences and the continuing increases of especially early-onset prostate cancer remain unclear.
Keywords: Prostatic Neoplasms, Incidence, Age-specific, Trends, International
Introduction
Worldwide prostate cancer is the second most frequently diagnosed cancer and the fifth leading cause of cancer death among men, with an estimated 1.1 million new cases diagnosed and 307,000 deaths in 20121. Patterns and trends in prostate cancer incidence have been influenced by screening, diagnostic ascertainment, and population risk factors which are not well understood. Initially an increasing use of transurethral resections of the prostate (TURPs) and, from the mid-to late-1980s, prostate-specific antigen (PSA) testing has influenced the observed incidence trends in a number of high-income countries2–4. Established risk factors for prostate cancer are limited to advancing age, black race, a family history of this malignancy, and certain genetic polymorphisms. There have been few studies of age-specific trends5, especially in low- and middle-income (LMIC) countries. Compiling and analyzing the age-specific trends may help quantify geographic variation, identify high-risk populations, suggest the degree of PSA testing uptake, and ultimately provide clues to etiology.
To this end, we analyzed the trends in overall and age-specific incidence rates of prostate cancer during a median period of 24 years in 43 populations across five continents using incidence data available from the International Agency for Research on Cancer (IARC) and certain cancer registry websites. We evaluated the trends and patterns in relation to geographical and temporal variations in the context of reported PSA testing and screening guidelines.
Materials and Methods
We extracted prostate cancer data as originally reported in the Cancer Incidence in Five Continents (CI5), CI5plus database6, which includes cases diagnosed during 1978–2007. The files with annual case counts and population estimates were updated and expanded with more recent data from cancer registry websites, e.g. the NORDCAN project data from the Nordic cancer registries7, the European Cancer Observatory (http://eco.iarc.fr), the United States Surveillance, Epidemiology and End Results (SEER) program8, as well as national incidence data from Australia (http://www.aihw.gov.au/), New Zealand (http://www.nzhis.govt.nz/), and the Republic of Korea (http://www.ncc.re.kr/english/infor/kccr.jsp). Population-based cancer registries were selected based on the following criteria: 1) inclusion in the CI5plus database6, which indicates achievement of data quality due to the strict editorial process; and 2) the availability of at least nine consecutive years of data till 2007.
Population-based data were available at the national level for 24 countries and regional level for 17 countries. To achieve better representativeness and larger numbers of prostate cancer cases, we combined data across regional registries with comparable data quality within 10 countries: the United States SEER program (9 registries: Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, Utah), Canada (9 registries: Alberta, British Columbia, Manitoba, New Brunswick, Newfoundland, Nova Scotia, Ontario, Prince Edward Island, Saskatchewan), France (6 registries: Doubs, Herault, Isere, Haut-Rhin, Somme, Tarn), Italy (6 registries: Modena, Parma, Ragusa, Romagna, Torino, Varese), Poland (3 registries: Cracow City, Kielce, Lower Silesia), Spain (4 registries: Granada, Murcia, Navarra, Tarragona), Switzerland (2 registries: Geneva, St Gall-Appenzell), India (2 registries: Chennai, Mumbai), Japan (3 registries: Miyagi, Nagasaki, Osaka), and China (2 registries: Shanghai, Hong Kong). Adequate historical data were available from a single regional registry for seven countries: Brazil, Goiania; Ecuador, Quito; Colombia, Cali; Philippines, Manila; Thailand, Chiang Mai; Germany, Saarland; and Uganda, Kyadondo County. US data were available for whites and blacks. In total, this analysis included 43 populations in 41 countries. For countries without national data, the number of registries included is shown in parentheses in each table and figure.
All registries had annual case counts to age 85+ years by 5-year age group. Most registries (n=33) had corresponding population data to age 85+ years, so that we calculated age-specific rates for 18 age groups. Four countries (Brazil (1), Poland (3), Singapore and Slovenia) did not have population data beyond ages 75–79 years, so we used an upper age category of 80+ for these populations. Likewise, we used ages 75+ years for six countries (Costa Rica, Ecuador (1), India (2), Philippines (1), Thailand (1) and Uganda (1)) without population data beyond age 70–74 years. We calculated age-adjusted rates per 100,000 man-years using the world (1960 Segi) standard9. To achieve more stable age-specific incidence rates, we calculated rates for four age groups 45–54, 55–64, 65–74, and 75+, each age-adjusted within using the world (1960 Segi) standard9.
Figures were prepared using a semi-log scale to facilitate the comparison of temporal trends and their relative magnitude. A slope of 10° indicates a change 1% per year10. In each figure and table, the countries were presented within continent/region in descending order of the most recent age-adjusted incidence rate. Rates based on case counts less than 10 were not shown in figures, nor were single data points. Given that data were available for 11 Northern European populations and that it is difficult to clearly present trends for more than five populations in a single plot, we subdivided Northern Europe into three finer groups: West, Nordic and East. Also given that African data were available only for Uganda (1), we hence presented them in the same plot as that for North America to facilitate comparison with US blacks (SEER 9).
We quantified the trends using joinpoint regression analysis11 via the Joinpoint statistical software12. Incidence rates were assumed to follow a Poisson distribution. Each joinpoint denotes a statistically significant change in the trend using a Monte Carlo permutation method. We used the default parameters, except for setting the minimum number of data points at either end of the data to four, to avoid over-fitting at truncation points. The direction and magnitude of changes in incidence rates were described by the annual percent change (APC), which was reported for countries with a minimum of five consecutive years of data with case counts ≥10 per annum. Average annual percent change (AAPC) was used to summarize the trends over the most recent decade for each population using the best-fit joinpoint model. Given PSA testing was introduced in the mid-1980s and early 1990s4, 13, we defined 1983–87, 1993–97, and 2003–07 roughly as pre-, peri-, and post-PSA testing eras for comparison with other countries, based on the inception and spread pattern in North America and Oceania.
Results
Temporal trends and recent patterns for all age groups combined
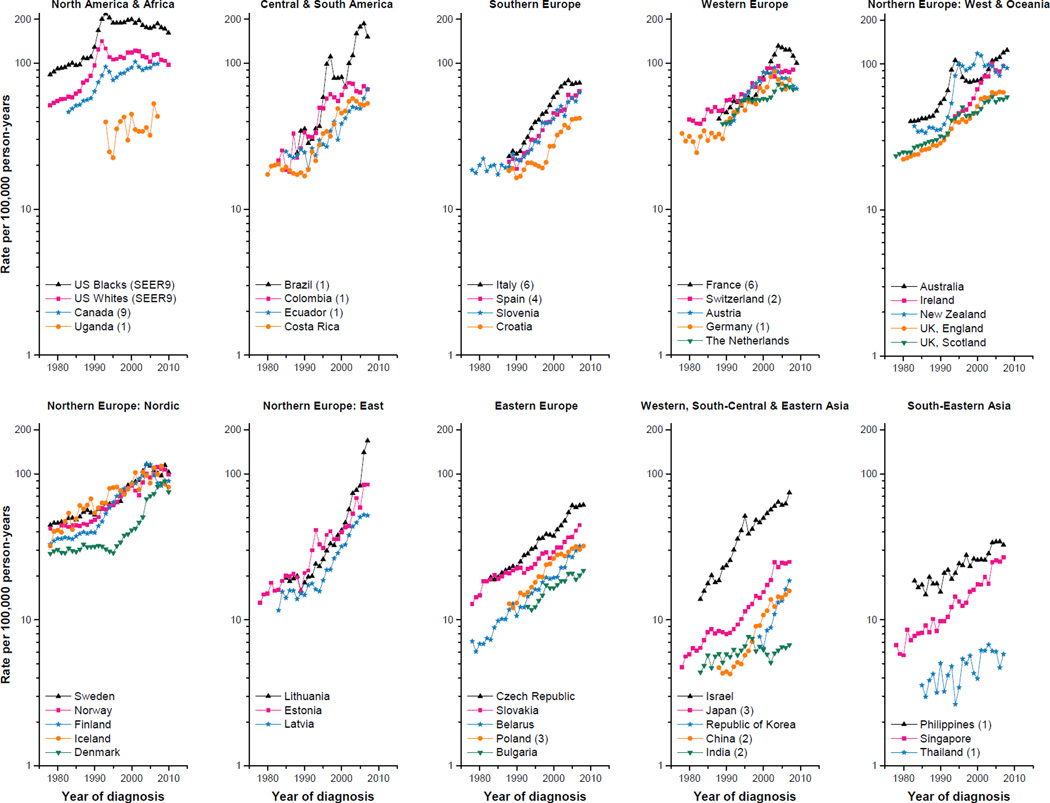
The annual number of prostate cancer cases and age-adjusted rates among the 43 populations across five continents are presented in Supplementary Table 1 and the age-adjusted rates are plotted in Figure 1. Incidence rates were consistently highest in North America and Oceania, and lowest in Asia. We semi-quantitatively summarized temporal patterns into five groups:
Continual increases. Incidence rates increased almost continually over the entire study period in ten populations, ranging from most rapidly in Brazil (1) and the Republic of Korea (APCs ≥10.0%); to rapidly in Spain (4), Belarus and Singapore (APCs of 5.0–9.9%); and to less rapidly in Bulgaria, Philippines (1), India (2), Thailand (1) and Uganda (1) (APCs<5.0%) (Table 1).
Step-wise increases. Rates rose in a step-wise fashion in six populations, among which four recently plateaued (Netherlands; UK, Scotland; Czech Republic and Japan (3)), while in two (UK, England and Slovakia), the rates continued to rise.
Slow rise then rapid increases. Twelve populations exhibited early slow changes, followed by an abrupt surge (APCs= 4.6–23.4%). There was no sign of recent abating in four populations (Ecuador (1), Slovenia, Lithuania and Estonia), while rates increased less rapidly in two populations (Croatia and China (2)) or plateaued in six (Latvia, Norway, Denmark, Sweden, Germany (1) and Costa Rica).
Moderate then slower increases. This pattern occurred in seven populations where rates increased almost continually (APCs=3.5–10.0%), followed by slower increases (Poland (3) and Israel) or a recent plateau (Colombia (1), Switzerland (2), Italy (6), Ireland and Iceland).
Rapid increase then a pronounced peak. Rates increased rapidly in eight populations (US blacks (SEER 9), US whites (SEER 9), Canada (9), Australia, New Zealand, France (6), Austria and Finland) with APCs>10% before reaching a pronounced peak, except in Austria (APC=8.6%). The highest incidence occurred earliest among US whites (SEER 9) in 1992 and more recently in France (6) and Finland in 2004. Subsequently, rates declined continually in US blacks (SEER 9), US whites (SEER 9), France (6) and Austria; plateaued in Canada (9), New Zealand and Finland; or resumed increasing in Australia.
Figure 1.
Temporal trends in age-adjusted (world 1960 Segi population) prostate cancer incidence rates per 100,000 man-years for 43 populations, all ages, 1978–2010
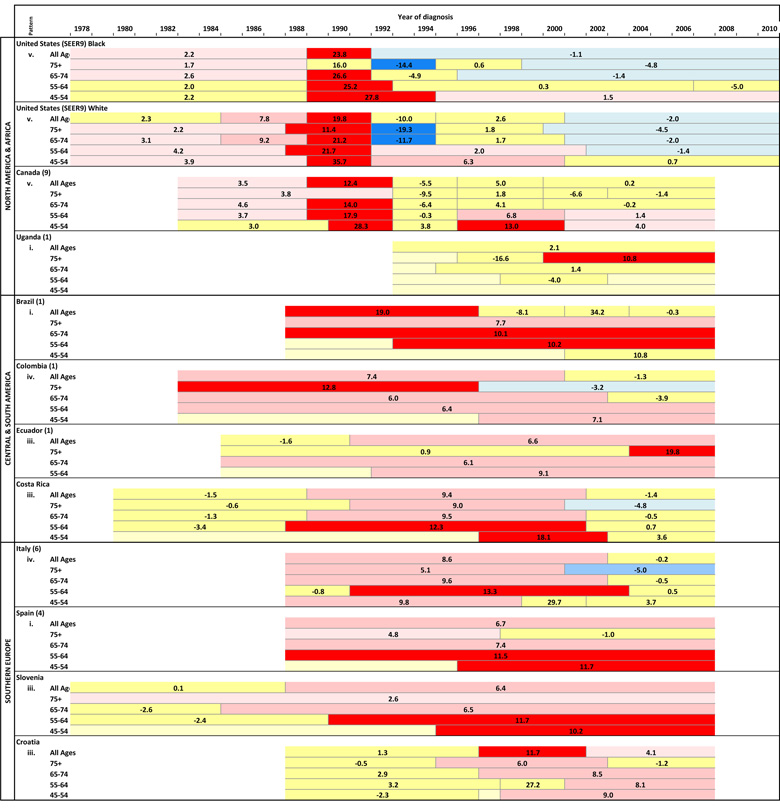
Table 1.
Annual percent changes (APCs) in overall and age-specific prostate cancer incidence rates by segments of year of diagnosis using the best-fit joinpoint regression models.
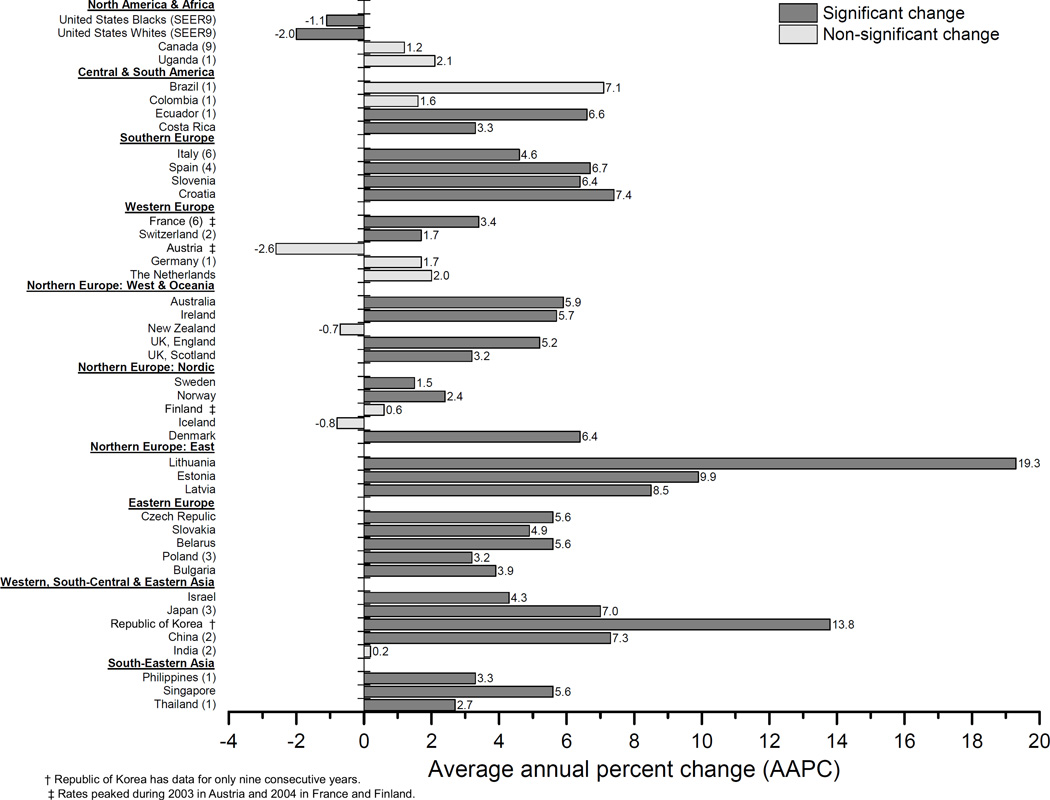
Global geographic variation in incidence rates has remained over 30-fold, increasing from 35 during 1983–1987 to 46 during 1993–1997 before falling to 31 during 2003–2007. The highest rates among the populations studied were consistently among US blacks (SEER 9), which have been five times higher than those in Uganda (1), which in turn have been five times higher than those in Thailand (1). During the most recent decade, rates increased in 30 of the 43 populations with AAPCs ranging from 1.5% in Sweden during 2001–2010 to 19.3% in Lithuania during 1998–2007 (Figure 2). Conversely, rates decreased among both US blacks and whites (SEER 9) with AAPCs of −1.1% and −2.0% during 2001–2010, respectively. In the remaining 10 countries, the rates did not change significantly during the past decade.
Figure 2.
Recent decade trends in overall prostate cancer incidence rates: average annual percent change (AAPC) for 43 populations, circa 1998–2010.
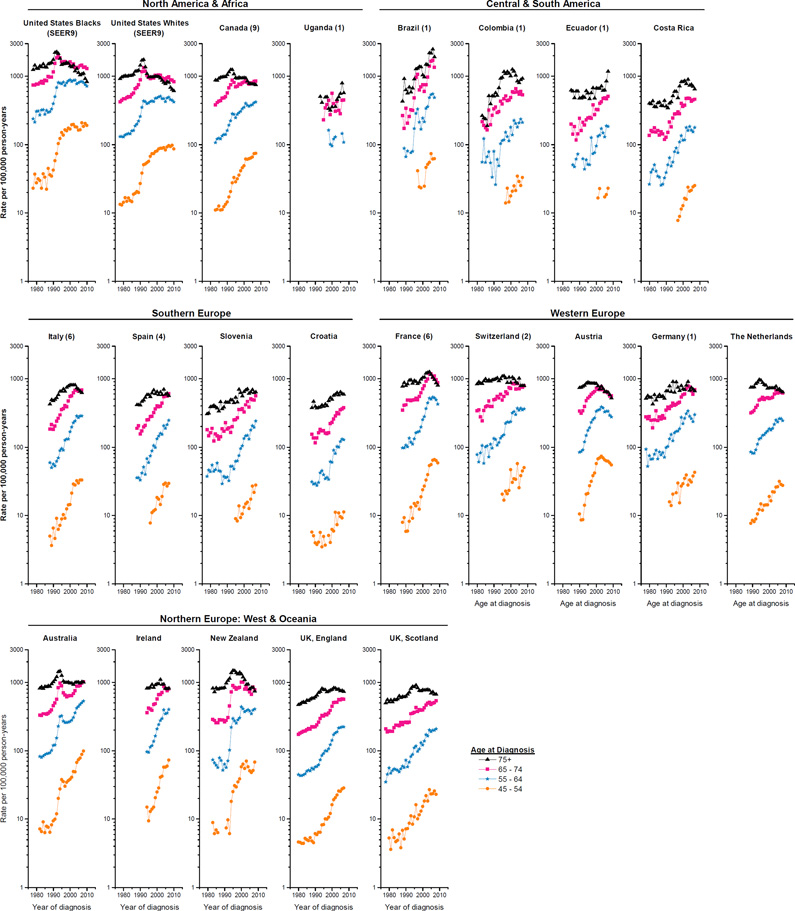
Age-specific temporal trends and recent patterns
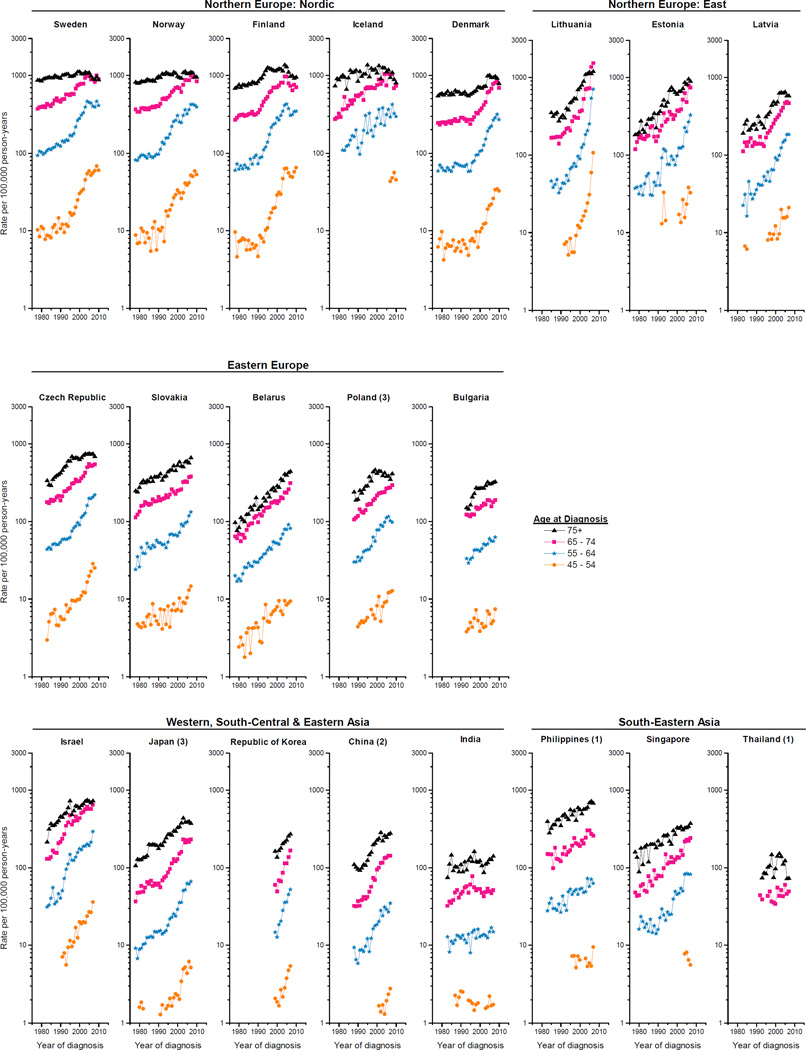
The corresponding annual age-specific counts and rates among the 43 populations are also presented in Supplemental Table 1. The trends generally mirrored those in the overall rates, with notable exceptions (Figure 3). For populations where the overall rates rose almost continually over the past several decades (pattern i), the rates at the younger ages generally increased more rapidly than those at older ages (Table 1). For populations with pattern ii, age-specific rates also generally rose in a step-wise fashion, except that rates clearly peaked in the oldest age group (age 75+) in the Netherlands; UK, Scotland and UK, England. For populations with pattern iii, the acceleration in the rising rates was apparent across most of the age groups, with the most rapid increases for ages <65 years in Lithuania (APCs>50%); in contrast, rates peaked among men aged 75+ in Norway, Denmark, Sweden and Costa Rica. Rates increased or plateaued across age groups in populations with pattern iv, except that rates peaked in the oldest age group in Colombia (1), Switzerland (2), Italy (6), Ireland and Iceland, but not in Poland (3) or Israel. Finally, in populations with pattern v, the age-specific rates generally peaked and stabilized/declined around the same time, with a tendency for peak incidence to occur during earlier calendar years among the older age groups and more recently among the younger age groups. In recent years, rates declined in Austria, US whites (SEER 9) at ages ≥55, US blacks (SEER 9) ages ≥65 and New Zealand ages ≥75; in contrast, rates continued to increase but less rapidly in Australia at ages <75, Canada (9) ages <65 and US blacks (SEER 9) ages 45–54.
Figure 3.
Temporal trends in prostate cancer incidence rates per 100,000 man-years for age groups 45–54, 55–64, 65–74, and 75+ years (age-adjusted within age groups, world 1960 Segi population) for 43 populations, 1978–2010.
Asia consistently had the lowest annual age-specific incidence rates (Figure 3). In recent years, geographic variation was more prominent among younger age groups compared with older ones. During1983–1987, the geographic variation was about 28-fold across the four age groups. By1993–1997, the variation had increased rapidly to 139 among those ages 45–54, 93 among those 55–64, and 51 among those 65–74, but decreased to 19 among those ages 75+. During 2003–2007, the range had declined to 102, 58, and 28 among the three younger age groups and rose slightly to 20 among the oldest.
Discussion
International trends in prostate cancer incidence showed five distinct patterns across the 43 populations assessed, ranging from substantial and almost constant rate increases over the entire study period to rapid increases followed by plateauing or declining rates in more recent years. Age-specific incidence trends generally mirrored those of the overall rates, with several notable exceptions. In populations with almost continual increases in the overall rates (pattern i), peak incidence occurred nonetheless among the oldest age group in a number of populations. In populations where incidence peaked (pattern v), the patterns varied considerably by age group, with the peak incidence most pronounced at older ages and often not present at younger ages. In the majority of populations included in this analysis, rates for ages 45–54 years did not clearly stabilize or decline. Global geographic and racial/ethnic variation was substantial for both overall and age-specific incidence rates, with rates consistently highest in US blacks (SEER 9) and lowest in Asia.
Changing temporal trends in prostate cancer incidence rates in high-income countries have been largely influenced by rising incidental detection of existing tumors via TURPs in patients with benign prostatic hyperplasia (BPH)3, and later by use of PSA testing since the mid- to late-1980s14. The US Food and Drug Administration (FDA) approved the PSA test to monitor disease progression in 1986 and then in 1994 approved its use for aiding early tumor detection in conjunction with digital rectal examination (DRE) among men aged 50 years or older15. Despite controversies over whether PSA testing is beneficial in reducing disease-specific mortality16, it has been widely used in many populations. However, the reported prevalence of PSA testing usually does not identify the purpose of its use, which ranges from early detection among asymptomatic men, to diagnostic testing in response to concerns about prostate diseases or urological symptoms, and to prostate cancer disease monitoring. Therefore, it is difficult to precisely disentangle the degree to which secular trends can be attributed to early detection. Furthermore, information on the prevalence of PSA testing is not available for all countries included in our analysis, and the available estimates are often not directly comparable due to different age groups, time periods, PSA time frames, the order of PSA tests, and data sources across populations. Data on age-specific use of PSA testing are even sparser.
Interpretation of observed geographic variations in prostate cancer incidence rates and elucidation of potential environmental factors have been hampered by differences in PSA testing. As an early detection and diagnostic tool, PSA has been used intensively by general practitioners (GPs) and urologists in many high-income countries since the late-1980s and early-1990s4 and has led to detection of a substantial number of early-stage prostate cancers and rapid increases in population-level incidence rates17. Congruent with the trend in age-adjusted incidence, the proportion of first-ever PSA tests rapidly increased from ~2% during 1988 and peaked at ~18% and ~14% respectively in 1992 among US white and black Medicare beneficiaries aged ≥65 years old13. By 2001, 75% of US men aged≥50 years reported ever-use of a PSA test18. Similarly, consistent with the rapid increases in age-adjusted rates during the 1990s and early 2000s, the annual rates of PSA testing derived from laboratory data rose rapidly from ~80 per 1000 men (all ages) in 1996 to ~190 in 2005 in Norway and from ~40 in 1995 to ~125 in 2002 in Sweden4.
The opportunistic PSA testing approaches that occurred in the vast majority of countries contrast with organized screening efforts in two European countries. A mass prostate cancer prevention project was launched in 1993 for men aged 45–75 years living in the Austrian state of Tyrol; over three-quarters of such men had at least one PSA test in the first five years of the project19. On a larger scale, Lithuania started a national Early Prostate Cancer Detection Program (EPCDP) in 2006 targeting men aged 50–75 years and younger men (ages ≥45 years) with a family history of prostate cancer20. The introduction of this population-based prostate cancer screening program coincides with the most rapid increases in overall and age-specific incidence rates. In other countries where uptake of the PSA test may have been slower, rates increased more gradually. In the UK, only 1% of men aged≥45 years had at least one test in 199421, and the prevalence increased to 4% in 199922 and 6% in 200723. In the Netherlands, 2% of men in the same age group had at least one test in 2002, and the prevalence increased to 5% in 201124. The discordance between relatively low uptake of PSA tests and step-wise increases in incidence may suggest a role of other unidentified risk factors.
Genetic and environmental factors may play a role in the persistent geographical incidence differences over the course of three decades despite different levels of PSA testing. Risk factors for prostate cancer still remain elusive. The fact that incidence rates are relatively high in black populations in Africa and the Caribbean, where PSA testing is not commonly used, supports the notion that genetic disposition modulates the risk25. Environmental risk factors related to western lifestyle have been proposed, but are far from established, including obesity, increased intake of fat and calories, dairy products and calcium, and the use of certain supplements26 that often confer small or minimal risks. Yet the interplay between genetic disposition and environmental factors has been supported by migrant studies. Black men of West African ancestry in the Caribbean Islands and UK connected by the Transatlantic Slave Trade have greater prostate cancer burden, compared with those in West Africa27. In the pre-PSA era, Asian immigrants and their offspring had lower prostate cancer risk than US whites while having higher risk than those in their native homeland28. Improved access to medical care may partially explain incidence differences, especially when comparing regions at different economic development levels. However, this is less likely for Japan, a country with comparable standards of care during our study period, where rates have remained consistently lower than those among US males over decades.
Incidence among men aged ≥75 years peaked in many populations and subsequent declining incidence rates since the 1990s coincided with the general agreement that men aged 75 years or older should not be screened29, 30. The underlying considerations for this general agreement are the long lead time of screen-detected tumors, competing causes of death and limited survival benefits if treatment is applied. However, the prevalence of PSA testing had not consistently decreased in this age group, as exemplified by data for the US and the Netherlands. The US National Health Interview Survey demonstrated that self-reported PSA screening rates in the past year increased from 28% and 23% in 2000 among men aged 80–84 and 85+ to 43% and 26% in 200531. Over 80% of US men aged ≥70 years in 2001 reported ever-use of a PSA test18. Similarly, the incidence rates of PSA testing increased from 29 per 1000 in 2002 to 97 in 2011 in the Netherlands among men aged ≥75 years based on a routine health care database24. Although reasons for testing were not determined, some testing could be ascribed to disease monitoring after prostate cancer diagnosis. In sum, the declining incidence rates at older ages may be partly due to diagnosis of prostate cancer at younger ages, given that positive screening rates declined with repeated PSA screening, as well as a decline in PSA screening use at older ages.
Prostate cancer has long been regarded as a disease of old men and risk factors for young patients with prostate cancer have rarely been examined, in part due to the smaller numbers of cases. Prevention trials provide limited data on the effect of PSA screening on the disease-specific mortality in men younger than 55 years. However, early-onset (age ≤55 years) prostate cancer recently has been determined to be a distinct clinicopathological phenotype, which is more aggressive and with a poorer prognosis32. Generally the prevalence of PSA testing is lower among younger compared to older men, while still fairly high in countries where testing is common. By 2001, 34% and 17% men aged 40–49 years reported ever use of a PSA test in the US18 and Canada33, respectively. In Australia, the largest increases in testing occurred in men younger than 55 years between 2001 and 2008, with an average prevalence of 12% for ages 45–5434. However, the increasing incidence rates in this young age group may not be fully explained by the intensive use of the PSA test. Contrary to the increasing incidence rates, self-reported use of PSA testing or DRE in the past two years among US men younger than 50 years dropped from 48% in 2002 to 42% in 200835. Among UK men ages 45–54 years, the prevalence of PSA use remained around 2% between 1999 and 200722, 23, while incidence rates increased rapidly. Future studies of early-onset tumors should focus on other risk factors, and PSA use in this young age group should be monitored.
Guidelines for prostate cancer early detection have evolved with accumulating evidence on screening efficacy for disease-specific mortality. There has been diversity in the recommendations put forth over time by different organizations within and across populations. In 1992, the American Urological Association (AUA) and the American Cancer Society (ACS)36 issued statements recommending yearly PSA testing and DRE for men starting at age 50 or at younger ages (≥40) for high-risk groups. The suggested age for an initial PSA test was lowered to 40 years by the AUA and European Association of Urology (EUA) in 2009 and 201037, 38. But later the AUA stated in their 2013 update that routine screening of men aged 40–54 years at average risk were not recommended39. Regarding the upper age limit, the ACS added restrictions to life expectancy of at least 10 years in 200240. The US Preventive Services Task Force (USPSTF) in 2008 recommended against screening for prostate cancer in men aged 75 years or older41, and it was echoed by the EAU in 2010. But later in 2013 the upper limit was further lowered to age 70 years by the AUA, and further PSA testing was not recommended for men with less than a 10- to 15-year life expectancy39. Yet in 2012, the USPSTF recommended against PSA-based screening for prostate cancer regardless of age42. In contrast to the progressively negative attitude toward PSA screening in the US and Europe, the Japanese Urological Association (JUA) recommended PSA screening for men aged≥ 50 years in 200843 and further lowered the initial age to 40 years for baseline PSA in 201044. Despite heterogeneous guidelines, shared decision between the patient and his physician based on balanced information of advantages and disadvantages of the screening tests has been widely accepted by urological societies worldwide37, 38, 43–45. Contemporary guidelines aim to limit the risk of overdiagnosis (i.e., cancers that would not be diagnosed in advance or otherwise have been detected without testing) and subsequent overtreatment, which may have a large impact on the quality of life of the patient46. To what extent guidelines influence screening behaviors of GPs, urologists, and the general population remains unclear. A recent study using US national hospital-based registry data reported incident prostate cancer uniformly decreased in risk strata, age and racial groups following the USPSTF 2012 guidelines47. However, discordances between guidelines and clinical practice have been revealed4, 24.
There are some limitations of this descriptive analysis. We may have obscured geographic variations within a country by using aggregated data to obtain more stable incidence rates. For example, rates were significantly higher in Austria, Tyrol during 1993–1999 compared with the other eight federal states that comprise Austria, primarily due to the mass prostate cancer prevention project48. In China, rates in Hong Kong historically have been higher than those in Shanghai, likely due to earlier adoption of western lifestyles or to differences in medical care practices. Secondly, the upper age-specific (ages 75+) rates may be slightly less comparable due to less granular age-adjustment and thus loss of precision for those ten countries lacking the full complement of data for the older 5-year age groups. Also, case ascertainment and population enumeration may be of lower quality among older age groups in certain populations and some researchers have restricted their analyses to those ages <75 years, but we included the entire age range given that prostate cancer is a disease of old men and that incidence rates declined at old ages in many populations in contrast to increasing rates as expected with improved data quality over time. Thirdly, joinpoint analysis is sensitive to parameter choice, and the statistical significance of an APC is subject to the number of data points included in a segment. Therefore, the pattern groupings of overall incidence rates may be subject to change if more data are added in the analysis. Lastly, although several prospective prostate cancer registries include detailed tumor characteristics49, no information on tumor stage was readily available in CI5plus, which may have helped to interpret our results. Furthermore, this analysis did not have data to present trends in overall and age-specific mortality rates of prostate cancer. In fact, decreasing overall prostate cancer mortality rates during the recent decade have been reported mainly for North America, Oceania, Western Europe and parts of Northern Europe where PSA testing was historically more intensively implemented, whereas rising prostate cancer mortality rates were evident primarily in Central and Eastern Europe, parts of Asia, and Africa50. The declining mortality rates may suggest that treatment and possibly earlier diagnosis have had an impact, whereas the rising rates may reflect increasing case diagnosis.
This analysis described the trends and patterns in overall and age-specific prostate cancer incidence rates in 43 populations. Five distinct patterns in the trends have been identified, some of which have been greatly influenced by the prevalence of PSA testing, the use of which has varied over time and across countries. Considerable geographic variation in prostate cancer incidence persists, and not all the patterns can be explained by screening. Determination of stage of disease at diagnosis and comparison of mortality patterns could help to understand the observed incidence rates. Future studies to elucidate the potential influence of environmental and host factors will need to take into account diagnostic and prognostic testing for prostate cancer. In particular, studies are needed to help understand the rising rates of early-onset prostate cancer globally.
Supplementary Material
Supplemental Table 1. Overall and age-specific annual prostate cancer cases and age-adjusted rates (world 1960 Segi population) per 100,000 man-years for 43 populations, 1978–2010.
Novelty and Impact.
We summarized prostate cancer incidence worldwide into five patterns of time trends. Notably, common peaks among men aged ≥75 years in most of the high-income populations may reflect declining PSA screening at older ages and diagnosis at younger ages. In contrast, rates for men aged 45–54 years did not clearly stabilize or decline in most populations, and PSA testing is not likely to fully explain the rapidly rising rates of early-onset prostate cancer.
Acknowledgments
Funding Information
This research was supported by the Intramural Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health
References
- 1.Ferlay JSI, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. GLOBOCAN 2012 v1.0, Cancer incidence and mortality worldwide: IARC CancerBase No. 11[Internet] Lyon, France: International Agency for Research on Cancer; 2013. [Google Scholar]
- 2.Etzioni R, Berry KM, Legler JM, Shaw P. Prostate-specific antigen testing in black and white men: An analysis of Medicare claims from 1991–1998. Urology. 2002;59:251–255. doi: 10.1016/s0090-4295(01)01516-3. [DOI] [PubMed] [Google Scholar]
- 3.Potosky AL, Kessier L, Gridley G, Brown CC, Horm JW. Rise in prostatic cancer incidence associated with increased rse of transurethral resection. Journal of the National Cancer Institute. 1990;82:1624–1628. doi: 10.1093/jnci/82.20.1624. [DOI] [PubMed] [Google Scholar]
- 4.Kvale R, Auvinen A, Adami HO, Klint A, Hernes E, Moller B, Pukkala E, Storm HH, Tryggvadottir L, Tretli S, Wahlqvist R, Weiderpass E, et al. Interpreting trends in prostate cancer incidence and mortality in the five Nordic countries. J Natl Cancer Inst. 2007;99:1881–1887. doi: 10.1093/jnci/djm249. [DOI] [PubMed] [Google Scholar]
- 5.Neppl-Huber C, Zappa M, Coebergh JW, Rapiti E, Rachtan J, Holleczek B, Rosso S, Aareleid T, Brenner H, Gondos A. Changes in incidence, survival and mortality of prostate cancer in Europe and the United States in the PSA era: additional diagnoses and avoided deaths. Ann Oncol. 2012;23:1325–1334. doi: 10.1093/annonc/mdr414. [DOI] [PubMed] [Google Scholar]
- 6.Ferlay JBF, Steliarova-Foucher E, Forman D. Cancer Incidence in Five Continents, CI5plus: IARC CancerBase No. 9 [Internet] Lyon, France: International Agency for Research on Cancer; 2014. [Google Scholar]
- 7.Engholm GFJ, Christensen N, Kejs AMT, Johannesen TB, Khan S, Milter MC, Ólafsdóttir E, Petersen T, Pukkala E, Stenz F, Storm HH. NORDCAN: Cancer Incidence, Mortality, Prevalence and Survival in the Nordic Countries, Version 7.0 (17.12.2014) Association of the Nordic Cancer Registries. Danish Cancer Society. Available from http://www.ancr.nu. [Google Scholar]
- 8.Bethesda, MD: National Cancer Institute; 2013. Apr, Surveillance Epidemiology and End Results (SEER) Program ( www.seer.cancer.gov). SEER*Stat Database: Incidence - SEER 9 Regs Research Data, Nov 2013 Sub (1973–2011)<Katrina/Rita Population Adjustment>- Linked To County Attributes - Total U.S., 1969–2012 Counties DCCPS, Surveillance Research Program, Surveillance Systems Branch. [Google Scholar]
- 9.Segi M. Cancer mortality for selected sites in 24 countries (1950–57) Sendai, Japan: Department of Public Health, Tohoku University of Medicine; 1960. [Google Scholar]
- 10.Devesa SS, Donaldson J, Fears T. Graphical Presentation of Trends in Rates. American Journal of Epidemiology. 1995;141:300–304. doi: 10.1093/aje/141.4.300. [DOI] [PubMed] [Google Scholar]
- 11.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 12.Bray F, Lortet-Tieulent J, Ferlay J, Forman D, Auvinen A. Prostate cancer incidence and mortality trends in 37 European countries: an overview. Eur J Cancer. 2010;46:3040–3052. doi: 10.1016/j.ejca.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 13.Legler JM, Feuer EJ, Potosky AL, Merrill RM, Kramer BS. The role of prostate-specific antigen (PSA) testing patterns in the recent prostate cancer incidence decline in the United States. Cancer Causes & Control. 1998;9:519–527. doi: 10.1023/a:1008805718310. [DOI] [PubMed] [Google Scholar]
- 14.Potosky AL, Miller BA, Albertsen PC, Kramer BS. The role of increasing detection of in the rising incidence of prostate-cancer. Journal of the American Medical Association. 1995;273:548–552. [PubMed] [Google Scholar]
- 15.Hankey BF, Feuer EJ, Clegg LX, Hayes RB, Legler JM, Prorok PC, Ries LA, Merrill RM, Kaplan RS. Cancer surveillance series: Interpreting trends in prostate cancer - Part I: Evidence of the effects of screening in recent prostate cancer incidence, mortality, and survival rates. Journal of the National Cancer Institute. 1999;91:1017–1024. doi: 10.1093/jnci/91.12.1017. [DOI] [PubMed] [Google Scholar]
- 16.Eckersberger E, Finkelstein J, Sadri H, Margreiter M, Taneja SS, Lepor H, Djavan B. Screening for Prostate Cancer: A Review of the ERSPC and PLCO Trials. Reviews in urology. 2009;11:127–133. [PMC free article] [PubMed] [Google Scholar]
- 17.Etzioni R, Gulati R, Falcon S, Penson DF. Impact of PSA screening on the incidence of advanced stage prostate cancer in the United States: A surveillance modeling approach. Medical Decision Making. 2008;28:323–331. doi: 10.1177/0272989X07312719. [DOI] [PubMed] [Google Scholar]
- 18.Sirovich BE, Schwartz LM, Woloshin S. Screening men for prostate and colorectal cancer in the United States - Does practice reflect the evidence? Jama-J Am Med Assoc. 2003;289:1414–1420. doi: 10.1001/jama.289.11.1414. [DOI] [PubMed] [Google Scholar]
- 19.Bartsch G, Horninger W, Klocker H, Reissigl A, Oberaigner W, Schonitzer D, Severi G, Robertson C, Boyle P. Prostate cancer mortality after introduction of prostate-specific antigen mass screening in the Federal State of Tyrol, Austria. Urology. 2001;58:417–424. doi: 10.1016/s0090-4295(01)01264-x. [DOI] [PubMed] [Google Scholar]
- 20.Smailyte G, Aleknaviciene B. Incidence of prostate cancer in Lithuania after introduction of the Early Prostate Cancer Detection Programme. Public Health. 2012;126:1075–1077. doi: 10.1016/j.puhe.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 21.Chamberlain J, Melia J, Moss S, Brown J. Report prepared for the Health Technology Assessment panel of the NHS Executive on the diagnosis, management, treatment and costs of prostate cancer in England and Wales. British journal of urology. 1997;79(Suppl 3):1–32. doi: 10.1111/j.1464-410x.1997.tb16914.x. [DOI] [PubMed] [Google Scholar]
- 22.Melia J, Moss S. Survey of the rate of PSA testing in general practice. Br J Cancer. 2001;85:656–657. doi: 10.1054/bjoc.2001.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams N, Hughes LJ, Turner EL, Donovan JL, Hamdy FC, Neal DE, Martin RM, Metcalfe C. Prostate-specific antigen testing rates remain low in UK general practice: a cross-sectional study in six English cities. BJU Int. 2011;108:1402–1408. doi: 10.1111/j.1464-410X.2011.10163.x. [DOI] [PubMed] [Google Scholar]
- 24.Hamoen EHJ, Reukers DFM, Numans ME, Barentsz JO, Witjes JA, Rovers MM. Discrepancies between guidelines and clinical practice regarding prostate-specific antigen testing. Family Practice. 2013;30:648–654. doi: 10.1093/fampra/cmt045. [DOI] [PubMed] [Google Scholar]
- 25.Parkin DM, Bray F, Ferlay J, Jemal A. Cancer in Africa 2012, Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2014;23:953–966. doi: 10.1158/1055-9965.EPI-14-0281. [DOI] [PubMed] [Google Scholar]
- 26.World Cancer Research Fund International. Continuous Update Project Report: Diet, Nutrition, Physical Activity, and Prostate Cancer. 2014 Available at: www.wcrf.org/sites/default/files/Prostate-Cancer-2014-Report.pdf. [Google Scholar]
- 27.Odedina FT, Akinremi TO, Chinegwundoh F, Roberts R, Yu D, Reams RR, Freedman ML, Rivers B, Green BL, Kumar N. Prostate cancer disparities in Black men of African descent: a comparative literature review of prostate cancer burden among Black men in the United States, Caribbean, United Kingdom, and West Africa. Infect Agent Cancer. 2009;10:1750–9378. doi: 10.1186/1750-9378-4-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cook LS, Goldoft M, Schwartz SM, Weiss NS. Incidence of adenocarcinoma of the prostate in Asian immigrants to the United States and their descendants. J Urol. 1999;161:152–155. [PubMed] [Google Scholar]
- 29.Albertsen PC, Hanley JA, Gleason DF, Barry MJ. Competing risk analysis of men aged 55 to 74 years at diagnosis managed conservatively for clinically localized prostate cancer. Jama. 1998;280:975–980. doi: 10.1001/jama.280.11.975. [DOI] [PubMed] [Google Scholar]
- 30.Fleming C, Wasson JH, Albertsen PC, Barry MJ, Wennberg JE. A decision analysis of alternative treatment strategies for clinically localized prostate cancer. Prostate Patient Outcomes Research Team. Jama. 1993;269:2650–2658. [PubMed] [Google Scholar]
- 31.Drazer MW, Huo D, Schonberg MA, Razmaria A, Eggener SE. Population-based patterns and predictors of prostate-specific antigen screening among older men in the United States. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:1736–1743. doi: 10.1200/JCO.2010.31.9004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salinas CA, Tsodikov A, Ishak-Howard M, Cooney KA. Prostate cancer in young men: an important clinical entity. Nature reviews Urology. 2014;11:317–323. doi: 10.1038/nrurol.2014.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beaulac JA, Fry RN, Onysko J. Lifetime and recent prostate specific antigen (PSA) screening of men for prostate cancer in Canada. Can J Public Health. 2006;97:171–176. doi: 10.1007/BF03405578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ranasinghe WKB, Kim SP, Lawrentschuk N, Sengupta S, Hounsome L, Barber J, Jones R, Davis P, Bolton D, Persad R. Population-based analysis of prostate-specific antigen (PSA) screening in younger men (<55 years) in Australia. BJU international. 2014;113:77–83. doi: 10.1111/bju.12354. [DOI] [PubMed] [Google Scholar]
- 35.Li J, German R, King J, Joseph D, Thompson T, Wu X-C, Ajani U, Tai E. Recent trends in prostate cancer testing and incidence among men under age of 50. Cancer Epidemiology. 2012;36:122–127. doi: 10.1016/j.canep.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 36.Mettlin C, Jones G, Averette H, Gusberg SB, Murphy GP. Defining and updating the american cancer society guidelines for the cancer-related checkup: Prostate and endometrial cancers. CA: a cancer journal for clinicians. 1993;43:42–46. doi: 10.3322/canjclin.43.1.42. [DOI] [PubMed] [Google Scholar]
- 37.Greene KL, Albertsen PC, Babaian RJ, Carter HB, Gann PH, Han M, Kuban DA, Sartor AO, Stanford JL, Zietman A, Carroll P. Prostate specific antigen best practice statement: 2009 update. J Urol. 2009;182:2232–2241. doi: 10.1016/j.juro.2009.07.093. [DOI] [PubMed] [Google Scholar]
- 38.Heidenreich A, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, Mottet N, Schmid HP, van der Kwast T, Wiegel T, Zattoni F. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol. 2011;59:61–71. doi: 10.1016/j.eururo.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 39.Carter HB, Albertsen PC, Barry MJ, Etzioni R, Freedland SJ, Greene KL, Holmberg L, Kantoff P, Konety BR, Murad MH, Penson DF, Zietman AL. Early detection of prostate cancer: AUA Guideline. J Urol. 2013;190:419–426. doi: 10.1016/j.juro.2013.04.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith RA, Cokkinides V, von Eschenbach AC, Levin B, Cohen C, Runowicz CD, Sener S, Saslow D, Eyre HJ. American Cancer Society Guidelines for the Early Detection of Cancer. CA: a cancer journal for clinicians. 2002;52:8–22. doi: 10.3322/canjclin.52.1.8. [DOI] [PubMed] [Google Scholar]
- 41.U.S. Preventive Services Task Force. Screening for prostate cancer: US preventive services task force recommendation statement. Ann Inter Med. 2008;149:185–191. doi: 10.7326/0003-4819-149-3-200808050-00008. [DOI] [PubMed] [Google Scholar]
- 42.Moyer VA. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Annals of internal medicine. 2012;157:120–134. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 43.Committee for Establishment of the Guidelines on Screening for Prostate C, Japanese Urological A. Updated Japanese Urological Association Guidelines on prostate-specific antigen-based screening for prostate cancer in 2010. International journal of urology : official journal of the Japanese Urological Association. 2010;17:830–838. doi: 10.1111/j.1442-2042.2010.02613.x. [DOI] [PubMed] [Google Scholar]
- 44.Ito K, Kakehi Y, Naito S, Okuyama A. Japanese Urological Association guidelines on prostate-specific antigen-based screening for prostate cancer and the ongoing cluster cohort study in Japan. International journal of urology : official journal of the Japanese Urological Association. 2008;15:763–768. doi: 10.1111/j.1442-2042.2008.02125.x. [DOI] [PubMed] [Google Scholar]
- 45.Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T, Zattoni F, Mottet N. EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol. 2014;65:124–137. doi: 10.1016/j.eururo.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 46.Sanda MG, Dunn RL, Michalski J, Sandler HM, Northouse L, Hembroff L, Lin XH, Greenfield TK, Litwin MS, Saigal CS, Mahadevan A, Klein E, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. New Engl J Med. 2008;358:1250–1261. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 47.Barocas DA, Mallin K, Graves AJ, Penson DF, Palis B, Winchester DP, Chang SS. The effect of the United States Preventive Services Task Force grade D recommendation against screening for prostate cancer on incident prostate cancer diagnoses in the US. J Urol. 2015 doi: 10.1016/j.juro.2015.06.075. [DOI] [PubMed] [Google Scholar]
- 48.Vutuc C, Schernhammer ES, Haidinger G, Waldhor T. Prostate cancer and prostate specific antigen (PSA) screening in Austria. Wiener Klinische Wochenschrift. 2005;117:457–461. doi: 10.1007/s00508-005-0395-y. [DOI] [PubMed] [Google Scholar]
- 49.Gandaglia G, Bray F, Cooperberg MR, et al. Prostate cancer registries: current status and future directions. Eur Urol. 2015 doi: 10.1016/j.eururo.2015.05.046. [DOI] [PubMed] [Google Scholar]
- 50.Center MM, Jemal A, Lortet-Tieulent J, Ward E, Ferlay J, Brawley O, Bray F. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012;61:1079–1092. doi: 10.1016/j.eururo.2012.02.054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Overall and age-specific annual prostate cancer cases and age-adjusted rates (world 1960 Segi population) per 100,000 man-years for 43 populations, 1978–2010.