Abstract
Objective
To understand how aminoglycosides such as gentamicin are used in a tertiary care setting. To familiarize otologists with the demographics and risk factors associated with gentamicin use at major medical centers to allow the possibility of early intervention.
Study Design
Retrospective review of existing clinical data.
Setting
University of Rochester Medical Center (URMC): including all associated hospitals (Strong Memorial Hospital, Highland Hospital, etc…)
Patients
All hospital inpatients who were prescribed intravenous gentamicin over a 4 year period starting in February 2011.
Interventions
None
Main Outcome Measures
Major patient populations receiving gentamicin and the associated diagnoses for which gentamicin was prescribed.
Results
A total of 5,257 patients were found to have received gentamicin. Three major populations of patients were found to have received gentamicin: 1) More than half the gentamicin exposures were children and 42% were under 2 years. 2) 18% of the exposures were young adults age 18–34 and in this population 88% were woman with most of these hospitalizations pregnancy related. 3) patients >55 were 19% of the exposures and most of these had serious infections. Disorders associated with patients receiving gentamicin included: Perinatal complications (1564); sepsis (1399); acute/chronic renal disease (1287); labor, delivery, or neonatal complications (1250); diabetes (949); and UTI/pyelonephritis (775).
Conclusions
Gentamicin is still widely used, and the neonatal population and young adult women are at especially high risk for gentamicin induced ototoxicity. Further data analysis should focus strategies to protect these populations by avoiding unnecessary exposures and by possible concurrent administration of protective medications such as metformin and aspirin.
Keywords: human, gentamicin, ototoxicity, aminoglycosides, hearing loss, vestibulopathy, dizziness
Introduction
Aminoglycoside antibiotics are one of the most commonly prescribed antibiotics worldwide because of their antimicrobial efficacy, widespread availability and low cost1. Aminoglycosides exert their bactericidal effects by binding to the bacteria’s 30S ribosomal subunit to block the initiation of protein synthesis, to cause the misreading of the mRNA, and to facilitate the premature termination of translation2. Aminoglycosides are poorly absorbed through the gastrointestinal tract and must be given parenterally or topically1. Aminoglycoside antibiotics were the first ototoxic agents known to cause irreversible inner ear toxicity and were added to the list of ototoxic drugs in the mid-1940s3,4. They are commonly combined in synergistic treatments with other antibiotics and used empirically to treat infections by Gram-negative rods (GNR)5. The reported incidence of significant ototoxicity in the literature varies widely and usually ranges from 2% to 25%1. Specifically, the incidence of cochleotoxicity has been reported to range from a few percent up to 33%, while balance can be effected in up to 15% of patients4,6–8.
Individual aminoglycosides differ in their ability to cause cochlear or vestibular toxicity. Together, gentamicin and streptomycin are the most common causes of bilateral vestibulopathy9,10. In fact, it is reported that there is no safe dose of systemically administered gentamicin. The onset of ototoxicity after parenteral use occurs unpredictably and should be considered uncontrollable11. Neither the serum peak and trough levels of gentamicin12 nor the number of doses11 can predict the development, severity, or outcome of ototoxicity. Although low or normal levels of gentamicin do not offer absolute protection to the inner ear, excessive levels are correlated with increased risk of ototoxicity1. Gentamicin is better known for its vestibular toxicity; however, there remains a large possibility of gentamicin induced hearing loss, especially with higher doses. A recent advancement made in aminoglycoside administration is once-daily dosing that involves the administration of a large dose, balanced by a larger interval between doses to achieve a higher peak and a lower trough1,13. However, the change from multiple daily dosing to once-daily dosing has not shown a significant improvement in rates of ototoxicity14.
Much research has been done to understand the mechanism of aminoglycoside ototoxicity: it is thought that aminoglycosides can disrupt mitochondrial protein synthesis, cause the overreaction of glutamatergic receptors, and cause the formation of free radical species that can lead to apoptotic cell death3. Specifically, aminoglycosides may induce the cell’s intrinsic apoptotic pathway causing condensation of nuclei in hair cells, create reactive oxygen species (ROS) that liberate cytochrome C from the mitochondria to activate apoptosis, or interact with transition metals, such as copper and iron to promote free radical formation3. Research into the mechanism of aminoglycoside-induced hearing and vestibular dysfunction has offered opportunities for medical intervention. For example, some of the oto-protective agents investigated include the anti-diabetic drug metformin, iron chelators, aspirin, and the antioxidant α-lipoic acid, each of which provide varying degrees of protection to the inner ear3,4,15,16.
Although the ototoxic and vestibulotoxic effects of gentamicin are well established, little research has been done to study their use in a hospital setting. Aminoglycoside-induced hearing loss and vestibular dysfunction are recognized as a problem in developing countries where the drugs are used frequently for their clinical effectiveness and affordability3. Although, many clinicians may believe that gentamicin is no longer used regularly as a first-line antibiotic in industrialized countries, ototoxicity is still a major concern. Although vestibulotoxicity is known to occur after gentamicin exposure, identifying which patients received gentamicin can be difficult. If neurotologists can be familiar with the demographics and risk factors associated with gentamicin use at major medical centers, clinicians could identify early on those at risk for bilateral vestibular loss and could initiate early intervention with a rescue medication. The authors are not aware of any studies that document how frequently it is used at a major medical center. The goal of our research is to retrospectively study the use of gentamicin in a hospital setting and to determine which patients are at high risk for exposure. Identifying such patients may help limit unnecessary exposures and identify populations for future studies looking at the potential oto-protective effects of metformin or aspirin.
Materials and Methods
This is a retrospective chart review study. The data for this study was obtained using i2b2, an open source informatics framework developed by the NIH-funded i2b2 Center at Harvard University to enable researchers to query clinical data from multiple clinical systems for research purposes and deployed at the University of Rochester. Using i2b2 and partnering with URMC’s Clinical and Translational Science Institute (CTSI), we constructed a database of all hospital in-patients who have been prescribed intravenous (IV) gentamicin at the University of Rochester Medical Center (URMC) including Strong Memorial Hospital, Highland Hospital and all affiliated hospitals in the URMC system, from February 2011 to February 2015. We excluded all patients who received non-systemic administrations of gentamicin (e.g. gentamicin sulfate ophthalmic solutions and gentamicin transtympanic injections) from our database query. The research protocol was reviewed and approved by the University of Rochester Research Subject Review Board and conducted according to the principles expressed in the Declaration of Helsinki.
The i2b2 database allowed for calculations of demographic data according to patient’s age and sex, and also allowed us to access their reported race, the length of their hospital stay, and their vital status (living or deceased). Using this tool, we could identify the number of male and females in each of our designated age groups (Fig. 1). Additionally, the i2b2 platform allowed us to identify patient sets using the International Classification of Diseases, Version 9 (ICD-9) diagnosis codes. We exported a de-identified file of the most frequent ICD-9 diagnosis codes for our entire patient set. Subsequently, using our clinical judgment for possible gentamicin indications and the already established diagnostic groupings of the ICD-9 codes, we assembled individual ICD-9 codes into more generalizable groups that represented similar pathological processes. For example, all of the ICD-9 codes that pertained to a diagnosis of sepsis were grouped under this indication (including, but not limited to, “unspecified septicemia,” “septicemia of the newborn,” and “sepsis.”) Only the most frequent and most relevant diagnoses to possible gentamicin prescription were included for the aggregated totals. Diagnosis frequency was also assessed for three particular age groups: less than 1 year olds, 18–44 year olds, and greater than 55 year olds. The frequency of the most popular diagnoses was extracted using the i2b2 database. A similar approach was applied when grouping the most common medications used with gentamicin. We placed a special emphasis towards studying other antibiotics prescribed concurrently with gentamicin and included all of the most frequently dosed antibiotics. All formulations of a particular drug, including various dosages and pharmacological manufacturing, were grouped according to their generic pharmacological class. We also included aspirin and metformin to our medication data, as their protective effects against aminoglycoside induced hearing loss have been implicated in previous studies.
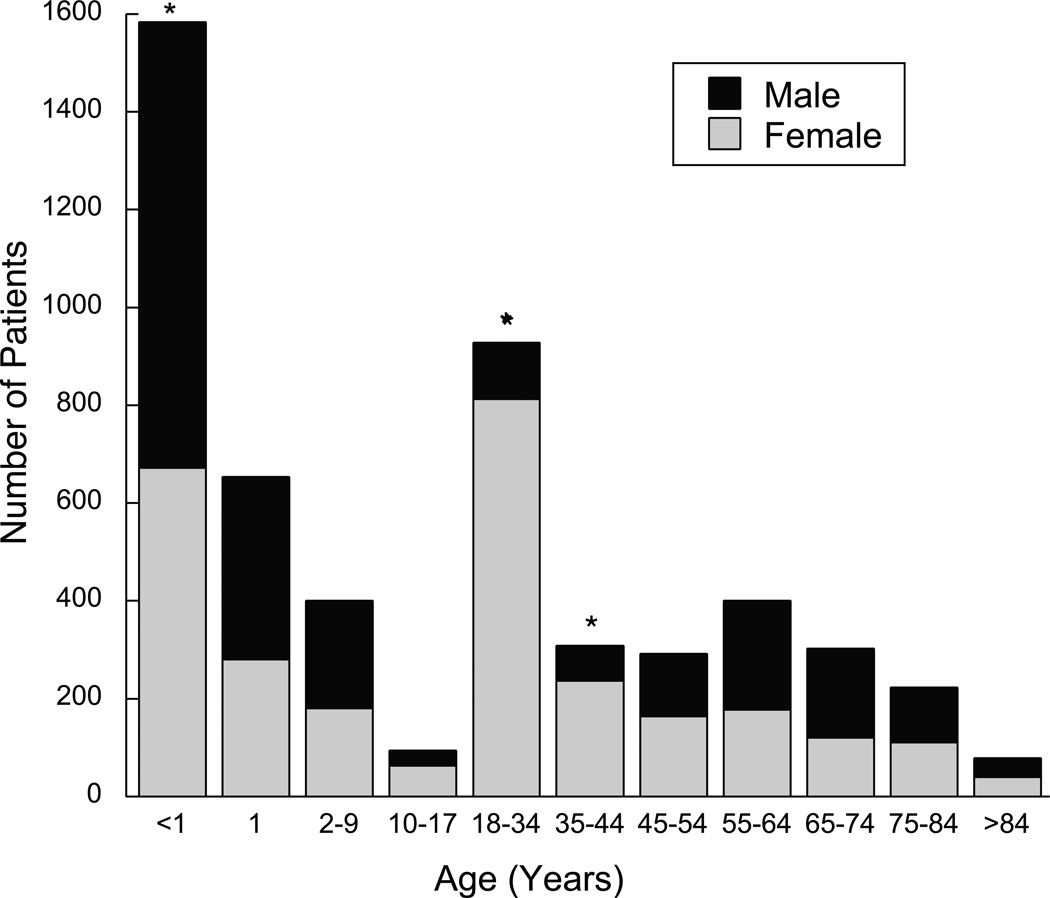
Figure 1.
Gentamicin use by age and gender. Each separate bar represents a single age group. Each bar is broken into the number of male and female patients that represent each age group. Three groups, <1, 18–34 years, and the 35–44 year old age group had a significantly different male vs. female proportion. Statistical significance p < 0.001 is marked with an asterisk (*).
Through random selection of electronic chart review of 60 patients prescribed gentamicin identified via the i2b2 system, we accessed the patient’s diagnostic history in order to assess the reasons for gentamicin prescription and to assess the physician’s thought process in prescribing gentamicin. Once a patient was randomly selected from our group of 5,267 patients, the encounter note in which gentamicin was prescribed was reviewed, including any labs, imaging, and consultations that were ordered during that visit. These data was not analyzed statistically, but allowed the authors to assess whether the indications for gentamicin use obtained through the i2b2 system correlated with the actual indications for gentamicin prescription and to ascertain common clinical reasons associated with gentamicin use.
A chi-squared test assessed the statistical significance of the differences of the number of male versus female patients in each patient age group (Fig. 1). P- values less than 0.001 were considered to be statistically significant. The researchers chose to represent significant data with a small p-value to decrease the likelihood of a type I error given the large number of parameters in our study. As a chart review study, it was imperative for us to show that the sex of patients in certain age groups, namely the 18–44 year olds, were truly different and not simply significant due to sampling error.
Results
There were a total of 5,267 patients prescribed at least one dose of (IV) gentamicin from February 2011 to February 2015. Of these patients, 2,409 (45.7%) were male and 2,858 (54.3%) were female, a statistically significant difference (p<0.001). The age group that comprised the largest fraction of patients was young children, 30% were less than 1-year and 42% under 2 years. Young adults (18–34 year olds) represented 18% of the exposed population. Patients >55 years old comprised 19.1% of this population. The ages with statistically different male vs. female populations included the <1 year olds, the 18–34 age group, and the 35–44 age group (P<0.001) (fig. 1). Of the 928 18–34 year olds, 115 (12.3%) were male and 813 were female (87.6%). Additionally, of the 309, 35–44 year olds, 72 (23.3%) were male and 237 (76.7%) were female. When combined, ages 18–44 comprised 24% of our patient population (1237); 85.0% were female.
The most common disorders associated with patients receiving gentamicin included: Perinatal complications (1564); sepsis (1399); acute/chronic renal disease (1287); labor, delivery, or neonatal complications (1250); diabetes (949); and UTI/pyelonephritis (775). The most common diagnoses by age are presented in figures 2–4. We found that 72% of patients less than 1 year old had a diagnosis of “observation for suspected infectious condition.” Of the patients age 18–44, 9 of the top 20 most frequent diagnoses were pregnancy related. Additionally, patients receiving gentamicin had a long hospital course: 45% of patients had a stay longer than 10 days and 38% had a stay between 5–10 days. Only 17% of patients had a hospital stay less than 5 days long. The medications most frequently administered concurrently with gentamicin are illustrated in figure 5. The two largest racial groups of our gentamicin patient population (70% white and 19% Black) are similar to the reported 2013 racial demographics of Monroe County, NY (72% white and 16% black). It should be noted that 8% of our patient population’s race was listed as “refused, other, or unknown” and not included in analysis.
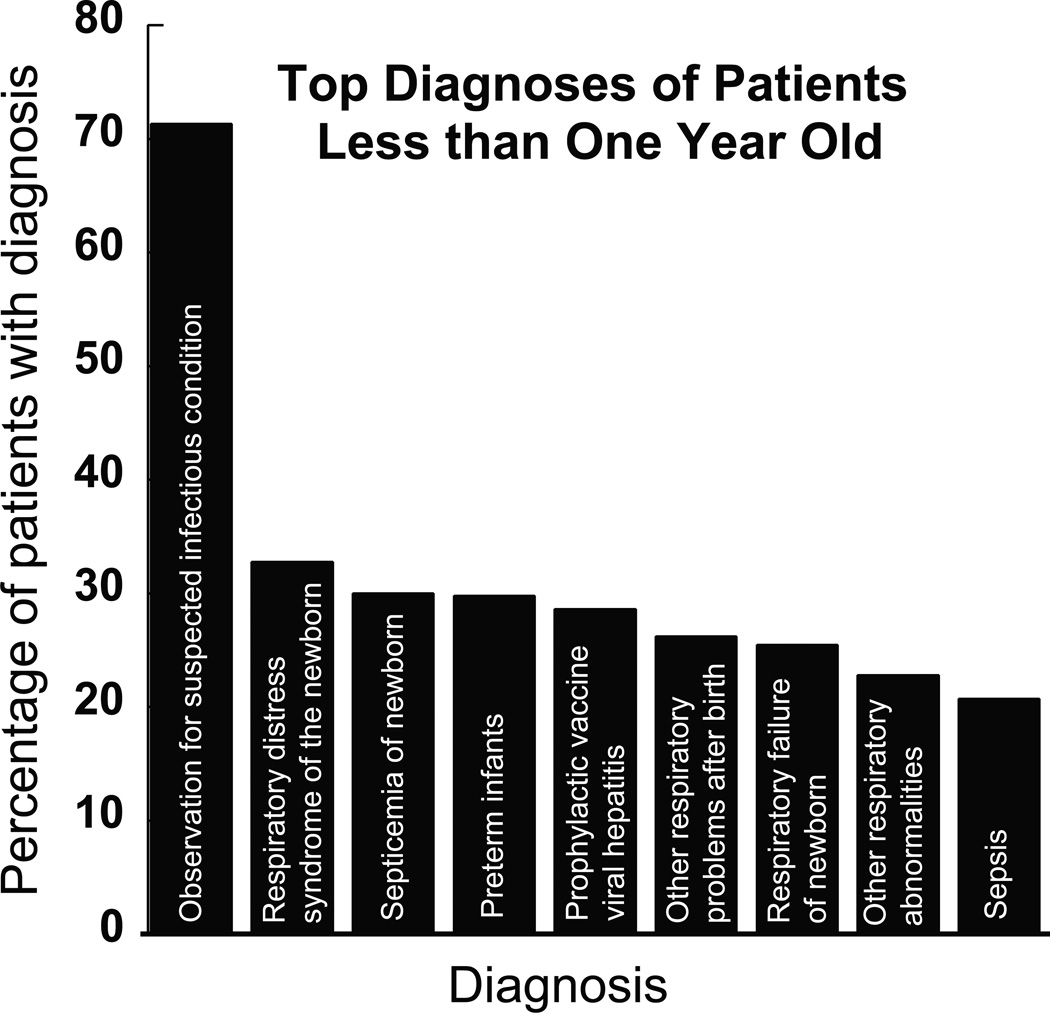
Figure 2.
The top 10 most frequent diagnoses of patients less than 1 year old who received gentamicin. The most common associated diagnosis was “observation for suspected infectious condition” (72%). Other common diagnoses in this age groups included respiratory distress syndromes and sepsis related conditions.
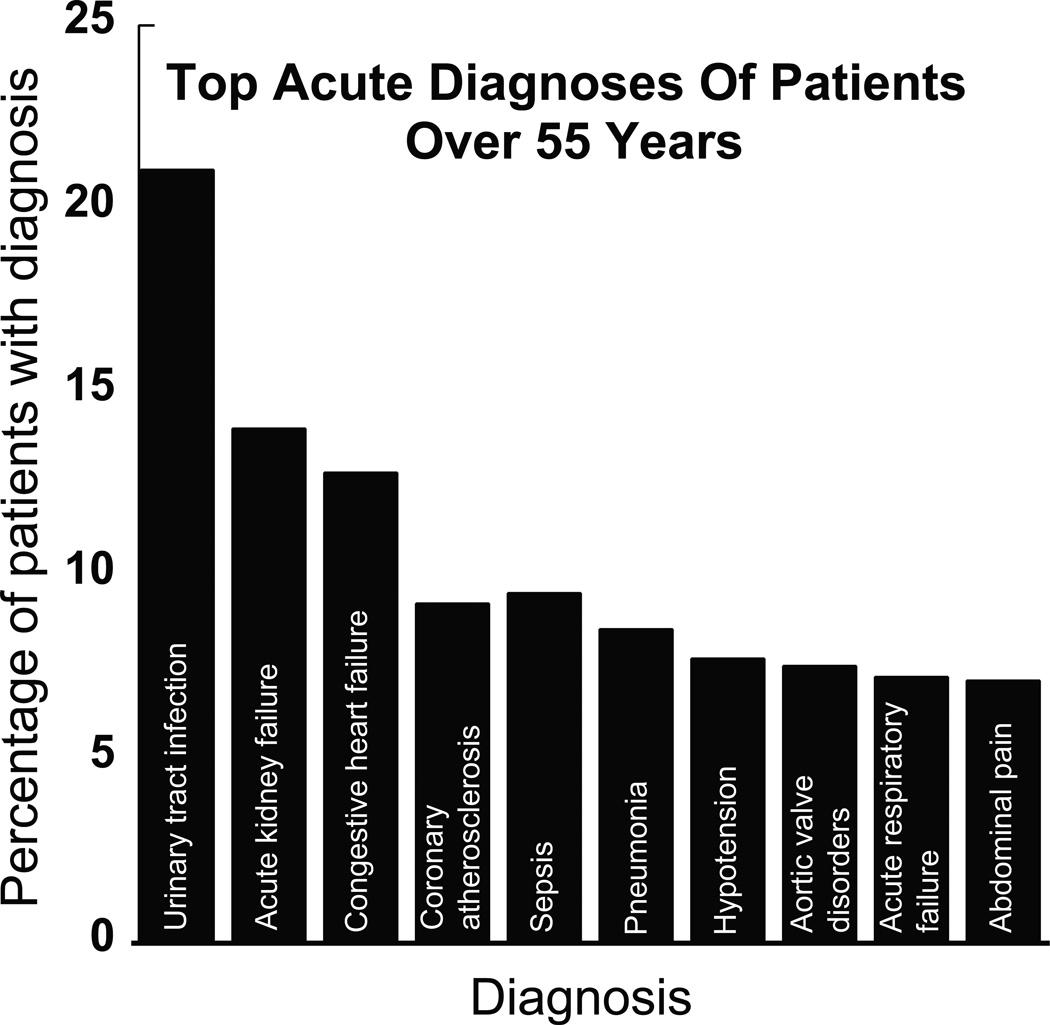
Figure 4.
The ten (10) most frequent acute diagnoses associated with patients 55 years or older that received gentamicin. The most frequent acute diagnosis was a urinary tract infection (21%). The most common diagnoses overall were hypertension and hyperlipidemia, 38% and 26% respectively (not shown). Of note, 11% of this patient population had a diagnosis of chronic renal failure (not shown).
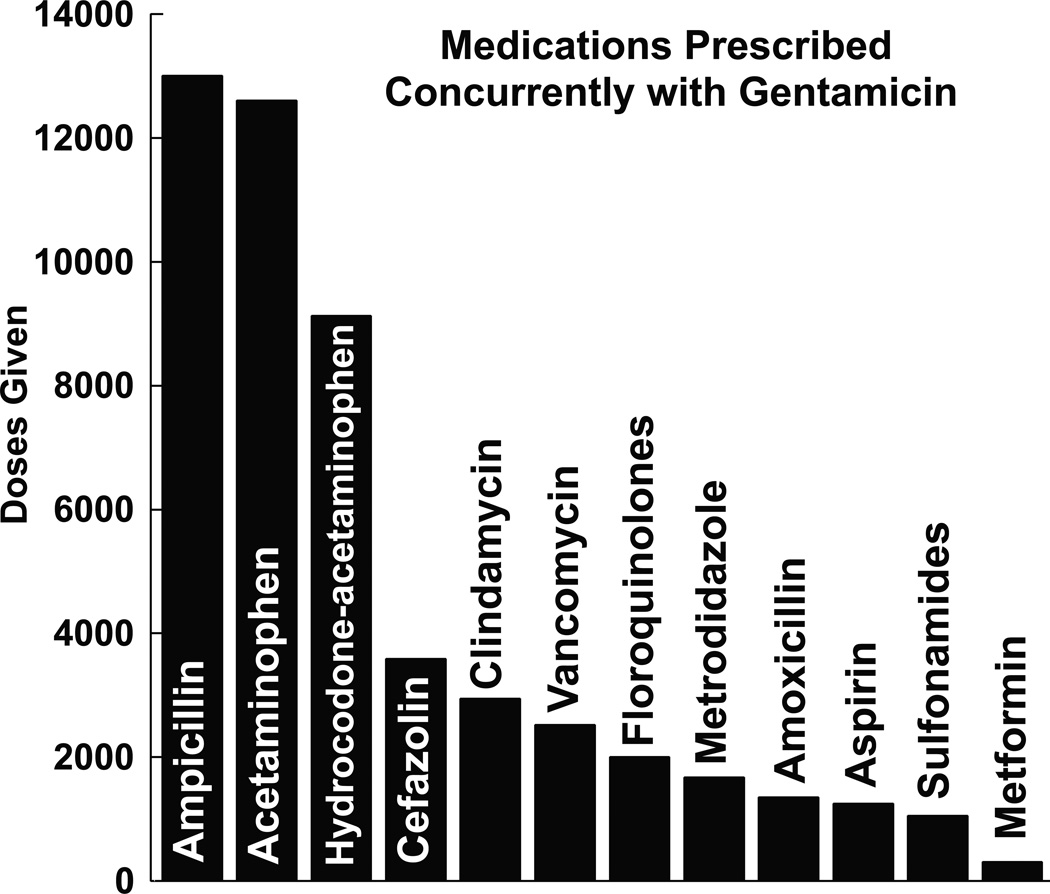
Figure 5.
Medications prescribed most frequently with gentamicin. Ampicillin, acetaminophen, and hydrocodone-acetaminophen were the three most used medications in our patient population. For reference, gentamicin was administered 10,872 times (not shown). This analysis does not consider the frequency at which these medications are dosed (i.e. q4h, qd or PRN) and only represents the total number of doses a medication was administered from February 2011 to February 2015 in our patient population. The number of patients (5,267) exposed to gentamicin was less than the total number gentamicin doses (10,827) because multiple doses were often given to a single patient.
Discussion
Our results demonstrated that gentamicin still has a major role in the modern day arsenal of antimicrobials. Patients exposed to gentamicin with highest frequency can be divided into three major populations: 1) neonates 2) obstetrical women 3) patients >55.
Analysis of the <1 year old age group also revealed a relatively homogenous patient population. In review of a representative sample of this population’s charts, we found that many young infants received gentamicin concurrently with presumed sepsis and perinatal complications. Gentamicin was frequently ordered in the midst of obtaining blood draws for bacterial culture; If cultures came back negative, i.e. sepsis ruled out, gentamicin dosing was discontinued. In cases with positive cultures, antibiotics were often changed to reflect specific pathogens. This finding is corroborated by our data looking at the most frequent diagnoses in this age group: 72% of patients less than year old had an associated diagnoses of “observation for suspected infectious condition.” For most patient charts that were reviewed by the authors, blood cultures ultimately came back negative and prophylactic gentamicin dosing was subsequently stopped. This data suggests that infants are exposed to gentamicin without a confirmed bacterial infection. It is not surprising that gentamicin was often chosen for initial prophylactic use given that it has good bactericidal gram-negative coverage, it is inexpensive, and can be used effectively in combination with other beta-lactam antibiotics (such as ampicillin).
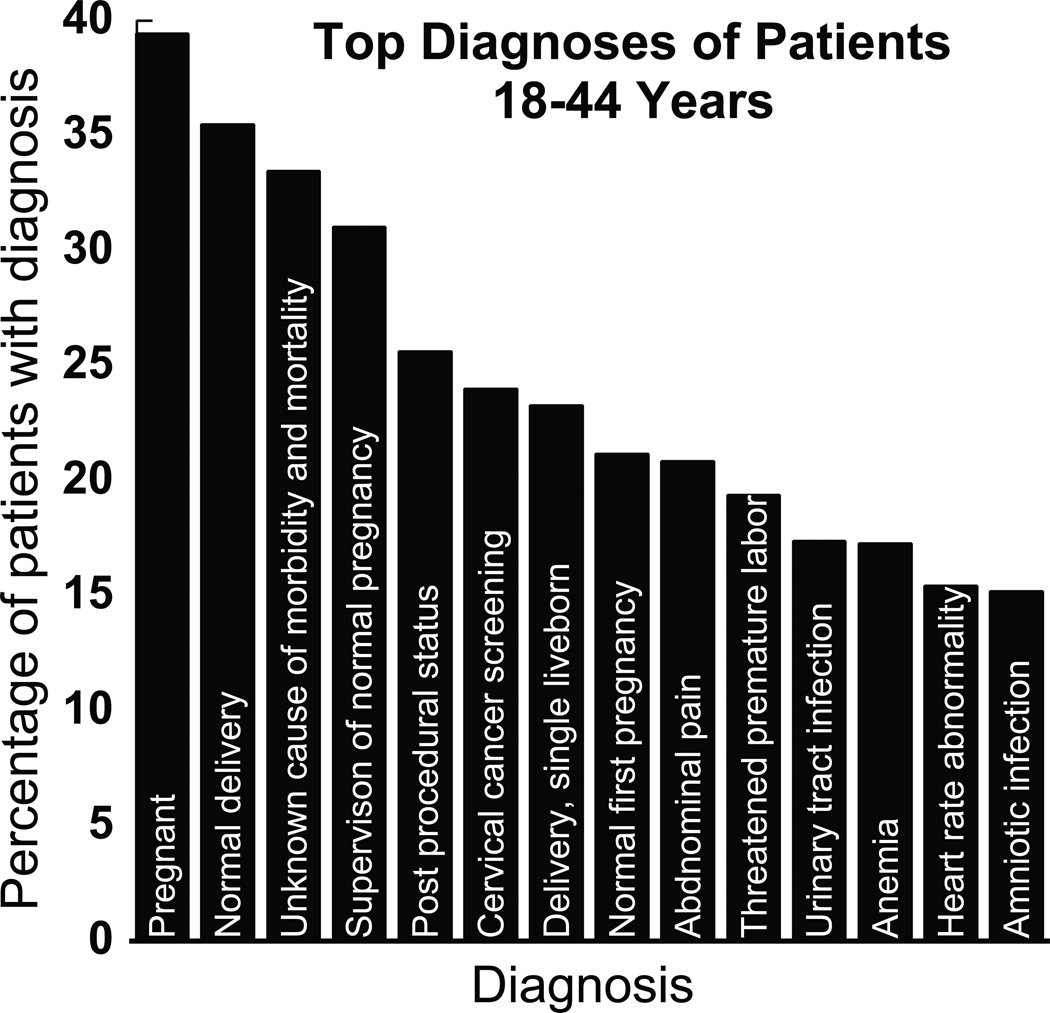
When analyzing the 18–44 year old population, we found that 85% of these patients were female. Analysis of a representative amount of female patient’s medical records suggests that these patients received gentamicin around the time of delivery. The association of gentamicin use and labor complications gathered from direct viewing of patient’s charts is consistent with our data received from the i2b2 database: high prevalence of pregnancy related diagnoses amongst the patients in this age group (Fig. 3). Interestingly, the top 5 most common pregnancy related diagnoses all involved normal pregnancies or births. This data illustrates that the majority of this patient population was pregnant while receiving gentamicin and suggests that pregnant women and their fetuses are at increased risk of gentamicin ototoxicity. The most frequent non-obstetrical infectious diagnosis in this age group was a urinary tract infection (17%). When looking at our data in aggregate, they show that 17% of our total patient population was given a diagnosis that falls under the umbrella of labor and delivery complications. This percentage is similar to the total percentage of women ages 18–44 that were given gentamicin (19.9%), suggesting that almost all of the women treated in this age group were treated for pregnancy related disorders. Pregnant women are being placed at high exposure risks for gentamicin-induced toxicity.
Figure 3.
Frequency of pregnancy related disorders in the 18–44 year old age group. Nine (9) of the top 20 most frequent diagnoses associated with patients in this age group were associated with pregnancy. The five (5) most frequent pregnancy related diagnoses involved a normal pregnant or normal delivery status. The most frequent non-pregnancy infectious related diagnosis in this group was a urinary tract infection.
Our third patient population, >55 years old, reveals a more heterogeneous population: The indications for gentamicin use are more varied compared with the first two patient populations identified. The medical records of this patient population reveal many diverse indications for gentamicin use and may pose as the most challenging patient population for pharmacologic intervention given the diversity of dosing indications. Study of the I2b2 data reveals that sepsis, UTI/pyelonephritis, and pneumonia represent at least 40% of gentamicin indications in this patient population. These patients’ disease course was often complicated by concurrent diagnosis of acute/chronic renal disease and diabetes. It is known that patients with diabetes are immunocompromised and are at a greater risk of UTI/pyelonephritis; additionally sepsis is often complicated by acute renal injury due to hypoperfusion of the kidney. 18% of this population had a formal diagnosis of diabetes. Interestingly, 11% of this patient population had a diagnosis of chronic kidney disease, despite the known nephrotoxicity of gentamicin. These patients are at an even higher risk for gentamicin-induced ototoxicity1.
Our demonstration that gentamicin is still widely used in a major medical health center suggests that these three patients populations, especially the neonatal population and young adult women, are at especially high risk for toxic exposure. Efforts should be aimed at targeting these most vulnerable patient populations. The infant population and the obstetrical patients are easily identifiable given the relative homogenous indications for gentamicin use in these populations. However, these patient populations are also the most difficult to study given the legal and ethical considerations surrounding these patients. Although gentamicin, and other aminoglycosides, has historically been the first line treatment for serious gram-negative infections, recent data has suggested that other anti-microbial agents would be equally effective. In a recent study by English and Williams, bacterial isolates of patients treated for GNR infections with aminoglycosides were sensitive to other antibiotics, showing that other medications could have been used5. The same study suggests that other antibiotics have similar efficacy to aminoglycosides: English and Williams found increased GNR sensitivities to ceftazidine and piperacillin and equivalent sensitivity to cefuroxime, ceftriaxone, and ciprofloxacin. These medications have safer toxicity profiles and do not carry the ototoxic and nephrotoxic effects of gentamicin5. These data suggest a questionable role of gentamicin in the modern antibiotic era; should these susceptible patient populations be exposed to gentamicin prophylactically before cultures return or are providers exposing these populations to potentially toxic drugs?
The paucity of good clinical evidence about the use of other possible anti-microbial agents clouds the risk/benefit ratio of using these non-aminoglycoside drugs in every day practice, especially in the most vulnerable of patient populations. While conducting randomized control trials of antibiotic efficacy in these patient populations is fraught with ethical and legal limitations, further studies should focus strategies to protect these populations by either avoiding unnecessary exposures and/or by concurrent administration of anti-oxidant protective medications. An easy and cost efficient way to protect patients from potential toxicities would be the addition of a “rescue” medication administered with each dosing of gentamicin. Metformin would one such option; It has a safe toxicity profile, it is inexpensive, and has been shown to increase hair cell viability following gentamicin dosing3. To this date, no clinical studies have been performed to verify the efficacy of such a strategy in humans. Before metformin can successfully be utilized as a rescue medication, it should be established that metformin does not interfere with the antimicrobial efficiency of gentamicin. Additionally, aspirin has long been known to curb the ototoxic effects of gentamicin: In 2006, it was shown that 1gm of aspirin could significantly reduce the number of patients who met criteria for hearing loss following gentamicin exposure without altering gentamicin serum levels4. Future studies should test the efficiency of aspirin with currently cardiovascular prophylactic dosing (81mg–325mg) to decrease the bleeding side effects witnessed in the study4. However, this controlled trial was instrumental in showing the possibility of prophylactic protection from gentamicin side effects.
A limitation of the data is the inability to define the indications for which each gentamicin dose was administered. While physicians must offer a diagnosis when prescribing a medication through electronic medical records, this information was not readily accessible using the current I2b2 program at URMC. In addition, patients commonly have multiple diagnoses that would make it hard to link gentamicin use with a specific indication. This information would have been vital to identify the exact ICD codes for which gentamicin had been administered in each encounter. Alternatively, we could only speculate by gathering the most populous diagnoses given to the pool of gentamicin patients during their hospital stay. We used our clinical judgment to infer the indications for gentamicin dosing and analyzed several patient charts to augment this clinical judgment. There are inherent judgment errors associated with this retrospective analysis but we feel that we could capture the global picture associated with gentamicin use, especially when cross-referencing these data to the demographics of the patients observed. Another limitation of this study is the inability to pinpoint the potential hearing and vestibular sequelae associated with gentamicin use. We did not have access to all of the newborn screening tests and any vestibular or hearing consultations that could have taken place outside of the URMC hospital systems in subsequent physician visits following gentamicin administration. Additionally, given the short time interval of this study, it is conceivable that many of these patients will have hearing and vestibular sequelae in the future that may yet be clinically relevant.
Although the ototoxic and vestibulotoxic effects of gentamicin are well established, little research has been done to study their use in a hospital setting. Our research has shown that gentamicin is still widely used at a major medical center and that three patient populations (neonates, women of reproductive age, and older adults) are at the greatest risk for developing gentamicin induced oto-toxicity. Future studies should be aimed at translating the previously identified in vitro oto-protective medications, such as metformin and aspirin, into patient care to reduce exposure risks. Other groups have taken a novel approach to aminoglycoside ototoxicity. Using apramycin, an aminoglycoside approved for use in veterinary medicine, Matt et al. has shown that researchers can separate an aminoglycoside’s antibacterial toxicity from its anti-mitochondrial effect (thought to be responsible for vestibular dysfunction).17 Their research provides a basis to investigate chemical modification of aminoglycosides that can maintain the strong antibacterial effects of these drugs while reducing their toxic side effects.17 Additionally, clinicians should continue to explore other options, such as vancomycin and cephalosporins, for prophylactically treating sepsis, especially in neonates, and limit gentamicin use to only microbes resistant to all other medications.
Acknowledgements
This study was supported by NIDCD K23 DC011298, R01 DC013580, and a Triological Society Career Scientist Award. Informatics assistance with i2b2 was obtained from the University of Rochester’s Clinical and Translational Sciences Institute, supported by Award Number Grant UL1 TR000042 from the National Center For Advancing Translational Sciences, National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Advancing Translational Sciences or the National Institutes of Health.
Footnotes
The Authors declare that they have no conflict of interest.
References
- 1.Rizzi MD, Hirose K. Aminoglycoside ototoxicity. Curr Opin Otolaryngol Head Neck Surg. 2007;15:352–357. doi: 10.1097/MOO.0b013e3282ef772d. [DOI] [PubMed] [Google Scholar]
- 2.Rybak LP, Ramkumar V. Ototoxicity. Kidney Int. 2007;72:931–935. doi: 10.1038/sj.ki.5002434. [DOI] [PubMed] [Google Scholar]
- 3.Chang J, Jung HH, Yang JY, Choi J, Im GJ, Chae SW. Protective role of antidiabetic drug metformin against gentamicin induced apoptosis in auditory cell line. Hear Res. 2011;282:92–96. doi: 10.1016/j.heares.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Huang WG, Zha DJ, et al. Aspirin attenuates gentamicin ototoxicity: from the laboratory to the clinic. Hear Res. 2007;226:178–182. doi: 10.1016/j.heares.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 5.English WP, Williams MD. Should aminoglycoside antibiotics be abandoned? Am J Surg. 2000;180:512–515. doi: 10.1016/s0002-9610(00)00539-0. discussion 515–516. [DOI] [PubMed] [Google Scholar]
- 6.Fausti SA, Henry JA, Helt WJ, et al. An individualized, sensitive frequency range for early detection of ototoxicity. Ear Hear. 1999;20:497–505. doi: 10.1097/00003446-199912000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Fee WE., Jr Aminoglycoside ototoxicity in the human. Laryngoscope. 1980;90:1–19. doi: 10.1288/00005537-198010001-00001. [DOI] [PubMed] [Google Scholar]
- 8.Lerner SA, Schmitt BA, Seligsohn R, Matz GJ. Comparative study of ototoxicity and nephrotoxicity in patients randomly assigned to treatment with amikacin or gentamicin. Am J Med. 1986;80:98–104. doi: 10.1016/0002-9343(86)90486-9. [DOI] [PubMed] [Google Scholar]
- 9.Selimoglu E. Aminoglycoside-induced ototoxicity. Curr Pharm Des. 2007;13:119–126. doi: 10.2174/138161207779313731. [DOI] [PubMed] [Google Scholar]
- 10.Selimoglu E, Kalkandelen S, Erdogan F. Comparative vestibulotoxicity of different aminoglycosides in the Guinea pigs. Yonsei Med J. 2003;44:517–522. doi: 10.3349/ymj.2003.44.3.517. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed RM, Hannigan IP, MacDougall HG, Chan RC, Halmagyi GM. Gentamicin ototoxicity: a 23-year selected case series of 103 patients. Med J Aust. 2012;196:701–704. doi: 10.5694/mja11.10850. [DOI] [PubMed] [Google Scholar]
- 12.Black FO, Pesznecker S, Stallings V. Permanent gentamicin vestibulotoxicity. Otol Neurotol. 2004;25:559–569. doi: 10.1097/00129492-200407000-00025. [DOI] [PubMed] [Google Scholar]
- 13.Al-Hamad A. Improving gentamicin dosing: a suggested approach to a simplified once-daily dosing schedule. J Infect Public Health. 2014;7:247–248. doi: 10.1016/j.jiph.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Contopoulos-Ioannidis DG, Giotis ND, Baliatsa DV, Ioannidis JP. Extended-interval aminoglycoside administration for children: a meta-analysis. Pediatrics. 2004;114:e111–e118. doi: 10.1542/peds.114.1.e111. [DOI] [PubMed] [Google Scholar]
- 15.Conlon BJ, Aran JM, Erre JP, Smith DW. Attenuation of aminoglycoside-induced cochlear damage with the metabolic antioxidant alpha-lipoic acid. Hear Res. 1999;128:40–44. doi: 10.1016/s0378-5955(98)00195-6. [DOI] [PubMed] [Google Scholar]
- 16.Song BB, Anderson DJ, Schacht J. Protection from gentamicin ototoxicity by iron chelators in guinea pig in vivo. J Pharmacol Exp Ther. 1997;282:369–377. [PubMed] [Google Scholar]
- 17.Matt T, Ng CL, Lang K, et al. Dissociation of antibacterial activity and aminoglycoside ototoxicity in the 4 monosustituted 2-deoxystreptamine apramycin. PNAS. 2012;109:10984–10989. doi: 10.1073/pnas.1204073109. [DOI] [PMC free article] [PubMed] [Google Scholar]