Abstract
Background
Tuberculosis (TB) during pregnancy in HIV-infected women is associated with poor maternal and infant outcomes. There are limited data on TB prevalence, optimal TB screening, and performance of rapid diagnostics in pregnant HIV-infected women.
Methods
We conducted a cross-sectional study among HIV-infected pregnant women seeking antenatal care in western Kenya. Following a standardized questionnaire, sputum smear microscopy for acid-fast bacilli (AFB), mycobacterial liquid culture, GeneXpert MTB/RIF (Xpert), urine lipoarabinomannan (LAM), and tuberculin skin testing (TST) were performed. We determined prevalence and correlates of culture-confirmed pulmonary TB, and compared diagnostic performance of World Health Organization (WHO) symptom screening and rapid diagnostic tests to sputum culture.
Results
Between July 2013 and July 2014, we enrolled 306 women. Among 288 women with a valid sputum culture result, 54% were on antiretroviral treatment, median CD4 cell count was 437 cell/mm3 (IQR 342–565), and prevalence of culture-confirmed pulmonary TB was 2.4% (CI 1.0–4.9%). Cough >2 weeks (p=0.04) and positive TST (≥5mm, p=0.03) were associated with pulmonary TB. Women with TB were 23-fold (95% CI 4.4–116.6) more likely to report a household member with TB symptoms (p=0.002). WHO symptom screen (43%), AFB smear (0%), Xpert (43%) and LAM (0%) had low sensitivity but high specificity (81%, 99%, 99% and 95%, respectively) for pulmonary TB.
Conclusion
HIV-infected pregnant women had appreciable prevalence of pulmonary TB despite modest immunosuppression. Current TB screening and diagnostic tools perform poorly in pregnant HIV-infected women. Adapted TB screening tools that include household member TB symptoms may be useful in this population.
Keywords: tuberculosis, HIV, pregnancy, Xpert, urine TB-LAM, screening
INTRODUCTION
Tuberculosis (TB) is a leading cause of morbidity and mortality among women of childbearing age, particularly in areas of high HIV prevalence.1,2 Late or missed diagnosis of TB among pregnant HIV-infected women is associated with poor maternal and infant outcomes.3,4 Identification and treatment of TB during antenatal care is an opportunity to link pregnant mothers to TB/HIV treatment and prevent morbidity and mortality in both mother and infant.5–8
The World Health Organization (WHO) recommends TB screening of HIV-infected individuals using a 4-part symptom screen including cough, fever, weight loss, and night sweats.9 However, the WHO TB symptom screen has performed poorly among HIV-infected pregnant women, perhaps because pregnancy may mask TB symptoms.10,11 The most commonly used diagnostic tests (acid-fast bacillus (AFB) smear microscopy and chest radiographs), perform poorly in the setting of HIV-infection,12 and clinicians may be reluctant to order radiographs in pregnancy.13 Newer rapid tests, including GeneXpert MTB/RIF® (Xpert, Cepheid, Sunnyvale, CA, USA) a DNA PCR-based test, and urine lipoarabinomannan (LAM) (Determine™ TB LAM; Alere, Waltham, MA, USA), an inexpensive lateral flow urine dipstick assay, may improve TB detection among HIV-infected pregnant women; however, performance characteristics in this population are undefined.
We aimed to determine the prevalence of culture-confirmed pulmonary TB, identify cofactors associated with TB, and assess the performance of the WHO TB symptom screen, Xpert, and LAM among HIV-infected pregnant women in western Kenya.
METHODS
Study Setting
We performed a cross-sectional study among HIV-infected pregnant women at two antenatal care clinics in the Nyanza region of western Kenya.
Participants
HIV-infected women 16 years or older accessing prevention of mother-to-child transmission (PMTCT) services as part of antenatal care were eligible for study enrollment. All participants were aware of their HIV diagnosis before enrollment, although diagnosis may have occurred on the day of enrollment. In Kenya, 92% of women seek antenatal care at least once during pregnancy.14 The Nyanza region has the highest prevalence of HIV in Kenya at 15% with HIV prevalence estimates in antenatal mothers ranging from 19–26%.15
Women were ineligible for enrollment if they were unable to provide consent in a study language (English or Dholuo), were currently on treatment for TB disease or latent TB infection (LTBI), or were treated for TB or LTBI within the prior year.
Procedures
Enrollment
We recruited consecutive HIV-infected pregnant women from two antenatal clinics and screened for study eligibility. Eligible participants who provided informed consent (written or, if illiterate, the consent was read and understanding confirmed with a thumb print) were interviewed by study staff using a structured interview tool that included questions on sociodemographic information, pregnancy history, HIV history (date of diagnosis, medications), TB and LTBI history, and the presence of TB symptoms in participants and their household members (as reported by participant). TB symptoms screen consisted of the WHO 4-part symptom screen (fever, any cough, weight loss, night sweats), as well as prolonged cough (>2 weeks), hemoptysis, and lymphadenopathy. Data extracted from clinic charts included medication history and CD4 cell count. The HIV status of participants was determined by antenatal clinic staff per Kenyan guidelines using a serial strategy of point-of-care rapid testing with the Determine™ HIV-1/2 (Abbott Japan Co Ltd, Tokyo, Japan) test performed on all samples, followed by SD Bioline® HIV 1/2 (Standard Diagnostics Inc, Kyonggi-do, Korea) for all positive or inconclusive samples.16 Uni-Gold™ HIV (Trinity Biotech PLC, Bray, Ireland) is used as a tie-breaker in the case of discordant Determine™ and SD Bioline® results.
Tuberculin Skin Tests (TST)
TSTs were performed using 5 tuberculin units (0.1ml) of purified protein derivative (RT 23 solution, Sanofi Pasteur) and read by study nurses using the “ball-point” technique and a ruler within 48–96 hours.17,18 A positive TST was defined as ≥5 mm of induration.19
Sputum Collection and TB Laboratory Testing
Participants were instructed on sputum collection and two expectorated sputa specimens were collected: one as a “spot” sample at the time of enrollment and a second as an early morning specimen collected by the subject upon awakening on the day of TST read. Sputum and urine samples were refrigerated and transported on ice at 4–8°C on a daily basis to the ISO 15189-accredited KEMRI/CDC Laboratory in Kisumu, Kenya. Specimens were decontaminated using N-acetyl-L-cysteine and sodium hydroxide and examined by AFB-smear microscopy using Ziehl-Neelsen technique. If one or more AFB per equivalent of 100 immersion fields was observed, the slide was considered positive and graded. After re-suspension with phosphate buffer, equal sample volumes were used to perform mycobacterial culture and Xpert. Mycobacterial culture was performed using a commercial broth method, MGIT Manual Mycobacterial Growth System (Becton-Dickinson, Franklin Lakes, NJ). Isolates were identified as M. tuberculosis using the Capilia TB Test Kit (TAUNS, Numazu, Japan). All smear and culture were performed on fresh samples. In general, one Xpert was performed on fresh sputum using the “spot” specimen. Xpert was performed on the frozen second sputum sample if the patient was unable to provide an initial spot sample or if the second sputum specimen was culture positive for M. tuberculosis. Urine was collected during the initial visit and LAM testing was performed within 8 hours of collection. Test results were interpreted using the reference scale card per the manufacturer’s instructions, with positive tests interpreted on a scale of 1–4 by intensity of the positive band.20
National Tuberculosis Guidelines
Per Kenyan national TB guidelines, it is recommended that all HIV-infected individuals, including women in antenatal care, undergo intensified case finding using the WHO 4-part TB symptom screen.21 Women with one or more symptoms receive further evaluation that may include chest radiograph and sputum collection for smear microscopy. However, sputum AFB culture, TSTs, Xpert and LAM were not routinely performed at the study sites. At the time of the study, isoniazid preventive therapy was not routinely provided at the study sites.
Study Endpoints and Statistical Analysis
Pulmonary TB was defined as at least one sputum culture positive for M. tuberculosis. Participants who met this definition were referred for TB care through the Kenya National Treatment Program. Urine LAM tests with the presence of a band of any intensity (grade ≥1, on a scale of 1–4) were considered positive. Univariate logistic regression and Fisher’s exact test were used as appropriate to assess the association between potential correlates and the outcome of pulmonary TB. The performance of the WHO TB symptom screen, AFB-smear, Xpert, and urine LAM were compared to culture using sensitivity, specificity, positive and negative predictive values, positive and negative likelihood ratios, and area under the receiver operating characteristic curve (AUC). All estimates were reported using 95% confidence intervals (CI), and all statistical tests were two-sided with α = 0.05. Analyses were performed using Stata 13 (StataCorp, College Station, TX).
Ethics Approval
This study was approved by the Kenyatta National Hospital-University of Nairobi Ethics and Research Committee and the University of Washington Institutional Review Board.
RESULTS
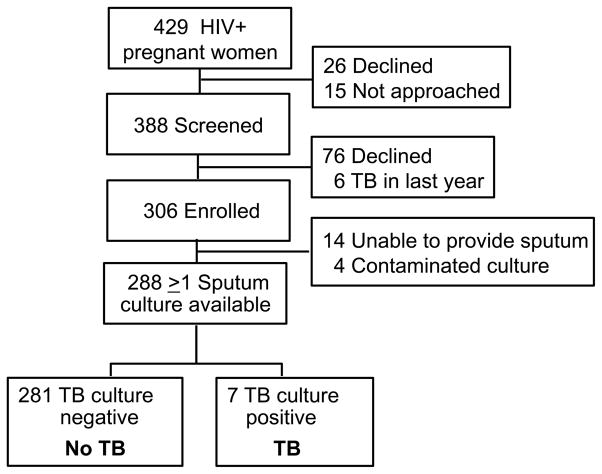
Between July 2013 and July 2014, 429 HIV-infected pregnant women attended routine antenatal care services at the two sites, and 388 were screened for study eligibility (Figure 1). Of women screened, 76 declined study participation and 6 were excluded from enrollment due to TB diagnosis in the preceding year. Of the 306 enrolled women, 18 were excluded from further analysis (14 women were unable to produce sputum, 4 women had contaminated cultures). Of the 18 women without evaluable sputum TB culture (either due to unable to produce sputum or culture contamination), 2 had TB symptoms (cough) and 2 reported TB exposures (though not within household). The remaining 288 women had one or more sputum samples with valid culture results for TB evaluation; of these 244 (85%) had two cultures performed.
Figure 1.
Study flow of HIV-infected pregnant women screened for pulmonary TB in western Kenya.
Median maternal age was 25 years (IQR 22–30), and median gestational age was 26 weeks (IQR 20–32) (Table 1). Most women (78%) had completed primary education, and 57% were employed. Twenty-seven percent of women were unaware of their HIV-status prior to the current pregnancy. Over one-half (54%) of participants were taking combination antiretroviral therapy (cART) prior to study enrollment. In general, participants were relatively immunocompetent with a median CD4 cell count of 437 cells/mm3 (IQR 342–565 cells/mm3); only 13.8% of subjects had a CD4 cell count ≤250 cells/mm3. Of 246 women who had a TST placed, only 85 (35%) women returned for TST reading between 48–96 hours. Eighteen (21%) had a positive TST ≥5 mm. Twenty-five (9%) women had a history of TB at a mean of 6.5 years prior to enrollment. Women reporting a history of TB were more likely to have a positive TST (OR 18.0, 95% CI 1.9–173.6). Of women who did not have their TSTs read within 48–96 hours, 79 returned before 48 hours (73 with TST <5 mm, 6 with TST ≥5 mm), 8 returned between 96–100 hours (3 with TST <5 mm, 5 with TST ≥5 mm), and 70 returned at >100 hours (64 with TST <5mm, 6 with TST ≥5 mm). Expanding the TST time to read cutoff to 45–100 hours, 28/145 (19%) women had a TST of > 5 mm, similar to the proportion of women with positive TSTs read between 48–96 hours (18/85, 21%).
Table 1.
Correlates of culture confirmed pulmonary TB among HIV-infected pregnant women
| Correlate | All patients N=288 n(%), or median (IQR) |
TB N=7 n(%), or median (IQR) |
No TB N=281 n(%), or median (IQR) |
OR | 95% CI | pa |
|---|---|---|---|---|---|---|
| Sociodemographic characteristics | ||||||
| Age (years) | 25 (22–30) | 21 (20–23) | 25 (22–30) | 0.84 | (0.69–1.02) | 0.07 |
| Gestational age (weeks) | 26 (20–32) | 24 (18–28) | 26 (20–32) | 0.96 | (0.87–1.06) | 0.39 |
| BMI (kg/m2) | 23.6 (21.9–25.8) | 21.3 (20.3–22.3) | 23.6 (21.9–25.8) | 0.78 | (0.59–1.03) | 0.08 |
| Education (years) | 8 (8–10) | 8 (7–10) | 8 (8–10) | 0.98 | (0.72–1.34) | 0.92 |
| Completed primary school | ||||||
| Yes | 225 (78.1) | 5 (71.4) | 220 (78.3) | 0.69 | (0.13–3.67) | 0.65 |
| No | 63 (21.9) | 2 (28.6) | 61 (21.7) | ref | ||
| Employed | ||||||
| Yes | 163 (56.6) | 3 (42.9) | 160 (56.9) | 0.57 | (0.12–2.58) | 0.47 |
| No | 125 (43.4) | 4 (57.1) | 121 (43.1) | ref | ||
| Currently married | ||||||
| Yes | 240 (83.3) | 7 (100.0) | 233 (82.9) | - | 0.61 | |
| No | 48 (16.7) | 0 (0.0) | 48 (17.1) | |||
| Residential Conditions | ||||||
| Persons in household | 4 (3–5) | 3 (2–3) | 4 (3–5) | 0.48 | (0.23–1.00) | 0.05 |
| Single room household | ||||||
| Yes | 100 (34.7) | 4 (57.1) | 184 (65.5) | 1.42 | (0.31–6.49) | 0.68 |
| No | 188 (65.3) | 3 (42.9) | 97 (34.5) | ref | ||
| HIV | ||||||
| CD4 cell count (cells/mm3)(N=239) | 437 (342–565) | 621 (439–888) | 430 (340–558) | 1.00 | (0.99–1.00) | 0.11 |
| ≤250 | 33 (13.8) | 0 (0.0) | 33 (14.2) | - | 1.00 | |
| >250 | 206 (86.2) | 6 (100.0) | 200 (85.8) | |||
| HIV status known prior to this pregnancy | ||||||
| Yes | 210 (72.9) | 6 (85.7) | 204 (72.6) | 2.26 | (0.27–19.11) | 0.68 |
| No | 78 (27.1) | 1 (14.3) | 77 (27.4) | ref | ||
| Current ART | ||||||
| PMTCT | 62 (20.3) | 3 (42.9) | 52 (18.5) | 2.19 | (0.47–10.12) | 0.10 |
| cART | 165 (53.9) | 4 (57.1) | 152 (54.1) | ref | ||
| None | 79 (25.8) | 0 (0) | 77 (27.4) | - | ||
| Current co-trimoxazole | ||||||
| Yes | 234 (81.2) | 5 (71.4) | 229 (81.5) | 0.57 | (0.11–3.01) | 0.62 |
| No | 54 (18.8) | 2 (28.6) | 52 (18.5) | ref | ||
| Partner’s HIV status | ||||||
| Positive | 133 (46.1) | 3 (42.9) | 130 (46.3) | 0.57 | (0.09–3.49) | 0.77 |
| Negative | 51 (17.7) | 2 (28.6) | 49 (17.4) | ref | ||
| Unknown | 104 (36.1) | 2 (28.6) | 102 (36.3) | 0.48 | (0.07–3.51) | |
| TB Symptoms and Exposure | ||||||
| History of TB (n=270) | ||||||
| Yes | 25 (9.3) | 1 (14.3) | 24 (9.1) | 1.66 | (0.19–14.37) | 0.645 |
| No | 245 (90.7) | 6 (85.7) | 239 (90.9) | ref | ||
| Cough | ||||||
| Yes | 43 (14.9) | 2 (28.6) | 41 (14.6) | 2.34 | (0.44–12.47) | 0.28 |
| No | 245 (85.1) | 5 (71.4) | 240 (85.4) | |||
| Fever | ||||||
| Yes | 14 (4.9) | 1 (14.3) | 13 (4.6) | 3.43 | (0.38–30.67) | 0.30 |
| No | 274 (95.1) | 6 (85.7) | 268 (95.4) | |||
| Weight loss | ||||||
| Yes | 3 (1.0) | 0 (0.0) | 3 (1.1) | - | 1.00 | |
| No | 285 (99.0) | 7 (100.0) | 278 (98.9) | |||
| Night sweats | ||||||
| Yes | 20 (6.9) | 1 (14.3) | 19 (6.8) | 2.30 | (0.26–20.08) | 0.40 |
| No | 268 (93.1) | 6 (85.7) | 262 (93.2) | ref | ||
| Any WHO TB symptom positiveb | ||||||
| Yes | 56 (19.4) | 3 (42.9) | 53 (18.9) | 3.22 | (0.70–14.85) | 0.14 |
| No | 232 (80.6) | 4 (57.1) | 228 (81.1) | ref | ||
| Cough >2 weeks | ||||||
| Yes | 14 (4.9) | 2 (28.6) | 12 (4.3) | 8.97 | (1.58–51.01) | 0.04 |
| No | 274 (95.1) | 5 (71.4) | 269 (95.7) | ref | ||
| Lymphadenopathy | ||||||
| Yes | 6 (2.1) | 1 (14.3) | 5 (1.8) | 9.2 | (0.92–91.2) | 0.14 |
| No | 282 (97.9) | 6 (85.7) | 276 (98.2) | ref | ||
| Hemoptysis | ||||||
| Yes | 1 (0.4) | 0 (0.0) | 1 (0.4) | - | 1.0 | |
| No | 287 (99.7) | 7 100.0 | 280 99.6 | |||
| TST ≥5mm (N=85) | ||||||
| Yes | 18 (21.2) | 3 (75.0) | 15 (18.5) | 13.2 | (1.28–135.88) | 0.03 |
| No | 67 (78.8) | 1 (25.0) | 66 (81.5) | ref | ||
| TB exposure | ||||||
| Yes | 44 (15.4) | 2 (28.6) | 42 (15.1) | 2.26 | (0.42–12.02) | 0.29 |
| No | 242 (84.6) | 5 (71.4) | 237 (84.9) | ref | ||
| Household TB contact (N=286) | ||||||
| Yes | 17 (5.9) | 1 (14.3) | 16 (5.7) | 2.74 | (0.31–24.14) | 0.35 |
| No | 269 (94.1) | 6 (85.7) | 263 (94.3) | ref | ||
| Household WHO TB symptom positive | ||||||
| Yes | 12 (4.2) | 3 (42.9) | 9 (3.2) | 22.67 | (4.40–116.57) | 0.002 |
| No | 276 (95.8) | 4 (57.1) | 272 (96.8) | ref | ||
Fisher’s exact for all categorical variables
Cough (any duration), fever, weight loss, or night sweats
Abbreviations: BMI, body mass index; ART, antiretroviral therapy; PMTCT, prevention of maternal to child transmission; cART, combination antiretroviral therapy; TST, tuberculin skin test; mm, millimeter.
The prevalence of pulmonary TB, defined by a positive sputum culture for M. tuberculosis, was 2.4% (CI 1.0–4.9%) (Figure 1). Compared to women without TB, women with pulmonary TB were more likely to report a cough lasting longer than 2 weeks (29% vs 4%, p=0.04) and have a positive TST ≥5 mm (75% vs 18%, p=0.03) (Table 1). Women with TB had 22.7-fold (95% CI 4.4–116.6) higher odds of reporting a household member with one of the 4 WHO TB symptoms compared to women without pulmonary TB (43% vs 3%, p<0.001).
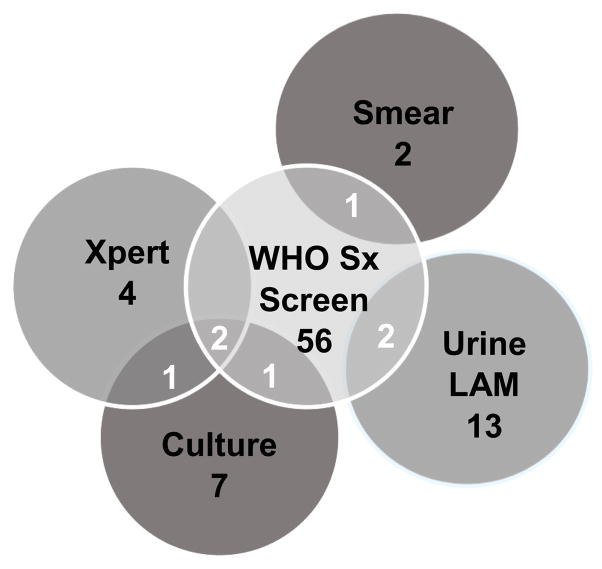
The WHO TB symptom screen identified 56 (19%) women with TB symptoms, 3 of whom had a positive culture for M. tuberculosis (Table 2). Most women (4/7, 57%) with positive sputum cultures for M. tuberculosis had a negative symptom screen (Figure 2). Overall, WHO screening had sensitivity of 42.9% (95% CI 9.9–81.6%), specificity 81.1% (95% CI 76.1–85.5%), positive predictive value 5.4% (95% CI 1.1–14.9%) and negative predictive value 98.3% (95% CI 95.6–99.5%) for identifying women with pulmonary TB. Twelve (4.2%) participants reported that a household member had one or more WHO TB symptoms. Of the 12 women who identified their partner as being a household TB contact; all reported that their partner was HIV positive. TB was diagnosed in 3 of these women (Table 1). Inclusion of participant report of either self or household member with a positive WHO TB symptom screen increased sensitivity to 71.4% (95% CI 29.0–96.3%), while maintaining high specificity of 80.1% (95% CI 74.9–84.6%) for pulmonary TB (Table 2).
Table 2.
Diagnostic accuracy of WHO TB symptom screen and rapid TB diagnostic tests compared to sputum culture for the diagnosis of pulmonary TB in HIV-infected pregnant women
| Screen or test | Positive among total N=288 (%) |
Positive among TB cases N=7 (%) |
Sensitivity % (95% CI) | Specificity % (95% CI) | PPV % (95% CI) | NPV % (95% CI) | AUC (95% CI) |
|---|---|---|---|---|---|---|---|
| Symptom screening | |||||||
| WHO TB symptoms | |||||||
| Cough | 43 (14.9) | 2 (28.6) | 28.6 (3.7–71.0) | 85.4 (80.7–89.3) | 4.7 (0.6–15.8) | 98.0 (95.3–99.3) | 0.57 (0.39–0.75) |
| Fever | 14 (4.9) | 1 (14.3) | 14.3 (0.4–57.9) | 95.4 (92.2–97.5) | 7.1 (0.2–33.9) | 97.8 (95.3–99.2) | 0.55 (0.41–0.69) |
| Weight loss | 3 (1.0) | 0 (0) | 0 (0.0–41.0) | 98.9 (96.9–99.8) | 0 (0.0–70.8) | 97.5 (95.0–99.0) | 0.49 (0.49–0.50) |
| Night sweats | 20 (6.9) | 1 (14.3) | 14.3 (0.4–57.9) | 93.2 (89.6–95.9) | 5.0 (0.1–24.9) | 97.8 (95.2–99.2) | 0.54 (0.40–0.68) |
| Any WHO TB symptom | 56 (19.4) | 3 (42.9) | 42.9 (9.9–81.6) | 81.1 (76.0–85.5) | 5.4 (1.1–14.9) | 98.3 (95.6–99.5) | 0.62 (0.42–0.82) |
| Cough >2 weeks | 14 (4.9) | 2 (28.6) | 28.6 (3.7–71.0) | 95.7 (92.7–97.8) | 14.3 (1.8–41.8) | 98.2 (95.8–99.4) | 0.62 (0.44–0.80) |
| Hemoptysis | 1 (0.4) | 0 (0) | 0 (0.0–40.9) | 99.6 (98.0–100) | 0 (0.0–97.5) | 97.6 (95.0–99.0) | 0.50 (0.49–0.50) |
| Lymphadenopathy | 6 (2.1) | 1 (14.3) | 14.3 (0.4–57.9) | 98.2 (95.9–99.4) | 16.7 (0.4–64.1) | 97.9 (95.4–99.2) | 0.56 (0.42–0.70) |
| Household TB symptom screen | 12 (4.2) | 3 (42.9) | 42.9 (9.9–81.6) | 96.8 (94.0–98.5) | 25.0 (5.5–57.2) | 98.6 (96.3–99.6) | 0.70 (0.50–0.90) |
| TB diagnostic test | |||||||
| Smear microscopy | 2 0.7 | 0 (0) | 0 (0.0–41.0) | 99.3 (97.5–99.9) | 0 (0.0–84.2) | 97.6 (95.0–99.0) | 0.50 (0.49–0.50) |
| Xpert | 4 (1.4) | 3 (42.9) | 42.9 (9.9–81.6) | 99.6 (98.0–100) | 75.0 (19.4–99.6) | 98.6 (96.4–99.6) | 0.71 (0.51–0.91) |
| Urine TB LAM (N=266) | 13 (4.9) | 0 (0) | 0 (0.0–70.8) | 95.1 (91.7–97.3) | 0 (0.0–24.7) | 98.8 (96.6–99.8) | 0.48 (0.46–0.49) |
| TST (N=85) | 18 (21.2) | 3 (75.0) | 75.0 (19.4–99.4) | 81.5 (71.3–89.2) | 16.7 (3.6–41.4) | 98.5 (92.0–100) | 0.78 (0.53–1.0) |
| Combined screening | |||||||
| WHO symptomsa | 61 (21.2) | 5 (71.4) | 71.4 (29.0–96.3) | 80.1 (74.9–84.6) | 8.2 (2.7–18.1) | 99.1 (96.9–99.9) | 0.76 (0.58–0.94) |
| WHO symptomsa and TST (N=129) | 72 (55.8) | 6 (100) | 100 (54.1–100) | 46.3 (37.3–55.6) | 8.3 (3.1–17.3) | 100 (93.7–100) | 0.73 (0.69–0.78) |
| WHO symptomsa and Xpert | 63 (21.9) | 6 (85.7) | 85.7 (42.1–99.6) | 79.7 (74.5–84.3) | 9.5 (3.6–19.6) | 99.6 (97.5–100) | 0.83 (0.69–0.97) |
| TST and Xpert (N=87) | 21 (24.1) | 5 (100) | 100 (47.8–100) | 80.5 (70.3–88.4) | 23.8 (8.2–47.2) | 100 (94.6–100) | 0.90 (0.86–0.95) |
| WHO symptomsa and Xpert or TST (N=131) | 74 (56.5) | 7 (100) | 100 (59.0–100) | 46.0 (37.0–55.1) | 9.5 (3.9–18.5) | 100 (93.7–100) | 0.73 (0.69–0.77) |
Extended WHO symptom screen including report of TB symptoms in participant or household member
Abbreviations: PPV, positive predictive value; NPV, negative predictive value; AUC, area under the curve; Xpert, GeneXpert MTB/RIF; TB LAM
Figure 2.
Overlap of TB screening and diagnostic tests for pulmonary TB in HIV-infected pregnant women
Xpert was positive in 4 women, and identified 3 of 7 women with sputum cultures positive for M. tuberculosis (Supplemental Table 1). Xpert had sensitivity 42.9% (95% CI 9.9–81.6%), specificity 99.6% (95% CI 98.0–100%), positive predictive value 75.0% (95% CI 19.4–99.4%) and negative predictive value 98.6% (95% CI 96.4–99.6%) (Table 2). Sputum smear microscopy was positive by Ziehl-Neelson staining in 2 women but did not identify any women with a sputum culture positive for M. tuberculosis. Sputum smear microscopy had sensitivity 0% (95% CI 0–41%), specificity 99.3% (95% CI 97.5–99.9%), positive predictive value 0% (95% CI 0–84.2%) and negative predictive value 97.6% (95% CI 95.0–99.0%). Urine LAM testing was performed on 266 women, and was grade 1 or 2 in 13 women (4.9%), and grade 2 in 2 women (0.8%). Using grade ≥1 as a threshold for a positive result, urinary LAM testing had sensitivity 0% (95% CI 0–70.8%), specificity 95.1% (95% CI 91.7–97.3%), positive predictive value 0% (95% CI 0–24.7%) and negative predictive value 98.8% (95% CI 96.6–99.8%) for pulmonary TB in this population. Of 11 women with positive LAM (grade ≥1) and available CD4 counts, only one had CD4 <400 (CD4=147).
Of the 7 women with culture-confirmed pulmonary TB, 3 had a positive symptom screen and 3 were Xpert positive (Figure 2). Two women with culture confirmed pulmonary TB were both positive by WHO TB symptom screen and Xpert. One woman with a positive AFB smear had TB symptoms (cough, night sweats); two women with a positive LAM had TB symptoms (one reported fever and night sweats, and one reported cough and night sweats). None of the smear or LAM positive women had positive TB sputum cultures. Women with positive smear microscopy or M. tuberculosis sputum culture were prescribed anti-tuberculosis therapy by the TB program.
In terms of overall performance of a single screen or test as measured by AUC, report of household symptoms (AUC 0.70, 95% CI 0.50–0.90), Xpert (AUC 0.71, 95% CI 0.51–0.91), and TST (AUC 0.78, 95% CI 0.53–1.0) performed similarly (Table 2) for pulmonary TB in this study. Using TST and Xpert yielded the highest combination of sensitivity and specificity (AUC 0.90, 95% CI 0.86–0.95).
DISCUSSION
We found a high burden of undiagnosed pulmonary TB disease among Kenyan HIV-infected pregnant women enrolled in antenatal care. Our estimate of pulmonary TB prevalence, 2.4% (CI 1.0–4.9%), is consistent with estimated TB prevalence in HIV-infected adults from a community-based study in western Kenya (2.1%)22 and in HIV-infected pregnant women in sub-Saharan Africa (0.3 to 6%).5,10,23–27 Notably, we observed a substantial burden of pulmonary TB disease in HIV-infected women during pregnancy in the absence of low CD4 cell counts and despite the use of cART. Compared to previous studies assessing the prevalence of culture-confirmed TB among pregnant HIV-infected women regardless of symptoms,10 our cohort had somewhat higher CD4 counts and a higher proportion of women on cART. Combination ART decreases the risk of TB by 67%, with increasing CD4 cell counts and duration of therapy associated with greater decline in risk.28 However, the risk of TB remains higher among HIV-infected individuals at all levels of immunosuppression compared to those without HIV.29–31
A novel finding of our study is that screening for the presence of WHO TB symptoms in household members was strongly associated with pulmonary TB. Inclusion of a positive WHO TB symptom screen in the participant or a household member increased the sensitivity of TB case finding to 71% without compromising specificity. Expansion of TB screening to include symptoms of household members may provide a mechanism to improve active TB case finding in HIV-infected pregnant women, and should be validated in larger cohorts. Importantly, presence of WHO TB symptoms in household members was more predictive than ascertaining a known TB contact in the household. Antenatal screening of women may provide a unique opportunity to diagnose not only pregnant women but others in the household through surrogate screening using the simple WHO symptom screen.
Intensified TB case finding using the WHO 4-part symptom screen of fever, cough, night sweats, or weight loss among pregnant women failed to identify more than half [4 of 7 (57%)] of the cases of culture-confirmed pulmonary TB. Low sensitivity of the WHO symptom screen (28–50%) for excluding TB has been observed in other studies of pregnant HIV-infected women that performed sputum culture independent of clinical symptoms.10,11,26 An individual participant data meta-analysis that included cohorts of HIV-infected cART-naïve individuals from sub-Saharan Africa and Southeast Asia found that the sensitivity of the WHO TB symptom screen was 79% overall and higher among individuals not previously screened for TB (88%).12 The sensitivity of the WHO symptom screen may be decreased in the context of cART, and was approximately 50% less sensitive among participants taking cART compared to cART-naïve individuals in two South African studies.32,33 TB symptoms may be less frequent among women compared to men,34 and pregnancy may further mask symptoms due to an overlap with pregnancy-related physiologic changes35 or relative suppression of Th1 pro-inflammatory cytokines.1,36 We did not screen for malnutrition, which may have impacted weight loss as a TB screening symptom in our cohort. However, there is no clear consensus on the most appropriate measure of malnutrition in pregnant women in general, or in HIV-infected pregnant women.37 TB symptom screening in pregnancy may require the addition of other symptoms such as fatigue, or inappropriately low weight gain in pregnancy. Despite low sensitivity, prolonged cough was associated with pulmonary TB in our cohort, which has been also been observed in pregnant women with TB disease in Tanzania.38
Although it has been suggested that Xpert may improve TB screening within antenatal care settings,39 we are unaware of published estimates regarding its performance in pregnant HIV-infected women. In our study, Xpert was less sensitive than the results of a meta-analysis that reported on test performance in HIV-infected individuals.40 In this same meta-analysis, Xpert performance was decreased among those who were smear negative to 67%. Our sensitivity estimate of 43% is within the range of sensitivities (40–81%) reported by studies evaluating smear-negative HIV-infected individuals41–43 and is most similar to a South African study evaluating the accuracy of Xpert compared to culture among HIV-infected outpatients regardless of symptoms prior to cART initiation.43 The use of cryopreserved samples in patients unable to provide “spot” samples may have contributed to the low sensitivity; however in a meta-analysis of Xpert performance, the use of cryopreserved samples led to only marginally decreased sensitivity and similar specificity.40
In contrast to sputum Xpert, urine LAM did not contribute to case detection of pulmonary TB within our study. Contrary to studies of Xpert and LAM among both hospitalized TB suspects and newly diagnosed HIV outpatients, there was no incremental benefit to the use of urine LAM to Xpert in our study cohort.44 In previous studies, LAM has performed best in highly immunocompromised individuals with very low CD4 counts,45 and the mild to modest immunosuppression observed in our sample may have resulted in lower test sensitivity. We did not actively investigate for extra-pulmonary TB (other than to ask participants about the presence of lymphadenopathy) and may have missed extra-pulmonary TB cases or sputum culture-negative cases associated with positive urine LAM testing. However, we are unaware of any women in our cohort who were diagnosed with either culture-negative or extrapulmonary TB.
In HIV-infected pregnant women, the sensitivities of screening tests, including WHO 4-part symptom screen, Xpert, and AFB sputum smear microscopy, were poor compared to liquid sputum culture in identifying women with pulmonary TB. This is of particular concern given the adverse effects of untreated TB on mother and infant. Additionally, the high proportion of false negative tests for pulmonary TB has implications for effective screening prior to the initiation of isoniazid preventive therapy (IPT). In our study more than half of the women found to have culture confirmed TB would have been offered IPT based on their negative WHO symptom screen. Although combining TST and Xpert maximized diagnostic accuracy to detect pulmonary TB [AUC 0.90 (95%CI 0.86–0.95)], there are multiple barriers to the widespread use of TST including the need for a return visit (35% compliance rate in our study) and refrigeration that may make it infeasible in low resource settings. With the inclusion of Xpert as a first line diagnostic in the most recent Kenya national guidelines in symptomatic HIV-infected individuals,21 and the ease of an extended symptom screen that includes household member TB symptoms, this combination screening approach [AUC 0.83 (95%CI 0.69–0.97)] may be a more viable option for determining who is safe for IPT and who requires further TB evaluation using sputum culture. Given the low sensitivity of the WHO 4-symptom screen in this population, and the risk of poor maternal and infant outcomes due to missed TB diagnosis, future research regarding the use of Xpert as a screening tool (including the cost-effectiveness of this approach) in PMTCT settings is needed.
Recent efforts have yielded promising results in developing TB diagnostics in high burden settings,46,47 including those that may perform well specifically in HIV-infected pregnant women,48 and point-of-care tests49 that may contribute to TB screening in HIV-infected individuals. Our results highlight the urgent need for improved TB diagnostics for use in HIV-infected pregnant women that have been rigorously evaluated in this vulnerable population.50
Our study had several limitations. We may have underestimated the burden of pulmonary TB by performing culture on a single sputum in 28% of patients. Subjects unable to spontaneously expectorate sputum did not undergo sputum induction, which may have resulted in further under-diagnosis of TB. We did not perform chest radiographs in subjects and may have missed radiographically-apparent cases of TB. Xpert testing was performed on only one of 2 sputum samples; for one positive culture from the 2nd sputum culture, Xpert was performed on a cryopreserved sputum sample to ensure adequate estimation of sensitivity. Our study had limited power for estimates of diagnostic performance. A strength of our study is the performance of diagnostic tests, including culture, in all participants regardless of symptoms.
In conclusion, we found a significant burden of undiagnosed pulmonary tuberculosis among HIV-infected pregnant women. Symptom screening and available diagnostic tests including sputum smear microscopy, Xpert and urinary LAM testing had poor performance in this population. Household TB symptom screening improved sensitivity to detect pulmonary TB. Untreated tuberculosis during pregnancy is associated with poor maternal and infant outcomes, and future studies to investigate optimal screening algorithms and novel tests in this vulnerable population are warranted.
Supplementary Material
Acknowledgments
SOURCE OF FUNDING: This work was supported by the National Institute of Allergy and Infectious Diseases and the National Institute of Child Health and Human Development at the National Institutes of Health [grant number K23 AI 85036-01 to DJH, K24 HD054314-06 to GJS, K12 HD000850 to LMC, T32 AI07140 to SML], the National Center for Research Resources at the National Institutes of Health [grant number UL1TR000423] and the Firland Foundation.
We thank the staff at the Ahero Sub-district Hospital and Bondo District Hospital antenatal clinics, KEMRI/CDC laboratory personnel, as well as our study staff and participants.
Footnotes
PRIOR REPORTS: Preliminary study results were presented at the Infectious Disease Society of America Meeting (Philadelphia, PA, USA), October 2014; International Union Against Tuberculosis and Lung Disease World Conference (Barcelona, Spain), November 2014; and the HIV Research in Women Symposium, Albert Einstein-Montefiore Center for AIDS Research (New Rochelle, NY, USA), December 2014.
CONFLICTS OF INTEREST: The authors report no conflicts of interest.
SML, LMC, BAR, DJH, GJS designed the study and analyses. SML, LMC, DJH, JK, DM conducted the study. SML, LMC, BAR, DJH conducted statistical analysis. All co-authors contributed to manuscript writing.
References
- 1.Mathad JS, Gupta A. Tuberculosis in pregnant and postpartum women: epidemiology, management, and research gaps. Clinical Infectious Diseases. 2012;55(11):1532–1549. doi: 10.1093/cid/cis732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walson JL, Brown ER, Otieno PA, et al. Morbidity among HIV-1-infected mothers in Kenya: prevalence and correlates of illness during 2-year postpartum follow-up. J Acquir Immune Defic Syndr. 2007;46(2):208–215. doi: 10.1097/QAI.0b013e318141fcc0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta A, Nayak U, Ram M, et al. Postpartum tuberculosis incidence and mortality among HIV-infected women and their infants in Pune, India, 2002–2005. Clinical Infectious Diseases. 2007;45(2):241–249. doi: 10.1086/518974. [DOI] [PubMed] [Google Scholar]
- 4.Toro PL, Schneider KL, Carter RJ, Abrams EJ, El-Sadr WM, Howard AA. Maternal and infant outcomes with concurrent treatment of tuberculosis and HIV infection in pregnant women. J Acquir Immune Defic Syndr. 2011;56(2):e63–e66. doi: 10.1097/QAI.0b013e318201e11d. [DOI] [PubMed] [Google Scholar]
- 5.Nachega J, Coetzee J, Adendorff T, et al. Tuberculosis active case-finding in a mother-to-child HIV transmission prevention programme in Soweto, South Africa. AIDS. 2003;17(9):1398–1400. doi: 10.1097/00002030-200306130-00018. [DOI] [PubMed] [Google Scholar]
- 6.Getahun H, Sculier D, Sismanidis C, Grzemska M, Raviglione M. Prevention, diagnosis, and treatment of tuberculosis in children and mothers: evidence for action for maternal, neonatal, and child health services. J Infect Dis. 2012;205 (Suppl 2):S216–227. doi: 10.1093/infdis/jis009. [DOI] [PubMed] [Google Scholar]
- 7.Gounder C, Wada N, Kensler C, et al. Active tuberculosis case-finding among pregnant women presenting to antenatal clinics in Soweto, South Africa. J Acquir Immune Defic Syndr. 2011;57(1):e77–84. doi: 10.1097/QAI.0b013e31821ac9c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deluca A, Chaisson RE, Martinson NA. Intensified case finding for tuberculosis in prevention of mother-to-child transmission programs: a simple and potentially vital addition for maternal and child health. J Acquir Immune Defic Syndr. 2009;50(2):196–199. doi: 10.1097/QAI.0b013e3181900201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings. 2011 http://whqlibdoc.who.int/publications/2011/9789241500708_eng.pdf.
- 10.Hoffmann CJ, Variava E, Rakgokong M, et al. High prevalence of pulmonary tuberculosis but low sensitivity of symptom screening among HIV-infected pregnant women in South Africa. PLoS One. 2013;8(4):e62211. doi: 10.1371/journal.pone.0062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kancheya N, Luhanga D, Harris J, et al. Integrating active tuberculosis case finding in antenatal services in Zambia. Int J Tuberc Lung Dis. 2014;18(12):1466–1472. doi: 10.5588/ijtld.14.0920. [DOI] [PubMed] [Google Scholar]
- 12.Getahun H, Kittikraisak W, Heilig CM, et al. Development of a standardized screening rule for tuberculosis in people living with HIV in resource-constrained settings: individual participant data meta-analysis of observational studies. PLoS Med. 2011;8(1):e1000391. doi: 10.1371/journal.pmed.1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ratnapalan S, Bona N, Chandra K, Koren G. Physicians’ perceptions of teratogenic risk associated with radiography and CT during early pregnancy. Am J Roentgenol. 2004;182(5):1107–1109. doi: 10.2214/ajr.182.5.1821107. [DOI] [PubMed] [Google Scholar]
- 14.Kenya National AIDS and STI Control Programme (NASCOP) Guidelines for prevention of mother to child transmission (PMTCT) of HIV/AIDS in Kenya. (4) 2012 http://www.faces-kenya.org/wp-content/uploads/2012/11/Guidelines-for-PMTCT-of-HIVAIDS-in-Kenya-1_2012.pdf.
- 15.Kenya National AIDS and STI Control Programme (NASCOP) Kenya AIDS Indicator Survey 2012: Final Report. 2014 http://www.nacc.or.ke/images/documents/KAIS-2012.pdf.
- 16.Kenya National AIDS and STI Control Programme (NASCOP) Guidelines for HIV Testing and Counselling in Kenya. 2010 http://nascop.or.ke/hiv_testing_&_counselling.php.
- 17.Cobelens F, Van Deutekom H, Draayer-Jansen I, Schepp-Beelen A, Van Gerven P, Mensen M. Tuberculin skin test reactions by time of reading among Dutch travellers. Int J Tuberc Lung Dis. 2003;7(8):758–763. [PubMed] [Google Scholar]
- 18.Tuberculin reaction size on five consecutive days. Bull World Health Organ. 1955;12(1–2):189–196. [PMC free article] [PubMed] [Google Scholar]
- 19.Targeted tuberculin testing and treatment of latent tuberculosis infection. Joint Statement of the American Thoracic Society (ATS) and the Centers for Disease Control and Prevention (CDC) Am J Respir Crit Care Med. 2000;161(4 Pt 2):S221–247. doi: 10.1164/ajrccm.161.supplement_3.ats600. [DOI] [PubMed] [Google Scholar]
- 20.Alere. Alere Determine™ TB LAM Ag Pakage Insert. 2013 Dec; http://www.alere.com/ww/en/product-details/determine-tb-lam.html.
- 21.Kenyan Ministry of Health, National AIDS and STI Control Program (NASCOP) Guidelines on use of antiretroviral drugs for treating and preventing HIV infection: a rapid advice. 2014 http://healthservices.uonbi.ac.ke/sites/default/files/centraladmin/healthservices/Rapid%20Advice%20Booklet%202014%2024%20June%2012%20noon_0.pdf.
- 22.van’t Hoog AH, Laserson KF, Githui WA, et al. High prevalence of pulmonary tuberculosis and inadequate case finding in rural western Kenya. Am J Respir Crit Care Med. 2011;183(9):1245–1253. doi: 10.1164/rccm.201008-1269OC. [DOI] [PubMed] [Google Scholar]
- 23.Pillay T, Khan M, Moodley J, et al. The increasing burden of tuberculosis in pregnant women, newborns and infants under 6 months of age in Durban, KwaZulu-Natal. S Afr Med J. 2001;91(11):983–987. [PubMed] [Google Scholar]
- 24.Kali PB, Gray GE, Violari A, Chaisson RE, McIntyre JA, Martinson NA. Combining PMTCT with active case finding for tuberculosis. J Acquir Immune Defic Syndr. 2006;42(3):379–381. doi: 10.1097/01.qai.0000218434.20404.9c. [DOI] [PubMed] [Google Scholar]
- 25.Gounder CR, Wada NI, Kensler C, et al. Active tuberculosis case-finding among pregnant women presenting to antenatal clinics in Soweto, South Africa. J Acquir Immune Defic Syndr. 2011;57(4):e77–84. doi: 10.1097/QAI.0b013e31821ac9c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Modi S, Cavanaugh S, Shiraishi R, et al. Symptom-based screening for tuberculosis among pregnant women living with HIV in Kenya. Conference of Retroviruses and Opportunistic Infections (CROI); 2014; Boston, Massachusetts. [Google Scholar]
- 27.Gupta A, Chandrasekhar A, Gupte N, et al. Symptom screening among HIV-infected pregnant women is acceptable and has high negative predictive value for active tuberculosis. Clin Infect Dis. 2011;53(10):1015–1018. doi: 10.1093/cid/cir605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lawn SD, Wood R, De Cock KM, Kranzer K, Lewis JJ, Churchyard GJ. Antiretrovirals and isoniazid preventive therapy in the prevention of HIV-associated tuberculosis in settings with limited health-care resources. Lancet Infect Dis. 2010;10(7):489–498. doi: 10.1016/S1473-3099(10)70078-5. [DOI] [PubMed] [Google Scholar]
- 29.Gupta A, Wood R, Kaplan R, Bekker LG, Lawn SD. Tuberculosis incidence rates during 8 years of follow-up of an antiretroviral treatment cohort in South Africa: comparison with rates in the community. PLoS One. 2012;7(3):e34156. doi: 10.1371/journal.pone.0034156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kufa T, Mabuto T, Muchiri E, et al. Incidence of HIV-associated tuberculosis among individuals taking combination antiretroviral therapy: a systematic review and meta-analysis. PLoS One. 2014;9(11):e111209. doi: 10.1371/journal.pone.0111209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lawn SD, Myer L, Edwards D, Bekker LG, Wood R. Short-term and long-term risk of tuberculosis associated with CD4 cell recovery during antiretroviral therapy in South Africa. AIDS. 2009;23(13):1717–1725. doi: 10.1097/QAD.0b013e32832d3b6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rangaka MX, Wilkinson RJ, Glynn JR, et al. Effect of antiretroviral therapy on the diagnostic accuracy of symptom screening for intensified tuberculosis case finding in a South African HIV clinic. Clin Infect Dis. 2012;55(12):1698–1706. doi: 10.1093/cid/cis775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmad Khan F, Verkuijl S, Parrish A, et al. Performance of symptom-based tuberculosis screening among people living with HIV: not as great as hoped. AIDS. 2014;28(10):1463–1472. doi: 10.1097/QAD.0000000000000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Long NH, Diwan VK, Winkvist A. Difference in symptoms suggesting pulmonary tuberculosis among men and women. J Clin Epidemiol. 2002;55(2):115–120. doi: 10.1016/s0895-4356(01)00455-3. [DOI] [PubMed] [Google Scholar]
- 35.Hamadeh MA, Glassroth J. Tuberculosis and pregnancy. Chest. 1992;101(4):1114–1120. doi: 10.1378/chest.101.4.1114. [DOI] [PubMed] [Google Scholar]
- 36.Singh N, Perfect JR. Immune reconstitution syndrome and exacerbation of infections after pregnancy. Clin Infect Dis. 2007;45(9):1192–1199. doi: 10.1086/522182. [DOI] [PubMed] [Google Scholar]
- 37.Ververs MT, Antierens A, Sackl A, Staderini N, Captier V. Which anthropometric indicators identify a pregnant woman as acutely malnourished and predict adverse birth outcomes in the humanitarian context? PLoS Curr. 2013;5 doi: 10.1371/currents.dis.54a8b618c1bc031ea140e3f2934599c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sheriff F, Manji K, Manji M, Chagani M. Pulmonary tuberculosis among pregnant mothers in Tanzania. Dar es Salaam Medical Student Journal. 2010;16:5–9. [Google Scholar]
- 39.Turnbull ER, Kancheya NG, Harris JB, Topp SM, Henostroza G, Reid SE. A model of tuberculosis screening for pregnant women in resource-limited settings ssing Xpert MTB/RIF. J Pregnancy. 2012;2012:565049. doi: 10.1155/2012/565049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steingart KR, Schiller I, Horne DJ, Pai M, Boehme CC, Dendukuri N. Xpert(R) MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2014;1:CD009593. doi: 10.1002/14651858.CD009593.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balcells ME, Garcia P, Chanqueo L, et al. Rapid molecular detection of pulmonary tuberculosis in HIV-infected patients in Santiago, Chile. Int J Tuberc Lung Dis. 2012;16(10):1349–1353. doi: 10.5588/ijtld.12.0156. [DOI] [PubMed] [Google Scholar]
- 42.Carriquiry G, Otero L, Gonzalez-Lagos E, et al. A diagnostic accuracy study of Xpert(R)MTB/RIF in HIV-positive patients with high clinical suspicion of pulmonary tuberculosis in Lima, Peru. PLoS One. 2012;7(9):e44626. doi: 10.1371/journal.pone.0044626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lawn SD, Brooks SV, Kranzer K, et al. Screening for HIV-associated tuberculosis and rifampicin resistance before antiretroviral therapy using the Xpert MTB/RIF assay: a prospective study. PLoS Med. 2011;8(7):e1001067. doi: 10.1371/journal.pmed.1001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shah M, Ssengooba W, Armstrong D, et al. Comparative performance of urinary lipoarabinomannan assays and Xpert MTB/RIF in HIV-infected individuals. AIDS. 2014;28(9):1307–1314. doi: 10.1097/QAD.0000000000000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lawn SD. Point-of-care detection of lipoarabinomannan (LAM) in urine for diagnosis of HIV-associated tuberculosis: a state of the art review. BMC Infect Dis. 2012;12:103. doi: 10.1186/1471-2334-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaforou M, Wright VJ, Oni T, et al. Detection of tuberculosis in HIV-infected and -uninfected African adults using whole blood RNA expression signatures: a case-control study. PLoS Med. 2013;10(10):e1001538. doi: 10.1371/journal.pmed.1001538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anderson ST, Kaforou M, Brent AJ, et al. Diagnosis of childhood tuberculosis and host RNA expression in Africa. N Engl J Med. 2014;370(18):1712–1723. doi: 10.1056/NEJMoa1303657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Naranbhai V, Moodley D, Chipato T, et al. The association between the ratio of monocytes: lymphocytes and risk of tuberculosis among HIV-infected postpartum women. J Acquir Immune Defic Syndr. 2014;67(5):573–575. doi: 10.1097/QAI.0000000000000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Drain PK, Mayeza L, Bartman P, et al. Diagnostic accuracy and clinical role of rapid C-reactive protein testing in HIV-infected individuals with presumed tuberculosis in South Africa. Int J Tuberc Lung Dis. 2014;18(1):20–26. doi: 10.5588/ijtld.13.0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ransohoff DF, Feinstein AR. Problems of spectrum and bias in evaluating the efficacy of diagnostic tests. N Engl J Med. 1978;299(17):926–930. doi: 10.1056/NEJM197810262991705. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.