Abstract
Accumulated research supports the idea that exercise could be an option of potential prevention and treatment for drug addiction. During the past few years, there has been increased interest in investigating of sex differences in exercise and drug addiction. This demonstrates that sex-specific exercise intervention strategies may be important for preventing and treating drug addiction in men and women. However, little is known about how and why sex differences are found when doing exercise-induced interventions for drug addiction. In this review, we included both animal and human that pulled subjects from a varied age demographic, as well as neurobiological mechanisms that may highlight the sex-related differences in these potential to assess the impact of sex-specific roles in drug addiction and exercise therapies.
Keywords: Sex differences, Drug addiction, Exercise, Animal and human studies, Neurobiological mechanisms
1. Introduction
Drug addiction is often referred to as a chronic brain disorder characterized by compulsive and uncontrollable drug seeking tendencies and usage, paired with the emergence of a negative emotional state when drug access is prohibited (Wakefield, 2015; Leshner, 1997). The neurobiology of drug addiction involves dysfunctions in specific neural pathways and neuropsychological pathology (Levy, 2013; Koob and Volkow, 2010). Numerous clinical and preclinical studies have found that sex differences emerge throughout all phases of drug addiction (Franconi et al., 2012; Becker and Hu, 2008; Anker and Carroll, 2011; Carroll and Anker, 2010). Studies of drug addiction in humans and animals have demonstrated that fluctuating levels of steroid hormones account for these sex differences. For example, estradiol facilitates positive subjective effects of drugs, while progesterone and its metabolite allopregnanolone attenuate drug-related responses and protect against the aversive effect of drugs in females (Becker et al., 2012; Carroll and Anker, 2010). Similarly, testosterone has been shown to modulate drug-related awarding effects and behavioral response differently in males and females (Menendez-Delmestre and Segarra, 2011; Bawor et al., 2014).
The most common treatments of drug addiction mainly involve pharmacological and social psychological approaches for symptom relief , not relapse prevention for drug addicts after detoxification (Winters et al., 2014; Bailey and Husbands, 2014). However, many forms of pharmacological treatments can also be addictive and malevolent to patients. Therefore, there is an urgency to seek more effective approaches as adjunctive treatment of drug addiction. Recently, increasing evidence from human and animal experiments indicates that exercise may be an option in terms of potential interventions in the treatment of addiction. Studies have demonstrated that exercise can effectively reduce the susceptibility to and craving for addictive drugs, producing significant effects on drug addiction prevention and rehabilitation (Lynch et al., 2013; Smith and Lynch, 2011; Zschucke et al., 2012). Although few human studies have focused on sex and hormonal differences related to the efficacy of exercise at preventing and treating drug abuse, limited preliminary findings suggest that sex differences exist in the usage of exercise as a prevention and treatment method for alcohol, marijuana, and other illicit drugs (Korhonen et al., 2009; Chen et al., 2010; Pate et al., 1996; Dolezal et al., 2013). Animal studies also showed sex differences in a variety of substances, but the sex-bias effects of exercise on the treatment of some drug addiction cases have been controversial (Cosgrove et al., 2002; Peterson et al., 2014; Smith et al., 2011; Smith et al., 2012; Thanos et al., 2010; Ehringer et al., 2009; Sanchez et al., 2014; Zlebnik et al., 2014b; Zhou et al., 2014). While there is no clear reason why men and women face drug addiction differently, extensive studies showed that sex hormones play critical roles in synaptic plasticity, neurogenesis and neurotransmitter systems which might be part of underlying mechanisms of drug addiction (Scarduzio et al., 2013; Li et al., 2011; Galea et al., 2014; Barth et al., 2015; Wetherington, 2010). As recently highlighted in Nature, men and women often have different profiles of drug safety and treatment efficacy (Pollitzer, 2013), we predict that men may experience different effects from exercises treating drug addiction compared to women. In order to elaborate on the sex differences in the benevolent effects of exercise-induced drug addiction treatment, in this review, we will discuss sex differences in drug addiction, exercise, the efficacy of exercise intervention for drug addiction, and its underlying neurobiological mechanisms. We believe that a better understanding of sex differences in exercise intervention for drug addiction prevention and recovery will provide a theoretical basis for novel sex-specific rehabilitations.
2. Sex differences in drug addiction
2.1 Animal studies
Sex differences in drug addiction have also been confirmed in animal studies, and this is reflected in laboratory animal models of drug addiction. The traditional animal models of drug addiction are framed by behavioral tests that emphasize the actions of drugs as positive reinforcers, such as the self-administration (SA) paradigm and conditioned place preference (CPP) paradigm. In the following sections, we will mainly discuss sex differences in the two commonly used animal models: SA and CPP.
As the most recognized animal model for evaluating various aspects of drug addiction, SA is highly correlated with human drug addiction behavior in humans (Panlilio and Goldberg, 2007; Henningfield et al., 1991). Extensive literature has focused on sex differences in SA (Fattore et al., 2008; Fattore et al., 2009). Researchers found that female rats learn to self-administer various drugs (e.g., cocaine, methylphenidates, amphetamine and heroin) faster, and are more sensitive to the rewarding effects than males (Kerstetter et al., 2008; Lynch and Carroll, 1999; Lynch, 2006). Moreover, female rats show a longer elevation of drug-seeking behavior during periods of abstinence and are more susceptible to the escalation of SA than males for cocaine, methamphetamine, cannabinoid and other illicit drugs (Roth and Carroll, 2004; Reichel et al., 2012; Fattore et al., 2010; Fattore et al., 2009), and are also likelier to have higher drug intake for maintaining SA extinction and reinstatement compared to males (Fattore et al., 2009; Lynch and Carroll, 2000; Fattore et al., 2007). These sex differences in drug addiction are partially regulated by sex hormones. For example, during the estrus phase, female rats (endogenous estradiol level is high) have more active drug-seeking behavior and consume greater amounts of drug than rats in non-estrus phase (estradiol level is low) (Kerstetter et al., 2008; Roth et al., 2002). Such estrogen-facilitated effects of drug addiction were further evidenced by studies of ovariectomized rats that expressed reduced acquisition, attenuated drug intake and longer extinction in SA compared to intact female rats with no difference from male rats (Fattore et al., 2007; Jackson et al., 2006). Taken together, these studies suggest that ovary hormones play a critical role in female addictive behaviors, and that females express greater vulnerability than males to develop an addicted phenotype such as acquisition, maintenance, craving, extinction and reinstatement.
As a popular animal model of drug addiction, CPP assesses the rewarding effects of multiple addicted phenotypes by measuring the learning process of drug addiction, making it fundamentally distinct from SA (Napier et al., 2013; Bardo and Bevins, 2000). CPP represents the conditioned reinforcing effects of drugs, which is closely related to environmental cues for drug craving behavior rather than the reinforcing effects of drugs or drug abuse behavior itself (Sanchis-Segura and Spanagel, 2006). Studies showed that female rats often required shorter training cycles and lower dosages of drugs ( including cocaine, morphine, nicotine, methylamphetamine, etc ) to acquire CPP compared to that in males (Russo et al., 2003b; Randall et al., 1998; Zakharova et al., 2009; Chen et al., 2003; Lenoir et al., 2015; Carroll and Anker, 2010). However, some studies showed no sex differences in the training cycle for CPP acquisition (Mathews and McCormick, 2007; Schindler et al., 2002; Hilderbrand and Lasek, 2014; Cummins et al., 2013; Bobzean et al., 2010). Such controversial findings can possibly be attributed to the type of drug, dosages of administration and age of the animals. Some other independent studies also reported that female animals needed higher dosage of drug (e.g., ethanol, nicotine, cocaine, morphine) to achieve CPP (Roger-Sanchez et al., 2012; Torres et al., 2009; Gioiosa et al., 2008). Despite the inconsistent results regarding CPP between males and females, it is generally accepted that the rewarding effect of drugs in CPP is greatly associated with levels of ovary hormones in females since ovariectomized female rats showed a reduction of drug induced CPP behavior compared to intact females (Russo et al., 2003a; Parada et al., 2012; Torres et al., 2009). Altogether, these contradicting results on sex effects related to CPP behavior are partially associated with drug types and dosages, animal species and ages, and the levels of steroid hormones.
Furthermore, most of drug addiction, withdraw and abstinence are associated with dysregulation of hypothalamic-pituitary adrenal (HPA) axis which directly associated with stress. While activation of HPA axis has been found to stimulate drug use and relapse, studies demonstrated that activation of ovarian hormone in females often have higher circulating levels of cortisol and greater release of adrenocorticotropic hormone in response to stress, while testosterone has been demonstrated to suppress the HPA axis in male rats (Bobzean et al., 2014; Handa et al. 1994; Sinha 2008).
2.2 Human studies
It is generally accepted that women exhibit lower rates of drug abuse and addiction than men, although the total number of female addictive drug users has increased in recent years (Bobzean et al., 2014). Indeed, men and women showed differences in the initiation and reasons for abusing drugs, drug-seeking and relapse during periods of abstinence, and treatment outcomes (Moeller, 2012; Kennedy et al., 2013; Greenfield et al., 2007; Becker et al., 2012). For example, female subjects with drug addiction show higher levels of craving, behavioral activation and motor impulsivity in drug/cue exposure than male individuals with drug addiction (Perry et al., 2013; Schlauch et al., 2013), and women become dependent on drugs more rapidly than men (Hernandez-Avila et al., 2004; Becker and Hu, 2008). Females are more inclined to begin taking drugs as self-medication to alleviate symptoms of mental disease (Becker et al., 2012). Once addicted to a drug (i.g., opiates, nicotine, alcohol, cocaine and the psychomotor stimulants), females tend to have more difficulty giving up drugs and are at greater risk for relapse following abstinence compared to their male counterparts (Becker and Hu, 2008; Lynch et al., 2002). Compared to male subjects in drug abuse treatment programs, female drug users often develop more severe withdrawal symptom, greater mood and anxiety disorders (Fox and Sinha, 2009; Doran, 2013; Back et al., 2011), use more combinations of different drugs and legal psychoactive substances and have an unwillingness to participate detoxification program during the treatment (Kissin et al., 2014; Ignjatova and Raleva, 2009; Bawor et al., 2014). Increasing evidence suggested that sex hormone related brain organization and activities may contribute significantly to the differences in drug addiction between men and women. For example, endogenous estrogen promotes drug-induced reward behavior in females (Quinones-Jenab and Jenab, 2010; Anker and Carroll, 2011), and facilitates drug addiction through interaction with dopaminergic system as well as opioid peptides (Segarra et al., 2010; Jacobs and D'Esposito, 2011). In addition, it has been reported that men and women show differences in the associations between the type of drug addiction and body mass index (BMI). Examples include male alcohol abusers and drug addicts who often have a lower BMI, while females do not (Pickering et al., 2011). Other studies also found that overweight men have higher rates of alcohol dependence and lower rates of nicotine dependence, whereas overweight women have lower incidence of alcohol dependence and higher risk for nicotine dependence (Vera-Villarroel et al., 2014; Barry and Petry, 2009).
In summary, females are more vulnerable to drug addiction with faster initiation of addiction, higher intake of drugs, and more frequent relapses. Females are more sensitive to drug acquisition, escalation of drug intake, maintenance, withdrawal, reinstatement, relapse and treatment. Although controversial results on sex and drug addiction have been associated with drug types, dosages, animal species and ages, steroid hormone levels, and stress, further investigations on sex-specific effects in drug addiction on a large scale are needed.
3. Sex differences in exercise and fitness
3.1 Animal studies
The majority of animal studies on drug addiction intervention through exercise were performed in rodents with three different exercises models, including a voluntary running wheel, forced treadmill running and forced swimming (Table 3 & 4). Among these, the running wheel was the most popular mode of active physical exercise, while the other two modes of exercise were passive ones and might involve more stress.
Table 3.
Human studies for sex differences in effects of exercise on drug addiction (single-sex)
|
Prevention effect
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Exercise schedule |
|||||||||
| Citation | Subjects | Sex | Age (years) | Drug | Type | Duration | Frequency | Intensity | Outcome of exercise |
| Terry-McEIrath et al.(2011) | HS | M | 18-25/26 | Alcohol/ Nicotine/ Cannabis | --- | --- | 1-5 days/wk | --- | Advantage |
| Terry-McEIrath et al.(2011) | MS | M | 15-18 | Alcohol/ Nicotine/ Cannabis | --- | --- | 1-5 days/wk | --- | Advantage or disadvantage |
| Mattila et al.(2012) | Conscripts | M | 18-29 | Nicotine | --- | 6 months | > 1 times/wk | Highly/Moderate /Light | Advantage or disadvantage |
| Henchoz et al.(2014) | Conscripts | M | 18-25 | Alcohol/ Nicotine/ Cannabis | --- | 12 months | 0-7 days/wk | --- | Advantage |
| Henchoz et al.(2014) | Conscripts | M | 18-25 | Alcohol/ Nicotine/ Cannabis | --- | 15 months | 0-7 days/wk | --- | Advantage or disadvantage |
|
Treatment effect | |||||||||
| Li at al. (2002) | Addicts | M | 18-52 | Heroin | Qigong | 10 days | Daily | --- | Advantage |
| Marcus et al. (1995) | Smokers | F | 22-56 | Nicotine | Cycle ergometer | 15 weeks | 3 days/wk | Vigorous | Advantage |
| Marcus et al. (1999) | Smokers | F | 18-65 | Nicotine | --- | 12 weeks | 3 days/wk | Vigorous | Advantage |
| Bock et al. (1999) | Smokers | F | Adults | Nicotine | --- | 12 weeks | 3 days/wk | Vigorous | Advantage |
| Marcus et al. (2005) | Smokers | F | 18-65 | Nicotine | Walking, etc | 8 weeks | 5 days/wk | Moderate | Advantage |
| Prapavessis et al. (2007) | Smokers | F | 18-62 | Nicotine | Cycle ergometer | 12 weeks | 3 days/wk | Vigorous | Advantage |
| Kinnunen et al. (2008) | Smokers | F | 18-55 | Nicotine | --- | 19 weeks | 2 days/wk | Moderate | Advantage |
| Vickers et al. (2009) | Smokers | F | 18-65 | Nicotine | --- | 10 weeks | 5 days/wk | --- | Advantage |
| Bock et al. (2010) | Smokers | F | --- | Nicotine | Yoga | 8 weeks | 2 days/wk | --- | Advantage |
| Williams et al. (2010) | Smokers | F | 18~65 | Nicotine | Brisk walking | 8 weeks | 3 days/wk | Moderate | Advantage |
| Williams et al. (2011) | Smokers | F | 18~65 | Nicotine | Brisk walking | 1 weeks | 3 days/wk | Moderate | Advantage |
| Korhonen et al. (2011) | Smokers | F | 18~55 | Nicotine | --- | 15 weeks | --- | Moderate | Advantage |
| Smits et al. (2012) | Smokers | F | Adults | Nicotine | --- | 15 weeks | 3 days/wk | Vigorous | Advantage |
| Li et al. (2013) | Addicts | F | 18-41 | Heroin | Tai Chi | 6 months | 3 days/wk | --- | Advantage |
HS: high school students; MS: middle school students
Table 4.
Human studies for sex differences in effects of exercise on drug addiction (double-sex)
|
Prevention effects
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Exercise schedule |
||||||||
| Citation | Subjects(age) | Drug | Type | Duration | Frequency | Intensity | Sex difference | Outcome of exercise |
| Aaron et al. (1995) | MS | Nicotine/Alcohol | --- | --- | --- | H, L, M | Yes | Less likely to smoke and use alcohol in females and males, respectively |
| Pate et al. (1996) | HS | Alcohol | 2 weeks | 2-7 days/wk | L, V | Yes | Less physical activity is associated with higher alcohol use in females, not in males | |
| Pate et al. (2000) | HS | Nicotine/Cocaine/Marijuana | --- | 12 months | --- | L, V | Yes | Exercise reduced usage of illegal drugs in males, not in females |
| Kulig et al.(2003) | HS | Nicotine/Alcohol/Marijuana | --- | --- | --- | V | Yes | Physical activities reduce use of illicit drugs in females |
| Moore et al. (2005) | MS | Nicotine/Alcohol/Marijuana | IS | --- | --- | --- | Yes | Reduction or increase in drug use in males and females are sports type dependent |
| Strohle et al. (2007) | Residents | Nicotine/Alcohol | --- | --- | 1-7 days/wk | --- | Yes | Reduced d rates of drugs abuse in females more than males |
| Korhonen et al.(2009) | Twins | Alcohol/Illicit drugs | --- | --- | 3-7 days/wk | --- | Yes | Persistent physical activity decreased illicit drug use more significant in females |
| Rodriguez et al. | HS | Nicotine | --- | --- | 3-7 days/wk | --- | NO | Physical excises significantly reduced tobacco use in both males and females. |
|
Treatment effect | ||||||||
| Chen et al. (2010) | --- | Q | 14 days | weekly | --- | Yes | Females showed more reduced craving, anxiety and withdrawal symptoms than | |
| Horn et al. (2011) | Nicotine | --- | 10 weeks | 1 day/wk | --- | Yes | Decreased the risk of continued smoking, particularly for males. | |
| Dolezal et al. (2013) | Methylamphetamine | TE | 8 weeks | 3 days/wk | M | Yes | Enhanced recovery from drug dependency mainly in men | |
HS: high school students; MS: middle school students; IS:Individual sports; Q: Qigong; TE:Treadmill exercise; H: High; L:Low; M: Moderate; V: Vigorous
Studies showed sex differences in all three types of exercise models. Females showed higher daily physical activity levels than males (Lightfoot, 2008). Moreover, a large number of studies showed that female rodents with drug addiction often ran more laps (longer distances) in wheel exercises than males within the same time frame (Eikelboom and Mills, 1988; Kanarek et al., 2009; Smith et al., 2011; Smith et al., 2012), suggesting females and males may be endowed with different exercise intensity tendencies when presented with voluntary wheel running exercises. This difference may result partially from females having a higher locomotive efficiency as compared to males (Rezende et al., 2006). Female mice have a greater capacity to increase their cardiac mass than males as an adaption in response to similar levels of exercises involving voluntary wheel running or even forced treadmill running (Konhilas et al., 2004), suggesting that females are more inclined to increase exercise capacity and hypertrophic response to exercise. For passive exercise models, male rats showed a small reduction of serum corticosteroid-binding globulin that was not found in female rats during a 10-day forced treadmill running regimen (Brown et al., 2007). It was also found that estrogen level increased in male rats muscle but not in females, while testosterone levels increased in muscle in both sexes after forced treadmill exercise (Aizawa et al., 2008). This may be due to the factor that aromatase, an estrogen synthase, increases in males and decreases in females after acute exercise (Aizawa et al., 2008). Compared to voluntary wheel running, which has reinforcing and rewarding properties, forced treadmill running is a more passive exercise and is commonly used for measurable interventions or treatment (Belke and Wagner, 2005; Greenwood et al., 2011; Brené et al., 2007; Leasure and Jones, 2008). In terms of examining the rehabilitation effect of forced treadmill running on brain injury, a study found that slow continuous training is effective only in male mice, while more intense interval training produces results in females (English et al., 2011). Sex differences in exercise performance are also observed in forced swimming. For instance, female rats are more active than males in water (Brotto et al., 2000) and swim at faster speeds with less sign of fatigue (Birren and Kay, 1958; Kay and Birren, 1958). However, it is unclear if the sex differences in forced swimming are related to the gender-specific differences in the response to stress or if females favor swimming as an exercise. It is worth noting that the swimming model is also used to detect psychological status (e.g., anxiety, depression and stress), cognition processes (e.g., learning and memory), as well as an intervention in treating drug relapse and addiction (Segat et al., 2014; Fadaei et al., 2015).
To conclude, female mice seem to have developed greater cardiovascular benefits in response to voluntary wheel running or even forced treadmill running compared to males. For brain injury rehabilitation, males respond better to forced treadmill continuous training, while more intense interval training is needed in females. Since females and males perform very differently among these exercise modes, it is important to include the consideration of the sex-specific exercise effectiveness on drug rehabilitation in animal experiments.
3.2 Human studies
Sex differences have been reported in different exercise models, including traditional aerobic exercises (walking, running, swimming and ball sports with modulate intensity), anaerobic exercises (strength training with high exercise intensity), and body-mind exercises (Yoga, Tai Chi and Qigong with very mild physical and mental exercise). Among these, aerobic exercises are most commonly used for intervening drug addiction (Table 3 & 4), while body-mind exercise is becoming a very popular practice in improving cognition (Man et al., 2010; Gothe et al., 2014).
Research has provided abundant evidence for sex-specific exercise-induced improvements in psychological and physiological well-being. For example, an eight week aerobic exercise intervention promoted higher levels of pulmonary function (forced vital capacity, forced expiratory volume and peak expiratory flow) and aerobic performance (peak oxygen consumption) in male cardiac patients compared to females (Temfemo et al., 2011). Another study found that the aerobic exercise-induced improvements in cardiovascular function were only seen in men, not in women, with particularly little benefit seen for postmenopausal women (Pierce et al., 2011). Recent research also suggests that enhanced cardiovascular structure and function, such as the development of ventricular hypertrophy and increase in VO2max, is greater in males rather than females when induced by long-term endurance exercises (Howden et al., 2015). However, other studies showed no sex differences in these exercise induced effects on a cardiovascular rehabilitation (Pina et al., 2014), while others reported that exercise might be a more important preventive factor for cardiovascular disease in women than men (Morita and Okita, 2013). Some of these different exercise effects on health between men and women are associated with sex-specific physiology in general, such as levels of maximal oxygen uptake, heart size, peak cardiac output (Uth, 2005; Wong et al., 2008; Winsley et al., 2009), and types of muscle fiber (Storey and Smith, 2012; Harris et al., 2012; Hicks et al., 2001). In general, there are sex differences in aerobic exercise associated with health improvements in both healthy people and individuals suffering from various diseases. It is unknown whether there are sex differences in aerobic exercise intervention's effect on drug addiction. On the other hand, studies showed that men often show a higher level of anaerobic exercise induced maximal hypoxia tolerance and higher level of peak post-exercise blood lactate concentration compares to women (Hill and Vingren, 2014; Hill and Smith, 1993). Studies also found that women often have a lower basal metabolism rates than men after the long-term yoga practice, which is likely to reduced arousal (Chaya et al., 2006). In addition, there some discrepancies in the prevalence, preference or attitudes about exercise between the sexes. For example, men are more likely to participate in more than one type of exercise for longer durations and more frequently than females (Stapleton et al., 2014; Deaner and Smith, 2013). A recent study in China suggests that women are more likely to practice Tai chi and dancing, while men involve in walking and jogging (Birdee et al., 2013). Moreover, studies showed that men are more likely to engage in team and/or combat exercises than women (Kirchengast, 2014; Deaner et al., 2012).
Taken together, results imply that women receive more beneficial effects from doing aerobics and body-mind exercises while men developed better capacity of hypoxia tolerance through anaerobic exercises. A greater understanding of sex differences in these exercise types may provide insight toward developing more effective exercise approaches and sex-specific treatments for drug addiction.
4. Sex differences in the efficacy of exercise intervention for drug addiction
Although extensive evidence shows that males and females are different in each phase of drug addiction as well as in various exercise activities, it is unclear whether such sex differences also exist for exercise interventions in drug addiction. During the past few decades, increasing evidence has suggested that exercises attenuate drug-seeking behaviors during drug initiation, escalation, extinction, abstinence and relapse (Lynch et al., 2013; Smith and Lynch, 2011). It is crucial to decrease the susceptibility to drugs (preventive effect) at an early stage of drug addiction and reduce the drug craving (therapeutic effect) at later stages in order to prevent relapse. Here, we will review recent studies on sex differences in the efficacy of exercise intervention for drug addiction from human trials and animal studies.
4.1 Animal studies
Although there are numerous studies on various exercise types for the prevention and treatment of drug addiction in animals, many of them recruited only one gender as shown in Table 1. The majority of the reports showed beneficial effects from voluntary and forced exercise activities as drug addiction prevention and treatment in male and female animal studies.
Table 1.
Animal studies for sex differences in effects of exercise on drug addiction (single-sex)
|
Prevention effect
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Exercise schedule |
||||||||||
| Citation | Subjects | Sex | Age /Weight | Drug | Model | Type | Duration | Frequency | Intensity | Outcome of exercise |
| Lett et al. (2002) | Rats | M | 184g | Morphine | CPP | VR | 8 days | 2-h/day, 1times/day | --- | Advantage |
| Eisenstein et al. (2007) | Rats | M | 250-275g | Morphine | CPP | VR | 3 weeks | 24-h/day | --- | Disadvantage |
| Xu et al. (2007) | Mice | M | 3 weeks | Morphine | CPP | VR | 2 months | 24-h/day | --- | Advantage |
| Chen et al. (2008) | Mice | M | 8 weeks | MDMA | CPP | FR | 4/8/12weeks | 1-h/day,5days/wk | 10~12m/min | Advantage |
| Hosseini et al. (2009) | Rats | M | 250-300g | Morphine | SA | FR | 11 or 30 days | 90 min/day, 1times/day | 15m/min | Advantage |
| EI Rawas et al. (2009) | Mice | M | 3 weeks | Heroin | CPP | VR | 2 months | 24-h/day, 1times/day | --- | Advantage |
| Solinas et al. (2009) | Mice | M | 3 weeks | Cocaine | CPP | VR | 2~3 months | 24-h/day | --- | Advantage |
| Fontes-Ribeiro et al. (2011) | Rats | M | 8 weeks | Amphetamine | CPP | FR | 8 weeks | 50min/day,5days/wk | 7~32 m/min | Advantage |
| Miladi-Gorji et al. (2011) | Rats | M | Adult | Morphine | * | VR | 10 days | 24-h/day | --- | Advantage |
| Smith and Pitts (2011) | Rats | M | 3 weeks | Cocaine | SA | VR | 6 weeks | 24-h/day | 4500m/day | Advantage |
| Mustroph et al. (2011) | Mice | M | 5 weeks | Cocaine | CPP | VR | 30 days | 24-h/day | 8300m/day | Advantage or Disadvantage |
| O'Dell et al. (2012) | Rats | M | Adult | METH | ** | VR | 6 weeks | 24-h/day | 8000m/day | Advantage |
| Smith and Pitts (2012) | Rats | M | 3 weeks | Heroin | SA | VR | 6 weeks | 24-h/day | 4500m/day | Advantage |
| McCulley et al. (2012) | Rats | M | 6 weeks | Alcohol | # | VR | 10 days | 24-h/day | --- | Advantage |
| Miller et al. (2012) | Rats | M | 200-250g | METH | SA | VR | 7~14days | 1-h/day, 1times/day | --- | Advantage or no effect |
| Hashemi et al. (2013) | Rats | M | 220-250g | Alcohol | &&& | FR | 2~8 weeks | 1-h/day,5days/wk | 17 m/min | Advantage |
| Renteria et al. (2013) | Rats | M | 200-250g | Cocaine | * | VR | 5~10 weeks | 24-h/day | --- | Advantage |
| Engelmann et al. (2014) | Rats | M | 200-250g | METH | SA | VR | 6 weeks | 24-h/day | --- | Advantage or Disadvantage |
| Geuzaine et al. (2014) | Mice | M | 4 weeks | Cocaine | CPP | VR | 10 weeks | 24-h/day | --- | Advantage |
| Smith et al. (2008) | Rats | F | 3 weeks | Cocaine | SA | VR | 6 weeks | 24-h/day | 10120 m/day | Advantage |
| Smith et al. (2008) | Rats | F | 3 weeks | Cocaine | CPP | VR | 6 weeks | 24-h/day | 9418 m/day | Disadvantage |
| Leasure et al. (2010) | Rats | F | 190-220g | Alcohol | & | VR | 14 days | 12-h/day, 1times/day | --- | Advantage |
| Smith and Witte (2012) | Rats | F | 3 weeks | Cocaine | SA | VR | 6 weeks | 24-h/day | 7700 m/day | Advantage or no effect |
| Zlebnik et al. (2014) | Rats | F | 90 days | Cocaine | * | VR | 21 days | 24-h/day | --- | Advantage |
In an attempt to understand the sex differences in exercise-induced effects on drug addiction, limited studies included both genders and reported controversial findings as shown in table 2. Female rats showed reduction in cocaine CPP after chronic forced treadmill exercise, while male rats showed completed inhibition of drug CPP after exercise (Thanos et al., 2010). On the other hand, another study showed a reduction in alcohol consumption in female rats post-exercise, not in male rats (Ehringer et al., 2009). Additionally, a different study showed an attenuation of cocaine SA under extended-access conditions after exercise in both sexes (Smith et al., 2011; Smith et al., 2012). In accordance with these results, a recent study found that a 10-day wheel exercise prior to alcohol exposure attenuated withdrawal-induced seizure susceptibility in both genders of rats (Devaud et al., 2012). Altogether, whether sex differences in the preventive effects of exercise on drug addiction remains unclear and further investigation in both genders is needed.
Table 2.
Animal studies for sex differences in effects of exercise on drug addiction (double-sex)
|
Prevention effects
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Exercise schedule |
||||||||||
| Citation | Subjects | Age | Drug | Model | Duration | Frequency | Intensity | Sex difference | Outcome of exercise | |
| Ehringer et al. (2009) | Mice | Adult | Alcohol | # | VR | 13 days | Daily, 24-h/day | --- | Yes | Reduced alcohol consummation significantly in females, while no differences were found in males. |
| Thanos et al. (2010) | Rats | 6 weeks | Cocaine | CPP | FR | 6 weeks | 5 days/week, 1-h/day | 10m/min | Yes | Blocked the formation of CPP in males, attenuated the CPP in females. |
| Smith et al. (2011) | Rats | 3 weeks | Cocaine | SA | VR | 6 weeks | Daily, 24-h/day | M: 3850m/day F: 9500m/day |
No | Weaken SA in both sexes, although the escalating intake was more in females than males. |
| Smith et al. (2012) | Rats | 3 weeks | Cocaine | SA | VR | 6 weeks | Daily, 24-h/day | M: 4000m/day F: 8500m/day |
No | Decreased reinstatement in both sexes, although females exhibited faster extinction of response than males. |
| Devaud et al. (2012) | Rats | 6 weeks | Alcohol | ## | VR | 10 days | Daily, 24-h/day | --- | No | Attenuated withdrawal-induced increases in seizure susceptibility in male and female rats. |
|
Treatment effects | ||||||||||
| Cosgrove et al. (2002) | Rats | Adult | Cocaine | SA | VR | 10 days | Daily, 24-h/day | --- | Yes | Suppressed SA in females, but not in males. |
| Brocardo et al. (2012) | Rats | 6 weeks | Alcohol | ## | VR | 10 days | Daily, 24-h/day | M: 2670m/day F: 5380m/day |
Yes | Reversed the depressive-like behaviors in males, but not in females. |
| Sanchez et al. (2014) | Rats | Adolescent | Nicotine | SA | VR | 10 days | Daily, 2-h/day | --- | Yes | Reduced SA in males, access to a wheel, either locked or unlocked, was sufficient to lower the seeking in females. |
| Peterson et al. (2014) | Rats | Adult | Cocaine | SA | VR | 14 days | Daily, 24-h/day 1,2,6,24-h/day |
--- | Yes | Male rats were more sensitive than females to exercise-induced attenuation of cocaine-seeking. |
| Zlebnik et al. (2014) | Rats | Adult | Cocaine | SA | VR | 14 days | Daily, 6-h/day | --- | Yes | Attenuated extinction responses and cocaine-primed reinstatement in females, but not in males. |
Unlimited access two-bottle choice (water or ethanol) model, and limited access drinking in the dark
Administrated by a nutritionally complete liquid diet
Although Table 1 shows how the majority of the studies on the therapeutic effects of exercise on drug addiction are conducted on male animals, Table 2 displays how voluntary wheel running showed reduction in both male and female rats, with females being more sensitive to the wheel running-induced reduction of cocaine SA than males (Cosgrove et al., 2002). This was supported by the discovery that female rats were more sensitive to the rewarding effects of the drug (Anker et al., 2011). In terms of exercise types, some research found that wheel running exercise reduced nicotine-seeking in male rats while female rats showed attenuated nicotine-seeking behavior by both running or an environmental enrichment (Sanchez et al., 2014). In addition, studies revealed that male rats showed a greater attenuation of cocaine-seeking with longer access to wheel running than that in females (Peterson et al., 2014), voluntary exercise mitigated extinction responses and cocaine-primed reinstatement in females but not males (Zlebnik et al., 2014b), and a 12-day wheel running exercise reversed the depressive-like behaviors in alcohol-exposed males, but not in females (Brocardo et al., 2012). Taken together, therapeutic effects seem to also be influenced by sex, which may be associated with different drug addiction models and exercise types.
4.2 Human studies
As shown in Table 3 and Table 4, human studies from the past two decades (1995 to 2014) show that various exercises may have preventive and therapeutic effects on drug addiction that can then be tailored by sex, with exercise-induced prevention and treatment for addictive drugs mainly limited to alcohol, tobacco and cannabis. Interestingly, the majority of published single-gender studies exploring preventive effects of exercise on drug addiction focused primarily on men while women were more involved in therapeutic studies on drug addiction. As shown in Table 3, in consistent with animal studies, exercises provide prevention and treatment effects on various drug addictions, regardless of exercise duration, intensity and type. However, the single gender studies are not able to display the sex effect on the exercise-induced reduction of drug addiction. Moreover, in many inclusive gender investigations, majority of the studies do not analyze and address the potential differences in the effects of exercise on drug addiction on men and women (Sinyor et al., 1982; Burling et al., 1992; Correia et al., 2005; Linke et al., 2012; Hallgren et al., 2014; Smelson et al., 2013; Escobedo et al., 1993; Collingwood et al., 2000; Werch et al., 2005; Charilaou et al., 2009), only limited reports have dissected the different impacts of exercise in drug addiction in men and women (Table 4).
Although the number of human studies on sex differences in exercise and drug addiction is limited, there were many researchers who paid attention to sex differences in the preventive effects of exercise on drug abuse (Table 4). Studies found a reversed relationship between drug dependence and exercise participation in both men and women (Rodriguez Garcia et al., 2014), while some revealed a much stronger association between exercise and substance abuse in women than men (Kulig et al., 2003; Strohle et al., 2007; Korhonen et al., 2009; Pate et al., 1996), and others showed greater preventive effects of exercise on drugs in men than women (Pate et al., 2000). Although the reasons behind these conflicting results remains unknown, it may be associated with the various demographic backgrounds of subjects, different types of addictive drugs, as well as different exercise activities (Aaron et al., 1995; Moore and Werch, 2005). Due to the limited number of human studies on sex differences in exercise intervention on drug addiction, it is still too early to provide a conclusive advice on gender-specific exercise recommendation for drug addiction prevention. Therefore, it is critical to extend further studies on exercise prevention in drug addiction in men and women, particularly in young populations when drugs are first introduced.
Exercise also showed sex-specific therapeutic effects on drug addiction as shown in Table 4. In only three studies that emphasized the potential sex specificity of exercise and drug addiction, data showed that long-term routine aerobic exercises reduced the risk of smoking, particularly in young men (Horn et al., 2011), and enhanced recovery from methamphetamine dependency in men. However, the study failed to show these results in women (Dolezal et al., 2013). Qigong, a typical body-mind exercise has been used as meditation therapy to reduce anxiety levels and withdrawal symptoms which are more common and severe in female substance abusers than males. Interestingly enough, individuals who maintained strong, disciplined mediation often had greater reductions in craving, anxiety, and withdrawal symptoms than those who practiced poor meditation (Chen et al., 2010) The study suggests that female drug abusers might be more sensitive to the meditative therapy then males since females often suffer more anxiety disorders and express more severe withdrawal symptoms, and that the quality of Qigong meditation practice could be associated with intervention in withdrawal symptoms. These limited findings of sex differences in exercise intervention in drug addiction treatment seem to suggest that men and women may have different responses to the therapeutic effects of exercise on drug addiction depending on type of exercises and addictive drugs.
5. Underlying neurobiological mechanisms for the sex-specific efficacy of exercise intervention for drug addiction
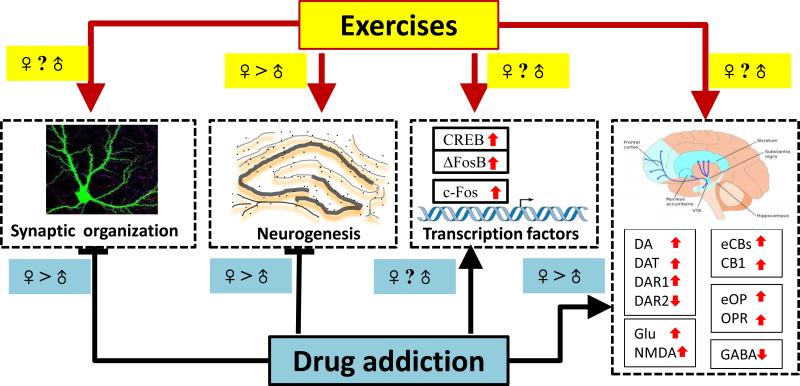
Long-term drug abuse led to corresponding compensatory changes in various neuronal functions, particularly the mesolimbic dopamine reward system (Feltenstein and See, 2013), including a series of molecular events induced by multiple neurotransmitters and their receptors in the ventral tegmental area (VTA), nucleus accumbens (NAc) and prefrontal cortex (PFC) (Nestler, 2004). These changes result in the impaired reward network processing, such as an addicted state, inhibitory feedback control and motivation (Baler and Volkow, 2006). On the other hand, there is considerable evidence that exercise can induce changes in neuroplasticity and regulate drug reward processing via the mesolimbic reward pathway (Zlebnik et al., 2014a; Greenwood et al., 2011; Werme et al., 2000; Werme et al., 2002b). Above all, the effects of drug addiction and exercise on these reward network signaling molecules are sex-dependent and regulated by steroid hormones (Miladi-Gorji et al., 2014; Berchtold et al., 2001; Peterson et al., 2014; Lynch et al., 2002; Becker and Hu, 2008). In this section, we present a brief overview of the sex differences in neural changes underlying the efficacy of exercise in drug addiction as indicated in Figure 1.
Figure 1. The neurobiology of sex difference in drug addiction and exercise intervention.
Drug addiction induces impairment of synaptic organization and neurogenesis, while exercise promotes synaptic plasticity and neurogenesis. In most of reports, males showed greater effect than females. Drug addiction is also altering several transcriptional factors and up-regulating neurotransmitters in specific brain regions, and exercise shared the similar signaling pathways to modulate and normalize the drug addiction related neuropathology. While there are no clear sex difference in exercise-induced regulation of synaptic plasticity and brain neurotransmitters, the sex differences in drug addiction- or exercise-induced alternation of transcriptional factors are brain regional dependent indicated as DA=dopamine; DAT=dopamine transporter; DAR=dopamine receptor; Glu=glutamate; NMDA=glutamate receptor; eCBs=endocannabinoids; CB1= cannabinoid receptor 1; eOP=endogenous opioid; OPR=opioid receptor; GABA=gamma-amino acid.
5.1 Synaptic plasticity and neurogenesis
5.1.1 Synaptic plasticity
Addictive drugs produce maladaptation via the mesocorticolimbic DA system, which includes changes of synaptic reorganization and synaptic function, such as long-term potentiation (LTP) and depression (Russo et al., 2010; Luscher and Malenka, 2011; Kauer and Malenka, 2007; Kalivas and O'Brien, 2008). Much research has been done on sex differences in synaptic plasticity. In general, normal female rats have higher densities of dendritic spines and large spines in the NAc than males (Forlano and Woolley, 2010). In animal models, female rats with cocaine addiction had higher densities of the spine density and spiculate protuberance of medium spiny neurons in NAc during abstinence compared to males (Wissman et al., 2011), the association between changes of dendritic spine plasticity and addicted behaviors was only found in females (Wissman et al., 2011). In addition, compared to males, female rats demonstrated higher sensitivity to drug-induced LTP deficits (An and Zhang, 2013), which can be modulated by sex hormones as an underlying synaptic plasticity through direct interaction with membrane receptors for estrogens and androgens in rats (Scarduzio et al., 2013), while a estradiol levels are higher in the female hippocampus compared to male animals (Brandt et al., 2013). Furthermore, studies also demonstrated that brain structure and synaptic formation/plasticity is highly dependent on ovary hormones, particularly estrogen, but are less dependent on testosterone (Bobzean et al., 2014; Mirbaha et al., 2009; Evans and Foltin, 2010). These results suggest that addictive drugs regulate synaptic plasticity differently in men and women, which may be related to sex hormones.
Although there is no direct evidence of sex differences between exercise and drug addiction-induced impairment of synaptic plasticity, it is well known that exercise alone induces modified synaptic plasticity and reorganization by triggering the producing of brain derived neurotrophic factor (BDNF) and insulin-like growth factor 1 in the hippocampus, interfacing with the regulating balance between the glutamatergic system and the GABAergic system, which together mediate neuronal excitability and synaptic transmission to enhance cognitive function (Gomez-Pinilla and Hillman, 2013; Wu et al., 2008; Farmer et al., 2004). In addition, voluntary exercise also stimulates histone deacetylase and DNA demethylation of BDNF promoter IV to regulate brain plasticity by engaging mechanisms of epigenetic regulation (Gomez-Pinilla et al., 2011). Recent studies found that short-term exercise enhanced hippocampal LTP and cognitive functions as well as increased numbers of spike LTP in morphine-dependent rats (Miladi-Gorji et al., 2014). Moreover, the 30-day intensive treadmill exercise increased dendritic spine density and arborization in mouse striatal, reducing drug-induced cognitive deficit (Toy et al., 2014). Altogether, it is theoretical possible that exercise-induced neuronal protective roles in drug addiction may be mediated partially by promoting synaptic plasticity, and we speculate that sex-specific neuroplasticity may be an underlying basis for the efficacy of exercise in treating drug addiction between men and women.
5.1.2 Neurogenesis
Animal studies have shown that females have high levels of cell proliferation in the dentate gyrus of the hippocampus, which is mediated by natural fluctuations of gonadal hormones, compared to their male counterpart (Tatar et al., 2013; Galea et al., 2013; Galea, 2008; Bowers et al., 2010). This kind of sex-specific neurogenesis in the hippocampus was also confirmed by other studies which reported that repeated exogenous estradiol administration altered neurogenesis and cell death in females, but not males (Barker and Galea, 2008). It was also reported that estradiol-induced neurogenesis and neuroprotection depended on the activation of either estrogen receptor subtype within the brain (Nilsen et al., 2000; Suzuki et al., 2007; Saleh et al., 2013). Young male rats showed delayed neurogenesis in the hippocampus after repeated exposure to cannabinoid compared to young female rats (Lee et al., 2014). Many studies have demonstrated that exercise can reduce the symptoms of drug addiction by ameliorating cognitive decline via the promotion of neurogenesis and activation of new neurons in the hippocampus. Such exercise-induced improvements of cognition were found more prominent in females than that in males (Engelmann et al., 2014; Leasure and Nixon, 2010; Chow et al., 2013). These differences in neurogenesis might be partly due to female animals having lower baseline values in terms of the number of newly proliferated cells and neuroblasts compared to males (Tzeng et al., 2014). Although exercise can reduce the symptoms of drug addiction by ameliorating cognitive decline via the promotion of neurogenesis, there is little direct evidence specifically focusing on sex differences in exercise-induced neurogenesis or exercise intervention on drug addiction.
Studies have shown that repeated exposure to addictive drugs causes the suppression of neurogenesis and enhancement of apoptosis in specific brain regions, which contribute to the formation of drug-taking or drug-seeking behaviors as well as future relapse (Engelmann et al., 2014; Mandyam et al., 2007; Noonan et al., 2010). Conversely, increased neurogenesis or gliogenesis in the brain's reward system may help reduce the vulnerability to relapse and promote recovery (Mandyam and Koob, 2012). Exercise has emerged as a potent intervening strategy for increasing neurogenesis and reducing apoptosis in mesocorticolimbic regions of brain, which promotes neuron health and reduces the risk of drug-related craving, mood disorders and cognitive impairment (Yau et al., 2014; Cotman et al., 2007; Maynard and Leasure, 2013; Engelmann et al., 2014; Mandyam et al., 2007; Leasure and Nixon, 2010). One study reported that treadmill running induced a higher level of neurogenesis and lower cell survival rate in the hippocampus of male mice compared to the female mice (Ma et al., 2012). However, it is unclear whether such a male favored exercise effect on neurogenesis is associated with sex hormones (Aizawa et al., 2008). Therefore, further investigations are needed for understand the sex differences in drug-related or exercise-induced neurogenesis in order to adequately develop sex-specific intervention on drug addiction.
5.2. Neurotransmitters and receptors
5.2.1. Dopamine and its receptor
Differing distributions of dopamine neurons in specific brain regions of animals have been observed between males and females. For example, male rats have more dopamine neurons in the substantia nigra, while females have more in the VTA and caudate nucleus (McArthur et al., 2007). Likewise, females have significantly higher levels of dopamine in the PFC, while males have higher levels of dihydroxyphenylacetic acid (dopamine metabolite) (Duchesne et al., 2009). This could be related to the fact that dopamine neurotransmission in the female rat is more tightly regulated by autoreceptors and transport in metabolic processes (Festa et al., 2004; Walker et al., 2006). In this way, dopamine receptors also appear to share similar characteristics. Male rats have 10% more DAR1 in the striatum and NAc than females (Becker and Hu, 2008), while females have few DAR2 in the striatum (Pohjalainen et al., 1998). Meanwhile, some research indicates that sex differences in the dopaminergic system are influenced by gonadal hormones. For example, in female rats, estrogen enhances dopamine release and dopamine-mediated behaviors by acting on the striatum and accumbens rapidly and directly, but this is not the case in males (Becker, 1999). Additionally, the reduction of endogenous sex hormones in castrated or ovariectomized rats showed a lessening of amphetamine-induced dopamine release (Becker and Hu, 2008; Becker, 1999). Moreover, estrogen receptors (ERs) including α and β subtypes are both important for sexual differentiation of the brain, and the proportion of the two subtypes regulate the expression of TH mRNA, which controls dopamine levels in the brain. Pendergast and colleagues found that female rodents had the same levels of ERα mRNA as males in the locus coeruleus, whereas ERβ mRNA expression was significantly lower compared to males (Pendergast et al., 2008), suggesting brain regional expression of ERs may contribute to sex differences in the dopamine system.
Although there is no direct evidence of sex differences related to dopamine and receptors mediating the efficacy of exercise in drug addiction, it is well documented that chronic exposure to addictive drugs results in the maladaptation of the mesolimbic dopamine reward circuitry, including the decrease of synaptic dopamine transporter (DAT) reuptake, increase of dopamine concentration, increase of levels of dopamine receptors 1 (DAR1) mRNA, decrease of levels of dopamine receptors 2 (DAR2) mRNA, and the increase of levels of tyrosine hydroxylase (TH) mRNA, a key limited enzyme in the synthesis process of dopamine (Volkow et al., 2007; Leyton and Vezina, 2014; O'Dell et al., 2012; Brenhouse et al., 2008; Ikemoto and Bonci, 2014). Exercise also up-regulates DAR1 signal transduction, down-regulates the level of DAR2 mRNA in the NAc (Foley and Fleshner, 2008; Greenwood et al., 2011; Roberts et al., 2012; Toy et al., 2014), reverses dopamine release and reduces the levels of TH expressing dopamine neurons in the midbrain, which is involved in ameliorating withdrawal symptoms and preventing relapse (Sobieraj et al., 2014; Chen et al., 2008). Based on this data, exercise can affect dopaminergic signaling at various levels, underlining its ability to reduce the susceptibility and craving for addictive drugs, and highlighting the possibility of a potential sex difference in exercise intervention for drug addiction via the dopamine system.
5.2.2 Endocannabinoids and their receptors
Endocannabinoids (eCBs), as a retrograde messenger, modulate the reward, motivation and relapse in drug addiction mainly through the activation or blockade of cannabinoid receptor 1 (CB1) (Bilbao, 2013; Friemel et al., 2014; Vlachou and Panagis, 2014). Studies in humans and rodents have shown evidence that females are more sensitive than males to the effects of cannabinoid. For example, female rats have shown more severe withdrawal symptoms, including motor activity dysfunction, anxiety and depression, which may be a reflection of differences in the eCBs system (Fattore and Fratta, 2010; Craft et al., 2013). The mechanisms underlying sex differences in cannabinoid effects appear to be associated with ovarian hormones, sexual dimorphism of the eCBs system and hypothalamic pituitary adrenal axis-eCBs interaction (Craft et al., 2013). For instance, intact female rats have lower levels of the CB1 binging in the hypothalamus and PFC, but higher levels in the amygdala than ovariectomized females and intact males (Riebe et al., 2010; López, 2010; Castelli et al., 2014). This suggests that the eCBs system is modulated by ovarian hormones. Moreover, early life stress such as maternal deprivation can alter the eCBs system to participate in the motivation and reward for drugs, which significantly downregulates CB1 levels in the male rats’ hippocampus, and upregulates CB1 receptor levels in the female hippocampus (Llorente-Berzal et al., 2013; Reich et al., 2009). In addition, male and female mice bred for high levels of voluntary wheel running differ from one another in response to antagonizing the CB1 receptor. For example, after injecting an antagonist of the CB1 receptor, male mice bred for high levels of voluntary wheel running showed a reduction of wheel revolutions over the entire period (10-120 min post-injection time), whereas their female counterparts showed a similar reduction only 70-120 min after injection (Keeney et al., 2012). This limited circumstantial evidence suggests a possible sex difference in eCBs system.
Recent studies showed that CB1 regulates the functional balance between GABAergic inhibitory and glutamatergic excitatory synaptic inputs into VTA and NAc, which modulates dopaminergic activity to establish the addictive processes (Maldonado et al., 2006; Mereu et al., 2013). Most studies reported that the primary rewarding effects and the motivation to seek different drugs of abuse were attenuated by the inactivation of CB1 (Maldonado et al., 2006; Maldonado et al., 2013). Exercise has also shown to be a positive impact on the eCBs system. For example, a large number of human studies and animal experiments have shown that moderate or high intensity exercises could increase the circulating levels of eCBs, which mediated some physiological and psychological adaptations (i.e., analgesia, sedation and a sense of wellbeing) (Sparling et al., 2003; Dietrich and McDaniel, 2004; Raichlen et al., 2013; Heyman et al., 2012). Animal studies have demonstrated that voluntary wheel running enhances the sensitivity of CB1 receptor stimulation to glutamate and GABA synapses in mice striatum for regulating the central reward pathway, and also increases the levels of CB1 in rat hippocampus for exercise-induced enhancement of progenitor cell proliferation (Hill et al., 2010; De Chiara et al., 2010). Furthermore, endocannabinoid signaling can be regulated by aerobic exercise and contributes to improving spatial memory (Tantimonaco et al., 2014; Ferreira-Vieira et al., 2014). The exercise-induced up-regulation of eCBs signaling involves neurogenesis, anti-apoptosis and synaptic plasticity (Tantimonaco et al., 2014), spatial memory improvement and BDNF expression in the hippocampus (Ferreira-Vieira et al., 2014), with possible implication for reward, depression and mood (Heyman et al., 2012; Tantimonaco et al., 2014). Based on the key role of eCBs in drug addiction, exercise-induced changes of eCBs signaling may contribute to exercise's ability to prevent and treat drug addiction.
5.2.3 Other neurotransmitters and receptors
Besides dopamine and the eCBs neurotransmitters described above, sex differences were also found in the endogenous opioid system (EOS), GABAergic system and glutamatergic system. Recent studies showed that female mice express higher level of dynorphin in striatum (Chen et al., 2009), whereas greater expression of proenkephalin mRNA in striatum and NAc was found in males (Torres-Reveron et al., 2007). Female mice also had higher levels of dynorphin and enkephalin immunoreactivity in the hippocampus compared to males (Van Kempen et al., 2013). The basal levels of dynorphin and enkephalin in the NAc, caudate putamen, and hippocampus varied during various phases of estrous cycle in female mice and rats (Roman et al., 2006; Van Kempen et al., 2013). For example, the levels of dynorphin and enkephalin immunoreactivity in rats are increased when estrogen levels are elevated (Torres-Reveron et al., 2008; Torres-Reveron et al., 2009). In addition, it is well known that women with opioid addiction have worse functional impairment, more psychiatric severity, and higher likelihoods of using opioids to cope with negative affect and pain than men (McHugh et al., 2013; Kennedy et al., 2013). However, animal studies showed that male mice had higher levels of expression of the morphine withdrawal symptoms than females, because GABA agonist baclofen attenuated morphine withdrawal signs observably only in females through reestablishing mu-opioid receptor binding sites (Diaz et al., 2006). In addition, previous evidence showed that GABA(A) receptors subunit genes might preferentially contribute to methamphetamine use disorder in women, but not in men (Lin et al., 2003). On the other hand, a very recent study found that metabotropic glutamate receptor 5 (mGluR5) activation is critical for the estradiol's impact on cocaine-induced behavioral sensitization in female rats (Martinez et al., 2014), suggesting the glutamatergic system may involve different behavioral responses to cocaine between males and females. Investigations also showed that chronic ethanol exposure increased three NMDA receptors subunits (NR1,NR2A and NR2B) levels in male rats hippocampus, but not in females (Devaud and Alele, 2004). These limited studies suggest a possible gender difference in EOS, GABAergic system and glutamatergic system.
In animal studies, addictive drugs increased the release of dopamine directly by activating opiate receptors in the NAc, indirectly inhibiting GABAnergic interneuron or enhancing glutamategic signal in the VTA (Nestler, 2005). These neurotransmitters are also regulated by exercise to improve psychological status and cognitive function related to brain health (Ma, 2008). Among these signal molecules, the endogenous opioid system is a common substrate in drug addiction (Trigo et al., 2010). Long-term active and passive exercise was able to increase the levels of endogenous opiate and their receptors (μ, δ, κ) in rats’ plasma and hippocampus, especially endogenous β-endorphin and its μ receptor (Chen et al., 2007; de Oliveira et al., 2010). A previous study reported that 6-week wheel running exercise reduced the physiological dependence and sensitivity of the central opiate receptor to opiate receptor agonist in rats (Smith and Yancey, 2003). Studies have also shown that forced and voluntary running decrease glutamate concentrations and increase GABA concentrations in the striatum and hippocampus (Jia et al., 2014; Guezennec et al., 1998; Biedermann et al., 2012), reduce the mRNA levels of mGluR5, NMDA and GABAA receptors in a rat model of cerebral ischemia or in a mouse model of Alzheimer's disease (Zhang et al., 2010; Revilla et al., 2014). This raises the possibility that exercise promotes plastic changes in glutamate receptors and GABA receptors. Therefore, exercise may have the ability to reduce drug craving by regulating the endogenous opioid system, and normalizing the levels of glutamate and GABA and their functioning. However, further research is needed to focus on sex differences in EOS, GABAergic system and glutamatergic system between exercise intervention and drug addiction.
5.3 Transcriptional factors
Altered patterns of gene expression are considered a pervasive mechanism by which drugs of abuse can produce a state of addiction. There are two key transcription factors to coordinate gene expression, including CREB and ΔFosB. The sex differences exist in the regulation of gene expression by these transcription factors.
5.3.1 CREB
Much progress has been made in recent years to the examined sex differences in CREB-BDNF signaling. One study showed that there was no sex difference in basal phosphorylation CREB (pCREB) protein levels in the rat NAc, but that cocaine administration-induced elevation of pCREB level in the NAc was higher in females than males (Nazarian et al., 2009). Other research showed that the levels of pCREB in the PFC increased in cocaine treated male rats, but not in female rats, which were positively correlated to CPP scores (Nygard et al., 2013). These conflicting results may be due to different brain regions. Moreover, previous research has found that male mice with deleted BDNF genes in the forebrain exhibit hyperactivity but normal depression-related behaviors, while females display normal locomotor activity but a striking increase in depression-like behavior (Monteggia et al., 2007). There is little research to elaborate the sex differences in other addictive drugs.
Earlier reports have shown that abnormal levels of BDNF within mesolimbic dopaminergic system may progressively facilitate the incubation of drug craving after prolonged withdrawal periods (Grimm et al., 2003; Wang et al., 2013; Ghitza et al., 2010). Both wheel running and treadmill exercise can enhance the activity of CREB to increase mRNA levels of BDNF in the rat hippocampus (Vaynman et al., 2003; Chen and Russo-Neustadt, 2005; Ji et al., 2014). Further research has found that exercise during chronic exposure to morphine ameliorated rats’ cognitive deficits and diminished the severity of dependency by up-regulating CREB-BDNF signaling (Miladi-Gorji et al., 2011). A very exciting line of research uncovered that repeated exposure to cocaine increased BDNF exon IV expression in the PFC. It also stipulated that wheel running during abstinence on subsequent cocaine-seeking can attenuate this increase dose dependently, while the efficacy of exercise was inversely associated with BDNF exon IV expression (Peterson et al., 2013). Furthermore, one human study demonstrated that exercise-induced elevation of serum BDNF concentration was more pronounced in men than in women (Schmidt-Kassow et al., 2012). Differences in CREB-BDNF signaling could partly be due to the regulation of gonadal steroids (e.g., progesterone, estradiol and testosterone) in the peripheral and central nervous system of human subjects and rodents (Pluchino et al., 2013). For example, estradiol could promote pCREB levels (Cheong et al., 2012). In particularly, fluctuating levels of estrogen along with the menstrual cycle affects CREB-BDNF signaling in the PFC and hippocampus, which have been implicated in the regulation of substance use disorders (Luine and Frankfurt, 2013; Carbone and Handa, 2013; Kuipers et al., 2013). In summary, the above results demonstrate that exercise-induced changes of CREB-BDNF signaling are likely to improve some drug addiction-induced deleterious behaviors, and CREB-BDNF signaling is different in males and females that exercise or are addicted to drugs. Whether the drug or exercise related CREB-BDNF signaling could be a sensitive target for developing new intervention on sex-specific treatment feature for drug addiction requires further investigations.
5.3.2 Immediately Early Genes
Among the many immediately early genes (IEGs) known to influence the addiction process, ΔFosB and cFos are classic molecular markers for long-term adaptation in brain reward pathways, especially in the striatum (McClung et al., 2004; Nestler, 2008). In regard to sex differences in Fos family protein, previous studies have shown that male rats expressed significantly higher levels of Fos protein within various brain regions during brain development (Olesen and Auger, 2005). A study using cocaine-induced CPP paradigm showed that female rats had a greater increase in caudate putamen ΔFosB levels than males (Nygard et al., 2013). Moreover, a very recent study revealed that acute administration with methamphetamine caused a greater activation of c-Fos receptor-positive cells in male mice than females in the hippocampus (Zuloaga et al., 2014). Gonadal hormones may be a major factor in these disparities, given that testosterone and estradiol induce Fos gene expression in adult and developmental rat brains, which are mediated by the binding of estrogen receptors (Giannakopoulou et al., 2001; Olesen and Auger, 2005). In addition, some studies have found that exercise-induced estrogen increases in OVX rats contributed to the improvement of emotional and cognitive disorders, which were induced by the lack of estrogen (Ben et al., 2010; Lu et al., 2014). These findings imply that exercise may alter levels of the sex hormones to influence Fos gene expression differently in males and females.
However, there are few studies on the sex differences of IEGs related to exercise. Research has found that chronic voluntary wheel running was rewarding and elevated the protein levels of ΔFosB in rat and mice NAc and striatum, which might reduce the frequency and severity of substance abuse disorders (Werme et al., 2002b; Greenwood et al., 2011). Housing mice in enriched environments with 30-day voluntary wheel running during forced abstinence completely eliminated behavioral sensitization and CPP to cocaine, and dramatically reduced the protein expression of c-Fos in the core of NAc and the infralimbic cortex (Solinas et al., 2008), which was in line with the results from cocaine SA in rats (Thiel et al., 2010). Collectively, these findings demonstrate that exercise may serve as a substitute or competition for drug abuse by changing ΔFosB or cFos immunoreactivity in the reward system to protect against later or previous drug use. Moreover, animals studies showed that sex differences in dopamine receptors and the protein levels of pCREB induced by addictive drugs indirectly influence the expression of Fos protein (Gross and Marshall, 2009), suggesting that sex differences in the transcription factor CREB and neurotransmitters system may contribute to sex differences related to Fos. Thus, sex differences in IEGs are likely to be involved in drug addiction and exercise.
6. Conclusions and future directions
As briefly reviewed above, a large number of human and rodent studies clearly show that there are sex differences in drug addiction and exercise. The sex differences are also found in the effectiveness of exercise on drug addiction prevention and treatment, as well as underlying neurobiological mechanisms. The postulate that exercise serves as an ideal intervention for drug addiction has been widely recognized and used in human and animal rehabilitation. Any sex differences in the efficacy of exercise intervention on drug addiction and rehabilitation remain understudied. Despite a small increase in animal research paying attention to how sex differences in the effectiveness of exercise reduce relapse vulnerability, little information is available to examine whether this effectiveness differs by sex among human subjects. The majority of the current studies were not inclusive and were performed on one gender, specifically males. As a recent article published in Nature by Pollitzer indicated, sex differences exist not only in basic cell biology, but also in clinical research, including research on drug effectiveness and side effects (Pollitzer, 2013). An important survey documenting the ratios of male-only versus female-only studies reported results that range from 3.7:1 in physiology to 5:1 in pharmacology and neuroscience (Beery and Zucker, 2011). Repeated attempts have been made to draw attention to the sex-dependent effects of exercise intervention on drug addiction, but the majority of human and rodent researchers still continue to use males exclusively for drug addiction and exercise studies. Thus, the tendency to ignore sex differences in human and rodent studies may limit the development of effective exercise intervention on drug abuse. Taken together in this review, we specify sex differences in types of various drug addictions, and exercise activities. We first discussed the different effects of active and passive exercise on drug rehabilitation among human and rodent subjects together. Then, we specifically summarized the preventive and therapeutic effects of exercise on drug addiction in human and animal studies. Lastly, we conducted deep speculation on several potential mechanisms that are the foundation for these differences, ranging from diversified molecular events induced by neurotransmitters and their receptors to neuroplasticity and neurogenesis.
To further understand the exercise conditions that most effectively reduce drug craving, we should consider the sex differences in drug addiction and exercise intervention between human and animal addicts. In particular, more studies on the neurobiological mechanism of exercise and its roles in preventing and treating drug addiction are needed.
Highlights.
sex differences in drug addiction from human to animals,
sex differences in exercise-based intervention to prevent and treat drug addiction from human to animal models
potential signal transduction pathways in exercise-based intervention in preventing and treating drug addiction
Acknowledgments
This work was supported by grants from the Shanghai Science and Technology Commission (NO.13490503600-CZ), also National Natural Science Foundation of China (NO.31171004-CZ), the American Health Assistance Foundation (G2006-118-RL), and the National Institutes of Health (R01AG032441-01-RL). We also thank Juliet Shen for editing and proofreading.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aaron DJ, Dearwater SR, Anderson R, Olsen T, Kriska AM, Laporte RE. Physical activity and the initiation of high-risk health behaviors in adolescents. Med Sci Sports Exerc. 1995;27:1639–1645. [PubMed] [Google Scholar]
- Aizawa K, Iemitsu M, Otsuki T, Maeda S, Miyauchi T, Mesaki N. Sex differences in steroidogenesis in skeletal muscle following a single bout of exercise in rats. J Appl Physiol. 2008;104:67–74. doi: 10.1152/japplphysiol.00558.2007. [DOI] [PubMed] [Google Scholar]
- Alaei H, Borjeian L, Azizi M, Orian S, Pourshanazari A, Hanninen O. Treadmill running reverses retention deficit induced by morphine. Eur J Pharmacol. 2006;536:138–141. doi: 10.1016/j.ejphar.2006.02.025. [DOI] [PubMed] [Google Scholar]
- An L, Zhang T. Spatial cognition and sexually dimorphic synaptic plasticity balance impairment in rats with chronic prenatal ethanol exposure. Behav Brain Res. 2013;256:564–574. doi: 10.1016/j.bbr.2013.09.017. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones. Curr Top Behav Neurosci. 2011;8:73–96. doi: 10.1007/7854_2010_93. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Zlebnik NE, Navin SF, Carroll ME. Responding during signaled availability and nonavailability of iv cocaine and food in rats: age and sex differences. Psychopharmacology (Berl) 2011;215:785–799. doi: 10.1007/s00213-011-2181-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SE, Lawson KM, Singleton LM, Brady KT. Characteristics and correlates of men and women with prescription opioid dependence. Addict Behav. 2011;36:829–834. doi: 10.1016/j.addbeh.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CP, Husbands SM. Novel approaches for the treatment of psychostimulant and opioid abuse-focus on opioid receptor-based therapies. Expert opinion on drug discovery. 2014;9:1333–1344. doi: 10.1517/17460441.2014.964203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baler RD, Volkow ND. Drug addiction: the neurobiology of disrupted self-control. Trends Mol Med. 2006;12:559–566. doi: 10.1016/j.molmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Barker J, Galea L. Repeated estradiol administration alters different aspects of neurogenesis and cell death in the hippocampus of female, but not male, rats. Neuroscience. 2008;152:888–902. doi: 10.1016/j.neuroscience.2007.10.071. [DOI] [PubMed] [Google Scholar]
- Barry D, Petry NM. Associations between body mass index and substance use disorders differ by gender: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Addict Behav. 2009;34:51–60. doi: 10.1016/j.addbeh.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth C, Villringer A, Sacher J. Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Frontiers in Neuroscience. 2015;9:37. doi: 10.3389/fnins.2015.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bawor M, Dennis BB, Anglin R, Steiner M, Thabane L, Samaan Z. Sex differences in outcomes of methadone maintenance treatment for opioid addiction: a systematic review protocol. Systematic reviews. 2014;3:45. doi: 10.1186/2046-4053-3-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacology Biochemistry and Behavior. 1999;64:803–812. doi: 10.1016/s0091-3057(99)00168-9. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Perry AN, Westenbroek C. Sex differences in the neural mechanisms mediating addiction: a new synthesis and hypothesis. Biology of sex differences. 2012;3:1–35. doi: 10.1186/2042-6410-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev. 2011;35:565–572. doi: 10.1016/j.neubiorev.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belke TW, Wagner JP. The reinforcing property and the rewarding aftereffect of wheel running in rats: a combination of two paradigms. Behav Processes. 2005;68:165–172. doi: 10.1016/j.beproc.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Ben J, Soares FM, Scherer EB, Cechetti F, Netto CA, Wyse AT. Running exercise effects on spatial and avoidance tasks in ovariectomized rats. Neurobiol Learn Mem. 2010;94:312–317. doi: 10.1016/j.nlm.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Berchtold NC, Kesslak JP, Pike CJ, Adlard PA, Cotman CW. Estrogen and exercise interact to regulate brain-derived neurotrophic factor mRNA and protein expression in the hippocampus. European Journal of Neuroscience. 2001;14:1992–2002. doi: 10.1046/j.0953-816x.2001.01825.x. [DOI] [PubMed] [Google Scholar]
- Biedermann S, Fuss J, Zheng L, Sartorius A, Falfan-Melgoza C, Demirakca T, Gass P, Ende G, Weber-Fahr W. In vivo voxel based morphometry: detection of increased hippocampal volume and decreased glutamate levels in exercising mice. Neuroimage. 2012;61:1206–1212. doi: 10.1016/j.neuroimage.2012.04.010. [DOI] [PubMed] [Google Scholar]
- Bilbao A. The role of the endocannabinoid system in addictive behavior. Addict Biol. 2013;18:904–907. doi: 10.1111/adb.12115. [DOI] [PubMed] [Google Scholar]
- Birdee GS, Cai H, Xiang YB, Yang G, Li H, Gao Y, Zheng W, Shu XO. T'ai chi as exercise among middle-aged and elderly Chinese in urban China. J Altern Complement Med. 2013;19:550–557. doi: 10.1089/acm.2012.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birren JE, Kay H. Swimming speed of the albino rat: I. Age and sex differences. Journal of Gerontology. 1958;13:374–377. doi: 10.1093/geronj/13.4.374. [DOI] [PubMed] [Google Scholar]
- Bobzean SA, Dennis TS, Addison BD, Perrotti LI. Influence of sex on reinstatement of cocaine-conditioned place preference. Brain Res Bull. 2010;83:331–336. doi: 10.1016/j.brainresbull.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Bobzean SA, Denobrega AK, Perrotti LI. Sex differences in the neurobiology of drug addiction. Exp Neurol. 2014;259:64–74. doi: 10.1016/j.expneurol.2014.01.022. [DOI] [PubMed] [Google Scholar]
- Bock BC, Marcus BH, King TK, Borrelli B, Roberts MR. Exercise effects on withdrawal and mood among women attempting smoking cessation. Addict Behav. 1999;24:399–410. doi: 10.1016/s0306-4603(98)00088-4. [DOI] [PubMed] [Google Scholar]
- Bock BC, Morrow KM, Becker BM, Williams DM, Tremont G, Gaskins RB, Jennings E, Fava J, Marcus BH. Yoga as a complementary treatment for smoking cessation: rationale, study design and participant characteristics of the Quitting-in-Balance study. BMC Complement Altern Med. 2010;10:14. doi: 10.1186/1472-6882-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers JM, Waddell J, McCarthy MM. A developmental sex difference in hippocampal neurogenesis is mediated by endogenous oestradiol. Biol Sex Differ. 2010;1:8. doi: 10.1186/2042-6410-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brager AJ, Hammer SB. Impact of wheel running on chronic ethanol intake in aged Syrian hamsters. Physiol Behav. 2012;107:418–423. doi: 10.1016/j.physbeh.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt N, Vierk R, Rune GM. Sexual dimorphism in estrogen-induced synaptogenesis in the adult hippocampus. Int J Dev Biol. 2013;57:351–356. doi: 10.1387/ijdb.120217gr. [DOI] [PubMed] [Google Scholar]
- Brené S, Bjørnebekk A, Åberg E, Mathé AA, Olson L, Werme M. Running is rewarding and antidepressive. Physiology & behavior. 2007;92:136–140. doi: 10.1016/j.physbeh.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Sonntag KC, Andersen SL. Transient D1 dopamine receptor expression on prefrontal cortex projection neurons: relationship to enhanced motivational salience of drug cues in adolescence. J Neurosci. 2008;28:2375–2382. doi: 10.1523/JNEUROSCI.5064-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocardo PS, Boehme F, Patten A, Cox A, Gil-Mohapel J, Christie BR. Anxiety- and depression-like behaviors are accompanied by an increase in oxidative stress in a rat model of fetal alcohol spectrum disorders: Protective effects of voluntary physical exercise. Neuropharmacology. 2012;62:1607–1618. doi: 10.1016/j.neuropharm.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Brotto LA, Barr AM, Gorzalka BB. Sex differences in forced-swim and open-field test behaviours after chronic administration of melatonin. Eur J Pharmacol. 2000;402:87–93. doi: 10.1016/s0014-2999(00)00491-x. [DOI] [PubMed] [Google Scholar]
- Brown DA, Johnson MS, Armstrong CJ, Lynch JM, Caruso NM, Ehlers LB, Fleshner M, Spencer RL, Moore RL. Short-term treadmill running in the rat: what kind of stressor is it? Journal of Applied Physiology. 2007;103:1979–1985. doi: 10.1152/japplphysiol.00706.2007. [DOI] [PubMed] [Google Scholar]
- Burling TA, Seidner AL, Robbins-Sisco D, Krinsky A, Hanser SB. Batter up! Relapse prevention for homeless veteran substance abusers via softball team participation. Journal of Substance Abuse. 1992;4:407–413. doi: 10.1016/0899-3289(92)90047-2. [DOI] [PubMed] [Google Scholar]
- Carbone DL, Handa RJ. Sex and stress hormone influences on the expression and activity of brain-derived neurotrophic factor. Neuroscience. 2013;239:295–303. doi: 10.1016/j.neuroscience.2012.10.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Anker JJ. Sex differences and ovarian hormones in animal models of drug dependence. Horm Behav. 2010;58:44–56. doi: 10.1016/j.yhbeh.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Castelli MP, Fadda P, Casu A, Spano MS, Casti A, Fratta W, Fattore L. Male and female rats differ in brain cannabinoid CB1 receptor density and function and in behavioural traits predisposing to drug addiction: effect of ovarian hormones. Curr Pharm Des. 2014;20:2100–2113. doi: 10.2174/13816128113199990430. [DOI] [PubMed] [Google Scholar]
- Charilaou M, Karekla M, Constantinou M, Price S. Relationship between physical activity and type of smoking behavior among adolescents and young adults in Cyprus. Nicotine Tob Res. 2009;11:969–976. doi: 10.1093/ntr/ntp096. [DOI] [PubMed] [Google Scholar]
- Chauvet C, Goldberg SR, Jaber M, Solinas M. Effects of environmental enrichment on the incubation of cocaine craving. Neuropharmacology. 2012;63:635–641. doi: 10.1016/j.neuropharm.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvet C, Lardeux V, Jaber M, Solinas M. Brain regions associated with the reversal of cocaine conditioned place preference by environmental enrichment. Neuroscience. 2011;184:88–96. doi: 10.1016/j.neuroscience.2011.03.068. [DOI] [PubMed] [Google Scholar]
- Chaya MS, Kurpad AV, Nagendra HR, Nagarathna R. The effect of long term combined yoga practice on the basal metabolic rate of healthy adults. BMC Complement Altern Med. 2006;6:28. doi: 10.1186/1472-6882-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Yang Y, Yeh T, Cherng C, Hsu H, Hsiao S, Yu L. Methamphetamine-induced conditioned place preference is facilitated by estradiol pretreatment in female mice. Chinese Journal of Physiology. 2003;46:169–174. [PubMed] [Google Scholar]
- Chen HI, Kuo YM, Liao CH, Jen CJ, Huang AM, Cherng CG, Su SW, Yu L. Long-term compulsive exercise reduces the rewarding efficacy of 3,4-methylenedioxymethamphetamine. Behav Brain Res. 2008;187:185–189. doi: 10.1016/j.bbr.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Chen J-X, Zhao X, Yue G-X, Wang Z-F. Influence of acute and chronic treadmill exercise on rat plasma lactate and brain NPY, L-ENK, DYN A1–13. Cellular and molecular neurobiology. 2007;27:1–10. doi: 10.1007/s10571-006-9110-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KW, Comerford A, Shinnick P, Ziedonis DM. Introducing qigong meditation into residential addiction treatment: a pilot study where gender makes a difference. J Altern Complement Med. 2010;16:875–882. doi: 10.1089/acm.2009.0443. [DOI] [PubMed] [Google Scholar]
- Chen MJ, Russo-Neustadt AA. Exercise activates the phosphatidylinositol 3-kinase pathway. Molecular brain research. 2005;135:181–193. doi: 10.1016/j.molbrainres.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Chen X, Grisham W, Arnold AP. X chromosome number causes sex differences in gene expression in adult mouse striatum. European Journal of Neuroscience. 2009;29:768–776. doi: 10.1111/j.1460-9568.2009.06610.x. [DOI] [PubMed] [Google Scholar]
- Cheong RY, Kwakowsky A, Barad Z, Porteous R, Herbison AE, Ábrahám IM. Estradiol acts directly and indirectly on multiple signaling pathways to phosphorylate cAMP-response element binding protein in GnRH neurons. Endocrinology. 2012;153:3792–3803. doi: 10.1210/en.2012-1232. [DOI] [PubMed] [Google Scholar]
- Chow C, Epp JR, Lieblich SE, Barha CK, Galea LA. Sex differences in neurogenesis and activation of new neurons in response to spatial learning and memory. Psychoneuroendocrinology. 2013;38:1236–1250. doi: 10.1016/j.psyneuen.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Collingwood TR, Sunderlin J, Reynolds R, Kohl HW. Physical training as a substance abuse prevention intervention for youth. Journal of drug education. 2000;30:435–452. doi: 10.2190/RVUE-9XW7-TYRQ-EJR8. [DOI] [PubMed] [Google Scholar]
- Correia CJ, Benson TA, Carey KB. Decreased substance use following increases in alternative behaviors: a preliminary investigation. Addict Behav. 2005;30:19–27. doi: 10.1016/j.addbeh.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Hunter RG, Carroll ME. Wheel-running attenuates intravenous cocaine self-administration in rats: sex differences. Pharmacology Biochemistry and Behavior. 2002;73:663–671. doi: 10.1016/s0091-3057(02)00853-5. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC, Christie L-A. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Craft RM, Marusich JA, Wiley JL. Sex differences in cannabinoid pharmacology: A reflection of differences in the endocannabinoid system? Life sciences. 2013;92:476–481. doi: 10.1016/j.lfs.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins ED, Griffin SB, Burgess KC, Peterson DJ, Watson BD, Buendia MA, Stanwood GD, Brown RW. Methylphenidate place conditioning in adolescent rats: an analysis of sex differences and the dopamine transporter. Behav Brain Res. 2013;257:215–223. doi: 10.1016/j.bbr.2013.09.036. [DOI] [PubMed] [Google Scholar]
- Darlington TM, McCarthy RD, Cox RJ, Ehringer MA. Mesolimbic transcriptional response to hedonic substitution of voluntary exercise and voluntary ethanol consumption. Behav Brain Res. 2014;259:313–320. doi: 10.1016/j.bbr.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Chiara V, Errico F, Musella A, Rossi S, Mataluni G, Sacchetti L, Siracusano A, Castelli M, Cavasinni F, Bernardi G. Voluntary exercise and sucrose consumption enhance cannabinoid CB1 receptor sensitivity in the striatum. Neuropsychopharmacology. 2010;35:374. doi: 10.1038/npp.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira MS, da Silva Fernandes MJ, Scorza FA, Persike DS, Scorza CA, da Ponte JB, de Albuquerque M, Cavalheiro EA, Arida RM. Acute and chronic exercise modulates the expression of MOR opioid receptors in the hippocampal formation of rats. Brain Res Bull. 2010;83:278–283. doi: 10.1016/j.brainresbull.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Deaner RO, Geary DC, Puts DA, Ham SA, Kruger J, Fles E, Winegard B, Grandis T. A sex difference in the predisposition for physical competition: males play sports much more than females even in the contemporary U.S. PloS one. 2012;7:e49168. doi: 10.1371/journal.pone.0049168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deaner RO, Smith BA. Sex differences in sports across 50 societies. Cross-Cultural Research. 2013;47:268–309. [Google Scholar]
- Devaud LL, Alele P. Differential effects of chronic ethanol administration and withdrawal on gamma-aminobutyric acid type A and NMDA receptor subunit proteins in male and female rat brain. Alcohol Clin Exp Res. 2004;28:957–965. doi: 10.1097/01.alc.0000128225.83916.40. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Walls SA, McCulley WD, 3rd, Rosenwasser AM. Voluntary wheel running attenuates ethanol withdrawal-induced increases in seizure susceptibility in male and female rats. Pharmacol Biochem Behav. 2012;103:18–25. doi: 10.1016/j.pbb.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz SL, Barros VG, Antonelli MC, Rubio MC, Balerio GN. Morphine withdrawal syndrome and its prevention with baclofen: Autoradiographic study of mu-opioid receptors in prepubertal male and female mice. Synapse. 2006;60:132–140. doi: 10.1002/syn.20279. [DOI] [PubMed] [Google Scholar]
- Dietrich A, McDaniel W. Endocannabinoids and exercise. Br J Sports Med. 2004;38:536–541. doi: 10.1136/bjsm.2004.011718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolezal BA, Chudzynski J, Storer TW, Abrazado M, Penate J, Mooney L, Dickerson D, Rawson RA, Cooper CB. Eight weeks of exercise training improves fitness measures in methamphetamine-dependent individuals in residential treatment. J Addict Med. 2013;7:122–128. doi: 10.1097/ADM.0b013e318282475e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran N. Sex differences in smoking cue reactivity: Craving, negative affect, and preference for immediate smoking. The American Journal on Addictions. 2013;23:211–217. doi: 10.1111/j.1521-0391.2014.12094.x. [DOI] [PubMed] [Google Scholar]
- Duchesne A, Dufresne MM, Sullivan RM. Sex differences in corticolimbic dopamine and serotonin systems in the rat and the effect of postnatal handling. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2009;33:251–261. doi: 10.1016/j.pnpbp.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Ehringer MA, Hoft NR, Zunhammer M. Reduced alcohol consumption in mice with access to a running wheel. Alcohol. 2009;43:443–452. doi: 10.1016/j.alcohol.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Eikelboom R, Mills R. A microanalysis of wheel running in male and female rats. Physiology & behavior. 1988;43:625–630. doi: 10.1016/0031-9384(88)90217-x. [DOI] [PubMed] [Google Scholar]
- Eisenstein SA, Holmes PV. Chronic and voluntary exercise enhances learning of conditioned place preference to morphine in rats. Pharmacol Biochem Behav. 2007;86:607–615. doi: 10.1016/j.pbb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- El Rawas R, Thiriet N, Lardeux V, Jaber M, Solinas M. Environmental enrichment decreases the rewarding but not the activating effects of heroin. Psychopharmacology (Berl) 2009;203:561–570. doi: 10.1007/s00213-008-1402-6. [DOI] [PubMed] [Google Scholar]
- Engelmann AJ, Aparicio MB, Kim A, Sobieraj JC, Yuan CJ, Grant Y, Mandyam CD. Chronic wheel running reduces maladaptive patterns of methamphetamine intake: regulation by attenuation of methamphetamine-induced neuronal nitric oxide synthase. Brain Struct Funct. 2014;219:657–672. doi: 10.1007/s00429-013-0525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English AW, Wilhelm JC, Sabatier MJ. Enhancing recovery from peripheral nerve injury using treadmill training. Ann Anat. 2011;193:354–361. doi: 10.1016/j.aanat.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobedo LG, Marcus SE, Holtzman D, Giovino GA. Sports participation, age at smoking initiation, and the risk of smoking among US high school students. JAMA. 1993;269:1391–1395. [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Does the response to cocaine differ as a function of sex or hormonal status in human and non-human primates? Horm Behav. 2010;58:13–21. doi: 10.1016/j.yhbeh.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadaei A, Gorji HM, Hosseini SM. Swimming reduces the severity of physical and psychological dependence and voluntary morphine consumption in morphine dependent rats. Eur J Pharmacol. 2015;747:88–95. doi: 10.1016/j.ejphar.2014.11.042. [DOI] [PubMed] [Google Scholar]
- Farmer J, Zhao X. v., Van Praag H, Wodtke K, Gage F, Christie B. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male sprague–dawley rats in vivo. Neuroscience. 2004;124:71–79. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Fattore L, Altea S, Fratta W. Sex differences in drug addiction: a review of animal and human studies. Womens Health (Lond Engl) 2008;4:51–65. doi: 10.2217/17455057.4.1.51. [DOI] [PubMed] [Google Scholar]
- Fattore L, Fadda P, Fratta W. Sex differences in the self-administration of cannabinoids and other drugs of abuse. Psychoneuroendocrinology. 2009;34:S227–236. doi: 10.1016/j.psyneuen.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Fattore L, Fratta W. How important are sex differences in cannabinoid action? Br J Pharmacol. 2010;160:544–548. doi: 10.1111/j.1476-5381.2010.00776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Spano M, Altea S, Angius F, Fadda P, Fratta W. Cannabinoid self-administration in rats: sex differences and the influence of ovarian function. Br J Pharmacol. 2007;152:795–804. doi: 10.1038/sj.bjp.0707465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Spano M, Altea S, Fadda P, Fratta W. Drug-and cue-induced reinstatement of cannabinoid-seeking behaviour in male and female rats: influence of ovarian hormones. Br J Pharmacol. 2010;160:724–735. doi: 10.1111/j.1476-5381.2010.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. Systems level neuroplasticity in drug addiction. Cold Spring Harb Perspect Med. 2013;3:a011916. doi: 10.1101/cshperspect.a011916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira-Vieira TH, Bastos CP, Pereira GS, Moreira FA, Massensini AR. A role for the endocannabinoid system in exercise-induced spatial memory enhancement in mice. Hippocampus. 2014;24:79–88. doi: 10.1002/hipo.22206. [DOI] [PubMed] [Google Scholar]
- Festa ED, Russo SJ, Gazi FM, Niyomchai T, Kemen LM, Lin SN, Foltz R, Jenab S, Quinones-Jenab V. Sex differences in cocaine-induced behavioral responses, pharmacokinetics, and monoamine levels. Neuropharmacology. 2004;46:672–687. doi: 10.1016/j.neuropharm.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Foley TE, Fleshner M. Neuroplasticity of dopamine circuits after exercise: implications for central fatigue. Neuromolecular medicine. 2008;10:67–80. doi: 10.1007/s12017-008-8032-3. [DOI] [PubMed] [Google Scholar]
- Fontes-Ribeiro C, Marques E, Pereira F, Silva A, Macedo TA. May exercise prevent addiction? Current neuropharmacology. 2011;9:45. doi: 10.2174/157015911795017380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlano PM, Woolley CS. Quantitative analysis of pre-and postsynaptic sex differences in the nucleus accumbens. Journal of Comparative Neurology. 2010;518:1330–1348. doi: 10.1002/cne.22279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Sinha R. Sex differences in drug-related stress-system changes: implications for treatment in substance-abusing women. Harvard review of psychiatry. 2009;17:103–119. doi: 10.1080/10673220902899680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franconi F, Campesi I, Occhioni S, Antonini P, Murphy MF. Sex and gender in adverse drug events, addiction, and placebo. Handb Exp Pharmacol. 2012;214:107–126. doi: 10.1007/978-3-642-30726-3_6. [DOI] [PubMed] [Google Scholar]
- Friemel CM, Zimmer A, Schneider M. The CB1 Receptor as an Important Mediator of Hedonic Reward Processing. Neuropsychopharmacology. 2014;39:2387–2396. doi: 10.1038/npp.2014.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea L, Leuner B, Slattery D. Hippocampal plasticity during the peripartum period: influence of sex steroids, stress and ageing. Journal of neuroendocrinology. 2014;26:641–648. doi: 10.1111/jne.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea LA. Gonadal hormone modulation of neurogenesis in the dentate gyrus of adult male and female rodents. Brain Res Rev. 2008;57:332–341. doi: 10.1016/j.brainresrev.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Galea LA, Wainwright SR, Roes MM, Duarte-Guterman P, Chow C, Hamson DK. Sex, Hormones and Neurogenesis in the Hippocampus: Hormonal Modulation of Neurogenesis and Potential Functional Implications. Journal of neuroendocrinology. 2013;25:1039–1061. doi: 10.1111/jne.12070. [DOI] [PubMed] [Google Scholar]
- Geuzaine A, Tirelli E. Wheel-running mitigates psychomotor sensitization initiation but not post-sensitization conditioned activity and conditioned place preference induced by cocaine in mice. Behav Brain Res. 2014;262:57–67. doi: 10.1016/j.bbr.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Zhai H, Wu P, Airavaara M, Shaham Y, Lu L. Role of BDNF and GDNF in drug reward and relapse: a review. Neuroscience & Biobehavioral Reviews. 2010;35:157–171. doi: 10.1016/j.neubiorev.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakopoulou M, Bozas E, Philippidis H, Stylianopoulou F. Protooncogene c-fos involvement in the molecular mechanism of rat brain sexual differentiation. Neuroendocrinology. 2001;73:387–396. doi: 10.1159/000054657. [DOI] [PubMed] [Google Scholar]
- Gioiosa L, Chen X, Watkins R, Klanfer N, Bryant CD, Evans CJ, Arnold AP. Sex chromosome complement affects nociception in tests of acute and chronic exposure to morphine in mice. Horm Behav. 2008;53:124–130. doi: 10.1016/j.yhbeh.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Hillman C. The influence of exercise on cognitive abilities. Compr Physiol. 2013;3:403–428. doi: 10.1002/cphy.c110063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Zhuang Y, Feng J, Ying Z, Fan G. Exercise impacts brain-derived neurotrophic factor plasticity by engaging mechanisms of epigenetic regulation. Eur J Neurosci. 2011;33:383–390. doi: 10.1111/j.1460-9568.2010.07508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorton LM, Vuckovic MG, Vertelkina N, Petzinger GM, Jakowec MW, Wood RI. Exercise effects on motor and affective behavior and catecholamine neurochemistry in the MPTP-lesioned mouse. Behav Brain Res. 2010;213:253–262. doi: 10.1016/j.bbr.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothe NP, Kramer AF, McAuley E. The Effects of an 8-Week Hatha Yoga Intervention on Executive Function in Older Adults. J Gerontol A Biol Sci Med Sci. 2014;69:1109–1116. doi: 10.1093/gerona/glu095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield SF, Brooks AJ, Gordon SM, Green CA, Kropp F, McHugh RK, Lincoln M, Hien D, Miele GM. Substance abuse treatment entry, retention, and outcome in women: A review of the literature. Drug Alcohol Depend. 2007;86:1–21. doi: 10.1016/j.drugalcdep.2006.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Le TV, Strong PV, Loughridge AB, Day HE, Fleshner M. Long-term voluntary wheel running is rewarding and produces plasticity in the mesolimbic reward pathway. Behav Brain Res. 2011;217:354–362. doi: 10.1016/j.bbr.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su T-P, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. The Journal of Neuroscience. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross NB, Marshall JF. Striatal dopamine and glutamate receptors modulate methamphetamine-induced cortical Fos expression. Neuroscience. 2009;161:1114–1125. doi: 10.1016/j.neuroscience.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guezennec CY, Abdelmalki A, Serrurier B, Merino D, Bigard X, Berthelot M, Pierard C, Peres M. Effects of prolonged exercise on brain ammonia and amino acids. Int J Sports Med. 1998;19:323–327. doi: 10.1055/s-2007-971925. [DOI] [PubMed] [Google Scholar]
- Hallgren M, Romberg K, Bakshi AS, Andreasson S. Yoga as an adjunct treatment for alcohol dependence: A pilot study. Complement Ther Med. 2014;22:441–445. doi: 10.1016/j.ctim.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Hammer SB, Ruby CL, Brager AJ, Prosser RA, Glass JD. Environmental modulation of alcohol intake in hamsters: effects of wheel running and constant light exposure. Alcohol Clin Exp Res. 2010;34:1651–1658. doi: 10.1111/j.1530-0277.2010.01251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa RJ, Burgess LH, Kerr JE, O'Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav. 1994;28:464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- Harris RC, Wise JA, Price KA, Kim HJ, Kim CK, Sale C. Determinants of muscle carnosine content. Amino Acids. 2012;43:5–12. doi: 10.1007/s00726-012-1233-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi Nosrat Abadi T, Vaghef L, Babri S, Mahmood-Alilo M, Beirami M. Effects of different exercise protocols on ethanol-induced spatial memory impairment in adult male rats. Alcohol. 2013;47:309–316. doi: 10.1016/j.alcohol.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Henchoz Y, Baggio S, N'Goran A A, Studer J, Deline S, Mohler-Kuo M, Daeppen JB, Gmel G. Health impact of sport and exercise in emerging adult men: a prospective study. Qual Life Res. 2014a;23:2225–2234. doi: 10.1007/s11136-014-0665-0. [DOI] [PubMed] [Google Scholar]
- Henchoz Y, Dupuis M, Deline S, Studer J, Baggio S, N'Goran AA, Daeppen JB, Gmel G. Associations of physical activity and sport and exercise with at-risk substance use in young men: A longitudinal study. Prev Med. 2014b;64:27–31. doi: 10.1016/j.ypmed.2014.03.022. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Cohen C, Heishman SJ. Drug self-administration methods in abuse liability evaluation. British journal of addiction. 1991;86:1571–1577. doi: 10.1111/j.1360-0443.1991.tb01750.x. [DOI] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Rounsaville BJ, Kranzler HR. Opioid-, cannabis-and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug Alcohol Depend. 2004;74:265–272. doi: 10.1016/j.drugalcdep.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Heyman E, Gamelin F-X, Goekint M, Piscitelli F, Roelands B, Leclair E, Di Marzo V, Meeusen R. Intense exercise increases circulating endocannabinoid and BDNF levels in humans—Possible implications for reward and depression. Psychoneuroendocrinology. 2012;37:844–851. doi: 10.1016/j.psyneuen.2011.09.017. [DOI] [PubMed] [Google Scholar]
- Hicks AL, Kent-Braun J, Ditor DS. Sex differences in human skeletal muscle fatigue. Exerc Sport Sci Rev. 2001;29:109–112. doi: 10.1097/00003677-200107000-00004. [DOI] [PubMed] [Google Scholar]
- Hilderbrand ER, Lasek AW. Sex differences in cocaine conditioned place preference in C57BL/6J mice. Neuroreport. 2014;25:105–109. doi: 10.1097/WNR.0000000000000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill DW, Smith JC. Gender difference in anaerobic capacity: role of aerobic contribution. Br J Sports Med. 1993;27:45–48. doi: 10.1136/bjsm.27.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill DW, Vingren JL. Effects of exercise mode and participant sex on measures of anaerobic capacity. J Sports Med Phys Fitness. 2014;54:255–263. [PubMed] [Google Scholar]
- Hill MN, Titterness AK, Morrish AC, Carrier EJ, Lee TTY, Gil-Mohapel J, Gorzalka BB, Hillard CJ, Christie BR. Endogenous cannabinoid signaling is required for voluntary exercise-induced enhancement of progenitor cell proliferation in the hippocampus. Hippocampus. 2010;20:513–523. doi: 10.1002/hipo.20647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn K, Dino G, Branstetter SA, Zhang J, Noerachmanto N, Jarrett T, Taylor M. Effects of physical activity on teen smoking cessation. Pediatrics. 2011;128:e801–811. doi: 10.1542/peds.2010-2599. [DOI] [PubMed] [Google Scholar]
- Hosseini M, Alaei HA, Naderi A, Sharifi MR, Zahed R. Treadmill exercise reduces self-administration of morphine in male rats. Pathophysiology. 2009;16:3–7. doi: 10.1016/j.pathophys.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Howden EJ, Perhonen M, Peshock RM, Zhang R, Arbab-Zadeh A, Adams-Huet B, Levine BD. Females have a blunted cardiovascular response to 1-year of intensive supervised endurance training. Journal of Applied Physiology. 2015 Apr 30; doi: 10.1152/japplphysiol.00092.2015. 2015 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignjatova L, Raleva M. Gender difference in the treatment outcome of patients served in the mixed-gender program. Bratisl Lek Listy. 2009;110:285–289. [PubMed] [Google Scholar]
- Ikemoto S, Bonci A. Neurocircuitry of drug reward. Neuropharmacology. 2014;76:329–341. doi: 10.1016/j.neuropharm.2013.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology. 2006;31:129–138. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- Jacobs E, D'Esposito M. Estrogen shapes dopamine-dependent cognitive processes: implications for women's health. The Journal of Neuroscience. 2011;31:5286–5293. doi: 10.1523/JNEUROSCI.6394-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J.-f., Ji S.-j., Sun R, Li K, Zhang Y, Zhang L.-y., Tian Y. Forced running exercise attenuates hippocampal neurogenesis impairment and the neurocognitive deficits induced by whole-brain irradiation via the BDNF-mediated pathway. Biochem Biophys Res Commun. 2014;443:646–651. doi: 10.1016/j.bbrc.2013.12.031. [DOI] [PubMed] [Google Scholar]
- Jia LL, Kang YM, Wang FX, Li HB, Zhang Y, Yu XJ, Qi J, Suo YP, Tian ZJ, Zhu Z, Zhu GQ, Qin DN. Exercise training attenuates hypertension and cardiac hypertrophy by modulating neurotransmitters and cytokines in hypothalamic paraventricular nucleus. PloS one. 2014;9:e85481. doi: 10.1371/journal.pone.0085481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, O'Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Kanarek RB, D'Anci KE, Jurdak N, Mathes WF. Running and addiction: precipitated withdrawal in a rat model of activity-based anorexia. Behav Neurosci. 2009;123:905–912. doi: 10.1037/a0015896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nature Reviews Neuroscience. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kay H, Birren JE. Swimming speed of the albino rat: II. Fatigue, practice and drug effects on age and sex differences. Journal of Gerontology. 1958;13:378–385. doi: 10.1093/geronj/13.4.378. [DOI] [PubMed] [Google Scholar]
- Keeney BK, Meek TH, Middleton KM, Holness LF, Garland T., Jr. Sex differences in cannabinoid receptor-1 (CB1) pharmacology in mice selectively bred for high voluntary wheel-running behavior. Pharmacol Biochem Behav. 2012;101:528–537. doi: 10.1016/j.pbb.2012.02.017. [DOI] [PubMed] [Google Scholar]
- Kennedy AP, Epstein DH, Phillips KA, Preston KL. Sex differences in cocaine/heroin users: drug-use triggers and craving in daily life. Drug Alcohol Depend. 2013;132:29–37. doi: 10.1016/j.drugalcdep.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstetter KA, Aguilar VR, Parrish AB, Kippin TE. Protracted time-dependent increases in cocaine-seeking behavior during cocaine withdrawal in female relative to male rats. Psychopharmacology (Berl) 2008;198:63–75. doi: 10.1007/s00213-008-1089-8. [DOI] [PubMed] [Google Scholar]
- Kinnunen T, Leeman RF, Korhonen T, Quiles ZN, Terwal DM, Garvey AJ, Hartley HL. Exercise as an adjunct to nicotine gum in treating tobacco dependence among women. Nicotine Tob Res. 2008;10:689–703. doi: 10.1080/14622200801979043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchengast S. Human sexual dimorphism--a sex and gender perspective. Anthropol Anz. 2014;71:123–133. doi: 10.1127/0003-5548/2014/0376. [DOI] [PubMed] [Google Scholar]
- Kissin WB, Tang Z, Campbell KM, Claus RE, Orwin RG. Gender-sensitive substance abuse treatment and arrest outcomes for women. J Subst Abuse Treat. 2014;46:332–339. doi: 10.1016/j.jsat.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konhilas JP, Maass AH, Luckey SW, Stauffer BL, Olson EN, Leinwand LA. Sex modifies exercise and cardiac adaptation in mice. Am J Physiol Heart Circ Physiol. 2004;287:H2768–2776. doi: 10.1152/ajpheart.00292.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T, Goodwin A, Miesmaa P, Dupuis EA, Kinnunen T. Smoking cessation program with exercise improves cardiovascular disease biomarkers in sedentary women. J Womens Health (Larchmt) 2011;20:1051–1064. doi: 10.1089/jwh.2010.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T, Kujala UM, Rose RJ, Kaprio J. Physical activity in adolescence as a predictor of alcohol and illicit drug use in early adulthood: a longitudinal population-based twin study. Twin Res Hum Genet. 2009;12:261–268. doi: 10.1375/twin.12.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuipers SD, Trentani A, van der Zee EA, den Boer JA. Chronic stress-induced changes in the rat brain: Role of sex differences and effects of long-term tianeptine treatment. Neuropharmacology. 2013;75:426–436. doi: 10.1016/j.neuropharm.2013.08.018. [DOI] [PubMed] [Google Scholar]
- Kulig K, Brener ND, McManus T. Sexual activity and substance use among adolescents by category of physical activity plus team sports participation. Arch Pediatr Adolesc Med. 2003;157:905–912. doi: 10.1001/archpedi.157.9.905. [DOI] [PubMed] [Google Scholar]
- López HH. Cannabinoid–hormone interactions in the regulation of motivational processes. Horm Behav. 2010;58:100–110. doi: 10.1016/j.yhbeh.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Larson EB, Carroll ME. Wheel running as a predictor of cocaine self-administration and reinstatement in female rats. Pharmacol Biochem Behav. 2005;82:590–600. doi: 10.1016/j.pbb.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Leasure JL, Jones M. Forced and voluntary exercise differentially affect brain and behavior. Neuroscience. 2008;156:456–465. doi: 10.1016/j.neuroscience.2008.07.041. [DOI] [PubMed] [Google Scholar]
- Leasure JL, Nixon K. Exercise neuroprotection in a rat model of binge alcohol consumption. Alcohol Clin Exp Res. 2010;34:404–414. doi: 10.1111/j.1530-0277.2009.01105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TTY, Wainwright SR, Hill MN, Galea LA, Gorzalka BB. Sex, drugs, and adult neurogenesis: Sex-dependent effects of escalating adolescent cannabinoid exposure on adult hippocampal neurogenesis, stress reactivity, and amphetamine sensitization. Hippocampus. 2014;24:280–292. doi: 10.1002/hipo.22221. [DOI] [PubMed] [Google Scholar]
- Lenoir M, Starosciak AK, Ledon J, Booth C, Zakharova E, Wade D, Vignoli B, Izenwasser S. Sex differences in conditioned nicotine reward are age-specific. Pharmacology Biochemistry and Behavior. 2015;132:56–62. doi: 10.1016/j.pbb.2015.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshner AI. Addiction is a brain disease, and it matters. Science. 1997;278:45–47. doi: 10.1126/science.278.5335.45. [DOI] [PubMed] [Google Scholar]
- Lett BT, Grant VL, Koh MT, Flynn G. Prior experience with wheel running produces cross-tolerance to the rewarding effect of morphine. Pharmacology Biochemistry and Behavior. 2002;72:101–105. doi: 10.1016/s0091-3057(01)00722-5. [DOI] [PubMed] [Google Scholar]
- Levy N. Addiction is Not a Brain Disease (and it Matters). Front Psychiatry. 2013;4:24. doi: 10.3389/fpsyt.2013.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyton M, Vezina P. Dopamine ups and downs in vulnerability to addictions: a neurodevelopmental model. Trends Pharmacol Sci. 2014;35:268–276. doi: 10.1016/j.tips.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DX, Zhuang XY, Zhang YP, Guo H, Wang Z, Zhang Q, Feng YM, Yao YG. Effects of Tai Chi on the protracted abstinence syndrome: a time trial analysis. Am J Chin Med. 2013;41:43–57. doi: 10.1142/S0192415X13500043. [DOI] [PubMed] [Google Scholar]
- Li J, Siegel M, Yuan M, Zeng Z, Finnucan L, Persky R, Hurn PD, McCullough LD. Estrogen enhances neurogenesis and behavioral recovery after stroke. Journal of Cerebral Blood Flow & Metabolism. 2011;31:413–425. doi: 10.1038/jcbfm.2010.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Chen K, Mo Z. Use of qigong therapy in the detoxification of heroin addicts. Altern Ther Health Med. 2002;8:50–59. [PubMed] [Google Scholar]
- Lightfoot JT. Sex hormones’ regulation of rodent physical activity: a review. International journal of biological sciences. 2008;4:126. doi: 10.7150/ijbs.4.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SK, Chen CK, Ball D, Liu HC, Loh EW. Gender-specific contribution of the GABA(A) subunit genes on 5q33 in methamphetamine use disorder. Pharmacogenomics J. 2003;3:349–355. doi: 10.1038/sj.tpj.6500203. [DOI] [PubMed] [Google Scholar]
- Linke SE, Rutledge T, Myers MG. Intermittent exercise in response to cigarette cravings in the context of an Internet-based smoking cessation program. Ment Health Phys Act. 2012;5:85–92. doi: 10.1016/j.mhpa.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorente-Berzal A, Assis MA, Rubino T, Zamberletti E, Marco EM, Parolaro D, Ambrosio E, Viveros MP. Sex-dependent changes in brain CB1R expression and functionality and immune CB2R expression as a consequence of maternal deprivation and adolescent cocaine exposure. Pharmacol Res. 2013;74:23–33. doi: 10.1016/j.phrs.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Lu J, Xu Y, Hu W, Gao Y, Ni X, Sheng H, Liu Y. Exercise ameliorates depression-like behavior and increases hippocampal BDNF level in ovariectomized rats. Neurosci Lett. 2014;573:13–18. doi: 10.1016/j.neulet.2014.04.053. [DOI] [PubMed] [Google Scholar]
- Luine V, Frankfurt M. Interactions between estradiol, BDNF and dendritic spines in promoting memory. Neuroscience. 2013;239:34–45. doi: 10.1016/j.neuroscience.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ. Sex differences in vulnerability to drug self-administration. Exp Clin Psychopharmacol. 2006;14:34–41. doi: 10.1037/1064-1297.14.1.34. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology (Berl) 1999;144:77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Reinstatement of cocaine self-administration in rats: sex differences. Psychopharmacology (Berl) 2000;148:196–200. doi: 10.1007/s002130050042. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Peterson AB, Sanchez V, Abel J, Smith MA. Exercise as a novel treatment for drug addiction: A neurobiological and stage-dependent hypothesis. Neurosci Biobehav Rev. 2013;37:1622–1644. doi: 10.1016/j.neubiorev.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Piehl KB, Acosta G, Peterson AB, Hemby SE. Aerobic exercise attenuates reinstatement of cocaine-seeking behavior and associated neuroadaptations in the prefrontal cortex. Biol Psychiatry. 2010;68:774–777. doi: 10.1016/j.biopsych.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology (Berl) 2002;164:121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Ma Q. Beneficial effects of moderate voluntary physical exercise and its biological mechanisms on brain health. Neurosci Bull. 2008;24:265–270. doi: 10.1007/s12264-008-0402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Hamadeh MJ, Christie BR, Foster JA, Tarnopolsky MA. Impact of treadmill running and sex on hippocampal neurogenesis in the mouse model of amyotrophic lateral sclerosis. PloS one. 2012;7:e36048. doi: 10.1371/journal.pone.0036048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado R, Robledo P, Berrendero F. Endocannabinoid system and drug addiction: new insights from mutant mice approaches. Curr Opin Neurobiol. 2013;23:480–486. doi: 10.1016/j.conb.2013.02.004. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Valverde O, Berrendero F. Involvement of the endocannabinoid system in drug addiction. Trends Neurosci. 2006;29:225–232. doi: 10.1016/j.tins.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Man DW, Tsang WW, Hui-Chan CW. Do older t'ai chi practitioners have better attention and memory function? J Altern Complement Med. 2010;16:1259–1264. doi: 10.1089/acm.2009.0462. [DOI] [PubMed] [Google Scholar]
- Mandyam CD, Koob GF. The addicted brain craves new neurons: putative role for adult-born progenitors in promoting recovery. Trends Neurosci. 2012;35:250–260. doi: 10.1016/j.tins.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandyam CD, Wee S, Eisch AJ, Richardson HN, Koob GF. Methamphetamine self-administration and voluntary exercise have opposing effects on medial prefrontal cortex gliogenesis. The Journal of Neuroscience. 2007;27:11442–11450. doi: 10.1523/JNEUROSCI.2505-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus BH, Albrecht AE, King TK, Parisi AF, Pinto BM, Roberts M, Niaura RS, Abrams DB. The efficacy of exercise as an aid for smoking cessation in women: a randomized controlled trial. Arch Intern Med. 1999;159:1229–1234. doi: 10.1001/archinte.159.11.1229. [DOI] [PubMed] [Google Scholar]
- Marcus BH, Albrecht AE, Niaura RS, Taylor ER, Simkin LR, Feder SI, Abrams DB, Thompson PD. Exercise enhances the maintenance of smoking cessation in women. Addict Behav. 1995;20:87–92. doi: 10.1016/0306-4603(94)00048-4. [DOI] [PubMed] [Google Scholar]
- Marcus BH, Lewis BA, Hogan J, King TK, Albrecht AE, Bock B, Parisi AF, Niaura R, Abrams DB. The efficacy of moderate-intensity exercise as an aid for smoking cessation in women: a randomized controlled trial. Nicotine Tob Res. 2005;7:871–880. doi: 10.1080/14622200500266056. [DOI] [PubMed] [Google Scholar]
- Marghmaleki VS, Alaei HA, Malekabadi HA, Pilehvarian A. Effect of Physical Activity on Symptoms of Morphine Addiction in Rats, after and before of Lesion of the mPFC Area. Iranian journal of basic medical sciences. 2013;16:1091. [PMC free article] [PubMed] [Google Scholar]
- Martinez LA, Peterson BM, Meisel RL, Mermelstein PG. Estradiol facilitation of cocaine-induced locomotor sensitization in female rats requires activation of mGluR5. Behav Brain Res. 2014;271:39–42. doi: 10.1016/j.bbr.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews I, McCormick C. Female and male rats in late adolescence differ from adults in amphetamine-induced locomotor activity, but not in conditioned place preference for amphetamine. Behavioural pharmacology. 2007;18:641. doi: 10.1097/FBP.0b013e3282effbf5. [DOI] [PubMed] [Google Scholar]
- Mattila VM, Raisamo S, Pihlajamaki H, Mantysaari M, Rimpela A. Sports activity and the use of cigarettes and snus among young males in Finland in 1999-2010. BMC Public Health. 2012;12:230. doi: 10.1186/1471-2458-12-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard ME, Leasure JL. Exercise enhances hippocampal recovery following binge ethanol exposure. PloS one. 2013;8:e76644. doi: 10.1371/journal.pone.0076644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur S, McHale E, Gillies GE. The size and distribution of midbrain dopaminergic populations are permanently altered by perinatal glucocorticoid exposure in a sex-region-and time-specific manner. Neuropsychopharmacology. 2007;32:1462–1476. doi: 10.1038/sj.npp.1301277. [DOI] [PubMed] [Google Scholar]
- McClung CA, Ulery PG, Perrotti LI, Zachariou V, Berton O, Nestler EJ. ΔFosB: a molecular switch for long-term adaptation in the brain. Molecular brain research. 2004;132:146–154. doi: 10.1016/j.molbrainres.2004.05.014. [DOI] [PubMed] [Google Scholar]
- McCulley WD, 3rd, Walls SA, Khurana RC, Rosenwasser AM, Devaud LL. Running wheel activity protects against increased seizure susceptibility in ethanol withdrawn male rats. Pharmacol Biochem Behav. 2012;100:485–489. doi: 10.1016/j.pbb.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh RK, Devito EE, Dodd D, Carroll KM, Potter JS, Greenfield SF, Connery HS, Weiss RD. Gender differences in a clinical trial for prescription opioid dependence. J Subst Abuse Treat. 2013;45:38–43. doi: 10.1016/j.jsat.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez-Delmestre R, Segarra AC. Testosterone is essential for cocaine sensitization in male rats. Physiol Behav. 2011;102:96–104. doi: 10.1016/j.physbeh.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mereu M, Tronci V, Chun LE, Thomas AM, Green JL, Katz JL, Tanda G. Cocaine-induced endocannabinoid release modulates behavioral and neurochemical sensitization in mice. Addict Biol. 2013;20:91–103. doi: 10.1111/adb.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miladi-Gorji H, Rashidy-Pour A, Fathollahi Y. Anxiety profile in morphine-dependent and withdrawn rats: effect of voluntary exercise. Physiol Behav. 2012;105:195–202. doi: 10.1016/j.physbeh.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Miladi-Gorji H, Rashidy-Pour A, Fathollahi Y, Akhavan MM, Semnanian S, Safari M. Voluntary exercise ameliorates cognitive deficits in morphine dependent rats: the role of hippocampal brain-derived neurotrophic factor. Neurobiol Learn Mem. 2011;96:479–491. doi: 10.1016/j.nlm.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Miladi-Gorji H, Rashidy-Pour A, Fathollahi Y, Semnanian S, Jadidi M. Effects of voluntary exercise on hippocampal long-term potentiation in morphine-dependent rats. Neuroscience. 2014;256:83–90. doi: 10.1016/j.neuroscience.2013.09.056. [DOI] [PubMed] [Google Scholar]
- Miller ML, Vaillancourt BD, Wright MJ, Jr., Aarde SM, Vandewater SA, Creehan KM, Taffe MA. Reciprocal inhibitory effects of intravenous d-methamphetamine self-administration and wheel activity in rats. Drug Alcohol Depend. 2012;121:90–96. doi: 10.1016/j.drugalcdep.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirbaha H, Tabaeizadeh M, Shaterian-Mohammadi H, Tahsili-Fahadan P, Dehpour AR. Estrogen pretreatment modulates morphine-induced conditioned place preference in ovariectomized mice. Pharmacology Biochemistry and Behavior. 2009;92:399–403. doi: 10.1016/j.pbb.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Moeller FG. Sex, stress, and drug cues in addiction. American Journal of Psychiatry. 2012;169:351–353. doi: 10.1176/appi.ajp.2012.12010041. [DOI] [PubMed] [Google Scholar]
- Monteggia LM, Luikart B, Barrot M, Theobold D, Malkovska I, Nef S, Parada LF, Nestler EJ. Brain-derived neurotrophic factor conditional knockouts show gender differences in depression-related behaviors. Biol Psychiatry. 2007;61:187–197. doi: 10.1016/j.biopsych.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Moore MJ, Werch CE. Sport and physical activity participation and substance use among adolescents. J Adolesc Health. 2005;36:486–493. doi: 10.1016/j.jadohealth.2004.02.031. [DOI] [PubMed] [Google Scholar]
- Morita N, Okita K. Is gender a factor in the reduction of cardiovascular risks with exercise training? Circ J. 2013;77:646–651. doi: 10.1253/circj.cj-12-0607. [DOI] [PubMed] [Google Scholar]
- Mustroph ML, Stobaugh DJ, Miller DS, DeYoung EK, Rhodes JS. Wheel running can accelerate or delay extinction of conditioned place preference for cocaine in male C57BL/6J mice, depending on timing of wheel access. Eur J Neurosci. 2011;34:1161–1169. doi: 10.1111/j.1460-9568.2011.07828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S. Conditioned ethanol aversion in rats induced by voluntary wheel running, forced swimming, and electric shock: an implication for aversion therapy of alcoholism. Integrative Physiological & Behavioral Science. 2004;39:95–104. doi: 10.1007/BF02734275. [DOI] [PubMed] [Google Scholar]
- Napier TC, Herrold AA, de Wit H. Using conditioned place preference to identify relapse prevention medications. Neurosci Biobehav Rev. 2013;37:2081–2086. doi: 10.1016/j.neubiorev.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarian A, Sun WL, Zhou L, Kemen LM, Jenab S, Quinones-Jenab V. Sex differences in basal and cocaine-induced alterations in PKA and CREB proteins in the nucleus accumbens. Psychopharmacology (Berl) 2009;203:641–650. doi: 10.1007/s00213-008-1411-5. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular mechanisms of drug addiction. Neuropharmacology. 2004;47:24–32. doi: 10.1016/j.neuropharm.2004.06.031. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Transcriptional mechanisms of addiction: role of ΔFosB. Philosophical Transactions of the Royal Society B: Biological Sciences. 2008;363:3245–3255. doi: 10.1098/rstb.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen J, Mor G, Naftolin F. Estrogen-regulated developmental neuronal apoptosis is determined by estrogen receptor subtype and the Fas/Fas ligand system. Journal of neurobiology. 2000;43:64–78. doi: 10.1002/(sici)1097-4695(200004)43:1<64::aid-neu6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Noonan MA, Bulin SE, Fuller DC, Eisch AJ. Reduction of adult hippocampal neurogenesis confers vulnerability in an animal model of cocaine addiction. The Journal of Neuroscience. 2010;30:304–315. doi: 10.1523/JNEUROSCI.4256-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygard SK, Klambatsen A, Hazim R, Eltareb MH, Blank JC, Chang AJ, Quinones-Jenab V, Jenab S. Sexually dimorphic intracellular responses after cocaine-induced conditioned place preference expression. Brain Res. 2013;1520:121–133. doi: 10.1016/j.brainres.2013.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell SJ, Galvez BA, Ball AJ, Marshall JF. Running wheel exercise ameliorates methamphetamine-induced damage to dopamine and serotonin terminals. Synapse. 2012;66:71–80. doi: 10.1002/syn.20989. [DOI] [PubMed] [Google Scholar]
- Olesen KM, Auger AP. Sex differences in Fos protein expression in the neonatal rat brain. J Neuroendocrinol. 2005;17:255–261. doi: 10.1111/j.1365-2826.2005.01302.x. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Goldberg SR. Self-administration of drugs in animals and humans as a model and an investigative tool. Addiction. 2007;102:1863–1870. doi: 10.1111/j.1360-0443.2007.02011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada M, Vargas EB, Kyres M, Burnside K, Pfaus JG. The role of ovarian hormones in sexual reward states of the female rat. Horm Behav. 2012;62:442–447. doi: 10.1016/j.yhbeh.2012.07.012. [DOI] [PubMed] [Google Scholar]
- Pate RR, Heath GW, Dowda M, Trost SG. Associations between physical activity and other health behaviors in a representative sample of US adolescents. Am J Public Health. 1996;86:1577–1581. doi: 10.2105/ajph.86.11.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate RR, Trost SG, Levin S, Dowda M. Sports participation and health-related behaviors among US youth. Arch Pediatr Adolesc Med. 2000;154:904–911. doi: 10.1001/archpedi.154.9.904. [DOI] [PubMed] [Google Scholar]
- Pendergast JS, Tuesta L, Bethea J. Oestrogen Receptor β Contributes to the Transient Sex Difference in Tyrosine Hydroxylase Expression in the Mouse Locus Coeruleus. Journal of neuroendocrinology. 2008;20:1155–1164. doi: 10.1111/j.1365-2826.2008.01776.x. [DOI] [PubMed] [Google Scholar]
- Perry RI, Krmpotich T, Thompson LL, Mikulich-Gilbertson SK, Banich MT, Tanabe J. Sex modulates approach systems and impulsivity in substance dependence. Drug Alcohol Depend. 2014;133:222–227. doi: 10.1016/j.drugalcdep.2013.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson AB, Abel JM, Lynch WJ. Dose-dependent effects of wheel running on cocaine-seeking and prefrontal cortex Bdnf exon IV expression in rats. Psychopharmacology (Berl) 2013;231:1305–1314. doi: 10.1007/s00213-013-3321-4. [DOI] [PubMed] [Google Scholar]
- Peterson AB, Hivick DP, Lynch WJ. Dose-dependent effectiveness of wheel running to attenuate cocaine-seeking: impact of sex and estrous cycle in rats. Psychopharmacology (Berl) 2014;231:2661–2670. doi: 10.1007/s00213-014-3437-1. [DOI] [PubMed] [Google Scholar]
- Pickering RP, Goldstein RB, Hasin DS, Blanco C, Smith SM, Huang B, Pulay AJ, Ruan WJ, Saha TD, Stinson FS. Temporal relationships between overweight and obesity and DSM-IV substance use, mood, and anxiety disorders: results from a prospective study. J Clin Psychiatry. 2011;72:1494–1502. doi: 10.4088/JCP.10m06077gry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce GL, Eskurza I, Walker AE, Fay TN, Seals DR. Sex-specific effects of habitual aerobic exercise on brachial artery flow-mediated dilation in middle-aged and older adults. Clin Sci (Lond) 2011;120:13–23. doi: 10.1042/CS20100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pina IL, Bittner V, Clare RM, Swank A, Kao A, Safford R, Nigam A, Barnard D, Walsh MN, Ellis SJ, Keteyian SJ, Investigators H-A. Effects of exercise training on outcomes in women with heart failure: analysis of HF-ACTION (Heart Failure-A Controlled Trial Investigating Outcomes of Exercise TraiNing) by sex. JACC Heart Fail. 2014;2:180–186. doi: 10.1016/j.jchf.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Pluchino N, Russo M, Santoro AN, Litta P, Cela V, Genazzani AR. Steroid hormones and BDNF. Neuroscience. 2013;239:271–279. doi: 10.1016/j.neuroscience.2013.01.025. [DOI] [PubMed] [Google Scholar]
- Pohjalainen T, Rinne JO, Någren K, SyvÄlahti E, Hietala J. Sex differences in the striatal dopamine D2 receptor binding characteristics in vivo. American Journal of Psychiatry. 1998;155:768–773. doi: 10.1176/ajp.155.6.768. [DOI] [PubMed] [Google Scholar]
- Pollitzer E. Biology: cell sex matters. Nature. 2013;500:23–24. doi: 10.1038/500023a. [DOI] [PubMed] [Google Scholar]
- Prapavessis H, Cameron L, Baldi JC, Robinson S, Borrie K, Harper T, Grove JR. The effects of exercise and nicotine replacement therapy on smoking rates in women. Addict Behav. 2007;32:1416–1432. doi: 10.1016/j.addbeh.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Quinones-Jenab V, Jenab S. Progesterone attenuates cocaine-induced responses. Horm Behav. 2010;58:22–32. doi: 10.1016/j.yhbeh.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Raichlen DA, Foster AD, Seillier A, Giuffrida A, Gerdeman GL. Exercise-induced endocannabinoid signaling is modulated by intensity. European journal of applied physiology. 2013;113:869–875. doi: 10.1007/s00421-012-2495-5. [DOI] [PubMed] [Google Scholar]
- Randall C, Kraemer PJ, Bardo M. Morphine-induced conditioned place preference in preweanling and adult rats. Pharmacology Biochemistry and Behavior. 1998;60:217–222. doi: 10.1016/s0091-3057(97)00585-6. [DOI] [PubMed] [Google Scholar]
- Reich CG, Taylor ME, McCarthy MM. Differential effects of chronic unpredictable stress on hippocampal CB1 receptors in male and female rats. Behav Brain Res. 2009;203:264–269. doi: 10.1016/j.bbr.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Chan CH, Ghee SM, See RE. Sex differences in escalation of methamphetamine self-administration: cognitive and motivational consequences in rats. Psychopharmacology (Berl) 2012;223:371–380. doi: 10.1007/s00213-012-2727-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renteria Diaz L, Siontas D, Mendoza J, Arvanitogiannis A. High levels of wheel running protect against behavioral sensitization to cocaine. Behav Brain Res. 2013;237:82–85. doi: 10.1016/j.bbr.2012.09.014. [DOI] [PubMed] [Google Scholar]
- Revilla S, Sunol C, Garcia-Mesa Y, Gimenez-Llort L, Sanfeliu C, Cristofol R. Physical exercise improves synaptic dysfunction and recovers the loss of survival factors in 3xTg-AD mouse brain. Neuropharmacology. 2014;81:55–63. doi: 10.1016/j.neuropharm.2014.01.037. [DOI] [PubMed] [Google Scholar]
- Rezende EL, Kelly SA, Gomes FR, Chappell MA, Garland T., Jr. Effects of size, sex, and voluntary running speeds on costs of locomotion in lines of laboratory mice selectively bred for high wheel-running activity. Physiol Biochem Zool. 2006;79:83–99. doi: 10.1086/498187. [DOI] [PubMed] [Google Scholar]
- Riebe CJ, Hill MN, Lee TT, Hillard CJ, Gorzalka BB. Estrogenic regulation of limbic cannabinoid receptor binding. Psychoneuroendocrinology. 2010;35:1265–1269. doi: 10.1016/j.psyneuen.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MD, Gilpin L, Parker KE, Childs TE, Will MJ, Booth FW. Dopamine D1 receptor modulation in nucleus accumbens lowers voluntary wheel running in rats bred to run high distances. Physiology & behavior. 2012;105:661–668. doi: 10.1016/j.physbeh.2011.09.024. [DOI] [PubMed] [Google Scholar]
- Rodriguez Garcia PL, Lopez Villalba FJ, Lopez Minarro PA, Garcia Canto E. Physical exercise, energy expenditure and tobacco consumption in adolescents from Murcia (Spain). Arch Argent Pediatr. 2014;112:12–18. doi: 10.5546/aap.2014.eng.12. [DOI] [PubMed] [Google Scholar]
- Roger-Sanchez C, Aguilar MA, Rodriguez-Arias M, Aragon CM, Minarro J. Age- and sex-related differences in the acquisition and reinstatement of ethanol CPP in mice. Neurotoxicol Teratol. 2012;34:108–115. doi: 10.1016/j.ntt.2011.07.011. [DOI] [PubMed] [Google Scholar]
- Roman E, Ploj K, Gustafsson L, Meyerson BJ, Nylander I. Variations in opioid peptide levels during the estrous cycle in Sprague-Dawley rats. Neuropeptides. 2006;40:195–206. doi: 10.1016/j.npep.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Roth ME, Carroll ME. Sex differences in the escalation of intravenous cocaine intake following long- or short-access to cocaine self-administration. Pharmacol Biochem Behav. 2004;78:199–207. doi: 10.1016/j.pbb.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Roth ME, Casimir AG, Carroll ME. Influence of estrogen in the acquisition of intravenously self-administered heroin in female rats. Pharmacology Biochemistry and Behavior. 2002;72:313–318. doi: 10.1016/s0091-3057(01)00777-8. [DOI] [PubMed] [Google Scholar]
- Russo S, Festa E, Fabian S, Gazi F, Kraish M, Jenab S, Quinones-Jenab V. Gonadal hormones differentially modulate cocaine-induced conditioned place preference in male and female rats. Neuroscience. 2003a;120:523–533. doi: 10.1016/s0306-4522(03)00317-8. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Dietz DM, Dumitriu D, Morrison JH, Malenka RC, Nestler EJ. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci. 2010;33:267–276. doi: 10.1016/j.tins.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Jenab S, Fabian SJ, Festa ED, Kemen LM, Quinones-Jenab V. Sex differences in the conditioned rewarding effects of cocaine. Brain Res. 2003b;970:214–220. doi: 10.1016/s0006-8993(03)02346-1. [DOI] [PubMed] [Google Scholar]
- Saleh MC, Connell BJ, Saleh TM. Resveratrol induced neuroprotection is mediated via both estrogen receptor subtypes, ERα and ERβ. Neurosci Lett. 2013;548:217–221. doi: 10.1016/j.neulet.2013.05.057. [DOI] [PubMed] [Google Scholar]
- Sanchez V, Moore CF, Brunzell DH, Lynch WJ. Effect of wheel-running during abstinence on subsequent nicotine-seeking in rats. Psychopharmacology (Berl) 2013;227:403–411. doi: 10.1007/s00213-012-2964-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez V, Moore CF, Brunzell DH, Lynch WJ. Sex differences in the effect of wheel running on subsequent nicotine-seeking in a rat adolescent-onset self-administration model. Psychopharmacology (Berl) 2014;231:1753–1762. doi: 10.1007/s00213-013-3359-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchis-Segura C, Spanagel R. Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict Biol. 2006;11:2–38. doi: 10.1111/j.1369-1600.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- Scarduzio M, Panichi R, Pettorossi VE, Grassi S. Synaptic long-term potentiation and depression in the rat medial vestibular nuclei depend on neural activation of estrogenic and androgenic signals. PloS one. 2013;8:e80792. doi: 10.1371/journal.pone.0080792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler CW, Bross JG, Thorndike EB. Gender differences in the behavioral effects of methamphetamine. Eur J Pharmacol. 2002;442:231–235. doi: 10.1016/s0014-2999(02)01550-9. [DOI] [PubMed] [Google Scholar]
- Schlauch RC, Breiner MJ, Stasiewicz PR, Christensen RL, Lang AR. Women Inmate Substance Abusers’ Reactivity to Visual Alcohol, Cigarette, Marijuana, and Crack-Cocaine Cues: Approach and Avoidance as Separate Dimensions of Reactivity. J Psychopathol Behav Assess. 2013;35:45–56. doi: 10.1007/s10862-012-9313-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Kassow M, Schädle S, Otterbein S, Thiel C, Doehring A, Lötsch J, Kaiser J. Kinetics of serum brain-derived neurotrophic factor following low-intensity versus high-intensity exercise in men and women. Neuroreport. 2012;23:889–893. doi: 10.1097/WNR.0b013e32835946ca. [DOI] [PubMed] [Google Scholar]
- Segarra AC, Agosto-Rivera JL, Febo M, Lugo-Escobar N, Menéndez-Delmestre R, Puig-Ramos A, Torres-Diaz YM. Estradiol: a key biological substrate mediating the response to cocaine in female rats. Horm Behav. 2010;58:33–43. doi: 10.1016/j.yhbeh.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segat HJ, Kronbauer M, Roversi K, Schuster AJ, Vey LT, Roversi K, Pase CS, Antoniazzi CT, Burger ME. Exercise modifies amphetamine relapse: Behavioral and oxidative markers in rats. Behav Brain Res. 2014;262:94–100. doi: 10.1016/j.bbr.2014.01.005. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinyor D, Brown T, Rostant L, Seraganian P. The role of a physical fitness program in the treatment of alcoholism. J Stud Alcohol. 1982;43:380–386. doi: 10.15288/jsa.1982.43.380. [DOI] [PubMed] [Google Scholar]
- Smelson D, Chen KW, Ziedonis D, Andes K, Lennox A, Callahan L, Rodrigues S, Eisenberg D. A pilot study of Qigong for reducing cocaine craving early in recovery. The Journal of Alternative and Complementary Medicine. 2013;19:97–101. doi: 10.1089/acm.2012.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Gergans SR, Iordanou JC, Lyle MA. Chronic exercise increases sensitivity to the conditioned rewarding effects of cocaine. Pharmacol Rep. 2008a;60:561–565. [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Lynch WJ. Exercise as a potential treatment for drug abuse: evidence from preclinical studies. Front Psychiatry. 2011;2:82. doi: 10.3389/fpsyt.2011.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Pennock MM, Walker KL, Lang KC. Access to a running wheel decreases cocaine-primed and cue-induced reinstatement in male and female rats. Drug Alcohol Depend. 2012;121:54–61. doi: 10.1016/j.drugalcdep.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Pitts EG. Access to a running wheel inhibits the acquisition of cocaine self-administration. Pharmacology Biochemistry and Behavior. 2011;100:237–243. doi: 10.1016/j.pbb.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Pitts EG. Wheel running decreases the positive reinforcing effects of heroin. Pharmacological Reports. 2012;64:960–964. doi: 10.1016/s1734-1140(12)70891-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Schmidt KT, Iordanou JC, Mustroph ML. Aerobic exercise decreases the positive-reinforcing effects of cocaine. Drug Alcohol Depend. 2008b;98:129–135. doi: 10.1016/j.drugalcdep.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Walker KL, Cole KT, Lang KC. The effects of aerobic exercise on cocaine self-administration in male and female rats. Psychopharmacology (Berl) 2011;218:357–369. doi: 10.1007/s00213-011-2321-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Witte MA. The effects of exercise on cocaine self-administration, food-maintained responding, and locomotor activity in female rats: importance of the temporal relationship between physical activity and initial drug exposure. Exp Clin Psychopharmacol. 2012;20:437–446. doi: 10.1037/a0029724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Yancey DL. Sensitivity to the effects of opioids in rats with free access to exercise wheels: μ-opioid tolerance and physical dependence. Psychopharmacology (Berl) 2003;168:426–434. doi: 10.1007/s00213-003-1471-5. [DOI] [PubMed] [Google Scholar]
- Smits JA, Zvolensky MJ, Rosenfield D, Marcus BH, Church TS, Frierson GM, Powers MB, Otto MW, Davis ML, DeBoer LB, Briceno NF. The efficacy of vigorous-intensity exercise as an aid to smoking cessation in adults with elevated anxiety sensitivity: study protocol for a randomized controlled trial. Trials. 2012;13:207. doi: 10.1186/1745-6215-13-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobieraj JC, Kim A, Fannon MJ, Mandyam CD. Chronic wheel running-induced reduction of extinction and reinstatement of methamphetamine seeking in methamphetamine dependent rats is associated with reduced number of periaqueductal gray dopamine neurons. Brain Struct Funct. 2014 Oct 2; doi: 10.1007/s00429-014-0905-7. 2014 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Chauvet C, Thiriet N, El Rawas R, Jaber M. Reversal of cocaine addiction by environmental enrichment. Proc Natl Acad Sci U S A. 2008;105:17145–17150. doi: 10.1073/pnas.0806889105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Thiriet N, El Rawas R, Lardeux V, Jaber M. Environmental enrichment during early stages of life reduces the behavioral, neurochemical, and molecular effects of cocaine. Neuropsychopharmacology. 2009;34:1102–1111. doi: 10.1038/npp.2008.51. [DOI] [PubMed] [Google Scholar]
- Sparling P, Giuffrida A, Piomelli D, Rosskopf L, Dietrich A. Exercise activates the endocannabinoid system. Neuroreport. 2003;14:2209–2211. doi: 10.1097/00001756-200312020-00015. [DOI] [PubMed] [Google Scholar]
- Stapleton JN, Martin Ginis KA, The, S.-S. C. I. R. G. Sex Differences in Theory-Based Predictors of Leisure Time Physical Activity in a Population-Based Sample of Adults With Spinal Cord Injury. Arch Phys Med Rehabil. 2014;95:1787–1790. doi: 10.1016/j.apmr.2014.03.021. [DOI] [PubMed] [Google Scholar]
- Storey A, Smith HK. Unique aspects of competitive weightlifting: performance, training and physiology. Sports Med. 2012;42:769–790. doi: 10.1007/BF03262294. [DOI] [PubMed] [Google Scholar]
- Strohle A, Hofler M, Pfister H, Muller AG, Hoyer J, Wittchen HU, Lieb R. Physical activity and prevalence and incidence of mental disorders in adolescents and young adults. Psychol Med. 2007;37:1657–1666. doi: 10.1017/S003329170700089X. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Gerhold LM, Böttner M, Rau SW, Dela Cruz C, Yang E, Zhu H, Yu J, Cashion AB, Kindy MS. Estradiol enhances neurogenesis following ischemic stroke through estrogen receptors α and β. Journal of Comparative Neurology. 2007;500:1064–1075. doi: 10.1002/cne.21240. [DOI] [PubMed] [Google Scholar]
- Tantimonaco M, Ceci R, Sabatini S, Catani MV, Rossi A, Gasperi V, Maccarrone M. Physical activity and the endocannabinoid system: an overview. Cellular and Molecular Life Sciences. 2014;71:2681–2698. doi: 10.1007/s00018-014-1575-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar C, Bessert D, Tse H, Skoff RP. Determinants of central nervous system adult neurogenesis are sex, hormones, mouse strain, age, and brain region. Glia. 2013;61:192–209. doi: 10.1002/glia.22426. [DOI] [PubMed] [Google Scholar]
- Temfemo A, Chlif M, Mandengue SH, Lelard T, Choquet D, Ahmaidi S. Is there a beneficial effect difference between age, gender, and different cardiac pathology groups of exercise training at ventilatory threshold in cardiac patients? Cardiol J. 2011;18:632–638. doi: 10.5603/cj.2011.0026. [DOI] [PubMed] [Google Scholar]
- Terry-McElrath YM, O'Malley PM. Substance use and exercise participation among young adults: parallel trajectories in a national cohort-sequential study. Addiction. 2011;106:1855–1865. doi: 10.1111/j.1360-0443.2011.03489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry-McElrath YM, O'Malley PM, Johnston LD. Exercise and substance use among American youth, 1991-2009. Am J Prev Med. 2011;40:530–540. doi: 10.1016/j.amepre.2010.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Stamos J, Robison LS, Heyman G, Tucci A, Wang GJ, Robinson JK, Anderson BJ, Volkow ND. Daily treadmill exercise attenuates cocaine cue-induced reinstatement and cocaine induced locomotor response but increases cocaine-primed reinstatement. Behav Brain Res. 2013;239:8–14. doi: 10.1016/j.bbr.2012.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Tucci A, Stamos J, Robison L, Wang GJ, Anderson BJ, Volkow ND. Chronic forced exercise during adolescence decreases cocaine conditioned place preference in Lewis rats. Behav Brain Res. 2010;215:77–82. doi: 10.1016/j.bbr.2010.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Engelhardt B, Hood LE, Peartree NA, Neisewander JL. The interactive effects of environmental enrichment and extinction interventions in attenuating cue-elicited cocaine-seeking behavior in rats. Pharmacol Biochem Behav. 2011;97:595–602. doi: 10.1016/j.pbb.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Pentkowski NS, Peartree NA, Painter MR, Neisewander JL. Environmental living conditions introduced during forced abstinence alter cocaine-seeking behavior and Fos protein expression. Neuroscience. 2010;171:1187–1196. doi: 10.1016/j.neuroscience.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Reveron A, Hurd YL, Dow-Edwards DL. Gender differences in prodynorphin but not proenkephalin mRNA expression in the striatum of adolescent rats exposed to prenatal cocaine. Neurosci Lett. 2007;421:213–217. doi: 10.1016/j.neulet.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Reveron A, Khalid S, Williams TJ, Waters EM, Drake CT, McEwen BS, Milner TA. Ovarian steroids modulate leu-enkephalin levels and target leu-enkephalinergic profiles in the female hippocampal mossy fiber pathway. Brain Res. 2008;1232:70–84. doi: 10.1016/j.brainres.2008.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Reveron A, Khalid S, Williams TJ, Waters EM, Jacome L, Luine VN, Drake CT, McEwen BS, Milner TA. Hippocampal dynorphin immunoreactivity increases in response to gonadal steroids and is positioned for direct modulation by ovarian steroid receptors. Neuroscience. 2009;159:204–216. doi: 10.1016/j.neuroscience.2008.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres OV, Natividad LA, Tejeda HA, Van Weelden SA, O'Dell LE. Female rats display dose-dependent differences to the rewarding and aversive effects of nicotine in an age-, hormone-, and sex-dependent manner. Psychopharmacology (Berl) 2009;206:303–312. doi: 10.1007/s00213-009-1607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toy WA, Petzinger GM, Leyshon BJ, Akopian GK, Walsh JP, Hoffman MV, Vučković MG, Jakowec MW. Treadmill exercise reverses dendritic spine loss in direct and indirect striatal medium spiny neurons in the 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) mouse model of Parkinson's disease. Neurobiology of Disease. 2014;63:201–209. doi: 10.1016/j.nbd.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trigo JM, Martin-Garcia E, Berrendero F, Robledo P, Maldonado R. The endogenous opioid system: a common substrate in drug addiction. Drug Alcohol Depend. 2010;108:183–194. doi: 10.1016/j.drugalcdep.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Tzeng WY, Chen LH, Cherng CG, Tsai YN, Yu L. Sex differences and the modulating effects of gonadal hormones on basal and the stressor-decreased newly proliferative cells and neuroblasts in dentate gyrus. Psychoneuroendocrinology. 2014;42:24–37. doi: 10.1016/j.psyneuen.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Uth N. Gender difference in the proportionality factor between the mass specific VO2max and the ratio between HR(max) and HR(rest). Int J Sports Med. 2005;26:763–767. doi: 10.1055/s-2005-837443. [DOI] [PubMed] [Google Scholar]
- Van Kempen TA, Kahlid S, Gonzalez AD, Spencer-Segal JL, Tsuda MC, Ogawa S, McEwen BS, Waters EM, Milner TA. Sex and estrogen receptor expression influence opioid peptide levels in the mouse hippocampal mossy fiber pathway. Neurosci Lett. 2013;552:66–70. doi: 10.1016/j.neulet.2013.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Interplay between brain-derived neurotrophic factor and signal transduction modulators in the regulation of the effects of exercise on synaptic-plasticity. Neuroscience. 2003;122:647–657. doi: 10.1016/j.neuroscience.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Vera-Villarroel P, Piqueras JA, Kuhne W, Cuijpers P, van Straten A. Differences between men and women in self-reported body mass index and its relation to drug use. Substance abuse treatment, prevention, and policy. 2014;9:1. doi: 10.1186/1747-597X-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers KS, Patten CA, Lewis BA, Clark MM, Ussher M, Ebbert JO, Croghan IT, Decker PA, Hathaway J, Marcus BH, Hurt RD. Feasibility of an exercise counseling intervention for depressed women smokers. Nicotine Tob Res. 2009;11:985–995. doi: 10.1093/ntr/ntp101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachou S, Panagis G. Regulation of brain reward by the endocannabinoid system: a critical review of behavioral studies in animals. Current pharmaceutical design. 2014;20:2072–2088. doi: 10.2174/13816128113199990433. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang G-J, Swanson JM, Telang F. Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Archives of neurology. 2007;64:1575–1579. doi: 10.1001/archneur.64.11.1575. [DOI] [PubMed] [Google Scholar]
- Wakefield J. DSM-5 substance use disorder: how conceptual missteps weakened the foundations of the addictive disorders field. Acta Psychiatrica Scandinavica. 2015 May 13; doi: 10.1111/acps.12446. 2015 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Walker QD, Ray R, Kuhn CM. Sex differences in neurochemical effects of dopaminergic drugs in rat striatum. Neuropsychopharmacology. 2006;31:1193–1202. doi: 10.1038/sj.npp.1300915. [DOI] [PubMed] [Google Scholar]
- Wang J, Fanous S, Terwilliger EF, Bass CE, Hammer RP, Nikulina EM. BDNF Overexpression in the Ventral Tegmental Area Prolongs Social Defeat Stress-induced Cross-Sensitization to Amphetamine and Increases ΔFosB Expression in Mesocorticolimbic Regions of Rats. Neuropsychopharmacology. 2013;38:2286–2296. doi: 10.1038/npp.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werch CC, Moore MJ, DiClemente CC, Bledsoe R, Jobli E. A multihealth behavior intervention integrating physical activity and substance use prevention for adolescents. Prev Sci. 2005;6:213–226. doi: 10.1007/s11121-005-0012-3. [DOI] [PubMed] [Google Scholar]
- Werme M, Lindholm S, Thorén P, Franck J, Brené S. Running increases ethanol preference. Behav Brain Res. 2002a;133:301–308. doi: 10.1016/s0166-4328(02)00027-x. [DOI] [PubMed] [Google Scholar]
- Werme M, Messer C, Olson L, Gilden L, Thorén P, Nestler EJ, Brené S. ΔFosB regulates wheel running. The Journal of Neuroscience. 2002b;22:8133–8138. doi: 10.1523/JNEUROSCI.22-18-08133.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werme M, Thorén P, Olson L, Brené S. Running and cocaine both upregulate dynorphin mRNA in medial caudate putamen. European Journal of Neuroscience. 2000;12:2967–2974. doi: 10.1046/j.1460-9568.2000.00147.x. [DOI] [PubMed] [Google Scholar]
- Wetherington CL. Sex differences and gonadal hormone influences in drug addiction and sexual behavior: progress and possibilities. Horm Behav. 2010;58:2–7. doi: 10.1016/j.yhbeh.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Williams DM, Dunsiger S, Whiteley JA, Ussher MH, Ciccolo JT, Jennings EG. Acute effects of moderate intensity aerobic exercise on affective withdrawal symptoms and cravings among women smokers. Addict Behav. 2011;36:894–897. doi: 10.1016/j.addbeh.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DM, Whiteley JA, Dunsiger S, Jennings EG, Albrecht AE, Ussher MH, Ciccolo JT, Parisi AF, Marcus BH. Moderate intensity exercise as an adjunct to standard smoking cessation treatment for women: a pilot study. Psychol Addict Behav. 2010;24:349–354. doi: 10.1037/a0018332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsley RJ, Fulford J, Roberts AC, Welsman JR, Armstrong N. Sex difference in peak oxygen uptake in prepubertal children. J Sci Med Sport. 2009;12:647–651. doi: 10.1016/j.jsams.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Winters KC, Tanner-Smith EE, Bresani E, Meyers K. Current advances in the treatment of adolescent drug use. Adolescent health, medicine and therapeutics. 2014;5:199–210. doi: 10.2147/AHMT.S48053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissman AM, McCollum AF, Huang GZ, Nikrodhanond AA, Woolley CS. Sex differences and effects of cocaine on excitatory synapses in the nucleus accumbens. Neuropharmacology. 2011;61:217–227. doi: 10.1016/j.neuropharm.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SY, Chan FW, Lee CK, Li M, Yeung F, Lum CC, Choy DT, Woo J. Maximum oxygen uptake and body composition of healthy Hong Kong Chinese adult men and women aged 20 - 64 years. J Sports Sci. 2008;26:295–302. doi: 10.1080/02640410701552658. [DOI] [PubMed] [Google Scholar]
- Wu A, Ying Z, Gomez-Pinilla F. Docosahexaenoic acid dietary supplementation enhances the effects of exercise on synaptic plasticity and cognition. Neuroscience. 2008;155:751–759. doi: 10.1016/j.neuroscience.2008.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Hou B, Gao Y, He F, Zhang C. Effects of enriched environment on morphine-induced reward in mice. Exp Neurol. 2007;204:714–719. doi: 10.1016/j.expneurol.2006.12.027. [DOI] [PubMed] [Google Scholar]
- Yau S.-y., Gil-Mohapel J, Christie BR, So K.-f. Physical Exercise-Induced Adult Neurogenesis: A Good Strategy to Prevent Cognitive Decline in Neurodegenerative Diseases? BioMed Research International. 2014 Apr 9; doi: 10.1155/2014/403120. 2014 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharova E, Wade D, Izenwasser S. Sensitivity to cocaine conditioned reward depends on sex and age. Pharmacol Biochem Behav. 2009;92:131–134. doi: 10.1016/j.pbb.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Jia J, Wu Y, Hu Y, Wang Y. The effect of treadmill training pre-exercise on glutamate receptor expression in rats after cerebral ischemia. Int J Mol Sci. 2010;11:2658–2669. doi: 10.3390/ijms11072658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Zhou C, Li R. Sex differences in exercise and drug addiction: A mini review of animal studies. Journal of Sport and Health Science. 2014;3:163–169. [Google Scholar]
- Zlebnik NE, Anker JJ, Carroll ME. Exercise to reduce the escalation of cocaine self-administration in adolescent and adult rats. Psychopharmacology (Berl) 2012;224:387–400. doi: 10.1007/s00213-012-2760-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlebnik NE, Anker JJ, Gliddon LA, Carroll ME. Reduction of extinction and reinstatement of cocaine seeking by wheel running in female rats. Psychopharmacology (Berl) 2010;209:113–125. doi: 10.1007/s00213-010-1776-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlebnik NE, Hedges VL, Carroll ME, Meisel RL. Chronic wheel running affects cocaine-induced c-Fos expression in brain reward areas in rats. Behav Brain Res. 2014a;261:71–78. doi: 10.1016/j.bbr.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlebnik NE, Saykao AT, Carroll ME. Effects of combined exercise and progesterone treatments on cocaine seeking in male and female rats. Psychopharmacology (Berl) 2014b;231:3787–3798. doi: 10.1007/s00213-014-3513-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zschucke E, Heinz A, Strohle A. Exercise and physical activity in the therapy of substance use disorders. ScientificWorldJournal. 2012 May 3; doi: 10.1100/2012/901741. 2012 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuloaga DG, Johnson LA, Agam M, Raber J. Sex Differences in Activation of the Hypothalamic-Pituitary-Adrenal Axis by Methamphetamine. Journal of neurochemistry. 2014;129:495–508. doi: 10.1111/jnc.12651. [DOI] [PMC free article] [PubMed] [Google Scholar]