Abstract
Purpose
Critically ill or injured children require prompt identification, rapid referral and quality emergency management. We undertook a study to evaluate the care pathway of critically ill or injured children to identify preventable failures in the care provided.
Methods
A year-long cohort study of critically ill and injured children was performed in Cape Town, South Africa, from first presentation to healthcare services until paediatric intensive care unit (PICU) admission or emergency department death, using expert panel review of medical records and caregiver interview. Main outcomes were expert assessment of overall quality of care; avoidability of severity of illness and PICU admission or death and the identification of modifiable factors.
Results
The study enrolled 282 children, 252 emergency PICU admissions, and 30 deaths. Global quality of care was graded good in 10% of cases, with half having at least one major impact modifiable factor. Key modifiable factors related to access to care and identification of the critically ill, assessment of severity, inadequate resuscitation, and delays in decision making and referral. Children were transferred with median time from first presentation to PICU admission of 12.3 hours. There was potentially avoidable severity of illness in 185 (74%) of children, and death prior to PICU admission was avoidable in 17/30 (56.7%) of children.
Conclusions
The study presents a novel methodology, examining quality of care across an entire system, and highlighting the complexity of the pathway and the modifiable events amenable to interventions, that could reduce mortality and morbidity, and optimize utilization of scarce critical care resources; as well as demonstrating the importance of continuity and quality of care.
Introduction
The ideal healthcare system would identify sick children early in their illness, and provide treatment that is safe, effective, patient centred, efficient, timely and equitable [1]. To focus quality improvement, we need to understand the current situation: the processes, time taken, and the nature and quality of care provided during each step. Quality of care and speed through health systems are related to the variety of conditions encountered [2], thus provision of optimal care to all children with a wide range of life threatening illness or injury may be extremely challenging [3]. Highlighting foci for effective quality improvement interventions in paediatric emergencies has the potential to provide significant benefit, especially in resource poor settings where the need is greatest, and the health services poorest [4], but also in more affluent countries where delivery of recommended care may still be very difficult [5]. The fundamental challenge of a “pathway to care” is that optimal results can only be expected if the entire process functions effectively.
We could find no studies in lower resource settings assessing the quality of care delivered to critically ill or injured children from the time of first presentation through to death or admission to intensive care. In the United Kingdom (UK), the Confidential Enquiry into Maternal and Child Health (CEMACH) has been in effect for more than 50 years, now including assessment of childhood mortality and morbidity [6–8]. There have been improvements in healthcare for mothers and children in the UK, which many attribute substantially to the CEMACH findings and recommendations [8–11], and widespread use of this process in other settings [12–18], such that it is now widely regarded as a framework for mortality review.
Although South Africa is considered a middle income country, and the Western Cape one of the wealthier provinces, there is much inequity in socio-economic and health status [19], with a third of the population living below international poverty lines, and a quarter of children living in informal and overcrowded houses, much as in many developing countries [20]. South Africa’s health system is divided, with the state funded public sector providing relatively resource constrained care to more than two thirds of the low income population, and a private sector, funded predominantly by medical insurance, serving the wealthier and formally employed population.
We undertook a study to identify preventable failures in the care of critically ill or injured children and thus identify key areas for interventions to optimise care. The objectives were to: i) describe the details of the entire care pathway to a paediatric intensive care unit (PICU) or death, ii) use expert clinical review to evaluate the quality of care along the pathway and identify preventable failures in the care provided.
Materials and Methods
Study Design
The study was conducted at the Red Cross War Memorial Children’s Hospital (RCWMCH), Cape Town, one of only two public tertiary hospitals managing children from throughout the Western Cape Province. Tygerberg Hospital in Cape Town has a smaller PICU, generally limited to medical patients (predominantly neonates) and post-surgical patients (elective), with a different referral area from RCWMCH although there is limited overlap. Referrals are primarily from the surrounding metropolitan area, with a paediatric population of 930 000 [20] by: approximately 600 independent general practitioners; 109 nurse-led clinics (Clinics); 45 doctor-led primary health centers (36 operate only in office hours; 9 are 24 hour) (Community Health Centers (CHC)); and 7 hospitals. Patients are transferred by the Emergency Medical Services (EMS) with 140 ambulances across the province.
The study was approved by the Faculty of Health Sciences Research Ethics Committee, University of Cape Town (UCT HREC 211/2011); and the Oxford Tropical Research Ethics Committee, Oxford University (OXTREC 29–11), as well as the Western Cape Department of Health and the City of Cape Town. The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. As per ethics approval, written informed consent for caregiver interview, and collection and analysis of the child's medical records was obtained from the parent or caregiver of every enrolled child in the study. The signed paper consent forms were securely stored with the study records. Patient identity was encrypted by the database, and paper records securely stored to retain anonymity and confidentiality.
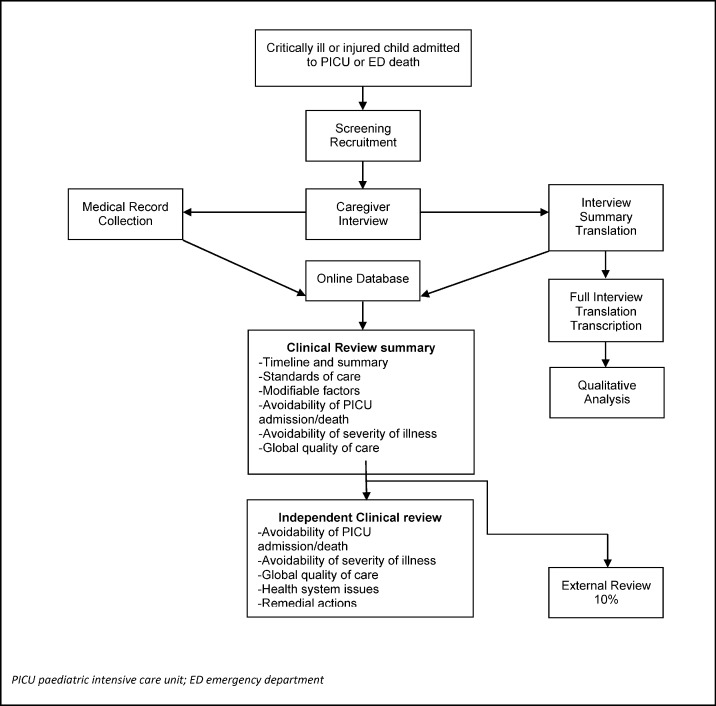
We recruited critically ill children on admission to PICU, or when they died in an emergency department (ED) (Fig 1). The inclusion criteria were: aged <13 years, emergency PICU admission during recruitment phases (alternate weeks) over a year; death in the RCWMCH-ED; or death in the ED of the immediate geographic referring facilities. Exclusion criteria were: elective PICU admission; inpatient for >5 days prior to PICU admission; children under palliative care, or those dead on arrival to the ED.
Fig 1. Flow Chart of Pathways to Care research project recruitment, data collection and clinical review process.
Following written informed consent, the caregiver of each child was interviewed, focusing on the caregiver’s narrative of the healthcare episode, access, timeline, and perceptions of care. Medical records were obtained from each healthcare facility and EMS transfer involved. Clinical outcomes collected for each child were the Paediatric Index of Mortality (PIM2) [21] score on admission to PICU; duration of stay in PICU and in RCWMCH; and the 30 day outcome.
Clinical Review
We developed a method of expert clinical review modelled on the CEMACH [6, 7]. A list of potential modifiable factors was compiled (S1 Table) based on those identified in the CEMACH, and the Child Healthcare Problem Identification Programme (CHIP) in South Africa [22]. We categorised the possible impact of these factors:
Major: factor which had clear negative impact on the outcome for the patient and worsened mortality or morbidity; directly and overwhelmingly important factor in the severity of illness/death
Moderate: factor which on its own had minimal negative impact on the outcome but may have caused some morbidity and/or extended the hospital/PICU stay
Near Miss: unplanned event that did not have major impact–but had the potential to do so—only a fortunate break in the chain of events prevented an injury, fatality or damage
No Defined Impact: factor which has no individual or cumulative negative impact on the outcome of this or future cases
Not known: cannot be established or estimated given facts known about scenario
Standards of care were developed for paediatric emergency care and referrals for facilities and EMS transfers using a modified Delphi technique with stakeholders (representing each healthcare level) prior to the study onset as there were no locally agreed care standards—these were categorised into critical, important and necessary (S2 Table). We also considered whether the health care prior to the acute episode was appropriate and if better care could have prevented the severity of illness or outcome.
One clinician (PH) summarized each case, developing a timeline of the pathway based on both the medical record and interview data. Three senior local clinicians, all leading clinicians and researchers in their fields, one each from paediatric intensive care (AA), emergency medicine (LW), and primary care (SR) then independently reviewed each case using all available information.
Outcomes for each child:
Overall global quality of care for the entire pathway
Avoidability of death
Avoidability of PICU admission
Avoidability of severity of illness on PICU admission
Number and type of modifiable factors
Presence of healthcare system issues
Outcomes for each healthcare contact/ transfer:
-
7
Number and type of modifiable factors
-
8
Compliance with consensus standards of care for facility level/ transfer
Conflicting assessments on outcomes 1 to 4 were resolved by discussion. A UK based paediatric emergency medicine specialist (IM) undertook external review of a random 10% of cases.
Sample Size
Pilot data suggested that the recruitment of 470 children would be possible, giving a precision of 4% based on a 95%CI on a proportion of 40% of children suffering from modifiable factors in their care. For our sample size calculation we did not assume a finite population. The estimate of the SE for the proportion is therefore SE(p) = sqrt ((p x (1-p) / n); where n is the required sample size. The precision defined is equal to 1.96 x SE(p)—that is half of the 95% Confidence Interval. A sample of 470 participants therefore would have provided a precision of 4.43% for a proportion of 40%. The expected annual emergency admissions to the RCWMCH ED for the study period were approximately 55 000. Correcting for this population size, a sample of 466 would have provided us with the same precision of 4.43% for the same proportion (40%).
Data Analysis
For demographics, diagnosis, pathway variables, and modifiable factors, we used descriptive statistics (median and interquartile ranges (IQR), or mean and standard deviation (where normally distributed) for continuous variables, and n(%) for the categorical variables). To assess agreement between each of the reviewers’ blinded assessments (of outcomes 1–4) and the final consensus assessment we estimated kappa statistics [23]. Analyses were performed using SPSS [24].
Results
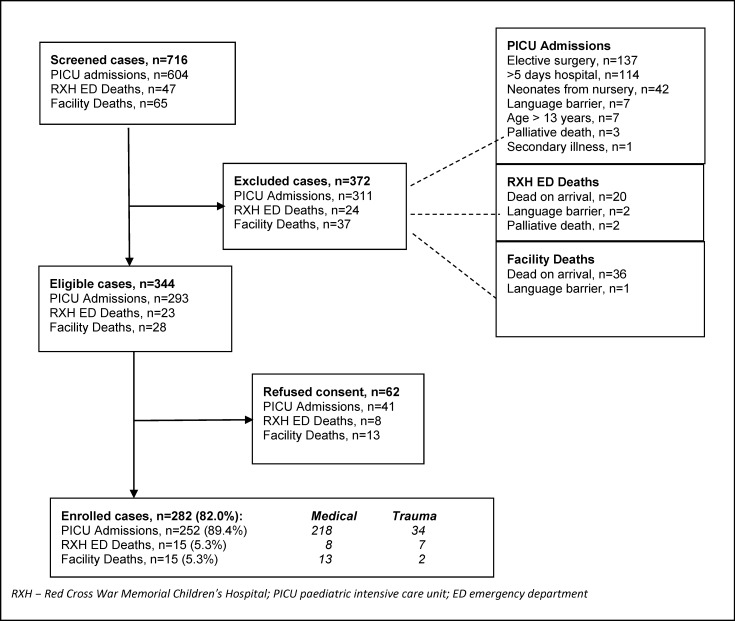
From 1st November 2011 to 31st October 2012, 716 cases were screened, of which 344 were eligible, and 282 (82.0%) agreed to participate (Fig 2). A total of 62 eligible caregivers (18.0%) refused to participate. There was a higher refusal rate in those caregivers whose children had already died at the time of recruitment (21/51, 41.2%). Most enrolled children were admitted to the PICU (252, 89.4%), with 15 deaths in RCWMCH-ED (5.3%) and 15 deaths at other EDs (5.3%). The majority of children (239, 84.8%) had medical illnesses; the rest were related to trauma.
Fig 2. Recruitment process and outcomes.
Participant Characteristics
Over half the children were male (164, 58.2%); 162/239 (67.8%) medical cases were predominantly under a year of age whereas 22/43 (51.2%) of trauma cases were older than 5 years (Table 1). The primary diagnoses of the 239 medical cases were respiratory (102, 42.7%) (mainly infection), followed by sepsis (42, 17.6%), and 97 (42.7%) of the medical cases were underweight for age (z<-2). Comparison of the cohort with the provincial census data [20] is shown in S3 Table. Data from the interviews showed less than a third of households (85, 31.1%) had an income over $300 per month. Transport to the nearest healthcare facility was on foot for 199 (70.5%) cases and to the nearest 24 hour facility (median 6.0 km (IQR 2.5–13.0) from home) via public transport for 154 (54.6%). Most children (254, 90.1%) lived within the Cape Town metropol.
Table 1. Demographics, acute referral timeline and clinical outcomes for the cohort of children.
| DEMOGRAPHICS | Medical (n = 239) | Trauma (n = 43) | Total (n = 282) |
|---|---|---|---|
| Gender: Male | 137(57.3%) | 27(62.8%) | 164(58.2%) |
| Age of Child Median (months) (IQR) | 4.8(2.2–20.2) | 63.5(23.5–63.5) | 7.8(2.5–33.6) |
| <1 month | 30(12 6%) | 0(0 0%) | 30(10 6%) |
| 1 month to 1 year | 132(55 2%) | 5(11 6%) | 137(48 6%) |
| 1 year to 5 years | 55(23 0%) | 16(37 2%) | 71(25 2%) |
| >5 years | 22(9 2%) | 22(51 2%) | 44(15 6%) |
| Distance from Health Facility | |||
| Nearest facility (km) Median(IQR) | 2 0(0 8–4 0) | 1 0(0 5–2 0) | 2 0(0 5–3 0) |
| Nearest 24 hour facility (km) Median(IQR) | 6 0(3 0–12 0) | 7 0(2 0–15 0) | 6 0(2 5–13 0) |
| Diagnosis | |||
| Traumaa | - | 43(100.0%) | 43(15.3%) |
| cardiacb | 30(12.6%) | - | 30(10.6%) |
| gastroenteritis | 13(5.4%) | - | 13(4.6%) |
| neurological-meningitis/epilepsyc | 20(8.4%) | - | 20(7.1%) |
| respiratory diseased | 102(42.7%) | - | 102(36.2%) |
| sepsis/ septic shocke | 42(17.6%) | - | 42(14.9%) |
| otherf | 32(13.4%) | - | 32(11.3%) |
| Expected weight for age (z-score)g | (n = 227) | (n = 32) | (n = 259) |
| z < -3 | 67(29 5%) | 0(0 0%) | 67(25 9%) |
| -3 < z < -2 | 30(13 2%) | 0(0 0%) | 30(11 6%) |
| z > -2 | 130(57 3%) | 32(100%) | 162(62 5%) |
| Length of pathway all children (Median (IQR)) | (n = 239) | (n = 43) | (n = 282) |
| Onset of illness to first presentation (days) | 2 (0–3.0) | 0 (0.0–0.0) | 1 (0.0–3.0) |
| First presentation to RCWMCH arrival (hours) | 4.4 (1.9–9.2) | 1.9 (1.0–5.2) | 4.2 (1.7–8.9) |
| First presentation to PICU admission (hours) | 13.8 (7.3–46.0) | 9.8 (6.3–16.0) | 12.3 (6.9–39.6) |
| RCWMCH arrival to PICU admission (hours)h | 5.0 (2.4–15.9) | 5.5 (3.1–8.1) | 5.0 (2.5–12.9) |
| EMS activation to destination facility (minutes)i | (n = 237) | (n = 55) | (n = 292) |
| 86.0 (56.0–124.0) | 80.0 (48.0–128.0) | 86.0 (54.0–124.0) | |
| Clinical Outcomes for PICU Admissions only | Medical (n = 218) | Trauma (n = 34) | Total (n = 252) |
| Outcome at 30 days | |||
| Death in/after PICU | 26(11.9%) | 2(5.9%) | 28(11.1%) |
| Discharge home | 150(68.8%) | 13(38.2%) | 163(64.7%) |
| Remain inpatient | 42(19.3%) | 19(55.9%) | 61(24.2%) |
| Median (IQR) | Median (IQR) | Median (IQR) | |
| Risk of mortalityj (PIM2%) | 6.9(1.8–18.2) | 7.6(4.6–12.6) | 6.9(2.0–16.6) |
| PICU Length of stay (hours) | 73.6 (43.0–159.4) | 94.5 (43.6–218.7) | 76.9 (43.0–164.0) |
| Total RCWMCH Length of stay (days) | 10.5(7.0–20.0) | 15.0(9.8–25.8) | 11.0 (7.0–21.0) |
IQR inter quartile range; RCWMCH Red Cross War Memorial Children’s Hospital; PICU paediatric intensive care unit; EMS emergency medical services
a trauma: road traffic accidents(28), burns (8) and other (7) non road traffic accident injury
b cardiac: congenital heart disease (17) and myocarditis/ cardiomyopathy (13)
c neurology includes meningitis (14), epilepsy(3);
d respiratory: infective (pneumonia/bronchiolitis) (82); obstructive airway/croup/asthma (13)
e sepsis/ septic shock: neonatal (18), older infants/ children (24)
f other includes: surgical (12),death unknown causes (7), overdose (3), drowning (2), renal failure, diabetic keto-acidosis, hepatic failure
g z-score—WHO Global Database on Child Growth and Malnutrition (data incomplete–no age/ weight z score for > 10 year olds)
h 32 patients went directly to PICU on arrival at RCWMCH (all had been previously accepted by PICU with a bed reserved for them); medical(31), trauma (1)
i EMS was not utilized by all cases but some cases had more than one EMS transfer
j on admission to PICU PIM2 score–Paediatric Index of Mortality [21]
Pathway Characteristics
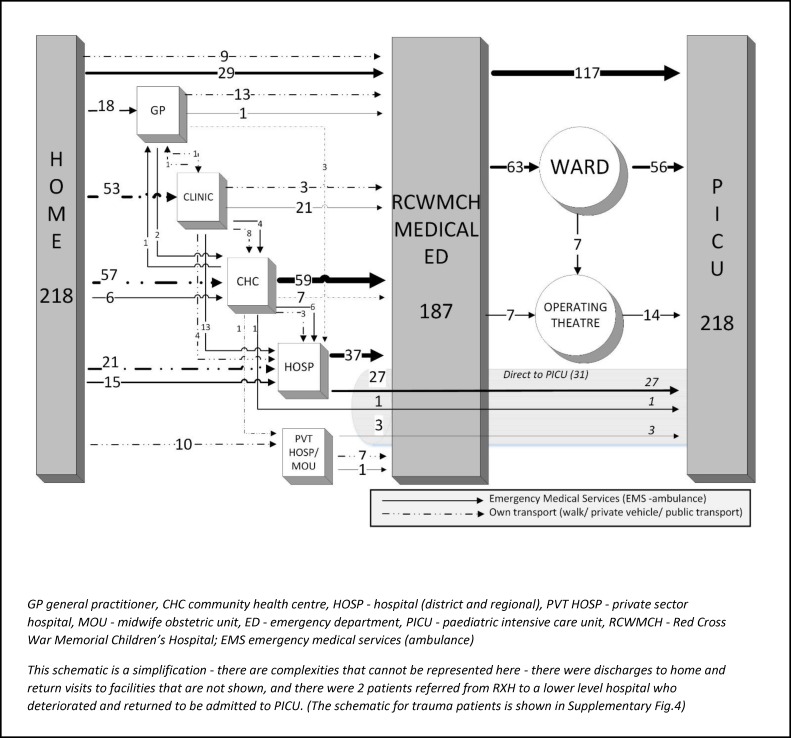
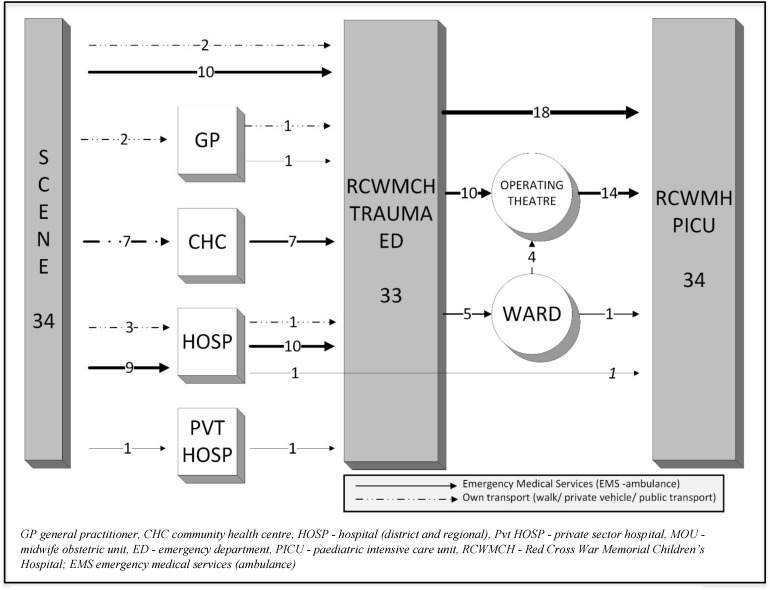
Over the study period data were collected from 641 consultations across 22 GPs, 32 Clinics, 6 CHCs, 20 Regional/District hospitals, 292 EMS transfers, and from RCWMCH. A fifth (52/239, 21.8%) of caregivers of children with medical problems had consulted elsewhere prior to their first presentation at a healthcare facility and only a third (92/282, 32.6%) of caregivers knew the number to call for EMS. Most children (140/239, 58.6%) with medical illness accessed a primary healthcare facility (GP, Clinic or CHC), while trauma cases were more often seen initially by EMS (25/43, 58.1%) rather than by primary health care (12/43, 27.9%). The majority presented outside working hours (148/239, 61.9% medical cases; 34/43, 79.1% trauma cases). Prior to arrival at RCWMCH, 170/282 (60.3%) children were seen at just a single facility. Children’s pathways from home through healthcare facilities to the PICU were often highly complex, particularly for medical cases (Figs 3 and 4).
Fig 3. Schematic of the referral pathway for medical patients admitted to Paediatric Intensive Care.
Fig 4. Schematic of the referral pathway for trauma patients admitted to Paediatric Intensive Care.
Timing of transitions through the pathway
All children with trauma presented to RCWMCH on the day of injury, whilst 76.6% (183/239) of medical cases presented within 3 days of illness onset (Table 1). Overall, 179 (75%) of medical patients arrived at RCWMCH within 9.2 hours of presenting to the first facility, and for trauma (32) (75%) of trauma patients within 5.2 hours. For those children admitted to PICU, 135/252 (53.6%) were admitted directly from the ED in a median of 4.1 hours (IQR 2.7–5.8). Other children were admitted to wards and had a longer and often overnight stay prior to PICU admission (S4 Table).
Child Based Outcomes
On admission to PICU, the median predicted risk of mortality (PIM2) for the medical cases was 6.9%, and 7.6% for trauma (Table 1). Total RCWMCH length of stay was longer for the trauma cases (median 15.0 days, IQR 9.8–25.8) than the medical cases (10.5 days, IQR 7.0–20.0). By day 30, almost two-thirds of the children had been discharged (163/252, 64.7%), a quarter remained in hospital (61/252, 24.2%) and the remainder had died (28/252, 11.1%).
The global quality of care was graded good in 29 (10.3%) of all cases, with the majority graded as fair (196, 69.5%) (Table 2). For the 30 children who died prior to PICU admission, death was considered avoidable or potentially avoidable in more than half (17, 56.7%). For the 252 admitted to PICU, admission was considered avoidable or potentially avoidable for 61 (24.2%), and the severity of illness/injury on admission avoidable or potentially avoidable in 185 (73.4%). Three quarters of cases (210, 74.5%) had clear or likely health system issues. A total of 3212 modifiable factors were identified for the 282 children, with half (141, 50.0%) having at least one major impact modifiable factor, whilst the majority (267, 94.7%) had at least one moderate impact factor. The two most frequent major impact modifiable factors associated with poor outcomes (i.e. poor global quality of care and avoidable: severity of illness, PICU admission and death) (S5 Table) were: inadequate initial assessment/interpretation of severity, and resuscitation not done or inadequate for a shocked patient. Others associated with poor outcomes were: delays in critical management decisions, inadequate access to emergency care or personnel, and referral delays.
Table 2. Outcomes of Expert Review.
| Medical | Trauma | Total | |
|---|---|---|---|
| Global Quality of Carea | (n = 239) | (n = 43) | (n = 282) |
| Poor | 55(23.0%) | 2(4.7%) | 57(20.2%) |
| Fair | 166(69.5%) | 30(69.8%) | 196(69.5%) |
| Good | 18(7.5%) | 11(25.6%) | 29(10.3%) |
| Avoidability of Death | (n = 21) | (n = 9) | (n = 30) |
| Not Avoidable | 6(28.6%) | 7(77.8%) | 13(43.3%) |
| Potentially Avoidable | 12(57.1%) | 2(22.2%) | 14(46.7%) |
| Avoidable | 3(14.3%) | 0(0.0%) | 3(10.0%) |
| Avoidability of PICU | (n = 218) | (n = 34) | (n = 252) |
| Not Avoidable | 161(73.9%) | 30(88.2%) | 191(75.8%) |
| Potentially Avoidable | 52(23.9%) | 4(11.8%) | 56(22.2%) |
| Avoidable | 5(2.3%) | 0(0.0%) | 5(2.0%) |
| Avoidability of Severity | (n = 218) | (n = 34) | (n = 252) |
| Not Avoidable | 49(22.5%) | 18(52.9%) | 67(26.6%) |
| Potentially Avoidable | 155(71.1%) | 15(44.1%) | 170(67.5%) |
| Avoidable | 14(6.4%) | 1(2.9%) | 15(6.0%) |
| System Issuesb | (n = 239) | (n = 43) | (n = 282) |
| No | 36(15.1%) | 36(83.7%) | 72(25.5%) |
| Possibly | 72(30.1%) | 3(7.0%) | 75(26.6%) |
| Yes | 131(54.8%) | 4(9.3%) | 135(47.9%) |
| Number of Modifiable Factorsc median (range; IQR) | |||
| Major Impact | 1 (0–16; 0–3) | 0 (0–8; 0–1) | 1 (0–16; 0–3) |
| Moderate Impact | 6 (0–19; 4–10) | 5 (0–13; 3–8) | 6 (0–19; 3–9) |
PICU paediatric intensive care unit; IQR inter quartile range
a grading of quality of care was performed relative to the expectations of reviewers: poor—health care which was clearly below the average expectations of the facility/health care provider (HCP); fair–health care of an average level expected of the facility/HCP; good–health care at an excellent level above average expectations
b System Issues—defined as potential healthcare interventions prior to the acute episode which could have had a positive impact on the health of the child prior to the acute critical illness. (e.g. missing long term deterioration at a prior consultation or inadequate follow up of a high risk baby)
c grading of Modifiable Factors: major (clear negative impact on the outcome for the patient), moderate (minimal negative impact on the outcome but likely caused some morbidity and/or extended the illness duration) (e.g. failure to administer a fluid bolus in a shocked child would be a major MF, delay in administration of antibiotics to a child with respiratory distress (of unclear aetiology) a moderate MF)
(a total of 3212 modifiable factors were identified for the entire cohort (comprising 477 (14.95) major, 1826 (56.9%) moderate, 44 (1.4%) near miss, 290 (9.0%) no defined impact and 575 (17.9%) unknown impact modifiable factors)
Facility Based Outcomes
The five most frequent major and medium impact factors for consultations at the main facilities are presented in Table 3. At both the clinics and 24hr CHCs, there were four common major impact modifiable factors: inadequate access to emergency care or personnel, inadequate assessment/interpretation of severity, resuscitation not done or inadequate and management of shock/ circulatory collapse. A similar pattern was noted in hospital EDs, where the four common major impact modifiable factors were: inadequate assessment/interpretation of severity, resuscitation not done or inadequate, delays in critical management decisions and issues with antibiotic therapy (additional details in S6–S8 Tables).
Table 3. Five most frequently identified major and moderate impact modifiable factors for the main facilities and for EMS transfers.
| Major Modifiable Factorsa | N(%b) | Moderate Modifiable Factorsa | N(%b) |
|---|---|---|---|
| City Health Clinic (n = 57 consultations) | |||
| Accessibility of Emergency Care area/ personnel | 12(21.1) | Accessibility of Emergency Care area/ personnel | 15(26.3) |
| Inadequate assessment at triage | 10(17.5) | Inadequate assessment at triage | 11(19.3) |
| Inadequate assessment/ interpretation of severity | 8(14.0) | Antibiotic therapy | 11(19.3) |
| Resuscitation not done/ inadequate for shocked patient | 7(12.3) | Ventilatory Management | 9(15.8) |
| Circulatory management | 4(7.0) | Explanation to caregiver | 9(15.8) |
| 24 Hour CHC (n = 103 consultations) | |||
| Resuscitation not done/ inadequate for shocked patient | 25(24.3) | Antibiotic therapy | 25(24.3) |
| Inadequate assessment/ interpretation of severity | 17(16.5) | Inadequate assessment/ interpretation of severity | 24(23.3) |
| Circulatory management | 14(13.6) | Explanation to caregiver | 24(23.3) |
| Accessibility of Emergency Care area/ personnel | 10(9.7) | Accessibility of Emergency Care area/ personnel | 22(21.4) |
| Missing key findings (history/ clinical) | 6(5.8) | Ongoing monitoring/management while awaiting transfer | 19(18.4) |
| Hospital (District & Regional) (n = 95) | |||
| Inadequate assessment/ interpretation of severity | 8(8.4) | Ongoing monitoring/management while awaiting transfer | 20(21.1) |
| Resuscitation not done/inadequate for shocked patient | 7(7.4) | Delay in disposal decisions | 20(21.1) |
| Accessibility of emergency care area/ personnel | 5(5.3) | Antibiotic therapy | 14(14.7) |
| Delay in critical management decisions | 5(5.3) | Accessibility of Emergency Care area/ personnel | 12(12.6) |
| Antibiotic therapy | 4(4.2) | Delay in critical management decisions | 12(12.6) |
| RCWMCH ED (n = 241 consultations) | |||
| Referral Delay | 11(4.6) | Ongoing monitoring/management while awaiting transfer | 106(44.0) |
| Resuscitation not done/inadequate for shocked patient | 11(4.6) | Referral Delay | 97(40.2) |
| Inadequate assessment/ interpretation of severity | 9(3.7) | Antibiotic therapy | 51(21.2) |
| Delay in critical management decisions | 7(2.9) | Delay in critical management decisions | 47(19.5) |
| Antibiotic therapy | 4(1.7) | Delay in disposal decisions | 34(14.1) |
| EMS (n = 292 transfers) | |||
| Inappropriate vehicle/ crew/ equipment | 20(6.8%) | Explanation to caregiver | 67(22.9%) |
| Response time delay | 19(6.5%) | Inappropriate vehicle/ crew/ equipment | 65(22.3%) |
| Inadequate stabilization for transfer | 10(3.4%) | Inadequate monitoring en route | 44(15.1%) |
| Inadequate assessment before transfer | 6(2.1%) | Response time delay | 43(14.7%) |
| Dispatch time delay | 6(2.1%) | Inadequate assessment before transfer | 43(14.7%) |
CHC community health centre; RCWMCH Red Cross War Memorial Children’s Hospital; ED Emergency Department; EMS emergency medical services
aModifiable Factor Impact: Major–factor which had clear negative impact on the outcome for the patient (worsened mortality or morbidity)—a directly and overwhelmingly important factor in the severity of illness/ death; Moderate–factor which on its own had minimal negative impact on the outcome but may have caused some morbidity and/ or extended the hospital/ PICU stay
b Percent of modifiable factor per consultations/ transfers at each facility/ EMS transfer
Agreement between the internal clinical reviewers and the consensus (after discussion) for the four main outcomes was moderate to substantial (kappa ranged from 0.471–0.864). The external reviewer generally rated care as better than the internal reviewers, with lower agreement (kappa ranged from 0.339–0.458; not estimable for avoidability of death (small numbers) and negative for global quality of care).
Each of the applicable standards (S2 Table) were applied to each facility visit and EMS transfer. A variable number of standards were applied to each site/transfer dependent on the context, diagnosis and information available. The rate of compliance with all standards, increased from primary health care sites (23.5% for GP, and 50.9% for clinics) through to hospital based sites (74.4% for district hospitals and 77.5% at RCWMCH ED) (S9 Table). Compliance with standards was higher for all types of EMS contacts (82.3% overall).
Discussion
This study evaluated the nature and the quality of care for critically ill or injured children within the setting of a metropolitan health service of a middle income country which is relevant to many other settings globally. Overall quality of healthcare was good in only a small minority of children; our findings demonstrate that the pathway to care from initial healthcare contact in community settings to PICU admission or death is complex and has considerable scope for improvement. The most frequent clinical failings were in the areas of assessment and initial resuscitation, compounded by delays in decision making and referral.
The methodology was based on the CEMACH approach, however, there are a number of unique features. This is the first study to review clinical care of a large number of critically ill children (with a wide range of diagnoses) in a resource constrained setting, with high enrolment rates and using a detailed review process, with the perspective of the family from caregiver interviews. The methodology in this study has provided information on multiple aspects of quality of care and has highlighted the complexity of quality analysis in a healthcare system which will have relevance to healthcare systems at all levels of resource availability.
Families of patients admitted to the study were poorer, more likely to be unemployed and to live in informal dwellings when compared to the provincial census data (S3 Table) [20]. This may have contributed to the difficulties in accessing healthcare as reflected by multiple visits to healthcare facilities prior to the onset of the life-threatening episode, delays in seeking help, and underutilization of emergency services.
Given that most families did not have access to private transport, the distance to after-hours healthcare facilities (the commonest site of acute presentations) is a significant challenge for improvement. Although individual facilities may be able to upscale after-hours resources, a system wide approach including empowering and educating parents about when, where and how to access care is indicated. Improving the ways parents can communicate concerns about acutely unwell children; alerting parents of high risk neonates on worrying signs and emergency access are two examples.
The high frequency of specific clinical failings in the pathway suggests further areas for interventions. Particular diagnoses such as respiratory tract infections were both common and inadequately managed, suggesting an educational intervention could be focussed on a relatively small group of conditions. The CEMACH enquiry [15] highlighted that failure, to recognise and manage serious infection was the most frequent avoidable factor in primary care. While poor recognition of illness severity in infants and small children is a common problem in EDs and district or regional hospitals [25], data from the primary care setting has highlighted the non-specificity of clinical signs in the early phases of severe sepsis [26]. Studies of children with meningococcemia [25, 27] and severe bacterial infection [28] in well-resourced settings, have identified recognition and management of seriously ill children as key issues. Education and training could focus on recognition of acutely unwell children, including recognition of ‘red flag’ features, measurement of vital signs and accurate triage scoring, and the value of parental and clinician ‘gut feeling’ (including the potential significance of repeat visits).
A body of evidence in hospitalised children in high resource settings has demonstrated the effectiveness of early warning systems for recognition of critically ill and deteriorating children [29–32]. Thus investment in quality of care improvement in CHCs and EDs (at all levels), in keeping with data from Kenya [33–36], would appear to be potentially more effective in improving outcomes, particularly as these centres saw many ill children.
A major concern is the duration of the pathway to PICU admission, which were consistently unacceptably long [37]. Pathways were complex, on occasion reflecting developing illness, but omission of certain steps could have substantially reduced the time taken. Referral delays could be reduced by optimizing system efficiencies, such as fast tracking patients directly to PICU, better prioritization of EMS services, and early warning systems in the hospital setting. A centralized paediatric emergency line, could be a useful avenue for emergency advice, as well as facilitating decision making and co-ordination of appropriate transfer and referral destination. The longest delays were in the EDs of the tertiary referral hospital, often a relating to access to the intensive care unit.
The study had some limitations. We enrolled a smaller sample of children than anticipated due to stringent exclusion criteria but since the proportion of children with good clinical care (10%), was lower than originally expected, recruitment of 282 children provided a precision of 4% (95% CI 7%-14%) consistent with what was felt to be clinically meaningful during study design. Reviewers were not blinded to the case outcome which may have introduced a bias when reviewing care, but we attempted to reduce this bias by clear, written definitions of categories, multiple reviews and consensus building. Agreement between different reviewers was initially moderate, but consensus was consistently achieved. The external reviewer (London based) consistently graded care more optimistically than the local reviewers which we attribute to incomplete insights to the local system. Although this might suggest a bias by the internal investigators to more stringent assessment of the system, we believe through the use of objective standards of care, and the systematic, blinded and multiparty review process we have minimized such bias.
Finally, there needs to be greater emphasis on an organization and systems approach rather than each step in isolation. Most systems focus on resource utilization efficiency (ensuring that the medical personnel throughout the system are fully utilized) rather than on “flow efficiency” (ensuring that the patient receives the most timely and effective care throughout the system) [2]. Major improvements could be achieved through simultaneous interventions at different levels of the healthcare system [38]. Policy makers and healthcare managers in this environment have already been struck by the implications and insights from this study. Further research is planned using elements of this process for routine quality assessment, and implementation of changes.
Conclusions
Using a novel methodology and approach we have plotted the pathways followed by critically ill children from first presentation through to PICU admission or death. We highlight the complexity of that pathway and focus attention on specific issues including access to care and the importance of continuity and quality of care at each step throughout a process. Frequently occurring modifiable events were identified at each step along the pathway which can inform future interventions. Any attempts at improvement of quality of care and patient outcome should be linked to well-conducted evaluations and will need to be focussed on entire systems and not simply on components of that system.
Supporting Information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
The authors are grateful to the caregivers of the children involved in this study, especially those recently bereaved. We thank all of the healthcare workers and facility managers for their willing participation and interest in the study. We are grateful to Sisters Rencia Gillespie and Wendy Rosenthal for their role in the data collection, and the interview team of Nontobeko Jacobs, Andile Mayekiso, and Janine Pheiffer. The online database was developed by Al Pirrie of Cache Ltd.
Data Availability
The database cannot be made available due to patient and health provider confidentiality. Data are available upon request. Requests for the data may be sent to the corresponding author (pwhodkinson@gmail.com).
Funding Statement
The study was funded by the Wellcome Trust (WT091107MA) and the Nuffield Department of Primary Care Health Sciences, Oxford University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Institute of Medicine Committee on Data Standards for Patient Safety. Achieving a New Standard for Care. Washington, DC: The National Academies Press; 2004. [PubMed] [Google Scholar]
- 2.Modig N, Åhlström P. This is Lean: Resolving the Efficiency Paradox. Stockholm: Rheologica Publishing; 2012. [Google Scholar]
- 3.Kissoon N, Carcillo JA, Espinosa V, Argent A, Devictor D, Madden M, et al. World Federation of Pediatric Intensive Care and Critical Care Societies: Global Sepsis Initiative. Pediatr Crit Care Med. 2011;12(5):494–503. 10.1097/PCC.0b013e318207096c [DOI] [PubMed] [Google Scholar]
- 4.Bhutta ZA, Das JK, Walker N, Rizvi A, Campbell H, Rudan I, et al. Interventions to address deaths from childhood pneumonia and diarrhoea equitably: what works and at what cost? Lancet. 2013;381(9875):1417–1429. 10.1016/S0140-6736(13)60648-0 [DOI] [PubMed] [Google Scholar]
- 5.Paul R, Neuman MI, Monuteaux MC, Melendez E. Adherence to PALS Sepsis Guidelines and Hospital Length of Stay. Pediatrics. 2013;130:e273–280. [DOI] [PubMed] [Google Scholar]
- 6.Lewis G. The Confidential Enquiry into Maternal and Child Health (CEMACH). Saving Mothers’ Lives: reviewing maternal deaths to make motherhood safer- 2003–2005 The Seventh Report on Confidential Enquiries into Maternal Deaths in the United Kingdom. London: CEMACH, 2007. 9780953353682. [Google Scholar]
- 7.Pearson GA, Ward-Platt M, Harnden A, Kelly D. Why children die: avoidable factors associated with child deaths. Arch Dis Child. 2011;96(10):927–931. 10.1136/adc.2009.177071 [DOI] [PubMed] [Google Scholar]
- 8.Weindling A. The confidential enquiry into maternal and child health (CEMACH). Arch Dis Child. 2003;88(12):1034–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drife J. Maternal mortality in well-resourced countries: is there still a need for confidential enquiries? Best Pract Res Cl Ob. 2008;22(3):501–15. [DOI] [PubMed] [Google Scholar]
- 10.Drife J. The value of confidential enquiries. S Afr J Obstet. 2012;18:32–36. [Google Scholar]
- 11.Papworth S, Cartlidge P. Learning from adverse events—the role of confidential enquiries. Semin Fetal Neonat. 2005;10(1):39–43. [DOI] [PubMed] [Google Scholar]
- 12.Committee on Child Abuse and Neglect, Committee on Injury Violence and Poison Prevention, Council on Community Pediatrics. American Academy of Pediatrics. Policy statement-child fatality review. Pediatrics. 2010;126(3):592–596. 10.1542/peds.2010-2006 [DOI] [PubMed] [Google Scholar]
- 13.Devlin B. Confidential enquiry into perioperative deaths. J Roy Soc Health. 1985;78(8): 698. [PMC free article] [PubMed] [Google Scholar]
- 14.Fleming PJ, Blair PS, Bacon C, Bensley D, Smith I, Taylor E, et al. Environment of infants during sleep and risk of the sudden infant death syndrome: results of 1993–5 case-control study for confidential inquiry into stillbirths and deaths in infancy. Confidential Enquiry into Stillbirths and Deaths Regional Coordinators and Researchers. BMJ. 1996;313(7051):191–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harnden A, Mayon-White R, Mant D, Kelly D, Pearson G. Child deaths: confidential enquiry into the role and quality of UK primary care. Br J Gen Pract. 2009;59(568):819–824. 10.3399/bjgp09X472520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mathews B, Kenny MC. Mandatory reporting legislation in the United States, Canada, and Australia: a cross-jurisdictional review of key features, differences, and issues. Child maltreatment. 2008;13(1):50–63. 10.1177/1077559507310613 [DOI] [PubMed] [Google Scholar]
- 17.Mohan G, Harrison BD, Badminton RM, Mildenhall S, Wareham NJ. A confidential enquiry into deaths caused by asthma in an English health region: implications for general practice. Brit J Gen Prac. 1996;46(410):529–532. [PMC free article] [PubMed] [Google Scholar]
- 18.Rimsza ME, Schackner RA, Bowen KA, Marshall W. Can Child Deaths Be Prevented? The Arizona Child Fatality Review Program Experience. Pediatrics. 2002;110: e11 [DOI] [PubMed] [Google Scholar]
- 19.Berry L, Biersteker L, Dawes A, Lake L, Smith C, (eds). South African Child Gauge 2013. Cape Town: Children's Institute, University of Cape Town, 2013. [Google Scholar]
- 20.Statistics South Africa. Census 2011 Municipal Report Western Cape. Pretoria: Statistics South Africa, 2012. 9780621414592. [Google Scholar]
- 21.Slater A, Shann F, Pearson G. Paediatric Index of Mortality Study G. PIM2: a revised version of the Paediatric Index of Mortality. Intensive Care Med. 2003;29(2):278–285. [DOI] [PubMed] [Google Scholar]
- 22.Child Healthcare Problem Identification Programme (ChIP). [Accessed 22 July 2015]. Available: http://www.childpip.org.za/.
- 23.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–74. [PubMed] [Google Scholar]
- 24.IBM Corp. IBM SPSS Statistics for Windows. 21.0 ed. Armonk, NY: IBM Corp; 2012. [Google Scholar]
- 25.Ninis N, Phillips C, Bailey L, Pollock JI, Nadel S, Britto J, et al. The role of healthcare delivery in the outcome of meningococcal disease in children: case-control study of fatal and non-fatal cases. BMJ 2005;330(7506):1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van den Bruel A, Thompson M, Buntinx F, Mant D. Clinicians' gut feeling about serious infections in children: observational study. BMJ. 2012;345:e6144 10.1136/bmj.e6144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nadel S, Britto J, Booy R, Maconochie I, Habibi P, Levin M. Avoidable deficiencies in the delivery of health care to children with meningococcal disease. J Accid Emerg Med. 1998;15(5):298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Launay E, Gras-Le Guen C, Martinot A, Assathiany R, Blanchais T, Mourdi N, et al. Suboptimal care in the initial management of children who died from severe bacterial infection: a population-based confidential inquiry. Pediatr Crit Care Med 2010;11(4):469–74. 10.1097/PCC.0b013e3181ce752e [DOI] [PubMed] [Google Scholar]
- 29.Tucker KM, Brewer TL, Baker RB, Demeritt B, Vossmeyer MT. Prospective evaluation of a pediatric inpatient early warning scoring system. J Spec Pediatr Nurs. 2009;14(2):79–85. 10.1111/j.1744-6155.2008.00178.x [DOI] [PubMed] [Google Scholar]
- 30.Egdell P, Finlay L, Pedley DK. The PAWS score: validation of an early warning scoring system for the initial assessment of children in the emergency department. Emerg Med J. 2008;25(11):745–9. 10.1136/emj.2007.054965 [DOI] [PubMed] [Google Scholar]
- 31.Seiger N, Maconochie I, Oostenbrink R, Moll HA. Validity of different pediatric early warning scores in the emergency department. Pediatrics. 2013;132(4):e841–50. 10.1542/peds.2012-3594 [DOI] [PubMed] [Google Scholar]
- 32.Brent AJ, Lakhanpaul M, Thompson M, Collier J, Ray S, Ninis N, et al. Risk score to stratify children with suspected serious bacterial infection: observational cohort study. Arch Dis Child. 2011;96(4):361–7. 10.1136/adc.2010.183111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barasa EW, Ayieko P, Cleary S, English M. A multifaceted intervention to improve the quality of care of children in district hospitals in Kenya: a cost-effectiveness analysis. PLoS Med. 2012;9(6):e1001238 10.1371/journal.pmed.1001238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Campbell H, Duke T, Weber M, English M, Carai S, Tamburlini G, et al. Global initiatives for improving hospital care for children: state of the art and future prospects. Pediatrics. 2008;121(4):e984–992. 10.1542/peds.2007-1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.English M. Child survival: district hospitals and paediatricians. Arch Dis Child. 2005;90(9):974–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.English M, Ntoburi S, Wagai J, Mbindyo P, Opiyo N, Ayieko P, et al. An intervention to improve paediatric and newborn care in Kenyan district hospitals: understanding the context. Implement Sci. 2009;4:42 10.1186/1748-5908-4-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mooney H. NHS must monitor time from presentation to treatment for all child patients, report says. BMJ. 2012;345:e5138 10.1136/bmj.e5138 [DOI] [PubMed] [Google Scholar]
- 38.Molyneux E, Ahmad S, Robertson A. Improved triage and emergency care for children reduces inpatient mortality in a resource-constrained setting. Bull World Health Organ. 2006;84(4):314–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
The database cannot be made available due to patient and health provider confidentiality. Data are available upon request. Requests for the data may be sent to the corresponding author (pwhodkinson@gmail.com).