Abstract
Background
Chronic alcohol abuse is associated with intestinal bacterial overgrowth, increased intestinal permeability, and translocation of microbial products from the intestine to the portal circulation and liver. Translocated microbial products contribute to experimental alcoholic liver disease.
Aim
To investigate the physiological relevance of the intestinal microbiota in alcohol-induced liver injury.
Methods
We subjected germ-free and conventional C57BL/6 mice to a model of acute alcohol exposure that mimics binge drinking.
Results
Germ-free mice showed significantly greater liver injury and inflammation after oral gavage of ethanol compared with conventional mice. In parallel, germ-free mice exhibited increased hepatic steatosis and upregulated expression of genes involved in fatty acid and triglyceride synthesis compared with conventional mice after acute ethanol administration. The absence of microbiota was also associated with increased hepatic expression of ethanol metabolizing enzymes, which led to faster ethanol elimination from the blood and lower plasma ethanol concentrations. Intestinal levels of ethanol metabolizing genes showed regional expression differences, and were overall higher in germ-free relative to conventional mice.
Conclusion
Our findings indicate that absence of the intestinal microbiota increases hepatic ethanol metabolism and the susceptibility to binge-like alcohol drinking.
Keywords: microbiome, alcoholic liver disease, microbiota, endotoxin, steatohepatitis
Introduction
Alcoholic liver disease is a leading cause of morbidity and mortality worldwide. Following absorption from the upper gastrointestinal tract, alcohol is primarily metabolized in hepatocytes. Ethanol oxidation results in generation of NADH, which inhibits fatty acid oxidation, but favors fatty acid and triglyceride synthesis in hepatocytes. Fatty liver disease can progress via hepatocellular damage and parenchymal inflammation to liver fibrosis and cirrhosis (European Association for the Study of L, 2012).
The intestinal microbiota contributes to alcoholic liver disease (Adachi et al., 1995). Patients with chronic alcohol abuse show increased intestinal permeability. Microbial products such as lipopolysaccharide (LPS or endotoxin) escape from the intestinal lumen through disrupted epithelial tight junction complexes and enter the circulation (Parlesak et al., 2000; Keshavarzian et al., 1994). Small intestinal bacterial overgrowth increases the amount of translocated pathogen associated molecular patterns (PAMPs) (Bode et al., 1984; Schnabl and Brenner, 2014), while alcohol-associated enteric dysbiosis (Mutlu et al., 2012; Yan et al., 2011; Bull-Otterson et al., 2013) contributes to a disruption of the protective mucosal barrier (Chen et al., 2015b). PAMPs reach the liver via the portal circulation, where they can contribute to hepatocyte damage and hepatic inflammation (Uesugi et al., 2001; Petrasek et al., 2013). Non-absorbable oral antibiotics decrease the amount of intestinal bacteria, stabilize the gut barrier and reduce experimental alcoholic liver disease (Chen et al., 2015b; Adachi et al., 1995). Maintaining intestinal eubiosis is beneficial in preclinical models of alcoholic liver disease and in patients with chronic alcohol abuse (Chen et al., 2015a; Kirpich et al., 2008). Together, these findings indicate that translocation of microbial products with subsequent immune activation in the liver is important for promoting alcoholic liver disease, suggesting that the microbiota is a driver of alcoholic liver disease. Consequently, its complete loss would be predicted to protect against disease. In the present study, we formally tested this hypothesis in germ-free mice exposed to a single (binge) dose of ethanol.
Material and Methods
Animal model
Germ-free C57BL/6 mice were bred and maintained at the gnotobiotic facility of the University of California, San Diego. Some of the germ-free mice were originally obtained from Germ-free & Gnotobiotic Mouse Facilities at the University of Michigan in Ann Arbor. Germ-free mice had access to water and autoclaved chow diet (2019S Teklad Global 19% Protein Extruded Rodent Diet, Harlan Laboratories; 23% calories from protein, 22% from fat, 55% from carbohydrates) ad libitum. Germ-free status was confirmed weekly by standard microbiological cultures of fecal extracts, using thioglycollate broth and potato dextrose broth for facultative anaerobes including fungi, and cooked meat broth, heart infusion broth, and Columbia blood agar for obligate anaerobes. As controls, C57BL/6 mice were bred under conventional, specific pathogen free conditions at the University of California, San Diego, and treated in parallel with germ-free mice using the same lot of diluted ethanol. Female mice were age-matched and had access to water and irradiated chow diet (5053 PicoLab Rodent Diet 20, Lab Diet; 25% calories from protein, 13% from fat, 62% from carbohydrates) ad libitum. Acute alcoholic liver injury was induced by a single gavage of 300 μl of 30% (vol/vol) ethanol at a dose of 3 g/kg. Control mice were gavaged with an isocaloric amount of dextrose (300 μl, 0.42 g/ml) at a dose of 5.2 g/kg. The entire experiment involving germ-free mice was performed in sterile isolators. Mice were removed from the isolators 9 hours after gavage and tissues were harvested. All animal studies were reviewed and approved by the Institutional Animal Care and Use Committee of the University of California, San Diego.
Histological analysis
Formalin fixed tissues were stained with hematoxylin and eosin, and analyzed by microscopy. For hepatic lipid accumulation analysis, frozen sections were cut and stained with Oil Red O (Chen et al., 2015b). F4/80 (eBioscience) immunofluorescence staining was performed as described (Chen et al., 2015a). Ten random fields per slide were chosen for quantification. Immunohistochemistry using an antibody directed against phospho-acetyl-CoA carboxylase (p-ACC; Cell Signaling) was performed following standard protocols.
Immunoblotting analysis
Liver lysates were prepared and immunoblotting was performed as described (Schnabl et al., 2001)) using primary antibodies against cytochrome p450 enzyme 2E1 (CYP2E1) (Millipore Corporation), sterol regulatory element binding transcription factor 1 (SREBP-1) (Santa Cruz) and β-Actin (Sigma-Aldrich).
Bacterial DNA isolation and bacterial qPCR
Cecal DNA extraction, bacterial qPCR for 16S rRNA, and analysis of total bacteria load was performed as described (Chen et al., 2015b).
Real-time PCR analysis
RNA was extracted from mouse tissues using Trizol (Invitrogen), and reverse transcription was performed using the High Capacity cDNA Reverse Transcription kit (ABI). Real-time qPCR was performed with Sybr Green supermix (BioRad) using primer sequences obtained from NIH qPrimerDepot. All mRNA levels are expressed relative to the value in conventional control mice.
Biochemical analyses
Plasma alanine aminotransferase (ALT) level was measured by Infinity ALT kit (Thermo Scientific). Plasma ethanol concentration was determined using the Ethanol Assay Kit (BioVision). Plasma and liver triglyceride levels were assessed using the Triglyceride Liquid Reagents Kit (Pointe Scientific). Hepatic alcohol dehydrogenase (ADH) activity was measured with a commercial enzyme kit (BioVision).
Statistical analysis
Mann-Whitney rank sum test was used for comparison of two groups. For analysis of four groups with normal distribution, one-way ANOVA analysis followed by Tukey test was performed; if samples were not normally distributed, the Kruskal-Wallis test with Dunns Post-test was performed. All data are presented as mean ± SEM, with p <0.05 considered as significant.
Results
Acute alcohol-induced liver injury is increased in germ-free mice
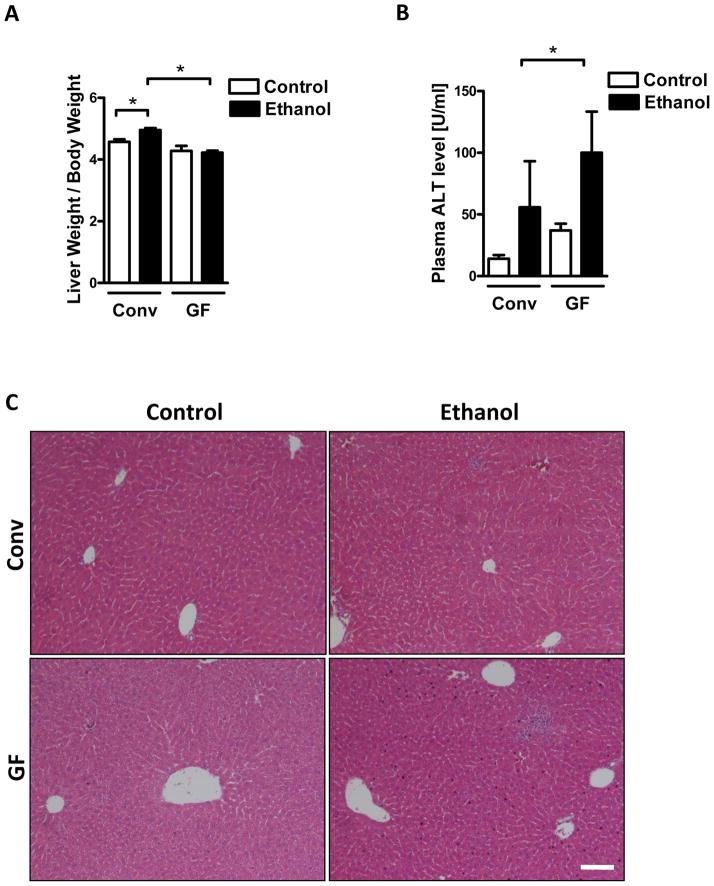
To determine the role of the intestinal microbiota in acute alcoholic liver injury, conventional and germ-free mice were gavaged once with alcohol, which models acute alcohol binge drinking, or an isocaloric dose of dextrose as a control. Liver weight was increased in conventional compared with germ-free mice following an alcohol binge (Table 1; Fig. 1A). Hepatic liver injury, as determined by plasma levels of alanine aminotransferase (ALT), was markedly higher in germ-free relative to conventional mice (Fig. 1B). Increased inflammatory cell infiltrates were observed on H&E stained liver sections in germ-free mice following ethanol gavage (Fig. 1C). These data show that the absence of a normal microbiota exacerbates the liver damage caused by acute alcohol exposure.
Table 1.
Body and liver weight of mice subjected to dextrose (control) gavage (n=8–10) or alcohol gavage (n=9–10). Conv, conventional; GF, germ-free.
| Control | Alcohol | |||
|---|---|---|---|---|
| Conv | GF | Conv | GF | |
| Body Weight (g) | 23.13 ± 0.42 | 24.84 ± 0.65 | 22.62 ± 0.37 | 24.35 ± 0.73 |
| Liver Weight (g) | 1.06 ± 0.02 | 1.06 ± 0.03 | 1.12 ± 0.03* | 1.03 ± 0.02 |
p < 0.05 as compared to GF mice fed alcohol
Figure 1. Germ-free mice display exacerbated acute alcoholic liver injury.
Conventional (Conv; n=10–15) and germ-free C57BL/6 mice (GF; n=7–16) were gavaged with ethanol (300μl, 30% vol/vol) or an isocaloric amount of dextrose (300μl, 0.42g/ml) once, and harvested after 9 h. (A) Liver to body weight ratio. (B) Plasma ALT level. (C) H&E staining of liver sections. Scale bar, 20μm. *p<0.05.
Absence of microbiota increases the hepatic inflammatory response in acute alcohol-induced liver injury
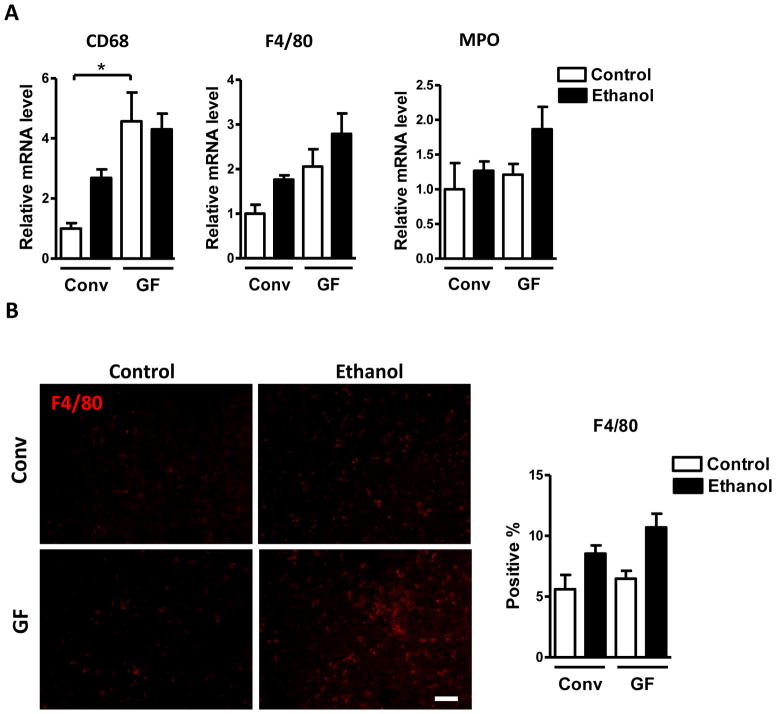
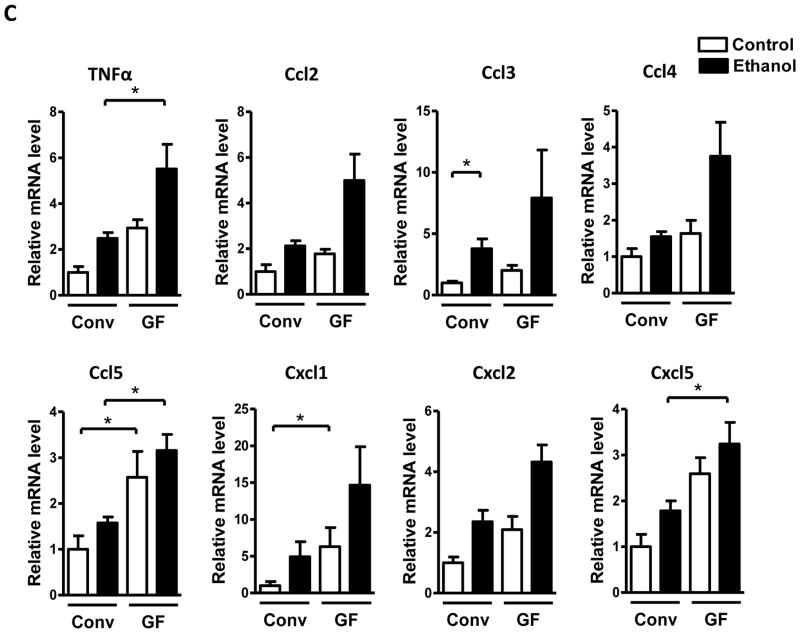
To further explore how the absence of the intestinal microbiota impacts hepatic inflammation in response to alcohol-induced liver injury, hepatic expression of several inflammatory cell markers, cytokines and chemokines was examined. Expression of the macrophage markers, CD68 and F4/80, as determined by qPCR, was higher in control (dextrose) and alcohol-gavaged germ-free compared with conventional mice, while mRNA expression of myeloperoxidase (MPO), a marker of neutrophils, was only slightly increased only after alcohol exposure in germ-free mice (Fig. 2A). Immunofluorescence staining for F4/80 confirmed the trend towards more hepatic macrophages in germ-free mice following ethanol binge (Fig. 2B). However, MPO positive cells were rarely seen in either of the groups after ethanol treatment (not shown). Chemokine (C-C motif) ligand (Ccl)-5 (also known as RANTES) was increased in both control and alcohol-binged germ-free relative to conventional mice (Fig. 2C). Tumor necrosis factor (TNF)-α and chemokine (C-X-C motif) ligand (Cxcl)-5 (ENA-78) were elevated in germ-free mice only after alcohol administration. Hepatic expression of Cxcl1 (KC/GRO-α) was higher in germ-free mice at baseline. Ccl2 (MCP-1), Ccl3 (MIP-1α) and Ccl4 (MIP-1β) Cxcl2 (MIP-2) showed a trend towards elevated expression in the liver of germ-free mice (Fig. 2C). Taken together, hepatic inflammatory cells and proinflammatory mediators are higher in the absence of microbiota at baseline and after acute alcohol exposure, thus underlining and extending the earlier histological observations in H&E stained sections.
Figure 2. Germ-free mice show elevated hepatic inflammation during acute alcoholic liver disease development.
Conventional (Conv; n=8–15) and germ-free C57BL/6 mice (GF; n=8–16) were gavaged with ethanol (300μl, 30% vol/vol) or an isocaloric amount of dextrose (300μl, 0.42g/ml) once, and harvested after 9 h. (A) Hepatic mRNA levels of the inflammatory cell markers, CD68, F4/80 and MPO. (B) Immunofluorescent analysis of F4/80 in the liver. Percentage of liver cells positive for F4/80 were counted on 10 randomly selected fields per slide (right panel; n= 4–5). (C) Hepatic mRNA levels of the indicated proinflammatory cytokines. Scale bar, 20μm. *p<0.05.
Enhanced hepatic steatosis in germ-free mice after alcohol binge
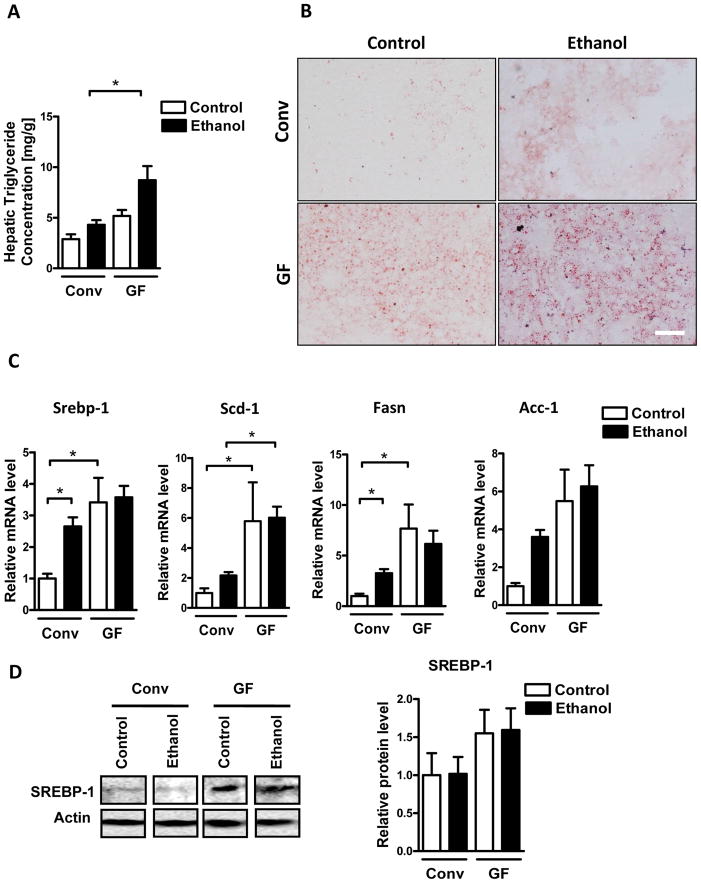
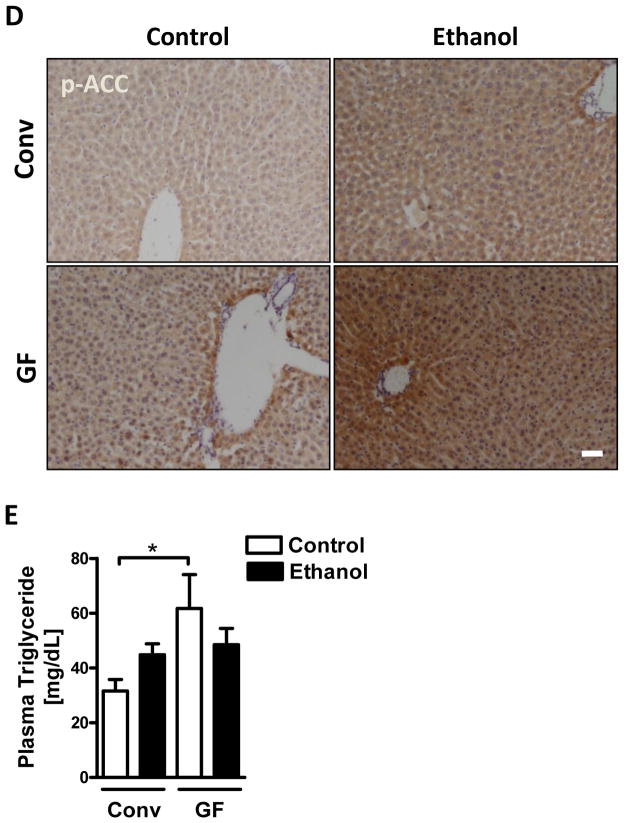
A hallmark of excessive ethanol consumption is the development of hepatic steatosis. Since steatosis could not be observed on H&E stained slides, we performed total hepatic triglyceride measurement and Oil red O staining to investigate any differences in fat accumulation between germ-free and conventional mice. Germ-free mice showed significantly increased levels of hepatic triglycerides after an alcohol binge (Fig. 3A and B). Consistent with enhanced hepatic steatosis, germ-free mice also showed elevated hepatic expression of genes involved in fatty acid and triglyceride synthesis, including sterol regulatory element binding transcription factor 1 (Srebp-1), stearoyl-Coenzyme A desaturase 1 (Scd-1), fatty acid synthase (Fasn), and acetyl-CoA carboxylase-1 (ACC-1) (Fig. 3C). Increased hepatic protein expression of SREBP-1 and increased phosphorylation of ACC in germ-free mice was confirmed by immunoblotting and immunohistochemistry, respectively (Fig. 3D). Moreover, plasma triglycerides were higher in controls, but similar in ethanol treated germ-free mice compared with conventional mice (Fig. 3E). This implies that increased hepatic steatosis is not the result of differences in lipid secretion or uptake, but rather a consequence of increased lipid synthesis by hepatocytes in germ-free mice. Together, these data suggest that increased hepatic steatosis might be an additional factor contributing to higher susceptibility of germ-free mice to alcohol-induced liver damage.
Figure 3. Exacerbated hepatic steatosis in germ-free mice after acute alcohol exposure.
Conventional (Conv; n=8–15) and germ-free C57BL/6 mice (GF; n=8–16) were gavaged with ethanol (300μl, 30% vol/vol) or an isocaloric amount of dextrose (300μl, 0.42g/ml) once, and harvested after 9 h. (A) Total hepatic triglyceride levels. (B) Oil Red O staining of liver sections. (C) Hepatic mRNA levels of Srebp-1, Scd-1, Fasn and Acc-1. (D) Immunoblot analysis and quantification of liver SREBP-1 (n=4–8), and immunohistochemistry of phospho-ACC (n=4–5). (E) Plasma triglyceride level. Scale bar, 20μm.*p<0.05.
Ethanol metabolism is enhanced in hepatocytes of germ-free mice
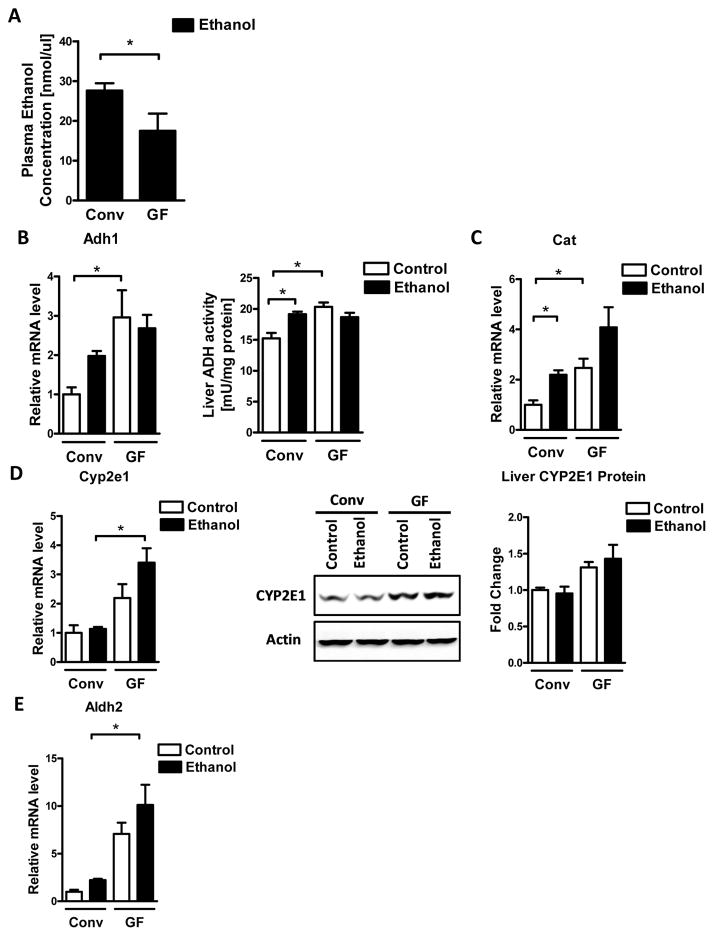
We next determined whether the absence of microbiota affects intestinal absorption and hepatic metabolism of ethanol. Plasma levels of ethanol were significantly lower in germ-free compared with conventional mice after acute intragastric alcohol administration (Fig. 4A). Alcohol dehydrogenase (Adh), CYP2E1, and catalase are the main hepatic enzymes that metabolize ethanol (Zakhari, 2006). Germ-free mice exhibited increases in Adh1 expression and Adh activity in the liver before alcohol exposure (Fig. 4B). Similarly, catalase (Cat) mRNA expression was higher in the liver of control germ-free mice, and only modestly induced after alcohol gavage (Fig. 4C). Cyp2e1 gene expression was significantly higher in germ-free mice after an alcohol binge, while CYP2E1 protein showed a trend towards higher expression in germ-free mice (Fig. 4D). Furthermore, expression of aldehyde dehydrogenase 2 (Aldh2), which catalyzes the transformation of acetaldehyde to acetic acid, was significantly higher in alcohol challenged germ-free mice (Fig. 4E). Taken together, these findings suggest that enhanced hepatic metabolism of ethanol leads to greater alcohol clearance from the blood, but is also associated with increased production of toxic alcohol metabolites in the liver that can contribute to increased liver injury in germ-free mice.
Figure 4. Germ-free mice metabolize ethanol faster than conventional mice.
Conventional (Conv; n=8–15) and germ-free C57BL/6 mice (GF; n=8–16) were gavaged with ethanol (300μl, 30% vol/vol) or an isocaloric amount of dextrose (300μl, 0.42g/ml) once, and harvested after 9 h. (A) Plasma ethanol levels. (B) Hepatic mRNA levels of Adh1, and liver ADH activity. (C) Hepatic mRNA levels of Cat. (D) Hepatic mRNA levels of Cyp2e1, and immunoblot analysis and quantification of liver CYP2E1 (n=5–8). (E) Hepatic mRNA levels of Aldh2. *p<0.05.
Expression of ethanol metabolizing enzymes in the gastrointestinal tract
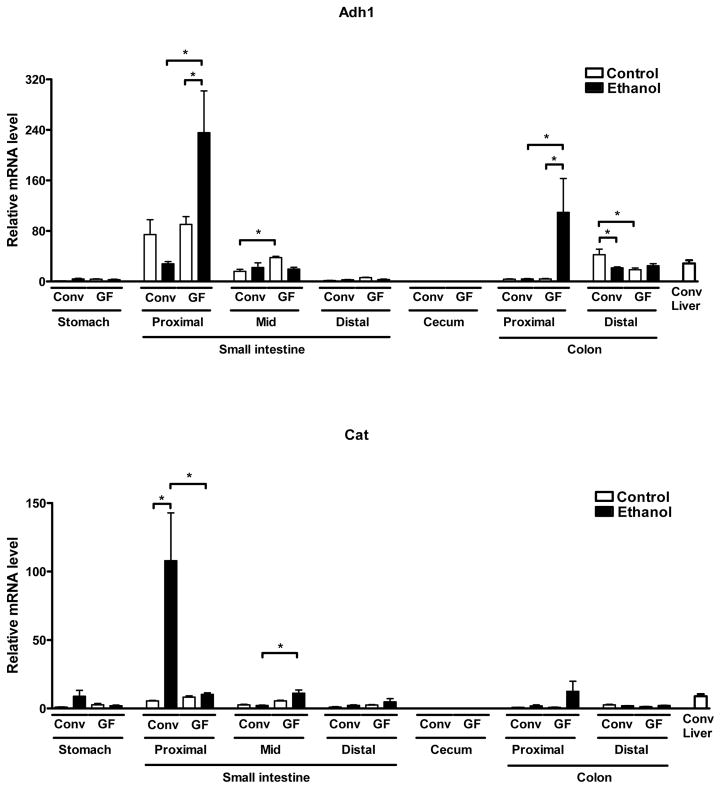
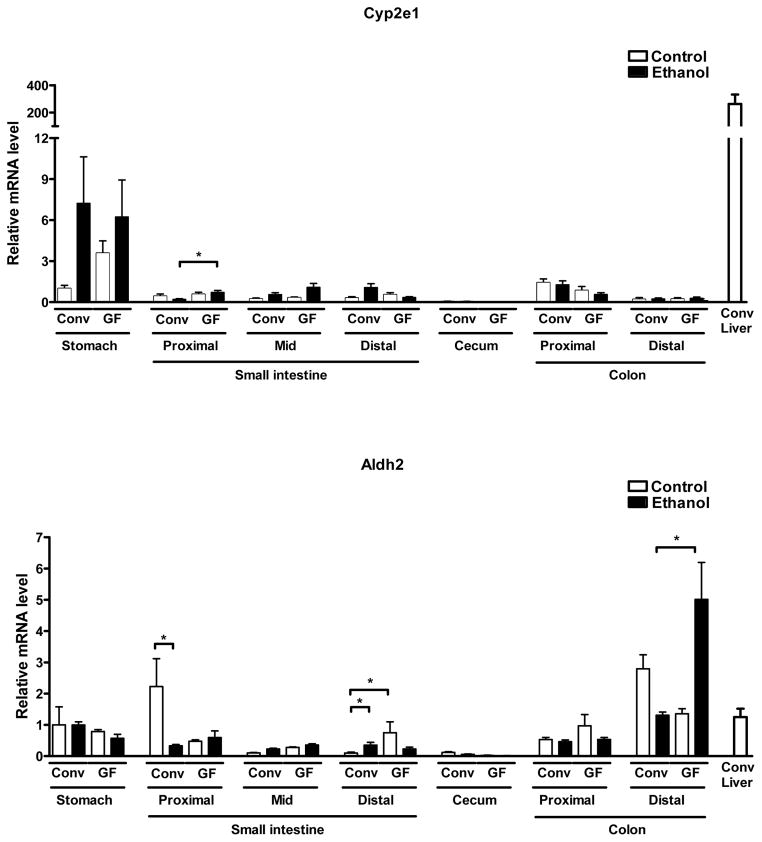
To investigate whether the absence of the microbiota might affect metabolism of ethanol not only in the liver but also in other organs, we assessed gene expression of alcohol-metabolizing enzymes along the gastrointestinal tract as the second most important site of ethanol metabolism. Adh1 showed the highest expression in the proximal small intestine of both conventional and germ-free mice, with further induction in germ-free but not conventional mice after an alcohol binge (Fig. 5). Germ-free mice also displayed marked induction of Adh1 in the proximal colon after alcohol exposure. Cat expression was significantly lower in the proximal, but higher in the mid small intestine of germ-free mice after alcohol exposure (Fig. 5). Cyp2e1 expression was highest in the stomach of both conventional and germ-free mice, but still relatively low when compared to hepatic expression in conventional mice. Cyp2e1 gene expression showed higher levels in the proximal small intestine of germ-free over conventional mice after alcohol exposure (Fig. 5). By comparison, the distal colon was the site with the highest expression of Aldh2 in the gastrointestinal tract of conventional and germ-free mice, with further induction after an alcohol binge in germ-free but modest suppression in conventional mice (Fig. 5). Thus, overall increased expression of ethanol-metabolizing enzymes in the gastrointestinal tract of germ-free mice might further contribute (beyond increased hepatic expression) to the lower blood alcohol levels in these mice after acute alcohol exposure.
Figure 5. Germ-free mice display elevated gastrointestinal expression of ethanol-metabolizing genes.
Conventional (Conv; n=8–15) and germ-free C57BL/6 mice (GF; n=8–16) were gavaged with ethanol (300μl, 30% vol/vol) or an isocaloric amount of dextrose (300μl, 0.42 g/ml) once, and harvested after 9 h. Expression levels of indicated ethanol-metabolizing genes were determined in stomach, proximal, mid and distal small intestine, cecum, proximal and distal colon. Hepatic expression in conventional mice is shown as comparison. *p<0.05.
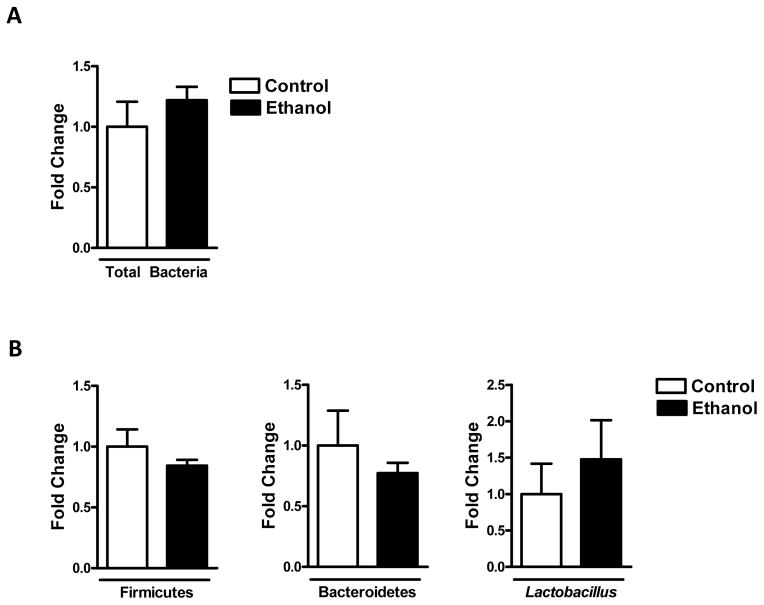
Single alcohol binge does not affect the intestinal microbiota
To determine whether acute ethanol treatment affects the composition and quantity of the intestinal microbiota, qPCR of bacteria in the cecum was performed. Total bacteria (Fig. 6A), relative abundance of the phyla Firmicutes and Bacteroidetes, and Lactobacillus were comparable between control and ethanol treated conventional mice (Fig. 6B).
Figure 6. Single ethanol treatment does not affect intestinal eubiosis.
Conventional mice (n=8) were gavaged with ethanol (300μl, 30% vol/vol) or an isocaloric amount of dextrose (300μl, 0.42 g/ml) once, and harvested after 9 h. Total bacteria (A) and relative abundance of Firmicutes, Bacteroidetes and Lactobacillus (B) were determined by qPCR in the cecum.
Discussion
This study demonstrates that germ-free mice are more susceptible to acute alcohol-induced liver injury. These findings are consistent with our previously reported observation that the commensal microbiota has hepatoprotective effects and prevents hepatotoxin-induced liver injury and fibrosis in mice (Mazagova et al., 2015). Together, these observations show that the intestinal commensal microbiota is important for promoting normal liver function in the face of acute injury. On the other hand, chronic alcoholic liver disease is associated with changes in the intestinal microbiota composition and in particular intestinal bacterial overgrowth. Dysbiosis contributes to disruption of the intestinal barrier, leading to translocation of microbial products to the portal circulation and liver (Chen et al., 2015b). This chronic and continuous stimulation of the immune system can ultimately result in disease progression. In that situation, reducing the intestinal bacterial load with non-absorbable antibiotics prevents alcoholic liver disease in preclinical models (Chen et al., 2015b; Adachi et al., 1995). However, while intestinal dysbiosis can lead to progression of alcoholic liver disease, our present data show that the complete absence of the microbiota is also problematic as it is associated with increased alcoholic liver injury. Therefore, the microbiota makes differential contributions to liver homeostasis and injury responses, depending on the duration of exposure and perhaps the nature of the stresses and injurious agents.
The observed phenotype in germ-free mice is likely caused by a combination of three factors, involving increases in the production of alcohol-derived toxic metabolites, steatosis, and inflammation.
Alcohol metabolism
Following absorption, ethanol is mostly metabolized in the liver by one of three major oxidative pathways (Zakhari, 2006). The first pathway converts ethanol into acetaldehyde by alcohol dehydrogenase (Adh) in the cytosol. Acetaldehyde is further oxidized into acetate by aldehyde dehydrogenase (Aldh) in mitochondria (Lee et al., 2006; Kwon et al., 2014). During these two steps, NADH+ is reduced to NADH, which causes an increased NADH/NAD+ ratio and changes the redox status of liver cells (Stewart et al., 2001). Another pathway occurs in microsomes, where ethanol can be oxidized to acetaldehyde by CYP2E1, which is the major p450 enzyme in this process (Misra et al., 1992). The third way to transform ethanol into acetaldehyde is dependent on catalase in peroxisomes (Zakhari, 2006). All these pathways lead to elevated acetaldehyde adduct formation, increased reactive oxygen species production, and an impaired redox state in the liver cells, finally causing alcoholic liver damage. While hepatic catalase and Adh were higher in control germ-free mice, Cyp2e1 gene expression was elevated in alcohol exposed germ-free mice. Increases in CYP2E1 may lead to more rapid ethanol oxidation, which could explain the lower blood alcohol levels observed in germ-free mice. Increased alcohol metabolism by CYP2E1 would also increase reactive oxygen species production and subsequent liver injury. This prediction is consistent with the observed protection of Cyp2e1 deficient mice from alcoholic liver disease (Lu et al., 2008).
Our results are consistent with another study demonstrating that germ-free mice have an accelerated and more efficient xenobiotic metabolism compared with conventional mice (Bjorkholm et al., 2009). In that work, expression of numerous genes involved in xenobiotic metabolism, including alcohol dehydrogenase, aldehyde dehydrogenase and several cytochrome P450 superfamily members, was markedly elevated in germ-free mice. Although a detailed mechanism was not shown at the time, it is possible that the gene induction resulted from the accumulation of physiologic androstane receptor (CAR)-ligands such as bilirubin, bile acids and steroid hormones in absence of metabolism of these ligands by intestinal bacteria in germ-free mice. Activation of CAR may lead to increased downstream activation of genes involved in xenobiotic metabolic pathways (Bjorkholm et al., 2009), including those important for alcohol metabolism, as we found in germ-free mice.
Steatosis
Hepatic steatosis shows a synergistic effect with alcohol on liver injury in experimental animal models (Xu et al., 2011) and patients (Loomba et al., 2009). Thus, increased hepatic steatosis is an additional pathogenic factor contributing to increased alcohol-induced liver injury in germ-free mice. Another study reported that germ-free mice have less hepatic triglycerides at baseline when compared to germ-free mice that were conventionalized (Parlesak et al., 2000). However, germ-free mice in our study had similar absolute levels of hepatic triglycerides as those in the prior study (Parlesak et al., 2000), whereas our conventional mice had much lower hepatic triglycerides than mice that were born in a germ-free environment but were later conventionalized (Parlesak et al., 2000). This might explain the baseline differences in hepatic triglycerides between our work and the prior report.
Inflammation
Microbial products such as LPS can activate acute inflammation, but they can also contribute to attenuate stress responses over extended periods, which is commonly known as tolerance (Enomoto et al., 1998). The persistent absence of the microbiota and the associated lack of this attenuation might therefore be associated with greater susceptibility to damage associated molecular patterns (DAMPs) in the liver. Although we did not detect differences in hepatic inflammation between conventional and germ-free mice in our previous investigations (Mazagova et al., 2015), we found significant differences in the present study between control (dextrose) and ethanol-gavaged germ-free and conventional mice. One possible explanation for these differences may be the use of different hepatic toxins (carbon tetrachloride (or CCl4) and thioacetamide (or TAA) vs ethanol) and the accompanying differences in the underlying mechanisms of toxic action. Taken together, elevated baseline levels of inflammatory mediators in germ-free mice is likely to contribute to increased liver damage following binge ethanol in these mice.
In summary, we have demonstrated a beneficial role of the commensal microbiota in protection against acute alcoholic liver disease. It will be an important next task to identify the specific microbial products and metabolites that confer the hepatoprotective effects and help to maintain liver homeostasis. Identification of such mediators might have clinical implications in the prevention or treatment of chronic alcoholic liver disease.
Acknowledgments
The manuscript was supported by NIH grants K08 DK081830, R01 AA020703, U01 AA021856, and by Award Number I01BX002213 from the Biomedical Laboratory Research & Development Service of the VA Office of Research and Development (to BS).
Abbreviations
- Adh
alcohol dehydrogenase
- ALT
alanine aminotransferase
- Ccl
chemokine (C-C motif) ligand
- Cxcl
chemokine (C-X-C motif) ligand
- conv
conventional
- Cyp2e1
cytochrome p450 enzyme 2e1
- GF
germ-free
- PAMPs
pathogen associated molecular patterns
- TLR
Toll-like receptor
- TNF-α
tumor necrosis factor-α
Footnotes
Conflict of interest: The authors declare the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
P.C., Y.M., M.M. and K-C.L. performed experiments; P.C., Y.M. and M.M. analyzed data; P.C., Y.M., M.M., L.E. and B.S. designed the experiments; P.C., Y.M., M.M., L.E. and B.S. wrote the article.
References
- Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology. 1995;108:218–224. doi: 10.1016/0016-5085(95)90027-6. [DOI] [PubMed] [Google Scholar]
- Bjorkholm B, Bok CM, Lundin A, Rafter J, Hibberd ML, Pettersson S. Intestinal microbiota regulate xenobiotic metabolism in the liver. PloS one. 2009;4:e6958. doi: 10.1371/journal.pone.0006958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode JC, Bode C, Heidelbach R, Durr HK, Martini GA. Jejunal microflora in patients with chronic alcohol abuse. Hepatogastroenterology. 1984;31:30–34. [PubMed] [Google Scholar]
- Bull-Otterson L, Feng W, Kirpich I, Wang Y, Qin X, Liu Y, Gobejishvili L, Joshi-Barve S, Ayvaz T, Petrosino J, Kong M, Barker D, McClain C, Barve S. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PLoS One. 2013;8:e53028. doi: 10.1371/journal.pone.0053028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Torralba M, Tan J, Embree M, Zengler K, Stärkel P, van Pijkeren JP, DePew J, Loomba R, Ho SB, Bajaj JS, Mutlu EA, Keshavarzian A, Tsukamoto H, Nelson KE, Fouts DE, Schnabl B. Supplementation of saturated long-chain fatty acids maintains intestinal eubiosis and reduces ethanol-induced liver injury in mice. Gastroenterology. 2015a;148:203–214. doi: 10.1053/j.gastro.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Stärkel P, Turner JR, Ho SB, Schnabl B. Dysbiosis-induced intestinal inflammation activates TNFRI and mediates alcoholic liver disease in mice. Hepatology. 2015b;61:883–894. doi: 10.1002/hep.27489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto N, Ikejima K, Bradford B, Rivera C, Kono H, Brenner DA, Thurman RG. Alcohol causes both tolerance and sensitization of rat Kupffer cells via mechanisms dependent on endotoxin. Gastroenterology. 1998;115:443–451. doi: 10.1016/s0016-5085(98)70211-2. [DOI] [PubMed] [Google Scholar]
- European Association for the Study of L. EASL clinical practical guidelines: management of alcoholic liver disease. J Hepatol. 2012;57:399–420. doi: 10.1016/j.jhep.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Keshavarzian A, Fields JZ, Vaeth J, Holmes EW. The differing effects of acute and chronic alcohol on gastric and intestinal permeability. The American journal of gastroenterology. 1994;89:2205–2211. [PubMed] [Google Scholar]
- Kirpich IA, Solovieva NV, Leikhter SN, Shidakova NA, Lebedeva OV, Sidorov PI, Bazhukova TA, Soloviev AG, Barve SS, McClain CJ, Cave M. Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol. 2008;42:675–682. doi: 10.1016/j.alcohol.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HJ, Won YS, Park O, Chang B, Duryee MJ, Thiele GE, Matsumoto A, Singh S, Abdelmegeed MA, Song BJ, Kawamoto T, Vasiliou V, Thiele GM, Gao B. Aldehyde dehydrogenase 2 deficiency ameliorates alcoholic fatty liver but worsens liver inflammation and fibrosis in mice. Hepatology. 2014;60:146–157. doi: 10.1002/hep.27036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SL, Chau GY, Yao CT, Wu CW, Yin SJ. Functional assessment of human alcohol dehydrogenase family in ethanol metabolism: significance of first-pass metabolism. Alcoholism, clinical and experimental research. 2006;30:1132–1142. doi: 10.1111/j.1530-0277.2006.00139.x. [DOI] [PubMed] [Google Scholar]
- Loomba R, Bettencourt R, Barrett-Connor E. Synergistic association between alcohol intake and body mass index with serum alanine and aspartate aminotransferase levels in older adults: the Rancho Bernardo Study. Aliment Pharmacol Ther. 2009;30:1137–1149. doi: 10.1111/j.1365-2036.2009.04141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Zhuge J, Wang X, Bai J, Cederbaum AI. Cytochrome P450 2E1 contributes to ethanol-induced fatty liver in mice. Hepatology. 2008;47:1483–1494. doi: 10.1002/hep.22222. [DOI] [PubMed] [Google Scholar]
- Mazagova M, Wang L, Anfora AT, Wissmueller M, Lesley SA, Miyamoto Y, Eckmann L, Dhungana S, Pathmasiri W, Sumner S, Westwater C, Brenner DA, Schnabl B. Commensal microbiota is hepatoprotective and prevents liver fibrosis in mice. FASEB journal. 2015;29:1043–1055. doi: 10.1096/fj.14-259515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra UK, Bradford BU, Handler JA, Thurman RG. Chronic ethanol treatment induces H2O2 production selectively in pericentral regions of the liver lobule. Alcoholism, clinical and experimental research. 1992;16:839–842. doi: 10.1111/j.1530-0277.1992.tb01878.x. [DOI] [PubMed] [Google Scholar]
- Mutlu EA, Gillevet PM, Rangwala H, Sikaroodi M, Naqvi A, Engen PA, Kwasny M, Lau CK, Keshavarzian A. Colonic microbiome is altered in alcoholism. Am J Physiol Gastrointest Liver Physiol. 2012;302:G966–G978. doi: 10.1152/ajpgi.00380.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlesak A, Schafer C, Schutz T, Bode JC, Bode C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol. 2000;32:742–747. doi: 10.1016/s0168-8278(00)80242-1. [DOI] [PubMed] [Google Scholar]
- Petrasek J, Iracheta-Vellve A, Csak T, Satishchandran A, Kodys K, Kurt-Jones EA, Fitzgerald KA, Szabo G. STING-IRF3 pathway links endoplasmic reticulum stress with hepatocyte apoptosis in early alcoholic liver disease. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:16544–16549. doi: 10.1073/pnas.1308331110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnabl B, Brenner DA. Interactions Between the Intestinal Microbiome and Liver Diseases. Gastroenterology. 2014;146:1513–1524. doi: 10.1053/j.gastro.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnabl B, Kweon YO, Frederick JP, Wang XF, Rippe RA, Brenner DA. The role of Smad3 in mediating mouse hepatic stellate cell activation. Hepatology. 2001;34:89–100. doi: 10.1053/jhep.2001.25349. [DOI] [PubMed] [Google Scholar]
- Stewart S, Jones D, Day CP. Alcoholic liver disease: new insights into mechanisms and preventative strategies. Trends in molecular medicine. 2001;7:408–413. doi: 10.1016/s1471-4914(01)02096-2. [DOI] [PubMed] [Google Scholar]
- Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34:101–108. doi: 10.1053/jhep.2001.25350. [DOI] [PubMed] [Google Scholar]
- Xu J, Lai KK, Verlinsky A, Lugea A, French SW, Cooper MP, Ji C, Tsukamoto H. Synergistic steatohepatitis by moderate obesity and alcohol in mice despite increased adiponectin and p-AMPK. J Hepatol. 2011;55:673–682. doi: 10.1016/j.jhep.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan AW, Fouts DE, Brandl J, Stärkel P, Torralba M, Schott E, Tsukamoto H, Nelson KE, Brenner DA, Schnabl B. Enteric Dysbiosis Associated with a Mouse Model of Alcoholic Liver Disease. Hepatology. 2011;53:96–105. doi: 10.1002/hep.24018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakhari S. Overview: how is alcohol metabolized by the body? Alcohol Res Health. 2006;29:245–254. [PMC free article] [PubMed] [Google Scholar]