Abstract
Hyaluronan (HA) is an integral component of the extracellular matrix. Its interactions with a cell surface receptor CD44 has been shown to play important roles in a variety of biological events including cell proliferation and metastasis. As multivalent CD44-HA binding is critical for downstream signaling, compounds that can selectively disrupt the complex formation of HA polysaccharide with CD44 can serve as useful probes of CD44 mediated cellular events as well as potential leads for novel therapeutics. Herein, we report the synthesis of several series of HA conjugates to target the HA binding pocket of CD44. As a small library of HA disaccharide derivatives failed to exhibit any inhibitory activities, we focused on HA tetrasaccharide based analogs. Traditional synthetic strategies towards HA oligosaccharides involve the construction of backbone from the corresponding monosaccharide building blocks, which can be quite tedious. In order to expedite the synthesis, we designed a new synthetic route taking advantage of the ability of hyaluronidase to generate large quantities of HA tetrasaccharide through digestion of HA polysaccharides. The HA tetrasaccharide obtained was utilized to prepare multiple S-linked HA analogs bearing aromatic groups at the reducing end glycan. One such compound containing an m-benzyl phenyl moiety exhibited significant inhibition of CD44-HA binding. Our approach provides a new direction towards the design of HA based CD44 antagonists.
Keywords: CD44, Hyaluronan, Inhibitor design, Synthesis
Introduction
Hyaluronan (HA) is a non-sulfated negatively charged linear polysaccharide, which is composed of repeating units of di-saccharide: D-glucuronic acid (β1→3) N-acetyl-D-glucosamine (β1→4) [1, 2]. Among many HA binding proteins [3], a major receptor of HA is CD44, a cell surface glycoprotein involved in signal transduction, cell adhesion and migration [4, 5]. The interactions of CD44 and HA have been shown to be important for tumor cell proliferation, metastasis and the development of multidrug resistance [6–9].
Due to the polymeric nature of HA, it can crosslink multiple CD44 receptors on cell surface, resulting in the activation of kinases and downstream cellular signaling [10]. The polyvalent interactions between HA and CD44 are critical for the formation of the signaling complexes. An HA oligosaccharide can potentially compete with endogenous HA polysaccharide for CD44 binding, resulting in disassembly of the signaling complexes [11]. This in turn can lead to the inhibition of CD44 dependent tumor cell proliferation and drug resistance [12, 8].
Reagents that can disrupt the binding of CD44 and HA can serve as useful probes of CD44 functions as well as potential leads for the development of novel therapeutics [12, 8, 11]. From culture broth of fungus, several glycolipids have been isolated that can inhibit CD44-HA binding with IC50 values around 20 μM, although the binding sites are not known [13–15]. Through fragment screening, Finzel and coworkers identified several tetrahydroisoquinolines, which bound with CD44 with Kd in the mM range [16]. Crystallographic characterization showed that these molecules bound to CD44 in a site adjacent to HA binding pocket, which interfered with CD44 interactions with polymeric HA. Besides these studies, an alternative approach to develop CD44 antagonists is to design HA analogs targeting HA binding pocket of CD44.
The crystal structures of hyaluronan binding domain (HABD) of CD44 and HA octasaccharides (HA8) have been reported [17]. HA8 binds to a groove on the surface of CD44 (Kd=125 μM) with four glycan units shown to make main contacts with CD44 [17]. The interactions are dominated by hydrogen bonds and hydrophobic interactions. Close examination of the crystal structure of CD44 HABD revealed the presence of an empty hydrophobic pocket formed by tyrosine 46 (Tyr46), Tyr83, isoleucine 111 (Ile111), threonine 116 (Thr116) and Tyr119 adjacent to the HA binding pocket (shown in orange color in Fig. 1). Studies have shown that binding affinities of carbohydrate ligands to receptors can be significantly improved by introducing aromatic groups to strengthen cation-π and hydrophobic-hydrophobic interactions with the proteins [18–20]. Herein, we explored the possibility of conjugating hydrophobic motifs into HA oligosac-charides to enhance CD44 binding affinity.
Fig. 1.
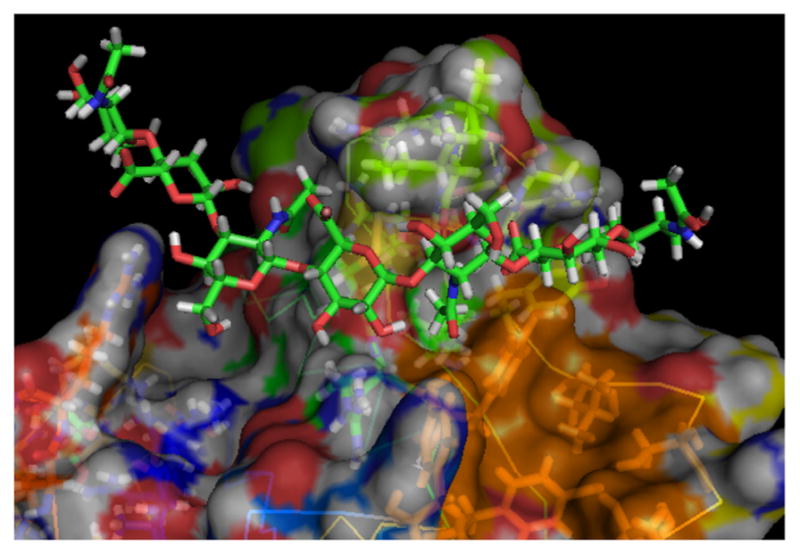
The hydrophobic pocket in CD44-HA8 co-crystal structure highlighted in orange color. The image was generated from the co-crystal structure (PDB code: 2JCQ) using Pymol
Result and discussion
Synthesis and CD44 binding of a HA disaccharide library
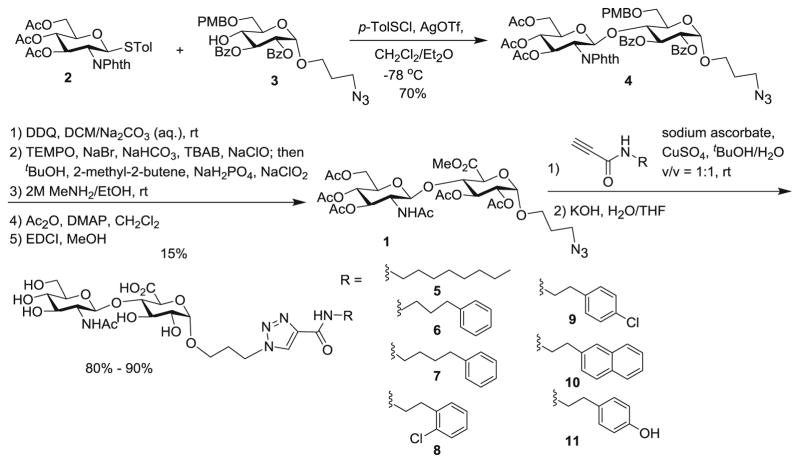
Due to the synthetic ease, our initial approach focused on a library of HA disaccharide (HA2) analogs, which were prepared from a common HA2 precursor 1. The synthesis of 1 started from coupling of monosaccharides 2 [21] and 3 producing disaccharide 4 in 70 % yield (Scheme 1). The PMB group in compound 4 was removed by DDQ oxidation, and the resulting primary hydroxyl group was oxidized to carboxylic acid [22]. Subsequent removal of benzoyl (Bz) and phthalimido (Phth) groups followed by acylation of the free hydroxyl groups and methyl ester protection led to disaccharide 1. Various hydrophobic functionalities were then conjugated to compound 1 through the copper catalyzed azide-alkyne cycloaddition reactions [23]. After ester hydrolysis, HA2 analogs 5–11 were obtained.
Scheme 1.
Synthesis of HA2 analogs
In order to assay CD44 binding by HA2 analogs, a competitive enzyme linked immunosorbent assay (ELISA) was established by incubating a CD44 HABD/IgGFc chimera onto anti-IgG antibody coated 96-well plates [24]. Compared to the direct absorption of CD44 onto the uncoated plates, this layout helped to orient the CD44 HABD for HA binding. HA poly-saccharide (MW~16 kDa) was functionalized with biotin (biotin-HA) [25] and the abilities of the HA2 analogs to inhibit biotin-HA binding to CD44 were measured by ELISA. Unfortunately, none of the HA2 showed any inhibitory effects. This was presumably due to the short length of the glycan units. In addition, docking of the disaccharides onto CD44 crystal structure showed that the aromatic groups on the di-saccharide analogs could not reach the hydrophobic pocket of interest. These considerations prompted us to investigate HA analogs with longer backbones.
Design, synthesis and screening of HA4 based conjugates
HA analog 12 was initially designed as its ring E can provide multiple potential sites including the C-3 (either axial or equatorial) and C-6 positions for modifications. The analogs were evaluated manually in silico inserting 12 into the co-crystal structure of CD44 HABD-HA8 (2JCQ) using Pymol and overlaying the HA4 portion of the backbone (glycan A → D in analog 12) onto HA8 in the structure. C-3-axial position of the E ring was deemed suitable for directing extra functionality into the hydrophobic pocket, while maintaining the hydrogen bond between the carboxylic group of glycan E and CD44. The C-3-equatorial and C-6 positions were also determined to be suitable functionalization sites with minimum impacts on HA4 interactions with CD44 HABD.
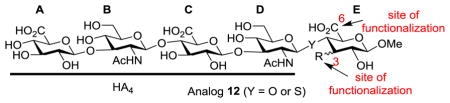
Assembly of oligosaccharides such as 12 can be a highly challenging task. While chemical syntheses of a variety of HA oligosaccharides have been accomplished [26–29], it is still very tedious to build up the oligosaccharide from the corresponding monosaccharide building blocks. To expedite the synthesis, we explored a new strategy, where a HA tetrasaccharide would be obtained through enzymatic digestion of HA polysaccharide and utilized for glyco-assembly.
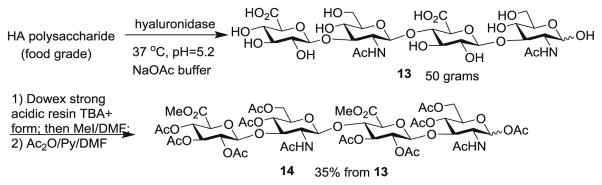
In order to prepare the HA tetrasaccharide, HA polysaccharide was treated with a hyaluronidase at pH 5.2 in sodium acetate buffer (Scheme 2) [30, 31]. This reaction was performed on an 80 g scale yielding 50 g of HA tetrasaccharide 13. Due to the low cost of hyaluronidase (~ $100/g) and HA polysaccharide ($2/g), this is an attractive approach to readily access the needed material. The HA tetrasaccharide 13 was methylated and acetylated yielding fully protected tetrasaccharide 14 in 35 % yield.
Scheme 2.
Synthesis of tetrasaccharide 14
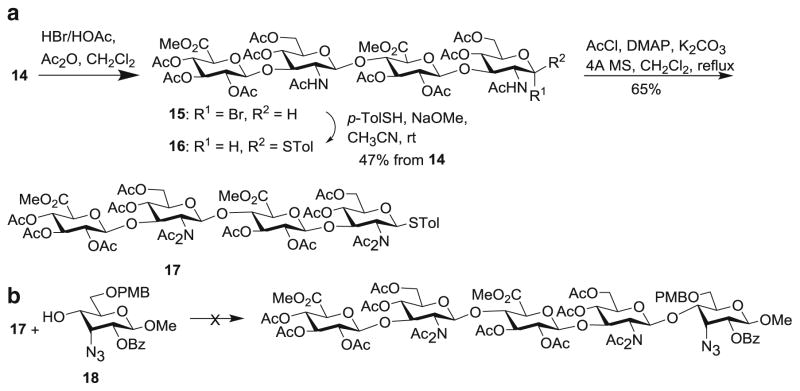
With HA tetrasaccharide 14 in hand, its conversion to analog 12 was explored by first transforming 14 to a glycosyl donor. Treatment of 14 with HBr and acetic acid generated the α-glycosyl bromide 15, which was converted to thioglycoside donor 16 (Scheme 3a). However, when either donor 15 or 16 was subjected to glycosylation, glycosyl oxazoline was obtained as the major side product with no desired glycosides, which is a common occurrence with 2-acetamide containing donors [32]. To overcome this problem, N,N-di-acetyl imide [33] donor 17 was prepared, which did not undergo productive glycosylations with several thiophilic promoters (Scheme 3b). Besides glycosyl bromide and thioglycoside, we examined the transformation of 14 to either glycosyl chloride or trichloroacetimidate donors, which were not successful.
Scheme 3.
aSynthesis of tetrasaccharide donor 17. b Unsuccessful glycosylation of 18 by donor 17
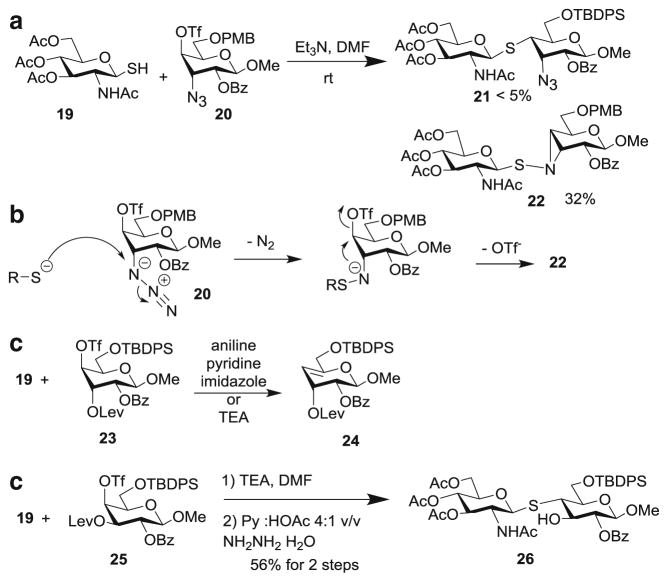
With the difficulties in formation of O-glycosides, the alternative of using S-linkage was explored. S-Glycosides have better hydrolytic stabilities than the corresponding O-glyco-sides, while maintaining similar conformations [34–36]. To establish the feasibility, a glycosyl thiol 19 was prepared as a model donor. Coupling reaction between 19 and triflate 20 failed to yield the desired thioglycoside 21 (Scheme 4a) . In stead, thioaziridine 22 was formed as the major product. This was presumably due to the attack of azide by the glycosyl thiolate to form S-N bond, followed by intramolecular displacement of the triflate (Scheme 4b). To avoid this problem, acceptor 23 was tested next, which gave mainly the elimination product 24. Interestingly, switching the configuration of 3-O-Lev from axial to equatorial (acceptor 25) led to the formation of the S-linked disaccharide 26 in 56 % yield.
Scheme 4.
Model study for formation of S-linked glycans
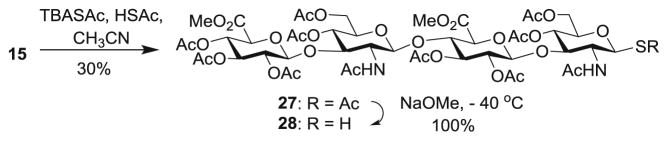
In order to prepare S-linked HA analogs, HA tetrasaccharide 15 was treated with tetrabutylammonium thioacetate (TBASAc) and thioacetic acid (HSAc) to form glycosyl thioacetate. HSAc was used to adjust the acidity of the reaction mixture to minimize the oxazoline formation. The S-acetate was removed selectively with NaOMe at −40 °C to give glycosyl thiol 28 in 30 % overall yield from 15. (Scheme 5).
Scheme 5.
Synthesis of glycosyl thiol 28
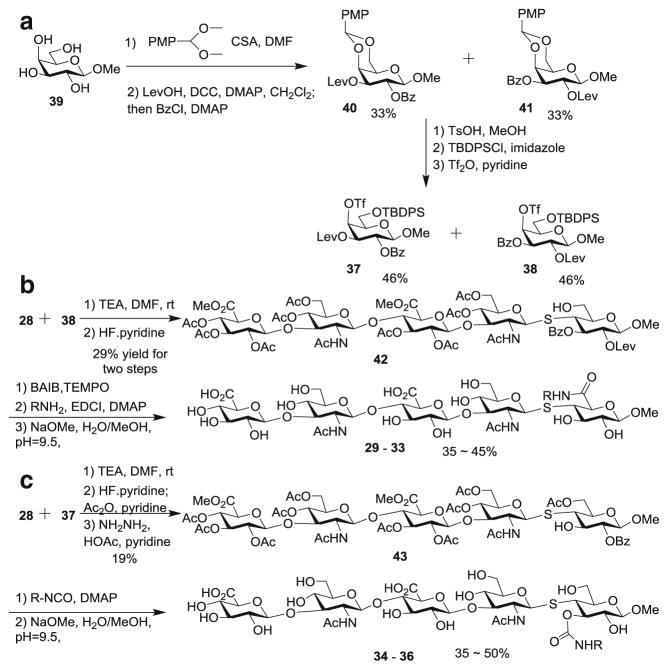
With the glycosyl thiol 28 in hand, two series of analogs (29–33 and 34–36) were designed based on the co-crystal structure of CD44 and HA8 [17]. Due to the difficulties encountered with the C-3 axial alloside (Scheme 4), we focused on functionalizing C-3 equatorial and C-6 positions of the reducing end glucoside or glucuronate. These analogs were manually overlaid onto HA8 in the crystal structure to maintain the conformation of the glycan backbone in the binding pocket (Figure S1). The strategically added functional groups were deemed to potentially be able to introduce new π-π stacking, hydrophobic interaction and/or H-bonding with CD44.
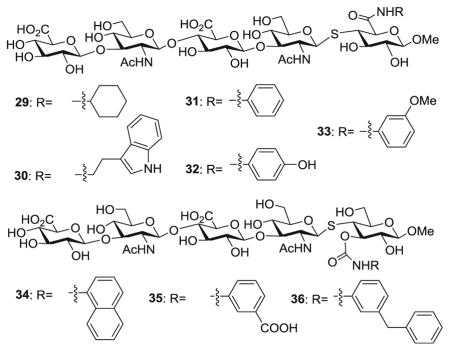
The synthesis of compounds 29–36 is outlined in Scheme 6. The triflate bearing galactosides 37 and 38 were prepared from commercially available methyl galactoside 39 and the two regio-isomers were separated (Scheme 6a). Glycosyl thiol 28 was coupled with galactoside 38 followed by TBDPS removal to generate 42 (Scheme 6b). The resulting free OH in 42 was oxidized to carboxylic acid [37], which was then coupled with a variety of amines. Subsequent ester hydrolysis produced analogs 29–33. Similarly, glycosyl thiol 28 was coupled with galactoside 37 and transformed to compound 43 (Scheme 6c). The 3-OH of 43 was conjugated to several isocyanates, which followed by deprotection led to conjugates 34–36.
Scheme 6.
Synthesis of HA conjugates 29–36
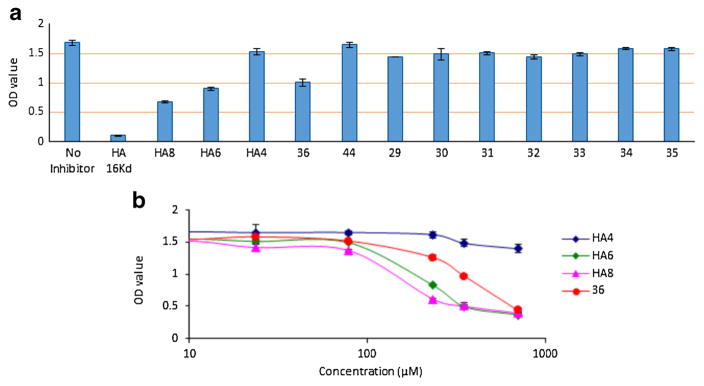
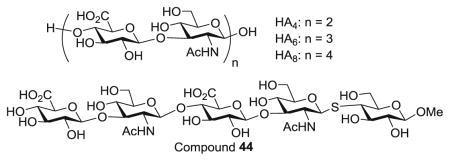
With the HA analogs 29–36 in hand, their abilities to inhibit the binding of biotinylated HA polysaccharide to CD44 HABD were analyzed using the competitive ELISA first at a single concentration (240 μM) together with several HA oligosaccharides. As shown in Fig. 2a, out of all synthetic compounds, 36 exhibited strongest inhibition indicating that the m-benzyl phenyl moiety linked to 3-O position of the reducing end glycan was beneficial for CD44 interactions. Compared to the unconjugated 44, the stronger affinity of 36 suggested the hydrophobic moiety was important. The inhibitory activity of 36 was stronger than that of HA4, comparable to HA6 but weaker than HA8 in this assay. Next, the abilities of 36, HA6 and HA8 to inhibit CD44-HA binding were measured at multiple concentrations, which yielded IC50 values of 322 μM (36), 213 μM (HA6) and 168 μM (HA8) (Fig. 2b).
Fig. 2.
a Comparison of activities of various HA analogs at 240 μM to inhibit CD44-HA binding through the competition ELISA. b Inhibition curves of compound 36, HA4, HA6 and HA8
Based on the modelling results, both the m-benzyl and phenyl groups in compound 36 can contact the hydrophobic pocket near the HA binding site. The phenyl group possesses hydrophobic interactions with Ile 111 (Figure S1H). The m-benzyl group has hydrophobic interactions with Tyr 119 and T-shaped π-π stacking with Tyr 46. The methylene group between two aromatic rings presents the two rings in an arrangement that both rings can fully contact this hydrophobic pocket. Compared to 36, compounds 29~35 have fewer hydrophobic contacts with the pocket than 36 (Figure S1, A~G).
Conclusion
Several series of HA conjugates have been designed to target the HA binding pocket of CD44 and to inhibit the interaction of CD44 with HA. The HA2 library did not exhibit any appreciable activities. As assembly of higher HA oligosaccharides could be very tedious, a new synthetic approach towards HA oligosaccharides was developed by taking advantage of the ability of hyaluronidase to produce HA tetrasaccharides by digesting HA polysaccharides. The HA tetrasaccharides generated by hyaluronidase were utilized to synthesize multiple HA conjugates. Compared to the traditional chemical synthesis [26–29], this strategy was more efficient as it utilized readily available sources of materials (HA polysaccharide and hyaluronidase) and obviated the need to assemble the tetrasaccharide through glycosylation reactions. Furthermore, with the simplified protective group patterns utilized, deprotection of the final product was much simpler than the lengthy and at times unpredictable deprotection procedures encountered in traditional synthesis [38, 28]. Among all conjugates synthesized, analog 36 bearing the m-benzyl phenyl carbamate moiety gave the strongest inhibition of CD44-HA polysaccharide binding. While its inhibitory activity is still modest, the structure of 36 provides a lead in designing HA based CD44 inhibitors, which demonstrates strategically placed aromatic rings can improve the potency. Further optimization of HA conjugates is underway to enhance the affinity.
Supplementary Material
Acknowledgments
We are grateful for financial support from the Department of Chemistry, Michigan State University as well as the National Institute of General Medical Sciences, NIH (R01GM072667) and the National Science Foundation (CHE-1507226).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10719-015-9597-3) contains supplementary material, which is available to authorized users.
Compliance with ethical standards The authors declare that there are no potential conflicts of interests. All authors agree with the submission.
References
- 1.Lapcik L, Jr, Lapcik L, De Smedt S, Demeester J, Chabrecek P. Hyaluronan: preparation, structure, properties, and applications. Chem Rev. 1998;98:2663–2684. doi: 10.1021/cr941199z. [DOI] [PubMed] [Google Scholar]
- 2.Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4:528–539. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- 3.Day AJ, Prestwich GD. Hyaluronan-binding proteins: tying up the giant. J Biol Chem. 2002;277:4585–4588. doi: 10.1074/jbc.R100036200. [DOI] [PubMed] [Google Scholar]
- 4.Lesley J. Hyaluronan binding by cell surface CD44. J Biol Chem. 2000;275:26967–26975. doi: 10.1074/jbc.M002527200. [DOI] [PubMed] [Google Scholar]
- 5.Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Cell Biol. 2003;4:33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 6.Misra S, Heldin P, Hascall VC, Karamano NK, Skandalis SS, Markwald RR, Ghatak S. Hyaluronan–CD44 interactions as potential targets for cancer therapy. FASEB J. 2011;278:1429. doi: 10.1111/j.1742-4658.2011.08071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toole BP. Hyaluronan-CD44 interactions in cancer: paradoxes and possibilities. Clin Cancer Res. 2009;15:7462–7468. doi: 10.1158/1078-0432.CCR-09-0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Misra S, Ghatak S, Zoltan-Jones A, Toole BP. Regulation of multidrug resistance in cancer cells by hyaluronan. J Biol Chem. 2003;278:25285–25288. doi: 10.1074/jbc.C300173200. [DOI] [PubMed] [Google Scholar]
- 9.Toole BP, Wight TN, Tammi MI. Hyaluronan-cell interactions in cancer and vascular disease. J Biol Chem. 2002;277:4593–4596. doi: 10.1074/jbc.R100039200. [DOI] [PubMed] [Google Scholar]
- 10.Bourguignon LY. Hyaluronan-mediated CD44 activation of Rho GTPase signaling and cytoskeleton function promotes tumor progression. Semin Cancer Biol. 2008;18:251–259. doi: 10.1016/j.semcancer.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asari A. Hyaluronan Today. Glycoforum; 2005. Novel functions of hyaluronan oligosaccharides. http://www.glycoforum.gr.jp/science/hyaluronan/hyaluronanE.html. [Google Scholar]
- 12.Toole BP, Ghatak S, Misra S. Hyaluronan oligosaccharides as a potential anticancer therapeutic. Curr Pharm Biotech. 2008;9:249–252. doi: 10.2174/138920108785161569. [DOI] [PubMed] [Google Scholar]
- 13.Harada H, Nakata T, Hirota-takahata Y, Tanaka I, Nakajima M, Takahashi M. F-16438s, novel binding inhibitors of CD44 and hyaluronic acid. J Antibiot. 2006;59:770–776. doi: 10.1038/ja.2006.101. [DOI] [PubMed] [Google Scholar]
- 14.Hirota-takahata Y, Harada H, Tanaka I, Nakata T, Nakajima M, Takahashi M. F-16438s, novel binding inhibitors of CD44 and hyaluronic acid. J Antibiot. 2006;59:777–784. doi: 10.1038/ja.2006.102. [DOI] [PubMed] [Google Scholar]
- 15.Hirota-Takahata Y, Harada H, Tanaka I, Nakata T, Nakajima M, Takahashi M. F-19848 A, a novel inhibitor of hyaluronic acid binding to cellular receptor CD44. J Antibiot. 2007;60:633–639. doi: 10.1038/ja.2007.81. [DOI] [PubMed] [Google Scholar]
- 16.Liu L-K, Finzel BC. Fragment-based identification of an inducible binding site on cell surface receptor CD44 for the design of protein – carbohydrate interaction inhibitors. J Med Chem. 2014;57:2714–27 25. doi: 10.1021/jm5000276. [DOI] [PubMed] [Google Scholar]
- 17.Banerji S, Wright AJ, Noble M, Mahoney DJ, Campbell ID, Day AJ, Jackson DG. Structures of the CD44-hyaluronan complex provide insight into a fundamental carbohydrate-protein interaction. Nat Struct Mol Biol. 2007;14:234–239. doi: 10.1038/nsmb1201. [DOI] [PubMed] [Google Scholar]
- 18.Cumpstey I, Salomonsson E, Sundin A, Leffler H, Nilsson UJ. Double affinity amplification of galectin–ligand interactions through arginine–arene interactions: synthetic, thermodynamic, and computational studies with aromatic diamido thiodigalactosides. Chem Eur J. 2008;14:4233–4245. doi: 10.1002/chem.200701932. [DOI] [PubMed] [Google Scholar]
- 19.Sörme P, Arnoux P, Kahl-Knutsson B, Leffler H, Rini JM, Nilsson UJ. Structural and thermodynamic studies on cation – π interactions in lectin – ligand complexes: High-affinity galectin-3 inhibitors through fine-tuning of an arginine – arene interaction. J Am Chem Soc. 2005;127:1737–1743. doi: 10.1021/ja043475p. [DOI] [PubMed] [Google Scholar]
- 20.Zeng Y, Rademacher C, Nycholat CM, Futakawa S, Lemme K, Ernst B, Paulson JC. High affinity sialoside ligands of myelin associated glycoprotein. Bioorg Med Chem Lett. 2011;21:5045–5049. doi: 10.1016/j.bmcl.2011.04.068. and references cited therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang L, Wang Z, Li X, Ye X, Huang X. Iterative one-pot syntheses of chitotetroses. Carbohydr Res. 2006;341:1669–1679. doi: 10.1016/j.carres.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang L, Teumelsan N, Huang X. A facile method for oxidation of primary alcohols to carboxylic acids and its application in glycosaminoglycan syntheses. Chem Eur J. 2006;12:5246–5252. doi: 10.1002/chem.200600290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hong V, Presolski SI, Ma C, Finn MG. Analysis and optimization of copper-catalyzed azide-alkyne cycloaddition for bioconjugation. Angew Chem Int Ed. 2009;48:9879–9883. doi: 10.1002/anie.200905087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamat M, El-boubou K, Zhu DC, Lansdell T, Lu X, Li W, Huang X. Hyaluronic acid immobilized magnetic nanoparticles for active targeting and imaging of macrophages. Bioconjugate Chem. 2010;21:2128–2135. doi: 10.1021/bc100354m. [DOI] [PubMed] [Google Scholar]
- 25.Pouyani T, Prestwich GD. Biotinylated hyaluronic acid: a new tool for probing hyaluronate-receptor interactions. Bioconjugate Chem. 1994;5:370–372. doi: 10.1021/bc00028a015. [DOI] [PubMed] [Google Scholar]
- 26.Huang L, Lu X, Huang X. Chemical syntheses of hyaluronic acid oligosaccharides. ACS Symp Ser. 2008;990:29–53. and references cited therein. [Google Scholar]
- 27.Mukherjee C, Liu L-K, Pohl N. Regioselective benzylation of 2-deoxy-2-aminosugars using crown ethers: application to a shortened synthesis of hyaluronic acid oligomers. Adv Synth Cat. 2014;356:2247–2256. doi: 10.1002/adsc.201400269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu X, Kamat MN, Huang L, Huang X. Chemical synthesis of a hyaluronic acid decasaccharide. J Org Chem. 2009;74:7608–7617. doi: 10.1021/jo9016925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dinkelaar J, Gold H, Overkleeft HS, Codee JDC, van der Marel GA. Synthesis of hyaluronic acid oligomers using chemoselective and one-pot strategies. J Org Chem. 2009;74:4208–4216. doi: 10.1021/jo9003713. [DOI] [PubMed] [Google Scholar]
- 30.Tawada A, Masa T, Oonuki Y, Watanabe A, Matsuzaki Y, Asari A. Large-scale preparation, purification, and characterization of hyaluronan oligosaccharides from 4-mers to 52-mers. Glycobiology. 2002;12:421–426. doi: 10.1093/glycob/cwf048. [DOI] [PubMed] [Google Scholar]
- 31.Mahoney DJ, Aplin RT, Calabro A, Hascall VC, Day AJ. Novel methods for the preparation and characterization of hyaluronan oligosaccharides of defined length. Glycobiology. 2001;11:1025–1033. doi: 10.1093/glycob/11.12.1025. [DOI] [PubMed] [Google Scholar]
- 32.Banoub J, Boullanger P, Lafont D. Synthesis of oligosaccharides of 2-amino-2-deoxy sugars. Chem Rev. 1992;92:1167–1195. [Google Scholar]
- 33.Castro-Palomino JC, Schmidt RR. N, N-Diacetyl-glucosamine and -galactosamine derivatives as glycosyl donors. Tetrahedron Lett. 1995;36:6871–6874. [Google Scholar]
- 34.Pachamuthu K, Schmidt RR. Synthetic routes to thiooligosaccharides and thioglycopeptides. Chem Rev. 2006;106:160–187. doi: 10.1021/cr040660c. [DOI] [PubMed] [Google Scholar]
- 35.Geyer A, Hummel G, Eisele T, Reinhardt S, Schmidt RR. Structural motifs of the dimeric Lewis glycolipids as determined by NMR spectroscopy and molecular dynamics simulations. Chem Eur J. 1996;2:981–988. [Google Scholar]
- 36.Witczak ZJ, Chhabra R, Chen H, Xie X-Q. Thiosugars II. A novel approach to thiodisaccharides the synthesis of 3-deoxy-4-thiocellobiose from levoglucosenone. Carbohydr Res. 1996;301:167–175. [Google Scholar]
- 37.van den Bos LJ, Litjens REJN, van den Berg RJBHN, Overkleeft HS, van der Marel GA. Preparation of 1-thio uronic acid lactones and their use in oligosaccharide synthesis. Org Lett. 2005;7:2007–2010. doi: 10.1021/ol050491y. [DOI] [PubMed] [Google Scholar]
- 38.Huang L, Huang X. Highly efficient syntheses of hyaluronic acid oligosaccharides. Chem Eur J. 2007;13:529–540. doi: 10.1002/chem.200601090. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.