Abstract
A 51-year-old male patient with chronic hepatitis B was referred to our hospital due to a 1-cm liver nodule on ultrasonography. Alpha-fetoprotein (AFP) was slightly elevated. The nodule showed prolonged enhancement on dynamic liver magnetic resonance imaging and appeared as a hyperintensity on both diffusion-weighted and T2-weighted imaging. The nodule was followed up because it was small and typical findings of hepatocellular carcinoma (HCC) were not observed in the dynamic imaging investigations. However, liver contrast-enhanced ultrasonography performed 1 month later showed enhancement during the arterial phase and definite washout during the delayed phase. Also, AFP had increased to over 200 ng/mL even though AST and ALT were decreased after administering an antiviral agent. He was presumptively diagnosed as HCC and underwent liver segmentectomy. Microscopy findings of the specimen indicated bile duct adenoma. After resection, the follow-up AFP had decreased to within the normal range. This patient represents a case of bile duct adenoma with AFP elevation mimicking HCC on contrast-enhanced ultrasonography.
Keywords: Adenoma, Bile duct, Hepatitis B, Alpha-fetoproteins, Ultrasonography
INTRODUCTION
Intrahepatic bile duct adenoma (BDA) is a rare benign liver tumor originated from the epithelium of the bile ducts. The incidence of BDA was reported 1.3% of primary liver tumors.1 Most of BDAs are less than 2cm and lack of symptom. Several imaging modalities such as computed tomography (CT) and magnetic resonance imaging (MRI) are used to detect BDA. Histopathologically, BDA is a kind of aggregation of proliferative noncystic bile ductules in background of connective tissue stroma with variable degrees of fibrosis and inflammation.2
We report a case of BDA with elevated alpha-fetoprotein (AFP) in a patient with chronic hepatitis B (CHB) assessed by various imaging modalities including contrast-enhanced ultrasonography (US). To our knowledge, this is the first case of BDA with elevated AFP mimicking hepatocellular carcinoma (HCC) on contrast-enhanced US in a patient with CHB.
CASE REPORT
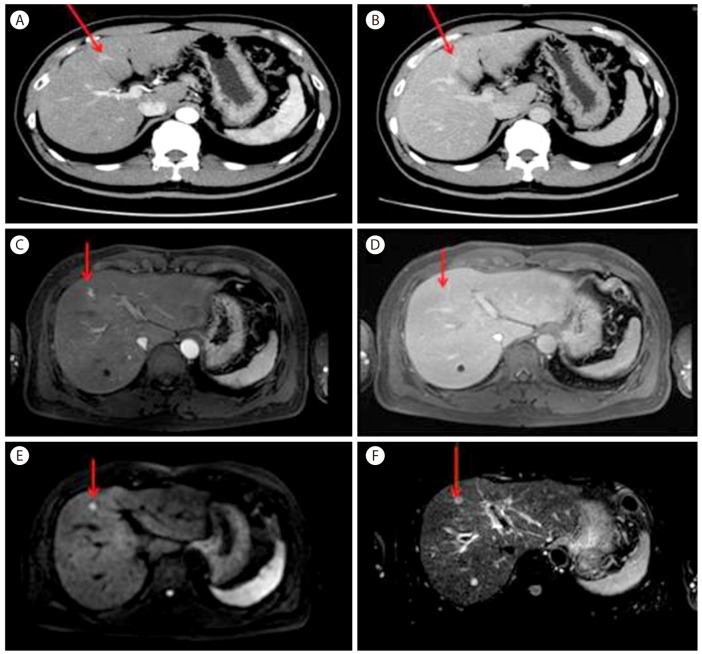
A 51-year-old male patient was referred to our hospital due to a liver nodule which was incidentally detected under abdominal US screening at a local clinic. He had been diagnosed as hepatitis B virus carrier 20 years ago but he had not received any check-up for hepatitis B or surveillance for HCC. He was a social drinker. He had no symptom and physical exam was non remarkable. On laboratory exam, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were elevated by 113 and 151 IU/L. On viral marker examination, HBsAg was positive, Anti-HBs negative, Anti-HCV negative, HBeAg positive, Anti-HBe negative, HBV DNA more than 170,000,000 IU/mL. On tumor markers, AFP was slightly elevated by 23 ng/mL but proteins Induced by vitamin K absence/antagonist-II was within normal range. On liver dynamic CT, about 1cm nodular lesion was detected on segment 4 in liver. It was well enhanced during arterial phase (Fig. 1A) and persistently enhanced during portal and hepatobiliary phase (Fig. 1B). We performed gadolinium-enhanced liver MRI to rule out the possibility of small HCC. On the MRI, about 0.8cm nodular lesion on segment 4 showed low signal intensity on T1-weighted images, enhancement during hepatic arterial phase (Fig. 1C), no definite washout during hepatobiliary phase (Fig. 1D), and high signal intensity on diffusion-weighted images (DWI) (Fig. 1E) and heavily T2-weighted images (Fig. 1F).
Figure 1. Liver dynamic computed tomography (CT) showed (A) early enhancement during the arterial phase and (B) prolonged enhancement during the delayed phase. Gadolinium-enhanced liver magnetic resonance imaging (MRI) showed (C) enhancement in the tumor during the T1 arterial phase, (D) isointensity in the tumor relative to normal liver parenchyma during the T1 delayed phase, (E) a high signal intensity on a diffusion-weighted image, and (F) a high signal intensity on a heavily T2-weighted image.
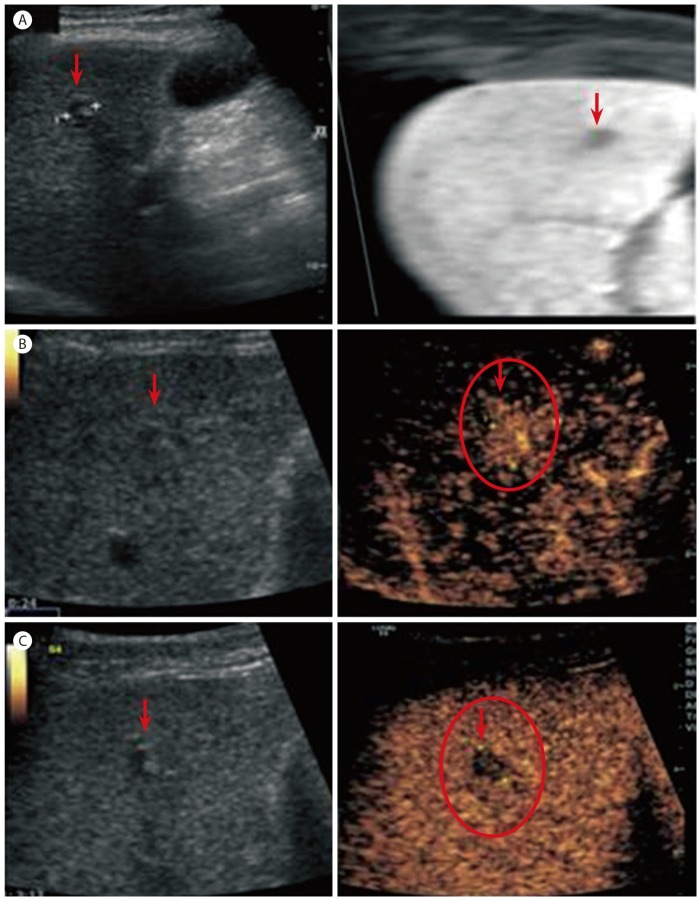
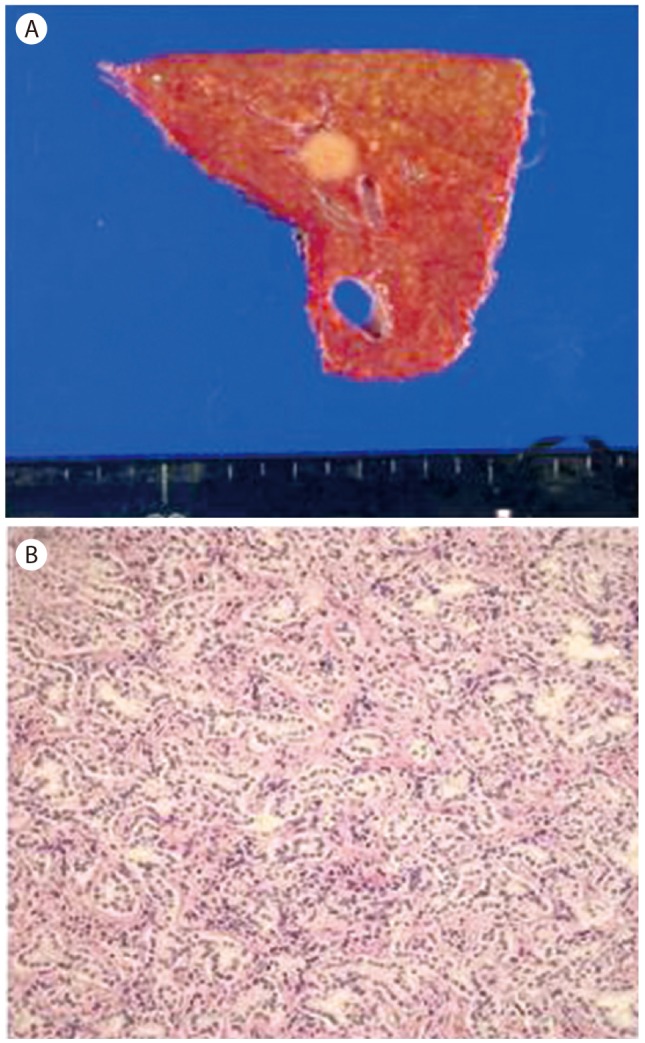
He started taking entecavir 0.5 mg per day for the treatment of immune reactive CHB. Although he had underlying disease of CHB and the rise of AFP, we decided to follow-up for the liver nodule because its size was small and dynamic imaging did not show typical features of HCC. A new imaging modality, contrast-enhanced liver US, was performed 1 month later. About 1 cm low echogenic nodule showing bull's eye pattern was found on segment 4 and it was confirmed by imaging fusion with volume navigation that the nodule on US was the same lesion observed on MRI (Fig. 2A). On contrast-enhanced US with microbubble contrast agent (Sono-Vue), it was well enhanced during arterial phase (Fig. 2B) and hypoechoic with definite washout during delayed phase (Fig. 2C). Moreover AFP was increased by 268 ng/mL after 1 month even though both AST and ALT were decreased after taking anti-viral agent. He was clinically presumptively diagnosed as HCC with the reasons that contrast enhanced US showed typical features of HCC, AFP was increased to over 200 ng/mL, and he had underlying disease of CHB. Then he underwent liver resection (S4 segmentectomy) for the purpose of both histological diagnosis and treatment. Gross findings of the specimen showed an 1cm well-circumscribed, non-capsulated, round yellowish nodule (Fig. 3A). Microscopically, it was a kind of proliferation of well-formed bile tubular ducts without cell atypia or mitotic activity in the background of connective tissue stroma (Fig. 3B). The final diagnosis was BDA on the basis of histology. Follow-up AFP was decreased to 44 ng/mL 1 week after the surgery, and within normal range by 4.5 ng/mL 6 months after the surgery. Also follow-up CT at 12 months showed no tumor recurrence.
Figure 2. (A) Ultrasonography revealed a hypoechoic nodule on segment 4, which was the same lesion observed in MRI, as confirmed by image fusion with volume navigation. Contrast-enhanced ultrasonography showed (B) enhancement in the tumor during the arterial phase and (C) washout during the delayed phase.
Figure 3. (A) Gross specimen appears as a 1-cm non-encapsulated, whitish, and spherical lesion. (B) Microscopy image shows proliferation of bile ductules with dense connective tissue stroma (H&E stain, ×200).
DISCUSSION
Intrahepatic BDA is a rare benign liver tumor originated from the epithelium of the bile ducts. BDA was usually found incidentally during autopsy or laparotomy.1,2,3,4 The incidence of BDA was reported 1.3% of primary liver tumors.1 Most of BDAs were ranged from 1 mm to 20 mm and had limitation on growth potential. Also they showed benign nature in follow-up period and no symptom.2,5 Although the pathogenesis of BDA has been still unclear, previous studies suggested that BDA is a peribiliary gland hamartoma6 or a result of reactive process to the focal injury.2 Macroscopically, BDA is a sub-capsular, well-demarcated, firm, and yellowish nodule.2 Histopathologically, BDAs consist of highly proliferative bile ductules in background of connective tissue stroma with variable degrees of fibrosis and inflammation without cell atypia or mitotic activity.7 Our case also showed asymptomatic BDA less than 2 cm. However our case was the first case of BDA accompanied by the elevation of AFP. Although AFP could be elevated in patients with CHB, there are two points which support that BDA induces AFP elevation. One point is that follow-up AFP was more increased even though AST and ALT were decreased after starting antiviral therapy. The other point is that AFP was decreased after resection of BDA.
Recently several imaging modalities such as dynamic CT and MRI are used to detect BDA. Most cases of BDAs showed hypervascular characteristics with prolonged enhancement on dynamic CT, MRI, and angiography because of the fibrous tissues within the tumor whereas HCC usually shows definite washout during delayed phase.8 However it is difficult to differentiate between small HCC and BDA because small HCC less than 2cm frequently shows atypical finding on imaging. Our case showed prolonged enhancement on dynamic CT and MRI, consistent with previously reported cases of BDAs. On DWI and T2-weighted imaging, the nodule showed high signal intensity. There have been just a few of reports regarding these image findings up to recently.3,9,10 An et al.11 reported a case of BDA using DWI for the first time in 2013. Our case undertook contrast-enhanced US as follow-up imaging modality 1 month later. To our knowledge, it was the first case to use this imaging modality for BDA. Our case showed early enhancement during arterial phase and wash-out during delayed phase suggesting HCC on contrast-enhanced US. This result was different from those with other dynamic imaging modalities. This difference probably occurred because the contrast enhanced US used microbubble as contrast agent and microbubble could be ruptured during delayed phase.12 Thus, BDA probably could show delayed washout on contrast-enhanced US whereas it showed prolonged enhancement on dynamic CT and MRI.
In summary, we experienced a case of BDA with elevated AFP in a patient with CHB assessed by various imaging modalities including contrast-enhanced US. In our case, AFP was highly elevated and decreased after BDA resection. Definite washout was observed on contrast-enhanced US whereas other imaging modalities showed prolonged enhancement. To our knowledge, this is the first case of BDA that undertook contrast-enhanced US and showed highly elevated AFP. Further cases of its diagnostic use with BDA are required to establish the image findings on contrast-enhanced US.
Abbreviations
- AFP
alpha-fetoprotein
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BDA
bile duct adenoma
- CHB
chronic hepatitis B
- CT
computed tomography
- DWI
diffusion-weighted imaging
- HCC
hepatocellular carcinoma
- MRI
magnetic resonance imaging
- US
ultrasonography
Footnotes
Conflicts of Interest: The authors have no conflicts to disclose.
References
- 1.HA. E. Atlas of tumor pathology, fascicle 25. Washington DC: Armed Forces Institute of Pathology; 1958. Tumors of the liver and intrahepatic bile duct; pp. 19–29. [Google Scholar]
- 2.Allaire GS, Rabin L, Ishak KG, Sesterhenn IA. Bile duct adenoma. A study of 152 cases. Am J Surg Pathol. 1988;12:708–715. doi: 10.1097/00000478-198809000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Tajima T, Honda H, Kuroiwa T, Yoshimitsu K, Irie H, Aibe H, et al. Radiologic features of intrahepatic bile duct adenoma: a look at the surface of the liver. J Comput Assist Tomogr. 1999;23:690–695. doi: 10.1097/00004728-199909000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Cho C, Rullis I, Rogers LS. Bile duct adenomas as liver nodules. Arch Surg. 1978;113:272–274. doi: 10.1001/archsurg.1978.01370150044007. [DOI] [PubMed] [Google Scholar]
- 5.Koga F, Tanaka H, Takamatsu S, Baba S, Takihara H, Hasegawa A, et al. A case of very large intrahepatic bile duct adenoma followed for 7 years. World J Clin Oncol. 2012;3:63–66. doi: 10.5306/wjco.v3.i4.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhathal PS, Hughes NR, Goodman ZD. The so-called bile duct adenoma is a peribiliary gland hamartoma. Am J Surg Pathol. 1996;20:858–864. doi: 10.1097/00000478-199607000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Iacobuzio-Donahue CA, Montgomery EA. In: Gastrointestinal and Liver Pathology. Goldblum JR, editor. Philadelphia, PA: Elsevier; 2005. pp. 600–602. [Google Scholar]
- 8.Yoshikawa J, Matsui O, Kadoya M, Gabata T, Arai K, Takashima T. Delayed enhancement of fibrotic areas in hepatic masses: CT-pathologic correlation. J Comput Assist Tomogr. 1992;16:206–211. doi: 10.1097/00004728-199203000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Maeda E, Uozumi K, Kato N, Akahane M, Inoh S, Inoue Y, et al. Magnetic resonance findings of bile duct adenoma with calcification. Radiat Med. 2006;24:459–462. doi: 10.1007/s11604-006-0044-z. [DOI] [PubMed] [Google Scholar]
- 10.Kim YS, Rha SE, Oh SN, Jung SE, Shin YR, Choi BG, et al. Imaging findings of intrahepatic bile duct adenoma (peribiliary gland hamartoma): a case report and literature review. Korean J Radiol. 2010;11:560–565. doi: 10.3348/kjr.2010.11.5.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.An C, Park S, Choi YJ. Diffusion-weighted MRI in intrahepatic bile duct adenoma arising from the cirrhotic liver. Korean J Radiol. 2013;14:769–775. doi: 10.3348/kjr.2013.14.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giannetti A, Franci L, Grechi C, Giangregorio F. Contrast-enhanced sonography in the diagnosis of hepatic hemangiomas: atypical appearance due to the washout of microbubbles. J Clin Ultrasound. 2013;41:361–365. doi: 10.1002/jcu.21939. [DOI] [PubMed] [Google Scholar]