Summary
Layer II (LII) of the medial entorhinal cortex (MEC) comprises grid cells that support spatial navigation. The firing pattern of grid cells might be explained by attractor dynamics in a network, which requires either direct excitatory connectivity between phase-specific grid cells or indirect coupling via interneurons. However, knowledge regarding local networks that support in vivo activity is incomplete. Here we identified essential components of LII networks in the MEC. We distinguished four types of excitatory neurons that exhibit cell-type-specific local excitatory and inhibitory connectivity. Furthermore, we found that LII neurons contribute to the excitation of contralateral neurons in the corresponding layer. Finally, we demonstrated that the medial septum controls excitation in the MEC via two subpopulations of long-range GABAergic neurons that target distinct interneurons in LII, thereby disinhibiting local circuits. We thus identified local connections that could support attractor dynamics and external inputs that likely govern excitation in LII.
Highlights
-
•
LII MEC excitatory neurons can be classified into four cell types
-
•
The four cell types exhibit specific local excitatory and inhibitory connectivity
-
•
LII neurons contribute to the excitation of contralateral LII neurons
-
•
Distinct septal GABAergic neurons exhibit cell-type-specific inhibition in LII MEC
Medial entorhinal cortex comprises grid cells that play an important role in spatial navigation. Fuchs et al. characterized essential excitatory and inhibitory components of the underlying networks. This is a prerequisite to develop realistic models to explain grid cell activity.
Introduction
The medial entorhinal cortex (MEC) is a major in- and output structure of the hippocampus and participates in processes supporting spatial navigation, learning, and memory (Bannerman et al., 2001, Howard et al., 2014, Steffenach et al., 2005, Suh et al., 2011). The superficial layer II (LII) and layer III (LIII) of the MEC are the origin of the perforant path terminating in the dentate gyrus and the temporo-ammonic pathway directly targeting CA1 neurons in the hippocampus.
Neurons located in the superficial layers of the MEC exhibit distinct spatial firing patterns. The most extensively studied are LII/III grid cells, which display a hexagonal firing pattern in two-dimensional environments (Hafting et al., 2005). The increasing information pertaining to many of the unique grid cell features contrasts with the sparse knowledge regarding the generation of their conspicuous firing pattern. Many types of network models were proposed that try to account for the generation of grid-like firing (Burak, 2014, Burgess and O’Keefe, 2011, Giocomo et al., 2011, McNaughton et al., 2006). However, even promising attractor models have been recently challenged, as they are not fully supported by empirical data. Thus, an important premise of attractor models is based on the presence of local connectivity between grid cells. In earlier models, this was implemented by direct excitatory connections between grid cells. Alternatively, a grid cell pattern can emerge in networks based on purely inhibitory local connections (Burak and Fiete, 2009). Grid-like firing also was generated in attractor models with grid cell communication mediated disynaptically via inhibitory interneurons (Couey et al., 2013, Pastoll et al., 2013, Roudi and Moser, 2014). These models were supported by empirical data that showed a lack of connectivity between stellate cells (Dhillon and Jones, 2000), but bidirectional connectivity between stellate cells and local inhibitory neurons (Couey et al., 2013, Pastoll et al., 2013).
Although electrophysiological recordings in vitro failed to establish excitatory connections between stellate cells (i.e., putative grid cells), there is the intriguing possibility that other excitatory neurons in LII might support grid-like firing by providing local excitation, as required by attractor models based on excitatory recurrent connectivity. Indeed, electrophysiological in vivo data support this notion as, upon morphological reconstruction, putative grid cells were found to comprise both stellate and pyramidal neurons (Domnisoru et al., 2013). The idea that both cell types could exhibit a grid cell firing pattern, although to a different degree, received further support from experimental work in which juxtacellularly labeled putative grid cells (Tang et al., 2014) and in vivo Ca2+ imaging in distinct cell types (Sun et al., 2015) were analyzed. However, it is not clear whether, and to which extent, pyramidal cells are connected within LII.
On the basis of electrophysiological properties measured in vitro, Alonso and Klink (1993) identified the existence of two cell types in LII, namely stellate and pyramidal-like cells. These findings were further extended by Canto and Witter (2012), who also distinguished between stellate and pyramidal cells but pointed out that there is a certain degree of variability within each cell class. The presence of at least two defined types of excitatory neurons is further supported by immunohistochemical evidence. Thus, calbindin (CB) and reelin (RE) expression in LII was correlated with the pyramidal and stellate phenotype, respectively (Kitamura et al., 2014, Ray et al., 2014, Varga et al., 2010). Interestingly, the expression pattern of the two markers exhibited a striking modular organization (Kitamura et al., 2014, Ray et al., 2014).
There is indication that the two types of excitatory neurons are differentially wired both locally as well as with respect to their downstream targets. Thus, inhibition onto stellate cells is provided by fast-spiking (FS), parvalbumin-positive (PV+) interneurons (Buetfering et al., 2014, Couey et al., 2013, Pastoll et al., 2013), while pyramidal cells are inhibited by cholecystokinin+ interneurons (Varga et al., 2010). Regarding the output projections of the two cell types, there is clear evidence that stellate/RE+ neurons constitute the perforant path and project to the dentate gyrus. The target area of pyramidal and/or CB+ neurons is still an issue of debate. While Varga et al. (2010) reported that CB+ neurons project to the contralateral MEC, a recent study proposed that CB+/WFS1+ neurons contribute to the temporo-ammonic pathway thereby directly targeting the CA1 region (Kitamura et al., 2014).
Finally, an important yet unresolved question pertains to the contribution of the external input in driving and/or modulating grid cell firing. Thus, inactivation of the medial septum (MS) disrupts the spatial periodicity of grid cell firing (Brandon et al., 2011, Koenig et al., 2011) without affecting the activity of other spatially tuned cells, such as boundary cells and head-direction cells. The septo-entorhinal pathway comprises cholinergic and GABAergic projections (Alonso and Köhler, 1984, Köhler et al., 1984). The latter received attention only lately. Thus, it is noteworthy that septal GABAergic neurons target FS and low threshold-spiking (LTS) interneurons in all layers of the MEC (Gonzalez-Sulser et al., 2014).
On the basis of these premises the following pressing questions arise: (1) Are pyramidal and/or CB+ neurons in LII directly interconnected and can thereby support some of the demands requested by attractor network models? (2) Are there yet other excitatory cell types in LII, and if so, how are they locally connected? (3) Are excitatory LII neurons differentially connected to inhibitory LII neurons? (4) And finally, do other brain regions that project to the MEC (e.g. the septum or contralateral MEC) contribute substantially to the recruitment of LII neurons, and if so, what are the mechanisms by which they do so?
Hence we revisited the dorsal MEC and analyzed electrophysiological and morphological properties of LII MEC neurons. Aided by connectivity measurements performed in mice expressing fluorescent proteins in defined neurons, we distinguished distinct excitatory cell types that exhibit cell-type-specific excitatory and inhibitory local connectivity. We addressed the long-range connectivity by combining retrograde tracer injections with optogenetic and electrophysiological analysis of target neurons. We identified and characterized two external inputs, namely excitatory projections from the contralateral MEC and inhibitory projections from the MS.
Results
Electrophysiological and Morphological Characterization of LII Excitatory Neurons
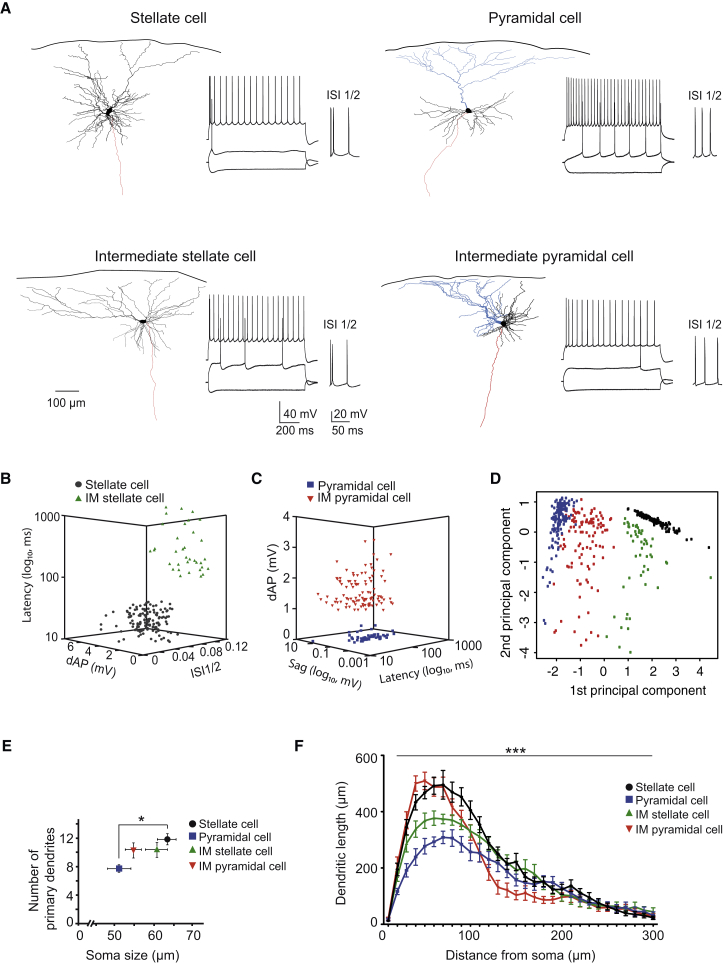
We identified four distinct types of excitatory neurons in LII, referred to hereafter as stellate cells, intermediate stellate cells, pyramidal cells, and intermediate pyramidal cells. Our classification is based on the following morphological and electrophysiological features: (1) hyperpolarizing and depolarizing sag potential, (2) burst firing (shorter ratio of ISI1/2) in response to depolarization, (3) depolarizing afterpotential (dAP), (4) latency to first spike, and (5) the presence of a main (apical) dendrite (Figure 1; Table S1). The first three criteria were used previously to differentiate between stellate and non-stellate cells in the MEC (Alonso and Klink, 1993, Canto and Witter, 2012). The most outstanding feature of stellate cells was the sag potential and burst firing, while pyramidal cells exhibited longer latency to first spike and more pronounced adaption of spike firing. In contrast to stellate cells, intermediate stellate cells displayed a significant longer latency to the first spike (>100 ms; Figures 1A and 1B; Table S1). Finally, the most prominent feature aiding in separating intermediate pyramidal cells from pyramidal cells was a clear dAP (>0.5 mV) in the former (Figures 1A and 1C; Table S1). Principal component analysis based on the five parameters resulted in a clear separation between stellate and pyramidal cells and underlined the “intermediate” distribution of the two intermediate cell types (Figure 1D).
Figure 1.
Morphological and Electrophysiological Features of Defined Excitatory LII Cell Types in Dorsal MEC
(A) Reconstruction of four representative neurons belonging to the indicated cell type (dendrites in black, apical dendrite in blue, axon in red) and their corresponding firing pattern upon somatic current injection (−200 to 600 pA). ISI1/2 plus dAP reveal differences between the four cell types: the stellate cell and intermediate stellate cell exhibit burstiness, the firing pattern of pyramidal cell and intermediate pyramidal cell displays adaptation, and dAP is absent in the pyramidal cell.
(B) Distribution of stellate (gray circles) and intermediate stellate cells (green triangles) when using latency to spike firing, ISI1/2, and dAP as distinction criteria.
(C) Distribution of pyramidal cells (blue squares) and intermediate pyramidal cells (red triangles) when using dAP, sag potential, and latency as distinction criteria.
(D) Principal component analysis based on the same electrophysiological parameters as used in (B) and (C) plus the presence of an apical dendrite. The plot shows the first two principal components, with component 2 representing predominantly latency while component 1 combines information from the four remaining variables. Note the clear separation between stellate (black) and pyramidal cells (blue), whereas both intermediate cells (IM stellate [green] and IM pyramidal [red] cells) display an “intermediate” distribution.
(E) Differences in soma size and numbers of primary dendrites of the four excitatory cell types (∗p < 0.05).
(F) Sholl analysis reveals difference between the four cell types when plotting dendritic length as a function of circular distance from the soma in 10-μm steps (two-way ANOVA, F(144,1813) = 4.43, ∗∗∗p < 0.001). Stellate and intermediate pyramidal cells exhibit locally (10–80 μm) a higher density of dendrites compared to pyramidal and intermediate stellate cells.
Abbreviations are as follows: ISI, interspike interval; dAP, depolarized afterpotential; and IM, intermediate. See also Figure S1 and Table S1.
A classification into four subtypes was further supported when morphological criteria were taken into account. Both the soma perimeter (63.38 ± 2.49 versus 51.18 ± 3.1 μm; p < 0.05) and number of primary dendrites (11.3 ± 0.8 versus 7.8 ± 0.59; p < 0.05, n = 12 and 8 cells, respectively; Figure 1E) were significantly different when comparing stellate cells and pyramidal cells. Values obtained for intermediate stellate and intermediate pyramidal cells were “intermediate” between those of stellate and pyramidal cells (soma perimeter: 60.72 ± 2.88 and 54.76 ± 2.16 μm; number of primary dendrites: 10.4 ± 1.1and 10.3 ± 1.1, n = 10 and 12 cells, respectively; Figure 1E). Furthermore, Sholl analysis of reconstructed cells revealed a significant difference regarding the dendritic distribution between the four cell types (two-way ANOVA followed by post hoc Bonferroni test; Figure 1F; Figures S1A and S1B).
A classification of these four cell types based on morphological and physiological features had a less clear counterpart when the immunocytochemical markers RE and CB/WFS1 were employed. Even though RE was expressed in all stellate cells and intermediate stellate cells, the marker could be detected also to a variable extent in cells belonging to the other two cell types. Similarly, WFS1 was detected in a large proportion of pyramidal cells and intermediate pyramidal cells, but the marker could be found also in some intermediate stellate cells (Table S1). On the basis of marker expression a defined cell type could not be further subdivided. In other words, the electrophysiological properties of intermediate pyramidal cells expressing RE did not differ from those of intermediate pyramidal cells expressing WFS1. In our hands, WFS1 was a more reliable marker than CB in post hoc analysis of biocytin-filled cells. The two markers colocalized almost completely in LII excitatory cells of dorsal MEC (97.9% ± 0.7% of 934 CB+/GAD67EGFP− neurons counted in 4 hemispheres from 2 GAD67EGFP mice).
Local Connectivity between Distinct Excitatory LII Neurons
As CB is preferentially expressed in pyramidal and intermediate pyramidal cells and RE marks stellate and intermediate stellate cells, we employed mice in which CB+ and RE+ neurons were fluorescently labeled to speed up the identification of excitatory neurons when measuring their putative connectivity (Figure S2).
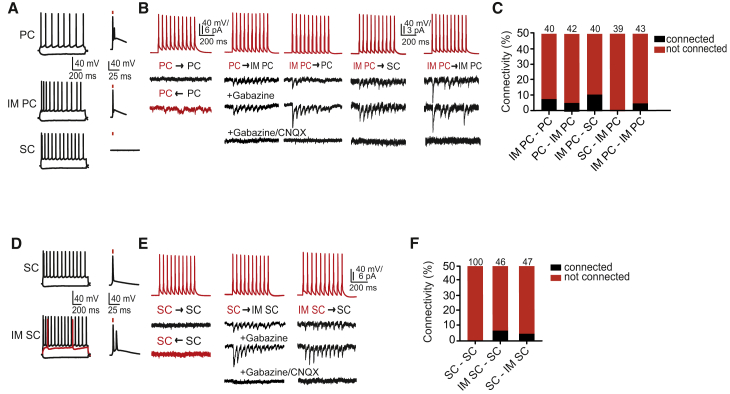
To identify CB+ neurons, we injected adeno-associated viral vector (AAV) DIO ChR2-mCherry into the MEC of CBCre mice. This resulted in specific mCherry expression in CB+ neurons (Figures S2A–S2D) that, based on their firing pattern, could be easily further classified as pyramidal and intermediate pyramidal cells (Figure 2A). Non-labeled neurons comprised stellate and intermediate stellate cells. We recorded unitary excitatory postsynaptic currents (uEPSCs) from pairs of neurons whose somata were located at a distance of <40 μm in LII. We elicited trains of presynaptic action potentials (10 spikes at 40 Hz), and searched in neighboring neurons for monosynaptic uEPSCs that were sensitive to the AMPA receptor inhibitor CNQX, but not to the GABAA receptor antagonist Gabazine. Direct connections between pairs of pyramidal cells or between pyramidal cells and stellate cells were extremely rare (1 of 56 pyramidal-pyramidal pairs, and 0 of 38 pyramidal-stellate pairs; Figures S3A and S3B). In contrast, intermediate pyramidal cells excited both pyramidal and stellate cells with higher probability (Figures 2A–2C; Figures S3A and S3B). Thus, the connectivity from intermediate pyramidal cells to pyramidal cells was 7.5% (3 of 40 pairs), and 4.8% in the opposite direction (2 of 42 pairs; Figures 2A–2C; Figures S3A and S3B). In pairs of intermediate pyramidal cells and stellate cells, the connectivity was 10.0% from intermediate pyramidal cells to stellate cells (4 of 40 pairs), but absent in the opposite direction (0 of 39 pairs; Figures 2A–2C; Figures S3A and S3B). Between intermediate pyramidal cell pairs, the connectivity was 4.7% (2 of 43 pairs; Figures 2A–2C; Figures S3A and S3B).
Figure 2.
Local Excitatory Connectivity in LII of the MEC
(A) Firing pattern (left) of indicated excitatory cells in CBCre mice. The PC and IM PC, but not the SC, are excited by ChR2 stimulation (right, stimulation is indicated by red bar above the spike).
(B) uEPSCs recorded at −70 mV in indicated cells elicited by a train of 10 action potentials in the presynaptic neuron (40 Hz) (action potential traces in red, upper row). The direction of tested connectivity is indicated by an arrow. uEPSCs are not blocked by Gabazine, but by Gabazine plus CNQX (both at a concentration of 10 μM).
(C) Summary graph of investigated connections between indicated cell types.
(D) Firing pattern of tested excitatory cells in Uchl1Cre mice. Representative examples showing activation of a SC and IM SC following ChR2 stimulation (stimulation is indicated by red bar above the spike).
(E) uEPSCs recorded at −70 mV in indicated cells elicited by a train of 10 action potentials in the presynaptic neuron (40 Hz) (action potential traces in red, upper row).
(F) Summary graph of investigated connections between indicated cell types. The numbers above the bars indicate the number of analyzed cell pairs.
Abbreviations are as follows: L, layer; IM, intermediate; MEC, medial entorhinal cortex; PC, pyramidal cell; SC, stellate cell; IM PC, intermediate pyramidal cell; and IM SC, intermediate stellate cell. See also Figures S2 and S3.
To aid the identification and study the two predominantly RE expressing cell types, namely stellate and intermediate stellate cells, we injected AAV DIO ChR2-mCherry into the MEC of Uchl1Cre mice, which resulted in fluorescently labeled RE+ neurons (Figures S2E and S2F). We concur with previous observations (Couey et al., 2013) that stellate cells (classified based on electrophysiological parameters) are not connected (0 of 100 pairs tested in both directions). However, the connectivity between intermediate stellate cells and stellate cells was 6.5% (3 of 46 pairs), and 4.3% in the opposite direction (2 of 47 pairs; Figures 2D–2F; Figures S3C and S3D).
Local Connectivity between Distinct Excitatory and Inhibitory LII Neurons
To test whether the four excitatory cell types in LII differ with respect to their inhibitory input, we recorded from pairs of excitatory cells and neighboring inhibitory neurons belonging to one of the three major interneuron subpopulations, namely PV+, somatostatin+ (SOM), and 5-HT3A+ neurons (Lee et al., 2010).
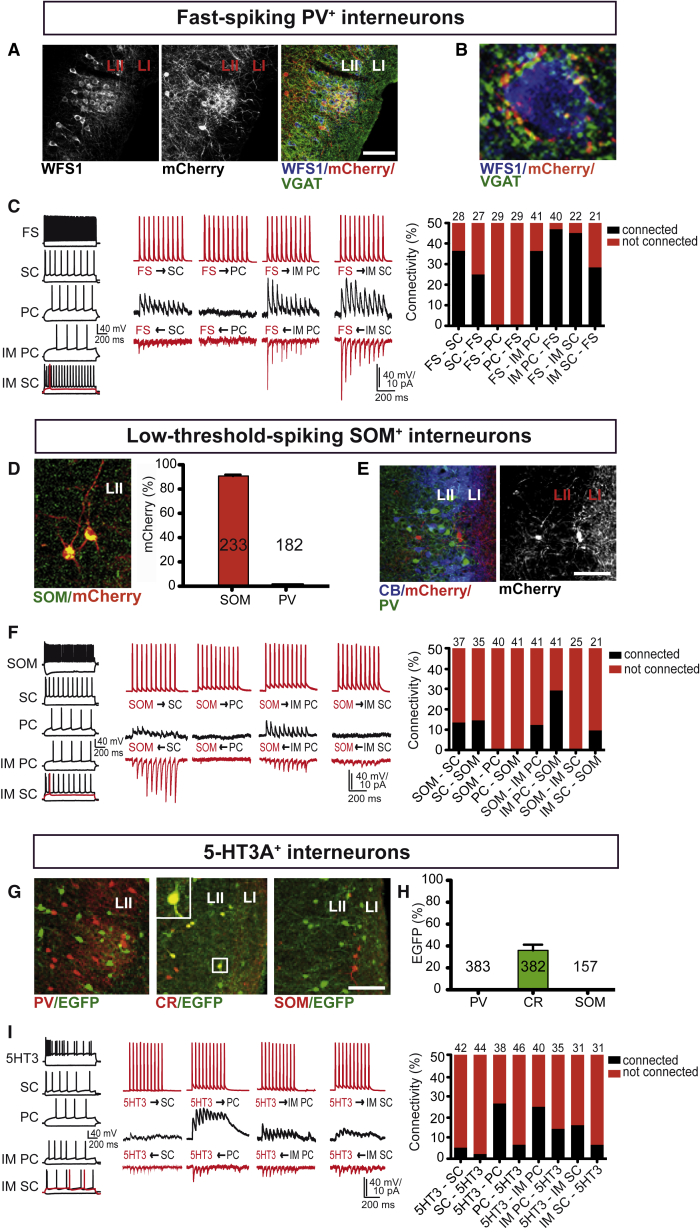
FS putative PV+ interneurons were reported to provide extensive inhibition onto stellate cells (Beed et al., 2013, Couey et al., 2013). PV immunohistochemistry, however, indicates that CB+/WFS1+ neurons must also be targeted by PV+ interneurons as evidenced by basket-like structures around CB+/WFS1+ cell bodies (Figures 3A and 3B; Figures S4A–S4C). To probe for the presence of monosynaptic connectivity between FS interneurons and all four excitatory cell types, we performed paired recordings in neurons identified by their firing pattern. We recorded unitary inhibitory PSCs (uIPSCs) at a holding potential of −50 mV in excitatory cells, and uEPSCs at −70 mV in FS interneurons, respectively. In agreement with previous data (Couey et al., 2013), connectivity from FS interneurons onto stellate cells was 35.7% (10 of 28 pairs), and 25.9% in the opposite direction (7 of 27 pairs; Figure 3C; Figure S5A). There was no connectivity in either direction between FS interneurons and pyramidal cells (0 of 29 pairs for each direction). Notably, FS interneurons inhibit both intermediate pyramidal cells and intermediate stellate cells, and receive excitatory input from both. Thus, in pairs of FS interneurons and intermediate pyramidal cells, the probability of monosynaptic uIPSCs was 36.6% (15 of 41 pairs), and that of uEPSC 47.5% (19 of 40 pairs; Figure 3C; Figure S5A). Connectivity from FS interneurons to intermediate stellate cells was 45.5% (10 of 22 pairs), and 28.6% (6 of 21 pairs) in the opposite direction (Figure 3C; Figure S5A).
Figure 3.
Local Inhibitory Connectivity in LII of the MEC
(A) WFS1+ neurons in an island (left) receive innervation from PV+ axons visualized by mCherry expression following AAV DIO ChR2-mCherry injection into the MEC of a PVCre mouse (middle). The merged picture shows immunostaining for VGAT, a marker of GABAergic terminals (right). Scale bar, 100 μm.
(B) Confocal image of PV+ and VGAT+ axon terminals surrounding the soma of a WFS1+ neuron in LII.
(C) Firing pattern (left) and representative traces (middle) of unitary IPSCs (uIPSCs, black) and unitary EPSCs (uEPSCs, red) recorded in FS interneurons and excitatory cells. uIPSCs were recorded at −50 mV, and uEPSCs at −70 mV in the respective postsynaptic neuron, and were elicited by a train of 10 action potentials (40 Hz) (train of 10 action potentials in red). The summary graph (right) shows the investigated connections between LII FS interneurons and excitatory cells.
(D) MCherry expression in SOM+ interneurons in LII following AAV DIO ChR2-mCherry injection into the MEC of a SOMCre mouse (left). Quantification of mCherry+ interneurons that expressed SOM or PV in MEC LII of SOMCre mice (right). The numbers indicate analyzed mCherry+ neurons from two mice.
(E) PV+ and SOM+ (labeled by mCherry following AAV DIO ChR2-mCherry injection into the MEC of a SOMCre mouse) interneurons in LII are localized preferentially around islands (left). mCherry expression reveals that fluorescently labeled axons of SOM+ interneurons are localized between islands and in LI (right). Scale bar, 100 μm.
(F) Firing pattern (left) and representative traces (middle) of uIPSCs (black) and uEPSCs (red) recorded in SOM+ interneurons and excitatory cells. Connectivity was tested by eliciting a train of 10 action potentials in the presynaptic neuron (train of action potentials in red). The summary graph (right) shows the quantitative evaluation of connectivity between SOM+ interneurons and indicated excitatory cells.
(G) PV (left), calretinin (middle), and SOM (right) immunostaining in MEC LII of a 5-HT3AEGFP mouse. The boxed double-labeled CR+/EGFP+ interneuron is shown at a higher magnification in the upper left corner. Scale bar, 100 μm.
(H) Quantification of EGFP+ interneurons expressing PV, CR, or SOM in LII of the MEC in 5-HT3AEGFP mice. The numbers indicate analyzed EGFP+ neurons from two or three mice.
(I) Firing pattern (left) and representative traces (middle) of uIPSCs (black) and uEPSCs (red) detected in 5-HT3AEGFP+ interneurons and excitatory cells. The summary graph (right) shows quantitative evaluation of investigated connections between indicated cell types. Data are represented as percentage of analyzed connections. The total number for the different cell pairs is indicated above the bars.
Abbreviations are as follows: PC, pyramidal cell; SC, stellate cell; IM PC, intermediate pyramidal cell; IM SC, intermediate stellate cell; CR, calretinin; 5-HT3A, 5-HT3A receptor; 5HT3, 5-HT3A+ interneuron; L, layer; PV, parvalbumin; SOM, somatostatin; and VGAT, vesicular glutamate transporter. See also Figures S4 and S5.
SOM+ and PV+ interneurons are virtually non-overlapping cell populations as revealed by double-labeling experiments upon injection of AAV DIO ChR2-mCherry into the MEC of SOMCre mice (Figure 3D). Also the axonal targeting pattern differed between the two interneuron populations. Thus, SOM+ axons extended preferentially between islands and in LI (Figure 3E). The firing pattern of all virally transduced fluorescent SOM+ interneurons was characterized by low-threshold firing and the presence of a prominent sag potential (Figure 3F; Figure S5B). In pairs of SOM+ interneurons and excitatory cells, we detected inhibitory input onto stellate cells and intermediate pyramidal cells (5 of 37 tested SOM+-stellate cell pairs, 5 of 41 tested SOM+-intermediate pyramidal cell pairs; Figure 3F; Figure S5B). In contrast, pyramidal cells and intermediate stellate cells received no monosynaptic uIPSCs from SOM+ interneurons (0 of 40 tested SOM+-pyramidal cell pairs, 0 of 25 SOM+-intermediate stellate cell pairs; Figure 3F; Figure S5B). Excitation onto SOM+ cells was provided by stellate cells (5 of 35 pairs), intermediate pyramidal cells (12 of 41 pairs), and intermediate stellate cells (2 of 21 pairs), but not pyramidal cells (0 of 41 pairs).
5-HT3A+ interneurons, identified with the help of 5-HT3AEGFP mice (Inta et al., 2008), constituted the third discrete interneuron cell population in LII of the MEC (Figures 3G and 3H), as reported for somatosensory cortex (Lee et al., 2010). The firing patterns of this interneuron cell population were reminiscent of what was found in the somatosensory cortex (Lee et al., 2010). We detected inhibitory monosynaptic input from 5-HT3AEGFP+ interneurons onto all four excitatory cell types, with the preferred target being intermediate pyramidal cells and pyramidal cells (Figure 3I). Thus, the connectivity from 5-HT3AEGFP+ interneurons to pyramidal cells was 26.3% (10 of 38 pairs), and that from 5-HT3AEGFP+ interneurons to intermediate pyramidal cells 25.0% (10 of 40 pairs; Figure 3I; Figure S5C). Finally, 5-HT3AEGFP+ interneurons received excitation from all four cell types, that is, stellate cells (1 of 44 pairs), pyramidal cells (3 of 46 pairs), intermediate pyramidal cells (5 of 35 pairs), and intermediate stellate cells (2 of 31 pairs).
CB+ Neurons Are a Source of Excitation to LII Neurons in the Contralateral MEC
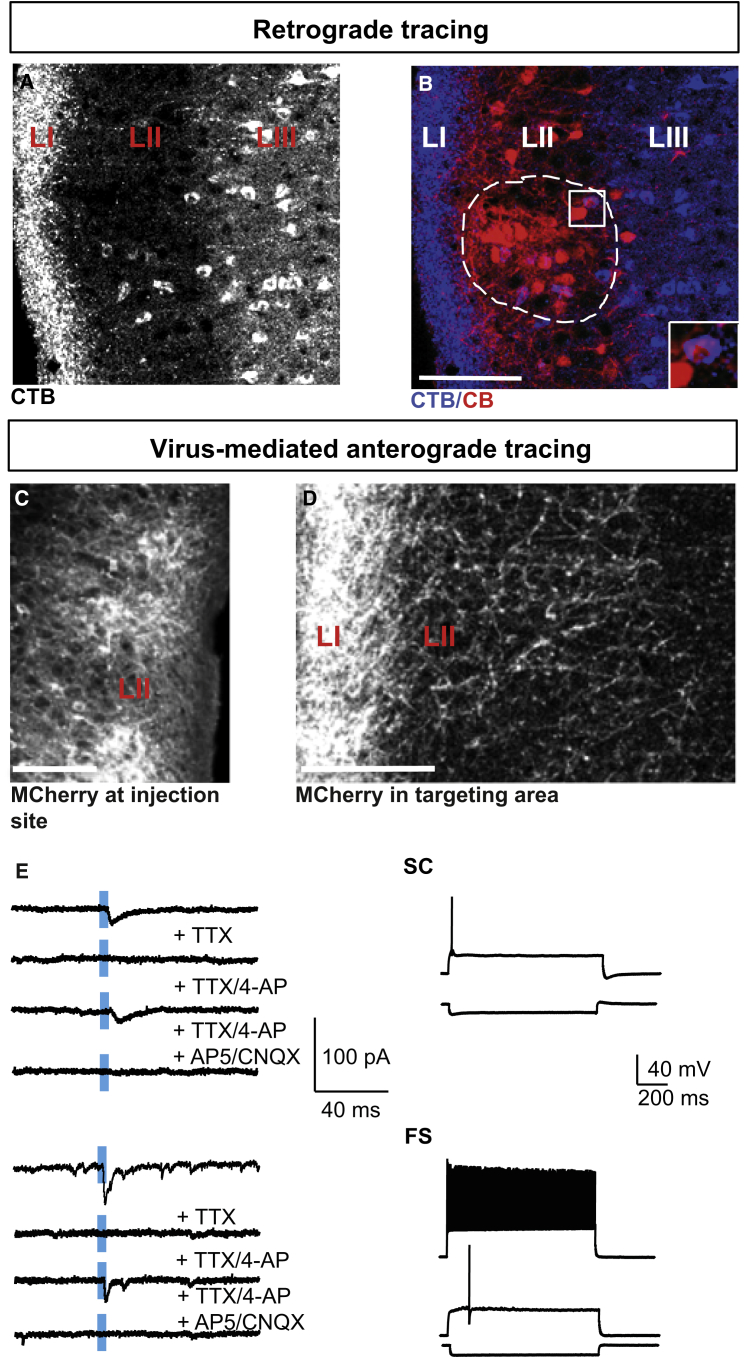
Varga et al. (2010) reported that CB+ neurons in LII project to the contralateral MEC. Two recent studies emphasized that CB+ neurons are organized in islands (Kitamura et al., 2014, Ray et al., 2014). Hence the following question was raised: Are CB+ neurons in islands the main source connecting the left and right MEC? We injected unilaterally the retrograde tracer cholera toxin subunit B (CTB) into the MEC of wild-type mice. Indeed, we could detect CTB+ neurons in LII of the contralateral MEC (Figure 4A). To further verify their identity, we performed immunohistochemical experiments. We found that 76.9% ± 2.8% CTB+ LII neurons were CB+ (626 CTB+ neurons in 5 hemispheres from 5 wild-type mice) and RE− (only 1% ± 0.6% was RE+, 384 CTB+ neurons in 5 hemispheres from 5 wild-type mice). Most CTB+/CB+ LII neurons (89.5% ± 1.6%) were localized in CB islands (Figure 4B). In deep LII, CTB+/CB+ neurons displayed a typical pyramidal-cell-like morphology, in contrast to more superficially localized CTB+/CB+ neurons that exhibited an oblique orientation of their soma and apical dendritic tree (Figure 1A; Figure S6), reminiscent of the previously described oblique pyramidal cells (Canto and Witter, 2012, Klink and Alonso, 1997). On the basis of electrophysiological properties, we classified the former CTB+ cells as pyramidal cells (Figure 1A) and the latter as intermediate pyramidal cells (Figure 1A). It should be pointed out though that the majority of CTB+ neurons were located in LIII (Figure 4A).
Figure 4.
LII CB+ Neurons Target Multiple Cell Types in LII of the Contralateral MEC
(A) Retrogradely labeled LII and LIII neurons following CTB injection into the contralateral MEC (sagittal section).
(B) Most retrogradely labeled neurons (blue) in LII were CB+ (red) and localized in CB+ islands (the confines of this island are indicated by a dashed line). The Inset is a higher magnification of the indicated area (white square) and shows a CTB+/CB+ neuron. Scale bar, 100 μm.
(C) Confocal image of injection site in LII following AAV DIO-ChR2-mCherry injection into the MEC of CBCre mice. Scale bar, 100 μm.
(D) Sagittal MEC section showing innervation of LII following AAV DIO-ChR2-mCherry injection into the contralateral MEC of CBCre mice. Scale bar, 100 μm.
(E) Synaptic responses and firing pattern of a targeted SC and a FS interneuron. ChR2-mCherry-expressing axons were stimulated by 5-ms laser pulses (blue bar) and EPSCs were recorded. Excitatory and monosynaptic inputs were identified in the presence of the indicated antagonists.
Abbreviations are as follows: L, layer; CTB, cholera toxin subunit B; MEC, medial entorhinal cortex; FS, fast-spiking interneuron; and SC, stellate cell. See also Figure S6 and Table S2.
To probe whether the interhemispheric MEC connectivity comprises also a GABAergic component, CTB was injected unilaterally into the MEC of GAD67EGFP mice. We did not detect retrogradely labeled GABAergic neurons (LII: 443 CTB+/GAD67− neurons; LIII: 1790 CTB+/GAD67− neurons, 3 hemispheres from 3 GAD67EGFP mice).
To detect target neurons of the contralaterally projecting CB+ neurons, we injected AAV DIO-ChR2-mCherry unilaterally into the MEC of CBCre mice (Figure 4C) and investigated the axonal projection pattern in the contralateral MEC (n = 3 mice; Figure 4D). Fluorescently labeled axons targeted LII and LI (Figure 4D).
To identify target neurons in LII of the contralateral MEC, we combined laser stimulation of ChR2-expressing axons and whole-cell recordings. Laser stimulation elicited reliable EPSCs in stellate, intermediate stellate, and FS cells, and less frequently in pyramidal and intermediate pyramidal cells and non-FS interneurons (Figure 4E; Table S2). To distinguish between direct (monosynaptic) and indirect (polysynaptic) responses, we blocked voltage-dependent Na+ channels with 1 μM tetrodotoxin (TTX), and K+ channels that are critical for axonal repolarization with 100 μM 4-aminopyridin (4-AP). We detected monosynaptic input from the contralateral MEC in stellate cells, intermediate stellate cells, and FS interneurons. The excitatory nature of the connections was further substantiated by the selective blockage with 10 μM CNQX and 50 μM D-AP5, but not with 10 μM Gabazine (n = 6 cells; Figure 4E; Table S2).
Excitatory and Inhibitory LII Neurons Project to the MS
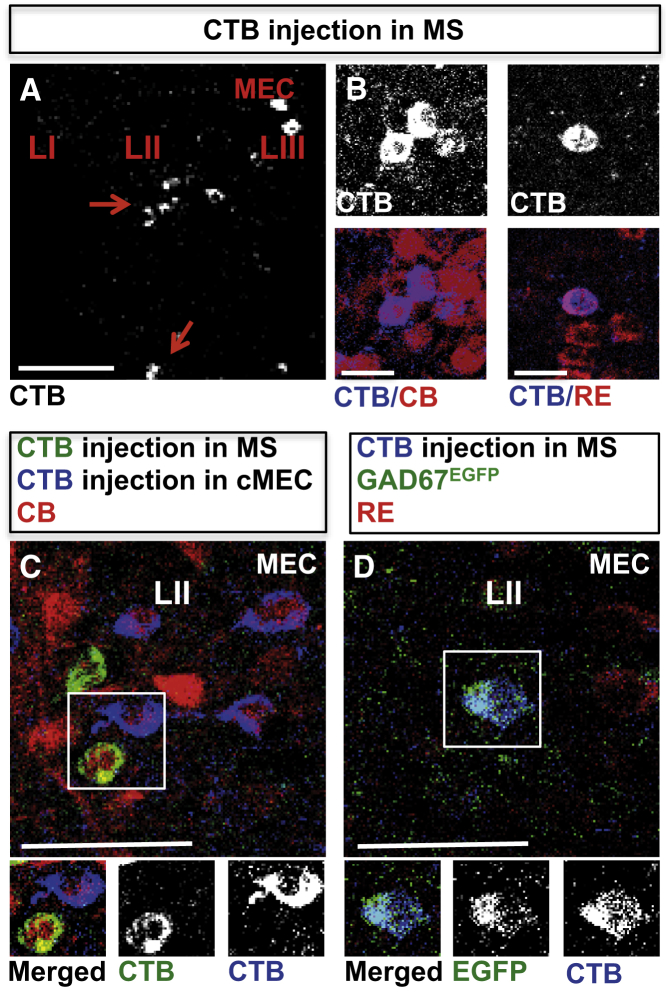
Given that injection of the retrograde tracer CTB into the contralateral MEC always led to the labeling of a small fraction of CB+ neurons within an island, we wondered whether CB+ neurons might also project to other brain areas. We chose to first investigate the MS as this structure is reciprocally connected with the MEC (Alonso and Köhler, 1984). Upon injection of CTB into the MS of GAD67EGFP mice, we detected labeled LII neurons that were often clustered (Figure 5A). Staining with CB or RE antibodies revealed that most CTB+ cells were excitatory CB+/GAD67− neurons (69% ± 2.2% CTB+/CB+/GAD67−; of these, 92.7% ± 1.8% CTB+/CB+/GAD67− were localized in CB islands; a total of 449 CTB+ neurons in 8 hemispheres from 4 GAD67EGFP mice were analyzed; Figure 5B). In addition, electrophysiological characterization indicated that all tested CTB+ cells exhibited an intermediate pyramidal cell phenotype (6 out of 6 cells from 3 mice). We detected a small population of RE+ cells that also project to the MS (6.3% ± 2.4% CTB+/RE+/GAD67−; 225 CTB+ neurons in 5 hemispheres from 3 GAD67EGFP mice; Figure 5B). These neurons were localized in the intermediate or ventral MEC.
Figure 5.
LII Neurons Project to the MS
(A) CTB+ neurons in MEC LII (red arrows) following tracer injection into the MS. Scale bar, 100 μm.
(B) CTB+/CB+ neurons located in CB islands (left two panels) and CTB+/RE+ cells (right two panels) following CTB injection into the MS. Scale bars, 20 μm.
(C) CB+ neurons (red) located in the same CB island can project to either the MS (green CTB labeling) or the contralateral MEC (blue CTB labeling). Boxed double-labeled neurons are shown below as a merged image and the single channel for green and blue CTB. Scale bar, 50 μm.
(D) GAD67EGFP+ neuron in LII co-labeled with CTB following tracer injection into the MS (upper panel). The boxed double-labeled neuron is shown below as a merged image and the single channel for EGFP and blue CTB. Scale bar, 50 μm.
Abbreviations are as follows: CB, calbindin; c, contralateral; CTB, cholera toxin subunit B; L, layer; MEC, medial entorhinal cortex; MS, medial septum; and RE, reelin.
We subsequently investigated whether a defined island provides input to both the contralateral MEC and the MS. To this end we injected green fluorescently labeled CTB into the MS and red fluorescently labeled CTB into the contralateral MEC. Notably, we could detect the two fluorochromes in CB+ neurons that were localized within the same island (n = 4 wild-type mice; Figure 5C).
Interestingly, CTB injection into the MS of GAD67EGFP mice revealed the existence not only of excitatory CTB+/EGFP− neurons in LII of the MEC but also of CTB+/EGFP+ cells, pointing to the presence of long-range GABAergic neurons in the MEC that project to the MS (6.1% ± 2.8% CTB+/GAD67+; a total of 449 CTB+ neurons in 8 hemispheres from 4 GAD67EGFP mice were analyzed; Figure 5D).
Distinct Populations of GABAergic Neurons from the MS Target the MEC
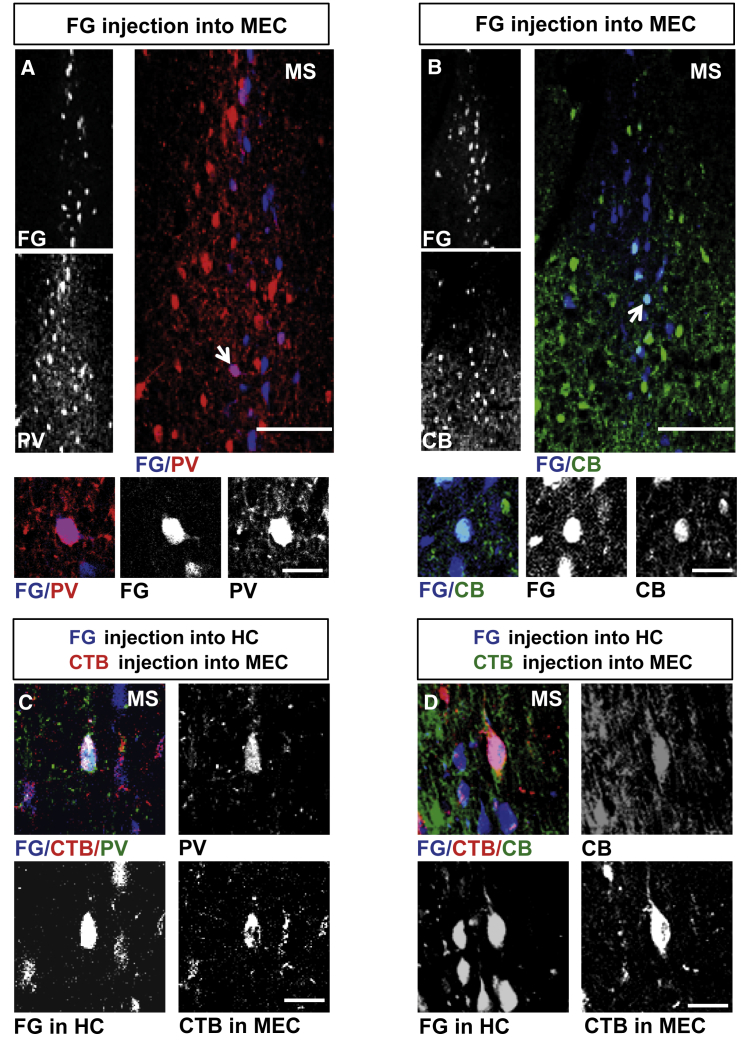
There is evidence that interareal connectivity via long-range GABAergic projections is often reciprocal (Caputi et al., 2013). Hence we injected the retrograde tracer fluorogold (FG) into the MEC of wild-type mice and detected FG+ neurons that were distributed throughout the dorsal-ventral extent of the MS (Figures 6A and 6B). Concomitant labeling with GABAergic neuron markers revealed PV+ neurons in the MS as one source for long-range GABAergic projections to the MEC (9.9% ± 4.7% FG+/PV+; 663 FG+ neurons in 4 wild-type mice; Figure 6A). We also identified retrogradely labeled CB+ neurons, providing evidence that more than one GABAergic subpopulation of the MS projects to the MEC (7.7% ± 0.6% FG+/CB+; 526 FG+ neurons in 4 wild-type mice; Figure 6B).
Figure 6.
Septal GABAergic Neurons Projecting to the MEC
(A) FG-labeled neurons in the MS (left, upper panel) following tracer injection into the MEC. PV staining of the same section (left, lower panel). The overlay is shown at higher magnification (right panel). Scale bar, 100 μm. Higher magnification images below show the double-labeled neuron indicated by the arrow in the right panel. Scale bar, 20 μm.
(B) FG-labeled neurons in the MS (left, upper panel) following tracer injection into the MEC. CB staining of the same section (left, lower panel). The overlay is shown at higher magnification (right panel). Scale bar, 100 μm. Higher magnification images below show the double-labeled neuron indicated by the arrow in the right panel. Scale bar, 20 μm.
(C) Image of a FG+/CTB+/PV+ neuron in the MS following FG (blue) injection into the hippocampus and CTB (red) injection into the MEC. Scale bar, 20 μm.
(D) Image of a FG+/CTB+/CB+ neuron in the MS following FG injection into the hippocampus and CTB injection into the MEC. Scale bar, 20 μm.
Abbreviations are as follows: CB, calbindin; CTB, cholera toxin subunit B; FG, fluorogold; HC, hippocampus; MEC, medial entorhinal cortex; MS, medial septum; and PV, parvalbumin.
Following injections of FG into the right hippocampus and CTB into the right MEC of the same mouse, we identified PV+ and CB+ neurons labeled with both retrograde tracers, suggesting that single GABAergic neurons in the MS can project to both target regions (7 FG+/CTB+/PV+ neurons in 3 mice, 430 FG+ and 107 CTB+ neurons; 3 FG+/CTB+/CB+ cells in 2 mice, 248 FG+ and 53 CTB+ neurons; Figures 6C and 6D).
GABAergic Long-Range Neurons Originating in the MS Target Distinct Inhibitory Neurons in LII
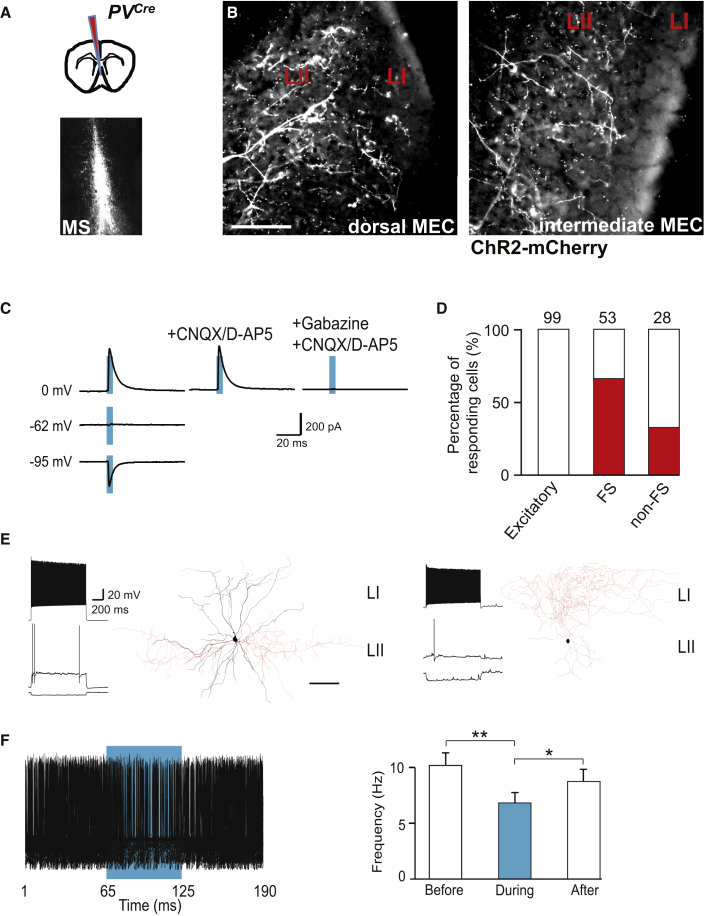
After establishing that PV+ and CB+ neurons originating in the MS project to the MEC, we next sought to determine the identity of the target cells. To this end we first injected AAV DIO ChR2-mCherry into the MS of PVCre mice. Virus injection resulted in specific expression of the fluorescent fusion protein ChR2-mCherry in PV+ neurons of the MS (Figure 7A). In the MEC, the projections of these neurons could be detected throughout all layers. MCherry-labeled axons formed a dense network in LII (Figure 7B). The GABAergic phenotype of the long-range axons was confirmed by their VGAT positivity (data not shown).
Figure 7.
Septal PV+ Neurons Inhibit Preferentially FS Interneurons in LII of the MEC
(A) Schematic drawing indicating the site of virus injection into the MS (top). MCherry expression following AAV DIO ChR2-mCherry injection into the MS of a PVCre mouse (coronal section; bottom).
(B) ChR2-mCherry+ axons in LII of the dorsal (left) and intermediate (right) MEC (sagittal sections). Scale bar, 50 μm.
(C) Responses of a targeted FS cell in MEC LII at the indicated potentials and in the presence of indicated antagonists. Blue bars show the duration of laser pulses.
(D) Histogram indicating percentage of responding neurons (red) in LII. The numbers above the bars indicate the number of analyzed cells.
(E) Representative firing pattern and reconstruction of a targeted FS (left) and a targeted non-FS GABAergic neuron (right). Dendrites are indicated in black and axons in red. Scale bar, 100 μm.
(F) Stimulation of septal PV+ long-range projections reduced spiking in LII FS neurons. Responding cells were depolarized to suprathreshold potentials, and long-range axons were stimulated with 60-ms pulses at 8 Hz. Superimposed traces of a representative cell (left) and histogram (right) showing significant reduction of the firing rate during 60-ms pulses (∗p < 0.05 and ∗∗p < 0.01). Data represent mean ± SEM.
Abbreviations are as follows: L, layer; MEC, medial entorhinal cortex; MS, medial septum; FS, fast-spiking interneuron; non-FS, non-fast-spiking interneuron; and PV, parvalbumin. See also Figures S7 and S8.
Subsequently we combined laser stimulation of ChR2-positive axons with patch-clamp recordings and morphological reconstruction of target cells in MEC LII. Despite the dense axonal plexus, none of the glutamatergic neurons belonging to the four cell types described above—stellate cells, intermediate stellate cells, pyramidal cells, and intermediate pyramidal cells—responded to laser stimulation (n = 99 cells from 19 mice; Figure 7D). Responses could be detected only in GABAergic neurons (Figures 7C and 7D). Target cells comprised both FS and non-FS GABAergic neurons. Of the analyzed FS cells, two-thirds responded to laser stimulation (66% out of 53 cells from 17 mice; Figures 7D and 7E), whereas only one-third of non-FS GABAergic neurons responded (32% out of 28 cells from 10 mice; Figures 7D and 7E). The GABAergic nature of responses was confirmed by the reversal potential (−59.5 ± 2.1 mV, n = 8 cells from 5 mice) and the blockage with Gabazine, but not with D-AP5 and CNQX (n = 7 out of 7 cells from 6 mice; Figure 7C). Similar results were obtained following viral infection of septal glutamatergic and GABAergic neurons. All recorded responses in LII were restricted to GABAergic interneurons and were inhibitory (Figure S7).
We tested how inhibition via PV+ long-range projections affected the activity of targeted FS neurons. We depolarized responding FS cells to suprathreshold potentials and activated long-range axons locally with 8-Hz laser pulses of 60-ms duration to simulate rhythmic burst firing of PV+ cells in the MS (Borhegyi et al., 2004, King et al., 1998, Li et al., 2014). All analyzed FS neurons in MEC LII reduced their firing rate during laser stimulation transiently (n = 11 cells from 5 mice; Figure 7F). The overall firing rate was reduced from 10.2 ± 1.1 Hz to 6.8 ± 0.9 Hz during laser stimulation (p < 0.01, repeated-measures ANOVA followed by post hoc Tukey’s test), while no persistent changes of the post-stimulation firing rate were seen (p > 0.05, repeated-measures ANOVA followed by post hoc Tukey’s test).
To determine the targets of septal long-range CB+ neurons, we injected AAV DIO ChR2-mCherry into the MS of CBCre mice. Virus injection resulted in specific expression of the fluorescent fusion protein ChR2-mCherry in CB+ neurons of the MS (Figure S8A). In the MEC, mCherry-labeled axons reached LII, and the GABAergic phenotype of the long-range axons was confirmed by their VGAT positivity that was visible in the transition zone between LI and LII (Figure S8B). Of note, responses could be detected only in LTS interneurons (80% out of 30 cells from 9 mice; Figure S8C).
Discussion
Local Excitatory and Inhibitory Network in LII
On the basis of electrophysiological parameters, we distinguished in addition to stellate and pyramidal cells two other excitatory cell types in LII, namely intermediate stellate and intermediate pyramidal cells. When scrutinizing previous studies, a certain extent of heterogeneity within the two cell classes can be inferred. Thus, in the study by Klink and Alonso (1997), both electrophysiologically identified stellate and pyramidal neurons exhibit some variability with respect to their morphology. Further morphological variance was reported in another study (Gatome et al., 2010). Finally, Canto and Witter (2012) reported that “sag” and “non-sag neurons” comprise at least five cell types when separated on morphological grounds. We searched for additional intrinsic parameters that would help to classify the different cell types in LII. While some parameters were clearly overlapping, we found that the sag, latency to first spike, dAP, and the ratio of ISI1/2 allowed a classification into four cell types. Thus, our analysis enabled us to further subdivide what was formerly denoted as “sag neurons” into stellate, intermediate stellate, and intermediate pyramidal cells. The most conspicuous parameters that helped to distinguish stellate cells from the two intermediate cell types are dAP, latency to spike firing, the initial burst, and the presence of an apical dendrite. Post hoc marker expression analysis of biocytin-filled cells helped to distinguish stellate and intermediate stellate cells from pyramidal and intermediate pyramidal cells in LII. It must be pointed out, however, that we detected co-expression of the two markers both in some intermediate stellate and some intermediate pyramidal neurons.
LII excitatory neurons can be distinguished based on their cell-type-specific excitatory and inhibitory connectivity. Thus, we detected excitatory connections between intermediate cell types that target directly stellate or pyramidal cells, but not between pairs of stellate cells or pyramidal cells. Previous studies emphasized the absence of excitatory connectivity in LII neurons, but concentrated on stellate cells (Couey et al., 2013) or cells with stellate-like appearance (Dhillon and Jones, 2000). The frequency of excitatory connections ranged from 4.3% (between stellate cells to intermediate stellate cells) to 10% (between intermediate pyramidal cells to stellate cells) and is comparable to what was reported for superficial layers in other brain areas (Feldmeyer et al., 2006, Holmgren et al., 2003, Mason et al., 1991).
The importance of inhibition in LII was emphasized by Couey et al. (2013), who highlighted the frequent coupling between FS interneurons and stellate cells. Strikingly, we found here that pyramidal cells were not inhibited either by FS or SOM+ interneurons, but exhibited recurrent connectivity with 5-HT3A+ interneurons. Cell-type-specific inhibitory connectivity in LII clearly separates pyramidal neurons not only from stellate and intermediate stellate cells but also from intermediate pyramidal cells. It is safe to assume that the connectivity between 5-HT3A+ interneurons and pyramidal cells is identical to that reported by Varga et al. (2010). The authors reported that excitatory CB+, but not RE+ neurons, are inhibited by cholecystokinin+ basket cells, known to be putative the 5-HT3A+ interneurons (Morales and Bloom, 1997). Of note, 5-HT3A+ interneuron connectivity with excitatory neurons in LII also supports the notion that stellate and intermediate stellate cells are distinct neuronal entities.
The presence of four excitatory cell types in LII raises the question as to their function in vivo. The cell-type-specific inhibitory pattern reported here allows the following conjecture. Since there is evidence that activation of FS cells in vivo inhibits grid cell firing most likely via monosynaptic connectivity (Buetfering et al., 2014), and given that FS interneurons inhibit stellate cells, intermediate stellate cells, and intermediate pyramidal cells, we infer that, at least based on the in vitro data reported here, all three cell types fulfill the criteria of putative grid cells. Furthermore, as pyramidal cells do not receive inhibition from FS interneurons, we suggest that grid cells that were identified based on marker expression (Sun et al., 2015, Tang et al., 2014) or morphology (Domnisoru et al., 2013) are very likely the here described intermediate pyramidal cells.
External Input to LII Neurons
We show here that LII neurons receive excitatory input from CB+ neurons located in the contralateral MEC and inhibitory input from septal long-range GABAergic neurons. Whereas the former excites glutamatergic cells and FS cells, the latter inhibits selectively GABAergic neurons. Needless to say, there are other projections that might be a source of excitation for LII neurons. These, however, were not considered in this study.
On the basis of previous retrograde tracing studies, it could be inferred that LIII neurons project to the contralateral MEC (Amaral et al., 1984, Steward and Scoville, 1976). On the basis of tracing and electrophysiological experiments, we provide evidence that CB+/WFS1+ neurons in LII are an important source of excitation for contralateral LII neurons. The contralateral projecting CB+/WFS1+ are very likely identical to the CB+ cells described by Varga et al. (2010). In the target area all major cell types were excited either directly or indirectly by axons of contralateral CB+ neurons. This promiscuous targeting in conjunction with the local excitatory connectivity accounts for the high probability of detecting cells in LII that exhibit either monosynaptic or polysynaptic responses upon optogenetic axonal stimulation of contralateral CB+ projections.
As indicated above, CB+ cells in LII form island-like structures that do not comprise, however, a homogenous cell population. Thus, not only are the islands composed of pyramidal cells and intermediate pyramidal cells with distinct electrophysiological features, but CB+ island cells target also different downstream areas, namely the contralateral MEC and the MS. At least based on anterograde and retrograde tracing experiments, we could not detect CB+ neurons targeting the CA1 region as previously reported by Kitamura et al. (2014), who detected such a projection in transgenic mice.
The reciprocal GABAergic septal-MEC circuit that we identified can be viewed as the pendant of the septal-hippocampal inhibitory pathway. Freund (1989) and Freund and Antal (1988) reported that septal PV+ cells project to the hippocampus where they target GABAergic neurons; they also described reciprocal connections linking these two brain structures (Takács et al., 2008, Tóth et al., 1993).
Long-range reciprocal GABAergic connections were also found between the hippocampus and the MEC (Melzer et al., 2012). Here we demonstrate that in the MEC, long-range septal GABAergic neurons target exclusively inhibitory neurons. Thus, there is increasing evidence that long-range GABAergic projections constitute a source of disinhibition in the target area.
For decades the septum has been considered the pacemaker of hippocampal and medial entorhinal cortical theta activity, thereby coordinating synchronous activity between distant brain areas (Buzsáki, 2002). In both brain regions distinct cell types fire at a preferred phase of the theta cycle (Mizuseki et al., 2009). Thus, lesions or pharmacological inactivation of the MS strongly reduce theta oscillations both in the hippocampus and the MEC (Jeffery et al., 1995, Mitchell et al., 1982, Petsche et al., 1962), leading to spatial memory deficits akin to those observed after hippocampal lesions (Bannerman et al., 2004, Winson, 1978). At least for the hippocampus, there are several reports directly linking the activity of PV+ cells in the septum with hippocampal theta activity that results from rhythmic disinhibition (Hangya et al., 2009). A similar mechanism may apply for the MEC. We identified here two distinct sources of GABAergic inputs to the MEC that are ideally suited to synchronize downstream target networks. Of note, from the connectivity pattern it can be concluded that both GABAergic septal projections cause disinhibition in the target area; however, differences in either the recruited networks or their timing is likely, given that the two projections differ with respect to their target cells: septal PV+ cells inhibit preferentially FS neurons in the MEC, while septal CB+ neurons inhibit LTS neurons.
Septal input to the MEC also controls the periodicity of grid cell firing as revealed experimentally upon pharmacological inactivation of the septum (Brandon et al., 2011, Koenig et al., 2011). However, so far it is not clear what exactly the contribution of the septal cholinergic is versus the septal GABAergic input to the MEC for the generation of grid cell firing and periodicity.
Conclusions
Our study led to the following main findings, each opening up new avenues that prompt further experimental and theoretical considerations. First, we identified four types of excitatory neurons in LII. Second, the distinct cell types exhibit cell-type-specific excitatory and inhibitory local connectivity. These local networks would meet requirements as proposed by current continuous attractor models for grid cells. Third, we demonstrate that CB+ neuron activation in LII leads to fast excitation of all major neuronal cell types in the contralateral LII. Finally, we show that the septum disinhibits neurons in LII via two distinct long-range GABAergic projections that exhibit cell-type-specific target selectivity. The exact role of these external sources of direct and indirect excitation for spatial firing and rhythmicity in the MEC warrants further investigations.
Experimental Procedures
Animals
We used wild-type C57Bl/6, GAD67EGFP (Tamamaki et al., 2003), CBCre (purchased from Taconic Biosciences), Uchl1Cre (obtained from the Mutant Mouse Regional Resource Center), PVCre (Hippenmeyer et al., 2005), SOMCre (Melzer et al., 2012), and 5-HT3AEGFP (Inta et al., 2008) mice. All procedures involving wild-type and genetical modified mice had ethical approval from the Regierungspräsidium Karlsruhe (AZ 35-9185.81/G-173-12) and (G-254-14).
Injection of Retrograde Tracer into the Mouse Brain
Eight-week-old male wild-type and GAD67EGFP mice were injected with 100 nl Cholera Toxin subunit B (Alexa Fluor 488 Conjugate or Alexa Fluor 555 Conjugate, Life Technology GmbH) or injected with 70 nl Fluorogold (0.5%, Fluorochrome). Animals were anesthetized with isoflurane, mounted in a stereotactic apparatus, and kept under isoflurane anesthesia during surgery.
For MEC injections, coordinates were 3.1 mm lateral from the midline, 0.1 mm anterior to the transverse sinus, and 1.8 mm below cortical surface; for dorsal hippocampal, 2.4 mm posterior to bregma, 2.0 mm lateral to the midline, and 1.5 below cortical surface; and for MS, 1 mm anterior to bregma and 4 mm below cortical surface.
Animals were perfused 5 to 12 days after injection and the brains processed with immunohistochemical methods. For details, see the Supplemental Experimental Procedures.
Injection of Recombinant Viruses into the Mouse Brain
We injected 8-week-old mice. Injections were performed as described above. 150 nl of recombinant virus were injected in entorhinal cortex or MS. For details, see the Supplemental Experimental Procedures.
Immunohistochemistry, Cell Identification, and Reconstruction of Biocytin-Labeled cells
These methods involved standard procedures described in the Supplemental Experimental Procedures.
Image Analysis
Confocal images were taken using a Zeiss LSM 700 microscope (Zeiss) from anatomically matched sections spanning the lateral-medial extent of the MEC or the rostro-caudal extent of the MS. Data are presented as mean ± SEM. For details, see the Supplemental Experimental Procedures.
Electrophysiology
Whole-cell recordings were performed at 30°C to 32°C using 300-μm sagittal slices containing the dorsal MEC from mice (6 to 12 weeks old).
For paired recordings, LII cells (from 83 mice) were visually identified, and cell pairs with less than 40-μm distances were patched with low Cl− potassium-based intracellular solution.
Classification of all cells was done based on different electrophysiological parameters reported by others previously (Alonso and Klink, 1993, Couey et al., 2013, Lee et al., 2010). Electrophysiological parameters for excitatory cells are summarized in Table S1.
Connectivity was tested with 40-Hz trains (10 pulses) with postsynaptic cells voltage clamped either at −70 mV to detect uEPSCs or at −50 mV to obtain uIPSCs. uEPSCs were verified with Gabazine/CNQX (both 10 μM), applied sequentially. uIPSCs were inhibited by Gabazine, but not by prior CNQX bath application.
See the Supplemental Experimental Procedures for long-range MEC-MEC or septal-MEC connection experiments.
Author Contributions
E.C.F. and A.C. performed and analyzed the tracing experiments. A.N., R.P., and S.M. performed and analyzed the electrophysiological experiments. H.M. designed and coordinated the study. H.M. wrote the final manuscript with the input of all co-authors.
Acknowledgments
We thank I. Preugschat-Gumprecht and R. Hinz for their technical assistance and Dr. T. Holland-Letz for the help with the principal component analysis. This work was supported by a European Research Council grant (GABAcellsAndMemory grant 250047, to H.M.), a German Ministry of Education and Research (BMBF) grant (01GQ1003A to H.M. and E.C.F.), and by DFG (grant MO 432/10-1 to H.M. and as part of the CRC 1134).
Published: December 17, 2015
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Supplemental Information includes Supplemental Experimental Procedures, eight figures, and two tables and can be found with this article online at http://dx.doi.org/10.1016/j.neuron.2015.11.029.
Supplemental Information
References
- Alonso A., Klink R. Differential electroresponsiveness of stellate and pyramidal-like cells of medial entorhinal cortex layer II. J. Neurophysiol. 1993;70:128–143. doi: 10.1152/jn.1993.70.1.128. [DOI] [PubMed] [Google Scholar]
- Alonso A., Köhler C. A study of the reciprocal connections between the septum and the entorhinal area using anterograde and retrograde axonal transport methods in the rat brain. J. Comp. Neurol. 1984;225:327–343. doi: 10.1002/cne.902250303. [DOI] [PubMed] [Google Scholar]
- Amaral D.G., Insausti R., Cowan W.M. The commissural connections of the monkey hippocampal formation. J. Comp. Neurol. 1984;224:307–336. doi: 10.1002/cne.902240302. [DOI] [PubMed] [Google Scholar]
- Bannerman D.M., Yee B.K., Lemaire M., Wilbrecht L., Jarrard L., Iversen S.D., Rawlins J.N., Good M.A. The role of the entorhinal cortex in two forms of spatial learning and memory. Exp. Brain Res. 2001;141:281–303. doi: 10.1007/s002210100868. [DOI] [PubMed] [Google Scholar]
- Bannerman D.M., Matthews P., Deacon R.M., Rawlins J.N. Medial septal lesions mimic effects of both selective dorsal and ventral hippocampal lesions. Behav. Neurosci. 2004;118:1033–1041. doi: 10.1037/0735-7044.118.5.1033. [DOI] [PubMed] [Google Scholar]
- Beed P., Gundlfinger A., Schneiderbauer S., Song J., Böhm C., Burgalossi A., Brecht M., Vida I., Schmitz D. Inhibitory gradient along the dorsoventral axis in the medial entorhinal cortex. Neuron. 2013;79:1197–1207. doi: 10.1016/j.neuron.2013.06.038. [DOI] [PubMed] [Google Scholar]
- Borhegyi Z., Varga V., Szilágyi N., Fabo D., Freund T.F. Phase segregation of medial septal GABAergic neurons during hippocampal theta activity. J. Neurosci. 2004;24:8470–8479. doi: 10.1523/JNEUROSCI.1413-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon M.P., Bogaard A.R., Libby C.P., Connerney M.A., Gupta K., Hasselmo M.E. Reduction of theta rhythm dissociates grid cell spatial periodicity from directional tuning. Science. 2011;332:595–599. doi: 10.1126/science.1201652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buetfering C., Allen K., Monyer H. Parvalbumin interneurons provide grid cell-driven recurrent inhibition in the medial entorhinal cortex. Nat. Neurosci. 2014;17:710–718. doi: 10.1038/nn.3696. [DOI] [PubMed] [Google Scholar]
- Burak Y. Spatial coding and attractor dynamics of grid cells in the entorhinal cortex. Curr. Opin. Neurobiol. 2014;25:169–175. doi: 10.1016/j.conb.2014.01.013. [DOI] [PubMed] [Google Scholar]
- Burak Y., Fiete I.R. Accurate path integration in continuous attractor network models of grid cells. PLoS Comput. Biol. 2009;5:e1000291. doi: 10.1371/journal.pcbi.1000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N., O’Keefe J. Models of place and grid cell firing and theta rhythmicity. Curr. Opin. Neurobiol. 2011;21:734–744. doi: 10.1016/j.conb.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Canto C.B., Witter M.P. Cellular properties of principal neurons in the rat entorhinal cortex. II. The medial entorhinal cortex. Hippocampus. 2012;22:1277–1299. doi: 10.1002/hipo.20993. [DOI] [PubMed] [Google Scholar]
- Caputi A., Melzer S., Michael M., Monyer H. The long and short of GABAergic neurons. Curr. Opin. Neurobiol. 2013;23:179–186. doi: 10.1016/j.conb.2013.01.021. [DOI] [PubMed] [Google Scholar]
- Couey J.J., Witoelar A., Zhang S.J., Zheng K., Ye J., Dunn B., Czajkowski R., Moser M.B., Moser E.I., Roudi Y., Witter M.P. Recurrent inhibitory circuitry as a mechanism for grid formation. Nat. Neurosci. 2013;16:318–324. doi: 10.1038/nn.3310. [DOI] [PubMed] [Google Scholar]
- Dhillon A., Jones R.S. Laminar differences in recurrent excitatory transmission in the rat entorhinal cortex in vitro. Neuroscience. 2000;99:413–422. doi: 10.1016/s0306-4522(00)00225-6. [DOI] [PubMed] [Google Scholar]
- Domnisoru C., Kinkhabwala A.A., Tank D.W. Membrane potential dynamics of grid cells. Nature. 2013;495:199–204. doi: 10.1038/nature11973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmeyer D., Lübke J., Sakmann B. Efficacy and connectivity of intracolumnar pairs of layer 2/3 pyramidal cells in the barrel cortex of juvenile rats. J. Physiol. 2006;575:583–602. doi: 10.1113/jphysiol.2006.105106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund T.F. GABAergic septohippocampal neurons contain parvalbumin. Brain Res. 1989;478:375–381. doi: 10.1016/0006-8993(89)91520-5. [DOI] [PubMed] [Google Scholar]
- Freund T.F., Antal M. GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. Nature. 1988;336:170–173. doi: 10.1038/336170a0. [DOI] [PubMed] [Google Scholar]
- Gatome C.W., Slomianka L., Lipp H.P., Amrein I. Number estimates of neuronal phenotypes in layer II of the medial entorhinal cortex of rat and mouse. Neuroscience. 2010;170:156–165. doi: 10.1016/j.neuroscience.2010.06.048. [DOI] [PubMed] [Google Scholar]
- Giocomo L.M., Moser M.B., Moser E.I. Computational models of grid cells. Neuron. 2011;71:589–603. doi: 10.1016/j.neuron.2011.07.023. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Sulser A., Parthier D., Candela A., McClure C., Pastoll H., Garden D., Sürmeli G., Nolan M.F. GABAergic projections from the medial septum selectively inhibit interneurons in the medial entorhinal cortex. J. Neurosci. 2014;34:16739–16743. doi: 10.1523/JNEUROSCI.1612-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafting T., Fyhn M., Molden S., Moser M.B., Moser E.I. Microstructure of a spatial map in the entorhinal cortex. Nature. 2005;436:801–806. doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- Hangya B., Borhegyi Z., Szilágyi N., Freund T.F., Varga V. GABAergic neurons of the medial septum lead the hippocampal network during theta activity. J. Neurosci. 2009;29:8094–8102. doi: 10.1523/JNEUROSCI.5665-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippenmeyer S., Vrieseling E., Sigrist M., Portmann T., Laengle C., Ladle D.R., Arber S. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol. 2005;3:e159. doi: 10.1371/journal.pbio.0030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren C., Harkany T., Svennenfors B., Zilberter Y. Pyramidal cell communication within local networks in layer 2/3 of rat neocortex. J. Physiol. 2003;551:139–153. doi: 10.1113/jphysiol.2003.044784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard L.R., Javadi A.H., Yu Y., Mill R.D., Morrison L.C., Knight R., Loftus M.M., Staskute L., Spiers H.J. The hippocampus and entorhinal cortex encode the path and Euclidean distances to goals during navigation. Curr. Biol. 2014;24:1331–1340. doi: 10.1016/j.cub.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inta D., Alfonso J., von Engelhardt J., Kreuzberg M.M., Meyer A.H., van Hooft J.A., Monyer H. Neurogenesis and widespread forebrain migration of distinct GABAergic neurons from the postnatal subventricular zone. Proc. Natl. Acad. Sci. USA. 2008;105:20994–20999. doi: 10.1073/pnas.0807059105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery K.J., Donnett J.G., O’Keefe J. Medial septal control of theta-correlated unit firing in the entorhinal cortex of awake rats. Neuroreport. 1995;6:2166–2170. doi: 10.1097/00001756-199511000-00017. [DOI] [PubMed] [Google Scholar]
- King C., Recce M., O’Keefe J. The rhythmicity of cells of the medial septum/diagonal band of Broca in the awake freely moving rat: relationships with behaviour and hippocampal theta. Eur. J. Neurosci. 1998;10:464–477. doi: 10.1046/j.1460-9568.1998.00026.x. [DOI] [PubMed] [Google Scholar]
- Kitamura T., Pignatelli M., Suh J., Kohara K., Yoshiki A., Abe K., Tonegawa S. Island cells control temporal association memory. Science. 2014;343:896–901. doi: 10.1126/science.1244634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klink R., Alonso A. Morphological characteristics of layer II projection neurons in the rat medial entorhinal cortex. Hippocampus. 1997;7:571–583. doi: 10.1002/(SICI)1098-1063(1997)7:5<571::AID-HIPO12>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Koenig J., Linder A.N., Leutgeb J.K., Leutgeb S. The spatial periodicity of grid cells is not sustained during reduced theta oscillations. Science. 2011;332:592–595. doi: 10.1126/science.1201685. [DOI] [PubMed] [Google Scholar]
- Köhler C., Chan-Palay V., Wu J.Y. Septal neurons containing glutamic acid decarboxylase immunoreactivity project to the hippocampal region in the rat brain. Anat. Embryol. (Berl.) 1984;169:41–44. doi: 10.1007/BF00300585. [DOI] [PubMed] [Google Scholar]
- Lee S., Hjerling-Leffler J., Zagha E., Fishell G., Rudy B. The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. J. Neurosci. 2010;30:16796–16808. doi: 10.1523/JNEUROSCI.1869-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L.B., Han L.N., Zhang Q.J., Sun Y.N., Wang Y., Feng J., Zhang L., Wang T., Chen L., Liu J. The theta-related firing activity of parvalbumin-positive neurons in the medial septum-diagonal band of Broca complex and their response to 5-HT1A receptor stimulation in a rat model of Parkinson’s disease. Hippocampus. 2014;24:326–340. doi: 10.1002/hipo.22226. [DOI] [PubMed] [Google Scholar]
- Mason A., Nicoll A., Stratford K. Synaptic transmission between individual pyramidal neurons of the rat visual cortex in vitro. J. Neurosci. 1991;11:72–84. doi: 10.1523/JNEUROSCI.11-01-00072.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton B.L., Battaglia F.P., Jensen O., Moser E.I., Moser M.B. Path integration and the neural basis of the ‘cognitive map’. Nat. Rev. Neurosci. 2006;7:663–678. doi: 10.1038/nrn1932. [DOI] [PubMed] [Google Scholar]
- Melzer S., Michael M., Caputi A., Eliava M., Fuchs E.C., Whittington M.A., Monyer H. Long-range-projecting GABAergic neurons modulate inhibition in hippocampus and entorhinal cortex. Science. 2012;335:1506–1510. doi: 10.1126/science.1217139. [DOI] [PubMed] [Google Scholar]
- Mitchell S.J., Rawlins J.N., Steward O., Olton D.S. Medial septal area lesions disrupt theta rhythm and cholinergic staining in medial entorhinal cortex and produce impaired radial arm maze behavior in rats. J. Neurosci. 1982;2:292–302. doi: 10.1523/JNEUROSCI.02-03-00292.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuseki K., Sirota A., Pastalkova E., Buzsáki G. Theta oscillations provide temporal windows for local circuit computation in the entorhinal-hippocampal loop. Neuron. 2009;64:267–280. doi: 10.1016/j.neuron.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M., Bloom F.E. The 5-HT3 receptor is present in different subpopulations of GABAergic neurons in the rat telencephalon. J. Neurosci. 1997;17:3157–3167. doi: 10.1523/JNEUROSCI.17-09-03157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastoll H., Solanka L., van Rossum M.C., Nolan M.F. Feedback inhibition enables θ-nested γ oscillations and grid firing fields. Neuron. 2013;77:141–154. doi: 10.1016/j.neuron.2012.11.032. [DOI] [PubMed] [Google Scholar]
- Petsche H., Stumpf C., Gogolak G. [The significance of the rabbit’s septum as a relay station between the midbrain and the hippocampus. I. The control of hippocampus arousal activity by the septum cells] Electroencephalogr. Clin. Neurophysiol. 1962;14:202–211. doi: 10.1016/0013-4694(62)90030-5. [DOI] [PubMed] [Google Scholar]
- Ray S., Naumann R., Burgalossi A., Tang Q., Schmidt H., Brecht M. Grid-layout and theta-modulation of layer 2 pyramidal neurons in medial entorhinal cortex. Science. 2014;343:891–896. doi: 10.1126/science.1243028. [DOI] [PubMed] [Google Scholar]
- Roudi Y., Moser E.I. Grid cells in an inhibitory network. Nat. Neurosci. 2014;17:639–641. doi: 10.1038/nn.3704. [DOI] [PubMed] [Google Scholar]
- Steffenach H.A., Witter M., Moser M.B., Moser E.I. Spatial memory in the rat requires the dorsolateral band of the entorhinal cortex. Neuron. 2005;45:301–313. doi: 10.1016/j.neuron.2004.12.044. [DOI] [PubMed] [Google Scholar]
- Steward O., Scoville S.A. Cells of origin of entorhinal cortical afferents to the hippocampus and fascia dentata of the rat. J. Comp. Neurol. 1976;169:347–370. doi: 10.1002/cne.901690306. [DOI] [PubMed] [Google Scholar]
- Suh J., Rivest A.J., Nakashiba T., Tominaga T., Tonegawa S. Entorhinal cortex layer III input to the hippocampus is crucial for temporal association memory. Science. 2011;334:1415–1420. doi: 10.1126/science.1210125. [DOI] [PubMed] [Google Scholar]
- Sun C., Kitamura T., Yamamoto J., Martin J., Pignatelli M., Kitch L.J., Schnitzer M.J., Tonegawa S. Distinct speed dependence of entorhinal island and ocean cells, including respective grid cells. Proc. Natl. Acad. Sci. USA. 2015;112:9466–9471. doi: 10.1073/pnas.1511668112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takács V.T., Freund T.F., Gulyás A.I. Types and synaptic connections of hippocampal inhibitory neurons reciprocally connected with the medial septum. Eur. J. Neurosci. 2008;28:148–164. doi: 10.1111/j.1460-9568.2008.06319.x. [DOI] [PubMed] [Google Scholar]
- Tamamaki N., Yanagawa Y., Tomioka R., Miyazaki J., Obata K., Kaneko T. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J. Comp. Neurol. 2003;467:60–79. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- Tang Q., Burgalossi A., Ebbesen C.L., Ray S., Naumann R., Schmidt H., Spicher D., Brecht M. Pyramidal and stellate cell specificity of grid and border representations in layer 2 of medial entorhinal cortex. Neuron. 2014;84:1191–1197. doi: 10.1016/j.neuron.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tóth K., Borhegyi Z., Freund T.F. Postsynaptic targets of GABAergic hippocampal neurons in the medial septum-diagonal band of broca complex. J. Neurosci. 1993;13:3712–3724. doi: 10.1523/JNEUROSCI.13-09-03712.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga C., Lee S.Y., Soltesz I. Target-selective GABAergic control of entorhinal cortex output. Nat. Neurosci. 2010;13:822–824. doi: 10.1038/nn.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winson J. Loss of hippocampal theta rhythm results in spatial memory deficit in the rat. Science. 1978;201:160–163. doi: 10.1126/science.663646. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.