Abstract
Chronic Q fever caused by Coxiella burnetii is uncommon in the United States and is most often associated with infective endocarditis. We present a 52-year-old woman with a history of aortic valve replacement and rheumatoid arthritis treated with Etanercept with chronic Q fever manifesting as prosthetic valve infective endocarditis. Explanted valve tissue showed organisms confirmed to be C. burnetii by PCR (polymerase chain reaction) sequencing. She subsequently reported consumption of unpasteurized cow milk which was the likely source of C. burnetii. She continues to do well 6 months after valve replacement on oral doxycycline and hydroxychloroquine.
Keywords: Coxiella burnetii endocarditis, Q fever endocarditis, Endocarditis and Etanercept, Unpasteurized milk infection
Introduction
Q fever is caused by the small gram-negative intracellular bacterium, Coxiella burnetii. It is rare and there are approximately 40 cases reported annually in the United States [1]. Chronic Q fever is also rare, occurring in <5% of persons with acute infection and most commonly occurs as infective endocarditis (IE) or vascular infection. We present a 52-year-old woman from Michigan with chronic Q fever presenting as prosthetic aortic valve infective endocarditis. PCR testing for bacterial organisms performed on the explanted cardiac valve tissue confirmed the diagnosis of C. burnetii IE [2]. Subsequent history identified consumption of raw milk from a shared cow in a local farm as the likely source of the pathogen.
Clinical history
A 52-year-old Caucasian woman, who is an educator from Kalamazoo, Michigan, was referred to the Cleveland Clinic with a chief complaint of fever, chills, and progressive dyspnea on exertion of several months duration. Her fever, chills, and night sweats were more prominent in the last two weeks prior to evaluation. She also complained of abdominal pain which had increased markedly in the last one month associated with nausea and non-bloody, non-bilious vomiting with reduced oral intake and involuntary weight loss of 25 pounds over this period. She has rheumatoid arthritis (RA) and has been taking Etanercept for the last 15 years. She underwent an aortic valve replacement due to aortic regurgitation 12 years prior to presentation at our institution. She denied exposure to sick contacts, invasive dental procedures, intravenous drug use, tick bites or recent travel. She has an indoor pet cat.
On examination, she appeared alert and well-oriented but was weak and fatigued. She had a blood pressure of 104/57 mmHg and was tachycardic with a pulse rate of 135 bpm. On cardiovascular exam, there was a 3/6 diastolic murmur appreciated in the left sternal area with the presence of normal S1 and S2. There was a well-healed median sternotomy scar from her prior open heart surgery. On abdominal examination, the spleen was palpable at 23 cm from the costal margin with tenderness to deep palpation in the left upper quadrant.
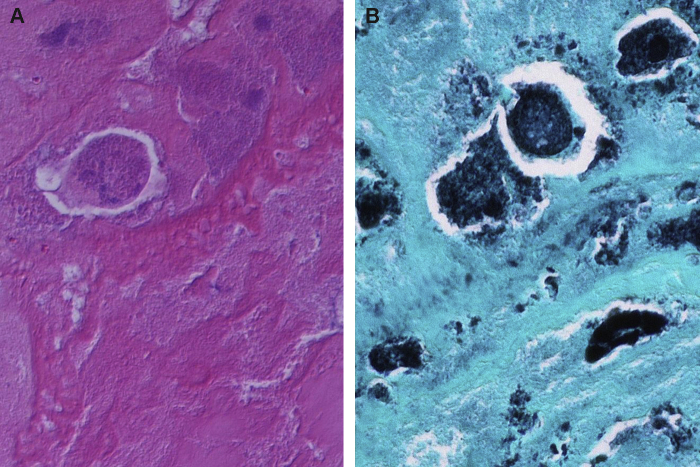
During the course of admission, laboratory tests showed a normocytic normochromic anemia with hemoglobin of 8.4 g/dL (reference range 12.1–15.1 g/dL) and a normal white blood cell count of 6.81 k/μl (reference range 4.5–11.0 k/μl). Her splenomegaly was initially thought to be neoplastic or rheumatologic in etiology and thus she was referred for a bone marrow biopsy. Pathologic review of her bone marrow specimen revealed a hypercellular marrow but no clonal process or ring granulomas were identified. A single blood culture (one of the four) was positive for growth of coagulase-negative Staphylococcus. This result prompted a transthoracic echocardiogram (TTE) which was followed by a transesophageal echocardiogram (TEE) given high clinical suspicion for IE. These tests showed moderate (2–3+) prosthetic aortic regurgitation and an aortic root abscess. She was started empirically on vancomycin and ceftriaxone and was scheduled for open heart surgery. The patient underwent aortic valve and root replacement with a 22-mm aortic homograft on day 3 of admission. Pathologic examination of the surgical specimen showed abundant fibrinous exudate with mild inflammation, mostly macrophages. A Gomori's methenamine silver stain demonstrated intracellular and extracellular small coccobacillary organisms (Fig. 1), but no microorganisms were apparent on the Gram and Periodic acid-Schiff stain. 16S rDNA sequencing of the valves came back positive for C. burnetii on post-operative day 10. Further retrospective history taking from the patient revealed that she had imbibed unpasteurized cow milk two years previously for a period of 2 months via cow sharing at a local farm in Michigan. C. burnetii titers were consistent with chronic Q fever (high titers to IgG phase 1 titers) (Table 1). She was treated with oral doxycycline (100 mg orally twice daily) and hydroxychloroquine (200 mg three times daily) after checking for glucose-6-phosphate dehydrogenase deficiency. At six months follow up, she has recovered with normal ophthalmologic evaluations (to assess for retinal toxicity) with plans to complete 18 months of treatment.
Fig. 1.
Histology of the aortic valve. (A) High power magnification of the hematoxylin-eosin stained section showed basophilic material within degenerated macrophages in a fibrinous background (original magnification 600×). (B) Small coccobacillary forms, mostly following the contours of macrophages consistent with intracellular localization and highly suggestive of bacterial organisms, were observed in the Gomori methenamine silver stain (original magnification 600×).
Table 1.
Serum serologies detecting phase 1 and phase 2 antibodies to Coxeilla antigen.
| IgG | IgM | IgA | |
|---|---|---|---|
| On admission: (reference <1:16) | |||
| Phase 1 | 1:1024 | 1:64 | >1:1024 |
| Phase 2 | 1:1024 | 1:256 | <1:16 |
| After 2 months of treatment: (reference <1:16) | |||
| Phase 1 | 1:1024 | 1:1024 | <1:16 |
| Phase 2 | 1:1024 | 1:1024 | <1:16 |
Discussion
Q fever is a zoonotic rickettsiosis caused by C. burnetii that is uncommon in the United States with approximately 40 cases reported annually [1]. It presents as an either acute or chronic infection and is manifested as a nonspecific flu-like illness in most patients. Acute infection produces a pneumonia or infectious hepatitis syndrome in its more severe form. Less than 5% of patients with documented acute infection develop chronic infection [3]. The chronic form of infection presents as an endovascular infection causing endocarditis, mycotic aneurysm, or infected vascular graft [3]. 1–5% of acute Q fever and up to 60–80% of chronic cases can develop endocarditis [4]. Chronic Q fever is uniformly fatal if not treated.
The largest documented outbreak of Q fever occurred in the Netherlands from 2007 to 2010 where more than 4000 cases were identified. The etiology of this outbreak was felt to be increased contact between goat farms and high-population density areas [5]. Aerosol transmission of bacteria from parturient animals and contact with bodily fluids of infected animals are the primary modes of transmission. Oral consumption of raw milk is considered a risk factor for transmission, but until recently was felt to play a minor role [3]. Raw milk consumption has been associated with seroconversion but there was insufficient evidence of it causing clinical infection [6]. Coxiella spores are extremely infectious and resistant to heat which emphasizes the need for pasteurization to effectively kill this species in milk products. Pasteurization for 30 min at 63 °C (145 °F) or 15 s at 72 °C (161 °F) can lead to eight decimal reductions in the number of Coxiella spores [6]. A recent outbreak in Michigan, where 5 cases were detected over a three month period in 2011, has been reported where the only predisposing condition common to all infections was consumption of unpasteurized milk [7]. Such consumption is common in farm workers, cow share owners, or those buying milk from bulk tanks.
In a French study of 92 patients with chronic Q fever between 1982 and 1990, the risk factors for infection include immunosuppression (20.2%), preexisting heart or vascular conditions (88.4%) and preexisting valvulopathy (88%) [4]. Prosthetic valves are associated with increased relative risk compared to the other factors for acquiring endocarditis amongst the chronic Q fever patients [8]. Studies show that there is equal involvement of both aortic and mitral valves [4].
Tumor necrosis factor (TNF) plays an important role in the immune response against this pathogen [9]. Experiments using an animal model consisting of knockout mice found that there was severe and fatal chronic Q fever in severe combined immunodeficiency (SCID) mice. Florid multiplication of viable microorganisms has been noted within the macrophages of infected mice [10]. The artificial immunosuppression of mice in these studies have led to postulation that administration of TNF blockers may put patients at greater risk for chronic Q fever. The literature regarding Q fever and biologics is sparse. One case has been reported of a RA patient on Etanercept who did not have any acute phase of illness and was diagnosed after valve cultures obtained at the time of open heart surgery came back positive [11]. Our patient was on Etanercept for approximately 15 years. Given its mechanism of TNFα inhibition, cytokine production by the macrophages and lymphocytes is decreased [9]. Since RA patients on immunotherapy are considered at increased risk of chronic infection, they need to be carefully monitored and counseled about decreasing risk of exposure [12].
The main symptoms of Q fever endocarditis are fever, fatigue, weight loss, night sweats, and hepatosplenomegaly [3]. A study from France which included 92 patients with chronic Q fever revealed anemia (60% of cases), increased erythrocyte sedimentation rate (100% of cases), and thrombocytopenia (50% of cases). Liver enzymes may also be elevated [4]. Blood cultures are typically negative given the special methods of culture required for isolation. Echocardiography shows cardiac vegetations only in 12% of cases [4].
C. burnetii demonstrates a unique characteristic called phase variation [5]. Phase 1 is the virulent form with phase 2 being the avirulent form which is demonstrated by serial passage in cell culture or embryonated hen eggs. Only phase 1 organisms affect humans but antibodies are formed for both phases which are detected using serology. Phase variation occurs due to the presence of lipopolysaccharide. Development of antibodies only occurs to the phase 2 antigen in the acute form of infection [13].
Chronic Q fever is established if infection persists for more than 6 months and there is a presence of IgG and IgA to phase 1 and phase 2 antigens. The presence of IgG phase 1 titer >1:800 along with IgA phase 1 titer of >1:25 is considered for the diagnosis of chronic disease [5]. However there have been reports of inadequate serologic response in chronic Q fever cases [14]. Studies have shown the overlap in both acute and chronic form serologic titers. Hence the use of serologic titers in distinguishing the stage of disease or outcome is problematic. They are also not a useful tool for deciding the duration of treatment [15]. Shortcomings of serologic testing and culture as well as prolonged turn around times for these tests highlights the potential benefit of PCR on pathologic specimens [2], [5]. Histologic examinations of infected cardiac valves show calcification, fibrosis, and typically pauci-inflammatory changes with very small or absent vegetations [16].
The usual medical treatment of chronic Q fever is doxycycline and hydroxychloroquine for a period of 24 and 18 months in cases of prosthetic valve and native valve endocarditis, respectively [8]. C. burnetii needs an acidic pH to replicate inside the cellular phagolysosomes. Hydroxychloroquine is lysosomotropic which increases the pH of the phagolysosomes, thus decreasing bacterial replication. Doxycycline is bactericidal in its activity and when combined with hydroxychloroquine is very effective. Cotrimoxazole is an alternative used in pregnancy and for children below 8 years of age. Rifampicin, Quinolones and Macrolides are not as effective and there is limited evidence for their usage as alternative regimens [17]. Cardiac surgery is usually recommended in cases of heart failure or valvular abscesses. Reports exist of cases with clinical and serological cure without adjunctive surgical treatment [3], [8]. Treatment and serologic follow-up are controversial with most experts agreeing that monitoring for up to five years may be appropriate [8].
Conflicts of interest
None declared.
References
- 1.Anderson A., Bijlmer H., Fournier P.E., Graves S., Hartzell J., Kersh G.J. Diagnosis and management of Q fever – United States, 2013: recommendations from CDC and the Q fever working group. MMWR Recomm Rep. 2013;62:1–30. [PubMed] [Google Scholar]
- 2.Shrestha N.K., Ledtke C.S., Wang H., Fraser T.G., Rehm S.J., Hussain S.T. Heart valve culture and sequencing to identify the infective endocarditis pathogen in surgically treated patients. Ann Thorac Surg. 2015;99:33–37. doi: 10.1016/j.athoracsur.2014.07.028. [DOI] [PubMed] [Google Scholar]
- 3.Maurin M., Raoult D. Q fever. Clin Microbiol Rev. 1999;12:518–553. doi: 10.1128/cmr.12.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brouqui P., Dupont H.T., Drancourt M., Berland Y., Etienne J., Leport C. Chronic Q fever. Ninety-two cases from france, including 27 cases without endocarditis. Arch Intern Med. 1993;153:642–648. doi: 10.1001/archinte.153.5.642. [DOI] [PubMed] [Google Scholar]
- 5.Schneeberger P.M., Wintenberger C., van der Hoek W., Stahl J.P. Q fever in the netherlands – 2007–2010: what we learned from the largest outbreak ever. Med Mal Infect. 2014;44:339–353. doi: 10.1016/j.medmal.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Cerf O., Condron R. Coxiella burnetii and milk pasteurization: an early application of the precautionary principle? Epidemiol Infect. 2006;134:946–951. doi: 10.1017/S0950268806005978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Signs K.A., Stobierski M.G., Gandhi T.N. Q fever cluster among raw milk drinkers in michigan, 2011. Clin Infect Dis. 2012;55:1387–1389. doi: 10.1093/cid/cis690. [DOI] [PubMed] [Google Scholar]
- 8.Million M., Thuny F., Richet H., Raoult D. Long-term outcome of Q fever endocarditis: a 26-year personal survey. Lancet Infect Dis. 2010;10:527–535. doi: 10.1016/S1473-3099(10)70135-3. [DOI] [PubMed] [Google Scholar]
- 9.Schoffelen T., den Broeder A.A., Nabuurs-Franssen M., van Deuren M., Sprong T. Acute and probable chronic Q fever during anti-TNFalpha and anti B-cell immunotherapy: a case report. BMC Infect Dis. 2014;14 doi: 10.1186/1471-2334-14-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andoh M., Naganawa T., Hotta A., Yamaguchi T., Fukushi H., Masegi T. SCID mouse model for lethal Q fever. Infect Immun. 2003;71:4717–4723. doi: 10.1128/IAI.71.8.4717-4723.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kampschreur L.M., Hoornenborg E., Renders N.H., Oosterheert J.J., Haverman J.F., Elsman P. Delayed diagnosis of chronic Q fever and cardiac valve surgery. Emerg Infect Dis. 2013;19:768–770. doi: 10.3201/eid1905.120353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schoffelen T., Kampschreur L.M., van Roeden S.E., Wever P.C., den Broeder A.A., Nabuurs-Franssen M.H. Coxiella burnetii infection (Q fever) in rheumatoid arthritis patients with and without anti-TNFalpha therapy. Ann Rheum Dis. 2014;73:1436–1438. doi: 10.1136/annrheumdis-2014-205455. [DOI] [PubMed] [Google Scholar]
- 13.Hackstadt T., Peacock M.G., Hitchcock P.J., Cole R.L. Lipopolysaccharide variation in coxiella burnetti: intrastrain heterogeneity in structure and antigenicity. Infect Immun. 1985;48:359–365. doi: 10.1128/iai.48.2.359-365.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barten D.G., Delsing C.E., Keijmel S.P., Sprong T., Timmermans J., Oyen W.J. Localizing chronic Q fever: a challenging query. BMC Infect Dis. 2013;13:413. doi: 10.1186/1471-2334-13-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Alarcon A. Q fever endocarditis: does serology predict outcome? Curr Infect Dis Rep. 2012;14:350–358. doi: 10.1007/s11908-012-0264-6. [DOI] [PubMed] [Google Scholar]
- 16.Lepidi H., Houpikian P., Liang Z., Raoult D. Cardiac valves in patients with Q fever endocarditis: microbiological, molecular, and histologic studies. J Infect Dis. 2003;187:1097–1106. doi: 10.1086/368219. [DOI] [PubMed] [Google Scholar]
- 17.Kersh G.J. Antimicrobial therapies for Q fever. Expert Rev Anti Infect Ther. 2013;11:1207–1214. doi: 10.1586/14787210.2013.840534. [DOI] [PMC free article] [PubMed] [Google Scholar]